Abstract
The co-occurrence of posttraumatic stress disorder (PTSD) and substance use is related to poorer outcome and increased dropout from trauma-focused treatment. Investigating PTSD and substance use can inform our intervention approaches. Exploring cannabis use in particular is especially important as rates of cannabis use are increasing with recent legalization trends. A better understanding of how substance use is associated with treatment processes and outcome for individuals with PTSD is needed to enhance care. In this study, both lifetime diagnoses of alcohol and drug use disorders and current alcohol and drug use severity were examined in 200 men and women with chronic PTSD who received either prolonged exposure (PE) or sertraline. No lifetime or current alcohol use variables predicted drop out, adherence, or poorer outcome. However, lifetime diagnosis of both an alcohol and drug disorder (OR = 3.42) and recent cannabis use (OR = 3.38) strongly predicted higher dropout. Recent cannabis use and drug use severity predicted poorer adherence to PE (β = −.22 to −.29), but not to sertraline. Drug use severity (β = −.22) also predicted worse treatment outcome, as did lifetime diagnosis of an alcohol and drug disorder (β = −.48). Overall, patients with drug use improved with treatment, but had less treatment retention, adherence, and symptom reduction. Strategies to increase engagement and retention may be indicated for these patients. Individuals who are using cannabis, or other drugs, may be at higher risk for not completing PTSD treatment, potentially prolonging the cycle of PTSD and substance use.
Keywords: PTSD, cannabis, marijuana, substance use, treatment
Considerable overlap between posttraumatic stress disorder (PTSD) and substance use exists (e.g., Chilcoat & Menard, 2003; Dass-Brailsford & Myrick, 2010; Ouimette & Brown, 2003). Epidemiological studies estimate that individuals with PTSD are two to four times more likely to have a substance use disorder than individuals without PTSD (Chilcoat & Breslau, 1998; Kessler et al., 1995; Pietrzak, Goldstein, Southwick, & Grant, 2011). Notably, diagnosis of an anxiety disorder, such as DSM-IV PTSD, predicts the transition from alcohol and substance use to dependence (Lopez-Quintero et al., 2011). In addition, the high rates of co-occurrence are particularly problematic given that individuals with co-occurring PTSD and substance use tend to have more complicated clinical presentations, such as more social issues, legal problems, and suicide attempts, and poorer treatment outcomes than individuals with either disorder alone (e.g., McCauley et al., 2012; Ouimette, Goodwin, & Brown, 2006; Read, Brown, & Kahler, 2004; Schafer & Najavits, 2007). Epidemiological findings show that individuals with comorbid substance use and PTSD report more severe PTSD and SUD symptoms than those with PTSD or SUD alone (Blanco et al., 2013). Individuals with PTSD and SUD comorbidity also report being more likely to use substances to relieve PTSD symptoms (Blanco et al., 2013), with 20% of individuals with PTSD reporting that they use substances to “self-medicate” PTSD symptoms (Leeies, Pagura, Sareen, & Bolton, 2010). Despite this evidence, in a recent review of treating substance use disorders (SUD) in the presence of comorbid PTSD, Hildebrand, Behrendt, and Hoyer (2015) concluded that across studies there was not a robust negative effect of comorbid PTSD on treatment outcome for SUD. Yet, as the authors themselves noted, their conclusions were limited by large differences in methods and treatments included. Thus, it is a continued empirical question of how PTSD and SUD influence each other and predict recovery.
Self-medication theory is commonly applied to explain the high co-occurrence between PTSD and substance use. According to this theory, trauma-exposed individuals use substances to mitigate distressing PTSD symptoms, and the accompanying decrease in distress is negatively reinforcing for continued and escalating substance use (e.g., Khantzian 1985; Saladin, Brady, Dansky, & Kilpatrick, 1995). Consistent with this theory, in a longitudinal sample of active duty military personnel, pre-deployment PTSD severity was associated with a higher likelihood of alcohol dependence at post-deployment (Kline et al., 2015). Higher PTSD symptoms on a given day also predict both same and next day alcohol use in men and women with co-occurring PTSD and alcohol use disorder (Simpson, Stappenbeck, Luterek, Lehavot, & Kaysen, 2014). Furthermore, substance use increases as PTSD worsens (Back et al., 2014; Bremner, Southwick, Darnell, & Charney, 1996), and PTSD symptoms worsen following decreased substance use (Back et al., 2014), suggesting that substance use may actually be effective in “self-medicating” PTSD symptoms. However, use of substances to cope with PTSD symptoms is unlikely to be effective in the long-term and can come at the cost of developing a substance use disorder or experiencing other negative consequences of substance use such as loss of work, interruptions to interpersonal relationships, and legal problems. Treating the underlying PTSD symptoms can alleviate distress and the accompanying substance use. Indeed, treatment-related improvements in PTSD symptoms have been shown to predict improvements in substance use (Back, Brady, Sonne, & Verduin, 2006; Hien et al., 2010; Read et al., 2004), although not all studies have found this relationship (Steindl, Young, Creamer, & Crompton, 2003). Taken together, individuals with PTSD may use substances as a coping strategy, and treating PTSD may help alleviate both trauma-related symptoms and substance use.
In regard to PTSD treatment, clinicians report that co-occurring PTSD and substance use is particularly difficult to treat, largely due to the challenge of integrating the different treatment components for both disorders (e.g., Back, Waldrop, & Brady, 2009). Clinicians are often reluctant to treat PTSD in individuals using substances due to concerns about dropout or exacerbation of substance use behavior (Coffey, Schumacher, Brimo, & Brady, 2005). Traditionally, treatment programs targeting PTSD in individuals with substance use, such as Seeking Safety (Najavits, 2002; 2007), put an increased emphasis on skill building and do not directly address traumatic events. More recently, integrated treatment programs that address substance use and PTSD symptoms concurrently by explicitly including a focus on the traumatic event itself have shown initial promise in decreasing both PTSD and substance use (Brady et al., 2001; Foa et al., 2013; Killeen, Back, & Brady, 2011; McGovern et al., 2009; Mills et al., 2012), although effect sizes are less robust compared to treatment trials examining individuals with PTSD without substance dependence. In addition, several studies have found higher rates of drop out from PTSD treatment in substance using samples (35% to 61%; Back et al., 2001; Foa et al., 2013; McGovern et al., 2009) compared to what is seen in general PTSD treatment trials (18–25%; Hembree et al., 2003; Imel et al., 2013).
Even less is known about how substance use affects adherence and outcome in standard evidence based treatments for PTSD that do not specifically address or incorporate techniques targeting substance use. In an archival analysis, veterans with PTSD and comorbid alcohol use disorders, both lifetime and current, were no more likely to dropout from cognitive processing therapy (CPT), a cognitive behavioral treatment for PTSD, than those without an alcohol use disorder, and they showed decreases in PTSD and depression symptoms following treatment (Kaysen et al., 2014). Although alcohol outcomes were not reported, this lends preliminary support to the notion that standard PTSD-focused treatment is effective and feasible in substance using patients. However, this study was not a randomized controlled trial, was limited by a lack of information on substance use other than alcohol, and assessed treatment adherence by only examining number of sessions completed. Additional research on how substance use effects treatment engagement and outcome is needed to help clinicians make informed decisions about for whom standard PTSD treatment is feasible and appropriate. It is also important to explore both current and lifetime substance use behavior to understand if it is active use of substances specifically that impairs treatment engagement and response, or if it is the general tendency to misuse substances at any point in one’s life that predicts impaired treatment processes.
There are several efficacious psychotherapeutic and pharmacological treatments available for individuals with PTSD, including a range of exposure-based cognitive behavioral therapies and antidepressant medications such as CPT, prolonged exposure (PE), eye movement and desensitization and reprocessing therapy (EMDR), sertraline, and fluoxetine (e.g., Watts et al., 2013). In particular, PE has strong empirical support for treatment of PTSD across various trauma types (e.g., Foa et al., 2005; Schnurr et al., 2007). Selective serotonin reuptake inhibitors (SSRIs), such as sertraline, also improve PTSD symptoms (e.g., Brady et al. 2000; Davidson et al., 2001; Stein et al., 2006). Pharmacotherapy clearly requires less time and emotional engagement than trauma-focused psychotherapy. In particular, PE requires actively approaching trauma-related memories and situations. Given that avoidant coping has been shown to predict substance use in a PTSD sample (Possemato et al., 2015), pharmacotherapy might show better efficacy compared to PE in substance using samples. Patient preference, or an individual’s choice when presented with different treatment options, also may be associated with treatment outcome (Feeny, Zoellner, Mavissakalian, & Roy-Byrne, 2009). Typically, individuals prefer psychotherapy over pharmacotherapy for the treatment of psychiatric disorders (McHugh, Whitton, Peckham, Weldge, & Otto, 2013), including PTSD (Angelo, Miller, Zoellner, & Feeny, 2008; Feeny et al., 2009) and substance use (Andreasson, Danielsson, & Wallhed-Finn, 2013). Furthermore, patients with problem drinking behavior are more likely to adhere to treatments that align with their own preferences (Robinson, Callister, Berry, & Dearing, 2008). Thus, both treatment modality and treatment preference (i.e., not receiving your preferred treatment) may be important effect modifiers to examine in patients with PTSD and substance use.
