Abstract
Skimmia anquetilia N.P. Taylor and Airy Shaw (Rutaceae) is a perennial, aromatic, gregarious wild ornamental shrub native to the Western Himalaya. The plant is used in the traditional medicinal system to treat copious health conditions like rheumatism, fever, inflammation, headache, influenza, body-ache, clearing of the nose, diabetes, lowering the body temperature, smallpox, wounds, burns, snake, and scorpion bites. Phytochemical and gas chromatography-mass spectrometer (GC-MS) analysis of S. anquetilia showed the presence of alkanes, alkenes, carboxylic acids, fatty acids, and their esters, simple coumarins, terpenes, phenylpropanoid, and so on. These active principles exhibit a wide array of pharmacological effects, including anti-inflammatory, antioxidant, anti-cancerous, anti-feedant, and antibacterial properties. Most pharmacological studies were based on the essential oil and the crude extracts of the plant and the bioactive compounds responsible for the bioefficacy have not been well-identified. Further investigations are required to transform the experience-based claims on the use of S. anquetilia in traditional medicine practices into evidence-based information. Detailed in-vitro and in-vivo studies on the mechanisms of action of pure bioactive compounds and more elaborate toxicity studies to ensure plant safety for human use should be conducted. This review recapitulates the current status of its use in the ethnobotany, phytochemistry, and pharmacological activities. It also offers a critical assessment of the plant’s existing information which would help to recuperate its potential as a source for drug development of lead molecules.
Keywords: Ornamental shrub, Phytochemistry, Pharmacological activities, Skimmia anquetilia, Traditional uses
Highlights
-
-
S. anquetilia is used as a folklore medicine for rheumatism, inflammation, smallpox, headache, fever, and as an antidote.
-
-
Over 130 bioactive phytoconstituents have been identified from S. anquetilia.
-
-
Terpenes, glycosides, and fatty acids were identified as major phytoconstituents.
-
-
Pharmacological studies such as antibacterial, anti-inflammatory, antioxidant, anticancer, anti-arthritic, etc., have been reported.
-
-
S. anquetilia is cytotoxic to cell lines such as MCF-7, HeLa, PC-3, and Caco-2.
Introduction
Medicinal plants have achieved broader recognition in recent times since these plants are natural products, they have minimal side effects and better effectiveness than their synthetic equivalents (Batiha et al., 2020). Approximately 80% of people in the world rely upon conventional medicine as a vital source of their basic medical care (Ekor, 2014). Most treatments use medicinal plant extracts and bioactive molecules (Michel et al., 2020). Medicinal plants are important sources of crude drugs that are used to treat various pathological conditions to maintain a status of well-being (Shakya, 2016). Medicinal plants have always been a potential source to treat various ailments, either in the form of traditional preparations or as pure active principles, and perhaps they are often the only source of medicine for the majority of folks in the developing nations (Taylor et al., 2001).
India has a long history of conventional medicinal systems and a lot of knowledge can be acquired from even a common man about herbal medicines of preventive and therapeutic significance. Out of 17,000–18,000 angiospermic species in India, over 7,000 plant species have been identified with medicinal properties (Kala et al., 2006) and of these, ∼1,748 plant species are being used as medicinal plants in the Himalayan region (Joshi et al., 2016). Medicinal plants have been reported to possess several biological activities such as anticancer (Mlilo and Sibanda, 2022), antimicrobial (Chan et al., 2022), anti-diabetic (Ahmed et al., 2022), anti-inflammatory (Rahmawati et al., 2021; Doe et al., 2022), antiviral (Shahrajabian et al., 2022), anti-feedant (Kerebba et al., 2022), antioxidant (Appiah-Opong et al., 2022), and anti-spasmolytic activities (Batiha et al., 2020; Hussain et al., 2020). These activities have been ascribed to their active principles viz: coumarins, glycosides, saponins, flavonoids, steroids, tannins, carotenoids, phenolics, phenols, alkaloids, terpenes, etc. (Nazir et al., 2020; Sarkar, 2020). One of the basic requirements for primary health care success is the accessibility and use of appropriate drugs. Conventional medicine is still the most inexpensive and easily available source of treatment in the primary healthcare system.
S. anquetilia is a medicinal plant that belongs to the genus Skimmia of the Rutaceae family (Saqib and Sultan, 2005). The plant is endemic to the Western Himalaya and is distributed in the mountain ranges of Afghanistan and Indian sub-continent: India, Pakistan, and Nepal (Nissar et al., 2018). In India, the plant originates in the subalpine region of the Garhwal (Gaur, 1999), Jammu and Kashmir, Uttar Pradesh, and Himachal Pradesh (Walters et al., 1986; Sharma et al., 1993). In the conventional medicinal system, different parts of the plant have been used for the treatment of various ailments in parts of the Western Himalayas of India, Nepal, and Pakistan (Epifano et al., 2015). The purpose of this review is to provide an up-to-date and comprehensive overview of the botany, phytochemistry, traditional uses, and pharmacological activities of S. anquetilia. Furthermore, the present knowledge obtained mainly from experimental studies was critically evaluated to provide evidence and validation for local and traditional uses of S. anquetilia and to suggest future research scenarios and prospective therapeutic uses for this plant.
Materials and methods
Searching strategies
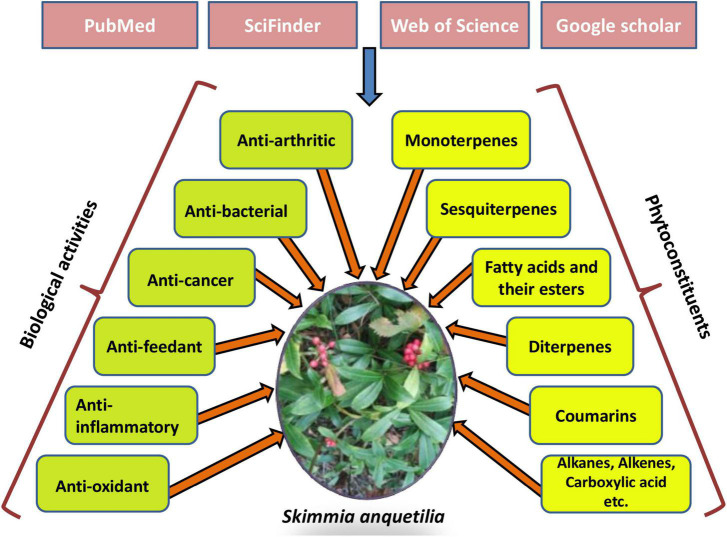
An extensive literature search related to the plant species S. anquetilia of the genus Skimmia was conducted to gather all relevant information about the traditional uses, phytochemicals, and pharmacological activities. Publicly accessible databases and primary sources were searched, including PubMed, SciFinder, Web of Science, Science Direct, Google Scholar, and so on (Figure 1). A large number of literature articles published from 1956 to 2022 were reviewed. The extracted data included vernacular plant names, plant descriptions, traditional uses, purified compounds, and pharmacological activities. The species name was validated using The Plant List (2013).1 All studies and reviews that investigated the ability of S. anquetilia to heal illnesses in a laboratory (in-vitro) and animals (in-vivo) were included as long as the effects were explicitly stated.
