Abstract
Objectives
In this systematic review, we aimed to evaluate the clinical features, therapeutic options, and outcomes of children with multisystem inflammatory syndrome in children (MIS-C) and to investigate whether MIS-C is a new variant of Kawasaki disease.
Materials and methods
Adhering to PRISMA principles, we searched for eligible studies between December 2019 and June 2020 through the following databases: PubMed, ISI Web of Science, SCOPUS, and Science Direct. Studies including original data of patients aged <21 years with MIS-C and descriptions of clinical signs, laboratory or radiological investigations were selected.
Results
A total of 84 studies were identified, for which 48 were eligible for full screening and only 13 studies (n=657) met our inclusion criteria. More than 70% of patients with MIS-C tested positive for severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2). The most common symptoms were gastrointestinal (80 to 100%) and most patients presented with fever for >4 days. Mucocutaneous manifestations are similar to Kawasaki disease presented in up to 64% in some studies. Almost all patients had significant elevations in inflammatory markers, and up to 50 to 100% had elevated troponin suggesting myocardial damage. Intravenous immunoglobulin (IVIG) was administered to 60% of patients in 12 studies and 80 to 100% in five studies. Steroids were administered to 10 to 95% of patients. The overall mortality rate was 0.9%.
Conclusion
The temporal association between novel coronavirus disease 2019 (COVID-19) onset and Kawasaki-like disease and MIS-C suggests a causal link. Both syndromes have similar cascades of symptoms and hyperinflammation, which likely explain their response to the same immunomodulatory agents. However, it is unclear yet why some children appear more susceptible to develop MIS-C.
Keywords: Children, COVID-19, Kawasaki disease, multisystem inflammatory syndrome in children, SARS-CoV-2
Introduction
In early January 2020, a new type of coronavirus was isolated (i.e., severe acute respiratory syndrome-coronavirus 2 [SARS-CoV-2]) responsible for the disease known later as novel coronavirus disease 2019 (COVID-19). It was recorded that the first death caused by COVID-19 was on January 9th, 2020 in Wuhan and, since then, nearly one million deaths have occurred worldwide.[1] Compared to adults, children with COVID-19 infection appear to have a milder clinical presentation and, additionally, there is scarcity in death reports among children.[2,3] However, the clinical course and patterns of COVID-19 presentations remain unclear among this population.
Kawasaki disease (KD) was first described in 1967. This disease describes a series of Japanese children suffering from acute febrile mucocutaneous syndrome associated with specific desquamation of the toes and fingers.[4] This syndrome is more common in boys than girls.[5] Nonetheless, the etiology is still unknown.[4] The current concept is that it is systemic inflammation in multiple organs resulting from an immune cascade of reactions enhanced as a response to exposure in the respiratory or gastrointestinal (GI) systems. It has been highlighted that infectious agents could be the primary triggering causes, as the peak of the incidence of this syndrome occurs mainly during winter and spring.[4]
In late April 2020, a description of a new novel syndrome in children and adolescents, namely multisystem inflammatory syndrome in children (MIS-C), with relation to SARS-CoV-2 infection took place worldwide.[6] This syndrome was first identified in Europe, and it was known initially as a pediatric inflammatory multisystem syndrome associated with SARS-CoV-2 and, then, the Centers for Disease Control and Prevention (CDC) issued the case definition of MIS-C.[7] These are the same or overlapping syndromes. The CDC case definition included a minimum of 24-h history of subjective or objective fever, clinically severe illness necessitating hospitalization, along with two or more organ system affected and laboratory evidence of inflammation, with no alternative diagnosis. Recently, the researchers have highlighted the relationship of KD which affects children, with the emerging coronavirus.[8] Also, Abuhammour and Dawoud[9] indicated that the laboratory results of the virus in COVID-19 patients with symptoms of cytokine storms, are very similar to KD syndrome that also leads to cytokine storm, and the direct cause is alerting the virus to the patient’s immune system, while the other reason, which is susceptibility in patients, is often linked to the genetic inheritance factor.
In this systematic review, we aimed to evaluate clinical features, diagnostic tests, current therapeutic management, and outcomes of MIS-C cases in children and adolescents reported in the literature and to evaluate the common points between the MIS-C and KD.
Patients and Methods
Compliance with the PRISMA principles
In the current analysis, we utilized the PRISMA principles to optimize the review process. The PRISMA statement involves a flow diagram and a checklist encompassing 27 items and these items are considered necessary for transparent reporting of systematic reviews.[10]
Search strategy
The following search term was utilized (((((COVID-19) OR coronavirus) OR SARS-CoV-2)) AND (((((((pediatrics) OR children) OR neonates) OR child) OR neonate) OR infant) OR infants) AND (MIS-C) or multi-system inflammatory syndrome) to retrieve all articles published between December 1st, 2019 and June 6th, 2020 through the following databases: PubMed, ISI Web of Science, SCOPUS, Science Direct, all resources excluding (book chapters, encyclopedias, conference abstracts, conference information, discussions, editorials, short communications, case report, case series) from inception, until the day the search was finalized (June 2020).
No language restrictions were applied. No attempts were made to contact the study authors for identifying missing and confusing data. A manual search of the references found in the selected articles and reviews was also performed.
Study selection and risk of bias
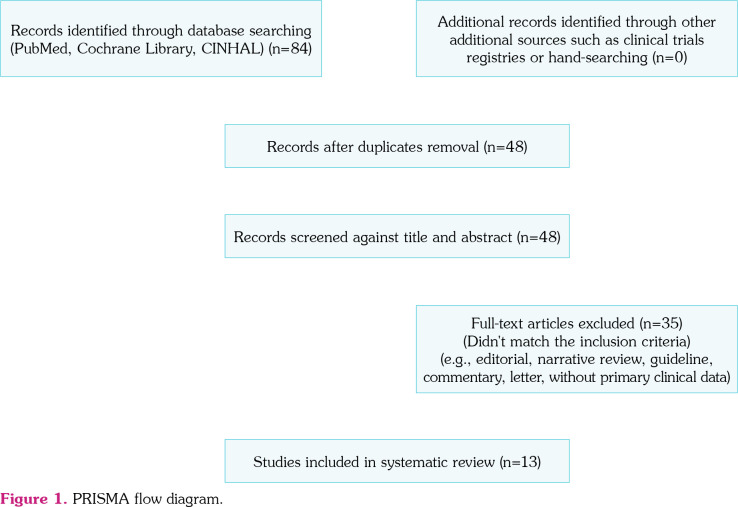
In keeping with the quality standards for reporting systematic reviews and meta-analysis of observational studies, two authors screened the titles and abstracts independently and in duplicate for potential eligibility. They subsequently read the full texts to determine final eligibility. An appraisal of the risk of bias for observational studies was conducted through the quality assessment tool published by the National Institutes of Health (NIH).[11] Independent risk of bias was assessed by at least two investigators. Discrepancies were resolved through discussion and consensus, and if necessary, the assistance of a third author was sought. Eligible studies fulfilled the following criteria: (i) studied patients younger than 21 years old; (ii) presented original data from cases of COVID-19 with a clinical MIS-C; and (iii) contained descriptions of clinical manifestations, laboratory tests, radiological examinations, or description of the therapeutic option (Figure 1).