Understanding effects of particular, commonly used substances can help elucidate clinically meaningful relationships that can inform intervention approaches. Two commonly used substances in individuals with PTSD are alcohol and cannabis. The high rates of use of these substances, in particular, are likely due to their perceived anxiolytic effects. For example, Bremner and colleagues (1996) found that Vietnam veterans reported using cannabis and alcohol to alleviate their hyperarousal symptoms (e.g., trouble sleeping, hypervigilance, exaggerated startle). Expectations for anxiety and tension reduction are some of the most commonly reported motivations for using alcohol (Ullman, Filipas, Townsend, & Starzynski, 2005) and cannabis (Green, Kavanaugh, & Young, 2003). In addition, acceptability and ease of access to cannabis has increased as there has been a national movement toward legalizing cannabis for both recreational and prescription use. One recent study found that individuals with a lifetime PTSD diagnosis were 3.3 times more likely to report lifetime cannabis use and were 2.6 times more likely to report lifetime alcohol abuse or dependence than those without PTSD (Cougle, Bonn-Miller, Vujanovic, Zvolensky, & Hawkins, 2011). Due to their high prevalence, examining the impact of lifetime and current use patterns for alcohol and cannabis on treatment outcome is particularly important for PTSD samples.
In this study, we explored alcohol and drug use behavior as a predictor of both treatment engagement (i.e., dropout, adherence) and treatment outcome for individuals receiving either PE or sertraline for chronic PTSD within the context of a randomized controlled trial using a doubly randomized preference design allowing for the examination of treatment preference (Zoellner, Roy-Byrne, Mavissakalian, & Feeny, 2017). We decided a priori to look at overall substance use as well as alcohol and cannabis specifically due to the documented high rates of use by individuals with PTSD. Throughout the manuscript, we use the term “substance use” for any alcohol or drug use behavior, “drug use” for any illegal drug use, and “cannabis” or “other drug” use for specificity. By including both current use and lifetime diagnoses for problem use, we sought to better understand how both recent behavior and lifetime propensity for substance use at a severity level that meets diagnostic threshold might impact treatment engagement and outcome. We hypothesized that higher alcohol or drug use severity and history of lifetime alcohol, drug, and cannabis use disorders, would predict higher treatment dropout, poorer PTSD treatment outcome, and lower PE homework adherence and sertraline adherence. In addition, for individuals with higher alcohol or drug use severity and those with lifetime diagnoses of alcohol or drug use disorders, we hypothesized that receiving PE over sertraline and receiving a nonpreferred treatment would be associated with higher dropout and worse outcome.
Method
Participants
Two hundred men (n = 49) and women (n = 151) with chronic PTSD were recruited through a wide range of sources, including clinical referrals and community advertising. Broad inclusion criteria and limited exclusion criteria were used in order to recruit a diagnostically-comorbid and clinically representative sample of individuals with primary chronic PTSD. Inclusion criteria included participants between 18 and 65 years of age and a current DSM-IV diagnosis of primary chronic PTSD. Exclusion criteria were derived based on standards of appropriate clinical care, where other problems/disorders that took precedence above PTSD were used as exclusion criteria. Exclusion criteria included: current diagnosis of schizophrenia or delusional disorder; medically unstable bipolar disorder, depression with psychotic features, or depression severe enough to require immediate psychiatric treatment (e.g., actively suicidal); severe self-injurious behavior or suicide attempt within the past three months; no clear trauma memory or trauma before age of three; current diagnosis of DSM-IV substance dependence within the previous three months (current DSM-IV substance abuse diagnosis and history of substance use dependence was allowed); ongoing intimate relationship with the perpetrator; unwilling or medically not advisable to stop current cognitive behavioral psychotherapy or antidepressant medication, based on condition assignment; previous non-response to adequate trial of either PE (8 sessions or more) or sertraline (150 mg/d; 8 wks); or medical contraindication for the initiation of sertraline (e.g., pregnancy/likely to become pregnant). Thus, eligible participants met general criteria that were designed to be consistent with standard clinical guidelines indicating treatment of PTSD as the primary diagnosis and that required participants to discontinue trauma focused CBT or antidepressant medications depending on assigned treatment condition. The exclusion for DSM-IV substance dependence was based on the notion that substance use behavior consistent with a dependence diagnosis would be severe enough to warrant primary intervention before implementing treatment for PTSD, given that at the time these data were collected there had been no published reports on the safety of implementing these treatments with substance dependent patients.
Participants had a mean age of 37.41 years and were primarily female (75.5%). Caucasian was the most frequently reported racial background (65.5%), followed by African American (21.5%). Index trauma exposure occurred on average twelve years prior to study enrollment (M = 11.97, SD = 12.69). Diverse trauma types were represented. Adult sexual assault (31%) and childhood physical or sexual assault (24%) were the most common index trauma types followed by adult non-sexual assault (22.5%), accident/natural disaster (13.5%), death/violence to a loved one (6.5%), and combat/war (2.5%).
Measure of PTSD Diagnosis and Severity
Posttraumatic Symptom Scale - Interview
(PSS-I; Foa et al., 1993). The PSS-I is a 17-item interviewer-administered measure that was used to assess PTSD symptom severity and DSM-IV diagnostic status. Items were rated on a scale based on frequency and severity of symptoms from 0 (not at all) to 3 (5 or more times per week/very much) in the past two weeks. In the present study, approximately 10% of the cases were rerated for diagnostic reliability. Overall, inter-rater reliability was high for PTSD severity (ICC = .985).
Measures of Adherence and Dropout
PE homework adherence
The Utility of Treatment Inventory (Foa, Hembree, & Dancu, 2002) assessed adherence with homework since the last session, rating usage of in vivo exposure and imaginal exposure during the past week on a scale from 1 (not at all) to 5 (more than 7 times). A mean adherence score was computed, separately for in vivo and imaginal practice, across completed sessions where homework was given. For those with no completed sessions with homework, a score of 1 was given.
Sertraline adherence
A single item was used to assess how often participants took sertraline each week at Sessions 2–10. Scores for each session ranged from 1 (not at all) to 5 (five or more days). A mean adherence score was computed across completed sessions. For those with no completed sessions where medication adherence was assessed, a score of 1 was given.
Dropout
To capture individuals who did not complete treatment, dropout was defined as completing 6 or fewer treatment sessions. This definition was chosen to capture an inadequate dose of either treatment, those who were unlikely to receive therapeutic benefit.
Substance Use Measures
Structured Clinical Interview for DSM-IV
(SCID-IV; First et al., 1995). The SCID-IV is interviewer administered and was used to assess comorbidity and exclusion criteria. Independent evaluators assessed current and lifetime criteria for abuse and dependence diagnoses for alcohol and six classes of drugs (i.e., cannabis, stimulants, opioids, cocaine, hallucinogens, and sedatives/anxiolytics), in addition to poly-substance use. In this study, to meet criteria for a substance use disorder, participants needed to meet either abuse or dependence criteria for one or more substances. Given the exclusion criteria of current substance dependence, only SCID-IV lifetime diagnoses of alcohol use disorder, any drug use disorder, and cannabis use disorder were included in these analyses. In the present study, approximately 10% of the cases were rerated for diagnostic reliability. Inter-rater reliability was good for current MDD (κ = .68, ppos = .88, pneg = .80), anxiety disorders (κ = 1.00, ppos = 1.00, pneg = 1.00), substance use disorders (ppos = .00, pneg = 1.00), and other diagnoses (ppos = 0.00, pneg = 1.00).
