Abstract
In Escherichia coli, high-abundance chemoreceptors are present in cellular amounts approximately 10-fold higher than those of low-abundance receptors. These two classes exhibit inherent differences in functional activity. As sole cellular chemoreceptors, high-abundance receptors are effective in methyl-accepting activity, in establishing a functional balance between the two directions of flagellar rotation, in timely adaptation, and in mediating efficient chemotaxis. Low-abundance receptors are not, even when their cellular content is increased. We found that the low-abundance receptor Trg acquired essential functional features of a high-abundance receptor by the addition of the final 19 residues of the high-abundance receptor Tsr. The carboxy terminus of this addition carried a methyltransferase-binding pentapeptide, NWETF, present in high-abundance receptors but absent in the low-abundance class. Provision of this docking site not only enhanced steady-state and adaptational methylation but also shifted the abnormal, counterclockwise bias of flagellar rotation toward a more normal rotational balance and vastly improved chemotaxis in spatial gradients. These improvements can be understood as the result of both enhanced kinase activation by the more methylated receptor and timely adaptation by more efficient methyl-accepting activity. We conclude that the crucial functional difference between the low-abundance receptor Trg and its high-abundance counterparts is the level of methyl-accepting activity conferred by the methyltransferase-docking site.
Chemotactic responses to an array of attractants and repellents are mediated in Escherichia coli by four well-characterized methyl-accepting chemotaxis proteins. These transmembrane receptors are related by a common domain organization, by significant sequence identity, and probably by a shared three-dimensional structure. References to the current body of information about these receptors can be found in several reviews (8, 11, 28). These chemoreceptors are the most extensively studied members of a large family of proteins that mediate tactic responses in a wide range of bacteria and archaea (17, 20, 36). The hallmarks of this family are a highly conserved region of ∼50 residues that is crucial for intracellular signaling through its control of a noncovalently associated histidine kinase, CheA, and bracketing regions containing methyl-accepting glutamyl residues that are covalently modified in the process of sensory adaptation. For the E. coli chemoreceptors Tsr, Tar, Trg, and Tap, two transmembrane segments, one near the amino terminus and the other approximately 40% of the way along the sequence, bracket a periplasmic, ligand-binding domain of ∼150 residues and connect it to a carboxy-terminal, cytoplasmic domain of ∼300 residues that contains the regions of kinase control and adaptational methylation.
Chemoreceptors are homodimers that form ternary complexes with CheA and an accessory protein, CheW (see references 8 and 28 for reviews of our understanding of the Che proteins). In a ternary complex, the receptor activates the kinase to establish a steady-state level of autophosphorylation. The availability of phosphorylated CheA (phospho-CheA) in turn determines the extent of phosphorylation of the response regulator CheY. Phospho-CheY binds to the flagellar switch to cause clockwise (CW) rotation of an otherwise counterclockwise (CCW)-rotating motor. An appropriate balance between CCW and CW flagellar rotation produces an alternation between runs and tumbles that creates a three-dimensional random walk as the cell swims. This balance requires a proper level of basal activation of the kinase to provide an appropriate phospho-CheY content. An increase in attractant occupancy at the ligand-binding site of a receptor reduces kinase activity and correspondingly the phospho-CheY concentration. The resulting reduced probability of CW rotation, and thus of tumbles, biases the random walk toward higher concentrations of attractant. The effects of changes in attractant occupancy at the ligand-binding site are transient because stimulated receptors are activated not only for kinase control but also at their methyl-accepting sites. Receptors experiencing increased attractant binding are activated to increase methylation, catalyzed by the methyltransferase CheR, to the extent necessary to balance the changes in occupancy and thus to restore kinase activity to its basal level. Some methyl-accepting sites are initially glutamines that are subsequently deamidated by CheB to create methyl-accepting glutamyls (15). An amide at a modification site is in large part the functional equivalent of a methyl ester (5, 6, 26).
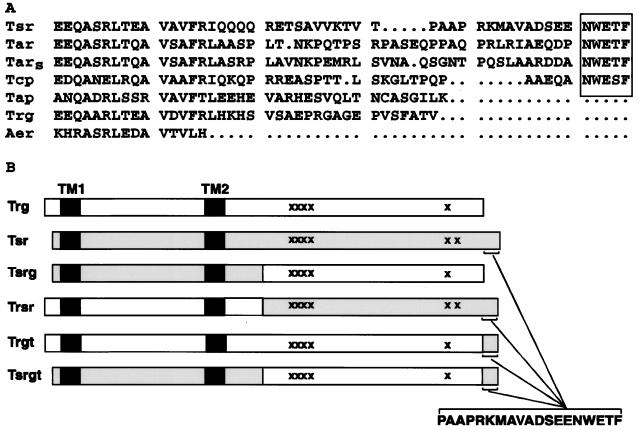
In wild-type E. coli, two high-abundance receptors, Tsr and Tar, are present in cellular amounts approximately 10-fold higher than those of two low-abundance receptors, Trg and Tap. Cells lacking high-abundance receptors exhibit abnormally low tumble frequencies and extended adaptation times, and the ability of the low-abundance receptors to mediate directed migration in spatial gradients is substantially compromised (9, 12, 14, 25, 34, 35). These phenotypes are not simply the result of reduced receptor content in cells lacking the numerically predominant high-abundance receptors, since increasing the cellular dosage of a low-abundance receptor in the absence of high-abundance receptors does not increase tumble frequency or improve the tactic response (9, 33). Instead, low-abundance receptors appear to be distinguished from high-abundance receptors by an inherent difference in activity. Characterization of hybrids between the low-abundance receptor Trg and the high-abundance receptor Tsr (9) or between the analogous pair of Tap and Tar (33) demonstrated that this difference resides in the cytoplasmic domain. Yet, it is in the cytoplasmic domains that receptors show the highest (∼60%) sequence identity and interact with the same sensory components: CheA, CheW, CheR, and CheB. However, Wu et al. (34) recently showed that a carboxy-terminal pentapeptide, NWETF, present on Tsr and Tar but absent from Trg and Tap (Fig. 1A), is a specific binding site for the methyltransferase CheR. A chemoreceptor recently discovered in E. coli, Aer (3, 29), is present in low abundance and also lacks the pentapeptide (Fig. 1A). To what extent is the absence of a CheR-binding pentapeptide in low-abundance receptors the basis of the functional differences between the receptor classes? To investigate this question, we grafted the 19-residue, carboxy-terminal segment of the high-abundance receptor Tsr, containing the methyltransferase-docking site, to the carboxyl terminus of the low-abundance receptor Trg and to the carboxyl terminus of a Tsr-Trg hybrid that is phenotypically a low-abundance receptor (9) and examined the functioning of these altered receptors in vivo.
