Abstract
Reductive cyclizations of carbonyl compounds, mediated by samarium(II) diiodide (SmI2, Kagan’s reagent), represent an invaluable platform to generate molecular complexity in a stereocontrolled manner. In addition to classical ketone and aldehyde substrates, recent advances in radical chemistry allow the cyclization of lactone and lactam-type substrates using SmI2. In contrast, acyclic esters are considered to be unreactive to SmI2 and their participation in reductive cyclizations is unprecedented. Here, we report a diastereoselective radical 1,4-ester migration process, mediated by SmI2, that delivers stereodefined alkene hydrocarboxylation products via radical cyclization of acyclic ester groups in α-carbomethoxy δ-lactones. Isotopic labeling experiments and computational studies have been used to probe the mechanism of the migration. We propose that a switch in conformation redirects single electron transfer from SmI2 to the acyclic ester group, rather than the “more reactive” lactone carbonyl. Our study paves the way for the use of elusive ketyl radicals, derived from acyclic esters, in SmI2-mediated reductive cyclizations.
Introduction
Radical cyclizations are privileged processes for the regio- and stereocontrolled construction of molecular complexity.1 In particular, cyclizations triggered by single electron transfer (SET)2 reduction of carbonyl compounds, using the archetypal SET reducing agent samarium(II) diiodide (SmI2, Kagan’s reagent),3 offer a radical umpolung strategy that couples carbonyl moieties with unsaturated functionalities and delivers decorated cyclic structures (Scheme 1A).4 For example, the facile intramolecular addition of ketyl radicals,5 generated upon treatment of ketones and aldehydes with SmI2,6 to alkenes continues to provide effective solutions for the synthesis of high-profile natural products and bioactive molecules.7 Recently, our group8 and others9 have exploited the use of coordinating additives (e.g., H2O, phosphoramides, ureas, amines, etc.) to modulate the reactivity of SmI2,10 and to achieve the SET reduction of more recalcitrant lactam, cyclic imide, and lactone derivatives, thus expanding the scope of SmI2-mediated reductive cyclizations to more “unusual” ketyl radicals. Acyclic esters, however, have long been considered to be unreactive to SmI2, and as such, they are often used as innocent chelating groups to direct SmI2 reactions.11 In an attempt to fill this synthetic gap, our group reported the use of SmI2·H2O·Et3N to reduce acyclic esters to the corresponding ketyl radical equivalents.12 However, in this system, the enhanced reducing power of SmI2 led to over-reduction of the ketyl radical intermediate thus precluding exploitation in radical cyclizations. To date, the reductive cyclization of acyclic esters with SmI2 remains unprecedented.13
Scheme 1. (A) SmI2-Mediated Radical Cyclization of Carbonyl Compounds; (B) Radical Cyclization of Acyclic Esters with SmI2 Underpins an Unusual Radical 1,4-Ester Shift (This Work).