Addiction Severity Index - Self Report
(ASI-SR; McGahan et al., 1986). The ASI-SR detects and measures the severity of various problem areas that are associated with alcohol and drug use (e.g., medical, legal, social, psychiatric). The ASI-SR produces two separate composite scores for alcohol and drug use that are arithmetically derived indices based on items that assess for problem severity over the past 30 days. Composite scores are continuous and range from 0 (no significant problem) to 1 (extreme problem). The ASI-SR and the clinician-administered ASI interview are strongly correlated (Rosen, 2000).
Cannabis use
Cannabis use is included in the ASI drug composite score and in the SCID-IV lifetime diagnoses. Given its relatively high prevalence in this sample, we conducted analyses examining cannabis use specifically. One item from the ASI-SR was used to measure presence or absence of cannabis use in the past 30 days (0 = no use; 1 = use).
Toxicology
Urine toxicology screens were obtained for a comprehensive list of substances including amphetamines, barbiturates, benzodiazepines, cocaine, methadone, opiates, phenylcyclidine, and cannabinoids. Specimens were tested at centralized laboratories using qualitative immunochemical testing to determine a positive or negative result for each substance. Screens were used to validate self-report of substance use and were only collected at baseline.
Procedure
Participants were recruited from two large metropolitan areas using a wide range of strategies including clinical referrals and community advertising. Institutional review boards at each site approved the studies. Interested participants were screened over the telephone and scheduled for an intake interview to determine eligibility. Participants gave written informed consent, and independent evaluators blinded to eventual treatment assignment conducted baseline diagnostic interviews (PSS-I, SCID-IV) and remained blind to treatment condition for the trial. Prior to randomization, participants viewed videotaped treatment rationales, which included information on efficacy of treatments, an analogy of how treatment works, treatment procedures, and possible side effects (Feeny, Zoellner, Mavissakalian, & Roy-Byrne, 2009). Videos were counterbalanced on order presented (sertraline, PE), background (psychiatrist, psychologist), and gender of clinician. After viewing rationales, participants indicated treatment preference in private. Prior to randomization, participants completed a physical exam with a study nurse who assessed indicators of physical health (e.g., weight, blood pressure), a pregnancy test for female participants, and a urine toxicology screen for presence of substances.
Eligible participants were then randomized using a computer-generated urn randomization sequence with stratification according to PTSD severity on the PSS-I (scores above and below 35) and current antidepressant status (yes/no). Participants were first randomly assigned to either choice or no choice of treatment. Those in the choice condition chose their treatment (PE or sertraline). Those in the no choice condition were once more randomized to a treatment condition (PE or sertraline).
Participants in the PE condition met with a therapist for 10 weekly sessions lasting between 90 – 120 min, using a standardized manual (Foa et al., 2002). Procedures included psychoeducation, breathing retraining, imaginal exposure, in vivo exposure, processing related to the exposure exercises, and between-session homework assignments. PE therapists were masters or PhD level, were trained in the delivery of PE, and attended weekly clinical supervision.
Participants in sertraline condition met with a psychiatrist for 10 weekly sessions up to 30 min each, with the first lasting 45 min, using a standardized treatment manual (Marshall et al., 2001). Sertraline dosage began at 25 mg/day and was increased to 200 mg/day when indicated and tolerated. Final dosage ranged from 12 to 300 mg/day, with an average final dosage of 115 mg/day (SD = 78.00). Study psychiatrists were board certified and experienced in the treatment of anxiety disorders and a Medical Director oversaw sertraline administration at each site.
Treatment sessions were recorded and 10% were reviewed by trained raters who assessed essential treatment components and protocol violations. Fidelity was excellent (PE: 90%; SER: 96%) and no protocol violations were observed. Raters also assessed therapist competence (e.g., engaged in interactive exchange with client) in PE on a 3-point scale (1 = Inadequate, 3 = Adequate or Better). Overall PE therapist competence was very good (M = 2.73, SD = .32).
Following treatment, participants completed self-report and interview measures at posttreatment and six-month follow-up assessments. Participants were paid $50 for completing posttreatment and follow-up assessments.
Data Analysis
Binary and logistic regressions were used to model the effects of current substance use severity, lifetime substance use diagnoses, and cannabis use on outcomes of dropout (yes/no dropped out before session 5 of PE or sertraline), adherence (mean completion of in vivo and imaginal exposure exercises or mean days of taking medication as prescribed), and PTSD symptom change (reductions in PSS-I scores at follow-up), with treatment type (PE or sertraline) and preference (receiving preferred treatment or not) included as moderators. All analyses were intent to treat. Multiple imputation (SPSS, version 19) was used to create five data sets (Rubin, 1996) for treatment outcome analyses, with pooled regression coefficients reported for posttreatment and follow-up analyses.
Given that ASI composite scores for alcohol and drug use severity were modestly correlated (r = .25), the ASI scores for alcohol and drug use were entered simultaneously in regression models. To explore effects of lifetime use diagnoses, composite substance use groups were constructed. Three dummy coded variables of lifetime alcohol diagnosis only (yes/no); lifetime drug use diagnosis only (yes/no); and lifetime diagnosis of both alcohol and drug use (yes/no) were entered simultaneously in regression equations to examine the differential effects of alcohol, drug, and both diagnoses on key outcome measures. Cannabis use in the last 30 days (yes/no) and having a lifetime diagnosis of cannabis as predictors were also examined. Due to sample size constraints, we did not examine cannabis in isolation but included individuals reporting cannabis use, either alone or in combination with alcohol or other drug use. Moderator analyses were run separately for treatment type (PE vs sertraline) and preference (match vs mismatch). When sample size of a predictor variable was below 10 subjects in a cell, moderation analyses were not conducted. Presence or absence of moderation effects, whether significant or not, were explicitly stated when these analyses were conducted. Patients who received their preferred treatment either by choice or randomization, were coded as receiving their preferred treatment (match).
Results
Substance Use in PTSD Treatment Seeking Sample
At pre-treatment, participants reported a range of drinking and drug use diagnoses, with 34.5% reporting a lifetime alcohol use disorder, 4.0% reporting current alcohol abuse disorder, 46.0% reporting a lifetime drug use disorder, and 5.5% reporting current drug abuse disorder. For those reporting lifetime drug use disorders, highest endorsement was for cannabis use disorder (40.2%), cocaine use disorder (25.0%), or poly drug use disorder (15.2%). Sixty-one percent (61.5%) reported alcohol use in the past 30 days and 21.0% reported use of a recreational drug in the past 30 days. The frequencies of reports of drug use in past 30 days included: 5.5% reporting opiate use, 4.0% reporting sedative use, 2.5% reporting cocaine use, 0.5% hallucinogen use, and 13.5% reporting cannabis use in the past 30 days. Notably, exploring the overlap of past month alcohol or drug use and lifetime diagnoses of alcohol or drug use disorders showed minimal overlap with 46 participants reporting both a lifetime diagnosis of an alcohol use disorder and alcohol use in the past 30 days, 15 participants reporting both a lifetime diagnosis of a drug use disorder and drug use in the past 30 days, and 9 participants reporting both a lifetime diagnosis of a cannabis use disorder and cannabis use in the past 30 days. For ASI composite scores, the mean for the alcohol scale was .07 (SD = .09; range = .00 – .42) and the mean for the substance use scale was .03 (SD = .06; range = .00 to .46).
Pre-treatment toxicology screens indicated that 4.0% of participants were positive for cocaine, 3.5% were positive for methadone/opiates, and 10% were positive for cannabinoids. No participants screened positive for phenylcyclidine, barbiturates, or amphetamines. The toxicology screens demonstrated moderate to high agreement with self-reported use for cannabis (r = .69) and cocaine (r = .49).
Table 1 depicts means and standard deviations for dropout, adherence, and PTSD severity for individuals with and without alcohol and drug use disorders. Table 2 shows these measures for those with and without cannabis use, specifically.
Table 1.