FIG. 1.
Carboxy-terminal amino acid sequences of natural chemoreceptors and diagrams of hybrid receptors. (A) Aligned carboxy-terminal amino acid sequences of E. coli Tsr, Tar, Trg, Tap, and Aer and of Salmonella typhimurium Tar (TarS) and Tcp deduced from the nucleotide sequences. The carboxy-terminal sequence conserved in high-abundance receptors is boxed. The carboxy-terminal sequence of Trg shown here differs from the sequence that we originally deduced (4) because, upon resequencing of the corresponding region of the gene, we found an additional C after position 1602 (the third base at codon 534) and an additional G after original position 1611. The revised gene sequence corresponds to the trg sequence determined in the Escherichia coli Genome Project and places the stop codon after codon 546 instead of after codon 537. Thus, Trg is 9 residues (GEPVSFATV) longer than originally deduced, and the 3 preceding residues are RGA instead of AER. (B) Primary structures of natural and hybrid chemoreceptors used in this study. The diagrams show transmembrane segments (TM1 and TM2), methyl-accepting sites (×), and the positions of fusion joints. Among the five methyl-accepting sites of Trg (23) and the six of Tsr (30), all but one, a site in the K1 peptide of Tsr, have been identified.
MATERIALS AND METHODS
Strains and plasmids.
CP177, a derivative of E. coli K-12, is ara-14 his-4 lacY1 leuB6 rpsL136 thi-1 thr-1(Am) tonA31 tsx-78 xyl-5 and contains a complete chromosomal deletion of trg linked to zdb::Tn5 (27). CP362, derived from CP177, carries additional deletions of tsr, tar, and tap (27). Plasmids pAL1, pCT1, pHF1, and pHF2 are derivatives of pHSe5 (21) in which tandem tac promoters and operators control the expression, respectively, of trg, tsr, trsr, and tsrg. pCT1 is our name for the tsr overexpression plasmid (10) created by insertion of an ∼2,500-bp BamHI-HindIII fragment containing tsr into pHSe5. pAL1 was created by oligonucleotide-directed mutagenesis of pGB1 (9) to change its EcoRI site to a BamHI site and its SacI site to a HindIII site and then replacement of the tsr-containing BamHI-HindIII fragment of pCT1 with the comparable, trg-containing fragment of the altered pGB1. See Feng et al. (9) for a description of the construction of trsr and tsrg. pAL75 carries trgt, in which the final 19 codons of tsr have been fused to the 3′ end of trg. It was constructed by creating sites for restriction endonucleases that create blunt ends at the center of their recognition sites. By PCR-based in vitro mutagenesis with appropriate mutagenic primers, we made the following changes: in pAL1 the final two codons of trg (GTGTGA, coding for Val and stop [termination of translation]) were converted to the Bst1107I recognition site (GTATAC, coding for Val and Tyr, respectively), and in pCT1 the sequence beginning 20 codons from the 3′ end of tsr was changed from ACGCCA (coding for Thr and Pro) to an StuI site (AGGCCT, coding for Arg and Pro, respectively). A combination of the trg-containing BamHI-Bst1107I fragment from the altered form of pAL1 with the StuI-BamHI fragment from the altered form of pCT1 produced pAL75. The resulting fusion gene, trgt, coded for all 546 residues of native Trg followed by the carboxy-terminal 19 residues of Tsr. pHF3, which carries tsrgt, was constructed by recombining the larger HindIII-EagI fragment from pHF2, which contained most of tsrg, and the smaller EagI-HindIII fragment from pAL75, which contained a 3′ fragment of trg fused to the final 19 tsr codons. All mutational changes and all constructs were confirmed by nucleotide sequencing.
Labeling with [methyl-3H]methionine.
Cells grown at 35°C to the mid-exponential phase in H1 minimal salts (13) containing the required amino acids at 1.0 mM, 0.2% ribose, 50 μg of ampicillin per ml, and 0, 20, or 30 μM isopropylthio-β-d-galactoside (IPTG) were harvested by centrifugation and submitted to at least five cycles of suspension and pelleting with chemotaxis buffer (10 mM potassium phosphate [pH 7.0], 0.1 mM EDTA). Labeling in vivo with 3H-methyl groups was performed essentially as described previously (7, 9). Samples representing ∼4.5 × 107 cells were submitted to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (11% polyacrylamide, 0.074% N,N′-methylenebisacrylamide [pH 8.2]) (23). The gel was stained with Coomassie brilliant blue, destained with 10% acetic acid, soaked in Amplify (Amersham Corp.), dried at 80°C for 50 min, placed on preflashed X-ray Hyperfilm (Amersham), and kept at −70°C until development of the film.
Behavioral assays.
Rotational phenotypes were determined with cells grown as described for the methylation assay. Cells were harvested, treated in a Waring blender to shear their flagella, tethered by use of antibodies to flagellin to the surface of a glass microscope slide, washed extensively in 10 mM potassium phosphate (pH 7.0)–0.1 mM EDTA–1 mM sodium succinate–1 μM methionine, placed on a microscope stage at 35°C, and recorded on videotape (27). The formation of chemotactic rings was assessed by use of plates containing 0.25% agar, 50 μg of ampicillin per ml, and tryptone broth or a mixture of minimal salts, required amino acids at 0.5 mM, 50 μg of ampicillin per ml, and either 0.05 mM galactose, 0.1 mM ribose, or 0.1 mM serine plus 1 mM glycerol. Plates were inoculated with highly motile, mid-exponential-phase cultures in tryptone broth, placed in a humid incubator at 35°C (14), and examined after appropriate times (4 to 8 h for tryptone plates; 12 to 17 h for minimal medium plates). Images were recorded with a digital camera.
