Abstract
Background:
There is growing recognition that reproductive factors are associated with increased risk of future cardiovascular disease (CVD). Infertility has been less well studied, although emerging data support its association with increased risk of CVD. Whether infertility is associated with future risk of heart failure (HF) is not known.
Objectives:
To examine the development of HF and HF subtypes in women with and without history of infertility.
Methods:
We followed post-menopausal women from the Women’s Health Initiative prospectively for the development of HF. Infertility was self-reported at study baseline. Multivariable cause-specific Cox models were used to evaluate the association of infertility with incident overall HF and HF subtypes (HF with preserved ejection fraction [HFpEF, LVEF ≥ 50%] vs reduced ejection fraction [HFrEF, LVEF < 50%]).
Results:
Among 38,528 post-menopausal women (mean age 63 ± 7 years), 5399 (14%) participants reported a history of infertility. Over a median follow-up of 15 years, 2373 developed incident HF, including 807 HFrEF and 1133 HFpEF. Infertility was independently associated with future risk of overall HF (HR 1.16, 95% CI 1.04–1.30, p=0.006). Notably, when examining HF subtypes, infertility was associated with future risk of HFpEF (HR 1.27, 95% CI 1.09–1.48, p=0.002), but not HFrEF (HR 0.97, 95% CI 0.80–1.18).
Conclusions:
Infertility was significantly associated with incident HF. This was driven by increased risk of HFpEF, but not HFrEF, and appeared independent of traditional CV risk factors and other infertility-related conditions. Future research should investigate mechanisms that underlie the link between infertility and HFpEF.
Keywords: infertility, heart failure, women
CONDENSED ABSTRACT
We sought to investigate the association of infertility with HF and HF subtypes in the Women’s Health Initiative. Among 38,528 post-menopausal women, history of infertility was significantly associated with risk of future HF. This was driven by greater risk of HFpEF and not HFrEF. Traditional cardiovascular risk factors, coronary heart disease, or other infertility-related conditions did not appear to explain this association.
INTRODUCTION
There is a growing recognition that sex-specific and reproductive factors including premature menopause and adverse pregnancy outcomes increase risk of future cardiovascular disease (CVD) (1–4). Early life events may provide a window into future CV risk and identify patients who would benefit from early prevention. Infertility is a reproductive factor that is consistently underrecognized with respect to CV risk in part due to paucity of rigorous data examining CV risk in women with a history of infertility. The risk of infertility should not be overlooked as it affects over 14% of women in the United States (5) and has been linked to higher risk of overall CVD risk in some studies although the available data are conflicting (6–9). In the Swedish Medical Birth Register, women who reported at least 5 years of infertility before a successful pregnancy had a 19% higher incidence of CVD compared with women without a history of infertility (6).
The association of infertility with heart failure (HF) has not been previously examined. HF is a major public health concern, affecting over 6 million individuals and accounting for more than 1 million hospital admissions per year in the United States (10). Notably, the prevalence of HFpEF, now the leading form of HF, is higher in women compared with men. Moreover, women with HFpEF are more likely to experience symptoms of congestion such as shortness of breath, exercise intolerance and report worse quality of life compared with men (11). Emerging data suggest that women may be more susceptible to developing HFpEF and that reproductive factors may play a role in mediating HF risk in women (12). A previous investigation in the Women’s Health Initiative (WHI) found that earlier age at menopause and nulliparity were both associated with higher risk of HF and HFpEF, respectively (13).
In this context, we sought to investigate the association of infertility with risk of HF and HF subtypes among women enrolled in the Women’s Health Initiative (WHI). Given prior associations of infertility with CVD, we hypothesized that infertility would be associated with incident HF, driven primarily by increased risk of HFrEF. We further postulated that the association between infertility and HF would be mediated in part by established atherosclerotic CVD risk factors.
METHODS
Study Population
The characteristics and enrollment of WHI participants have been previously described (14,15). Briefly, the WHI is a national prospective study of 161,808 post-menopausal women recruited across 40 clinical centers between 1993 and 1998. Participants were enrolled in either the observational study or one or more of the three clinical trials. We included a subset of 44,174 WHI participants with centrally adjudicated HF outcomes from the HF sub cohort. The HF sub cohort includes all women randomized to the hormone trial of WHI (n=27,347) as well as all Black (n=11,880) and Hispanic (n=4,947) participants from the observational study and other clinical trial components.
We excluded subjects with prevalent HF (n=669), missing infertility data (n= 587), missing follow-up data (n=297), and missing key covariates (n=4093), yielding a final sample of 38,528 participants. For analyses examining 10-year estimated atherosclerotic cardiovascular disease (ASCVD) risk score, we excluded prevalent CVD (n=2884), missing infertility data (n=587), and missing covariates (n=3884), yielding a final sample of 36,819 participants.
All participants provided informed consent and study protocols were approved by the appropriate institutional review boards.
Clinical Assessment
All participants underwent comprehensive medical history including detailed reproductive history, physical examination, and anthropometry at screening, and interim medical history was queried twice each year for the duration of follow-up. The primary exposure of interest was history of infertility. Infertility was defined as the inability to conceive after one or more years of trying to become pregnant independent of eventual pregnancy outcome (16). Self-reported history of infertility and specific cause of infertility were ascertained via interviews and questionnaires at baseline. Causes of infertility included hormones or ovulation, tubes or uterus (including endometrial polyps, fibroids, intrauterine or peritubal adhesions, tubal occlusion, or salpingitis), endometriosis, problems with partner, or unexplained infertility. Selected covariates included age at screening, self-reported race/ethnicity, body mass index (BMI), systolic blood pressure, hypertension treatment, diabetes mellitus (DM), hyperlipidemia, smoking status, baseline coronary heart disease (CHD), irregular menses, thyroid disease, and early menopause. DM was defined as a fasting glucose >126 mg/dL or use of diabetes medication. Irregular menses was defined as lack of regular monthly menstruation. Thyroid disease included hypothyroidism, hyperthyroidism, or mixed thyroid disease (both hypothyroidism and hyperthyroidism). Early menopause was defined as menopause at or before the age of 45.
Heart Failure Outcomes
All HF events including confirmed cases of HF hospitalization and patient-reported hospitalization for HF or CHD were sent to University of North Carolina for adjudication by trained physicians. Available medical records for HF hospitalizations were reviewed, and HF hospitalizations were classified into 1 of 5 categories (definite acute decompensated HF, possible acute decompensated HF, chronic stable HF, unclassifiable, or HF unlikely) based on the algorithm used in the Atherosclerosis Risk in Communities (ARIC) study (17). The primary outcome of interest was incident HF, and secondary outcomes included HF with reduced ejection fraction (HFrEF) and HF with preserved ejection fraction (HFpEF). A designation of definite acute decompensated HF or possible acute decompensated HF was considered incident HF. HF with a left ventricular ejection fraction <50% was defined as HFrEF, and HF with LVEF ≥50% was classified as HFpEF. If no LVEF was available or if the HF case was designated as recovered LVEF, it was designated as unclassified HF.