Lifetime Alcohol and Drug Use Disorders and Adherence and PTSD Severity Outcomes
| No AUD or Drug Dx (SCID-IV) n = 108a M (SD) or % |
AUD Dx Only (SCID-IV) n = 31a M (SD) or % |
Drug Dx Only (SCID-IV) n = 23 M (SD) or % |
AUD and Drug Dx (SCID-IV) n = 38a M (SD) or % |
|
|---|---|---|---|---|
| Drop Out | 24.1% | 32.3% | 30.4% | 55.3% |
| Adherence | ||||
| Sertraline (mean) | 2.15 (0.92) | 2.26 (1.49) | 1.82 (0.67) | 2.37 (1.39) |
| PE In vivo (mean) | 3.02 (1.16) | 2.85 (0.97) | 2.76 (1.27) | 2.75 (1.09) |
| PE Imaginal (mean) | 2.64 (1.01) | 2.82 (0.99) | 2.25 (1.19) | 2.27 (1.09) |
| PTSD Severity (PSS-I) | ||||
| Pre-treatment | 29.00 (6.70) | 28.00 (5.33) | 29.17 (7.24) | 32.71 (6.56) |
| Post-treatment | 9.85 (8.79) | 12.67 (11.26) | 9.56 (8.79) | 18.28 (13.41) |
| 6-month follow-up | 6.97 (7.43) | 7.53 (8.63) | 12.44 (11.79) | 14.29 (12.02) |
| Pre- to 6-month | 3.11 | 2.86 | 1.71 | 1.90 |
| ES (Cohen's d) |
Note. AUD = Alcohol Use Disorder; SUD = Substance Use Disorder. SCID = Structured Clinical Interview for DSM-IV Lifetime Diagnosis. PSS-I = Posttraumatic Symptom Scale–Interview. Means are original data, not imputed values. Effect sizes for PTSD severity were calculated using pooled means and standard deviations.
Sample size varied for this predictor, with one participant missing alcohol use diagnoses on the SCID at baseline.
Table 2.
Lifetime Cannabis Use Disorder Status and Past 30-day Use and Adherence and PTSD Severity
| No CUD (SCID) n = 162a M (SD) or % |
CUD (SCID) n = 37a M (SD) or % |
No CB Last 30 n = 163a M (SD) or % |
CB Last 30 Days n = 27a M (SD) or % |
|
|---|---|---|---|---|
| Early Drop Out | 28.4% | 48.6% | 27.0% | 55.6% |
| Adherence | ||||
| Sertraline (mean) | 2.19 (1.10) | 2.16 (1.20) | 2.18 (1.04) | 2.62 (1.53) |
| PE In vivo (mean) | 3.02 (1.10) | 2.42 (1.15) | 3.09 (1.05) | 2.20 (1.18) |
| PE Imaginal (mean) | 2.66 (1.05) | 2.08 (1.04) | 2.68 (1.01) | 2.04 (1.23) |
| PTSD Severity (PSS-I) | ||||
| Pre-treatment | 29.14 (6.48) | 31.00 (7.00) | 29.61 (6.57) | 28.22 (6.70) |
| Post-treatment | 10.14 (9.29) | 19.21 (12.99) | 10.92 (10.27) | 14.00 (10.91) |
| 6-month follow-up | 7.95 (8.82) | 13.24 (10.33) | 8.46 (9.11) | 10.18 (11.19) |
| Pre- to 6-month | 2.16 | 1.72 | 2.14 | 2.00 |
| ES (Cohen's d) |
Note. CUD = Cannabis Use Disorder; SCID = Structured Clinical Interview for DSM-IV Lifetime Diagnosis; CB = Cannabis Use yes/no; PSS-I = Posttraumatic Symptom Scale-Interview. Means are original data, not imputed values. Effect sizes for PTSD severity were calculated using pooled means and standard deviations.
Sample size varied for this predictor with one participant missing cannabis use diagnosis on the SCID at baseline and 10 participants missing self-reported cannabis use on the ASI-SR at baseline.
Dropout Prior to Likely Therapeutic Benefit
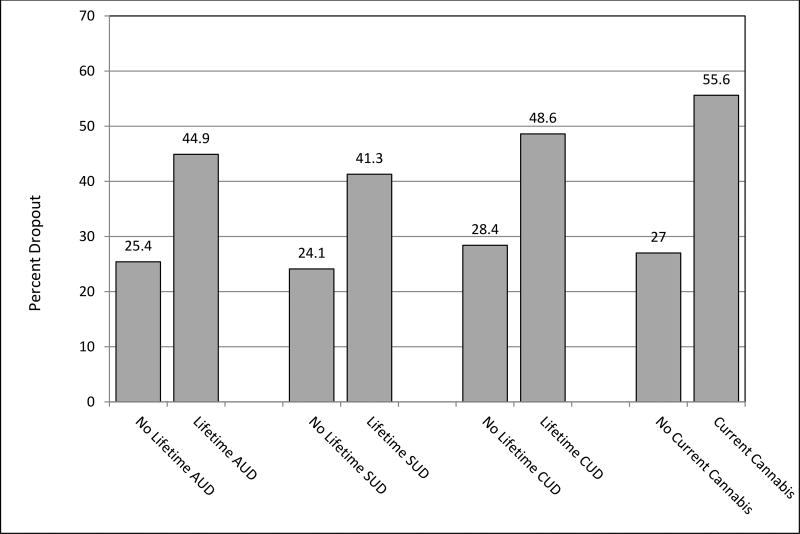
We first examined pre-treatment alcohol and drug use, both recent alcohol or drug use severity (ASI composites) and lifetime diagnoses of alcohol or drug use disorders, as predictors of dropout, including treatment type and preference as potential effect moderators. See Figure 1 for differential dropout rate for those with a lifetime alcohol, drug, or cannabis use disorder compared to those without. Dropout rates are also presented in Table 1 by lifetime substance use disorder diagnosis group (no lifetime diagnosis, alcohol use disorder only, drug use disorder only, and both alcohol and drug use disorder) and in Table 2 for those with and without current cannabis use and those with and without a lifetime cannabis use disorder.
Figure 1.
Drop out rates by alcohol and substance use
Note. AUD = Alcohol Use Disorder, SUD = Substance Use Disorder, CUD = Cannabis Use Disorder
Examining the role of current alcohol and drug use severity (ASI composite scores) on dropout, there were no significant effects of severity of alcohol use behavior in the last 30 days, but the effect of drug use severity in the last 30 days was significant B = 9.37, Wald = 9.46, p = .002, such that individuals with higher drug use in the last 30 days showed higher dropout from treatment than individuals with lower drug use.1 This effect was not significantly moderated by treatment type or preference.
Examining the role of lifetime diagnosis (SCID-IV; an alcohol only lifetime diagnosis, a drug use only lifetime diagnosis, and a lifetime alcohol and drug use diagnosis) on dropout there was a main effect for lifetime diagnoses of both an alcohol and drug use disorder, B = 1.36, Wald = 11.77, p = .001, with individuals with lifetime diagnoses of both alcohol and drug use being much more likely to drop out of treatment (55.3%) than other individuals (26.5%; OR = 3.42).
To examine whether cannabis use affected treatment dropout, separate logistic regression analyses were conducted with cannabis use in the last 30 days and lifetime diagnosis of a cannabis use disorder (SCID-IV) as predictor variables. Cannabis use in the last 30 days predicted dropout, with individuals reporting recent cannabis use being much more likely to dropout (55.6% vs 27%), B = 1.22, Wald = 8.19, p = .004 (OR = 3.38). Similarly, a lifetime diagnosis of a cannabis use disorder significantly predicted dropout, with those having a lifetime diagnosis being more likely to dropout (48.6% vs 28.4%), B = 0.44, Wald = 5.47, p = .02 (OR = 2.39).
Thus, across measures, alcohol, drug, and cannabis use was associated with higher patient dropout prior to receiving a full therapeutic dose of treatment. Individuals with a lifetime history of both alcohol and drug use disorders, those with a lifetime diagnosis of a cannabis use disorder, and those with current drug use, and current cannabis use specifically, had higher dropout.
Treatment Adherence
Prolonged exposure
For individuals in PE, in the regression model including composite scores for both alcohol and drug use severity in the past 30 days, there were no significant main effects of alcohol use severity (ASI) on either in vivo or imaginal exposure adherence. However, for drug use severity (ASI), there was an effect for in vivo, β = −.28, t(111) = −3.01, p = .003 and for imaginal adherence, β = −.26, t(111) = −2.77, p = .007, with higher drug use severity in the past 30 days predicting worse adherence. Treatment preference was not a significant moderator.
For lifetime alcohol and drug use disorders (SCID-IV), there was no significant effect of lifetime diagnoses (alcohol disorder only, drug use disorder only, or alcohol and drug use disorder) for in vivo or imaginal adherence.
Examining cannabis use, there was an effect of cannabis use in the past 30 days on both in vivo, β = −.29, t(110) = −3.14, p = .002, and imaginal, β = −.22, t(110) = −2.32, p = .022, exposure adherence. Similarly, there was an effect of lifetime cannabis use disorder on both in vivo, β = −.21, t(115) = −2.27, p = .03, and imaginal exposure adherence, β = −.21, t(115) = −2.27, p = .03. In sum, current drug use, current cannabis use, and lifetime cannabis use were associated with worse adherence to PE.