Figure 1. PRISMA flow diagram.
Data extraction
A structured data extraction form was piloted and, then, used to extract data from the reports of all included studies in duplicate and independently by two authors. Discrepancies in extracted data were resolved through discussion. The following data were extracted, when available, from each selected article: site, publication date, and study design, number of cases, sex, age, clinical manifestations, laboratory tests, radiological examinations, therapeutic management, and outcomes (hospitalization, intensive care unit [ICU] admission, or death).
Results
Study identification
A total of 84 potentially relevant articles were identified by the search strategy. Following duplicate removal, title and/or abstract screening ensued, 48 studies were eligible for full-text screening. Of these, 35 studies were excluded, as they did not match the inclusion criteria. We identified 13 studies[12-24] for our systematic review from five countries across six continents (Table 1).
Table 1. Description of the study and demographic data.
| Reference | Description of the study | Site | Sample size | Age (year) | Sex (%) | |
| Capone et al.,[12] | Single-center retrospective | New-York | 33 | 8.6* | M | 20.61 |
| Whittaker et al.,[13] | Retrospective analysis 8 hospitals | UK | 58 | 9* | F | 66 |
| Dufort et al.,[14] | Descriptive analyses of patients who met New-York State Department of Health case definition of MIS-C 1.3.2020-10.5.2020 multi centre |
New-York | 99 | 31% (0-5) 42% (6-12) 26% (13-21) |
M | 54 |
| Pouletty et al.,[15] | Multicenter cohort April 2020 | Paris | 16 | 10* | M | 50 |
| Belhadjer et al.,[16] | Retrospective study for all children with MIS-C and acute left ventricular dysfunction in the context of Sars-CoV- 2 pandemic in 12 hospitals in France and one hospital in Switzerland | France & Switzerland | 35 children | 10* | M | 51 |
| Toubiana et al.,[17] | Prospective observational study single centre | France | 21 children | 7.9* | M | 43 |
| Verdoni et al.,[18] | Retrospective analysis of patients diagnosed with KD during the pandemic of COVID-19 single centre | Italy | 10 patients | 7.5** | M | 70 |
| Feldstein et al.,[19] | Prospective and retrospective surveillance of patients with MIS-C who were admitted to participating health centres and confirmed SARS-COV-2 infection 15/3/2020-20/5/2020 | USA | 186 patients who were not included in previous surveillances | 8.3* | M | 62 |
| Ramcharan et al.,[20] | Retrospective study of patients refereed for cardiovascular evaluation as confirmed pediatric inflammatory multisystem syndrome associated with SARS-CoV-2 between 10/4/2020-9/5/2020 single centre | UK | 15 | 8.8* | M | 73 |
| Miller et al.,[21] | Retrospective chart review of 44 patients who were hospitalized with a diagnosis of MIS-C at the children’s hospital 18/4/2020-22/5/2020 single centre | Columbia | 44 | 7.3* | M | 45 |
| Riollano-Cruz et al.,[22] | Retrospective analysis of 15 cases with MIS-C Related to COVID-19 presented to a tertiary care centre between 24.4.2020-19.6.2020 single centre | New-York | 15 | 12** | M | 73 |
| Cheung et al.,[23] | Prospective analysis 18/4-5.5.2020 for less than 21 years and presented with clinical syndrome prolonged fever, systemic inflammation, shock, end organ dysfunction, KD had evidence of recent severe respiratory syndrome coronavirus -2 infection single centre | New-York | 17 | 8* | M | 8 |
| Belot et al.,[24] | Surveillance of pediatric inflammatory multisystem syndrome cases in France 1.3.2020-17.5.2020 with confirmed/probable/possible/or non CoV cases multi centre | France | 156 (79 confirmed, 16 probables, 13 possible, 48 excluded) Total included 108 |
8* | M/F ratio | 0.96 |
| * Median; ** Mean; UK: United Kingdom; USA: United State of America. | ||||||
Characteristics of included studies
Thirteen studies investigated in this review were all observational and no randomized trials were identified of any interventions that directly addressed the included study populations. The included studies were published between December 2019 and June 2020 and encompassed the children (<21 years). A total of 657 descriptions of pediatric were obtained, including 359 males (54.6%). Of these 13 studies, seven studies took a form of retrospective analysis of the included cases,[12,13,16,18,20-22] whereas other included studies were prospective in nature[14,15,17,19,23] and one single study took a form of surveillance methodology.[24] Except for six multi-center studies,[13-16,19,24] all others were unicentric studies. Almost half of the included studies (n=6) were conducted in the United State of America,[12,14,19,21-23] three in France,[15,17,24] two in the United Kingdom,[13,20] one in France and Switzerland,[16] and one in Italy.[18]
Clinical manifestations
Patients with evidence of COVID-19 infection or recent exposure to COVID-19 infection in the absence of testing were included. Of these, 12 studies revealed that more than 70% of the included patients tested positive for SARS-CoV-2 infection by reverse transcriptase-polymerase chain reaction (RT-PCR), antibody testing, or both.[12-17,19-24] Across nine studies, most of the patients had fever present for more than four days,[12-16,20-23] and the most common presenting symptoms included GI symptoms which presented by 80 to 100% of the cases presented in eight studies,[12-15,20-23] these symptoms include vomiting, abdominal pain, and/or diarrhea. In addition, KD symptoms including mucocutaneous manifestations such as conjunctivitis and rash with neurological findings were common in these children who were diagnosed to have MIS-C. Neurological findings in nine of the included studies were common in these children who were diagnosed with MIS-C. The most common neurological findings were headache followed by aseptic meningitis, meningeal irritation, stiff neck, and cranial nerve palsy.[12-15,17,21-24] It was reported in the present review that 22 to 64% of the included cases presented with signs and symptoms similar to complete KD,[12,13,15,17,22,23] whereas 29.4 to 70% of the included cases admitted with some of KD features (incomplete KD).[16-20,22] One single study indicated that 36% of the patients present with signs and symptoms of KD or atypical KD.[14] Of note, four studies revealed that 50 to 76% of the included cases were diagnosed with KDSS (Table 2).[12,15,17,18] Universally, results of the laboratory testing revealed the significant elevation in the inflammatory markers including but not limited to C-reactive protein and ferritin. Another major finding was that troponin levels were elevated in many patients (50 to 100% of the patients in the included studies), suggesting myocardial damage as presented in Table 3. The majority of the included cases in the present review were previously healthy children. Nonetheless, it was noted that overweight (6 to 39%), obesity (8 to 39%), and asthma (5.2 to 26.7%) were the most common comorbidities among these included cases (Table 2).