The capillary assay was performed essentially as described by Adler (1). Cells were grown as described for the methylation assay, harvested by centrifugation at 1,600 × g for 10 min, submitted to three cycles of gentle suspension and pelleting designed to avoid shearing flagella, suspended to 5 × 106 cells per ml in chemotaxis buffer at 30°C, and placed in 0.5-ml portions in small chambers created by glass U tubes resting on a glass plate and covered by a glass coverslip (20 by 50 mm). The glass plate formed the bottom of a plastic, lid-covered box suspended in a constant-temperature water bath (30°C). After 10 min of equilibration, capillaries containing chemotaxis buffer alone or buffer plus attractant were inserted into each chamber in sequence. After 45 min, capillaries were removed in sequence from the chambers and emptied into 1 ml of ice-cold tryptone broth, the samples were diluted further as necessary, and an appropriate volume was mixed with 3 ml of molten 0.8% soft agar containing tryptone broth and spread on freshly made tryptone plates. Plates were incubated overnight at 35°C, colonies were counted, and the number of cells that had accumulated in capillaries was calculated.
RESULTS
Adding a CheR-docking site to Trg and Tsrg.
We added a 19-codon sequence corresponding to the final 19 residues of the high-abundance receptor Tsr to the 3′ end of the trg gene, creating a form of Trg with the CheR-binding site, NWETF, attached by a 14-residue linker segment to the carboxyl terminus of the low-abundance receptor (Fig. 1B). In a parallel construct, we added the same sequence to Tsrg, a hybrid receptor (9) in which the amino-terminal 257 residues of Tsr are fused to the carboxy-terminal 281 residues of Trg at a junction just within the cytoplasmic domain, 43 residues from the lysine that marks the end of the second transmembrane segment of Tsr (Fig. 1B). We refer to the 19-codon segment and the 19-residue peptide as the tsr-tail and the Tsr-tail, respectively; to the new gene constructs as trgt (trg plus tail) and tsrgt; and to their products as Trgt and Tsrgt. For the studies described here, all relevant genes were introduced into a common plasmid vector. This vector placed the introduced gene under the control of tandem tac promoters and operators and carried a copy of the lacI gene to provide tight control of gene expression. It was possible to create a cellular content of the receptor proteins approximating that produced from a single chromosomal gene by growth in minimal salts medium in the absence of IPTG for constructs with tsr-derived 5′ segments and in the presence of 20 or 30 μM IPTG for those with trg-derived 5′ segments. All characterizations of receptor function were performed with cells grown in this way. Immunoblots of samples from such cells revealed that the addition of the Tsr-tail had no detectable effect on receptor content or stability in vivo (data not shown).
Methylation.
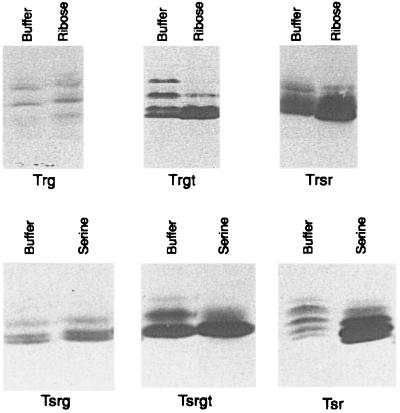
We assessed patterns of steady-state and adaptational methylation in vivo by using polyacrylamide gel electrophoresis, immunoblotting, and fluorography to examine electrophoretic patterns of receptors labeled with 3H-methyl groups (Fig. 2). In such analyses, Trg appears in multiple electrophoretic forms corresponding to multiply methylated species (22, 23). Increased methylation of the cellular population of receptor molecules in 3H-methyl-labeled cells results in more intensity on the fluorograph and a relative increase in more rapidly migrating, more methylated forms of the receptors. Decreased methylation reduces the intensity and shifts the distribution toward more slowly migrating, less methylated forms. Parallel immunoblots for the fluorographs shown in Fig. 2 showed no significant difference in the cellular contents of Trg and Trgt or of Tsrg and Tsrgt (data not shown); thus, the relative intensities of the fluorographic patterns reflect the relative extents of methylation of Trg versus Trgt and of Tsrg versus Tsrgt. Introduction of the Tsr-tail resulted in significant increases in the levels of steady-state methylation of both Trg and Tsrg, as documented by comparison of the Trg and Trgt patterns and of the Tsrg and Tsrgt patterns in Fig. 2, buffer lanes. Note that the addition of the Tsr-tail resulted in a slightly slower electrophoretic migration, corresponding to a larger polypeptide chain; thus, the entire electrophoretic pattern of Trgt was shifted to a slightly higher position on the gel relative to Trg, and the same was true for the Tsrgt-Tsrg pair. The increase in steady-state methylation caused by adding the Tsr-tail to Trg was similar to the increase caused by replacing 87% of the cytoplasmic domain of Trg (281 residues) with the comparable segment of Tsr (compare the Trgt and Trsr patterns in Fig. 2, buffer lanes). Thus, enhanced methylation was a function not of the methyl-accepting sites themselves, which were from a high-abundance receptor in Trsr and from a low-abundance receptor in Trgt, but rather of the pentapeptide-containing carboxy-terminal sequence, present in both Trsr and Trgt.
FIG. 2.
Patterns of methylation in unstimulated and stimulated cells. The panels are fluorographs of sodium dodecyl sulfate-polyacrylamide gels loaded with samples of 3H-methyl-labeled cells containing the indicated protein as the sole detectable chemoreceptor. Only the segments of the fluorographs including the labeled chemoreceptors are shown. Cells were mixed with buffer, 10 mM ribose, or 10 mM serine. Exposure times were 100 h for the top panels, corresponding to genes with trg-derived 5′ segments, and 5 h for the bottom panels, corresponding to genes with tsr-derived 5′ segments.
