Adolescent, nonsexually acquired genital ulcers may be associated with coronavirus disease 2019 (COVID-19) vaccination, highlighting the need for further research in aphthous ulcer management in adolescents.
BACKGROUND:
Nonsexually acquired genital ulcers have been described among girls who are prepubertal after various viral illnesses due to mucosal inflammation from an immunologic response. Until recently, nonsexually acquired genital ulcers have only been associated with viral infections.
CASE:
We present a case of an adolescent girl developing nonsexually acquired genital ulcers after both her first and second coronavirus disease 2019 (COVID-19) vaccine doses. Her course followed an expected timeline for severity and resolution of ulcers.
CONCLUSIONS:
Aphthous ulcers may arise from inflammatory effects of COVID-19 vaccination. Clinical monitoring after COVID-19 vaccination from all formulations should include assessment for nonsexually acquired genital ulcers if vaginal pain is reported.
Teaching Points
Adolescent girls are particularly prone to developing nonsexually acquired genital ulcers after viral illnesses; they may also occur after coronavirus disease 2019 (COVID-19) vaccination.
Nonsexually acquired genital ulcers follow a similar course of painful eruption followed by healing when associated with viral illnesses and COVID-19 vaccination, lending them to the same treatment of supportive care, pain management, and optional steroid supplementation in severe cases.
The benefits of COVID-19 vaccination outweigh any side effects associated with the development of nonsexually acquired genital ulcers.
Nonsexually acquired genital ulcers most commonly appear in early puberty both before and after menarche, with a mean age of 14 years.1 Lesions typically present as acute, painful vulvar aphthous ulcers preceded by nonspecific viral symptoms, including fever, fatigue, and body aches.2 The classic appearance of a vulvar aphthous ulcer is a shallow, well-demarcated lesion that is often larger than 1 cm. The ulcer typically has an overlying fibrinous exudate or eschar and may be associated with swelling of the surrounding vulvar tissue. Ulcers occasionally may present with symmetrical “kissing” lesions on the opposite labia.1 Health care professionals who are not familiar with the appearance of nonsexually acquired genital ulcers often misdiagnose the condition as a herpes simplex virus (HSV) ulcer or labial abscess and mistake the eschar for necrotic tissue. The mainstays of treatment include pain management and supportive care with sitz baths. Topical and oral steroids are recommended for more severe lesions based on expert opinion,3,4 but no comparative trials have established their superiority over supportive care alone.
CASE
A 15-year-old girl, previously healthy with no prior symptoms associated with autoimmune disorders, presented to a community urgent care clinic with complaints of vaginal pain and “vaginal lesions.” She had not experienced symptoms associated with coronavirus disease 2019 (COVID-19) that would necessitate testing and reported feeling well before her first dose of Pfizer-BioNTech mRNA (Pfizer) COVID-19 vaccine 4 days before presentation. One day postvaccination, she reported a low-grade fever lasting for 48 hours. She noticed a sore on her vagina 3 days postvaccination and presented to urgent care on day 4. The acute care health care professional was concerned for a labial abscess and collected Neisseria gonorrhoeae and Chlamydia trachomatis polymerase chain reaction tests and blood cultures.
The patient was transferred to a tertiary care children's hospital for further evaluation. Her medical and surgical history were noncontributory. She reported no allergies and was taking no medications. The patient began menarche 1 year prior. Her menses were monthly and light, requiring only pads. She reported no sexual activity, and a pregnancy test result was negative. The patient was well-appearing and afebrile, with an isolated tachycardia of 101. A gynecologic specialist was consulted, and the patient’s labia were evaluated, noting a single superficial ulceration on the medial aspect of the right labia minora consistent with nonsexually acquired genital ulcers (Fig. 1). Based on institutional data noting uniformly negative HSV associations,1 our current protocols do not include HSV, cytomegalovirus, or Epstein-Barr virus cultures or serologic testing when nonsexually acquired genital ulcers are diagnosed. The patient was discharged to home with instructions to perform daily sitz bath soaks and to use oral analgesics and topical anesthetics for pain. Topical steroids were prescribed to limit inflammation. She was seen in clinic 1 week after the initial evaluation, with no reports of pain and resolution of the ulcer (Fig. 2). Previous cultures and sexually transmitted infection test results were negative.