FIGURE 1.
Several databases used to access various phytoconstituents and pharmacological activities of Skimmia anquetilia.
Inclusion and exclusion criteria
The literature published up to 2022 was used in this review paper to assess the biological efficacy of S. anquetilia concerning six health conditions related to humans. The search was limited to studies published in the English language, independent of the sample size or prospective development period.
Data extraction
The titles, abstracts, and full articles were used to make a preliminary assessment of the publications by the researchers. Manuscripts that met the study’s predetermined addition and exemption criteria were selected and included. The papers were then used to collect the necessary data on experimental design (animal model and extraction methods), interventions delivered, and treatment findings.
Collection and identification of plant specimen
The whole plant of S. anquetilia N.P. Taylor and Airy Shaw was obtained from the Doodhpathri area (geographical coordinates 74o33′35.81″N and 33o52′10.87″E) of Budgam district, Kashmir, India at an altitude of 2,814 m above msl. The voucher specimen No. 3152-(KASH) was identified and deposited at the Herbarium, Department of Botany, University of Kashmir. The plant specimen collection did not include endangered or protected species.
Vernacular names
S. anquetilia is known as “Nair” in Garhwal and “Patrang,” “Nar,” “Barru” or “Shalangli” in Punjab, “Nairpatti,” “Nayalpatti” or “Nihar” in Kumaun, “Kasturchara” or “Gurlpatta” in Jaunsar, “Naer Patar,” “Inga,” “Patar,” “Nar,” or “Near” in Kashmir (Goel et al., 1989), and “Kedarpatti” in other hilly areas of India (Negi et al., 2011).
Plant description
S. anquetilia is an aromatic, perennial, evergreen, gregarious, usually dioeciously, or monoclinous shrub (Gondwal et al., 2018), often cultivated for decorative purposes (Kumar et al., 2012; Figure 2). It has an erect, aerial, cylindrical, faintly fissured, densely branched, glabrous, yellowish, 1.5 m tall, and a 5′ wide stem. Leaves are alternate, simple, often crowded at the tips of branches, petiolate, unicostate, reticulate venation, coriaceous, marginate, oblanceolate, glands with essential oils, narrowly cuneate at base, acute at apex; terminal, paniculate, peduncle; with corymbose inflorescences. The root is tapering, thick, and branched. Flowers are yellowish, complete, indorous, hypogynous, pedicellate, and ebracteate; calyx (4 or 5), connate; petals (5), imbricate; stamens (5), filaments subulate, anthers ellipsoid, with fleshy drupaceous berry fruit, bright red-colored, ovoid (Nissar et al., 2016, 2021).
FIGURE 2.
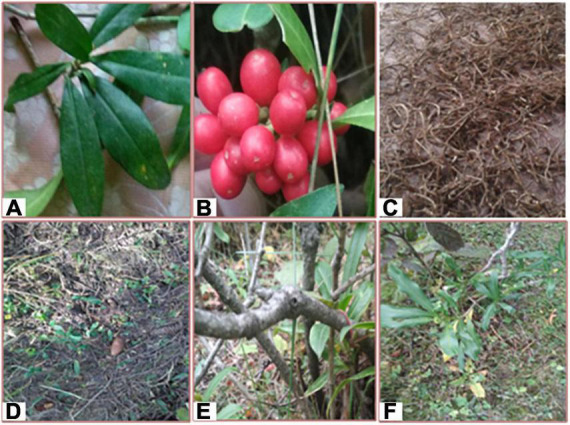
(A) Leaves of Skimmia anquetilia, (B) Berry of S. anquetilia, (C) Dried root of S. anquetilia, (D) Plant in nature, (E) Stem of S. anquetilia, (F) Whole plant of S. anquetilia.
Ethnobotany of S. anquetilia
Ethnobotany is a field of research that applies indigenous plant knowledge to health care. In India S. anquetilia has long been used in Ayurveda and Unani system of medicine, which describes its numerous uses (Table 1). The paste prepared from the roots of S. anquetilia has been used as an antidote against scorpion and snake bites (Bhattarai, 1992; Ahmed et al., 2004; Qureshi et al., 2009; Gondwal et al., 2012b). The powder of the plant bark has been found effective in curing wounds and burns (Negi et al., 2011; Singh and Rawat, 2011). The cold infusion of fresh leaves has been used to treats smallpox (Namiki, 1990; Saqib and Sultan, 2005; Prakash et al., 2011; Kumar et al., 2012), headache, and fever (Bhattarai, 1992; Gaur et al., 1993). The smoke of dried leaves has been used for freshness (Bhattarai, 1992; Gaur et al., 1993; Kunwar et al., 2010), and air purification (Chopra et al., 1956; Chauhan et al., 2017). Some tribal sections of India use its leaves in the preparation of curries (Anon, 1966; Chauhan et al., 2017), an alcoholic drink “Soor” high in calories (Rana et al., 2004), and as a flavoring agent (Anon, 1966; Chauhan et al., 2017). A paste prepared with the mixture of leaves of S. anquetilia and turmeric has been used to treat inflammation and rheumatism (Negi et al., 2011; Singh and Rawat, 2011). In Nepal, leaf infusion is taken for headache and for freshness, leaves are aromatic and used for headache and general fever (Baral and Kurmi, 2006). Personal communications with locals highlighted that that the leaf extract is used to treat diabetes in the Kupwara district of Kashmir valley. Moreover, its dried leaf powder has been used as an insecticidal and pesticidal agent (Bhattarai, 1992). The whole plant has been used in anesthesia and to treat several other health complications like pneumonia, paralysis, and lung cancer (Wani et al., 2016).
TABLE 1.