Stimulation of a receptor by an attractant results in increased receptor methylation in the course of adaptation. In cells containing only a single receptor type, these changes were striking for Tsr (compare the buffer and serine lanes for Tsr in Fig. 2) but modest for Trg (compare the buffer and ribose lanes) or Tsrg (compare the buffer and serine lanes). The addition of the Tsr-tail to Trg and to Tsrg resulted in enhanced changes in methylation in the course of adaptation after stimulation (Fig. 2). For instance, stimulation by the attractant ribose, recognized through an interaction of the occupied binding protein with the periplasmic domain of Trg, resulted in a just detectable increase in the intensity of the two most rapidly migrating bands in the Trg pattern, with little other apparent change, whereas Trgt exhibited a substantial increase in radioactivity and a distinct shift from slower to more rapidly migrating bands. As noted for steady-state methylation, the addition of the Tsr-tail to Trg had essentially the same effect of enhancing adaptational methylation as did the replacement of 87% of the cytoplasmic domain of the low-abundance receptor with the high-abundance sequence, again indicating that the crucial feature was the methyltransferase-docking site, not the specific methyl-accepting sites. For the fusion protein Tsrg, the addition of the Tsr-tail to the Trg-derived cytoplasmic domain enhanced the extent of change in methylation after stimulation by the attractant serine, recognized by the Tsr periplasmic domain (Fig. 2). However, the magnitude of this change was not as great as that exhibited by intact Tsr (Fig. 2). In summary, the methyltransferase-docking site at the carboxyl terminus of the Trg cytoplasmic domain enhanced both steady-state and adaptational methylation of both intact Trg and the Tsr-Trg hybrid.
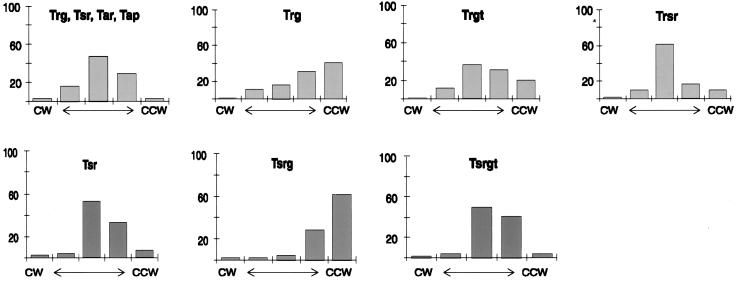
Rotational bias.
As sole cellular chemoreceptors, Trg and Tsrg are unable to establish the normal balance between CCW and CW flagellar rotation (9). A strong bias toward CCW rotation and thus toward runs is likely to contribute to ineffective taxis. We determined rotational bias by observing tethered cells and found that the addition of the Tsr-tail to Tsrg shifted the considerable CCW bias to a rotational distribution almost identical to that of cells containing only Tsr (Fig. 3, bottom row). The addition of the Tsr-tail to Trg resulted in a significant shift from a less extreme CCW bias to the balanced rotational phenotype of cells containing both high- and low-abundance receptors (Fig. 3, top row). The addition of the Tsr-tail is almost as effective as the replacement of 87% of the cytoplasmic domain of Trg with the comparable segment of Tsr to create Trsr (9) (Fig. 3, top row, rightmost panel).
FIG. 3.
Rotational phenotypes. The six strains used for the analysis in Fig. 2 plus one with a deletion of chromosomal trg (CP177) but containing plasmid pAL1 carrying trg were tethered and analyzed for rotational phenotypes by observing at least 100 rotating cells, each for 10 to 15 s, and classifying the behavior into one of five categories (displayed from left to right in each histogram as follows: exclusively CW, predominantly CW with occasional reversals, reversing frequently with no evident directional bias, predominantly CCW with occasional reversals, and exclusively CCW) (31). The data are averages for two independent determinations that yielded very similar distributions. Patterns for strains harboring plasmid-borne genes coding for receptors with the ligand-binding domain of Trg (top row) and patterns for receptors with the ligand-binding domain of Tsr (bottom row) are shown.
Tactic responses.
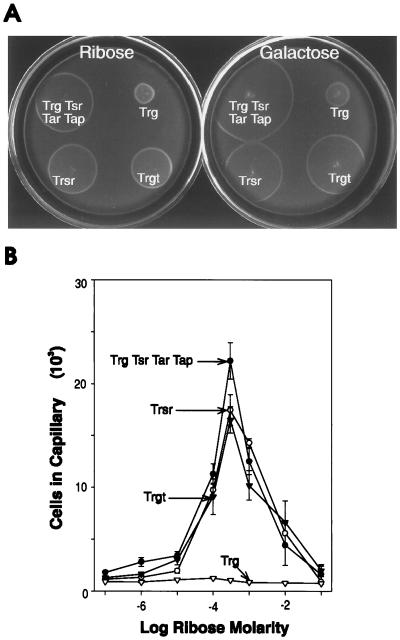
We examined the ability of the receptors of interest to mediate migration in spatial gradients by testing for the formation of chemotactic rings on semisolid agar plates and for migration up gradients formed by the diffusion of an attractant from the mouth of a capillary tube. In the plate assay, cells are inoculated into semisolid agar containing minimal salts and a low concentration of a metabolizable attractant that can serve as a source of carbon and energy. As the cells multiply, they deplete the attractant at the site of inoculation, creating a gradient to which chemotactic cells respond by migrating outward toward high attractant concentrations. The chemotactic cells in the expanding ring multiply as they metabolize the attractant and leave behind an area depleted of the attractant. The combination of a sharp gradient created by cellular metabolism, incubation times of many hours, and amplification by continued growth of cells that make correct decisions makes the formation of chemotactic rings a particularly effective assay for detecting even minimal tactic abilities. In contrast, the capillary assay exposes cells to a diffusion gradient from the capillary mouth and assesses accumulation after 45 min in a buffer that does not support cell division. These stringent conditions provide an effective assay for comparing the relative effectiveness of cells that exhibit significant tactic responses. Thus, the response to ribose of cells containing only Trg was detectable as the formation of a slow-moving chemotactic ring in the plate assay (Fig. 4A) but was not detected in the capillary assay (Fig. 4B).