Statistical Analysis
Baseline characteristics were summarized for participants with and without history of infertility. Results are reported as means (standard deviations) and medians (interquartile ranges) for continuous variables and percentages for dichotomous variables. Between-group differences were examined using Student’s t-test, chi square test, or Wilcoxon rank-sum test as appropriate.
In our primary analysis, we examined the association of infertility with incident HF and HF subtypes. Cumulative incidence rates of overall HF, HFrEF, and HFpEF were estimated using a cumulative incidence function, accounting for competing risks of death, other HF subtype, and unclassified HF. Between group differences in the cumulative incidence rates were formally tested using Gray’s test. We then performed cause-specific Cox proportional hazards models fitted for overall HF, and separately for HFpEF and HFrEF. Models were adjusted for age at screening, BMI, systolic blood pressure, hypertension treatment, DM, hyperlipidemia, smoking status (current and former), baseline CHD, and cohort (observational study or clinical trial). Secondary models were further adjusted for race/ethnicity. In sensitivity analyses we reclassified HF subtypes using the cutpoint of LVEF <40% vs ≥40%. We also performed sensitivity analyses excluding women with history of CHD to explore potential ischemic mechanisms driving the relationship between infertility and HF. Finally, to examine whether eventual pregnancy outcome modified the association between infertility and HF, we performed sensitivity analyses excluding women who never became pregnant. For all Cox regressions, we confirmed that the proportional hazards assumption was met using Schoenfeld residuals. We performed multiple imputation techniques to impute missing covariate data for the n=4093 women in our study with missing covariate data. A fully conditional specification (FCS) regression model with all clinical covariates list above was used and we refit models from the primary analyses. Results from 20 iterations were properly combined using PROC MIANALYZE and they were consistent with the primary findings (Supplemental Table 1).
To better understand the link between infertility and HF, we evaluated the association of infertility with established CHD risk factors (age, BMI, race/ethnicity, systolic blood pressure, hypertension treatment, DM, smoking status, and hyperlipidemia) using both age-adjusted and stepwise logistic regression (age and cohort forced in, entry P<0.10, retention P<0.05). We also examined the association of infertility with traditional atherosclerotic CVD risk as captured by the 10-year ASCVD risk score using univariate logistic regression models. For ASCVD risk score analyses, we performed multiple imputation to impute missing total cholesterol and high-density lipoprotein values due to significant missingness (18). Total cholesterol and high-density lipoprotein along with all CHD risk factors were used in an FCS regression model and the final results were summarized from 20 iterations of the imputation process using PROC MIANALYZE. All cross-sectional models were also adjusted for cohort to reduce selection bias from the HF sub cohort.
In exploratory analyses, we examined the association of infertility-related risk factors with HF outcomes. We first added known risk factors for infertility (irregular menses, thyroid disease, waist circumference, and early menopause) into the primary stepwise logistic regression model. We then assessed the association of infertility risk factors with incident HF using Cox proportional hazards models. Finally, we further adjusted the primary analyses evaluating the association of infertility with incident HF for the presence of irregular menses, thyroid disease, waist circumference, and early menopause.
All tests were two-sided and a P-value of <0.05 was considered statistically significant. Statistical analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
Of 38,528 women included for analysis, 5399 (14%) reported a history of infertility. The mean age at enrollment was 63 ± 7 years, 50% of women identified as non-Hispanic White, 33% were Black, and 15% were Hispanic (Table 1, baseline characteristics for n=36,819 included in the 10-year ASCVD analysis are presented in Supplemental Table 2). Compared with women without a history of infertility, women with infertility were more likely to be former or current smokers (former smoking: 42% vs 38%, p<0.001, current smoking: 11% vs 10%, p=0.04), have a history of irregular menses (24% vs 15%, p<0.001), thyroid disease (24% vs 18%, p<0.001), and early menopause (35% vs 30%, p<0.001).
Table 1.
Baseline Demographics and Clinical Characteristics
| Total cohort N=38,528 | Infertility N=5399 | No Infertility N=33,129 | p-value | |
|---|---|---|---|---|
|
| ||||
| Age, years | 63 ± 7 | 63 ± 7 | 63 ± 7 | 0.68 |
| Race/Ethnicity | ||||
| Non-Hispanic White, n (%) | 19,213 (50%) | 2884 (53%) | 16329 (49%) | <0.001 |
| Black, n (%) | 12,826 (33%) | 1663 (31%) | 11163 (34%) | <0.001 |
| Hispanic, n (%) | 5588 (15%) | 718 (13%) | 4870 (15%) | 0.01 |
| Traditional CV risk factors | ||||
| Systolic blood pressure, | ||||
| mmHg | 129 ± 18 | 129 ± 18 | 129 ± 18 | 0.95 |
| Hypertension treatment, n (%) | 11,499 (30%) | 1577 (29%) | 9922 (30%) | 0.27 |
| BMI, kg/m2 | 29.6 ± 6.3 | 29.4 ± 6.2 | 29.6 ± 6.3 | 0.04 |
| DM, n (%) | 2790 (7%) | 382 (7%) | 2408 (7%) | 0.61 |
| Total cholesterol, mg/dL | 220 ± 45 | 218 ± 45 | 221 ± 45 | 0.01 |
| HDL cholesterol, mg/dL | 56 ± 15 | 56 ± 15 | 56 ± 14 | 0.01 |
| Hyperlipidemia, n (%) | 5542 (14%) | 786 (15%) | 4756 (14%) | 0.69 |
| Former smoker, n (%) | 14,808 (38%) | 2259 (42%) | 12,549 (38%) | <0.001 |
| Current smoker, n (%) | 3906 (10%) | 590 (11%) | 3316 (10%) | 0.04 |
| Coronary heart disease, n (%) | 1097 (3%) | 166 (3%) | 931 (3%) | 0.28 |
| 10-year ASCVD Risk, % | 7 (10) | 7 (10) | 7 (10) | 0.26 |
| Infertility-related risk factors | ||||
| Irregular menses, n (%) | 6424 (17%) | 1368 (24%) | 5056 (15%) | <0.001 |
| Premature menopause, n (%) | 11669 (30%) | 1872 (35%) | 9797 (30%) | <0.001 |
| Waist circumference, cm | 90 ±14 | 89 ±14 | 90 ± 14 | 0.38 |
| Thyroid disease, n (%) | 7342 (19%) | 1278 (24%) | 6064 (18%) | <0.001 |
Values are means (standard deviations) or medians (inter-quartile ranges) unless otherwise noted.
