Abstract
Objectives
Biological medications have been used with an increasing frequency to treat rheumatological diseases. Autoimmune events can be induced by these drugs, such as psoriasiform lesions, alopecia, lupus and, vasculitis, which more often affects the skin (small-sized vessels) and eventually other organs. In this review, we describe the clinical profile of patients with vasculitis induced by the main biological agents used in rheumatology.
Patients and methods
We performed a systematic review following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) recommendations. The PubMed database was used for searching eligible articles. We included case reports, case series, and letter to the editor of patients on anti-tumor necrosis factor-alpha (anti-TNF-a) molecules, as well as tocilizumab, ustekinumab, secukinumab, rituximab, and abatacept, who had vasculitis induced by these agents.
Results
Eighty-one articles were included for final analysis (n=89). Twenty-seven patients were using infliximab, 20 adalimumab, 18 etanercept, seven secukinumab, four certolizumab, four rituximab, three golimumab, three ustekinumab, two abatacept, and one tocilizumab. Unspecific leukocytoclastic vasculitis (LCV) was the most common type of vasculitis (n=37), followed by anti-neutrophil cytoplasmic antibody (ANCA)- associated vasculitis (n=16). The medication was replaced with another biological molecule in 23 cases, with only four relapses. In six cases, the biological was maintained, but vasculitis worsened/persisted in one case, being necessary drug removal.
Conclusion
Infections, infusion reaction, cancer, and autoimmune events are well-known side effects of biological therapy. This review demonstrates that vasculitis is another adverse effect of this type of therapy, particularly the anti-TNF-a molecules, and LCV the most reported type of vasculitis.
Keywords: Biological therapy, drug-induced event, vasculitis
Introduction
Biological therapy has been incorporated into clinical practice, particularly in the management of rheumatological diseases. These medications have a good safety profile, as they target specific proteins or receptors involved in the pathogenesis of the disease. On the other hand, numerous immunological adverse events have also been reported with these drugs.[1]
The first immunological adverse effect secondary to biological therapy reported in the literature was a lupus-like condition in a patient with rheumatoid arthritis (RA) using infliximab.[2] Later on, several reports with other inhibitors of tumor necrosis factor-alpha (anti-TNF-α) and other biological classes were described with the development of lupus, cutaneous vasculitis, sarcoidosis, demyelinating diseases, interstitial lung disease, psoriasis, inflammatory eye disease, autoimmune hepatitis, among others.[3]
Drug-associated vasculitis is a well-defined entity and had its nomenclature recognized by the 2012 Chapel Hill International Consensus Conference.[4] The most affected organ is the skin, with the involvement of small-sized vessels. However, pulmonary, renal, hepatic, central nervous system involvement may occur. A significant difference between drug-induced and idiopathic vasculitis is that, in most cases, the removal of causal factor (drug) is enough to reverse the autoimmune phenomenon. Classical vasculitis-inducers are propylthiouracil, levamisole, hydralazine, minocycline, montelukast, and statins.[5]
In this review, we describe the cases of vasculitis induced by the main biological agents used in rheumatology, emphasizing the clinical profile of these patients, the most frequently involved molecules, and the outcome of this complication.
Patients and Methods
We performed a systematic review in accordance with the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).[6]
Inclusion criteria
• Case reports of patients using biological medications mostly used in rheumatology (anti-TNF-α agents as well as tocilizumab, ustekinumab, secukinumab, rituximab and abatacept) and with a diagnosis of drug-induced vasculitis. Of note, vasculitis secondary to these agents used for other indications than rheumatological conditions were also recorded.
• A clear causal and temporal relationship between the onset of biological agent and the pathological condition was considered necessary to inclusion, regardless the timeframe for its development. Also, the reintroduction of medication with relapse of vasculitis favors the diagnosis.
• Histopathological confirmation was required in cases of cutaneous involvement alone.
Exclusion criteria
• Presence of an alternative clinical condition that may explain the onset of vasculitis; e.g., an infectious disease, overlap of autoimmune conditions suspected, when vasculitis persists despite discontinuation of medication
• Case reports of patients diagnosed with vasculitis associated with lupus-like condition
• Studies other than a case report.
Strategy for article search
The MEDLINE (PubMed) database was used to search for potentially eligible articles published in English, Spanish or Portuguese until November 2020. To identify all relevant articles published about the proposed theme (case report, case series and letter to the editor), the following descriptors were used: "Vasculitis" combined with “Biological Therapy”, “Anti-TNF”, “Etanercept”, “Infliximab”, “Adalimumab”, “Certolizumab”, “Golimumab”, “Rituximab”, “Abatacept”, “Tocilizumab”, “Ustekinumab” and “Secukinumab”.
The reference lists of the included studies were also checked for additional relevant studies.
Data extraction and analysis
Studies were selected based on their titles and abstracts and those that met the eligibility criteria were separated for further analysis.
Two authors read the full articles selecting those that were compatible with the selection criteria. In case of disagreement between the authors, a third author was consulted.
Data extraction was made using a standard form and a descriptive analysis of the data was performed.
Results
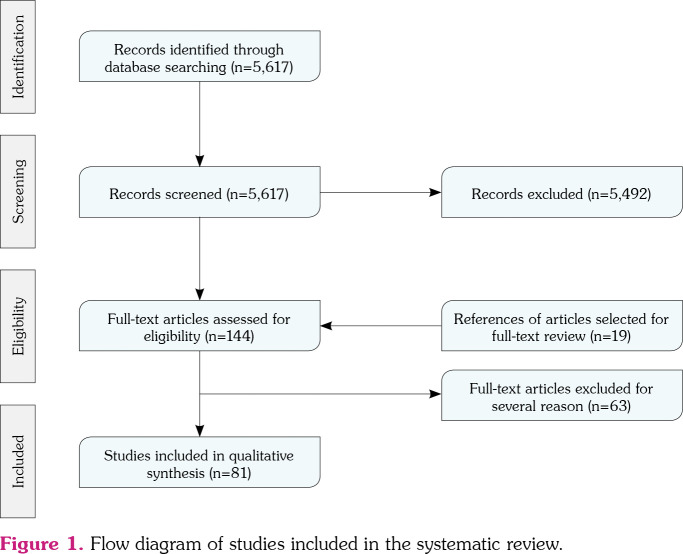
We identified a total of 5,617 articles in the initial search; however, 5,492 were excluded by title and abstract screening and 125 were selected for full-text reviewing. Additionally, 19 articles were retrieved from secondary references. Finally, 81 studies fulfilled the selection criteria (Figure 1), with a total of 89 patients reported.