FIG. 4.
Chemotactic responses to Trg-linked attractants mediated by intact and hybrid receptors. (A) Formation of chemotactic rings on semisolid agar plates. The images were taken 12 h after inoculation of plates containing the indicated attractant with CP177/pAL1 (Trg, Tsr, Tar, Tap), CP362/pAL1 (Trg), CP362/pAL75 (Trgt), and CP362/pHF1 (Trsr). (B) Accumulation in capillaries. The strains used in panel A were assayed at 30°C for 45 min. The points are averages for more than four replicates, and the error bars represent standard deviations. Points with no error bars had standard deviations within the size of the symbol.
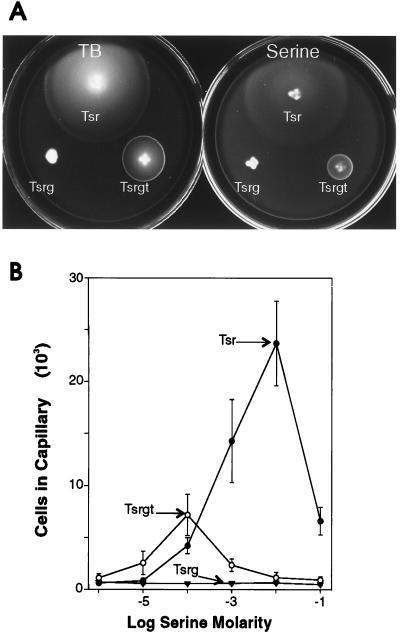
The representative data shown in Fig. 4 illustrate that, as assessed by both assays, the addition of the Tsr-tail to Trg greatly enhanced effective taxis in cells lacking high-abundance receptors. The improved responses were similar to those mediated by the hybrid Trsr, in which most of the cytoplasmic domain is from Tsr, and were only modestly smaller than Trg-mediated responses in the presence of high-abundance receptors. As shown in Fig. 4B, Trgt in the absence of other chemoreceptors mediated accumulation in the capillary assay almost as effectively as Trg in the presence of high-abundance receptors, a striking improvement over the lack of any accumulation by cells containing only Trg. The addition of the Tsr-tail to the hybrid Tsrg improved the ability of this hybrid receptor to mediate migration in spatial gradients (Fig. 5). The Tsrg hybrid exhibited no ability to mediate taxis toward serine in either assay, whereas cells containing the Tsrgt variant responded in both assays. However, the Tsrgt-mediated responses were different from the responses mediated by intact Tsr, being both smaller in magnitude and more limited in concentration range. Specifically, on semisolid agar plates containing either tryptone or serine plus glycerol, Tsr-mediated responses resulted in a fast-moving ring with the unusual feature of being much more diffuse than the characteristically sharp rings observed for responses mediated by other receptors (compare Tsr-mediated responses shown in Fig. 5A to Trg-mediated responses shown in Fig. 4A). In contrast, rings formed on plates containing tryptone or serine plus glycerol by cells containing Tsrgt were sharper, although more slowly moving, than Tsr-mediated rings. In the capillary assay, Tsrgt-containing cells responded well at low serine concentrations but did not respond over the extended concentration range characteristic of taxis mediated by intact Tsr.
FIG. 5.
Chemotactic responses to Tsr-linked attractants mediated by intact and hybrid receptors. (A) Formation of chemotactic rings on semisolid agar plates. The images were taken 8 h after inoculation of plates containing tryptone (TB) or serine-glycerol (Serine) with CP362/pCT1 (Tsr), CP362/pHF2 (Tsrg), or CP362/pHF3 (Tsrgt). (B) Accumulation in capillaries. The strains used in panel (A) were assayed at 30°C for 45 min. The points are averages for more than four replicates, and the error bars represent standard deviations. Points with no error bars had standard deviations within the size of the symbol.
DISCUSSION
Converting the low-abundance receptor Trg to the functional equivalent of a high-abundance receptor.
We found that the low-abundance receptor Trg acquired essential functional features of a high-abundance receptor by the addition of the Tsr-tail. As sole cellular chemoreceptors, high-abundance receptors are effective in methyl-accepting activity, in establishing a functional run-tumble balance, in rapid adaptation, and in mediating efficient chemotaxis; low-abundance receptors are not, even when their cellular content is increased (9, 33). Replacing most of the cytoplasmic domain of the low-abundance receptor Trg or Tap with the ∼60% identical segment of the high-abundance receptor Tsr or Tar created low-abundance receptors that have the essential features of high-abundance receptors (9, 33). We created a similarly functional low-abundance receptor by adding the Tsr-tail to the complete Trg sequence; this result implied that the crucial contribution of the carboxy-terminal 294 residues of Tsr was made by the final 19 amino acids. This segment, missing in low-abundance receptors, ends with the pentapeptide NWETF, identified by Wu et al. (34) as a binding site for the methyltransferase CheR. In high-abundance receptors, deletion or alteration of this pentapeptide reduced substantially methyl-accepting activity in vivo (24, 33) and in vitro (18, 19); the addition of an NWETF-containing tail to the low-abundance receptor Tap increased the number of electrophoretic forms of the low-abundance receptor, implying enhanced methylation in vivo (33).