Abbreviations: ASCVD = atherosclerotic cardiovascular disease, DM = diabetes, HDL = high-density lipoprotein, HF = heart failure, HFpEF = heart failure with preserved ejection fraction, HFrEF = heart failure with reduced ejection fraction, LVEF = left ventricular ejection fraction.
Over a median follow-up time of 15 years (Q1-Q3: 8–20), 2373 women developed incident HF, including 807 HFrEF cases and 1133 HFpEF cases.
History of infertility predicts risk of future heart failure
We examined the association of history of infertility with HF outcomes (Central Illustration). Cumulative incidence plots show greater risk of future HF among women with vs without history of infertility (Figure 1). In both age-adjusted and multivariable-adjusted Cox models, infertility was independently associated with future risk of overall HF. Specifically, women with a history of infertility had nearly 20% increased risk of developing HF compared with those without infertility (HR 1.16, 95% CI 1.04–1.30, p=0.006, Table 2). When examining HF subtypes, we found that infertility was significantly associated with future risk of HFpEF (HR 1.27, 95% CI 1.09–1.48, p = 0.002), but not HFrEF (HR 0.97, 95% CI 0.80–1.18, p=0.78) (Figure 2).
Central Illustration. Infertility is associated with future HFpEF, but not HFrEF.
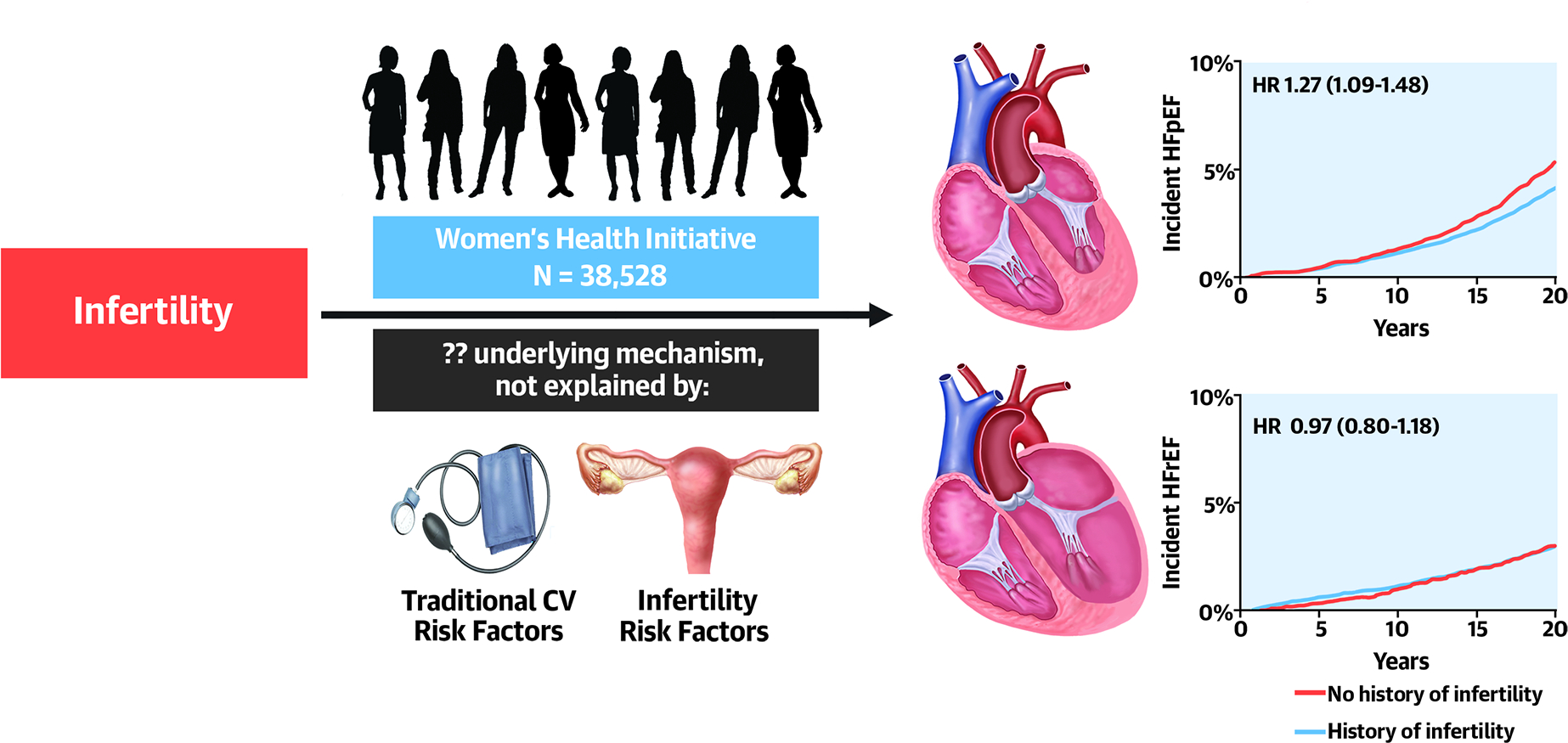
Women with a history of infertility had greater risk of incident heart failure, specifically heart failure with preserved ejection fraction. This association did not appear to be explained by traditional cardiovascular risk factors or infertility-related factors. Abbrevations: HF = heart failure, HFpEF = heart failure with preserved ejection fraction, HFrEF = heart failure with reduced ejection fraction, HR = hazard ratio
Figure 1. Incident Heart Failure Among Women With and Without Infertility.