Sertraline
For sertraline, there were no main effects of last 30-day substance use measures (severity of alcohol use [ASI], severity of drug use [ASI], presence of cannabis use [yes/no]) or of any of the lifetime diagnoses (alcohol, drug, cannabis disorders) on adherence. There was no moderating effect of preference for the relationship between ASI alcohol or drug use and sertraline adherence.
PTSD Severity at Post-Treatment and Six-Month Follow-up
Neither pre-treatment current alcohol use severity nor drug use severity (ASI composites) showed an effect on post-treatment PTSD severity (PSS-I) or at six-month follow-up, controlling for pre-treatment PTSD severity. There was no moderating effect of treatment type or preference.
Examining lifetime disorders (alcohol use disorder only, drug use disorder only, and both alcohol and drug use disorder), a lifetime diagnosis of both an alcohol and drug use disorder predicted higher PTSD severity at post-treatment, β = .48, t(199) = 2.56, p = .01, but lifetime diagnosis of an alcohol use only or drug use only disorder did not. Of note, all three substance use groups demonstrated reductions in PTSD symptoms with treatment, with all three groups reporting PTSD symptoms below clinical levels at post-treatment (See Table 1). At 6-month follow up, a lifetime diagnosis of both an alcohol and drug use disorder, β = .37, t(199) = 1.98, p = .05, and a lifetime drug use disorder only predicted worse PTSD symptoms β = .51, t(199) = 2.30, p = .02, controlling for pre-treatment symptoms, but diagnosis of an alcohol use disorder only did not. There were no moderating effects of treatment type or preference.
Cannabis use, in the last 30 days, did not predict PTSD symptoms at either post-treatment or 6-month follow up. Individuals with a lifetime diagnosis of a cannabis use disorder reported slightly higher post-treatment PTSD symptoms compared to those without a lifetime diagnosis, controlling for pre-treatment PTSD severity, β = .22, t(199) = 3.15, p = .002. However, the presence of a lifetime diagnosis of cannabis use disorder did not predict PTSD symptoms at six-month follow-up, controlling for pre-treatment severity.
Discussion
Overall, substance use, particularly drug use, may impact treatment processes for individuals with PTSD, especially patient dropout. No studies have looked at the impact of drug use, including cannabis use, on treatment outcomes following standard PTSD treatments. There is high co-occurrence of PTSD and cannabis use (Cougle et al., 2011) and increasing trends to legalize cannabis for the treatment of PTSD despite this lack of research. In this study, current cannabis use and lifetime history of cannabis use disorder predicted almost twice the dropout across both treatments and predicted slightly worse homework adherence in PE. However, it did not strongly impact how likely a patient was to take sertraline in the previous week. In addition, history of a cannabis use disorder predicted moderately worse PTSD outcome at post-treatment. Treatment type, PE or sertraline, did not significantly alter the effect of drug use, cannabis or more general drug use, on dropout or treatment response. Similarly, receiving one’s preferred treatment also did not change the effects of drug use on dropout, treatment engagement (i.e., homework completion, medication adherence), or treatment outcome. Thus, drug use, and specifically cannabis use, was an important behavior that negatively impacted both treatment retention and engagement in exposure therapy.
Drug use, both current severity of use and lifetime diagnosis of both an alcohol and drug use disorder, predicted higher dropout from treatment. Rates of dropout were highest for patients who had lifetime diagnoses of both alcohol and drug use disorders, followed by those with a lifetime diagnosis of either an alcohol use disorder only or a drug use disorder only. This potentially reflects a severity effect, as those with diagnoses of both alcohol and drug use may represent higher severity of disorder or functional impairment. Differential severity effects may also explain the lack of significant effects for current alcohol use severity on dropout, despite the significant effect of current drug use severity on dropout. It is likely the patients in this study currently using alcohol reflect those using at normative, recreational levels and are likely not as high severity as those currently using illicit drugs, which included cannabis at the time of data collection. This is consistent with existing studies highlighting high dropout from PTSD treatment for patients with substance use disorders (e.g., Brady et al., 2001; Mills et al., 2012). Notably, observed rates of dropout for those reporting alcohol or drug use, ranging from 30–55%, are in line with rates of dropout seen in substance using samples in treatment programs that are both trauma and non-trauma focused (e.g., Hien et al., 2010) and are considerably higher than dropout rates reported in standard PTSD treatment trials (Imel et al., 2013). This suggests that retention in PTSD treatment is a considerable challenge for patients with a history of using both alcohol and drugs or drugs only and extends this across psychotherapy and SSRIs, as treatment modality did not change effects of drug use on dropout. The higher risk for dropout applies to individuals who were using drugs in the last 30 days, and to individuals with lifetime diagnoses of combined alcohol and drug use disorders. Thus, the detrimental effect of substance use on treatment completion was robust across current and historical measures of substance use. This speaks to the need for simpler and potentially abbreviated treatments that can produce symptom change rapidly, given that patients with substance use are difficult to retain even in treatments as brief as 10 weeks. It is worth noting that several standard treatments for PTSD, such as PE and CPT, have been shown to be effective when delivered in a twice a week format (e.g., Resick et al., 2002; Zoellner et al., 2017) and delivery of these approaches in the more intense dose of 5–6 weeks of therapy may be a better fit for substance using patients.
This is the first study that we know of to examine two divergent treatment approaches, psychotherapy and pharmacotherapy, as they relate to substance use and PTSD recovery. Paralleling the effect on dropout, there were consistent small to moderate effects of current drug use on adherence for patients in PE, both with in vivo and imaginal exposure homework. Engagement in trauma-focused treatment may be a challenge for these patients. Notably, we did not see the effect of alcohol use on PE adherence. This is likely due to the patients in the current study using alcohol in a manner more consistent with normative “social” or “moderate” drinking behavior. Thus, alcohol may not have detrimental effects on social engagement and behavioral activation in these patients (Peele & Brodsky, 2000), which is important given that motivation to engage in activities and experience activation of emotions is integral to completing therapy homework. Consistent with self-medication theory (Saladin et al., 1995), drug use, including cannabis use, may help patients avoid distressing symptoms, thus making it more difficult for these patients to complete things like in vivo and imaginal exposure homework, which can trigger strong emotional responses. Notably, we did not see detrimental effects of drug use on routinely taking sertraline; a treatment approach that is characterized by less burden, time, and effort compared to a cognitive behavioral therapy such as PE. Indeed, pharmacological approaches do not require the same level of engagement as trauma-focused psychotherapy, potentially making them easier for some patients. This is not to say that trauma-focused treatment is contraindicated for those with substance use. Rather that patients with current drug use, whether it be use of cannabis or use of another drug, are less likely to achieve adherence with exposure-based psychotherapy approaches, and may benefit from direct intervention around adherence; an indication that does not necessarily apply to medication treatments. Given that patients generally prefer psychotherapy (Andreasson et al., 2013; Feeny et al., 2009), this has important clinical utility in guiding treatment decisions, and future research should explore the role of substance use in preference for different PTSD treatment modalities.
It should also be noted that neither current alcohol nor drug use severity predicted worse PTSD severity at post-treatment or follow-up, although lifetime diagnoses of both an alcohol and drug use disorder predicted slightly worse outcome at post-treatment and at six month follow-up. Further, diagnosis of a lifetime drug use disorder predicted worse outcome at six month follow-up. It should be noted that patients with a drug use disorder history did still make large clinical gains, just to a lesser extent than those without a history of a drug use diagnosis. This lends support to the notion that PTSD treatment can be successfully implemented with individuals with substance use behavior, which has great clinical utility given that past research shows that improvements in PTSD symptoms predict subsequent improvements in substance use behavior (e.g., Back et al., 2006; Hien et al., 2010). Although the base rate of drug use was low in this study, cannabis use, in particular, was relatively common. This is perhaps not surprising given the overall increasing popularity of cannabis, particularly for individuals with PTSD (Cougle et al., 2011). Notably, we observed both distal and proximal cannabis effects, such that even a history of problematic use was associated with higher drop out, slightly poorer exposure adherence in PE, and slightly worse PTSD outcome. Exploring the degree of overlap between participants endorsing lifetime diagnosis of a cannabis use disorder and those with current use, it emerged that the majority of participants with cannabis use either had a lifetime diagnosis or current use but not both. Thus, the present findings highlight an important role for the presence of past cannabis use disorders in predicting treatment outcomes. This is consistent with longitudinal studies demonstrating a deleterious effect of cannabis use (Wilkinson, Stefanovics, & Rosenheck, 2015) or effects of cannabis related symptoms such as withdrawal (Bonn-Miller, Boden, Vujanovic, & Drescher, 2013) on PTSD therapy outcomes. Why individuals using cannabis would show worse adherence to PE but not sertraline is an open question. This is the first study we are aware of to examine cannabis effects on adherence in both a psychotherapy and a pharmacotherapy for PTSD. Individuals who chose to use cannabis may have a more developed or engrained pattern of seeking to avoid distressing stimuli, such as approaching reminders and memories with exposure, particularly if cannabis is used to facilitate this avoidance. They may also be less motivated or invested in the need to do additional exposure exercises if they perceive their cannabis use as being a way of managing PTSD symptoms. Additional research on this topic, particularly studies that tease apart motivations for cannabis use and daily relationships between cannabis and PTSD symptoms, are needed. It is worth noting that at the time these data were collected cannabis was an illegal substance in the states in which these data were collected. Thus, patients using cannabis may have looked functionally more impaired and more similar to those using other illicit drugs than those using alcohol, a legal substance.