Herein, we present the first SmI2-mediated radical cyclizations of acyclic esters: methyl esters embedded within substituted-δ-lactones 1 undergo SET reduction by SmI2, to form elusive acyclic ester ketyl radicals (Scheme 1B). These undergo efficient intramolecular addition to a pendant alkene, outcompeting both the unproductive back electron transfer (BET) to the metal center and overreduction of the ketyl radical by SmI2. Fragmentation of the resultant spirocyclic tetrahedral intermediate (vide infra) provides stereocontrolled access to alkene hydrocarboxylation products 3. Isotopic labeling studies and DFT calculations have been used to explore the unique diastereoselective radical 1,4-ester migration process.14
Design of Reactivity
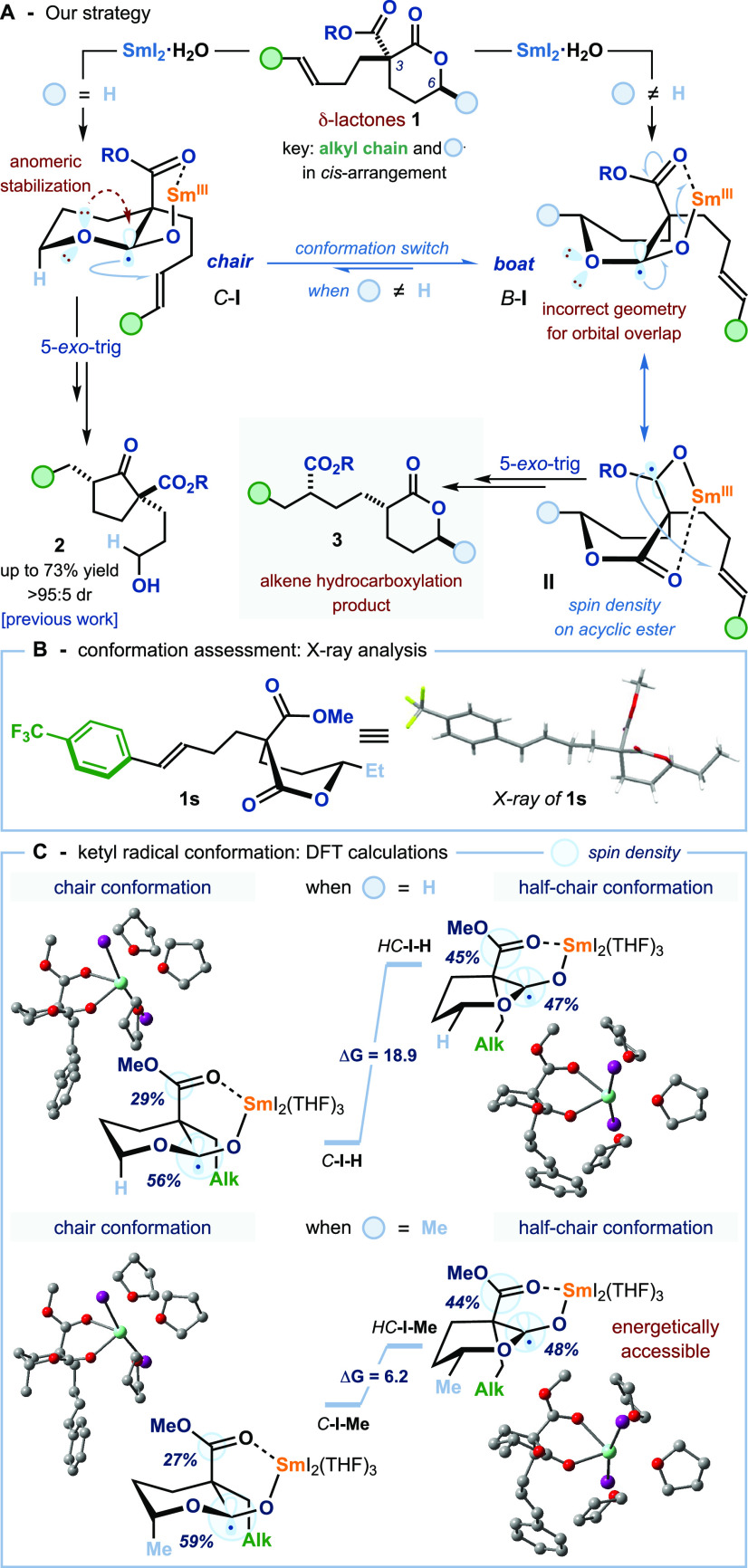
We have previously highlighted the important role of conformation in the stabilization of the ketyl radical intermediates in the SmI2-mediated reductive cyclizations of lactones.8a We proposed that SET from SmI2·H2O to 3-carboxyl-3-alkyl-disubstituted δ-lactones, generating Sm(III)-radical intermediate I, is facilitated by the ability of the lactone and its ketyl radical anion to access a chair conformation C-I (Scheme 2A, left-hand side). This conformation grants the correct orbital orientation to permit the anomeric stabilization15 of the pseudoaxial radical in I, and fosters a diastereoselective 5-exo-trig cyclization pathway that delivers pentanone products 2, after collapse of the resultant tetrahedral intermediate.
Scheme 2. Radical Cyclization of Lactones Enabled by SmI2·H2O: (A) Our Strategy: Conformational Distortion of Sm(III)-Ketyl Radical Intermediates Drives the Cyclization of Acyclic Esters; (B) Conformation Assessment of δ-Lactones 1 by X-ray Analysis; (C) Computational Studies on Ketyl Radical Anions I (ΔG Values Reported in kJ/mol).
Percentages refer to distribution of spin density.
In our quest for the first SmI2-mediated radical cyclizations of acyclic esters, we envisaged that a remote substituent on the lactone ring in 1 could be used to direct reductive ketyl-radical generation to the acyclic ester group. Specifically, we postulated that, by forcing lactones 1, or the ketyl radicals formed upon their reduction, into a boat conformation (B-I), the correct geometry for orbital overlap between the carbon-centered radical and the lone pair on the adjacent oxygen atom would be disrupted. This would destabilize ketyl radical I and drive radical relocation to the acyclic ester moiety, generating Sm(III)-ketyl radical intermediate II (Scheme 2A, right-hand side). This switch would convert the former ancillary coordinating group into a noninnocent reactive functionality. We envisaged that the key conformational switch could be achieved by introducing an alkyl group at the C6 position of the lactone ring, anti to the ester group. This substituent should favor a boat conformation for the lactone16 and the ketyl radical (B-I), by destabilizing chair ketyl radical C-I. Thus, desired ketyl radical II would result from relocation of the spin density and undergo 5-exo-trig cyclization, providing hydrocarboxylation products 3 after an unusual radical 1,4-ester migration.