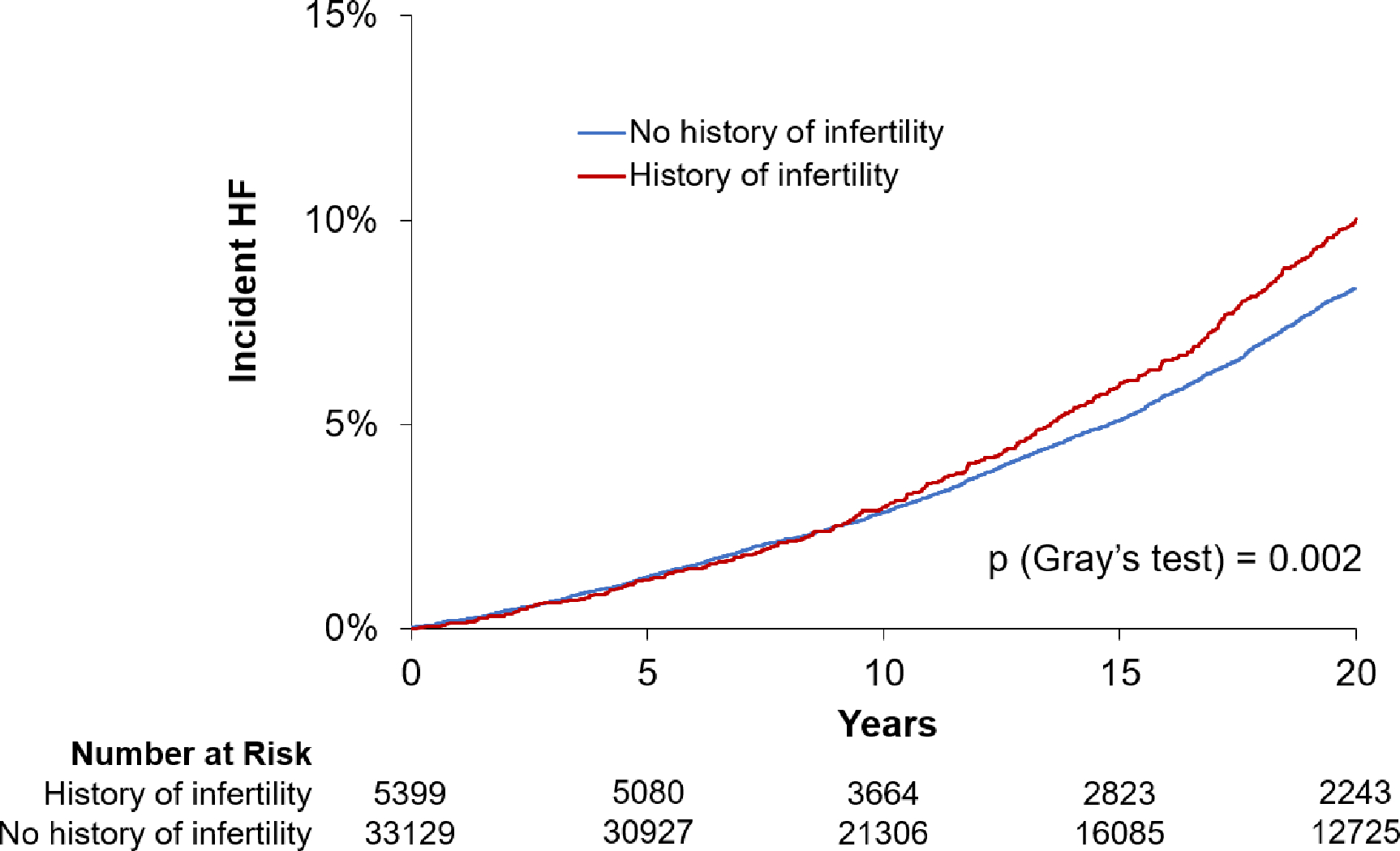
The cumulative incidence plots reflect incident heart failure among women with history of infertility (red line) and without history of infertility (blue line).
Table 2.
Association of infertility with incident heart failure and heart failure subtypes
| Incident HF events | Age-adjusted |
Multivariable-adjusted |
|||
|---|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) p-value | |||
|
| |||||
| Overall HF | 2373 | 1.16 (1.04–1.29) | 0.008 | 1.16 (1.04–1.30) | 0.006 |
| HFpEF | 1133 | 1.26 (1.08–1.47) | 0.003 | 1.27 (1.09–1.48) | 0.002 |
| HFrEF | 807 | 0.98 (0.80–1.19) | 0.88 | 0.97 (0.80–1.18) | 0.78 |
MV model adjusted for age, BMI, systolic blood pressure, hypertension treatment, DM, hyperlipidemia, smoking status, baseline CHD, and cohort. Covariates ascertained at study baseline. Abbreviations: CI = confidence interval, HF = heart failure, HFpEF = heart failure with preserved ejection fraction, HFrEF = heart failure with reduced ejection fraction, HR = hazard ratio.
Figure 2. Association of infertility with incident HF and HF subtypes.
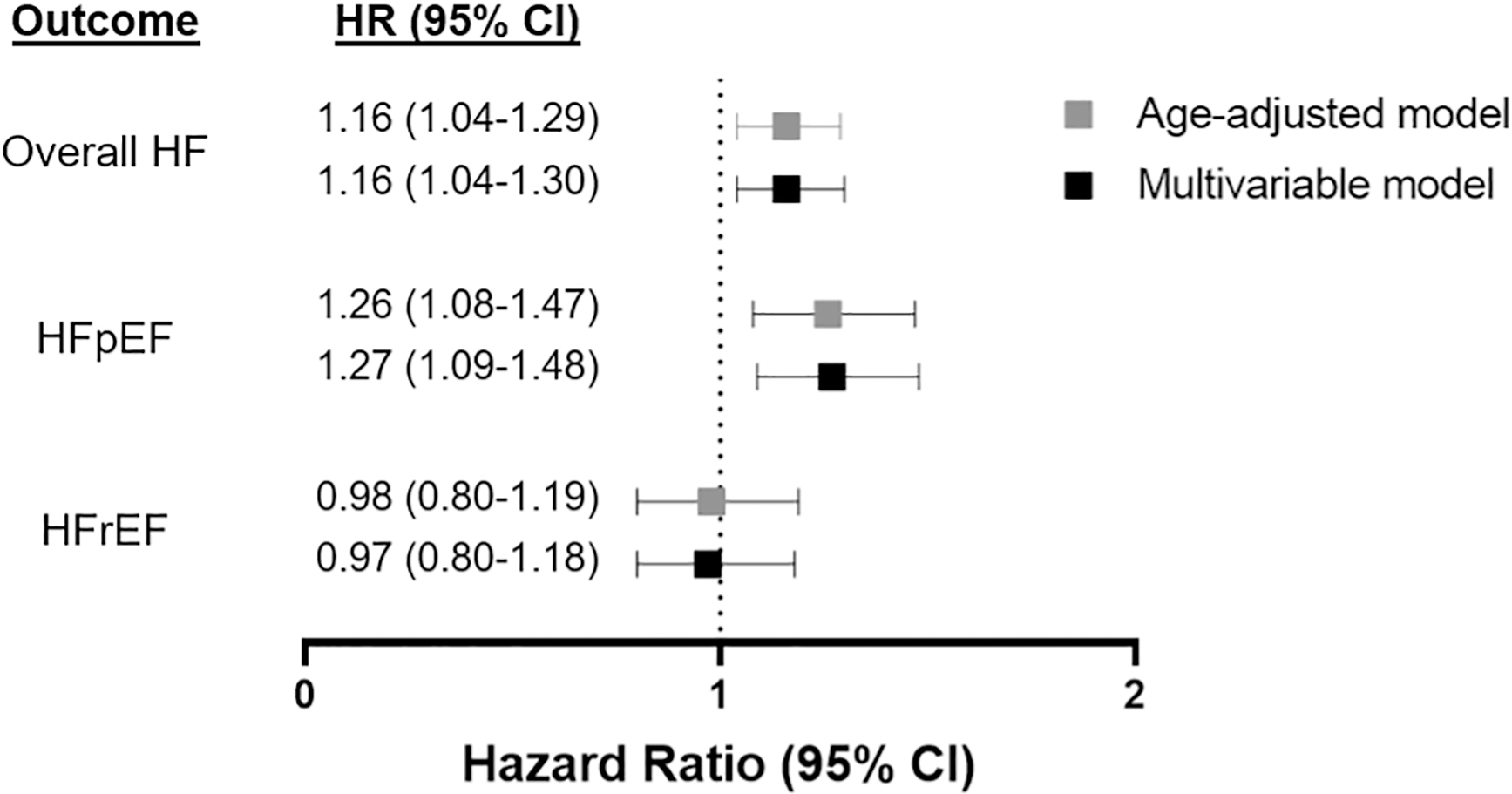
Women with a history of infertility had increased hazards of HF and HFpEF. Age-adjusted and multivariable-adjusted Cox proportional hazards models begin follow-up at study enrollment. Multivariable-adjusted models were adjusted for age at screening, BMI, systolic blood pressure, hypertension treatment, DM, hyperlipidemia, smoking status (current and former), baseline CHD, and cohort (observational study or clinical trial). Abbreviations: CI = confidence interval, HF = heart failure, HFpEF = heart failure with preserved ejection fraction, HFrEF= heart failure with reduced ejection fraction, HR = hazard ratio
Results were consistent after further adjustment for race/ethnicity (Supplemental Table 3, stratified results in Supplemental Table 4), and reclassification of HF subtypes using the LVEF 40% cut-point (Supplemental Table 5). In sensitivity analyses after exclusion of women with prevalent CHD, we found similar results with a 16% increased risk of future HF development among those with infertility (HR 1.16, 95% CI 1.04–1.30, p=0.009), again limited to increased risk of HFpEF and not HFrEF (Supplemental Table 6). Finally, the association of infertility with HF and HFpEF persisted even after exclusion of women who never became pregnant (Supplemental Table 7).
Among women with infertility, the proportion of women with a history of infertility due to hormones or ovulation was slightly higher in those who developed incident HF compared with those who did not, although these differences were not statistically significant (7.4% vs 5.9%, p=0.18). Other reasons for infertility including tubes or uterus, endometriosis, problems with partner, or unexplained infertility were similar between the two groups (Supplemental Table 8).
Infertility is not associated with 10-year atherosclerotic cardiovascular disease risk