Fig. 1. Ulcerative lesions (arrowhead) on the vestibular mucosa 4 days after the first coronavirus disease 2019 (COVID-19) vaccine dose.
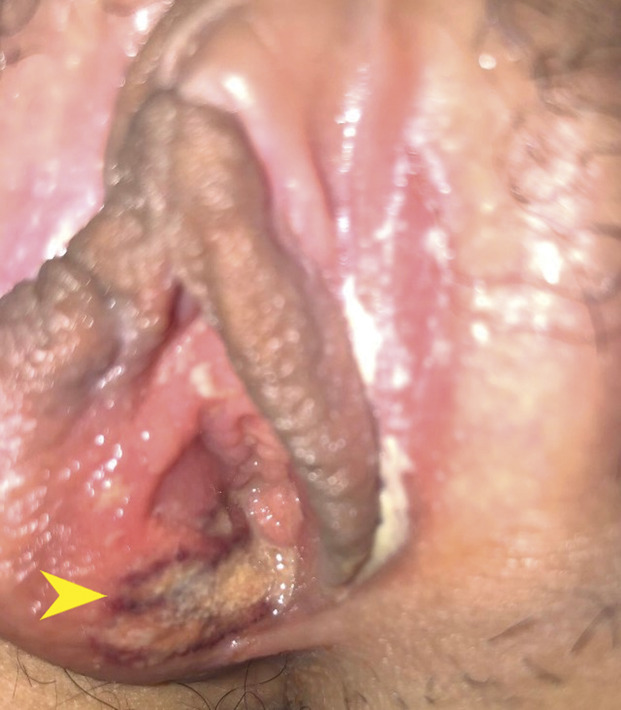
Scott. Aphthous Ulcers After COVID-19 Vaccination. Obstet Gynecol 2022.
Fig. 2. Healed mucosal surface (arrowhead) 1 week after initial presentation.
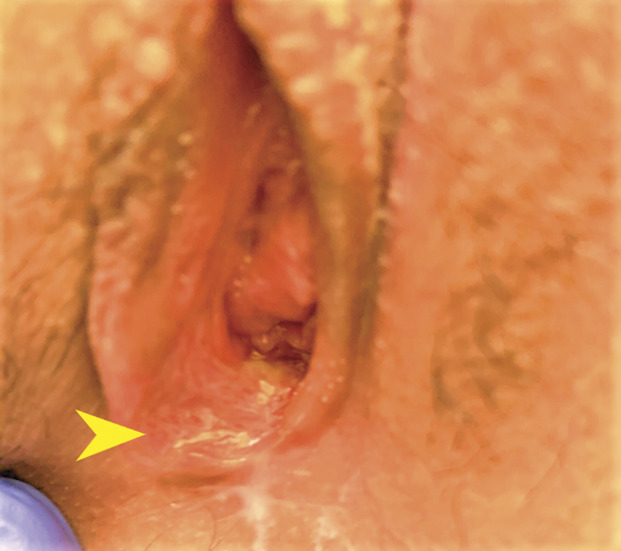
Scott. Aphthous Ulcers After COVID-19 Vaccination. Obstet Gynecol 2022.
The patient received her second dose of COVID-19 vaccine 21 days after the initial dose. Three days after her second vaccine dose, she reported recurrent pain and was seen in clinic. A new ulcer on the right labia was noted (Fig. 3). She reported no fever or prodromal symptoms. One week after her clinic visit, she reported that the second ulcer and pain had resolved with supportive care only.
Fig. 3. New ulcers (arrowhead) 3 days after the second coronavirus disease 2019 (COVID-19) vaccine dose.