Computational and structural studies on the parent lactones support our proposal that the introduction of an alkyl group at the C6 position of the lactone ring, anti to the ester group, can lead to a conformational switch: our DFT calculations indicate that lactone 1-H preferentially adopts a half-chair conformation while trisubstituted lactone 1a bearing a C6-Me substituent prefers a boat conformation18—the latter being consistent with the X-ray crystal structure obtained for derivative 1s (Scheme 2B). Our findings are consonant with literature crystallographic data for related lactone structures.17,18
Furthermore, computational studies on the ketyl radical intermediates derived from lactones 1-H and 1a support our proposal, although the conformational switch is more subtle, involving a switch from a chair to half-chair conformation rather than a switch from chair to boat conformation. Calculations suggest that, while both ketyl radicals favor chair conformations (C-I-H and C-I-Me), the presence of the axial C6-Me substituent in C-I-Me brings it closer in energy to a half-chair conformation HC-I-Me (Scheme 2C). Crucially, in the energetically accessible half chair conformation HC-I-Me, anomeric stabilization of the ketyl radical derived from the lactone carbonyl is suboptimal and increased spin density resides at the acyclic ester group; this is consistent with formation of the desired ketyl radical II (cf.Scheme 2A). Interestingly, the axial C6-Me in C-I-Me is also likely to block the approach of the alkene moiety to the radical thus disfavoring cyclization.
Results and Discussion
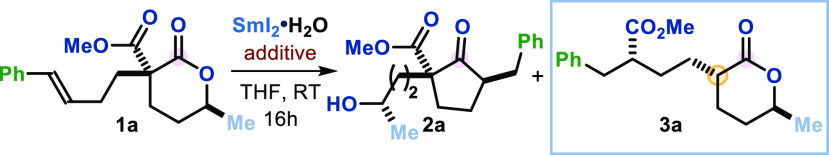
The feasibility of our design plan was assessed using 6-methyl-substituted δ-lactone 1a (Table 1). First, 1a was exposed to SmI2·H2O, the reagent system previously used for the reductive radical cyclization of lactones and the formation of cyclopentanones 2 (entry 1). Under these conditions, the reaction delivered a complex mixture that contained a 1:1.6 ratio of cyclopentanone 2a and the desired radical 1,4-ester migration product 3a, resulting from radical cyclization of the acyclic ester group. The use of HMPA as an additive10 (entry 2) led to a cleaner reaction and increased the preference for product 3a, while increasing the equivalents of SmI2 resulted in the complete consumption of starting material 1a (entry 3). By reducing the amount of both H2O and HMPA (entries 4–6), increased chemoselectivity was observed and the amount of 3a increased. Optimal conditions for the radical cyclization of the acyclic ester group were obtained when the protocol with SmI2·H2O·HMPA was performed at −78 °C:under these conditions, radical 1,4-ester migration product 3a was isolated in 76% yield as a 3:1 mixture of diastereoisomers (entry 7). Crucially, the use of tripyrrolidinophosphoric acid triamide (TPPA) as a nontoxic alternative to HMPA, under the conditions reported in entry 7, provided migration product 3a with similar levels of efficiency and selectivity (66% yield, 4:1 dr).18 The diastereomeric mixture obtained for 3a arises from a protonation event at the C3-carbon of the lactone ring (vide infra), whereas the radical 1,4-ester migration, and construction of the new stereocenter, takes place with complete diastereocontrol.
Table 1. Optimization of the Radical 1,4-Ester Migration.
The conversion of starting material, the products ratio and the diastereoisomeric ratio of 3a were determined by 1H NMR analysis of the crude reaction mixture.
Reaction performed at −78 °C. The yellow circle within compound 3a denotes the stereocenter that gives rise to the diastereoisomeric mixture. HMPA: hexamethylphosphoramide. THF: tetrahydrofuran. ND: not determined, due to the complexity of the reaction mixture.
To evaluate the scope of the radical 1,4-ester migration, an array of δ-lactones 1, adorned with different alkyl substituents at the C6-position, was submitted to the optimized Sm(II)-conditions (Figure 1). Primary alkyl substituents (methyl-, ethyl-, n-butyl-, benzyl-, and neopentyl-) were well tolerated, and ester migration products 3a–e were obtained in good yield. Likewise, lactones 1f–j, bearing bulkier secondary and tertiary alkyl substituents (including cyclohexyl- and tetrahydropyranyl-groups), efficiently underwent the ester radical cyclization process to deliver products 3f–j. When evaluating the effects on reactivity brought about by substitution on the arene moiety of 1, we found that both electron-donating (methyl-, naphthyl-, phenyl-, and methoxy-) and electron-withdrawing (fluoro-, chloro-, bromo-, and trifluoromethyl-) functionalities, at all positions of the aromatic ring, were compatible with our SmI2-conditions (products 3k–t). The relative stereochemistry of the major and minor diastereoisomers of the product of 1,4-ester migration 3p was confirmed by X-ray crystallographic analysis.18 Furthermore, a heteroaryl substituted olefin and a trisubstituted styrene derivative successfully participated in the 1,4-ester migration protocol to give thiophene derivative 3u and product 3v, respectively. C6-Substituted δ-lactones tailored with terminal (1w) or α,α-dialkyl-substituted (1x) pendant alkenes proved unsuitable for the SmI2-mediated 1,4-ester migration reaction. Furthermore, phenylalkynyl-derivative 1y also failed to deliver the corresponding 1,4-ester migration product. These observations suggest that the generation of a stabilized benzylic radical intermediate—upon ketyl radical cyclization—facilitates the transformation.