We evaluated the association of infertility with traditional CV risk factors. In stepwise regression models, we found that BMI, White race, and smoking status were independently associated with infertility (p<0.05, Table 3). Specifically, former and current smoking were both associated with nearly 1.2-fold higher odds of self-reported history of infertility compared with non-smokers (former smoker: OR 1.19, 95% CI 1.12–1.27, p<0.001; current smoker: OR 1.14, 95% CI 1.03–1.26, p=0.01). White race was associated with 1.2-fold higher odds of infertility (OR 1.10, 95% 1.03–1.19, p=0.009). By contrast, systolic blood pressure, hypertension treatment, DM, and hyperlipidemia were not associated with infertility (p>0.05 for all). We further examined the association of infertility and traditional CV risk as captured by 10-year estimated ASCVD risk and found no significant association (OR 0.95, 95% CI 0.67–1.34, p=0.77).
Table 3.
Cross-sectional associations of traditional cardiovascular and infertility-related risk factors with infertility
| Age-adjusted analysis | Stepwise Regression* | |||
|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
|
| ||||
| Traditional CV risk factors | ||||
| Age | 0.99 (0.96–1.02) | 0.47 | 0.98 (0.95–1.01) | 0.21 |
| BMI | 0.97 (0.94–1.00) | 0.04 | 0.96 (0.93–0.99) | 0.01 |
| Non-Hispanic White race/ethnicity | 1.16 (1.09–1.25) | <0.001 | 1.10 (1.03–1.19) | 0.009 |
| Black race | 0.91 (0.85–0.98) | 0.008 | - | - |
| Hispanic ethnicity | 0.93 (0.85–1.01) | 0.10 | - | - |
| Systolic blood pressure | 1.01 (0.98–1.04) | 0.62 | - | - |
| Hypertension treatment | 0.98 (0.92–1.05) | 0.56 | - | - |
| DM treatment | 0.99 (0.88–1.11) | 0.85 | - | - |
| Former smoker | 1.18 (1.11–1.25) | <0.001 | 1.19 (1.12–1.27) | <0.001 |
| Current smoker | 1.10 (1.00–1.21) | 0.049 | 1.14 (1.03–1.26) | 0.01 |
| Hyperlipidemia | 1.03 (0.95–1.12) | 0.50 | - | - |
| Infertility-related risk factors | ||||
| Irregular menses | 1.88 (1.75–2.01) | <0.001 | 1.84 (1.72–1.97) | <0.001 |
| Early menopause | 1.28 (1.20–1.36) | <0.001 | 1.26 (1.18–1.34) | <0.001 |
| Waist circumference | 0.99 (0.96–1.01) | 0.31 | - | - |
| Thyroid disease | 1.38 (1.29–1.48) | <0.001 | 1.33 (1.24–1.42) | <0.001 |
OR: odds ratio per dichotomous variable or 1 standard deviation increase in continuous variable.
Stepwise model: age and cohort forced, entry at P<0.10, retention at P<0.05.
Abbreviations: BMI = body mass index, CI = confidence interval, CV = cardiovascular, DM = diabetes mellitus.
Risk factors for infertility do not explain association between infertility and heart failure
To better understand the link between infertility and HF, we explored the association of infertility-related risk factors with incident HF. We first confirmed the association between established infertility-related risk factors with infertility in our sample. Addition of infertility-related risk factors to the stepwise model demonstrated significant associations between irregular menses, early menopause, and thyroid disease with infertility (irregular menses: OR 1.84, 95% CI 1.72–1.97, p<0.001; early menopause: OR 1.26, 95% CI 1.18–1.34, p<0.001; thyroid disease: OR 1.33, 95% CI 1.24–1.42, p<0.001, Table 3). Waist circumference was not associated with infertility status (p=0.31).