There is some evidence that cannabis can enhance fear extinction learning (Rabinak et al., 2013; Rabinak & Phan, 2014); and given that exposure therapy may utilize extinction processes, cannabis could potentially enhance exposure therapy outcomes. Thus, cannabis has been posited as a potential novel approach to PTSD treatment (e.g., Bowers & Ressler, 2015; Das et al., 2013; Rabinak et al., 2013), but clinical data on this theory are lacking. Importantly, a facilitation effect of cannabis on extinction was not observed in the present data as evidenced by worse PE adherence and slightly worse treatment outcome for patients with cannabis use; however, cannabis use during exposure was not systematically assessed to examine the pattern between cannabis and changes in exposure distress. The patients in this trial who reported cannabis use also reported worse adherence with PE, suggesting they got a smaller “dose” of exposure than individuals not endorsing cannabis use. In addition, although extinction enhancement effects have been shown for cannabis, so have effects such as decreased motivation and cognition (Volkow et al., 2015). These detrimental effects would have expected negative impacts on PTSD treatment engagement and outcome and should not be discounted. It is important to note that standard PTSD treatments, such as PE and sertraline, were effective with patients with PTSD and cannabis use, as shown by large decreases in symptoms regardless of substance use but that there may be specific considerations around retention and adherence that could enhance outcomes. Clinically, cannabis use should be queried by clinicians and potentially incorporated into a treatment plan by conceptualizing how it may impact attendance and adherence specifically and problem solving to decrease any detrimental impact.
Results of this study should be interpreted with several limitations in mind. This trial excluded for alcohol and drug dependence due to concerns about appropriate clinical care given the previously unanswered question of whether it was safe and effective to treat PTSD when substance dependence is present. In more recent years, data has emerged showing that this exclusion is not necessary as patients with an alcohol use disorder benefit from PE even when receiving it in combination with a placebo treatment for alcohol use (e.g., Foa et al., 2013). However, we did not exclude for substance abuse, current substance use behavior, or for past disorders. This enabled us to examine individuals who are currently drinking and using drugs and those with a history of a substance diagnosis. Rates of drug use were relatively low, as were composite scores representing severity of use. The observed findings may be even more robust in more severe samples. In addition, our relatively low rates of drug use made it difficult to test differential relationships between specific substances, such as looking at use of specific drugs other than cannabis. Future research should elucidate unique relationships with different drugs. That said, patients in this trial were selected for having a primary diagnosis of PTSD in line with good clinical practice and thus this sample may closely resemble patients likely to receive PTSD treatment in clinical settings (Bedard-Gilligan et al., 2015). Other individual factors likely related to alcohol and drug use, such as some personality traits, impaired social support and interpersonal relationships, legal issues, and poorer occupational functioning could explain the relationship between substance use and the observed outcomes. This study did not specifically test mediators, including the role of dropout or adherence as an effect mediator between substance use and outcomes. Larger sample sizes and careful development of rubrics of engagement across distinct therapeutic modalities are needed to test this hypothesis. This study did not examine changes in substance use with PTSD treatment, including any changes in toxicology results. Lastly, small cell sizes precluded our ability to look at treatment type and preference in some of our moderation analyses.
Taken together, these findings are encouraging that standard, empirically-supported treatments for PTSD can be implemented successfully with individuals who report alcohol, cannabis use, or other drug use. Given the substantial overlap in PTSD and substance use behavior, and in particular cannabis, the present study provides clinicians with reassurance that exposure-based therapies and selective serotonin-reuptake inhibitors are safe and feasible for substance using patients. However, it also points to specific clinical considerations for these patients. Most notably drug use, particularly cannabis use, was associated with dropout, suggesting that when this substance use behavior is present special emphasis should be placed on retaining patients in treatment and that the field should focus on designing and emphasizing treatments that are brief and low burden in order to potentially increase retention for these individuals.
Acknowledgments
This research was funded in part by grants from the National Institute of Mental Health R01 MH066347 (PI: Zoellner) and R01 MH066348 (PI: Feeny), the National Institute on Alcohol Abuse and Alcoholism R34 AA022966 (PI: Bedard-Gilligan), and the National Institute on Drug Abuse R34 DA040034 (PI: Bedard-Gilligan). Sertraline was supplied by Pfizer, Inc, New York, New York at no cost. NIMH or Pfizer had no role in the study design, collection, analysis or interpretation of the data, writing the manuscript, or the decision to submit the paper for publication.
Footnotes
This research was presented at the 29th Annual Meeting of the International Society of Traumatic Stress Studies in Philadelphia, PA.
The variable of race was significantly correlated with both treatment dropout and substance use behavior variables. Models including race as a covariate did not change the pattern of significant findings for any predictors.
Contributor Information
Michele Bedard-Gilligan, Department of Psychiatry and Behavioral Sciences, University of Washington.
Natalia Garcia, Department of Psychology, University of Washington.
Lori A. Zoellner, Department of Psychology, University of Washington
Norah C. Feeny, Department of Psychology, Case Western Reserve University
References
- Andréasson S, Danielsson AK, Wallhed-Finn S. Preferences regarding treatment for alcohol problems. Alcohol and Alcoholism. 2013;48:694–699. doi: 10.1093/alcalc/agt067. [DOI] [PubMed] [Google Scholar]
- Angelo FN, Miller HE, Zoellner LA, Feeny NC. “I need to talk about it”: A qualitative analysis of trauma-exposed women’s reasons for treatment choice. Behavior Therapy. 2008;39:13–21. doi: 10.1016/j.beth.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back SE, Dansky BS, Carroll KM, Foa EB, Brady KT. Exposure therapy in the treatment of PTSD among cocaine-dependent individuals: Description of procedures. Journal of Substance Abuse Treatment. 2001;21(1):35–45. doi: 10.1016/S0740-5472(01)00181-7. [DOI] [PubMed] [Google Scholar]
- Back SE, Brady KT, Sonne SC, Verduin ML. Symptom improvement in co-occurring PTSD and alcohol dependence. Journal of Nervous and Mental Disease. 2006;194(9):690–696. doi: 10.1097/01.nmd.0000235794.12794. [DOI] [PubMed] [Google Scholar]