Figure 1. Flow diagram of studies included in the systematic review.
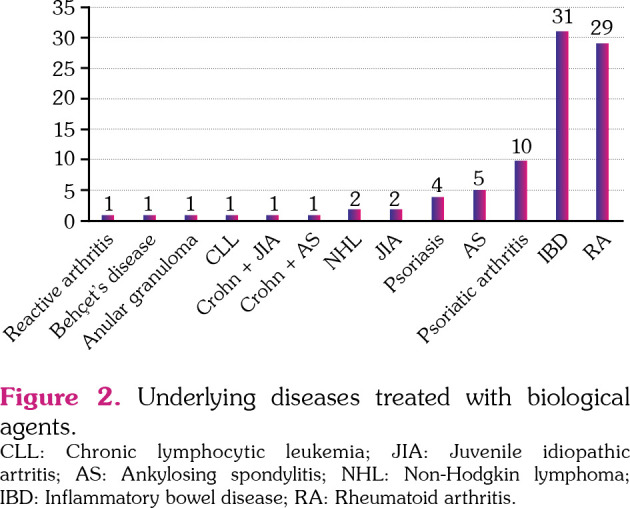
Most patients were women (56 [62.9%]) and median age of the entire population was 44 years (range, 16 to 82). Anti-TNF-α agents were involved in the majority of the cases, with infliximab and adalimumab being the most common agents reported (Table 1). The most common underlying diseases were inflammatory bowel disease (IBD) with 31 cases (34.8%) and RA with 29 cases (32.6%). Other underlying diseases are described in Figure 2.
Table 1. Demographic data and types of biological agents causing vasculitis.
| Descending order | ||||
| Demography | n | % | Median | Range |
| Age (year) | 44 | 16-82 | ||
| Sex | ||||
| Female | 56 | |||
| Male | 33 | |||
| Biological agent | ||||
| Infliximab | 27 | 30.3 | ||
| Adalimumab | 20 | 22.4 | ||
| Etanercept | 18 | 20.2 | ||
| Secukinumab | 7 | 7.9 | ||
| Rituximab | 4 | 4.5 | ||
| Certolizumab | 4 | 4.5 | ||
| Golimumab | 3 | 3.4 | ||
| Ustekinumab | 3 | 3.4 | ||
| Abatacept | 2 | 2.2 | ||
| Tocilizumab | 1 | 1.1 | ||
Figure 2. Underlying diseases treated with biological agents. CLL: Chronic lymphocytic leukemia; JIA: Juvenile idiopathic artritis; AS: Ankylosing spondylitis; NHL: Non-Hodgkin lymphoma; IBD: Inflammatory bowel disease; RA: Rheumatoid arthritis.
Leukocytoclastic vasculitis (LCV) was the most common type of vasculitis, observed in 37 cases (41.6%), followed by 16 cases (18%) of anti-neutrophil cytoplasmic antibody (ANCA)- associated vasculitis, 13 Henoch-Schönlein purpura (HSP), five Takayasu arteritis (TA), three Behçet's disease (BD), three cutaneous lymphocytic vasculitis, two giant cell arteritis (GCA), two hypocomplementemic urticarial vasculitis, one cutaneous medium-sized vessel vasculitis, one retinal vasculitis, one erythema elevatum diutinum, one cutaneous and intestinal vasculitis, one peripheral neuropathy secondary to necrotizing vasculitis, one digital vasculitis, one cutaneous polyarteritis nodosa (cPAN), and one granulomatous vasculitis in the tongue (Table 2). The time frame from the start of therapy and vasculitis appearance ranged from immediately after medication infusion to 10 years of drug use. Causative agents were maintained in six cases with worsening in one patient, despite the use of cyclophosphamide pulse therapy. In the remaining five cases, one improved with topical corticosteroid, one improved with prednisone, one improved after reducing the dose of the biological, one improved with colchicine and one had spontaneous improvement. Reintroduction of the same medication was reported in 10 patients, with seven recurrences. Medication was replaced with another biological agent in 23 cases, with only four relapses (Table 3). From the entire population, 61 patients (68.5%) required corticosteroids use to improve their condition.
Table 2. Types of vasculitis induced by biological therapy.
| Induced vasculitis | Total of cases reported |
| Leucocytoclastic vasculitis | 37 |
| ANCA-associated vasculitis | 16 |
| Henoch-Schönlein purpura | 13 |
| Takayasu arteritis | 5 |
| Behçet's disease | 3 |
| Lymphocytic cutaneous vasculitis | 3 |
| Giant cell arteritis | 2 |
| Hypocomplementemic urticarial vasculitis | 2 |
| Erythema elevatum diutinum | 1 |
| Retinal vasculitis | 1 |
| Cutaneous and intestinal vasculitis | 1 |
| Peripheral neuropathy secondary to necrotizing vasculitis | 1 |
| Cutaneous medium-sized vessel vasculitis | 1 |
| Digital vasculitis | 1 |
| Cutaneous polyarteritis nodosa | 1 |
| Granulomatous vasculitis in the tongue | 1 |
| Total | 89 |
| ANCA: Anti-neutrophil cytoplasmic antibody. | |
Table 3. Outcome of the patients with vasculitis induced by a biological molecule in whom the drug was maintained or rechallenged with the same or other biological agent.
| n | |
| Total of patients who had the drug suspended and later rechallenged with an alternative biologic agent | |
| No. of patients | 23 |
| Recurrence of the vasculitis | 4 |
| No recurrence of the vasculitis | 19 |
| Total of patients who had the drug suspended and later rechallenged with the same biologic agent | |
| No. of patients | 10 |
| Recurrence of the vasculitis | 7 |
| No recurrence of the vasculitis | 3 |
| Outcome of patients who had the medication maintained | |
| No. of patients | 6 |
| Worsened of the vasculitis | 1 |
| Improved of the vasculitis | 5 |
Anti-tumor necrosis factor-alpha therapy
We found 72 cases of vasculitis induced by anti-TNF-α agents. The agents most associated in descending order were infliximab (n=27), adalimumab (n=20), etanercept (n=18), certolizumab (n=4), and golimumab (n=3). The most common underlying diseases in these patients were Crohn's disease (CD), RA, and spondyloarthritis. A total of 63.8% of the patients were women with a median age of 48 (range, 16 to 82) years.[7-72]
In addition, LCV was the most frequent type of vasculitis induced by infliximab (14/27) followed by ANCA-associated vasculitis (5/27) and TA (2/27). Totally, 41 patients had their medication halted, whereas it was maintained in four. Biological agents were changed in 20 cases with only four recurrences and rechallenge with the same agent was attempted in seven cases with five recurrences.