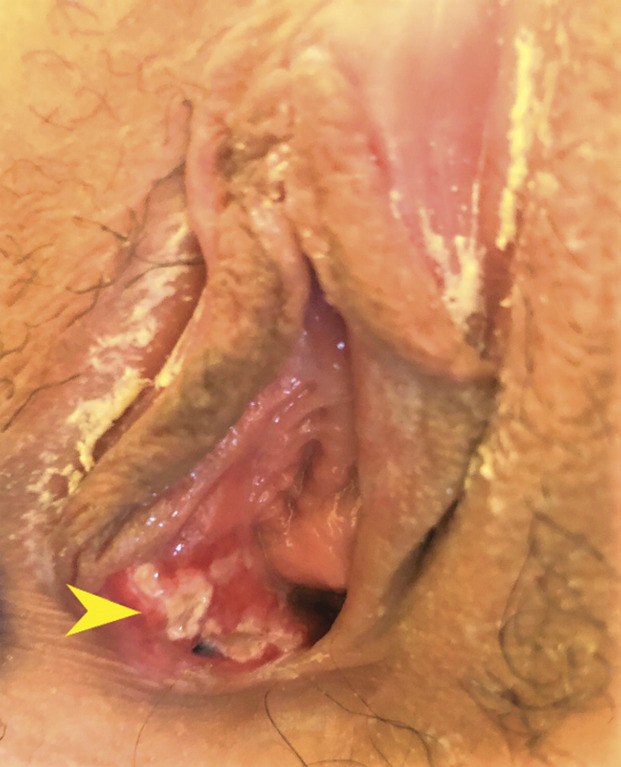
Scott. Aphthous Ulcers After COVID-19 Vaccination. Obstet Gynecol 2022.
DISCUSSION
Review of the literature documents various viral illnesses preceding nonsexually acquired genital ulcers, including two cases (one in adolescence) of vulvar aphthous ulcers after infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).1,5–8 The pathogenesis of vulvar aphthosis is thought to be due to a nonspecific inflammatory response to various systemic viral illnesses.1 In general, younger age is associated with a relatively greater immunologic response9; this coupled with the influence of reproductive hormones10 puts adolescent girls at particular risk for developing nonsexually acquired genital ulcers. Unlike Behçet's disease, which involves ulcers erupting on multiple organ sites and can take months to years to diagnose,11 almost all instances of nonsexually acquired genital ulcers are the first occurrence of mucosal lesions. The percentage of patients with nonsexually acquired genital ulcers who are ultimately diagnosed with Behçet's disease is currently unknown.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is an RNA virus known to affect multiple organ systems through activation of a systemic inflammatory response.12 Case reports of nonsexually acquired genital ulcers after COVID-19 vaccination in nonimmunocompromised patients have been described in the literature.13,14 The individual in one case experienced fatigue, body aches, and insomnia 12 hours after the vaccination, lasting for 1 day.13 It is currently unknown whether compromised immune status would influence the development of nonsexually acquired genital ulcer eruptions; immune compromise would render an individual more likely to become infected but less likely to mount a secondary immunologic response. The possibility of nonsexually acquired genital ulcers associated with COVID-19 vaccination is suggested in this case by the development of a unique ulcer soon after each injection of a two-dose series. Although a viral infection could explain the first presentation,1,2 the second eruption's lack of associated prodromal symptoms along with its proximity to the second vaccine dose makes an association plausible.
The COVID-19 vaccine's mechanism of action and previously documented side effects support a potential association with genital mucosal ulceration. The Pfizer COVID-19 vaccine produces immunogenicity through the presentation of the virus' mRNA spike protein to accelerate antibody production within human cells.15 Previous reports have identified associations between vaccine administration and inflammation of organs, including the brain, eyes, and skin.16–18 Given the systemic immune response, the link between vulvar aphthosis and COVID-19 vaccination has a plausible physiologic basis.