Table 2. Clinical presentation of the included cases.
| Reference | Covid-19 test/results | Clinical manifestations | Comorbidities | ||
| Capone et al.,[12] | All patients had evidence of SARS-CoV-2 infection (+ serology in 91%), | Median of 4 days' fever, 97% had gastrointestinal symptoms & involvement of other organ system, 64% had symptoms fulfilling complete criteria of KD, 76% of patients complete KD criteria had shock (n=16). Myocardial dysfunction (58%) | Overweight | 2 | 6 |
| Obesity | 12 | 39 | |||
| Whittaker et al.,[13] | In total 78% had evidence of current or prior SARS-CoV-2, infection PCR + in 26%, SARS-CoV- IgG + 83% |
All patients had fever 3-19 days, 10% sore throat, 26% headache, vomiting 45%, abdominal pain 53%, diarrhea 52%, erythematous rash 52%, conjunctival injection 45%, respiratory symptoms 21%, lymphadenopathy 16%, swollen hands & feet 16%, mucous membrane changes and red cracked lips 29% |
Asthma | 3 | 5.2 |
| Neuro-disability | 1 | 1.7 | |||
| Sickle-cell trait | 1 | 1.7 | |||
| Alopecia | 1 | 1.7 | |||
| Dufort et al.,[14] | 96% were classified as having a confirmed case and 4% having a suspected case. 24% had a COVID-19 compatible illness a median of 21 days before hospitalization, 38% had exposure to a person with confirmed COVID-19 like illness, 22% had direct contact with a person who had a clinically compatible COVID-19 like illness PCR + 51%, Serology + 99% | All presented with subjective fever or chills, 63% had fever on admission, 97% had tachycardia,78% had tachypnea, 32% hypotension 80% had GI symptoms, 60% had rash, 56% had conjunctival injection, 27% had mucosal changes, (Dermatologic 62%, mucucutaneous 61%), lower respiratory 40%, 61% had GI and either dermatologic or mucocutaneous symptoms | Obesity | 29 | 80.5 |
| Pouletty et al.,[15] | SARS-COVID was detected in 11 (96%). 31% (5 ) cases had documented recent contact with a quantitative PCR-positive individual PCR + 69%, Serology + 87% | 44% cardiac involvement Fever (100%) Respiratory signs 2 (12%) Gastrointestinal signs 13 (81%) Neurological signs 9 (56%)Skin rash 13 (81%) Hands and feet erythema/oedema 11 (68%) Conjunctivitis 15 (94%) Dry cracked lips 14 (87%) Cervical lymphadenopathy 6 (37%) Hemodynamic failure 11 (69%) Complete Kawasaki disease: 10 (62%) Kawasaki disease shock syndrome 7 (44%) | Overweight | 4 | 25 |
| Asthma | 2 | 12.5 | |||
| Belhadjer et al.,[16] | 88% patients tested positive for SARS CoV-2 infection PCR +40% Serology + 86% |
All children presented with fever and asthenia, gastrointestinal symptoms were prominent 80%, left ventricular ejection fraction less than 30% in one third | Overweight | 6 | 17 |
| Asthma | 3 | 8.5 | |||
| Lupus | 1 | 3 | |||
| Toubiana et al.,[17] | 90% had evidence of recent SARS-CoV-2 infection detected IgG antibodies against SARS-CoV-2. Two patients were negative. PCR +38% Serology +90% |
52% presented with KD complete criteria syndrome, polymorphous skin rash 76%, 76% conjunctivitis injection 81% myocarditis. All had noticeable GI symptoms (abdominal pain, vomiting, and diarrhea) 95%, irritability 57%. 29% headache confusion, meningeal irritation. Pericardial effusion 48%. LVF 10%-57%. | NA | ||
| Verdoni et al.,[18] | PCR +20% Serology +80% | Five (50%) of 10 patients were diagnosed with incomplete Kawasaki disease, presenting with three or fewer clinical criteria associated with additional laboratory criteria (n=1) or an abnormal echocardiography (n=4). In these patients, echocardiography revealed left ventricular function depression, mitral valve regurgitation, and pericardial effusion; they also required inotropic support. Chest x-ray, done in all patients in group 2, was positive in five (50%) patients for minimal mono or bilateral infiltrates. Five (50%) of 10 patients in group 2 met the criteria for KDSS because of hypotension and clinical signs of hypoperfusion. Two (20%) patients had diarrhoea and meningeal signs, four (40%) had only diarrhoea, and two (20%) had only meningeal signs. | NA | ||
Table 3. Inflammation markers and prognosis of the included cases.