Figure 1.

Scope of the method. Reaction conditions: 1 (1 equiv.), SmI2 (0.1 M in THF, 2.5 equiv.), H2O (16 equiv.), HMPA (10 equiv.), in THF (0.5 mL/0.1 mmol of substrate), at −78 °C under a nitrogen atmosphere. Isolated yields. The diastereoisomeric ratio was determined by 1H NMR analysis of the crude reaction mixture. The yellow circle within general product structure 3 denotes the stereocenter at which there is a diastereoisomeric mixture.
A 13C-isotope labeling experiment was used to track the migration event and shed light on the mechanism of the SmI2-mediated radical 1,4-ester shift. Analogue 1a-13C, bearing a 13C-labeled acyclic ester group, was submitted to the optimized SmI2-mediated conditions. Labeled product 3a-13C was isolated in 68% yield (Scheme 3A) with 13C-enrichment solely at the carboxylic carbon of the migrated ester unit (cf.Scheme 3C, bottom left). This confirms that a Sm(III)-ketyl radical, formed from the acyclic ester, engages the alkene moiety in a radical cyclization event.
Scheme 3. Mechanistic Studies and Proposed Reaction Pathway.

The diastereoisomeric ratios were determined by 1H NMR analysis of the crude reaction mixture.
We next explored the behavior of related acetate and malonate derivatives 4—in which there are no chemoselectivity challenges—with the aim of further probing the role of the lactone ring in the reactivity of 1. Exposure of methyl hexenoate derivative 4a to the standard SmI2-conditions gave no reaction (Scheme 3B). Whereas, treatment of malonate 4b with SmI2·H2O·HMPA, under standard reaction conditions, gave cyclopentanol derivative 5 in low yield as a mixture of four diastereoisomers. Cyclopentanol 5 is formed by ester radical cyclization, collapse of a tetrahedral intermediate, and further reduction of the cyclopentanone intermediate. In both cases, no 1,4-ester migration product 6 was observed. The latter experiment confirms that the SET reduction of acyclic esters is not limited to lactone-containing substrates; however, the lactone moiety is key to drive productive fragmentation of the spirocyclic intermediate—generated after the radical cyclization event—to give products 3; 1,4-ester migration in lactones 1 crucially generates a more stable cyclic Sm(III)-enolate species (pKa values for δ-valerolactone = 25.2 and ethyl acetate = 27.5, in DMSO).19
A plausible mechanistic pathway for the SmI2-mediated radical 1,4-ester migration is outlined in Scheme 3C. SET from SmI2·H2O·HMPA to lactones 1 forms ketyl radicals III. The substituent at C6 on the lactone ring disfavors the radical adopting the chair conformation necessary for anomeric stabilization and relocation of the spin density to the acyclic ester gives ketyl radicals IV. Facile, diastereoselective 5-exo-trig radical cyclization, SET reduction, and protonation of the ensuing radical species generate spirocyclic intermediates V that collapse to form cyclic Sm(III)-enolates VI. Protonation of VI delivers 1,4-ester migration products 3.
Conclusion
SmI2, in the presence of H2O and HMPA, mediates the unprecedented reductive radical cyclization of acyclic esters. Varying the substitution on the lactone ring in α-carbomethoxy δ-lactones allows a switch in conformation that redirects SET from SmI2 to the acyclic ester group, rather than to the “more reactive” lactone carbonyl. 5-Exo-trig cyclization of the ketyl radical derived from the ester group results in a diastereoselective radical 1,4-ester migration, and the formation of stereodefined alkene hydrocarboxylation products. The protocol tolerates a range of functional groups, and the migration event proceeds with complete diastereocontrol. In addition to describing the first SmI2-mediated ketyl-olefin couplings of acyclic esters, and an unusual radical 1,4 ester migration, our studies showcase how control of conformation can be used to alter the chemoselectivity of radical processes.
Acknowledgments
We thank the BBSRC (DTP Studentship to C.M.), the Indonesia Endowment Fund for Education (LPDP Scholarship to I.A.), the EPSRC (iCAT CDT Studentship to E.P.; EP/S023755/1), and the University of Manchester (Dean’s Award to Á.P. and Lectureship to G.E.M.C.). N.K. acknowledges the assistance given by Research IT, and the use of the Computational Shared Facility and the HPC Pool funded by the Research Lifecycle Programme at The University of Manchester. Finally, we thank the Leigh group for their help with NMR experiments, and Niklas Radhoff for assistance with early synthetic studies.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications Web site. The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.2c05972.