With input from our rheumatology experts in this case, completion of the vaccine series was recommended given that nonsexually acquired genital ulcers do not result in long-term sequelae, in contrast to the severe sequelae of COVID-19. Although ulcers did recur after the second vaccine dose, they also resolved as expected. The protection provided by COVID-19 vaccination continues to outweigh this temporary side effect based on this reassuring outcome. If patients are identified in the future with nonsexually acquired genital ulcers after an initial COVID-19 vaccine dose in a two-dose series, close monitoring to assess for recurrence and severity after the second injection is warranted.
Finally, current treatment for nonsexually acquired genital ulcers is mainly supportive, including pain management with frequent sitz baths, topical anesthetics, and oral analgesics, but may also include topical or systemic steroids depending on the severity of the lesions and symptoms. Currently, it is unknown whether systemic steroids for treatment of nonsexually acquired genital ulcers would affect COVID-19 vaccine efficacy. Impairment of circulating T cells has been demonstrated within 48 hours after a control group of healthy individuals received intravenous steroids.19 A case report noted impaired neutralizing antibodies to the Moderna COVID-19 vaccine in a man of elderly age who was taking chronic steroids for myasthenia gravis.20 This is in contrast to studies that noted no significant alteration in efficacy from killed virus vaccines among participants with other autoimmune disorders taking chronic steroids.21 It is unknown whether the short-term steroid bursts used for treatment of some nonsexually acquired genital ulcers would alter the efficacy of COVID-19 vaccines in patients who are immunocompetent or immunocompromised. Currently there is not enough knowledge to suggest alterations in current steroid regimens.
In conclusion, this case report describes a potential association of a two-dose COVID-19 vaccine series with vulvar aphthosis in an adolescent. Further research is needed to determine whether this association is identified in other adolescent girls receiving the Pfizer COVID-19 vaccine series as well as other COVID-19 vaccine formulations. The natural course of ulcer resolution in this case was self-limited and followed the same time course as seen after viral infections. This potential new association with nonsexually acquired genital ulcers reminds health care professionals that little is known about the efficacy of current ulcer management. Future studies to determine the efficacy of steroids on the course of nonsexually acquired genital ulcers may inform health care professionals on the benefits of steroid use in the short term balanced against any potentially blunted vaccine response.
Footnotes
Financial Disclosure The authors did not report any potential conflicts of interest.
The authors thank Dr. Kendra Hutchens for her assistance with editing.
Each author has confirmed compliance with the journal's requirements for authorship.
Peer reviews and author correspondence are available at http://links.lww.com/AOG/C792.
REFERENCES
- 1.Huppert JS, Gerber MA, Deitch HR. Vulvar ulcers in young females: a manifestation of aphthosis. J Pediatr Adolesc Gynecol 2006;19:195–204. doi: 10.1016/j.jpag.2006.02.006 [DOI] [PubMed] [Google Scholar]
- 2.Farhi D, Wendling J, Molinari E. Non–sexually related acute genital ulcers in 13 pubertal girls: a clinical and microbiological study. Arch Dermatol 2009;145:38–45. doi: 10.1001/archdermatol.2008.519 [DOI] [PubMed] [Google Scholar]
- 3.Farag Ahmed ES. Nonsexually acquired acute genital ulceration: a commonly misdiagnosed condition. Gulf J Dermatol Venereol 2018;25:13 [Google Scholar]
- 4.Dixit S, Bradford J, Fischer G. Management of nonsexually acquired genital ulceration using oral and topical corticosteroids followed by doxycycline prophylaxis. J Am Acad Dermatol 2013;68:797–802. doi: 10.1016/j.jaad.2012.10.014 [DOI] [PubMed] [Google Scholar]