Traditional uses of Skimmia anquetilia.
| Place | Part used | Mode of use | Traditional use | References |
| NA | Dried leaves | Smoke | To purify air | Chopra et al., 1956 |
| Some tribal hilly areas of Himalaya | Fresh leaves | NA | Used in curries and as flavoring agent | Anon, 1966 |
| NA | Dried leaves | Powder | Used as pesticide, insecticide | Bhattarai, 1992 |
| NA | Fresh leaves | Infusion | Treatment of fever, freshness, headache, smallpox | Bhattarai, 1992 |
| Nepal | Dried leaves and flowers | Smoke | Burned leaves and flowers are used for air purification and keep off evil spirits in the intention of accelerating the patient’s healing. | Bhattarai, 1992 |
| Tons valley of Gaharwal Himalaya | Leaves | NA | Energy rich alcoholic drink known as “Soor” is prepared | Rana et al., 2004 |
| Far-Western Nepal | Leaves | Infusion | Used for freshness and to treat headache | Kunwar et al., 2010 |
| NA | Bark | Powder | To heal burns and wounds | Negi et al., 2011; Singh and Rawat, 2011 |
| Rawain valley of Utarkashi, Uttarakhand | Leaves | Paste | A mixture of fresh leave and turmeric is used to treat rheumatism and inflammation | Negi et al., 2011; Singh and Rawat, 2011 |
| NA | Root | Paste | Used to treat scorpion, snake bites | Gondwal et al., 2012b |
| Pir-Panjal Range of Himalayas | Whole plant | NA | Paralysis, pneumonia, lung cancer, anesthesia | Wani et al., 2016 |
NA, not available.
Phytochemistry
The isolation and identification of secondary metabolites are vital to discover novel drugs to treat diseases. Several studies have been conducted on the phytoconstituents of S. anquetilia (Epifano et al., 2015). Over 130 compounds such as fatty acids and their esters, alkanes, alkenes, carboxylic acids, terpenes (such as monoterpenes, diterpenes, sesquiterpenes), phenylpropanoids, etc., have been identified using the GC-MS technique. Most of the phytochemicals have been identified from the essential oils of different parts of S. anquetilia. For instance, seventy compounds have been identified using the GC-MS technique from the essential oils of fruit pulp and seeds of S. anquetilia (Prakash et al., 2011). Common compounds found in both essential oils were fatty acids and their esters, whereas compounds such as α-cadinol, α-terpineol, selinene, neo-isolongifolene, linalool, cis-Z-α-bisabolene oxide, and aromadendrene were found to be the main difference among them (Prakash et al., 2011). Furthermore, more than fifty compounds have been identified from the essential oils of flowers and leaves of S. anquetilia and the majority of compounds were found to be monoterpenes and sesquiterpenes such as β-phellandrene (18.4, 1.8%), geijerene (15.0, 2.0%), germacrene B (2.0, 11.6%), linalyl acetate (11.2, 7.3%), linalool (9.4, 9.5%), α-terpineol (4.4, 5.6%), and pregeijerene (5.6, 0.2%) (Gondwal et al., 2012b). To date, only six glycosides (simple coumarins) namely, skimminan (Sharma et al., 2008a), ulopterol, skimmin, osthol, esculetin, and scopoletin (Sharma et al., 2008a,b) have been isolated through column chromatography and identified via nuclear magnetic resonance (NMR), correlation spectroscopy (COSY), heteronuclear multiple quantum coherence (HMQC), heteronuclear multiple bond correlation (HMBC), and nuclear overhauser effect spectroscopy (NOESY) techniques from the methanolic leaf extract of S. anquetilia. The identified phytoconstituents of S. anquetilia are presented in Table 2.
TABLE 2.
Significant bioactive compounds isolated from Skimmia anquetilia.
| Class | Bioactive compound(s) | Plant part(s) | References |
| Alkanes (Straight-chain) | Tetracosane, nonacosane, docosane, heneicosane, heptacosane | Seeds and fruit pulp | Prakash et al., 2011 |
| Alkene | 1-Octadecene | Seeds | Prakash et al., 2011 |
| Benzoic acid esters | Dibutyl phthalate | Fruit pulp | Prakash et al., 2011 |
| Carboxylic acid | Ethylhexanoic acid | Seeds | Prakash et al., 2011 |
| Carboximidic acid | Octadecanamide | Fruit pulp | Prakash et al., 2011 |
| Cyclohexenones | Cryptone | Leaves, seeds and fruit pulp | Prakash et al., 2011; Chauhan et al., 2017 |
| Fatty acids and esters of fatty acids | Neryl acetate, octadecanoic acid, tridecanoic acid, oleic acid, n-hexadecanoic acid, methyl oleate, methyl linolenate, hexadecanoic acid,1,1-dimethylethyl ester, hexadecanoic acid, methyl ester, dodecanoic acid, hexadecanoic acid, butyl ester, heptadecanoic acid, tetradecanoic acid, geranyl acetate | Leaves, seeds, Stem bark, and root bark | Mathela et al., 1992; Prakash et al., 2011; Gondwal et al., 2012b; Wani et al., 2016; Chauhan et al., 2017 |
| Glycosides | Skimminan {7,8-dihdroxy-6-[3′- β-D-glucopyranosyloxy-2′(ξ)-hydroxy-3′ methylbutyl]-coumarin}, Skimmin (7-O-β-D-glucopyranosylumbelliferon), osthol [7-methoxy-8-(3-methylbut-2-enyl)-2-chromenone], esculetin (6,7-dihydroxy-chromen-2-one), scopoletin (7-hydroxy-6-methoxy-2H-1-benzopyran-2-one) | Leaves | Sharma et al., 2008a |
| Phenylpropanoid | Asarone | Seeds and fruit pulp | Prakash et al., 2011 |
| Monoterpenes | β-phellandrene, linalool, myrcene, α-terpineol, geraniol, linalyl acetate, sabinene, β-myrcene, nerol, (S)-(+)-carvone acetate, 1,8-cineole, α-phellandrene, α-pinene, terpinen-4-ol, p-cymene, β-pinene, terpinolene, Z-β-ocimene, E-β-ocimene, α-thujene, camphene, terpinen-4-ol, γ–terpinene, linalyl propionate, D-limonene, terpinyl acetate, Limonene, citral, α-terpinyl acetate, cuminic alcohol, cumaldehyde, (+)-4-carene, β-fenchol, Cis-geraniol, Cis-ocimene, Trans-geraniol | Leaves, flower, stem bark, root bark, seeds, and fruit pulp | Sharma et al., 1966; Sarin, 1977; Gulati, 1982; Goel et al., 1989; Mathela et al., 1992; Prakash et al., 2011; Gondwal et al., 2012b; Wani et al., 2016; Chauhan et al., 2017 |
| Diterpene | Thunbergene, phytol | Leaves, stem bark, and root bark | Prakash et al., 2011; Wani et al., 2016 |