Second, we examined the association of infertility risk factors with incident HF (Supplemental Table 9). We found that thyroid disease was associated with future risk of overall HF (HR 1.11, 95% CI 1.01–1.22, p=0.03) but not incident HFpEF or HFrEF (p=0.06 and 0.70, separately). Early menopause was associated with future risk of overall HF (HR 1.11, 95% CI 1.02–1.21, p=0.02) and HFrEF (HR 1.17, 95% CI 1.01–1.35, p=0.04), but not HFpEF. Also, Early menopause was associated with a 11% increase in the incidence of overall HF (HR 1.11, 95% CI: 1.02–1.21, p=0.02) and a 17% increase in the incidence of HFrEF only (HR 1.17, 95% CI: 1.01–1.35, p=0.04). Irregular menses was not associated with future risk of overall HF or HF subtypes.
Finally, the relationship between infertility and future risk of HF and HFpEF persisted even after further adjustment for irregular menses, early menopause, and thyroid disease (overall HF: HR 1.15, 95% CI 1.03–1.29, p=0.01; HFpEF: HR 1.26, 95% CI 1.08–1.47, p=0.003, Supplemental Table 10).
DISCUSSION
In a large prospective cohort of 38,528 post-menopausal women, we demonstrate that history of infertility was significantly associated with incident HF. Our principal findings are two-fold. First, women with infertility had a higher risk of developing HF, driven primarily by greater risk of future HFpEF and not HFrEF. Second, the association between infertility and HF did not appear to be explained by established CV risk factors or infertility-related risk factors, highlighting the need for further investigation into the underlying mechanistic drivers.
Emerging data suggest that a woman’s reproductive period provides an important window into her lifetime CV risk. Premature menopause and adverse pregnancy outcomes including hypertensive disorders of pregnancy and gestational diabetes have now been recognized as independent risk factors for future atherosclerotic CVD (1). By contrast, infertility, a condition that affects over 14% of women, has not been rigorously studied with respect to its impact on future CVD, and the limited available data are conflicting. In 863,324 women with self-reported fertility data in the Swedish Medical Birth Register, women with infertility for 5 or more years prior to successful pregnancy had a 20% greater risk of incident CVD compared with women without a history of infertility (6). A cross-sectional analysis of 744 women who participated in the National Health and Nutrition Examination Survey also found that self-reported infertility was significantly associated with cardiovascular events (19). By contrast, previous studies from the WHI and the Study of Women’s Health Across the Nation (SWAN) did not find independent associations of infertility with CVD events (7,8). Given the rising prevalence of HF, particularly HFpEF, among women, increasing attention has been directed toward identifying reproductive risk factors that contribute to future HF development. Recent studies have demonstrated higher risk of HF among women with history of preeclampsia, particularly recurrent preeclampsia, as well as nulliparity and shorter total reproduction duration (13,20). We now present evidence supporting infertility as an independent risk factor for the development of HF among women without previous history of HF.
Contrary to our hypothesis, the increased risk of HF in women with a history of infertility was not explained by traditional atherosclerotic CV risk factors such as hypertension, obesity, and DM or greater risk of ischemic heart disease. First, adjustment for traditional CV risk factors or exclusion of women with prevalent CHD did not attenuate the association between infertility and HF. Second, we found no association between infertility and 10-year ASCVD risk score, nor was infertility associated with observed CHD events in a previous WHI analysis (7). Finally, infertility was associated with greater risk of HFpEF and not HFrEF; the opposite would be expected for a process driven by ischemic heart disease. Taken together, these findings suggest that the mechanisms mediating HF risk in infertility are orthogonal to traditional atherosclerotic CVD.
We separately examined whether risk factors for infertility, rather than infertility itself, explained the association of infertility with risk of HF. Menstrual cycle irregularity and premature menopause are both well-established risk factors for infertility and have been consistently shown to increase risk of future CVD in cross-sectional and prospective studies (21). Thyroid disease, another risk factor for the development of infertility, is strongly associated with CVD including HF and CHD (22,23). We confirmed independent associations between irregular menses, early menopause, and thyroid disease with infertility, as well as associations between thyroid disease with incident HF. However, further adjustment for these infertility-related risk factors did not attenuate the association between infertility and incident HF, which argues that they do not mediate the association between infertility and HF.
Taken together, these findings support the novel association of infertility with incident HFpEF, which appears independent of traditional cardiovascular risk factors, ischemic heart disease, or infertility-associated factors. The current paradigm of HFpEF development proposes that cardiometabolic comorbidities induce a pro-inflammatory state that contributes to downstream myocardial structural and functional alterations characteristic of HFpEF (24,25). In our exploratory analyses, infertility due to hormonal or ovulatory dysfunction, but not other causes of infertility, was more commonly reported among women who developed HF compared with those who did not. Hypothalamic pituitary axis dysregulation and associated estrogen deficiency have previously been implicated in the development of HF and CVD and are being explored as a potential targets for treatment of HF (26). Specifically, estradiol is a key regulator of inflammatory processes and lower concentrations of estradiol is thought to trigger systemic inflammation, which promotes production of reactive oxygen species, impaired nitric oxide signaling, renin-angiotensin-aldosterone system activation, and endothelial and vascular inflammation (27). Collectively, these changes are postulated to contribute to downstream cardiac structural changes including increased collagen synthesis and diastolic dysfunction, key factors in the pathogenesis of HFpEF (28,29). This hypothesis has been supported by both pre-clinical and clinical studies. For example, in rodent studies, bilateral ovariectomy triggered collagen deposition and myocardial hypertrophy while low-dose estrogen replacement attenuated these changes (28). Clinical studies have consistently demonstrated acceleration of CVD and HF risk following menopause, including premature menopause, raising the hypothesis that estrogen deficiency plays an important role in mediating CV risk (2). In post-menopausal women, estrogen deficiency, in the form of a higher testosterone/estradiol ratio, has been associated with higher risk of incident CVD, CHD, and HF (30), and estrogen replacement therapy was associated with significant reduction in mortality among postmenopausal women with advanced HF (31). However, estrogen replacement therapy administered in randomized trials increased CV events, highlighting the complex relationship between sex hormones and CVD (32,33). Beyond estrogen deficiency, shared cardiometabolic risk factors such as obesity and diabetes may be also explain the association between infertility and HFpEF. For example, polycystic ovary syndrome, a common cause of infertility, is strongly associated with increased cardiometabolic risk (34). Moreover, in a Framingham Heart Study of 1,968 women, infertility was strongly associated with BMI and waist circumference (35), and a smaller study of 130 women with and without unexplained infertility found elevated high sensitivity C- reactive protein, triglycerides, low-density lipoprotein cholesterol, and lower high-density lipoprotein levels among women with history of infertility compared with controls (36). Notably, we did not find associations between BMI, waist. circumference, or hyperlipidemia with infertility in our sample. However, these parameters were ascertained at the time of study enrollment when participants were post-menopausal and not contemporaneous with time of infertility. It is conceivable that the cardiometabolic risk profile between women with and without infertility varies early in life but may converge later in life, particularly as many women experience postpartum weight retention following pregnancy. Whether the association of infertility with HFpEF is causal or due to shared risk factors warrants further investigation.