In the present study, we have documented enhanced methylation in vivo of Trgt, a low-abundance receptor with the NWETF-containing tail of a high-abundance receptor. Parallel studies in vitro have shown that Trgt has a 10-fold-higher initial rate of methylation than native Trg (2). These enhancements are likely to reflect increased local concentrations of methyltransferase created by the CheR-binding pentapeptide. In addition, the enhancements might also depend on other features of the Tsr-tail, for instance, the provision of a flexible linker to allow pentapeptide-bound CheR to reach methyl-accepting sites on the same or neighboring receptors (18, 19). In any case, the conversion of Trg into a receptor with the functional features of a high-abundance receptor, particularly the ability to mediate effective chemotaxis as a sole cellular chemoreceptor, can be understood as a consequence of more effective methylation. These notions and their implications, as well as some related matters, are considered in more detail below.
Improving the function of the hybrid receptor Tsrg.
The hybrid Tsrg, containing most of the Trg cytoplasmic domain and the periplasmic and transmembrane domains of Tsr, functions like a low-abundance receptor. As documented in this study and a previous study (9), as the sole chemoreceptor in a cell, Tsrg is unable to establish a normal rotational phenotype and does not mediate taxis in spatial gradients. In our previous study (9), the tsrg gene, under the control of the tsr promoter, had been transferred by homologous recombination and plasmid resolution from a plasmid construct to a site on the chromosome in the lac operon (27). In the present study, tsrg was transferred to a different plasmid in which it was under the control of tandem tac promoters and the product of an accompanying lacI gene. Using this construct, we confirmed the inability of Tsrg to mediate chemotaxis, but other aspects of the phenotype differed from those reported for the chromosomally integrated hybrid gene. With the plasmid-borne construct, we observed modest changes in the levels of methylation after exposure to the attractant serine or the repellent leucine or phenol that were not apparent in the previous study (9), and the rotational phenotype was substantially biased to CCW, in contrast to the earlier observation of a CW bias. Since we confirmed the identity and integrity of the plasmid-borne hybrid by complete nucleotide sequencing, we are confident that the current observations provide the best definition of the phenotypic characteristics of Tsrg: reduced steady-state and adaptational methylation, a strong CCW bias, and an inability to mediate taxis in spatial gradients, as assayed either on plates or with capillary tubes.
The addition of the Tsr-tail to Tsrg improved each of these features, but in a somewhat different pattern than that observed with the addition of the same tail to intact Trg. For the tail-containing forms of both Tsrg and Trg, steady-state and adaptational methylation was enhanced. However, whereas Trgt as a sole cellular receptor mediated chemotactic responses to ribose gradients that were nearly the same as the responses of wild-type cells or of cells containing Trsr, Tsrgt appeared to mediate serine taxis in a different, apparently less effective manner than intact Tsr (Fig. 5), even though the rotational bias established by Tsrgt was nearly identical to that established by Tsr (Fig. 3). This apparently lower effectiveness of Tsrgt could reflect disruptions or mismatches caused by combining three receptor fragments. An interesting possibility is that such disruptions caused Tsrgt to mediate responses in spatial gradients over a narrower range of serine concentrations than Tsr. Early studies (32) noted that Tsr-mediated accumulations in capillaries occur over an extended range of serine concentrations, suggesting either recognition by more than one site with different affinities or some other deviation from single-site, single-binding-isotherm recognition. Sensitivity over an extended concentration range is consistent with the diffuse rings on semisolid agar plates characteristic of Tsr-mediated responses to serine, since extended sensitivity would mean a greater distance between threshold and saturation along the spatial gradient created by cellular metabolism. A reduced sensitivity to serine, reflecting recognition by a single, high-affinity binding site, could result in a pattern of responses in the capillary assay much like the usual one at low serine concentrations, but reaching a peak (receptor saturation) at a lower concentration. Such a reduced sensitivity would also sharpen the rings formed in response to the serine gradient created in semisolid agar plates and reduce the extent of increased methylation necessary to balance receptor saturation. Since Tsrgt-mediated responses exhibited these features (Fig. 3 and 5), it is possible that some feature of the Tsr cytoplasmic domain not provided by the comparable segment of Trg contributes to the extended Tsr-mediated sensitivity to serine. Thus, the cytoplasmic domains of the two receptors may have functional differences in addition to the striking difference conferred by the CheR-docking site.
A parallel construct designed with low-abundance receptor Tap.
Weerasuriya et al. (33) exchanged the final 5 residues of the low-abundance receptor Tap for the final 23 residues of the high-abundance receptor Tar, providing Tap with the NWETF pentapeptide at the end of a linker. Comparisons of cells containing as a sole receptor either Tap or the fusion protein Tapl (Tap lengthened) provided indications that methylation was more extensive for Tapl, but there were no improvements in the extreme CCW bias or the lack of chemotaxis exhibited on semisolid agar plates by cells containing only Tap. It is not clear why these results are so different from our observations with Trgt. It is possible that the 5 residues removed from Tap were not effectively replaced by the Tar residues put in their place or that the cellular dosage of Tapl was not optimal in cells induced by a high concentration of IPTG. However, even without pentapeptide-containing tails, Trg and Tap have functional differences. Both are ineffective in mediating taxis when present as sole cellular chemoreceptors, but to different degrees. Trg mediates the formation of modest rings on appropriate semisolid agar plates (Fig. 4A), but Tap does not (33). The difference in activities of derivatives carrying CheR-docking sites may simply parallel the difference in activities of the native proteins.
Enhanced methylation as the key to improved receptor function.
Can enhanced steady-state and adaptational methylation of Trgt in vivo (Fig. 2) account for the conversion of a low-abundance receptor to the functional equivalent of a high-abundance receptor? Specifically, how could enhanced methylation shift the rotational bias and thus the tumble frequency to values closer to the normal wild-type values, and how could it improve so dramatically migration up the diffusion gradients of the capillary assay? The low tumble frequency of cells containing only Trg indicates a low level of phospho-CheY and implies a low steady-state activity of the kinase CheA. Therefore, the low-abundance receptor Trg might have been inherently and significantly less effective at kinase activation than high-abundance receptors. However, recent tests in vitro (2) have demonstrated that Trg activates CheA approximately as effectively (within a factor of two) as the high-abundance receptor Tar, and the addition of the 19-residue, pentapeptide-containing tail does not alter this activation. In contrast, the initial rate of CheR-catalyzed methylation of Trg in vitro was only 1/20 the rate of Tar methylation, and the addition of the Tsr-tail to Trg increased the rate 10-fold. Thus, the in vitro results indicate that the major activity difference between the low-abundance receptor Trg and the high-abundance receptor Tar is not kinase activation but rather methyl-accepting ability. In addition, the in vitro studies suggest a methylation-related origin for low kinase activation in cells containing only Trg, since kinase activation by Trg was found to be a function of the extent of covalent modification at methyl-accepting sites (2), an influence previously documented for Tar (5).