| Sesquiterpenes | Pregeijerene, elemol, dictamnol, α-humulene, pregeijerene B, geijerene, germacrene D, (E,E)-farnesyl acetate, epi-cubebol, δ-cadinene, α-cadinene, γ-elemene, Cis-nerolidol, germacrene B, nerolidol, (E)-nerolidol, β-longipinene, (+)-ledene, β-caryophyllene, β-gurjunene, γ-eudesmol, epi-α-muurolol, β-eudesmol, selin-11-en-4- α–ol, α-cadinol, bulnesol, α-bisabolol, E-farnesol, E-farnesyl acetate, α-farnesene, selinene, β-humulene, β-elemene, α-santalol, viridiflorol, vetiverol, longifolenaldehyde, caryophyllene oxide, cedrenol, ledol, (-)-spathulenol, aromadendrene, (+)-farnesol, nerolidyl acetate | Leaves, flower, stem bark, and root bark | Mathela et al., 1992; Prakash et al., 2011; Gondwal et al., 2012b; Wani et al., 2016; Chauhan et al., 2017 |
| Other compounds | Isogeijerene C, dehydrosabina ketone, C12H18 isomer, 8-epi-dictamnol, < N-methyl > methyl anthranilate, 10-epi- γ-eudesmol, bicyclovetivenol, tricyclo [4.4.1.1 (3,8)] dodeca-4,9-diene, trans-Z-α-bisabolene epoxide, pyrethrone, longipinane (E)-, neo-isolongifolene, dimethylethyl ester, diepicedrene-1-oxide, didecenyl succinic anhydride, cyclofenchene, Cis-Z-α-bisabolene epoxide, Cis-linaloloxide, 1-(4-butoxy-2,6 dimethylphenyl) ethanone, dipropyl phthalate, 2,3-dichlorobi phenyl, Trans-2,4-decadienol | Leaves and flower | Gondwal et al., 2012b; Chauhan et al., 2017 |
Pharmacology of S. anquetilia
The varied traditional uses of S. anquetilia have contributed to the initiation of several pharmacological studies. Preceding research shows that the S. anquetilia extracts exhibit a wide array of bioactivities, viz: antibacterial (Sharma et al., 2008a), antioxidant, anti-inflammatory, and other activities like anti-feedant and anticancer activities (Gondwal et al., 2012a; Kumar et al., 2012; Negi et al., 2012; Wani et al., 2016; Table 3). At the same time, a wide array of in-vivo and in-vitro models has been used to evaluate the pharmacological properties of S. anquetilia. Evidence-based laboratory analysis indicates that petroleum ether, chloroform, ethyl-acetate, methanolic, and aqueous extracts of S. anquetilia possess several promising pharmacological properties.
TABLE 3.
Summary of bioefficacy of Skimmia anquetilia.
| Biological efficacy | Plant part(s) evaluated | Test system | Tested substance | References |
| Anti-arthritic | Leaves | In-vitro | Ethyl-acetate extract | Verma et al., 2020 |
| Antibacterial | Leaves | In-vitro | Methanolic extract and active compounds isolated; Skimminan, Skimmin | Sharma et al., 2008a |
| Leaves | In-vitro | Methanol extract | Nabi et al., 2022a | |
| Root | In-vitro | n-Hexane, ethyl-acetate, and methanol extract | Nabi et al., 2022b | |
| Anticancer | Leaves/stem bark/root bark | In-vitro | Essential oil | Wani et al., 2016 |
| Anti-feedant | - | In-vitro | - | Negi et al., 2012 |
| Flowers, leaves | In-vitro | Essential oil | Gondwal et al., 2012a | |
| Anti-inflammatory | Leaves | In-vivo | Petroleum ether, chloroform, ethyl-acetate, methanol, and aqueous extract | Kumar et al., 2012 |
| Leaves | In-vitro | Petroleum ether, chloroform, ethyl-acetate, methanol, and aqueous extract | Kumar et al., 2012 | |
| Leaves | In-vitro | Ethyl-acetate extract | Verma et al., 2020 | |
| Antioxidant | Seeds and fruit pulp | In-vitro | Aqueous extract | Prakash et al., 2011 |
| Leaves/flowers | In-vitro | Aqueous extract and essential oil | Gondwal et al., 2012b | |
| Leaves | In-vitro | n-Hexane, dichloromethane, ethyl-acetate, butanol, methanol, and aqueous fractions | John et al., 2014 |
Antibacterial activity
The methanol leaf extract and isolated active constituents, namely; skimminan and skimmin of S. anquetilia exhibited broad-spectrum antibacterial activity by inhibition of Agrobacterium tumifaciens, Pseudomonas syringae, and Pactobacterium carotovorum (Gram-negative plant pathogens) at a dose of 200 μg/disc using disc diffusion method. Results showed that the methanol extract and skimminan exhibited inhibitory activities against all three pathogens, whereas skimmin was only effective against A. tumifaciens. The highest zone of inhibition (12.6 ± 0.8) was exhibited by methanol extract against A. tumifaciens (Sharma et al., 2008a). Recently, (Nabi et al., 2022a,b) reported the antibacterial activity of methanol leaf extract and n-hexane, ethyl-acetate, and methanol root extracts of S. anquetilia against Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumoniae, Salmonella typhi, and Staphylococcus aureus at different concentrations (10, 20, 40, 80, and 160 mg/ml) using the agar well diffusion assay. The methanolic leaf extract showed the highest zone of inhibition against E. coli (19.0 ± 0.57), followed by P. aeruginosa (18.0 ± 0.57) and K. Pneumoniae (17.0 ± 0.57) at 160 mg/ml. Among the root extracts, ethyl acetate extract showed the highest zone of inhibition against P. aeruginosa (18.0 ± 1.0), followed by S. aureus (17.0 ± 1.0). Furthermore, the minimum inhibitory concentration (MIC) of methanol leaf extract against P. aeruginosa (2 mg/ml) and ethyl acetate root extract against S. aureus (4 mg/ml) demonstrated therapeutically significant antibacterial activity.
Anticancer activity
Among the 35,000 plant species screened against cancer, about 3,000 have demonstrated potential anticancer activities (Surendran et al., 2021). Wani et al. (2016) determined the anticancer activity of the essential oils extracted from the leaf, stem bark, and root bark of S. anquetilia. The study was carried out on four different cell lines viz: MCF-7 (Breast), HeLa (Cervix), PC-3 (Prostate), and Caco-2 (Colon) using sulphorhodamine (SRB) assay. The stem bark essential oil was found to be the most active against all tested human cancer cell lines with IC50 values ranging from 2.71 to 6.21 μg/ml. Leaf essential oil (IC50 3.01 to 114.50 μg/ml) and root bark essential oil (IC50 14.88 to 49.04 μg/ml) exhibited cytotoxic activity against tested human cancer cell lines.