Additional experiments, experimental procedures, characterization data, CCDC numbers for X-ray crystal structures, and spectra for all new compounds. (PDF)
Author Contributions
† I.A. and E.P. contributed equally.
The authors declare no competing financial interest.
Supplementary Material
References
- For recent reviews about the topic, see:; a Plesniak M. P.; Huang H.-M.; Procter D. J. Radical cascade reactions triggered by single electron transfer. Nature Rev. Chem. 2017, 1, 0077. 10.1038/s41570-017-0077. [DOI] [Google Scholar]; b Miyabe H.; Kawashima A.; Yoshioka E.; Kohtani S. Progress in Enantioselective Radical Cyclizations. Chem. -Eur. J. 2017, 23, 6225. 10.1002/chem.201603124. [DOI] [PubMed] [Google Scholar]; c Huang H.-M.; Garduño-Castro M. H.; Morrill C.; Procter D. J. Catalytic cascade reactions by radical relay. Chem. Soc. Rev. 2019, 48, 4626. 10.1039/C8CS00947C. [DOI] [PubMed] [Google Scholar]
- For selected reviews on SET and radical chemistry, see:; a Gansäuer A.; Bluhm H. Reagent-Controlled Transition-Metal-Catalyzed Radical Reactions. Chem. Rev. 2000, 100, 2771–2788. 10.1021/cr9902648. [DOI] [PubMed] [Google Scholar]; b Studer A.; Curran D. P. Catalysis of Radical Reactions: A Radical Chemistry Perspective. Angew. Chem. Int, Ed. 2016, 55, 58–102. 10.1002/anie.201505090. [DOI] [PubMed] [Google Scholar]; c Yan M.; Lo J. C.; Edwards J. T.; Baran P. S. Radicals: Reactive Intermediates with Translational Potential. J. Am. Chem. Soc. 2016, 138, 12692–12714. 10.1021/jacs.6b08856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Girard P.; Namy J.-L.; Kagan H. B. Divalent lanthanide derivatives in organic synthesis. 1. Mild preparation of SmI2 and YbI2 and their use as reducing or coupling agents. J. Am. Chem. Soc. 1980, 102, 2693–2698. 10.1021/ja00528a029. [DOI] [Google Scholar]; For selected reviews of SmI2 chemistry, see:; b Szostak M.; Fazakerley N. J.; Parmar D.; Procter D. J. Cross-Coupling Reactions Using Samarium(II) Iodide. Chem. Rev. 2014, 114, 5959–6039. 10.1021/cr400685r. [DOI] [PubMed] [Google Scholar]; c Molander G. A.; Harris C. R. Sequencing Reactions with Samarium(II) Iodide. Chem. Rev. 1996, 96, 307–338. 10.1021/cr950019y. [DOI] [PubMed] [Google Scholar]; d Flowers R. A. II. Mechanistic Studies on the Roles of Cosolvents and Additives in Samarium(II)-Based Reductions. Synlett 2008, 2008, 1427–1439. 10.1055/s-2008-1078414. [DOI] [Google Scholar]
- Péter Á.; Procter D. J. Cascades, Catalysis and Chiral Ligand Control with SmI2; The Rebirth of a Reagent. Chimia 2019, 74, 18. 10.2533/chimia.2020.18. [DOI] [PubMed] [Google Scholar]
- Péter Á.; Agasti S.; Knowles O.; Pye E.; Procter D. J. Recent advances in the chemistry of ketyl radicals. Chem. Soc. Rev. 2021, 50, 5349. 10.1039/D0CS00358A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SmI2-mediated enantioselective reductive cyclizations:; a Kern N.; Plesniak M. P.; McDouall J. J. W.; Procter D. J. Enantioselective cyclizations and cyclization cascades of samarium ketyl radicals. Nat. Chem. 2017, 9, 1198. 10.1038/nchem.2841. [DOI] [PubMed] [Google Scholar]; Radical cyclizations enabled by SmI2-catalysis:; b Huang H.-M.; McDouall J. J. W.; Procter D. J. SmI2-catalyzed cyclization cascades by radical relay. Nat. Catal. 2019, 2, 211. 10.1038/s41929-018-0219-x. [DOI] [Google Scholar]; c Huang H.-M.; He Q.; Procter D. J. SmI2-Catalyzed Radical Cascade Cyclizations that Construct Quaternary Stereocenters. Synlett 2020, 31, 45. 