Adalimumab was the cause of vasculitis in 20 cases. Twelve of 20 patients had CD, six of 20 patients had RA, one patient had granuloma annulare, and one patient had ulcerative colitis (UC). The vasculitis types were LCV (9/20), HSP (5/20), ANCA-associated vasculitis (3/20), TA (2/20), and GCA (1/20). Nineteen patients discontinued the medication, there was no information about drug withdrawal in one patient. Five patients had their medications resumed, two without recurrence of vasculitis (using corticosteroids), three with recurrence of the vasculitis (only one using prednisone). Four patients had their medications replaced without new event (two with ustekinumab, one with infliximab and one with etanercept). Nine patients used systemic corticosteroids, three used pulse therapy with cyclophosphamide and methylprednisolone (one associated with sulfamethoxazole-trimethoprim and one with plasmapheresis), one methotrexate and prednisone and, one colchicine and prednisone. In five cases, improvement occurred after drug suspension and information was not available in one.
Eighteen patients had etanercept-induced vasculitis, and 11 of these had RA. Also, ANCA- associated vasculitis (6/18) was the most common type of vasculitis followed by HSP (5/18). Two patients developed LCV, two lymphocytic cutaneous vasculitis, one GCA, one urticarial hypocomplementemic vasculitis, and one digital vasculitis. Two patients initially maintained etanercept. Rechallenge with the same agent was attempted in two cases, all of them with recurrences. Five patients had their medication changed, two of them with recurrence of the vasculitis-one using prednisone. Six patients used only systemic corticosteroids, two received pulse therapy with methylprednisolone and cyclophosphamide, one prednisolone and cyclophosphamide pulse therapy, one prednisolone and dapsone, two antihistamine drugs (one associated with dapsone), one cyclophosphamide, one prednisone + rituximab, one pulse therapy with methylprednisolone followed by prednisone plus azathioprine and methotrexate, one methylprednisolone 1 g/day for three days followed by prednisone 1 mg/kg daily plus colchicine, one improved after drug withdrawal, and one had missing data.
Four patients (three with RA and one with CD + ankylosing spondylitis [AS]) developed vasculitis using certolizumab. Two developed LCV, one ANCA-associated vasculitis, and one urticarial hypocomplementemic vasculitis. All patients discontinued certolizumab, one evolved with spontaneous improvement after suspension of the drug and the others improved after: (i) use of oral methylprednisolone + rituximab (ANCA vasculitis); (ii) prednisone + dapsone; (iii) oral prednisolone + topical corticosteroids + antihistamine. One patient started another biological agent (rituximab), without recurrence of vasculitis.
Vasculitis secondary to golimumab was reported in three patients (two with AS and one with RA). One patient developed LCV, one ANCA-associated vasculitis and the other one TA. All patients had their biological suspended and all used systemic corticosteroid (one associated with methotrexate and one with cyclophosphamide pulse therapy), evolving with good clinical response.
Secukinumab
We found seven cases of vasculitis associated with secukinumab use. Four had psoriatic arthritis, two cutaneous psoriasis, and one AS. Three patients developed BD, two LCV and the remaining had cutaneous and intestinal vasculitis and HSP. All suspended the biological agent, two used oral corticosteroid therapy, one used prednisone + colchicine, one prednisone + cyclosporine, one pulse therapy with methylprednisolone for three days, one colchicine and one methotrexate + methylprednisolone + ranitidine + antihistamine. In two patients, another biological agent was introduced without recurrence (etanercept and infliximab).[73-78]
Rituximab
Four patients developed LCV due to rituximab, two had non-Hodgkin lymphoma, one chronic lymphocytic leukemia, and one RA. All of them had their medication suspended, two with improvement only after drug withdrawal and two with systemic corticosteroid (single dose of intravenous dexamethasone and prednisolone). One patient was exposed again to the drug with recurrence of skin lesions.[79-82]
Abatacept
Two patients developed vasculitis while using abatacept, one with psoriatic arthritis and one with juvenile idiopathic arthritis (JIA). One patient developed erythema elevatum diutinum with improvement only with topical corticosteroids and the other one developed cutaneous medium vessel vasculitis with improvement after drug withdrawal and prednisone use.[83,84]
Ustekinumab
Three patients with CD developed paradoxical vasculitis during ustekinumab use, two developed LCV and one cutaneous polyarteritis nodosa. In one case, the medication was maintained and after the introduction of colchicine the condition improved. In two cases, the medication was discontinued and reintroduced with recurrence in one case, despite the initial improvement with prednisone and with no recurrence in the other one.[85,86]
Tocilizumab
A case of LCV was described in a patient with RA using tocilizumab. The condition improved after drug withdrawal and introduction of prednisone. Abatacept was introduced without recurrence.[87]
Discussion
Despite the excellent safety profile, paradoxical autoimmune events, including vasculitis, secondary to biological molecules have been increasingly described, as demonstrated in this review. These complications usually occur between one month and one year after the initiation of the drug.[88] Still, there are reports of vasculitis initiated soon after its infusion.[35,58] Its actual incidence is not well known, as sometimes it is difficult to differentiate it from the manifestations of the underlying disease itself.
The mechanisms related to biologicalinduced vasculitis are poorly understood. Some hypotheses have been suggested, such as deposit of anti-TNF-α/TNF-α immunocomplexes in small capillaries, leading to the activation of the complement system and triggering a hypersensitivity reaction; imbalance in the cytokine pool, as it occurs after blocking a cytokine, when other pro-inflammatory cytokines may prevail leading to the emergence of a new disease.[88]
In 2006, the Study Group on Autoimmune Diseases (GEAS) of the Spanish Society of Internal Medicine created the BIOGEAS, a multicenter registry to collect data on biological agents in autoimmune diseases.[89] A subproject of this registry was designed to evaluate the data on autoimmune manifestations secondary to these molecules. In this registry, until May 2017, there were 291 cases of biological-induced vasculitis described. The most common underlying disease was RA/JIA observed in 232 (80%) cases, followed by IBD (n=27) and psoriasis/psoriatic arthritis (n=10). The most frequent biological drugs involved were anti-TNF-α therapy (n=273 [94%]), particularly etanercept (n=119) and infliximab (n=108).[90] The most described disorders were isolated cutaneous vasculitis (n=187), isolated neurological involvement (n=34), ANCA-related vasculitis (n=12), large-vessel vasculitis (n=7), HSP (n=7), and polyarteritis nodosa (n=3).[90] These findings are similar to what we found in our review with anti-TNF therapy being the biological agent most associated with vasculitis (80.8x94%), although infliximab (30.3%) and adalimumab (22.4%) were the most frequent agents in our review. Only isolated cases have been described in association of other biologics; therefore, it is not possible to specify the incidence and prevalence of this condition. In BIOGEAS, four cases were described after administration of rituximab and two after tocilizumab. No events were related to the other agents studied in our research.