- Back SE, Waldrop AE, Brady KT. Treatment Challenges Associated with Comorbid Substance Use and Posttraumatic Stress Disorder: Clinicians’ Perspectives. American Journal of Addiction. 2009;18:15–20. doi: 10.1080/10550490802545141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back SE, Killeen TK, Teer AP, Hartwell EE, Federline A, Beylotte F, Cox E. Substance use disorders and PTSD: An exploratory study of treatment preferences among military veterans. Addictive Behaviors. 2014;39:369–373. doi: 10.1016/j.addbeh.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedard-Gilligan M, Duax Jakob J, Stines Doane L, Jaeger J, Eftekhari A, Feeny N, Zoellner L. An Investigation of Depression, Trauma History and Severity in Individuals enrolled in a Treatment Trial for Chronic PTSD. Journal of Clinical Psychology. 2015;71:725–740. doi: 10.1002/jclp.22163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco C, Xu Y, Brady K, Pérez-Fuentes G, Okuda M, Wang S. Comorbidity of posttraumatic stress disorder with alcohol dependence among US adults: results from National Epidemiological Survey on Alcohol and Related Conditions. Drug and Alcohol Dependence. 2013;132(3):630–638. doi: 10.1016/j.drugalcdep.2013.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonn-Miller MO, Boden MT, Vujanovic AA, Drescher KD. Prospective investigation of the impact of cannabis use disorders on posttraumatic stress disorder symptoms among veterans in residential treatment. Psychological Trauma: Theory, Research, Practice, and Policy. 2013;5(2):193–200. doi: 10.1037/a0026621. [DOI] [Google Scholar]
- Brady K, Pearlstein T, Asnis GM, Baker D, Rothbaum B, Sikes CR, Farfel GM. Efficacy and safety of sertraline treatment of posttraumatic stress disorder: a randomized controlled trial. Journal of the American Medical Association. 2000;283(14):1837–1844. doi: 10.1001/jama.283.14.1837. [DOI] [PubMed] [Google Scholar]
- Brady KT, Dansky BS, Back SE, Foa EB, Carroll KM. Exposure therapy in the treatment of PTSD among cocaine-dependent individuals: Preliminary findings. Journal of Substance Abuse Treatment. 2001;21(1):47–54. doi: 10.1016/S0740-5472(01)00182-9. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Southwick SM, Darnell A, Charney DS. Chronic PTSD in Vietnam combat veterans: course of illness and substance abuse. The American Journal of Psychiatry. 1996;153:369–75. doi: 10.1176/ajp.153.3.369. [DOI] [PubMed] [Google Scholar]
- Buckner JD, Keough ME, Schmidt NB. Problematic alcohol and cannabis use among young adults: The roles of depression and discomfort and distress tolerance. Addictive Behaviors. 2007;32:1957–1963. doi: 10.1016/j.addbeh.2006.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilcoat HD, Menard C. Trauma and Substance Abuse. Washington, DC: American Psychological Association; 2003. Epidemiological investigations: Comorbidity of posttraumatic stress disorder and substance use disorder. [DOI] [Google Scholar]
- Chilcoat HD, Breslau N. Posttraumatic stress disorder and drug disorders: testing causal pathways. Archives of General Psychiatry. 1998;55:913–917. doi: 10.1001/archpsyc.55.10.913. [DOI] [PubMed] [Google Scholar]
- Cougle JR, Bonn-Miller MO, Vujanovic AA, Zvolensky MJ, Hawkins KA. Posttraumatic stress disorder and cannabis use in a nationally representative sample. Psychology of Addictive Behaviors. 2011;25:554. doi: 10.1037/a0023076. [DOI] [PubMed] [Google Scholar]
- Coffey SF, Schumacher JA, Brimo ML, Brady KT. Exposure therapy for substance abusers with PTSD: Translating research to practice. Behavior Modification. 2005;29:10–38. doi: 10.1177/0145445504270855. [DOI] [PubMed] [Google Scholar]
- Dass-Brailsford P, Myrick AC. Psychological trauma and substance abuse: The need for an integrated approach. Trauma, Violence, & Abuse. 2010;11:202–213. doi: 10.1177/1524838010381252. [DOI] [PubMed] [Google Scholar]
- Davidson JR, Rothbaum BO, van der Kolk BA, Sikes CR, Farfel GM. Multicenter, double-blind comparison of sertraline and placebo in the treatment of posttraumatic stress disorder. Archives of General Psychiatry. 2001;58(5):485–492. doi: 10.1001/archpsyc.58.5.485. [DOI] [PubMed] [Google Scholar]
- Feeny NC, Zoellner LA, Mavissakalian MR, Roy-Byrne PP. What would you choose? Sertraline or prolonged exposure in community and PTSD treatment seeking women. Depression and Anxiety. 2009;26(8):724–731. doi: 10.1002/da.20588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition, SCID-I/P Version 2.0. New York, NY: Biometrics Research Department, New York State Psychiatric Institute; 1995. [Google Scholar]
- Foa EB, Hembree EA, Dancu CV. Prolonged exposure (PE) manual: Revised version. 2002 Unpublished manuscript. [Google Scholar]
- Foa EB, Riggs DS, Dancu CV, Rothbaum BO. Reliability and validity of a brief instrument for assessing post- traumatic stress disorder. Journal of Traumatic Stress. 1993;6:459–473. doi: 10.1002/jts.2490060405. [DOI] [Google Scholar]
- Foa EB, Hembree EA, Cahill SP, Rauch SA, Riggs DS, Feeny NC, Yadin E. Randomized trial of prolonged exposure for posttraumatic stress disorder with and without cognitive restructuring: outcome at academic and community clinics. Journal of Consulting and Clinical Psychology. 2005;73(5):953–964. doi: 10.1037/0022-006X.73.5.953. [DOI] [PubMed] [Google Scholar]
- Foa EB, Yusko DA, McLean CP, Suvak MK, Bux DA, Oslin D, O’Brien CP, Imms P, Riggs DS, Volpicelli J. Concurrent naltrexone and prolonged exposure therapy for patients with comorbid alcohol dependence and PTSD: a randomized clinical trial. The Journal of the American Medical Association. 2013;310:488–495. doi: 10.1001/jama.2013.8268. [DOI] [PubMed] [Google Scholar]
- Green B, Kavanagh D, Young R. Being stoned: a review of self-reported cannabis effects. Drug and Alcohol Review. 2003;22:453–460. doi: 10.1080/09595230310001613976. [DOI] [PubMed] [Google Scholar]
- Hien DA, Jiang H, Campbell AN, Hu MC, Miele GM, Nunes EV. Do treatment improvements in PTSD severity affect substance use outcomes? A secondary analysis from a randomized clinical trial in NIDA's Clinical Trials Network. American Journal of Psychiatry. 2010;167:95–101. doi: 10.1176/appi.ajp.2009.09091261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hembree EA, Foa EB, Dorfan NM, Street GP, Kowalski J, Tu X. Do patients drop out prematurely from exposure therapy for PTSD? Journal of Traumatic Stress. 2003;16:555–562. doi: 10.1023/B:JOTS.0000004078.93012.7d. [DOI] [PubMed] [Google Scholar]
- Imel ZE, Laska K, Jakupcak M, Simpson TL. Meta-analysis of dropout in treatments for posttraumatic stress disorder. Journal of Consulting and Clinical Psychology. 2013 Jun 3;81:394–404. doi: 10.1037/a0031474. 2013, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaysen D, Schumm J, Pedersen ER, Seim RW, Bedard-Gilligan M, Chard K. Cognitive processing therapy for veterans with comorbid PTSD and alcohol use disorders. Addictive Behaviors. 2014;39:420–427. doi: 10.1016/j.addbeh.2013.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Archives of General Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- Khantzian EJ. The self-medication hypothesis of addictive disorders: Focus on heroin and cocaine dependence. The American Journal of Psychiatry. 1985;142:1259–1264. doi: 10.1176/ajp.142.11.1259. [DOI] [PubMed] [Google Scholar]
- Killeen T, Back SE, Brady KT. The use of exposure-based treatment among individuals with PTSD and co-occurring substance use disorders: Clinical considerations. Journal of Dual Diagnosis. 2011;7:194–206. doi: 10.1080/15504263.2011.620421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeies M, Pagura J, Sareen J, Bolton JM. The use of alcohol and drugs to self-medicate symptoms of posttraumatic stress disorder. Depression and Anxiety. 2010;27(8):731–736. doi: 10.1002/da.20677. [DOI] [PubMed] [Google Scholar]
- Lopez-Quintero C, de los Cobos JP, Hasin DS, Okuda M, Wang S, Grant BF, Blanco C. Probability and predictors of transition from first use to dependence on nicotine, alcohol, cannabis, and cocaine: results of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) Drug and Alcohol Dependence. 2011;115(1):120–130. doi: 10.1016/j.drugalcdep.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall RD, Beebe KL, Oldham M, Zaninelli R. Efficacy and safety of paroxetine treatment for chronic PTSD: A fixed-dose, placebo-controlled study. American Journal of Psychiatry. 2001;158:1982–1988. doi: 10.1176/appi.ajp.158.12.1982. [DOI] [PubMed] [Google Scholar]