10.1055/s-0039-1690196. [DOI] [Google Scholar]; d Agasti S.; Beattie N. A.; McDouall J. J. W.; Procter D. J. SmI2-Catalyzed Intermolecular Coupling of Cyclopropyl Ketones and Alkynes: A Link between Ketone Conformation and Reactivity. J. Am. Chem. Soc. 2021, 143, 3655. 10.1021/jacs.1c01356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For reviews of SmI2 in natural product synthesis, see:; a Edmonds D. J.; Johnston D.; Procter D. J. Samarium(II)-iodide-mediated cyclizations in natural product synthesis. Chem. Rev. 2004, 104, 3371–3403. 10.1021/cr030017a. [DOI] [PubMed] [Google Scholar]; b Nicolaou K. C.; Ellery S. P.; Chen J. S. Samarium diiodide mediated reactions in total synthesis. Angew. Chem., Int. Ed. 2009, 48, 7140–7165. 10.1002/anie.200902151. [DOI] [PMC free article] [PubMed] [Google Scholar]; For selected examples of SmI2 in natural product synthesis, see:; c Mukaiyama T.; Shiina I.; Iwadare H.; Saitoh M.; Nishimura T.; Ohkawa N.; Sakoh H.; Nishimura K.; Tani Y-i.; Hasegawa M.; Yamada K.; Saitoh K. Asymmetric Total Synthesis of Taxol®. Chem. -Eur. J. 1999, 5, 121–161. . [DOI] [Google Scholar]; d Beemelmanns C.; Reissig H.-U. A Short Formal Total Synthesis of Strychnine with a Samarium Diiodide Induced Cascade Reaction as the Key Step. Angew. Chem., Int. Ed. 2010, 49, 8021–8025. 10.1002/anie.201003320. [DOI] [PubMed] [Google Scholar]; e Cha J. Y.; Yeoman J. T. S.; Reisman S. E. A Concise Total Synthesis of (−)-Maoecrystal Z. J. Am. Chem. Soc. 2011, 133, 14964–14967. 10.1021/ja2073356. [DOI] [PubMed] [Google Scholar]; f Fazakerley N. J.; Helm M. D.; Procter D. J. Total Synthesis of (+)-Pleuromutilin. Chem. -Eur. J. 2013, 19, 6718–6723. 10.1002/chem.201300968. [DOI] [PubMed] [Google Scholar]; g Breitler S.; Carreira E. M. Total Synthesis of (+)-Crotogoudin. Angew. Chem., Int. Ed. 2013, 52, 11168–11171. 10.1002/anie.201305822. [DOI] [PubMed] [Google Scholar]; h Cai L.; Zhang K.; Kwon O. Catalytic Asymmetric Total Synthesis of (−)-Actinophyllic Acid. J. Am. Chem. Soc. 2016, 138, 3298–3301. 10.1021/jacs.6b00567. [DOI] [PMC free article] [PubMed] [Google Scholar]; i Gong J.; Chen H.; Liu X.-Y.; Wang Z.-X.; Nie W.; Qin Y. Total Synthesis of Atropurpuran. Nat. Commun. 2016, 7, 12183. 10.1038/ncomms12183. [DOI] [PMC free article] [PubMed] [Google Scholar]; j Farney E. P.; Feng S. S.; Schäfers F.; Reisman S. E. Total Synthesis of (+)-Pleuromutilin. J. Am. Chem. Soc. 2018, 140, 1267. 10.1021/jacs.7b13260. [DOI] [PMC free article] [PubMed] [Google Scholar]; k Leung J. C.; Bedermann A. A.; Njardarson J. T.; Spiegel D. A.; Murphy G. K.; Hama N.; Twenter B. M.; Dong P.; Shirahata T.; McDonald I. M.; Inoue M.; Taniguchi N.; McMahon T. C.; Schneider C. M.; Tao N.; Stoltz B. M.; Wood J. L. Total Synthesis of (±)-Phomoidride D. Angew. Chem., Int. Ed. 2018, 57, 1991–1994. 10.1002/anie.201712369. [DOI] [PubMed] [Google Scholar]; l Classen M. J.; Böcker M. N. A.; Roth R.; Amberg W. M.; Carreira E. M. Enantioselective Total Synthesis of (+)-Euphorikanin A. J. Am. Chem. Soc. 2021, 143, 8261. 10.1021/jacs.1c04210. [DOI] [PubMed] [Google Scholar]; m Péter Á.; Crisenza G. E. M.; Procter D. J. Asymmetric Total Synthesis of (−)-Phaeocaulisin A. J. Am. Chem. Soc. 2022, 144, 7457–7464. 10.1021/jacs.2c02188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selected contributions.; a Parmar D.; Duffy L. A.; Sadasivam D. V.; Matsubara H.; Bradley P. A.; Flowers R. A. II; Procter D. J. Studies on the mechanism, selectivity and synthetic utility of lactone reduction using SmI2 and H2O. J. Am. Chem. Soc. 2009, 131, 15467. 