Anti-inflammatory activity
Although various anti-inflammatory drugs have been discovered and are in clinical use, the inflammation condition is still challenging. Most of the existing drugs are opioids and non-steroidal anti-inflammatory drugs (NSAIDs), but they produce many side effects. Hence, the discovery of novel drugs is necessary. Plants possess various phytoconstituents that have displayed anti-inflammatory properties with few side effects (Virshette et al., 2019). Phytoconstituents, for instance, tannins, saponins, alkaloids, flavonoids, and phytosterols, have shown promising anti-inflammatory activities (Abdul-Nasir-Deen et al., 2020). The anti-inflammatory effect of S. anquetilia has been reported previously. Kumar et al. (2012) evaluated the anti-inflammatory activity of S. anquetilia leaf extract (LESA) by in-vitro and in-vivo methods using the human red blood cell (HRBC) membrane stabilization model and the carrageenan-induced rat paw edema model. The authors indicated that the anti-inflammatory effects of LESA revealed the concentration-dependent activity. For HRBC membrane stabilizing agent, S. anquetilia methanol extract exhibited the highest anti-inflammatory effect compared to the other leaf extracts and showed a result value of 68.50 ± 1.57. The chloroform, ethyl-acetate, and methanol leaf extract of S. anquetilia at a dose of 400 mg/kg showed 58.22, 60.17, and 67.53% inhibition respectively in albino rats. The methanol extract showed a maximum anti-inflammatory activity of 67.53% at a 400 mg/kg dose against the standard drug Diclofenac (10 mg/kg) (Kumar et al., 2012). Recently, Verma et al. (2020) reported the anti-inflammatory activity of ethyl-acetate leaf extract (EESA) of S. anquetilia by in-vitro methods using the HRBC membrane stabilization model at doses of 50, 100, 200, and 400 mg/ml. The EESA extract exhibited concentration-dependent inhibition, and the maximum inhibitory effect found was 90.70% at 400 mg/ml in comparison with the standard drug diclofenac sodium which showed 94.88% protection.
Antioxidant activity
Prakash et al. (2011) have analyzed 2,2-diphenyl-1 picryalhydrazide (DPPH) radical scavenging activity, reducing power assay, and chelating activity of Fe2+ of aqueous extracts of seeds and fruit pulp of S. anquetilia using butylated hydroxytoluene (BHT), catechin, and gallic acid as standards. The results of the study revealed that both extracts exhibited moderate in-vitro antioxidant potential. Gondwal et al. (2012b) determined the antioxidant efficiency of essential oils of leaves and flowers of S. anquetilia by reducing power, chelating properties of Fe+2, and 2′2′-diphenylpicrylhadrazyl (DPPH) radical-scavenging assay. DPPH radical scavenging activity was higher in the leaf essential oil and extract, whereas the maximum chelating activity was observed in the flower’s essential oil and aqueous extract and the highest reducing power was shown by flower essential oil and leaf extract. John et al. (2014) have determined the antioxidant activity of crude methanol extract, n-hexane, dichloromethane, ethyl-acetate, n-butanol, and aqueous fractions of S. anquetilia leaves by eight distinct assays viz: 2,2’-azinobis-(3-ethylbenzothiazoline-6-sulpohonic acid) (ABTS) radical cation scavenging activity, the ferric reducing antioxidant power (FRAP), 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity, total phenolic contents (TPC), total flavonoid contents (TFC), total antioxidant activity by phosphomolybdenum method, superoxide anion radical scavenging activity, and metal chelating activity. They opined that the ethyl-acetate fraction showed the highest total phenolic content, 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulpohonic acid) (ABTS) radical cation scavenging activity, the ferric reducing antioxidant power (FRAP), and the DPPH radical scavenging activity. Dichloromethane fraction showed the highest antioxidant activity. The highest superoxide anion radical scavenging activity was displayed by the aqueous fraction. The crude methanolic extract exhibited the highest total flavonoid contents.
Anti-feedant activity
Anti-feedants are substances with anti-feedant characteristics that, at low concentrations, act on the pest’s extremely specific receptor cells. Anti-feedant sensory-linked neurons either dissuade or inhibit insect feeding (feeding suppressant effect), or limit the functionality of a feeding stimulant receptor of herbivores, or the capacity to attach directly to normal feeding cues like carbohydrates and amino acids (Purrington, 2003). The essential oils from leaves and flowers of S. anquetilia showed suppression in the potential of egg-laying by Caryedon serratus, damaging the beetle for groundnut seeds at a 1.5% concentration. The suppression increased with the increase in oil concentration with no interference with the further development of larvae in adults (Gondwal et al., 2012a). The same effects of S. anquetilia extracts on Lepidoptera (forest pests) have been reported by Negi et al. (2012).
Anti-arthritic activity
The production of autoantigens in certain arthritic diseases may be due to the denaturation of protein and membrane lysis action. Denaturation of proteins causes the production of autoantigens in conditions such as rheumatic arthritis, cancer, and diabetes, which are considered inflammatory conditions. Therefore, by inhibition of protein denaturation, inflammatory activity can be inhibited. The anti-arthritic activity of ethyl-acetate leaf extract (EESA) of S. anquetilia at concentrations 50, 100, 200, and 400 mg/ml was determined using the protein denaturation assay. The results were compared with standard acetylsalicylic acid (100 mg/ml). The EESA extract showed dose-dependent inhibition of protein denaturation, the maximum inhibition of protein denaturation was found 92.41% at 400 mg/ml in comparison to the standard which showed 96.21% inhibition at 100 mg/ml (Verma et al., 2020).
Future prospects
S. anquetilia is proving to be an unreliable option for the future. Various active compounds viz, alkanes, alkenes, coumarins, carboxylic acids, fatty acids, and esters of fatty acids, terpenes (monoterpenes, diterpenes, sesquiterpenes), etc., have been reported to be the major bioactive compounds in this plant (Bhatt et al., 2021). The varied bioactivities including anti-arthritic (Verma et al., 2020), anticancer (Wani et al., 2016), anti-inflammatory, antibacterial (Nabi et al., 2022a,b), antioxidant, and anti-feedant (Gondwal et al., 2015) activities have been studied with potential findings. Despite the positive outcome, most of the studies are based on the in-vitro models and mechanisms of action are not well-studied. Various traditional medicinal sources indicate that this plant has been used to treat diabetes, smallpox, burn injuries, etc., but, no pharmacological studies have been conducted to validate these activities. Furthermore, in some areas, there is inadequate information and limited research is available. Therefore, further studies concerning the basic chemical composition of phytoconstituents and the mechanisms involved in traditional uses are needed. The pharmacological activities must be experimented to the next levels for generation of novel drugs. This might prove helpful to use its immense therapeutic efficacy as a potent phytomedicine. Thus, systemic research experiments must be carried out for the development of drugs and medicines for their better economic and therapeutic utilization.