| Reference | Inflammation markers and other abnormalities | ICU & mortality | Prognosis & outcome |
| Capone et al.,[12] | Marked inflammation , 48% had coronary abnormalities, (15% had coronary artery aneurysm and 9% had coronary artery dilatation). | 79% of patients were admitted ICU, No death | 18% required mechanical ventilation, most patients exhibited rapid clinical improvement, Mild cardiac dysfunction was still present at time of discharge only in 9 out of 19 who had impaired function during hospitalization, Median length of hospitalization=4 days. |
| Whittaker et al.,[13] | All patients had evidence of a marked inflammatory state (C-reactive protein, neutrophilia, and ferritin), tropoinin conc were elevated in 68%, NT-proBNP in 83%, evidence of left ventricular dysfunction on ECG 62% of the 29 children developed shock | 50% of patients were admitted to ICU One death | 22% only required supportive care, 22% developed acute kidney injury, shock requiring inotropic support 47%, and mechanical ventilation (43%), 2 children required extracorporeal membrane oxygenation for severe myocardial dysfunction |
| Dufort et al.,[14] | 66% had lymphopenia, 74 out of 82 (90%) had elevated proBNP levels, 63 out of 89 (71%) had elevated troponin, 100% had elevated C-reactive protein levels, 86 out of 94 (91%) had elevated D-dimer levels | 80% of patients were admitted to ICU 2% died (none of them received IVIG, Steroids, or immune- modulators) | 10% received mechanical ventilation 53% evidence of myocarditis, 52% had some degree of ventricular dysfunction, 32% had pericardial effusion, and 9% had a documented coronary-artery-aneurysm, 36% had KD or KD like syndrome, Median length of hospitalization stay was 6 days On date of publishing 77% of patients were discharged |
| Pouletty et al.,[15] | Ten patients (62%) fulfilled the American Heart Association (AHA) definition of complete KD. Inflammatory biomarkers were highly elevated in all patients. Acute renal failure was observed in nine cases (56%), Serum cytokines were elevated in tested patients | 43% were admitted to the ICU No death | 43% patients required fluid resuscitation. Respiratory assistance for ICU patients included oxygen therapy (n=4, 57%) for a median time of 2 days, non-invasive ventilation (n=3, 42%) or invasive ventilation (n=2, 28%). Cardiac ultrasound was abnormal and Myocardial enzymes were elevated in 11 patients. |
| Belhadjer et al.,[16] | All presented with a severe inflammatory state Inflammatory markers were suggestive of cytokine storm (C-reactive protein, D-dimer, and interleukin. Mild-moderate elevation in troponin, NT-proBNP or BNP elevation was present in all children. 10/35 depressed left ventricular systolic function <30%. 17% dilatation of coronary arteries | 83% were admitted to the ICU directly and 17% were transferred to ICU after one day from admission No death | 28% required mechanical circulatory assistance with ECMO, 66% required invasive mechanical ventilator support 80% were in cardiogenic shock required inotropic support & left ventricular function was restored in 25/35 patients of those discharged from the ICU, all patients treated with ECMO were successfully weaned. Two-thirds had respiratory distress requiring invasive mechanical ventilator support. 28/35 discharged. Seven patients were still in the hospital or with LV dysfunction. |
| Toubiana et al.,[17] | High level of inflammatory markers. Echocardiography detected coronary artery abnormalities in eight (38%). Transient kidney failure was observed in 11 (52%) patients. local patchy shadowing, and interstitial abnormalities were present in eight (44%) patients. Moderate increases in serum alanine transaminases and g-glutamyltransferase levels occurred in 62% and 76% of patients, respectively high sensitivity cardiac troponin 81%. B-type natriuretic peptide 78%. All patients had high level of inflammatory markers. 81% had lymphopaenia 95% increased D-dimer. 81% increased tropoinin. 78% increased B-type NP | 81% of patients required ICU No death | Outcome was favorable in all patients, Moderate coronary artery dilations were detected in 24% of patients during hospital stay, all patients were discharged home Median (range) length of hospital stay (days) 8 (5-17). Fluid resuscitation 11 (52%) Mechanical ventilation 11 (52%). |
| Verdoni et al.,[18] | Increased inflammatory markers ESR, CRP, and ferritin Full blood count showed a mean white cell count of 10.8¥109 per L (SD 6.1), with increased neutrophil percentage in eight patients lymphopenia in eight patients, and thrombocytopenia in eight patients. Hyponatraemia was observed in eight patients and a slight increase in transaminases was recorded in seven patients (aspartate aminotransferase, alanine aminotransferase, Hypertriglyceridaemia was shown in seven (87%) of eight tested patients in group 2, fibrinogen was high in nine (90%) of 10 patients as was D-dimer in eight (80%) of 10 patients. Laboratory criteria predicted intravenous immunoglobulin-resistance in seven (70%) of 10 patients. MAS was diagnosed in five (50%) of 10 patients. Troponin I was elevated in five (55%) of nine tested patients. creatine phosphokinase in one (10%) of 10 patients, and proBNP in all 10 patients. | Most patients (148 [80%]) were cared for in an intensive care unit 4 (2%) had died | Inotropic treatment in 20%, Response to treatment 100%. (48%) receiving vasoactive support. Most patients (170 [91%]) had at least one echocardiogram. Coronary-artery aneurysms identified on the basis of a z score of 2.5 or higher in the left anterior descending or right coronary artery were documented in 8% of the patients (15 of 186) and in 9% of those with echocardiograms (15 of 170). Respiratory insufficiency or failure occurred in 109 patients (59%). 85 (78%) of these patients had no underlying respiratory conditions. Overall, 37 patients (20%) received invasive mechanical ventilation and 32 (17%) received noninvasive mechanical ventilation. Most patients (132 [71%]) had involvement of at least four organ systems. The most commonly involved organ systems were the gastrointestinal (171 [92%]), cardiovascular (149 [80%]), hematologic (142 [76%]), mucocutaneous (137 [74%]), and respiratory (131 [70%]) systems. 37 (20%) received invasive mechanical ventilation. Eight patients (4%) received extracorporeal membrane oxygenation (ECMO) support. A total of 130 patients (70%) had been discharged alive, 52 (28%) were still hospitalized. The median length of hospitalization was 7 days among the patients who were discharged alive and 5 days (range, 2 to 5) among those who died. The 4 patients who died were 10 to 16 years of age; 2 of the patients had diagnoses of underlying conditions, and 3 received ECMO support |
| Feldstein et al.,[19] | 92% elevation in at least four biomarker indicating inflammation (73% BNP, 50% troponin). Respiratory failure or insufficiency 59%, 92% had 4 elevated inflammatory markers (Majority had elevated ESR, C-reactive protein, lymphocytopenia, neutrophilia, ferritin,....) | Most patients (148 [80%]) were cared for in an intensive care unit and 4 (2%) had died | 37 (20%) received invasive mechanical ventilation. Eight patients (4%) received ECMO support. a total of 130 patients (70%) had been discharged alive, 52 (28%) were still hospitalized, the median length of hospitalization was 7 days (interquartile range, 4 to 10) among the patients who were discharged alive and 5 days (range, 2 to 5) among those who died |
| Ramcharan et al.,[20] | Elevated inflammatory markers. 100% troponin, 7 had chest abnormalities including pleural effusion, consolidation, cardiomegaly. Fourteen patients had chest radiographs; 7 were normal, 7 had abnormalities including pleural effusions (5), consolidation (3), and cardiomegaly (2). Six patients had abdominal ultrasound due to persistent gastrointestinal symptoms, showing no abnormalities. During their admission, two patients had non-coronary CT angiograms and one had a MRI whole body, due to persisting inflammation despite treatment, all of which showed no evidence of vasculitis. 60% had cardiac abnormalities | 67% of patients needed ICU with a median stay of 4 days There were no deaths. | 8 (53%) needed respiratory support, half of them required mechanical ventilation and others required high-flow nasal cannula support, median hospital stay 12 days. Ten patients (67%) needed fluid resuscitation. Nine required epinephrine to support LV dysfunction. Median inpatient stay was 12 days (IQR 9-13 days). All 15 patients were discharged home clinically well with normal/improving biochemical and cardiac parameters. |