Sokumbi et al.[91] performed a 13-year retrospective cohort with 345 patients using anti-TNF-α therapy, and they identified eight cases of vasculitis induced by this molecule. The main indication for this therapy was RA (50%). Similar to our analysis, the mean age at diagnosis was 48.5 (range, 18 to 70) years and the female-male ratio was 3:1. Skin involvement occurred in five patients (63%) - four had palpable purpura, two ulcerated lesions, and one erythematous macules or blisters. Peripheral nerve involvement was present in four patients (two mononeuritis multiplex, one asymmetric polyneuropathy and one dysesthesia) and, only one patient had renal involvement with microscopic hematuria and proteinuria. Similar to the present review, the most frequent organ involved was the skin and the agents most commonly used were infliximab (n=5), followed by etanercept (n=2) and adalimumab (n=1).
Henoch-Schönlein purpura is a systemic vasculitis that presents with purpura, arthralgia, and gastrointestinal and renal involvement. It affects children more than adults, usually after an upper respiratory tract infection. The clinical course is more severe in adults with higher tendency to progress to renal failure. In this systematic review, 13 patients were diagnosed with HSP after onset of biological therapy, the youngest patient was 19 years old. Of these patients, 11 had their medication suspended, and all evolved with an improvement in their clinical condition. Twelve patients used systemic corticosteroids.
In our review, we did not include the cases from BIOGEAS registry nor from Sokumbi et al.’s[91] study, as we could not retrieve details of clinical manifestations of the patients. In addition, the BIOGEAS registry evaluated not only vasculitis, but also other types of induced autoimmune events.
In general, paradoxical reactions to biological therapy have a good prognosis with complete resolution of the symptoms in 75% of the cases after drug interruption.[88] Curiously, not all instances require drug withdrawal. Thus, in non-severe cutaneous vasculitis, the medication can be maintained and the patient is closely monitored. On the other hand, in case of life-threatening vasculitis, in addition to suspension of the drug, corticosteroid pulse therapy and even other immunosuppressive agents may be necessary.
There are no consistent data on risk factors that favor the appearance of biological-induced vasculitis. Still, it has been suggested that the positivity of ANCA and cryoglobulins during such therapy may be a marker for developing this complication.[88] Further studies are needed to identify risk factors and genetic background, which may allow identifying those patients at higher risk for these immunological events.
Nonetheless, our review has some limitations. Although we made efforts to select only articles with an evident causal relationship between the drug and autoimmune event and included only case reports with biopsies, whenever possible, we cannot ensure that this association is true or is just an overlap of autoimmune disorders or even a rare manifestation of the underlying disease. Temporal relationship, underlying illness in remission, improvement after drug withdrawal, and recurrence with reintroduction are some of the factors that support the diagnosis of biological-induced vasculitis.
In conclusion, although rare, vasculitis secondary to biological agents is a reality. Exclusion of underlying disease activity itself, infectious complications, or a paraneoplastic phenomenon allows us to confirm this entity. Depending on the severity of the manifestation, the biological therapy may or may not be suspended, as its termination can lead to the reactivation of the underlying disease. Further studies addressing to predisposing factors and biomarkers for the development of such complication are urgently needed.
Footnotes
Conflict of Interest: The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Author Contributions: Conception, analysis, drafting: C.S.C.D., A.S.P., M.B.S.
Financial Disclosure: The authors received no financial support for the research and/or authorship of this article.
References
- 1.Ingrasciotta Y, Cutroneo PM, Marcianò I, Giezen T, Atzeni F, Trifirò G. Safety of biologics, including biosimilars: Perspectives on current status and future direction. Drug Saf. 2018;41:1013–1022. doi: 10.1007/s40264-018-0684-9. [DOI] [PubMed] [Google Scholar]
- 2.Charles PJ, Smeenk RJ, De Jong J, Feldmann M, Maini RN. Assessment of antibodies to doublestranded DNA induced in rheumatoid arthritis patients following treatment with infliximab, a monoclonal antibody to tumor necrosis factor alpha: Findings in open-label and randomized placebo-controlled trials. Arthritis Rheum. 2000;43:2383–2390. doi: 10.1002/1529-0131(200011)43:11<2383::AID-ANR2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 3.Ramos-Casals M, Roberto-Perez-Alvarez, DiazLagares C, Cuadrado MJ, Khamashta MA, BIOGEAS Study Group Autoimmune diseases induced by biological agents: A double-edged sword. Autoimmun Rev. 2010;9:188–193. doi: 10.1016/j.autrev.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 4.Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, et al. 2012 revised International Chapel Hill Consensus conference nomenclature of vasculitides. Arthritis Rheum. 2013;65:1–11. doi: 10.1002/art.37715. [DOI] [PubMed] [Google Scholar]