Conclusion
S. anquetilia is probably a possible herbal treatment for various diseases. The plant provides several promising perspectives for both traditional as well as modern medicine. S. anquetilia is a wealthy source of essential oils containing various important bioactive compounds but there is inadequate information concerning the basic chemical composition and their mechanisms involved. Most of the plant parts have been used in traditional medicine including leaves, stem, flower, fruit, and root bark. Therefore, determining research analysis of the bioactive constituents is needed, particularly its pharmacological properties and toxicity in terms of both in-vitro as well as in-vivo test systems to authenticate the safety of such plant-based phytochemicals and to develop standard novel drugs.
Author contributions
MN: conceptualization and writing—review and editing the manuscript. NT and BAG: reviewing and editing the manuscript. All authors contributed to the article and approved the submitted version.
Acknowledgments
The support provided by the Department of Environmental Science, University of Kashmir during the present study is highly acknowledged.
Abbreviations
- GC-MS
gas chromatography-mass spectroscopy
- NMR
nuclear magnetic resonance
- COSY
correlation spectroscopy
- HMQC
heteronuclear multiple quantum coherence
- HMBC
heteronuclear multiple bond correlation
- NOESY
nuclear overhauser effect spectroscopy
- HRBC
human red blood cell
- SRB
sulphorhodamine
- NSAIDs
non-steroidal anti-inflammatory drugs
- DPPH
2,2-diphenyl-1-picrylhydrazyl
- BHT
butylated hydroxytoluene
- ABTS
2,2′-azinobis-(3-ethylbenzothiazoline-6-sulpohonic acid)
- FRAP
ferric reducing antioxidant power
- TPC
total phenolic contents
- TFC
total flavonoid contents
- MIC
minimum inhibitory concentration.
Footnotes
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Abdul-Nasir-Deen A. Y., Boakye Y. D., Osafo N., Agyare C., Boamah D., Boamah V. E., et al. (2020). Anti-inflammatory and wound healing properties of methanol leaf extract of Physalis angulata L. South Afr. J. Bot. 133 124–131. 10.1016/j.sajb.2020.06.030 [DOI] [Google Scholar]
- Ahmed E., Arshad M., Ahmad M., Saeed M., Ishaque M. (2004). Ethnopharmacological survey of some medicinally important plants of Galliyat areas of NWFP, Pakistan. Asian J. Plant Sci. 3 410–415. 10.3923/ajps.2004.410.415 [DOI] [Google Scholar]
- Ahmed S. R., El-sherei M. M., Michel C. G., Musa A., Al-Sanea M. M., Qasim S. (2022). Botanical description, bioactivity guided isolation and in Silico mode of action of anti-diabetic constituents of Pterocarpus dalbergioides flowers. S. Afr. J. Bot. 147 163–175. 10.1016/j.sajb.2021.12.008 [DOI] [Google Scholar]
- Anon (1966). The Wealth of India-Raw Material (Vol-IX: Rh-So). Publication and Information, Directorate, 7. New Delhi: CSIR, 3–4. [Google Scholar]
- Appiah-Opong R., Agyemang K., Dotse E., Atchoglo P., Owusu K. B. A., Aning A., et al. (2022). Anti-plasmodial, cytotoxic and antioxidant activities of selected Ghanaian medicinal plants. J. Evid. Based Integr. Med. 27:2515690X211073709. 10.1177/2515690X211073709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baral S. R., Kurmi P. P. (2006). A Compendium of Medicinal Plants in Nepal. Kathmandu: Mrs Rachana Publishers, 534. [Google Scholar]
- Batiha G. E. S., Alkazmi L. M., Wasef L. G., Beshbishy A. M., Nadwa E. H., Rashwan E. K. (2020). Syzygium aromaticum L. (Myrtaceae): traditional uses, bioactive chemical constituents, pharmacological and toxicological activities. Biomolecules 10:202. 10.3390/biom10020202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt M. D., Kunwar R. M., Abbasi A. M., Bussmann R. W., Paniagua-Zambrana N. Y. (2021). “Skimmia anquetilia Tayl. & Airy Shaw. Skimmia arborescens T. Anderson ex Gamble Skimmia laureola (DC.) Sieb. & Zucc. ex Walp. Skimmia melanocarpa Rehder & EH Wilson Skimmia multinervia Huang Rutaceae,” in Ethnobotany of the Himalayas, eds Kunwar R. M., Sher H., Bussmann R. W. (Cham: Springer International Publishing; ), 1861–1868. 10.1007/978-3-030-57408-6_228 [DOI] [Google Scholar]
- Bhattarai N. K. (1992). Medical ethnobotany in the Karnali zone. Nepal. Econ. Bot. 46 257–261. 10.1007/BF02866624 [DOI] [Google Scholar]
- Chan S. M., Fong V. Y., Koo S. Y., Singh T. R., Tang E. H., Thoo L. T., et al. (2022). Antibacterial activity of selected medicinal plants from Malaysia. Asia Pac. J. Sci. Technol. 27 AST–27. [Google Scholar]
- Chauhan R. S., Nautiyal M. C., Dhyani A., Bahuguna Y. M., Rawat S., Tava A., et al. (2017). Variability in volatile composition of Skimmia anquetilia NP Taylor & Airy Shaw. J. Essent. Oil Bear. Plants 20 1167–1171. 10.1080/0972060X.2017.1377119 [DOI] [Google Scholar]
- Chopra R. N., Nayar S. N., Chopra I. C. (1956). Glossary of Indian Medicinal Plants. New Delhi: CSIR Publication, 228. [Google Scholar]
- Doe P., Danquah C. A., Ohemeng K. A., Opare A. E., Sharif A., Akua-Abora D., et al. (2022). Analgesic, anti-inflammatory, and anti-pyretic activities of Crinum pedunculatum R. Br. Bulb extracts. Pharmacogn. Res. 14 24–29. 10.5530/pres.14.1.5 [DOI] [Google Scholar]
- Ekor M. (2014). The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 4:177. 10.3389/fphar.2013.00177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epifano F., Fiorito S., Genovese S., Granica S., Vitalini S., Zidorn C. (2015). Phytochemistry of the genus Skimmia (Rutaceae). Phytochemistry 115 27–43. 10.1016/j.phytochem.2015.02.014 [DOI] [PubMed] [Google Scholar]