10.1021/ja906396u. [DOI] [PubMed] [Google Scholar]; b Parmar D.; Price K.; Spain M.; Matsubara H.; Bradley P. A.; Procter D. J. Reductive Cyclization Cascades of Lactones Using SmI2–H2O. J. Am. Chem. Soc. 2011, 133, 2418. 10.1021/ja1114908. [DOI] [PubMed] [Google Scholar]; c Parmar D.; Matsubara H.; Price K.; Spain M.; Procter D. J. Lactone radical cyclizations and cyclization cascades mediated by SmI2–H2O. J. Am. Chem. Soc. 2012, 134, 12751. 10.1021/ja3047975. [DOI] [PubMed] [Google Scholar]; d Szostak M.; Sautier B.; Spain M.; Behlendorf M.; Procter D. J. Selective Reduction of Barbituric Acids Using SmI2/H2O: Synthesis, Reactivity, and Structural Analysis of Tetrahedral Adducts. Angew. Chem., Int. Ed. 2013, 52, 12559. 10.1002/anie.201306484. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Huang H.-M.; Procter D. J. Radical-radical cyclization cascades of barbiturates triggered by electron-transfer reduction of amide-type carbonyls. J. Am. Chem. Soc. 2016, 138, 7770. 10.1021/jacs.6b04086. [DOI] [PubMed] [Google Scholar]; f Just-Baringo X.; Clark J.; Gutmann M. J.; Procter D. J. Selective synthesis of cyclooctanoids by radical cyclization of seven-membered lactones: Neutron diffraction study of the stereoselective deuteration of a chiral organosamarium intermediate. Angew. Chem., Int. Ed. 2016, 55, 12499. 10.1002/anie.201606792. [DOI] [PMC free article] [PubMed] [Google Scholar]; g Huang H.-M.; Procter D. J. Dearomatizing radical cyclizations and cyclization cascades triggered by electron-transfer reduction of amide-type carbonyls. J. Am. Chem. Soc. 2017, 139, 1661. 10.1021/jacs.6b12077. [DOI] [PubMed] [Google Scholar]; h Huang H.-M.; Procter D. J. Radical heterocyclization and heterocyclization cascades triggered by electron transfer to amide-type carbonyls. Angew. Chem., Int. Ed. 2017, 56, 14262. 10.1002/anie.201708354. [DOI] [PubMed] [Google Scholar]; i Huang H.-M.; McDouall J. J. W.; Procter D. J. Radical Anions from Urea-type Carbonyls: Radical Cyclizations and Cyclization Cascades. Angew. Chem., Int. Ed. 2018, 57, 4995. 10.1002/anie.201800667. [DOI] [PubMed] [Google Scholar]
- Shi S.; Szostak M. Aminoketyl Radicals in Organic Synthesis: Stereoselective Cyclization of Five- and Six-Membered Cyclic Imides to 2-Azabicycles Using SmI2–H2O. Org. Lett. 2015, 17, 5144–5147. 10.1021/acs.orglett.5b02732. [DOI] [PubMed] [Google Scholar]
- a Dahlén A.; Hilmersson G. Samarium(II) Iodide Mediated Reductions – Influence of Various Additives. Eur. J. Inorg. Chem. 2004, 2004, 3393. 10.1002/ejic.200400442. [DOI] [Google Scholar]; For relevant studies, see:; b Shabangi M.; Flowers R. A. II. Electrochemical Investigation of the Reducing Power of SmI2 in THF and the Effect of HMPA Cosolvent. Tetrahedron Lett. 1997, 38, 1137. 10.1016/S0040-4039(97)00008-7. [DOI] [Google Scholar]; c Enemærke R. J.; Hertz T.; Skrydstrup T.; Daasbjerg K. Evidence for Ionic Samarium(II) Species in THF/HMPA Solution and Investigation of Their Electron-Donating Properties. Chem. -Eur. J. 2000, 6, 3747–3754. . [DOI] [PubMed] [Google Scholar]; d Prasad E.; Flowers R. A. Mechanistic Impact of Water Addition to SmI2: Consequences in the Ground and Transition State. J. Am. Chem. Soc. 2005, 127, 18093–18099. 10.1021/ja056352t. [DOI] [PubMed] [Google Scholar]
- Szostak M.; Spain M.; Choquette K. A.; Flowers R. A. II; Procter D. J. Substrate-Directable Electron Transfer Reactions. Dramatic Rate Enhancement in the Chemoselective Reduction of Cyclic Esters Using SmI2–H2O: Mechanism, Scope, and Synthetic Utility. J. Am. Chem. Soc. 2013, 135, 15702. 10.1021/ja4078864. [DOI] [PubMed] [Google Scholar]; For synthetic examples, refer to refs (6a) and (8a).