Gene-encoded Trg, containing methyl ester-mimicking glutamines at two of its five methyl-accepting sites, activated kinase almost as well as gene-encoded Tar, which had the same number of glutamines at its sites of adaptional modification. In contrast, Trg with only glutamates at these sites was much less active. Since the inherently low methyl-accepting activity of Trg would result in low steady-state methylation in cells containing only Trg, kinase activation would be correspondingly low, not because of an inherent defect in Trg-mediated activation of kinase but because low methylation would create a receptor state ineffective at activation. Since the stimulation of a receptor by an attractant acts to reduce kinase activity, a low steady-state level of autophosphorylation could significantly reduce receptor effectiveness by shrinking the dynamic range over which ligand occupancy could modulate kinase activity. Crucially, with low rates of methylation, adaptation would not be complete in behaviorally relevant time spans; thus, gradient sensing would be seriously compromised. We suspect that these two phenomena are the important contributors to the ineffective taxis of cells containing only Trg. In any case, our observations of Trg and its pentapeptide-carrying derivative in vivo (this study) and in vitro (2) provide a strong basis for concluding that the most important origin for the functional differences between the low-abundance receptor Trg and its high-abundance counterparts is the difference in methyl-accepting activity conferred by the CheR-docking pentapeptide that is present on the high-abundance receptors and absent from Trg.
ACKNOWLEDGMENTS
This work was supported by grant GM29963 from the National Institutes of Health to G.L.H.
We thank Michael Manson for introducing us to the idea of fusing the carboxy-terminal segment of a high-abundance receptor to a low-abundance receptor.
REFERENCES
- 1.Adler J. A method for measuring chemotaxis and use of the method to determine optimum conditions for chemotaxis by Escherichia coli. J Gen Microbiol. 1973;74:77–91. doi: 10.1099/00221287-74-1-77. [DOI] [PubMed] [Google Scholar]
- 2.Barnakov A N, Barnakova L A, Hazelbauer G L. Comparison in vitro of a high- and a low-abundance chemoreceptor of Escherichia coli: similar kinase activation but different methyl-accepting activities. J Bacteriol. 1998;180:6713–6718. doi: 10.1128/jb.180.24.6713-6718.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bibikov S I, Biran R, Rudd K E, Parkinson J S. A signal transducer for aerotaxis in Escherichia coli. J Bacteriol. 1997;179:4075–4079. doi: 10.1128/jb.179.12.4075-4079.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bollinger J, Park C, Harayama S, Hazelbauer G L. Structure of the Trg protein: homologies with and differences from other sensory transducers of Escherichia coli. Proc Natl Acad Sci USA. 1984;81:3287–3291. doi: 10.1073/pnas.81.11.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borkovich K A, Alex L A, Simon M I. Attenuation of sensory receptor signaling by covalent modification. Proc Natl Acad Sci USA. 1992;89:6756–6760. doi: 10.1073/pnas.89.15.6756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dunten P, Koshland D E., Jr Tuning the responsiveness of a sensory receptor via covalent modification. J Biol Chem. 1991;266:1491–1496. [PubMed] [Google Scholar]
- 7.Engström P, Hazelbauer G L. Multiple methylation of methyl-accepting chemotaxis proteins during adaptation of E. coli to chemical stimuli. Cell. 1980;20:165–171. doi: 10.1016/0092-8674(80)90244-5. [DOI] [PubMed] [Google Scholar]
- 8.Falke J J, Bass R B, Butler S L, Chervitz S A, Danielson M A. The two-component signaling pathway of bacterial chemotaxis: a molecular view of signal transduction by receptors, kinases, and adaptation enzymes. Annu Rev Cell Dev Biol. 1997;13:457–512. doi: 10.1146/annurev.cellbio.13.1.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feng X, Baumgartner J W, Hazelbauer G L. High- and low-abundance chemoreceptors in Escherichia coli: differential activities associated with closely related cytoplasmic domains. J Bacteriol. 1997;179:6714–6720. doi: 10.1128/jb.179.21.6714-6720.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gegner J A, Graham D R, Roth A F, Dahlquist F W. Assembly of an MCP receptor, CheW, and kinase CheA complex in the bacterial chemotaxis signal transduction pathway. Cell. 1992;70:975–982. doi: 10.1016/0092-8674(92)90247-a. [DOI] [PubMed] [Google Scholar]
- 11.Hazelbauer G L. Bacterial chemoreceptors. Curr Opin Struct Biol. 1991;2:505–510. [Google Scholar]
- 12.Hazelbauer G L, Engström P. Parallel pathways for transduction of chemotactic signals in Escherichia coli. Nature (London) 1980;283:98–100. doi: 10.1038/283098a0. [DOI] [PubMed] [Google Scholar]
- 13.Hazelbauer G L, Harayama S. Mutants in transmission of chemotactic signals from two independent receptors of E. coli. Cell. 1979;16:617–625. doi: 10.1016/0092-8674(79)90035-7. [DOI] [PubMed] [Google Scholar]