Limitations
This study has several limitations worth discussion. First, infertility status was self-reported, which may have resulted in some misclassification. Nevertheless, self-report has previously been validated as a highly sensitive method for ascertaining infertility (37). Second, the WHI enrolled post-menopausal women between 1993 and 1998 and thus most of the pregnancies in this cohort pre-dated contemporary fertility treatments such as in vitro fertilization. As such, we were unable to examine the effect of fertility treatment on the risk of HF among women with infertility, an important area for future study. Third, granular data on duration of infertility prior to pregnancy and reasons for nulliparity were not available in the WHI. Sensitivity analyses restricted to women with infertility and subsequent reported history of pregnancy still showed greater risk of HF, however we were not powered to examine women who never became pregnant. Fourth, baseline covariates including traditional CV risk factors including BMI, WC, and blood pressure were ascertained at study enrollment, and do not reflect CV risk at the time of infertility or across the life course. Information on cardiometabolic risk profile including anthropomorphic data, vital signs, and laboratory values including hormone levels obtained at the time of infertility may provide important insights into the mechanisms driving the association of infertility with HF but were not available in the WHI. Fourth, while CHD did not appear to explain the association between infertility and HF, microvascular disease, a key factor in HFpEF pathogenesis, was not specifically examined. Finally, this is an observational study, limiting our ability to make causal inferences. Nevertheless, this study is strengthened by the availability of comprehensive reproductive data from a large and diverse patient sample with generalizability across different racial/ethnic groups, which allows us to uniquely evaluate long term HF risk among women with a history of infertility.
CONCLUSION
In summary, history of infertility was significantly associated with risk of future HF, driven by increased risk of HFpEF but not HFrEF. Traditional CV risk as ascertained by 10-year ASCVD risk score was not associated with infertility. While traditional CV risk factors and infertility-related risk factors were independently associated with incident HF, they did not explain the association between infertility with incident HF and HFpEF. Our findings highlight the need for rigorous studies investigating the mechanisms by which infertility may lead to future HFpEF and overall CV risk.
Supplementary Material
CLINICAL PERSPECTIVES.
Competency in Medical Knowledge:
Infertility is associated with an increased risk of heart failure, specifically HFpEF, and this association is not explained by conventional cardiovascular risk factors, coronary disease, or other infertility-related conditions.
Translational Outlook:
Future studies are needed to identify the mechanisms that underlie the association between infertility and heart failure.
Funding:
The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts 75N92021D00001, 75N92021D00002, 75N92021D00003, 75N92021D00004, 75N92021D00005. E.S.L is supported by the American Heart Association (853922). J.E.H. is supported by NIH R01-HL134893, R01-HL140224, and K24-HL153669.
ABBREVIATIONS
- ASCVD
atherosclerotic cardiovascular disease
- BMI
body mass index
- CHD
coronary heart disease
- CVD
cardiovascular disease
- DM
diabetes mellitus
- HF
heart failure
- HFrEF
heart failure with reduced ejection fraction
- HFpEF
heart failure with preserved ejection fraction
- LVEF
left ventricular ejection fraction
- WHI
Women’s Health Initiative
Footnotes
Disclosures: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Emily S. Lau, Cardiology Division, Massachusetts General Hospital, Boston, MA; Cardiovascular Research Center Center, Massachusetts General Hospital, Boston, MA.
Dongyu Wang, CardioVascular Institute and Division of Cardiology, Department of Medicine, Beth Israel Deaconness Medical Center, Boston, MA.
Mary Roberts, Department of Family Medicine, The Warren Alpert Medical School of Brown University, Providence, RI.
Christy N. Taylor, Department of Medicine, Massachusetts General Hospital, Boston, MA.
Gayathree Murugappan, Department of Obstetrics and Gynecology, Stanford University Medical Center, Stanford, CA.
Aladdin H. Shadyab, Herbert Wertheim School of Public Health and Human Longevity Science, University of California, San Diego, La Jolla, CA.
Peter F. Schnatz, Department of Obstetrics and Gynecology, The Reading Hospital/Tower Health, Reading, PA.
Leslie V. Farland, Department of Epidemiology and Biostatistics, University of Arizona; Department of Obstetrics and Gynecology, College of Medicine-Tucson, Tucson, AZ.
Malissa J. Wood, Cardiology Division, Massachusetts General Hospital, Boston, MA.
Nandita S. Scott, Cardiology Division, Massachusetts General Hospital, Boston, MA.
Charles B. Eaton, Department of Family Medicine, The Warren Alpert Medical School of Brown University, Providence, RI.
Jennifer E. Ho, CardioVascular Institute and Division of Cardiology, Department of Medicine, Beth Israel Deaconness Medical Center, Boston, MA.