- Gaur R. D. (1999). Flora of the District Garhwal Northwest Himalaya. Srinagar Garhwal: Trans Media. [Google Scholar]
- Gaur R. D., Bhatt K. C., Tiwari J. K. (1993). An ethnobotanical study of Uttar Pradesh Himalaya in relation to veterinary medicines. J. Indian Bot. Soc. 72 139–144. [Google Scholar]
- Goel C. L., Shiva M. P., Mehra S. N., Rai Y. C., Badola K. C. (1989). Production of essential oil from S. laureola (S. orborescens) in Uttar Pradesh. Indian Perfum. 33 161–164. [Google Scholar]
- Gondwal M., Gautam B. P. S., Kishore N. (2018). Egg-laying behaviour of Caryedon serratus (Olivier) on the essential oils of Skimmia anquetilia. Trends Insect Mol. Biol. Biotechnol. 2018, 233–249. 10.1007/978-3-319-61343-7_11 [DOI] [Google Scholar]
- Gondwal M., Joshi Nee, Pant G., Pratap Singh Gautam B., Gondval M. (2015). Chemical constituents and biological activities of genus “Skimmia”. Nat. Prod. J. 5 91–102. 10.2174/2210315505999150610165122 [DOI] [Google Scholar]
- Gondwal M., Prakash O., Punetha H., Kanaujia S., Pant A. K. (2012a). Effect of essential oils of Skimmia anquetilia N.P. Taylor & Airy Shaw on fecundity, growth and development of Caryedon serratus. Int. J. Biol. Pharm. Allied Sci. 1 124–132. [Google Scholar]
- Gondwal M., Prakash O., Vivekanand, Pant A. K., Padalia R. C., Mathela C. S. (2012b). Essential oil composition and antioxidant activity of leaves and flowers of Skimmia anquetilia NP Taylor & Airy Shaw. J. Essent. Oil Res. 24 83–90. 10.1080/10412905.2012.646034 [DOI] [Google Scholar]
- Gulati B. C. (1982). “Skimmia laureola: oil of Skimmia laureola Sieb. & Zucc. ex. Walp,” in : Cultivation and Utilization of Aromatic Plants, eds Atal C. K., Kapur B. M. (New Delhi: Publication & Information Division, CSIR; ), 668. [Google Scholar]
- Hussain M. K., Khan M. F., Khatoon S., Al-Sehemi A. G., Saquib M. (2020). Chromenes: Phytomolecules with Immense Therapeutic Potential: Plant-derived Bioactives. Berlin: Springer, 185–204. 10.1007/978-981-15-2361-8_8 [DOI] [Google Scholar]
- John P., Ahmad I., Aziz-ur-Rehman, Riaz T., Abbasi M. A. (2014). In vitro evaluation of antioxidant activity of Skimmia anquetilia leaves extracts. Int. Res. J. Pharm. 5, 143-150. 10.7897/2230-8407.050330 [DOI] [Google Scholar]
- Joshi R. K., Satyal P., Setzer W. N. (2016). Himalayan aromatic medicinal plants: a review of their ethnopharmacology, volatile phytochemistry, and biological activities. Medicines 3:6. 10.3390/medicines3010006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kala C. P., Dhyani P. P., Sajwan B. S. (2006). Developing the medicinal plants sector in northern India: challenges and opportunities. J. Ethnobiol. Ethnomed. 2 1–15. 10.1186/1746-4269-2-3216393342 [DOI] [Google Scholar]
- Kerebba N., Oyedeji A. O., Byamukama R., Kuria S. K., Oyedeji O. O. (2022). Evaluation for feeding deterrents against Sitophilus zeamais (Motsch.) from Tithonia diversifolia (Hemsl.) A. Gray. J. Biol. Act. Prod. Nat. 12 77–93. 10.1080/22311866.2021.2023046 [DOI] [Google Scholar]
- Kumar V., Bhat Z. A., Kumar D., Khan N. A., Chashoo I. A. (2012). Evaluation of anti-inflammatory potential of leaf extracts of Skimmia anquetilia. Asian Pac. J. Trop. Biomed. 2 627–630. 10.1016/S2221-1691(12)60109-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunwar R. M., Shrestha K. P., Bussmann R. W. (2010). Traditional herbal medicine in far-west Nepal: a pharmacological appraisal. J. Ethnobiol. Ethnomed. 6:35. 10.1186/1746-4269-6-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathela C. S., Melkani A. B., Pant A. K. (1992). Reinvestigation of Skimmia laureola essential oil. Indian Perfum. 36 217–217. [Google Scholar]
- Michel J., Abd Rani N. Z., Husain K. (2020). A review on the potential use of medicinal plants from Asteraceae and Lamiaceae plant family in cardiovascular diseases. Front. Pharmacol. 11:852. 10.3389/fphar.2020.00852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlilo S., Sibanda S. (2022). An ethnobotanical survey of the medicinal plants used in the treatment of cancer in some parts of Matebeleland, Zimbabwe. S. Afr. J. Bot. 146 401–408. 10.1016/j.sajb.2021.11.022 [DOI] [Google Scholar]
- Nabi M., Zargar M. I., Tabassum N., Ganai B. A., Wani S. U. D., Alshehri S., et al. (2022a). Phytochemical profiling and antibacterial activity of methanol leaf extract of Skimmia anquetilia. Plants 11:1667. 10.3390/plants11131667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabi M., Tabassum N., Ganai B. A. (2022b). Phytochemical screening and antibacterial activity of Skimmia anquetilia N.P. Taylor and Airy Shaw: A first study from Kashmir Himalaya. Front. Plant Sci. 13:937946. 10.3389/fpls.2022.937946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namiki M. (1990). Antioxidants/antimutagens in food. Crit. Rev. Food Sci. Nutr. 29 273–300. 10.1080/10408399009527528 [DOI] [PubMed] [Google Scholar]
- Nazir N., Zahoor M., Nisar M. (2020). A review on traditional uses and pharmacological importance of genus Elaeagnus species. Bot. Rev. 86 247–280. 10.1007/s12229-020-09226-y [DOI] [Google Scholar]
- Negi D. S., Kumar A., Negi N., Lal Tamta M. (2012). Himalayan plant species as pesticidal agents. Mini Rev. Organ. Chem. 9 143–150. 10.2174/157019312800604670 [DOI] [Google Scholar]