- 5.Grau RG. Drug-induced vasculitis: New insights and a changing lineup of suspects. Curr Rheumatol Rep. 2015;17:71–71. doi: 10.1007/s11926-015-0545-9. [DOI] [PubMed] [Google Scholar]
- 6.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann Intern Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 7.Arora H, Madani S, Debelenko LV, McGrath EJ, Mutyala R, Guglani L. Limited granulomatosis with polyangiitis in an adolescent with Crohn’s disease on infliximab therapy: Cause or coincidence. Clin Respir J. 2015;9:506–511. doi: 10.1111/crj.12168. [DOI] [PubMed] [Google Scholar]
- 8.Asahina A, Ohshima N, Nakayama H, Shirai A, Juji T, Matsui T. Henoch-Schönlein purpura in a patient with rheumatoid arthritis receiving etanercept. Eur J Dermatol. 2010;20:521–522. doi: 10.1684/ejd.2010.0977. [DOI] [PubMed] [Google Scholar]
- 9.Ashok D, Dubey S, Tomlinson I. C-ANCA positive systemic vasculitis in a patient with rheumatoid arthritis treated with infliximab. Clin Rheumatol. 2008;27:261–264. doi: 10.1007/s10067-007-0712-0. [DOI] [PubMed] [Google Scholar]
- 10.Bernardes C, Carvalho D, Saiote J, Ramos J. Leukocytoclastic vasculitis complicating adalimumab therapy for Crohn's disease: Report of three cases. Gastroenterol Hepatol. 2018;41:442–443. doi: 10.1016/j.gastrohep.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 11.Broshtilova V, Iliev E, Gantcheva M. Etanerceptinduced Wegener granulomatosis in a patient with rheumatoid arthritis. Dermatol Ther. 2013;26:73–76. doi: 10.1111/j.1529-8019.2012.01538.x. [DOI] [PubMed] [Google Scholar]
- 12.Chandra T, Tabanor-Gayle JA, Lakshminarayanan S. Adalimumab-induced anti-neutrophilic cytoplasmic antibody vasculitis: A rare complication of an increasingly common treatment. e5598Cureus. 2019;11 doi: 10.7759/cureus.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chebli JMF, de Oliveira Moreira B, da Rocha Ribeiro TC. An unusual cause of skin Rash in crohn's disease. Gastroenterology. 2018;155:618–620. doi: 10.1053/j.gastro.2018.01.061. [DOI] [PubMed] [Google Scholar]
- 14.Condamina M, Diaz E, Jamart C, Loget J, Durlach A, Salmon JH, et al. Severe attack of HenochSchönlein purpura with neurological involvement during adalimumab treatment for Crohn's disease. J Crohns Colitis. 2020;14:538–542. doi: 10.1093/ecco-jcc/jjz164. [DOI] [PubMed] [Google Scholar]
- 15.Cury DB, de Souza AW, Vianna GA, Odashiro D, Farias A, Moss AC. Cutaneous vasculitis in a patient with Crohn’s disease treated with adalimumab. E1-E2Inflamm Bowel Dis. 2017;23 doi: 10.1097/MIB.0000000000000982. [DOI] [PubMed] [Google Scholar]
- 16.Devos SA, Van Den Bossche N, De Vos M, Naeyaert JM. Adverse skin reactions to anti-TNFalpha monoclonal antibody therapy. Dermatology. 2003;206:388–390. doi: 10.1159/000069965. [DOI] [PubMed] [Google Scholar]
- 17.Downes MR, Prendiville S, Kiely C, Lenane P, Mulligan N. Cutaneous reactions to adalimumab administration. Ir Med J. 2011;104:122–123. [PubMed] [Google Scholar]
- 18.Duffy TN, Genta M, Moll S, Martin PY, Gabay C. Henoch Schönlein purpura following etanercept treatment of rheumatoid arthritis. S106Clin Exp Rheumatol. 2006;24(2 Suppl 41) [PubMed] [Google Scholar]
- 19.Fadahunsi AW, Garcia-Rosell M, Pattanaik D. Hypocomplementemic urticarial vasculitis syndrome possibly secondary to etanercept use. J Clin Rheumatol. 2015;21:274–275. doi: 10.1097/RHU.0000000000000269. [DOI] [PubMed] [Google Scholar]
- 20.Florez H, Morlà R, Castellanos-Moreira R, Sanmartí R. Sustained response to rituximab in a TNFi-induced ANCA-vasculitis developed in a patient with rheumatoid arthritis. e15-e16Semin Arthritis Rheum. 2018;47 doi: 10.1016/j.semarthrit.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 21.Fujikawa K, Kawakami A, Hayashi T, Iwamoto N, Kawashiri SY, Aramaki T, et al. Cutaneous vasculitis induced by TNF inhibitors: A report of three cases. Mod Rheumatol. 2010;20:86–89. doi: 10.1007/s10165-009-0232-7. [DOI] [PubMed] [Google Scholar]
- 22.Funada M, Nawata M, Nawata A, Miyamoto T, Saito K, Tanaka Y. Rapidly progressive glomerulonephritis after introduction of certolizumab pegol: A case report. Mod Rheumatol Case Rep. 2021;5:11–15. doi: 10.1080/24725625.2020.1798061. [DOI] [PubMed] [Google Scholar]
- 23.Ginsberg S, Rosner I, Slobodin G, Boulman N, Rozenbaum M, Kaly L, et al. Etanercept treatmentrelated c-ANCA-associated large vessel vasculitis. Clin Rheumatol. 2016;35:271–273. doi: 10.1007/s10067-015-3134-4. [DOI] [PubMed] [Google Scholar]
- 24.Goulão J, Cunha H, Anes I, Bártolo E, Furtado C, Serrano P, et al. Urticarial vasculitis due do infliximab. J Eur Acad Dermatol Venereol. 2008;22:882–883. doi: 10.1111/j.1468-3083.2007.02480.x. [DOI] [PubMed] [Google Scholar]
- 25.Hawryluk EB, Linskey KR, Duncan LM, Nazarian RM. Broad range of adverse cutaneous eruptions in patients on TNF-alpha antagonists. J Cutan Pathol. 2012;39:481–492. doi: 10.1111/j.1600-0560.2012.01894.x. [DOI] [PubMed] [Google Scholar]
- 26.Hirohama D, Hoshino J, Hasegawa E, Yamanouchi M, Hayami N, Suwabe T, et al. Development of myeloperoxidase-antineutrophil cytoplasmic antibodyassociated renal vasculitis in a patient receiving treatment with anti-tumor necrosis factor-a. Mod Rheumatol. 2010;20:602–605. doi: 10.1007/s10165-010-0339-x. [DOI] [PubMed] [Google Scholar]
