SUMMARY
Background
Metastatic castration-resistant prostate cancer (mCRPC) is driven by activated androgen receptors and elevated intratumoural androgens. Apalutamide and abiraterone, which suppress the androgen signalling axis in different ways, was investigated as a combination treatment in mCRPC.
Methods
ACIS was a randomised, phase 3, placebo-controlled, double-blind, multinational study in mCRPC. Inclusion criteria: chemotherapy-naive men (≥18 years) with mCRPC, receiving ongoing androgen deprivation therapy, Eastern Cooperative Oncology Group performance status of 0 or 1, Brief Pain Inventory-Short Form Question #3 (worst pain in last 24 hours) score ≤3. Patients previously treated with androgen biosynthesis signalling inhibitors were excluded. Patients were randomised 1:1 (centralised interactive web response system; permuted block randomisation scheme; block size 4) to apalutamide (240 mg once daily) and abiraterone acetate (1000 mg once daily) plus prednisone (5 mg twice daily) (henceforth “apalutamide plus abiraterone-prednisone”) or placebo and abiraterone acetate plus prednisone (henceforth “abiraterone-prednisone”) administered orally. Primary endpoint: radiographic progression-free survival (rPFS; intention-to-treat population). Safety was reported (safety population). This completed study is registered with ClinicalTrials.gov, number NCT02257736.
Findings
982 men were randomised from December 10, 2014, to August 30, 2016 (492, apalutamide plus abiraterone-prednisone; 490, abiraterone-prednisone). At primary analysis (median 25·7 months’ follow-up, interquartile range [IQR] 23·0–28·9), apalutamide plus abiraterone-prednisone extended median rPFS by 6 months versus abiraterone-prednisone (22·6 (95% CI 19·5–27·4) vs 16·6 (95% CI 13·9–19·3) months [hazard ratio (HR) 0·69 (95% confidence internal [CI] 0·58–0·83); p<0·0001]). At the updated rPFS analysis (final analysis for overall survival; median 54·8 months’ follow-up, IQR 51·5–58·4), apalutamide plus abiraterone-prednisone extended rPFS by 7·4 months (24·0 [95% CI 19·7–27·5] vs 16·6 [95% CI 13·9–19·3] months; HR 0·70 [95% CI 0·60–0·83]; p<0·0001). Grade 3 or 4 treatment-emergent adverse events (TEAEs) were reported in 60% (294/490) of patients receiving apalutamide plus abiraterone-prednisone versus 51% (250/489) receiving abiraterone-prednisone. The most common grade 3-4 TEAE was hypertension for apalutamide plus abiraterone-prednisone (17% [82/490]) and abiraterone-prednisone (10% [49/489]). The most common serious TEAEs were pneumonia (4% [18/490] and 2% [10/489]), haematuria (1% [6/490] and 3% [13/489]), and urinary tract infection (2% [12/490] and 1% [6/489], respectively). TEAEs associated with death occurred in 3% (17/490) and 8% (37/489), respectively.
Interpretation
Despite the use of an active and established therapy as the comparator, apalutamide plus abiraterone-prednisone demonstrated improvement in rPFS.
Funding
Funded by Janssen Research & Development.
Introduction
Progression to metastatic castration-resistant prostate cancer (mCRPC) occurs in the majority of patients with advanced prostate cancer who initially respond to androgen deprivation therapy (ADT).1 Failure of ADT may occur because of persistent androgen receptor (AR) signalling despite castrate levels of testosterone resulting from extragonadal steroidogenesis and AR gene amplification2 and/or overexpression.3 Current standard of care for mCRPC comprises therapies that target a single androgen signalling mechanism, including a CYP17 inhibitor and AR antagonists.