REFERENCES
- 1.Arnett DK, Blumenthal RS, Albert MA et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019;140:e596–e646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Honigberg MC, Zekavat SM, Aragam K et al. Association of Premature Natural and Surgical Menopause With Incident Cardiovascular Disease. Jama 2019;322:2411–2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tooher J, Thornton C, Makris A, Ogle R, Korda A, Hennessy A. All Hypertensive Disorders of Pregnancy Increase the Risk of Future Cardiovascular Disease. Hypertension (Dallas, Tex : 1979) 2017;70:798–803. [DOI] [PubMed] [Google Scholar]
- 4.Søndergaard MM, Hlatky MA, Stefanick ML et al. Association of Adverse Pregnancy Outcomes With Risk of Atherosclerotic Cardiovascular Disease in Postmenopausal Women. JAMA cardiology 2020;5:1390–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Infertility Workup for the Women’s Health Specialist: ACOG Committee Opinion, Number 781. Obstetrics and gynecology 2019;133:e377–e384. [DOI] [PubMed] [Google Scholar]
- 6.Parikh NI, Cnattingius S, Mittleman MA, Ludvigsson JF, Ingelsson E. Subfertility and risk of later life maternal cardiovascular disease. Human reproduction (Oxford, England) 2012;27:568–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parikh NI, Jeppson RP, Berger JS et al. Reproductive Risk Factors and Coronary Heart Disease in the Women’s Health Initiative Observational Study. Circulation 2016;133:2149–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cairncross ZF, Ahmed SB, Dumanski SM, Nerenberg KA, Metcalfe A. Infertility and the Risk of Cardiovascular Disease: Findings From the Study of Women’s Health Across the Nation (SWAN). CJC open 2021;3:400–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farland LV, Grodstein F, Srouji SS et al. Infertility, fertility treatment, and risk of hypertension. Fertility and sterility 2015;104:391–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heidenreich PA, Albert NM, Allen LA et al. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circulation Heart failure 2013;6:606–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dewan P, Rørth R, Raparelli V et al. Sex-Related Differences in Heart Failure With Preserved Ejection Fraction. Circulation Heart failure 2019;12:e006539. [DOI] [PubMed] [Google Scholar]
- 12.Lam CSP, Arnott C, Beale AL et al. Sex differences in heart failure. European Heart Journal 2019;40:3859–3868c. [DOI] [PubMed] [Google Scholar]
- 13.Hall PS, Nah G, Howard BV et al. Reproductive Factors and Incidence of Heart Failure Hospitalization in the Women’s Health Initiative. Journal of the American College of Cardiology 2017;69:2517–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Curb JD, McTiernan A, Heckbert SR et al. Outcomes ascertainment and adjudication methods in the women’s health initiative. Annals of Epidemiology 2003;13:S122–S128. [DOI] [PubMed] [Google Scholar]
- 15.Design of the Women’s Health Initiative Clinical Trial and Observational Study. Controlled Clinical Trials 1998;19:61–109. [DOI] [PubMed] [Google Scholar]
- 16.Definitions of infertility and recurrent pregnancy loss: a committee opinion. Fertility and sterility 2020;113:533–535. [DOI] [PubMed] [Google Scholar]
- 17.Rosamond WD, Chang PP, Baggett C et al. Classification of heart failure in the atherosclerosis risk in communities (ARIC) study: a comparison of diagnostic criteria. Circulation Heart failure 2012;5:152–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parikh NI, Kapphahn K, Hedlin H et al. Effects of reproductive period duration and number of pregnancies on midlife ECG indices: a secondary analysis from the Women’s Health Initiative Clinical Trial. BMJ Open 2018;8:e019129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gleason JL, Shenassa ED, Thoma ME. Self-reported infertility, metabolic dysfunction, and cardiovascular events: a cross-sectional analysis among U.S. women. Fertility and sterility 2019;111:138–146. [DOI] [PubMed] [Google Scholar]
- 20.Honigberg MC, Riise HKR, Daltveit AK et al. Heart Failure in Women With Hypertensive Disorders of Pregnancy: Insights From the Cardiovascular Disease in Norway Project. Hypertension (Dallas, Tex : 1979) 2020;76:1506–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Solomon CG, Hu FB, Dunaif A et al. Menstrual cycle irregularity and risk for future cardiovascular disease. The Journal of clinical endocrinology and metabolism 2002;87:2013–7. [DOI] [PubMed] [Google Scholar]
- 22.Redmond GP. Thyroid dysfunction and women’s reproductive health. Thyroid : official journal of the American Thyroid Association 2004;14 Suppl 1:S5–15. [DOI] [PubMed] [Google Scholar]
- 23.Flynn RW, Macdonald TM, Jung RT, Morris AD, Leese GP. Mortality and vascular outcomes in patients treated for thyroid dysfunction. The Journal of clinical endocrinology and metabolism 2006;91:2159–64. [DOI] [PubMed] [Google Scholar]
- 24.Savji N, Meijers WC, Bartz TM et al. The Association of Obesity and Cardiometabolic Traits With Incident HFpEF and HFrEF. JACC Heart failure 2018;6:701–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eaton CB, Pettinger M, Rossouw J et al. Risk Factors for Incident Hospitalized Heart Failure With Preserved Versus Reduced Ejection Fraction in a Multiracial Cohort of Postmenopausal Women. Circulation Heart failure 2016;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takefuji M, Murohara T. Corticotropin-Releasing Hormone Family and Their Receptors in the Cardiovascular System. Circulation journal : official journal of the Japanese Circulation Society 2019;83:261–266. [DOI] [PubMed] [Google Scholar]
- 27.Murphy E Estrogen signaling and cardiovascular disease. Circ Res 2011;109:687–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao Z, Wang H, Jessup JA, Lindsey SH, Chappell MC, Groban L. Role of estrogen in diastolic dysfunction. Am J Physiol Heart Circ Physiol 2014;306:H628–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beale AL, Meyer P, Marwick TH, Lam CSP, Kaye DM. Sex Differences in Cardiovascular Pathophysiology: Why Women Are Overrepresented in Heart Failure With Preserved Ejection Fraction. Circulation 2018;138:198–205. [DOI] [PubMed] [Google Scholar]
- 30.Zhao D, Guallar E, Ouyang P et al. Endogenous Sex Hormones and Incident Cardiovascular Disease in Post-Menopausal Women. Journal of the American College of Cardiology 2018;71:2555–2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lindenfeld J, Ghali JK, Krause-Steinrauf HJ et al. Hormone replacement therapy is associated with improved survival in women with advanced heart failure. Journal of the American College of Cardiology 2003;42:1238–45. [DOI] [PubMed] [Google Scholar]
- 32.Manson JE, Hsia J, Johnson KC et al. Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med 2003;349:523–34. [DOI] [PubMed] [Google Scholar]
- 33.Anderson GL, Limacher M, Assaf AR et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. Jama 2004;291:1701–12. [DOI] [PubMed] [Google Scholar]
- 34.Osibogun O, Ogunmoroti O, Michos ED. Polycystic ovary syndrome and cardiometabolic risk: Opportunities for cardiovascular disease prevention. Trends in cardiovascular medicine 2020;30:399–404. [DOI] [PubMed] [Google Scholar]
- 35.Mahalingaiah S, Sun F, Cheng JJ, Chow ET, Lunetta KL, Murabito JM. Cardiovascular risk factors among women with self-reported infertility. Fertility research and practice 2017;3:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verit FF, Yildiz Zeyrek F, Zebitay AG, Akyol H. Cardiovascular risk may be increased in women with unexplained infertility. Clinical and experimental reproductive medicine 2017;44:28–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dick ML, Bain CJ, Purdie DM, Siskind V, Molloy D, Green AC. Self-reported difficulty in conceiving as a measure of infertility. Human reproduction (Oxford, England) 2003;18:2711–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