- 27.Horai Y, Ishikawa H, Iwanaga N, Izumi Y, Matsuoka Y, Miura S, et al. Development of hypocomplementemic urticarial vasculitis during certolizumab pegol treatment for rheumatoid arthritis: A case report. J Clin Pharm Ther. 2020;45:1179–1182. doi: 10.1111/jcpt.13117. [DOI] [PubMed] [Google Scholar]
- 28.Horai Y, Satoru O, Lapalme-Remis S, Sumiyoshi R, Nakashima Y, Suzuki T, et al. Takayasu arteritis developing during treatment of ulcerative colitis with infliximab. Mod Rheumatol. 2013;23:572–576. doi: 10.1007/s10165-012-0684-z. [DOI] [PubMed] [Google Scholar]
- 29.Iwahashi C, Ono H, Haruta M, Minami T, Mashimo H, Shimojo H, et al. New onset or exacerbation of uveitis with infliximab: Paradoxical effects. e000250BMJ Open Ophthalmol. 2019;4 doi: 10.1136/bmjophth-2018-000250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jarrett SJ, Cunnane G, Conaghan PG, Bingham SJ, Buch MH, Quinn MA, et al. Anti-tumor necrosis factor-alpha therapy-induced vasculitis: Case series. J Rheumatol. 2003;30:2287–2291. [PubMed] [Google Scholar]
- 31.Karoui S, Bibani N, Ben Gorbel I, Serghini M, Mlika M, Braham A, et al. Leukocytoclastic vasculitis: A rare adverse effect secondary to infliximab. E4-5Inflamm Bowel Dis. 2011;17 doi: 10.1002/ibd.21309. [DOI] [PubMed] [Google Scholar]
- 32.Katoh N, Kubota M, Shimojima Y, Ishii W, Matsuda M, Akamatsu T, et al. Takayasu's arteritis in a patient with Crohn's disease: An unexpected association during infliximab therapy. Intern Med. 2010;49:179–182. doi: 10.2169/internalmedicine.49.2491. [DOI] [PubMed] [Google Scholar]
- 33.Kiyohara H, Hisamatsu T, Matsuoka K, Naganuma M, Kameda H, Seta N, et al. Crohn's disease in which the patient developed aortitis during treatment with adalimumab. Intern Med. 2015;54:1725–1732. doi: 10.2169/internalmedicine.54.3853. [DOI] [PubMed] [Google Scholar]
- 34.LaConti JJ, Donet JA, Cho-Vega JH, Sussman DA, Ascherman D, Deshpande AR. Henoch-Schönlein purpura with adalimumab therapy for ulcerative colitis: A case report and review of the literature. Case Rep Rheumatol. 2016;2016:2812980–2812980. doi: 10.1155/2016/2812980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laresche C, Locatelli F, Biver-Dalle C, Nachury M, Heyd B, Koch S, et al. Severe Henoch-Schönlein purpura complicating infliximab therapy for ulcerative colitis. E20-E22Cutis. 2017;99 [PubMed] [Google Scholar]
- 36.Lee A, Kasama R, Evangelisto A, Elfenbein B, Falasca G. Henoch-Schönlein purpura after etanercept therapy for psoriasis. J Clin Rheumatol. 2006;12:249–251. doi: 10.1097/01.rhu.0000239901.34561.5e. [DOI] [PubMed] [Google Scholar]
- 37.Leydet-Quilici H, Luc M, Armingeat T, Pham T, Lafforgue P. Giant cell arteritis during adalimumab treatment for rheumatoid arthritis. Joint Bone Spine. 2007;74:303–304. doi: 10.1016/j.jbspin.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 38.Mangat P, Whittle S, Cleland L, Limaye V. Digital vasculitis: A late complication of anti-tumour necrosis factor alpha therapy. Clin Rheumatol. 2008;27:1593–1595. doi: 10.1007/s10067-008-1015-9. [DOI] [PubMed] [Google Scholar]
- 39.Mariani N, So A, Aubry-Rozier B. Two cases of Takayasu's arteritis occurring under anti-TNF therapy. Joint Bone Spine. 2013;80:211–213. doi: 10.1016/j.jbspin.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 40.Marques I, Lagos A, Reis J, Pinto A, Neves B. Reversible Henoch-Schönlein purpura complicating adalimumab therapy. J Crohns Colitis. 2012;6:796–799. doi: 10.1016/j.crohns.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 41.McCain ME, Quinet RJ, Davis WE. Etanercept and infliximab associated with cutaneous vasculitis. Rheumatology (Oxford) 2002;41:116–117. doi: 10.1093/rheumatology/41.1.116. [DOI] [PubMed] [Google Scholar]
- 42.McIlwain L, Carter JD, Bin-Sagheer S, Vasey FB, Nord J. Hypersensitivity vasculitis with leukocytoclastic vasculitis secondary to infliximab. J Clin Gastroenterol. 2003;36:411–413. doi: 10.1097/00004836-200305000-00010. [DOI] [PubMed] [Google Scholar]
- 43.Muñoz Villafranca C, Izu Belloso RM, Bravo Rodriguez MT, Basterra Olabarrieta S. Adalimumab vasculitis in a patient with Crohn's disease. Gastroenterol Hepatol. 2013;36:296–297. doi: 10.1016/j.gastrohep.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 44.Muto J, Usami J, Watanabe D. Leukocytoclastic vasculitis and dermal perivascular hemophagocytosis associated with adalimumab therapy for rheumatoid arthritis. Am J Dermatopathol. 2018;40:57–59. doi: 10.1097/DAD.0000000000000959. [DOI] [PubMed] [Google Scholar]
- 45.Numakura T, Tamada T, Nara M, Muramatsu S, Murakami K, Kikuchi T, et al. Simultaneous development of sarcoidosis and cutaneous vasculitis in a patient with refractory Crohn's disease during infliximab therapy. BMC Pulm Med. 2016;16:30–30. doi: 10.1186/s12890-016-0193-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Orpin SD, Majmudar VB, Soon C, Azam NA, Salim A. Adalimumab causing vasculitis. Br J Dermatol. 2006;154:998–999. doi: 10.1111/j.1365-2133.2006.07195.x. [DOI] [PubMed] [Google Scholar]
- 47.Ortiz-Sierra MC, Echeverri AF, Tobón GJ, Cañas CA. Developing of granulomatosis with polyangiitis during etanercept therapy. Case Rep Rheumatol. 2014;2014:210108–210108. doi: 10.1155/2014/210108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pàmies A, Castro S, Poveda MJ, Fontova R. Leucocytoclastic vasculitis associated with golimumab. Rheumatology (Oxford) 2013;52:1921–1923. doi: 10.1093/rheumatology/ket125. [DOI] [PubMed] [Google Scholar]