| Miller et al.,[21] | Overall, the majority of cases at admission had markedly elevated inflammatory markers. ESR CRP and mildly decreased albumin Transaminases were elevated in 52.3% and lipase was elevated >3 times. Upper limit of normal in only one patient. Findings included mesenteric adenitis (n=2), biliary sludge or acalculous cholecystitis (n=6), and ascites (n=6). In three patients, US or MRI revealed bowel wall thickening (n=3), Of these patients, one had intense RLQ abdominal pain, fever and rash with MRI findings of severe concentric mural thickening, edema, and hyper-enhancement of a short segment of terminal ileum with extensive mesenteric fat edema, as well as similar mural thickening in the rectosigmoid colon | No death | Intubation 2.3%, one required renal replacement therapy. 2.3%, Discharged on publishing 97.7%. Only 25% required supplemental oxygen and one was intubated. |
| Riollano-Cruz et al.,[22] | Elevated inflammatory markers in all patients, including 15 (100%) with elevated CRP and D-dimer, and 13 (87%) with elevated ferritin levels and 14 (93%) ESR. Levels of procalcitonin were checked in 13 patients and were elevated in 9 (60%) cases. At admission, lymphopenia was present in 13 (87%) patients, thrombocytopenia in 6 (40%), hypoalbuminemia in 8 (53%), and elevated fibrinogen in 14 (93%) patients. 100% interleukin-6, and interleukin 8. And 87% severe cardiac involvement. The most common findings Four (27%) patients presented with only depressed left ventricular function, and 3 (20%) with depressed biventricular function. Three patients presented with coronary artery abnormalities, including one patient with dilation and 2 (13%) with ectasia. One patient had ventricular tachycardia and ventricular ectopy, and another one had diffuse ST elevations on ECG. | 14 patients needed ICU One death required ECMO | 20% required intubation and mechanical ventilation., One patient required an intra-aortic balloon pump to treat cardiogenic shock, all except one were discharged, none required mechanical circulatory support, and one patient required renal replacement therapy. Three patients (20%) required intubation and mechanical ventilation, and an additional 5 (33%) patients required noninvasive mechanical ventilation. The child who died required ECMO during the nine days of admission. Eight (53%) patients needed vasopressor and vasoactive therapy, and one patient required an intra-aortic balloon pump to treat cardiogenic shock. Nine had gradual normalization of D-dimer, BNP, and troponin levels during admission. Thirteen remained admitted for a range of 6-13 days (mean 8 days) and have had continued improvement in inflammatory parameters upon outpatient follow-up. One patient expired on day nine after admission and one remains admitted. |
| Cheung et al.,[23] | All had elevated inflammatory markers (lymphopenia 71%, elevated troponin 82%, NT proBNP 100%). ECG showed nonspecific abnormalities | 88% of patients were admitted to the ICU No death | Most patients had improved function on follow-up echocardiogram. One patient had a medium-sized aneurysm of the left anterior descending coronary artery. Length of hospitalization 3-18 (7.1) days, All patients discharged home, vasoactive support in 59% |
| Belot et al.,[24] | KLD and myocarditis were the most prevalent clinical features and were associated with 61% and 70% of the cases, respectively. Seritis and features of MAS were also overrepresented with a frequency of 22% and 23%. | Critical care support was required in 67% of cases one death | 73% of the patients who were admitted to the intensive care unit required vasopressors and 43% required mechanical ventilation. |
| ICU: Intensive care unit; SARS-CoV-2: Severe acute respiratory syndrome-coronavirus 2; KD: Kawasaki disease; ECMO: Extracorporeal membrane oxygenation; BNP: B-type natriuretic peptide; ESR: Erythrocyte sedimentation rate; CRP: C-reactive protein; SD: Standard deviation; LV: Left ventricular; IQR: Interquartile range; US: Ultrasound; MRI: Magnetic resonance imaging; RLQ: Right lower quadrant; ECG: Electrocardiogram; KLD: Kawasaki like disease; MAS: Macrophage activation syndrome. | |||
There are overlapping features between MIS-C and KD symptoms suggesting that this new condition may be a new variant of KD, triggered by SARS-CoV-2 infection (Table 5). However, the striking and significant elevation in the inflammatory markers is higher in the MIS-C in comparison to KD.
Table 5. Differences in clinical features of typical KD and included cases in the present review.
| Kawasaki disease | Results of the included studies MIS-C | Clinical feature |
| Gastrointestinal symptoms | Have more gastrointestinal manifestation 80-97% except one single study (60%) | 30% |
| Cardiac dysfunction (mainly left ventricular dysfunction) | Have greater risk of left ventricular dysfunction 48-100% | 1.5-7% & approaches 20% if not treated |
| Shock | Have greater risk of shock 47-80% (mentioned in nine out of 13 studies) | 3% |
| Age (years) | Median (7.3-12) | <5 |
| Inflammation | Have a more profound form of inflammation | Less profound form of inflammation |
| KD: Kawasaki disease; MIS-C: Multisystem inflammatory syndrome in children. | ||
Therapeutic management and outcomes
Several children in the majority of the included studies developed severe hypotension requiring admission to the ICUs. Of note, more than 79% of the included patients in eight studies were admitted to the ICU,[12,14,16-19,22,23] whereas 38 to 67% of other patients in four studies needed ICU admission.[13,15,20,24] Mechanical ventilation was required for the included patients in 12 studies (10 to 66%).[12-22,24] Most of the patients who were admitted to ICU required inotropes and vasopressors (Table 3).
Across 12 studies, the majority of affected children (more than 60%) were managed therapeutically with intravenous immunoglobulin (IVIG).[12-23] In five studies, 80 to 100% of the included cases received IVIG.[12,16,17,21,22] Steroids were used in all studies ranging from 10 to 95% of the included patients. In 12 studies, 66 to 100% of the included cases were treated with IVIG as first-line therapy as a single dose[12-23] of whom four studies indicated that 1 to 62% required a second dose of IVIG doses.[15,16,17,20] Steroids were used for the included cases in 12 studies as first-line therapy or adjunctive therapy ranging from 20 to 100%.[12-23] It is worth noting that death occurred in two of the included cases in one study[14] and neither of them received IVIG, systemic glucocorticosteroids, or immunomodulators. On the other hand, acetylsalicylic acid was used in a few studies,[12,22,23] whereas anticoagulant was used in a single study.[23] Remdesivir was used in one single study in 13% of the included children.[22] In two studies, the broad-spectrum antibiotics were used in all included patients.[20,22] Most patients responded favorably to therapeutic management with an improvement in the vital signs and cardiac dysfunction, few cases in six studies[12,13,14,19,21,22] required additional therapies such as biological modulating drugs. Clinical outcomes of death, discharged, or still hospitalized were described for all cases in the included studies (Table 4). Of these, the majority of cases were discharged when studies were submitted. Overall mortality rates have been low with six deaths (0.9% among included cases) occurred among the included studies. It was reported that 13 patients from the included cases in the present review died representing a range of 1 to 66%.[13,14,18,19,22,24]
Table 4. Therapeutic management.