- 14.Hazelbauer G L, Park C, Nowlin D M. Adaptational “crosstalk” and the crucial role of methylation in chemotactic migration by Escherichia coli. Proc Natl Acad Sci USA. 1989;86:1448–1452. doi: 10.1073/pnas.86.5.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kehry M R, Bond M W, Hunkapiller M W, Dahlquist F W. Enzymatic deamidation of methyl-accepting chemotaxis proteins in Escherichia coli catalyzed by the cheB gene product. Proc Natl Acad Sci USA. 1983;80:3599–3603. doi: 10.1073/pnas.80.12.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krikos A, Conley M P, Boyd A, Berg H C, Simon M I. Chimeric chemosensory transducers of Escherichia coli. Proc Natl Acad Sci USA. 1985;82:1326–1330. doi: 10.1073/pnas.82.5.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Le Moual H, Koshland D E., Jr Molecular evolution of the C-terminal cytoplasmic domain of a superfamily of bacterial receptors involved in taxis. J Mol Biol. 1996;261:568–585. doi: 10.1006/jmbi.1996.0483. [DOI] [PubMed] [Google Scholar]
- 18.Le Moual H, Quang T, Koshland D E., Jr Methylation of the Escherichia coli chemotaxis receptors: intra- and interdimer mechanisms. Biochemistry. 1997;36:13441–13448. doi: 10.1021/bi9713207. [DOI] [PubMed] [Google Scholar]
- 19.Li J, Li G, Weis R M. The serine chemoreceptor from Escherichia coli is methylated through an inter-dimer process. Biochemistry. 1997;36:11851–11857. doi: 10.1021/bi971510h. [DOI] [PubMed] [Google Scholar]
- 20.Morgan D G, Baumgartner J W, Hazelbauer G L. Proteins antigenically related to methyl-accepting chemotaxis proteins of Escherichia coli detected in a wide range of bacterial species. J Bacteriol. 1993;175:133–140. doi: 10.1128/jb.175.1.133-140.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muchmore D C, McIntosh L P, Russell C B, Anderson D E, Dahlquist F W. Expression and nitrogen-15 labeling of proteins for proton and nitrogen-15 nuclear magnetic resonance. Methods Enzymol. 1989;177:44–73. doi: 10.1016/0076-6879(89)77005-1. [DOI] [PubMed] [Google Scholar]
- 22.Nowlin D M, Nettleton D O, Ordal G W, Hazelbauer G L. Chemotactic transducer proteins of Escherichia coli exhibit homology with methyl-accepting proteins from distantly related bacteria. J Bacteriol. 1985;163:262–266. doi: 10.1128/jb.163.1.262-266.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nowlin D M, Bollinger J, Hazelbauer G L. Sites of covalent modification in Trg, a sensory transducer of Escherichia coli. J Biol Chem. 1987;262:6039–6045. [PubMed] [Google Scholar]
- 24.Okumura H, Nishiyama S-I, Sasaki A, Homma M, Kawagishi I. Chemotactic adaptation is altered by changes in the carboxy-terminal sequence conserved among the major methyl-accepting chemoreceptors. J Bacteriol. 1998;180:1862–1868. doi: 10.1128/jb.180.7.1862-1868.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oosawa K, Imae Y. Glycerol and ethylene glycol: members of a new class of repellents of Escherichia coli chemotaxis. J Bacteriol. 1983;154:104–112. doi: 10.1128/jb.154.1.104-112.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park C, Dutton D P, Hazelbauer G L. Effects of glutamines and glutamates at sites of covalent modification of a methyl-accepting transducer. J Bacteriol. 1990;172:7179–7187. doi: 10.1128/jb.172.12.7179-7187.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park C, Hazelbauer G L. Mutations specifically affecting ligand interaction of the Trg chemosensory transducer. J Bacteriol. 1986;167:101–109. doi: 10.1128/jb.167.1.101-109.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parkinson J S. Signal transduction schemes in bacteria. Cell. 1993;72:857–871. doi: 10.1016/0092-8674(93)90267-t. [DOI] [PubMed] [Google Scholar]
- 29.Rebbapragada A, Johnson M S, Harding G P, Zuccarelli A J, Fletcher H M, Zhulin I B, Taylor B L. The Aer protein and the serine chemoreceptor Tsr independently sense intracellular energy levels and transduce oxygen, redox, and energy signals for Escherichia coli behavior. Proc Natl Acad Sci USA. 1997;94:10541–10546. doi: 10.1073/pnas.94.20.10541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rice M S, Dahlquist F W. Sites of deamidation and methylation in Tsr, a bacterial chemotaxis sensory transducer. J Biol Chem. 1991;266:9746–9753. [PubMed] [Google Scholar]
- 31.Slocum M K, Halden N F, Parkinson J S. Hybrid Escherichia coli sensory transducers with altered stimulus detection and signaling properties. J Bacteriol. 1987;169:2938–2944. doi: 10.1128/jb.169.7.2938-2944.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Springer M S, Goy M F, Adler J. Sensory transduction in Escherichia coli: two complementary pathways of information processing that involve methylated proteins. Proc Natl Acad Sci USA. 1977;74:3312–3316. doi: 10.1073/pnas.74.8.3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weerasuriya S, Schneider B M, Manson M D. Chimeric chemoreceptors in Escherichia coli: signaling properties of Tar-Tap and Tap-Tar hybrids. J Bacteriol. 1998;180:914–920. doi: 10.1128/jb.180.4.914-920.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu J, Li J, Li G, Long D G, Weis R M. The receptor binding site for the methyltransferase of bacterial chemotaxis is distinct from the sites of methylation. Biochemistry. 1996;35:4984–4993. doi: 10.1021/bi9530189. [DOI] [PubMed] [Google Scholar]
- 35.Yamamoto K, Macnab R M, Imae Y. Repellent response functions of the Trg and Tap chemoreceptors of Escherichia coli. J Bacteriol. 1990;172:383–388. doi: 10.1128/jb.172.1.383-388.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang W, Brooun A, McCandless J, Banda P, Alam M. Signal transduction in the archaeon Halobacterium salinarium is processed through three subfamilies of 13 soluble and membrane-bound transducer proteins. Proc Natl Acad Sci USA. 1996;93:4649–4654. doi: 10.1073/pnas.93.10.4649. [DOI] [PMC free article] [PubMed] [Google Scholar]