- a Szostak M.; Spain M.; Procter D. J. Electron transfer reduction of unactivated esters using SmI2-H2O. Chem. Commun. 2011, 47, 10254. 10.1039/c1cc14014k. [DOI] [PubMed] [Google Scholar]; b Szostak M.; Spain M.; Procter D. J. On the Role of Pre- and Post-Electron Transfer Steps in the SmI2/amine/H2O-Mediated Reduction of Esters: New Mechanistic Insights and Kinetic Studies. Chem. – Eur. J. 2014, 20, 4222. 10.1002/chem.201400295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The literature contains two examples of rearrangement reactions triggered by the intramolecular radical cyclization of acyclic esters to alkenes. However, these require the use of a specialized allylsamarium(II) bromide complex and they are limited to specific homoallylic systems:; a Tu Y.; Zhou L.; Yin R.; Lv X.; Flowers R. A. II; Choquette K. A.; Liu H.; Niu Q.; Wang X. Study on the coupling of acyclic esters with alkenes – the synthesis of 2-(2-hydroxyalkyl)cyclopropanols via cascade cyclization using allylsamarium bromide. Chem. Commun. 2012, 48, 11026–11028. 10.1039/c2cc34630c. [DOI] [PubMed] [Google Scholar]; b Shen M.; Tu Y.; Xie G.; Niu Q.; Mao H.; Xie T.; Flowers R. A. II; Lv X.; Wang X. Allylsamarium Bromide-Mediated Cascade Cyclization of Homoallylic Esters. Synthesis of 2-(2-Hydroxyalkyl)cyclopropanols and 2-(2-Hydroxyethyl)bicyclo[2.1.1]hexan-1-ols. J. Org. Chem. 2015, 80, 52–61. 10.1021/jo501797w. [DOI] [PubMed] [Google Scholar]
- For examples of radical processes involving the migration of acyl- and cyano-groups, see:; a Best W. M.; Cook A. P. F.; Russell J. J.; Widdowson D. A. Reactions potentially related to coenzyme B12 dependent rearrangements: observations on the radical [1,2]-acyl and -thiol ester migrations. J. Chem. Soc., Perkin Trans. 1 1986, 1139–1143. 10.1039/p19860001139. [DOI] [Google Scholar]; b Beckwith A. L. J.; O’Shea D. M.; Gerba S.; Westwood S. W. Cyano or acyl group migration by consecutive homolytic addition and β-fission. J. Chem. Soc., Chem. Commun. 1987, 666–667. 10.1039/C39870000666. [DOI] [Google Scholar]
- For a recent discussion about the anomeric effect, see:; a Juaristi E.; Bandala Y. Anomeric Effect in Saturated Heterocyclic Ring Systems. Adv. Heterocycl. Chem. 2012, 105, 189–222. 10.1016/B978-0-12-396530-1.00002-4. [DOI] [Google Scholar]; b Wiberg K. B.; Bailey W. F.; Lambert K. M.; Stempel Z. D. The Anomeric Effect: It’s Complicated. J. Org. Chem. 2018, 83, 5242–5255. 10.1021/acs.joc.8b00707. [DOI] [PubMed] [Google Scholar]; c Alabugin I. V.; Kuhn L.; Krivoshchapov N. V.; Mehaffy P.; Medvedev M. G. Anomeric effect, hyperconjugation and electrostatics: lessons from complexity in a classic stereoelectronic phenomenon. Chem. Soc. Rev. 2021, 50, 10212. 10.1039/D1CS00564B. [DOI] [PubMed] [Google Scholar]
- Selected studies:; a Lavie D.; Kirson I.; Glotter E.; Snatzke G. Conformational Studies on Certain 6-Membered Ring Lactones. Tetrahedron 1970, 26, 2221–2228. 10.1016/S0040-4020(01)92801-7. [DOI] [Google Scholar]; b Ammon H. L.; DeShong P.; Simpson D. Structure of a Lactone, (3α,4α,6α)-4-(tert-Butyldimethylsiloxy)-3,4,5,6-tetrahydro-6-methyl-3-(2-oxopropyl)-2H-pyran-2-one. Acta Crystallogr. C 1991, 47, 2482–2484. 10.1107/S0108270191006030. [DOI] [PubMed] [Google Scholar]; c Brandänge S.; Färnbäck M.; Leijonmarck H.; Sundin A. Highly Diastereoselective Hydrogenations Leading to β-Hydroxy δ-Lactones in Hydroxy-Protected Form. A Modified View of δ-Lactone Conformations. J. Am. Chem. Soc. 2003, 125, 11942–11955. 10.1021/ja036002b. [DOI] [PubMed] [Google Scholar]; d Weber F.; Brückner R. Conformational Analysis of δ-Lactones by DFT Calculations: The Parent Compound and its Monomethyl and Selected Dimethyl Derivatives. Chem. -Eur. J. 2013, 19, 1288–1302. 10.1002/chem.201202988. [DOI] [PubMed] [Google Scholar]
- Geibel I.; Dierks A.; Schmidtmann M.; Christoffers J. Formation of δ-Lactones by Cerium-Catalyzed, Baeyer–Villiger-Type Coupling of β-Oxoesters, Enol Acetates, and Dioxygen. J. Org. Chem. 2016, 81, 7790–7798. 10.1021/acs.joc.6b01441. [DOI] [PubMed] [Google Scholar]
- See Supporting Information for full computational data, optimization studies, and X-ray CCDC numbers.
- Nakamura S.; Hirao H.; Ohwada T. Rationale for the Acidity of Meldrum’s Acid. Consistent Relation of C-H Acidities to the Properties of Localized Reactive Orbital. J. Org. Chem. 2004, 69, 4309–4316. 10.1021/jo049456f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.