| Reference | Treatment | |||
| IVIG | Aspirin | Corticosteroids | Others | |
| Capone et et al.,[12] | 100% of patients received IVIG | - | 70% of patients received corticosteroids | 24% of cases were treated with biologic modifying medication. 76% requiring vasoactive medications |
| Whittaker et al.,[13] | 71% of patients received IVIG | - | 64% of patients received corticosteroids | Three patients received anakinra. Eight patients received infliximab. 60% needed two agent of medication, 16% needed three agents. |
| Dufort et al.,[14] | 70% of patients received IVIG | - | 64% of patients received corticosteroids | 48% patients received both IVIG and systemic glucocorticosteroids. 72% received empiric systemic antibacterial therapy. 62% received vasopressor support |
| Pouletty et al.,[15] | 93% of patients received IVIG (31% single IVIG 62% required second line of treatment | 93% (Aspirin was added to IVIG either at anti-inflammatory doses (30 to 80 mg/kg/day) in seven (47%) patients or as an antiaggregant in eight (53%) patients | 25% of patients received corticosteroids & 10% required steroids with second line treatment | Two patients received IL-1 receptor antagonist, one patient received hydroxychloroquine for initial suspicion of systemic lupus erythematosus. Six patients required inotropic supports |
| Belhadjer et al.,[16] | 25/35 of patients received IVIG. One patient needed repeated IVIG infusion | 12 patients received corticosteroids | Three patients needed IL I antagonist. 23/35 needed therapeutic dose of heparin. 28/35 inotropic support | |
| Toubiana et al.,[17] | All 21 patient received high dose of IVIG. Five patients (24%) needed second infusion with corticosteroids in four of them | All patients needed low dose aspirin | 7 patients received coticosteroids | (86%) patients received empirical broad spectrum antibiotic treatment. Including third generation cephalosporin. Vasoactive and inotropic agents 15 (71%). |
| Verdoni et al.,[18] | 100% of patients received IVIG | 80% received corticosteroids as adjunctive therapy | ||
| Ramcharan et al.,[20] | Ten patients received intravenous immunoglobulin (IVIG), of whom two received a second dose | Eleven patients (73%) were discharged on low dose aspirin with two requiring high doses initially | Five patients received IV methylprednisolone followed by oral prednisolone, Intravenous hydrocortisone was used for refractory hypotension in eight patients | All patients were treated with broad spectrum antibiotics for at least five days. Ten (67%) required inotropes/vasopressors. Norepinephrine was used in eight (53%) with additional support using vasopressin in three to treat systemic hypotension. One patient was transferred from an external hospital on milrinone, which was weaned off. |
| Miller et al.,[21] | 81.8% of patients received IVIG | 95.5% of patients received corticosteroids | 18.2% of patients received anakinra. 90.1% anticoagulation | |
| Riollano-Cruz et al.,[22] | 80% of patients received IVIG | 20% of patients received | All patients were treated with broad-spectrum | |
| corticosteroids | antibiotics for possible septic shock and toxic shock syndrome. 60% required inotropic or vasopressor support. 13% remdesivir. Additionally, all patients received prophylactic anticoagulation with enoxaparin which continued until two weeks' post-discharge. Twelve (80%) patients received one to three intravenous doses of the anti-interleukin -6 (anti-IL-6) antibody tocilizumab; one of those patients also received SARS-CoV-2 convalescent plasma transfusion. 13% received Anakinra. Two patients (13%) were treated with Remdesivir; one completed five days and the other one nine days of treatment. | |||
| Cheung et al.,[23] | 76% of patients received IVIG | Aspirin 24% | 14 patients received steroids | One patient received tocilizumab enoxaparin |
| (71% methylprednisolone 21% hydrocortisone) | 59% prophylaxis 6% treatment | |||
| Belot et al.,[24] | NA | NA | NA | NA |
| IVIG: Intravenous immunoglobulin; IV: Intravenous; NA: Not available. | ||||
Discussion
In this review article, we described the main clinical manifestations for patients younger than 21 years who presented with MIS-C, and were infected with or had a recent exposure to COVID-19 patients reported in 13 observational studies published in the literature during between December 2019 and June 2020. Among the included patients, more than 70% tested positive for SARS-CoV-2 infection, and it was observed that the severity of the COVID-19 infection among the positive cases in the included studies was mild to moderate, with the majority of patients responded favorably to therapeutic management and with only six reported deaths. In general, COVID-19 has a favorable clinical course in children and usually progresses with a mild course.[2,3] Nonetheless, in April 2020, a new life-threatening condition in children named MIS-C emerged.[8,25] In most of the included studies, the temporal association between the onset of the SARS-CoV-2 pandemic worldwide with Kawasaki-like disease and MIS-C suggests a causal link.
As presented by Riphagen et al.,[26] hyperinflammatory shock is a common component in MIS-C. From the clinical manifestations presented in the recruited studies, it was evident that MIS-C has a cascade of symptoms which include fever, abdominal pain, diarrhea, vomiting, mucocutaneous manifestations, and elevation of inflammatory biomarkers. However, KD affects predominantly young children <5 years of age, whereas the median age in our review is (7 to 12) years.[27] Until now, there is no available test used to diagnose KD in children, with the diagnosis based solely on the clinical manifestations according to the American Heart Association (AHA) criteria.[28] This disease is considered among the most common causes of heart disease in children, including coronary heart disease, which can lead to sudden death in some children.[28] Physicians have noted some clinical similarities between MIS-C and KD; however, these clinical signs can be noticed in many infectious diseases in children and are not specific for any one diagnosis. The main question raised in this review is whether MIS-C is a new spectrum of KD, suggesting that it is a KD with SARS-CoV-2 as the main triggering factor leading to a new spectrum of KD.
While children with COVID-19 present with upper respiratory tract symptoms, MIS-C is distinguished by fever, GI symptoms such as vomiting, significant elevation in the inflammatory markers, and mucocutaneous manifestations such as rash. In this review, it was evident that all of the included patients showed a confirmed elevation of the inflammatory markers in addition to some other features related to KD such as fever, GI symptoms, and mucocutaneous manifestation. Among the included studies, six studies reported that around 29 to 64% of the patients presented with features consistent with KD,[1,4,6,10,12,13] while around 40 to 61% of patients in other studies were diagnosed with incomplete KD.[7,9,13,14] In addition, three studies reported that 48%, 44%, and 50% of the included patients met the criteria for KD shock syndrome (KDSS). This may support the claim that MIS-C is a new variant of KD with a striking elevation of inflammatory markers in comparison to KD. The triggering factor in both is the SARS-CoV-2 virus. The present reports indicate that MIS-C typically manifests three to four weeks after SARS-CoV-2 infection.[19,29] This may justify why some children in the present review had positive antibodies to SARS-CoV-2, but negative RT-PCR at the time of MIS-C assessment.[12,13,17]
The prevalence of cardiac manifestations in children with MIS-C was dominant (48 to 100%). Many of the included cases in the present review had an initial echocardiogram that was normal and showed depressed ejection fraction or dilation/aneurysm of the coronary arteries after few days. The results revealed that the most common cardiac abnormality, on echocardiogram, was a depressed ejection fraction. In line with these outcomes, a recent research has shown that adult cases who recently recovered from COVID-19 had myocardial inflammation in addition to ongoing cardiac involvement.[30] Thus, pediatric patients undergoing evaluation for MIS-C must have a baseline assessment echocardiogram, electrocardiogram, and their cardiac function and artery changes should be followed through repeating imaging. Additionally, close follow-up is important, since the long-term implications of MIS-C cardiac involvement are still unknown.