- Negi V. S., Maikhuri R. K., Vashishtha D. P. (2011). Traditional healthcare practices among the villages of Rawain valley, Uttarkashi, Uttarakhand, India. Ind. J. Trad. Knowl. 10 533–537. [Google Scholar]
- Nissar S., Majid N., Raja W. Y., Nawchoo I. A., Bhat Z. A. (2021). Pharmacognostic and physico-chemical characterization of different parts of Skimmia anquetilia: a perspective for the development of quality control. Proc. Natl. Acad. Sci. U.S.A. 91 615–625. 10.1007/s40011-021-01259-6 [DOI] [Google Scholar]
- Nissar S., Majid N., Rather A. M., Nawchoo I. A., Mohi-Ud-Din G. G. (2018). A detailed review on morphotaxonomy and chemoprofiling of Skimmia anquetilia N.P. Taylor and Airy Shaw. Acta Sci. Microbiol. 1 56–60. [Google Scholar]
- Nissar S., Nawchoo I. A., Mohi-Ud-Din G. G., Majid N. (2016). Impact of altitude and habitat variability on morphological attributes and resource allocation patterns in Skimmia anquetilia in Kashmir Himalaya. Imp. J. Interdiscip. Res. 2 1443–1448. [Google Scholar]
- Prakash O., Gondwal M., Pant A. K. (2011). Essential oils composition and antioxidant activity of water extract from seeds and fruit pulp of Skimmia anquetilia N.P. Taylor & Airy Shaw. Indian J. Nat. Prod. Resour. 2, 435–441. [Google Scholar]
- Purrington C. B. (2003). Encyclopedia of applied plant sciences. Second. Prod. 2003, 1140–1145. 10.1016/B0-12-227050-9/00127-7 [DOI] [Google Scholar]
- Qureshi R. A., Ghufran M. A., Gilani S. A., Yousaf Z., Abbas G., Batool A. (2009). Indigenous medicinal plants used by local women in southern Himalayan regions of Pakistan. Pak J Bot. 41 19–25. [Google Scholar]
- Rahmawati L., Park S. H., Kim D. S., Lee H. P., Aziz N., Lee C. Y., et al. (2021). Anti-inflammatory activities of the ethanol extract of Prasiola japonica, an edible freshwater green algae, and its various solvent fractions in LPS-induced macrophages and carrageenan-induced paw edema via the AP-1 pathway. Molecules 27:194. 10.3390/molecules27010194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rana T. S., Datt B., Rao R. R. (2004). Soor: a traditional alcoholic beverage in Tons valley, Garhwal Himalaya. Indian J. Tradit. Knowl. 3 59–65. [Google Scholar]
- Saqib Z., Sultan A. (2005). Ethnobotany of Palas valley, Pakistan. Ethnobot. Leaflets 2004 1350–1357. [Google Scholar]
- Sarin Y. K. (1977). “Skimmia leaf: a potent source of essential oil from hill area of U.P,” in Advances in Essential Oil Industry, eds Kapoor L. D., Krishna R. (New Delhi: Today and Tomorrow’s Printers and Publishers; ), 95. [Google Scholar]
- Sarkar S. (2020). Common medicinal plants and their importance. Res. Rev. J. Bot. 9 1–9. [Google Scholar]
- Shahrajabian M. H., Sun W., Cheng Q. (2022). The importance of flavonoids and phytochemicals of medicinal plants with antiviral activities. Mini Rev. Organ. Chem. 19 293–318. 10.2174/1570178618666210707161025 [DOI] [Google Scholar]
- Shakya A. K. (2016). Medicinal plants: future source of new drugs. Int. J. Herb. Med. 4 59–64. [Google Scholar]
- Sharma B. D., Balakrishnan N. P., Rao R. R., Hajra P. K. (1993). Flora of India, Botanical Survey of India. 2. New Delhi: Deep Printers, 117. [Google Scholar]
- Sharma M. L., Nigam M. C., Handa K. L., Rao P. R. (1966). Chemical and gas chromatographic investigation on linalool and linalyl acetate bearing plants in India. Indian Oil Soap J. 31 303–307. [Google Scholar]
- Sharma R. K., Negi D. S., Gibbons S., Otsuka H. (2008a). Chemical and antibacterial constituents of Skimmia anquetelia. Planta med. 74 175–177. 10.1055/s-2008-1034281 [DOI] [PubMed] [Google Scholar]
- Sharma R. K., Negi P., Negi N., Negi D. S., Otsuka H. (2008b). New coumarin from Skimmia anquetelia. J. Indian Chem. Soc. 85 1055–1056. 10.1002/chin.200918196 [DOI] [Google Scholar]
- Singh G., Rawat G. S. (2011). Ethnomedicinal survey of Kedarnath wildlife sanctuary in Western Himalaya, India. Indian J. Fundam. Appl. Sci. 1 35–46. 10.15373/2249555X/APR2013/134 [DOI] [Google Scholar]
- Surendran S., Prabha A. C., Ramasubbu R., Krishnaraj M. V. (2021). Humboldtia Vahl (Fabaceae): a review on ethnobotany, phytochemistry and pharmacology. Phytomed. Plus 1:100080. 10.1016/j.phyplu.2021.100080 [DOI] [Google Scholar]
- Taylor J. L. S., Rabe T., McGaw L. J., Jäger A. K., Van Staden J. (2001). Towards the scientific validation of traditional medicinal plants. Plant Growth Regul. 34 23–37. 10.1023/A:1013310809275 [DOI] [Google Scholar]
- The Plant List (2013). A Working List of all Plant Species Version 1.1. Available online at: http://www.theplantlist.org/ (accessed January 1, 2013). [Google Scholar]
- Verma P., Singh B., Kaur A., Kumar V. (2020). In-vitro anti-inflammatory and anti-arthritic activities of ethyl acetate extract of Skimmia anquetilia leaves. J. Med. Herbs Ethnomed. 6 42–44. 10.25081/jmhe.2020.v6.6221 [DOI] [Google Scholar]
- Virshette S. J., Patil M. K., Somkuwar A. P. (2019). A review on medicinal plants used as anti inflammatory agents. J. Pharmacogn. Phytochem. 8 1641–1646. [Google Scholar]
- Walters S. M., Brady A., Brickell C. D., Cullen J., Green P. S., et al. (1986). The European Garden Flora. I. Cambridge: Cambridge University Press, 430. [Google Scholar]
- Wani T. A., Kumar N., Khan J. (2016). In-vitro cytotoxic activity of Skimmia anquetilia Taylor & Airy Shaw essential oils on various human cancer cell lines. Int. J. Res. Pharm. Chem. 6 89–94. [Google Scholar]