- 5.Lehman J, Bruce A, Wetter D. Reactive nonsexually related acute genital ulcers: review of cases evaluated at Mayo Clinic. J Am Acad Dermatol 2010;63:44–51. doi: 10.1016/j.jaad.2009.08.038 [DOI] [PubMed] [Google Scholar]
- 6.Falkenhain-López D, Agud-Dios M, Ortiz-Romero P. COVID-19-related acute genital ulcers. J Eur Acad Dermatol Venereol 2020;34:e655–6. doi: 10.1111/jdv.16740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dominguez-Santas M, Diaz-Guimaraens B, Fernandez-Nieto D. Minor aphthae associated with SARS-CoV-2 infection. Int J Dermatol 2020;59:1022–3. doi: 10.1111/ijd.15004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christl J, Alaniz VI, Appiah L, Buyers E, Scott S, Huguelet PS. Vulvar aphthous ulcer in an adolescent with COVID-19. J Pediatr Adolescgynecol 2021;34:418–20. doi: 10.1016/j.jpag.2021.02.098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fi Kumar R, Burns EA. Age-related decline in immunity: implications for vaccine responsiveness. Expert Rev Vaccin 2008;7:467–79. doi: 10.1586/14760584.7.4.467 [DOI] [PubMed] [Google Scholar]
- 10.Markle JG, Fish EN. SeXX matters in immunity. Trends Immunology 2014;35:97–104. doi: 10.1016/j.it.2013.10.006 [DOI] [PubMed] [Google Scholar]
- 11.Bulur I, Onder M. Behçet disease: new aspects. Clin Dermatol 2017;35:421–34. doi: 10.1016/j.clindermatol.2017.06.004 [DOI] [PubMed] [Google Scholar]
- 12.Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LF. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol 2020;20:363–74. doi: 10.1038/s41577-020-0311-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drucker A, Corrao K, Gandy M. Vulvar aphthous ulcer following Pfizer-BioNTech COVID-19 vaccine–a case report. J Pediatr Adolesc Gynecol 2021;35:165–6. doi: 10.1016/j.jpag.2021.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wojcicki AV, O'Brien KLF. Vulvar aphthous ulcer in an adolescent after Pfizer-BioNTech (BNT162b2) COVID-19 vaccination. J Pediatr Adolesc Gynecol 2022;35:167–70. doi: 10.1016/j.jpag.2021.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jeyanathan M, Afkhami S, Smaill F, Miller MS, Lichty BD, Xing Z. Immunological considerations for COVID-19 vaccine strategies. Nat Rev Immunol 2020;20:615–32. doi: 10.1038/s41577-020-00434-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khayat-Khoei M, Bhattacharyya S, Katz J, Harrison D, Tauhid S, Bruso P, et al. COVID-19 mRNA vaccination leading to CNS inflammation: a case series. J Neurol 2022;269:1093–106. doi: 10.1007/s00415-021-10780-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maleki A, Look-Why S, Manhapra A, Foster CS. COVID-19 recombinant mRNA vaccines and serious ocular inflammatory side effects: real or coincidence? J Ophthalmic Vis Res 2021;16:490–501. doi: 10.18502/jovr.v16i3.9443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fernandez‐Nieto D, Hammerle J, Fernandez‐Escribano M, Moreno‐del Real CM, Garcia‐Abellas P, Carretero‐Barrio I, et al. Skin manifestations of the BNT162b2 mRNA COVID‐19 vaccine in healthcare workers. ‘COVID‐arm’: a clinical and histological characterization. J Eur Acad Dermatol Venereol 2021;35:e425–7. doi: 10.1111/jdv.17250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haynes BF, Fauci AS. The differential effect of in vivo hydrocortisone on the kinetics of subpopulations of human peripheral blood thymus-derived lymphocytes. J Clin Invest 1978;61:703–7. doi: 10.1172/JCI108982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Golding B, Lee Y, Golding H, Khurana S. Pause in immunosuppressive treatment results in improved immune response to SARS-CoV-2 vaccine in autoimmune patient: a case report. Ann Rheum Dis 2021;80:1359–61. doi: 10.1136/annrheumdis-2021-220993 [DOI] [PubMed] [Google Scholar]
- 21.Nessib DB, Fazaa A, Miladi S, Sellami M, Ouenniche K, Souabni L, et al. Do immunosuppressive agents hamper the vaccination response in patients with rheumatic diseases? A review of the literature. Therapie 2021;76:215–9. doi: 10.1016/j.therap.2020.08.002 [DOI] [PubMed] [Google Scholar]