Kawasaki disease typically presents with high fever and peeling skin with inflammation.[31] The overlapping features between MIS-C and KD suggest that they may share a similar pathophysiology and likely explains why these patients respond to similar therapies. As described in this review, most children respond to similar therapies.[32] It was observed in the recruited studies that more than 60% of the affected children received IVIG.[12-23] Acetylsalicylic acid was used in a few studies.[12,15,17,20,23] Corticosteroids, on the other hand, were used in almost all studies ranging from 10 to 95% of the included patients.[12-23] This indicates that some patients with severe COVID-19 may need to start the treatment protocol for KD, as they show a favorable response to IVIG. Initiating such treatment protocol may prevent the occurrence of the cytokine storm and subsequent multi-organ failure. Although the mortality rate in the present reviews is low (0.9%), it is much higher than the reported mortality rate (0.09%) observed in pediatric patients with COVID-19.[33]
More research is needed to understand why some children may be more susceptible to develop MIS-C. This disease is rare, in a single published report, the estimated incidence of laboratory-confirmed SARS-CoV-2 infection in individuals aged <21 years was 322/100,000 and the incidence of MIS-C was 2/100,00014. However, the long-term sequelae from this condition are currently not clear. The results of the present review are consistent with the previous report.[8] In a recent study, the two most common systemic involvements in their study were cardiac and GI. Additionally, older age was found to be an independent predictor for pediatric ICU admission. Moreover, respiratory, renal, and neurological involvement rates were significantly higher in patients admitted to ICUs.[8]
This review summarizes the clinical presentation of a new childhood disease that is most likely linked to SARS-CoV-2 infection. Multisystem inflammatory syndrome in children is a severe systemic infection characterized by inflammation, fever, abdominal symptoms, conjunctivitis, and rash. Children would present with signs and symptoms of MIS-C in most of the cases three to four weeks after COVID-19 infection and many would progress rapidly into shock and cardiorespiratory failure. The results of the present review are consistent with the previous report.[8] The majority of include cases of the present review had higher inflammatory markers with higher cardiac involvement. Moreover, most of them suffering from cytokine storm that is reflected clinically by heart failure, GI, neurological, and renal features associated with a high level of inflammatory markers, such as C-reactive protein, ferritin, interleukin 1 (IL1), IL6 at the time of admission or shortly after the admission. It is worth noting that patients who were admitted to the ICU units had a higher level of systemic inflammatory markers and had a more severe disease course. Interestingly, most of these patients had a good recovery by the time of discharge. Nonetheless, long-term follow-up is required to assess the impact of such a condition on organ functions.
The overlapping features between MIS-C and KD symptoms suggest that this new condition may be a new variant of KD triggered by SARS-CoV-2 infection. The clinical features and laboratory findings and pathogenesis, which involves mainly cytokine storm, of MIS-C in the present review appear to be similar to KD; however, we found that GI and cardiac involvement and the risk of developing shock state were more common and severe in MIS-C than KD. Moreover, while 80% of KD are encountered in children younger than five years; MIS-C extends to older children with a median age of seven to 12 years old. Additionally, both KD and MIS-C respond to the same management which includes high-dose IVIG.
This review has several limitations. First, we were rigorous in our search, but there is a possibility we may have missed studies. Second, our review is mainly descriptive, and international statistical conclusions cannot be drawn from it. However, we believe that this report is clinically important and may affect the patient care in the clinical setting.
In conclusion, this review discusses the clinical presentation of a new childhood disease that is most likely linked to SARS-CoV-2 infection. Multisystem inflammatory syndrome in children is a rare and dangerous systemic infection characterized by hyperinflammation, fever, and abdominal symptoms, and the potential long-term sequelae are currently unknown. The similarities between MIS-C and KD suggest that MIS-C may be a new variant of KD triggered by the SARS-CoV-2 virus. However, MIS-C is more severe than KD and this may be due to significantly higher inflammatory markers than KD. Parents should seek medical advice and physicians should be vigilant not to miss MIS-C in any child presenting with fever or history of fever along with confirmed COVID-19 infection or history of exposure to individuals with COVID-19 infection. We strongly believe, despite the presence of few differences between MIS-C and KD, MISC is a spectrum of KD, but to a more severe extent.
Footnotes
Conflict of Interest: The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Author Contributions: Conceptualized and designed the study, designed the data collection instrument, collected data, carried out the initial analyses, drafted the initial manuscript, reviewed and revised the manuscript: K.A.H.; Carried out the initial analyses, drafted the initial manuscript, reviewed and revised the manuscript: R.A.F., Q.M.; Carried out the initial analyses, drafted the initial manuscript, reviewed and revised the manuscript: T.D.; Conceptualized and designed the study, coordinated and supervised data collection, and critically reviewed the manuscript for important intellectual content: W.A.H.
Financial Disclosure: The authors received no financial support for the research and/or authorship of this article.