- McCauley JL, Killeen T, Gros DF, Brady KT, Back SE. Posttraumatic stress disorder and co-occurring substance use disorders: Advances in assessment and treatment. Clinical psychology: Science and Practice. 2012;19:283–304. doi: 10.1111/cpsp.12006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGahan PL, Griffith JA, Parente R, McLellan AT. Composite Scores for the Addiction Severity Index. Philadelphia, PA: Department of Veterans Affairs Medical Center; 1986. [Google Scholar]
- McGovern MP, Lambert-Harris C, Acquilano S, Xie H, Alterman AI, Weiss RD. Cognitive behavioral therapy for co-occurring substance use and posttraumatic stress disorders. Addictive Behaviors. 2009;34:892–897. doi: 10.1016/j.addbeh.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh RK, Whitton SW, Peckham AD, Welge JA, Otto MW. Patient preference for psychological vs pharmacologic treatment of psychiatric disorders: a meta-analytic review. The Journal of Clinical Psychiatry. 2013;74:595–602. doi: 10.4088/JCP.12r07757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills KL, Teesson M, Back SE, Brady KT, Baker AL, Hopwood S, Ewer PL. Integrated exposure-based therapy for co-occurring posttraumatic stress disorder and substance dependence: a randomized controlled trial. Journal of American Medical Association. 2012;308(7):690–699. doi: 10.1001/jama.2012.9071. [DOI] [PubMed] [Google Scholar]
- Najavits LM. Seeking safety: A treatment manual for PTSD and substance abuse. Guilford Press; 2002. [DOI] [PubMed] [Google Scholar]
- Najavits LM. Seeking Safety: An evidence-based model for substance abuse and trauma/PTSD. Therapist's guide to evidence-based relapse prevention. 2007:141–167. [Google Scholar]
- Ouimette PE, Brown PJ. Trauma and substance abuse: Causes, consequences, and treatment of comorbid disorders. American Psychological Association; 2003. [DOI] [Google Scholar]
- Ouimette P, Goodwin E, Brown PJ. Health and well being of substance use disorder patients with and without posttraumatic stress disorder. Addictive Behaviors. 2006;31:1415–1423. doi: 10.1016/j.addbeh.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Peele S, Brodsky A. Exploring psychological benefits associated with moderate alcohol use: a necessary corrective to assessments of drinking outcomes? Drug and Alcohol Dependence. 2000;60(3):221–247. doi: 10.1016/s0376-8716(00)00112-5. [DOI] [PubMed] [Google Scholar]
- Pietrzak RH, Goldstein RB, Southwick SM, Grant BF. Prevalence and Axis I comorbidity of full and partial posttraumatic stress disorder in the United States: results from Wave 2 of the National Epidemiologic Survey on Alcohol and Related Conditions. Journal of Anxiety Disorders. 2011;25(3):456–465. doi: 10.1016/j.janxdis.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Possemato K, Maisto SA, Wade M, Barrie K, McKenzie S, Lantinga LJ, Ouimette P. Ecological momentary assessment of PTSD symptoms and alcohol use in combat veterans. Psychology of Addictive Behaviors. 2015;29(4):894. doi: 10.1037/adb0000129. [DOI] [PubMed] [Google Scholar]
- Rabinak CA, Angstadt M, Sripada CS, Abelson JL, Liberzon I, Milad MR, Phan KL. Cannabinoid facilitation of fear extinction memory recall in humans. Neuropharmacology. 2013;64:396–402. doi: 10.1016/j.neuropharm.2012.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read JP, Brown PJ, Kahler CW. Substance use and posttraumatic stress disorders: Symptom interplay and effects on outcome. Addictive Behaviors. 2004;29:1665–1672. doi: 10.1016/j.addbeh.2004.02.061. [DOI] [PubMed] [Google Scholar]
- Resick PA, Nishith P, Weaver TL, Astin MC, Feuer CA. A comparison of cognitive-processing therapy with prolonged exposure and a waiting condition for the treatment of chronic posttraumatic stress disorder in female rape victims. Journal of Consulting and Clinical Psychology. 2002;70(4):867–879. doi: 10.1037/0022-006X.70.4.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JH, Callister LC, Berry JA, Dearing KA. Patient- centered care and adherence: Definitions and applications to improve outcomes. Journal of the American Academy of Nurse Practitioners. 2008;20:600–607. doi: 10.1111/j.1745-7599.2008.00360.x. [DOI] [PubMed] [Google Scholar]
- Rosen CS, Henson BR, Finney JW, Moos RH. Consistency of self- administered and interview- based Addiction Severity Index composite scores. Addiction. 2000;95:419–425. doi: 10.1046/j.1360-0443.2000.95341912.x. [DOI] [PubMed] [Google Scholar]
- Rubin DB. Multiple imputation after 18+ years. Journal of the American Statistical Association. 1996;91(434):473–489. [Google Scholar]
- Saladin ME, Brady KT, Dansky BS, Kilpatrick DG. Understanding comorbidity between PTSD and substance use disorders: Two preliminary investigations. Addictive Behaviors. 1995;20:643–655. doi: 10.1016/0306-4603(95)00024-7. [DOI] [PubMed] [Google Scholar]
- Schäfer I, Najavits LM. Clinical challenges in the treatment of patients with posttraumatic stress disorder and substance abuse. Current Opinion in Psychiatry. 2007;20:614–618. doi: 10.1097/YCO.0b013e3282f0ffd9. [DOI] [PubMed] [Google Scholar]
- Schnurr PP, Friedman MJ, Engel CC, Foa EB, Shea MT, Chow BK, Turner C. Cognitive behavioral therapy for posttraumatic stress disorder in women: A randomized controlled trial. Journal of the American Medical Association. 2007;297(8):820–830. doi: 10.1001/jama.297.8.820. [DOI] [PubMed] [Google Scholar]
- Simpson TL, Stappenbeck CA, Luterek JA, Lehavot K, Kaysen DL. Drinking motives moderate daily relationships between PTSD symptoms and alcohol use. Journal of Abnormal Psychology. 2014;123(1):237–247. doi: 10.1037/a0035193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein DJ, Ipser JC, Seedat S. Pharmacotherapy for post traumatic stress disorder (PTSD) The Cochrane Library. 2006 doi: 10.1002/14651858.CD002795.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steindl SR, Young RM, Creamer M, Crompton D. Hazardous alcohol use and treatment outcome in male combat veterans with posttraumatic stress disorder. Journal of Traumatic Stress. 2003;16(1):27–34. doi: 10.1023/A:1022055110238. [DOI] [PubMed] [Google Scholar]
- Ullman SE, Filipas HH, Townsend SM, Starzynski LL. Trauma exposure, posttraumatic stress disorder and problem drinking in sexual assault survivors. Journal of Studies on Alcohol and Drugs. 2005;66:610–619. doi: 10.15288/jsa.2005.66.610. [DOI] [PubMed] [Google Scholar]
- Watts BV, Schnurr PP, Mayo L, Young-Xu Y, Weeks WB, Friedman MJ. Meta-analysis of the efficacy of treatments for posttraumatic stress disorder. The Journal of Clinical Psychiatry. 2013;74(6):541–550. doi: 10.4088/JCP.12r08225. [DOI] [PubMed] [Google Scholar]
- Wilkinson ST, Stefanovics E, Rosenheck RA. Marijuana use is associated with worse outcomes in symptom severity and violent behavior in patients with posttraumatic stress disorder. The Journal of Clinical Psychiatry. 2015;76(9):1–478. doi: 10.4088/JCP.14m09475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Swanson JM, Evins AE, DeLisi LE, Meier MH, Gonzalez R, Baler R. Effects of cannabis use on human behavior, including cognition, motivation, and psychosis: a review. JAMA Psychiatry. 2016;73(3):292–297. doi: 10.1001/jamapsychiatry.2015.3278. [DOI] [PubMed] [Google Scholar]
- Zoellner LA, Roy-Byrne PB, Mavissakalian M, Feeny NC. Doubly randomized preference trial in PTSD: Prolonged Exposure versus sertraline. 2017 doi: 10.1176/appi.ajp.2018.17090995. Manuscript submitted for publication. [DOI] [PubMed] [Google Scholar]
- Zoellner LA, Telch M, Foa EB, Farach FJ, McLean CP, Gallop R, Gonzalez-Lima F. Enhancing extinction learning in posttraumatic stress disorder with brief daily imaginal exposure and methylene blue: a randomized controlled trial. The Journal of Clinical Psychiatry. 2017;78(7):e782–e789. doi: 10.4088/JCP.16m10936. [DOI] [PubMed] [Google Scholar]
- Zvolensky MJ, Marshall EC, Johnson K, Hogan J, Bernstein A, Bonn-Miller MO. Relations between anxiety sensitivity, distress tolerance, and fear reactivity to bodily sensations to coping and conformity marijuana use motives among young adult marijuana users. Experimental Clinical Psychopharmacology. 2009;7:31–42. doi: 10.1037/a0014961. [DOI] [PMC free article] [PubMed] [Google Scholar]