- 49.Parekh K, Ching D, Rahman MU, Stamp LK. Onset of Wegener's granulomatosis during therapy with golimumab for rheumatoid arthritis: A rare adverse event. Rheumatology (Oxford) 2010;49:1785–1787. doi: 10.1093/rheumatology/keq101. [DOI] [PubMed] [Google Scholar]
- 50.Pastore S, Londero M, Gortani G, Abate MV, Marchetti F, Di Leo G, et al. Infliximab-related vasculitis in patients affected by ulcerative colitis. J Pediatr Gastroenterol Nutr. 2010;51:226–228. doi: 10.1097/MPG.0b013e3181e5e198. [DOI] [PubMed] [Google Scholar]
- 51.Pinheiro RR, Lencastre A. Henoch-Schönlein purpura during anti-TNF-a therapy: A fortuitous event or an indication to stop therapy. Eur J Dermatol. 2017;27:304–305. doi: 10.1684/ejd.2017.2979. [DOI] [PubMed] [Google Scholar]
- 52.Pontikaki I, Shahi E, Frasin LA, Gianotti R, Gelmetti C, Gerloni V, et al. Skin manifestations induced by TNF-alpha inhibitors in juvenile idiopathic arthritis. Clin Rev Allergy Immunol. 2012;42:131–134. doi: 10.1007/s12016-011-8262-2. [DOI] [PubMed] [Google Scholar]
- 53.Rahman FZ, Takhar GK, Roy O, Shepherd A, Bloom SL, McCartney SA. Henoch-Schönlein purpura complicating adalimumab therapy for Crohn's disease. World J Gastrointest Pharmacol Ther. 2010;1:119–122. doi: 10.4292/wjgpt.v1.i5.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reitblat T, Reitblat O. Appearance of ANCA - associated vasculitis under tumor necrosis factor-alpha inhibitors treatment. Am J Case Rep. 2013;14:80–82. doi: 10.12659/AJCR.883841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Richette P, Dieudé P, Damiano J, Lioté F, Orcel P, Bardin T. Sensory neuropathy revealing necrotizing vasculitis during infliximab therapy for rheumatoid arthritis. J Rheumatol. 2004;31:2079–2081. [PubMed] [Google Scholar]
- 56.Rolle AS, Zimmermann B, Poon SH. Etanerceptinduced Henoch-Schönlein purpura in a patient with ankylosing spondylitis. J Clin Rheumatol. 2013;19:90–93. doi: 10.1097/RHU.0b013e3182863027. [DOI] [PubMed] [Google Scholar]
- 57.Rosen DR, Cologne KG, Popek SM, Sung BC, Ortega AE, Senagore AJ. Recurrent drug-induced ANCA vasculitis in a patient with Crohn's colitis treated with infliximab: A potential contraindication to immunosuppressive therapy. Am Surg. 2012;78:1406–1408. [PubMed] [Google Scholar]
- 58.Sehgal R, Stratman EJ, Cutlan JE. Biologic agentassociated cutaneous adverse events: A single center experience. Clin Med Res. 2018;16:41–46. doi: 10.3121/cmr.2017.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Seton M. Giant cell arteritis in a patient taking etanercept and methotrexate. J Rheumatol. 2004;31:1467–1467. [PubMed] [Google Scholar]
- 60.Shivaji UN, Awasthi AK, Aherne R. Cutaneous vasculitis caused by anti-tumor necrosis factor therapy: A case report. e1-2Clin Gastroenterol Hepatol. 2016;14 doi: 10.1016/j.cgh.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 61.Simms R, Kipgen D, Dahill S, Marshall D, Rodger RS. ANCA-associated renal vasculitis following antitumor necrosis factor alpha therapy. e11-4Am J Kidney Dis. 2008;51 doi: 10.1053/j.ajkd.2007.10.043. [DOI] [PubMed] [Google Scholar]
- 62.Srivastava MD, Alexander F, Tuthill RJ. Immunology of cutaneous vasculitis associated with both etanercept and infliximab. Scand J Immunol. 2005;61:329–336. doi: 10.1111/j.1365-3083.2005.01570.x. [DOI] [PubMed] [Google Scholar]
- 63.Stokes MB, Foster K, Markowitz GS, Ebrahimi F, Hines W, Kaufman D, et al. Development of glomerulonephritis during anti-TNF-alpha therapy for rheumatoid arthritis. Nephrol Dial Transplant. 2005;20:1400–1406. doi: 10.1093/ndt/gfh832. [DOI] [PubMed] [Google Scholar]
- 64.Wendling D, Streit G, Lehuédé G, Toussirot E, Aubin A, Petitjean A. Cutaneous lymphocytic vasculitis during TNFalpha antagonist therapy for polyarthritis. Joint Bone Spine. 2006;73:215–216. doi: 10.1016/j.jbspin.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 65.Woody M, Warren D, Speck L, Jackson J. Leukocytoclastic vasculitis drug reaction to certolizumab pegol. Proc (Bayl Univ Med Cent) 2017;30:213–214. doi: 10.1080/08998280.2017.11929591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.da Silva Cendon Duran C, Barreto Santiago M. Cutaneous vasculitis during secukinumab treatment. Eur J Case Rep Intern Med. 2020;7:001815–001815. doi: 10.12890/2020_001815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Iwasaki S, Watanabe T, Tsuji T, Otsuka T, Makita K, Fukasawa Y, et al. Infliximab-induced granulomatous vasculitis with amyloid deposition in the tongue of a patient with Behçet disease. J Clin Rheumatol. 2020 doi: 10.1097/RHU.0000000000001567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kishimoto K, Kawashima K, Fukunaga M, Kotani S, Sonoyama H, Oka A, et al. Intermittent purpura development associated with leukocytoclastic vasculitis induced by infliximab for Crohn's disease. Intern Med. 2021;60:385–389. doi: 10.2169/internalmedicine.5340-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lahita RG, Vernace MA. Vasculitis, vitiligo, thyroiditis, and altered hormone levels after anti-tumor necrosis factor therapy. J Rheumatol. 2011;38:579–580. doi: 10.3899/jrheum.100968. [DOI] [PubMed] [Google Scholar]