References
- 1.Lu X, Zhang L, Du H, Zhang J, Li YY, Qu J, et al. SARS-CoV-2 infection in Children. N Engl J Med. 2020;382:1663–1665. doi: 10.1056/NEJMc2005073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.CDC COVID-19 Response Team Coronavirus Disease 2019 in Children - United States, February 12-April 2, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:422–426. doi: 10.15585/mmwr.mm6914e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in china. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kawasaki T. Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children. Arerugi. 1967;16:178–222. [PubMed] [Google Scholar]
- 5.PubMed Health Kawasaki Disease. NHLBI Health Topics. 11 June 2014. Archived from the original on 11 September 2017. Retrieved 26 August 2016. [Google Scholar]
- 6.Guidance - paediatric multisystem inflammatory syndrome temporally associated with COVID-19. Royal College of Paediatrics and Child Health. Available at: https://www.rcpch.ac.uk/resources/guidance-paediatric-multisystem-inflammatorysyndrome -temporally-associated-covid-19. [Accessed: July 8, 2020] [DOI] [PubMed]
- 7. Available at: https://www.cdc.gov/mis-c/ [Accessed: May 14, 2020]
- 8.Haslak F, Yıldız M, Adrovic A, Şahin S, Barut K, Kasapçopur Ö. A recently explored aspect of the iceberg named COVID-19: Multisystem inflammatory syndrome in children (MIS-C) Turk Arch Pediatr. 2021;56:3–9. doi: 10.5152/TurkArchPediatr.2020.20245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abuhammour W, Dawoud T. What can we learn from Kawasaki disease to treat COVID-19 patients. EC Paediatrics. 2020;9:27–28. [Google Scholar]
- 10.National Heart, Lung, and Blood Institute Quality assessment tool for observational cohort and cross-sectional studies. National Institutes of Health (2014) Available at: https://www.nhlbi.nih.gov/healthtopics/study-quality-assessment-tools. [Accessed: 2017]
- 11.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J Clin Epidemiol. 2009;62:1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 12.Capone CA, Subramony A, Sweberg T, Schneider J, Shah S, Rubin L, et al. Characteristics, cardiac involvement, and outcomes of multisystem inflammatory syndrome of childhood associated with severe acute respiratory syndrome coronavirus 2 infection. J Pediatr. 2020;224:141–145. doi: 10.1016/j.jpeds.2020.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whittaker E, Bamford A, Kenny J, Kaforou M, Jones CE, Shah P, et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. 2020;324:259–269. doi: 10.1001/jama.2020.10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dufort EM, Koumans EH, Chow EJ, Rosenthal EM, Muse A, Rowlands J, et al. Multisystem inflammatory syndrome in children in New York state. N Engl J Med. 2020;383:347–358. doi: 10.1056/NEJMoa2021756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pouletty M, Borocco C, Ouldali N, Caseris M, Basmaci R, Lachaume N, et al. Paediatric multisystem inflammatory syndrome temporally associated with SARS-CoV-2 mimicking Kawasaki disease (KawaCOVID-19): A multicentre cohort. Ann Rheum Dis. 2020;79:999–1006. doi: 10.1136/annrheumdis-2020-217960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Belhadjer Z, Méot M, Bajolle F, Khraiche D, Legendre A, Abakka S, et al. Acute heart failure in multisystem inflammatory syndrome in children in the context of global SARS-CoV-2 pandemic. Circulation. 2020;142:429–436. doi: 10.1161/CIRCULATIONAHA.120.048360. [DOI] [PubMed] [Google Scholar]
- 17.Toubiana J, Poirault C, Corsia A, Bajolle F, Fourgeaud J, Angoulvant F, et al. Kawasaki-like multisystem inflammatory syndrome in children during the covid-19 pandemic in Paris, France: Prospective observational study. m2094BMJ. 2020;369 doi: 10.1136/bmj.m2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verdoni L, Mazza A, Gervasoni A, Martelli L, Ruggeri M, Ciuffreda M, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: An observational cohort study. Lancet. 2020;395:1771–1778. doi: 10.1016/S0140-6736(20)31103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feldstein LR, Rose EB, Horwitz SM, Collins JP, Newhams MM, Son MBF, et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383:334–346. doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramcharan T, Nolan O, Lai CY, Prabhu N, Krishnamurthy R, Richter AG, et al. Paediatric inflammatory multisystem syndrome: Temporally associated with SARS-CoV-2 (PIMS-TS): Cardiac features, management and short-term outcomes at a UK tertiary paediatric hospital. Pediatr Cardiol. 2020;41:1391–1401. doi: 10.1007/s00246-020-02391-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller J, Cantor A, Zachariah P, Ahn D, Martinez M, Margolis KG. Gastrointestinal symptoms as a major presentation component of a novel multisystem inflammatory syndrome in children that is related to coronavirus disease 2019: A single center experience of 44 cases. Gastroenterology. 2020;159:1571–1574. doi: 10.1053/j.gastro.2020.05.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Riollano-Cruz M, Akkoyun E, Briceno-Brito E, Kowalsky S, Reed J, Posada R, et al. Multisystem inflammatory syndrome in children related to COVID19: A New York City experience. J Med Virol. 2021;93:424–433. doi: 10.1002/jmv.26224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheung EW, Zachariah P, Gorelik M, Boneparth A, Kernie SG, Orange JS, et al. Multisystem inflammatory syndrome related to COVID-19 in previously healthy children and adolescents in New York City. JAMA. 2020;324:294–296. doi: 10.1001/jama.2020.10374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Belot A, Antona D, Renolleau S, Javouhey E, Hentgen V, Angoulvant F, et al. SARS-CoV-2-related paediatric inflammatory multisystem syndrome, an epidemiological study, France, 1 March to 17 May 2020. Euro Surveill. 2020;25:2001010–2001010. doi: 10.2807/1560-7917.ES.2020.25.22.2001010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Multisystem inflammatory syndrome in children and adolescents with Covid-19. World Health Organisation. published May 2020. Available at: https://emergency.cdc.gov/han/2020/han00432.asp. (Accessed: August 2020).
- 26.Riphagen S, Gomez X, Gonzales-Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020 doi: 10.106/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Correction to: Diagnosis, treatment, and long-term management of Kawasaki disease: A scientific statement for health professionals from the American Heart Association e181-e184.Circulation. 2019;140 doi: 10.1161/CIR.0000000000000703. [DOI] [PubMed] [Google Scholar]
- 28.Dajani AS, Taubert KA, Gerber MA, Shulman ST, Ferrieri P, Freed M, et al. Diagnosis and therapy of Kawasaki disease in children. Circulation. 1993;87:1776–1780. doi: 10.1161/01.cir.87.5.1776. [DOI] [PubMed] [Google Scholar]
- 29.Godfred-Cato S, Bryant B, Leung J, Oster ME, Conklin L, Abrams J, et al. COVID-19-associated multisystem inflammatory syndrome in children - United States, March-July 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1074–1080. doi: 10.15585/mmwr.mm6932e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Puntmann VO, Carerj ML, Wieters I, Fahim M, Arendt C, Hoffmann J, et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:1265–1273. doi: 10.1001/jamacardio.2020.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kil HR, Yu JW, Lee SC, Rhim JW, Lee KY. Changes in clinical and laboratory features of Kawasaki disease noted over time in Daejeon, Korea. Pediatr Rheumatol Online J. 2017;15:60–60. doi: 10.1186/s12969-017-0192-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCrindle BW, Rowley AH, Newburger JW, Burns JC, Bolger AF, Gewitz M, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: A scientific statement for health professionals from the American Heart Association. e927-e999Circulation. 2017;135 doi: 10.1161/CIR.0000000000000484. [DOI] [PubMed] [Google Scholar]
- 33.Hoang A, Chorath K, Moreira A, Evans M, Burmeister-Morton F, Burmeister F, et al. COVID19 in 7780 pediatric patients: A systematic review. EClinicalMedicine. 2020;24:100433–100433. doi: 10.1016/j.eclinm.2020.100433. [DOI] [PMC free article] [PubMed] [Google Scholar]