- 70.Song Y, Shi YH, He C, Liu CQ, Wang JS, Zhao YJ, et al. Severe Henoch-Schönlein purpura with infliximab for ulcerative colitis. World J Gastroenterol. 2015;21:6082–6087. doi: 10.3748/wjg.v21.i19.6082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Martín Varas C, Heras M, Saiz A, Coloma R, Calle L, Callejas R, et al. Antineutrophil cytoplasmic antibodies associated vasculitis in patient with Crohn's disease treated with adalimumab. Nefrologia. 2017;37:560–561. doi: 10.1016/j.nefro.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 72.Martínez-Taboada VM, Val-Bernal JF, Pesquera LC, Fernández-Llanio NE, Esteban JM, Blanco R, et al. Demyelinating disease and cutaneous lymphocitic vasculitis after etanercept therapy in a patient with rheumatoid arthritis. Scand J Rheumatol. 2006;35:322–323. doi: 10.1080/03009740600557249. [DOI] [PubMed] [Google Scholar]
- 73.Villani A, De Fata Salvatores G, Nappa P, Megna M, Fabbrocini G, Napolitano M. Cutaneous leucocytoclastic vasculitis during secukinumab treatment. G Ital Dermatol Venereol. 2019 doi: 10.23736/S2784-8671.18.06203-X. [Online ahead of print] [DOI] [PubMed] [Google Scholar]
- 74.Chelli C, Loget J, Vanhaecke C, Durlach A, Gagneux-Lemoussu L, Soriano C, et al. Cutaneous vasculitis with gut involvement during secukinumab treatment for psoriatic arthritis. adv00077Acta Derm Venereol. 2020;100 doi: 10.2340/00015555-3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Reverte M, Etienne M, Fouchard M, Doucet L, Brenaut E, Misery L. Occurrence of Henoch-Schönlein purpura in a patient treated with secukinumab. e455-e457J Eur Acad Dermatol Venereol. 2019;33 doi: 10.1111/jdv.15776. [DOI] [PubMed] [Google Scholar]
- 76.Barrado-Solís N, Rodrigo-Nicolás B, De la MorenaBarrio I, Pérez-Pastor G, Sanchis-Sánchez C, TomásCabedo G, et al. Report of two cases of Behçet's disease developed during treatment with secukinumab. e587-e589J Eur Acad Dermatol Venereol. 2020;34 doi: 10.1111/jdv.16454. [DOI] [PubMed] [Google Scholar]
- 77.Dincses E, Yurttas B, Esatoglu SN, Melikoglu M, Hamuryudan V, Seyahi E. Secukinumab induced Behçet’s syndrome: A report of two cases. omz041Oxf Med Case Reports. 2019;2019 doi: 10.1093/omcr/omz041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Perkovic D, Simac P, Katic J. IgA vasculitis during secukinumab therapy. Clin Rheumatol. 2021;40:2071–2073. doi: 10.1007/s10067-020-05364-1. [DOI] [PubMed] [Google Scholar]
- 79.Kandula P, Kouides PA. Rituximab-induced leukocytoclastic vasculitis: A case report. Arch Dermatol. 2006;142:246–247. doi: 10.1001/archderm.142.2.246. [DOI] [PubMed] [Google Scholar]
- 80.Kim MJ, Kim HO, Kim HY, Park YM. Rituximabinduced vasculitis: A case report and review of the medical published work. J Dermatol. 2009;36:284–287. doi: 10.1111/j.1346-8138.2009.00639.x. [DOI] [PubMed] [Google Scholar]
- 81.Dereure O, Navarro R, Rossi JF, Guilhou JJ. Rituximabinduced vasculitis. Dermatology. 2001;203:83–84. doi: 10.1159/000051713. [DOI] [PubMed] [Google Scholar]
- 82.Abe K, Itoh M, Asahina A. Rituximab-induced vasculitis: Does the immune complex of rituximab play a key role in developing paradoxical adverse events. e311-e312J Dermatol. 2019;46 doi: 10.1111/1346-8138.14872. [DOI] [PubMed] [Google Scholar]
- 83.Golmia A, Grinblat B, Finger E, Klieman C, Assir F, Scheinberg M. The development of erythema elevatum diutinum in a patient with juvenile idiopathic arthritis under treatment with abatacept. Clin Rheumatol. 2008;27:105–106. doi: 10.1007/s10067-007-0743-6. [DOI] [PubMed] [Google Scholar]
- 84.Holt MH, Liu V, Fairley J. Medium-vessel vasculitis presenting as multiple leg ulcers after treatment with abatacept. JAAD Case Rep. 2018;4:811–813. doi: 10.1016/j.jdcr.2018.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Costa-Moreira P, Lopes S, Santos AL, Pedrosa AF, Andrade P, Portugal R, et al. Leukocytoclastic vasculitis related to ustekinumab in a Crohn's disease patient: First case report and literature review. J Crohns Colitis. 2020;14:274–276. doi: 10.1093/ecco-jcc/jjz128. [DOI] [PubMed] [Google Scholar]
- 86.Chugh R, Proctor DD, Little A, Myung P, Cowper S, Imaeda S, et al. Cutaneous vasculitis after ustekinumab induction in Crohn's disease. e30-e31Inflamm Bowel Dis. 2021;27 doi: 10.1093/ibd/izaa285. [DOI] [PubMed] [Google Scholar]
- 87.Sakaue S, Sumitomo S, Kubo K, Fujio K, Yamamoto K. Tocilizumab-induced leucocytoclastic vasculitis in a patient with rheumatoid arthritis. Rheumatology (Oxford) 2014;53:1529–1530. doi: 10.1093/rheumatology/keu008. [DOI] [PubMed] [Google Scholar]
- 88.Gutiérrez-González LA. Biological therapy-Induced systemic vasculitis. Curr Rheumatol Rep. 2016;18:39–39. doi: 10.1007/s11926-016-0588-6. [DOI] [PubMed] [Google Scholar]
- 89.Ramos-Casals M, Brito-Zerón P, Muñoz S, Soria N, Galiana D, Bertolaccini L, et al. Autoimmune diseases induced by TNF-targeted therapies: analysis of 233 cases. Medicine (Baltimore) 2007;86:242–251. doi: 10.1097/MD.0b013e3181441a68. [DOI] [PubMed] [Google Scholar]
- 90.Pérez-De-Lis M, Retamozo S, Flores-Chávez A, Kostov B, Perez-Alvarez R, Brito-Zerón P, et al. Autoimmune diseases induced by biological agents. A review of 12,731 cases (BIOGEAS Registry) Expert Opin Drug Saf. 2017;16:1255–1271. doi: 10.1080/14740338.2017.1372421. [DOI] [PubMed] [Google Scholar]
- 91.Sokumbi O, Wetter DA, Makol A, Warrington KJ. Vasculitis associated with tumor necrosis factor-a inhibitors. Mayo Clin Proc. 2012;87:739–745. doi: 10.1016/j.mayocp.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]