Abiraterone acetate, which suppresses the androgen biosynthesis pathways,4 plus prednisone, added to ADT significantly extends overall survival (OS) and radiographic progression-free survival (rPFS) in patients with mCRPC.5-7 Abiraterone acetate plus prednisone/prednisolone (hereafter, abiraterone-prednisone) is approved for treating mCRPC based on the COU-AA-301 and COU-AA-302 studies, and metastatic castration-sensitive prostate cancer (mCSPC) based on LATITUDE.6-10 Despite the survival benefit of abiraterone-prednisone in patients with mCRPC,5-7 resistance invariably develops, predominately through AR gene amplification and overexpression.11,12 Similarly, apalutamide, which binds to and directly antagonises the AR,13 plus ADT, significantly delays progression to mCRPC and improves OS in both non-metastatic CRPC (SPARTAN study) and mCSPC (TITAN study), and is approved in multiple countries for both disease states.14-18
Androgen annihilation therapy, such as the combination of apalutamide and abiraterone-prednisone, involves dual inhibition of the androgen signalling axis using therapies that suppress androgen signalling in different ways,4,13,15,16,19 and may delay resistance and improve outcomes in patients with mCRPC.4,20,21 The apalutamide and abiraterone acetate plus prednisone combination (hereafter, apalutamide plus abiraterone-prednisone) has not been studied extensively in mCRPC, apart from a phase 1b study which found evidence of antitumour activity with a good tolerability profile.22 The current phase 3 trial evaluated clinical benefit of apalutamide plus abiraterone-prednisone versus placebo and abiraterone-prednisone (hereafter abiraterone-prednisone) in patients with chemotherapy-naive mCRPC.
Methods
Study design and participants
ACIS (completed; ClinicalTrials.gov, NCT02257736) was a randomised, placebo-controlled phase 3 multinational, double-blind study of patients with chemotherapy-naive mCRPC receiving ongoing ADT conducted at 167 sites in 17 countries in North America, Europe, the Asia-Pacific region, Africa, and South America. The institutional review board at each institution approved the study protocol, which was conducted in accordance with current International Council for Harmonisation Good Clinical Practice guidelines, and according to Declaration of Helsinki principles.
Eligible patients were adult men, aged ≥18 years, with mCRPC. Patients had prostate adenocarcinoma with metastatic disease as documented by technetium-99m bone scans, computed tomography (CT) or magnetic resonance imaging (MRI) scans, and castration resistance. If lymph nodes were the only evidence of metastasis, they had to be ≥2 cm in diameter at the longest point for trial inclusion. Prostate cancer progression was documented by prostate-specific antigen (PSA) according to the Prostate Cancer Clinical Trials Working Group 2 (PCWG2), radiographic progression of soft tissues according to Response Evaluation Criteria In Solid Tumors (RECIST), version 1.1 modified based on PCWG2, or radiographic progression of bone metastases according to PCWG2.23 Castration resistance was defined as three rises of PSA ≥1 week apart and last PSA ≥2 ng/mL during continuous androgen deprivation therapy. Patients had an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 or 1 and scored ≤3 on the Brief Pain Inventory-Short Form (BPI-SF) Question #3 (worst pain in last 24 hours). Patients with prior chemotherapy for prostate cancer (unless administered in the adjuvant/neoadjuvant setting) and those previously treated with androgen biosynthesis inhibitors and/or AR inhibitors were excluded. Patients who received a first-generation antiandrogen must have had at least a 6-week washout prior to randomisation and must have shown continuing disease (PSA) progression (an increase in PSA) after the washout period. All patients provided written informed consent.
Patients’ laboratory values at screening had to meet the following criteria: haemoglobin ≥9·0 g/dL (≥90·0 g/L), independent of transfusion and/or growth factors within 3 months prior to randomisation; platelet count ≥100 × 109/L (≥100,000/μL) independent of transfusion and/or growth factors within 3 months prior to randomisation; serum albumin ≥3·0 g/dL (≥30·0 g/L); serum creatinine <2·0 × upper limit of normal (ULN); serum potassium ≥3·5 mmol/L; serum total bilirubin ≤1·5 × ULN (Note: In patients with Gilbert’s syndrome, if total bilirubin was >1·5 × ULN, measure direct and indirect bilirubin and if direct bilirubin was ≤1·5 × ULN, patient may be eligible); and aspartate aminotransferase or alanine aminotransferase <2·5 × ULN.
Patients with a history of any of the following were not eligible: seizure or known condition that may predispose to seizure (including but not limited to prior stroke, transient ischaemic attack, loss of consciousness within 1 year prior to randomisation, brain arteriovenous malformation; or intracranial masses such as schwannomas and meningiomas that are causing oedema or mass effect); any prior malignancy (other than adequately treated basal cell or squamous cell skin cancer, superficial bladder cancer, or any other cancer in situ currently in complete remission) within 5 years prior to randomisation; severe or unstable angina, myocardial infarction, symptomatic congestive heart failure, arterial or venous thromboembolic events (eg, pulmonary embolism, cerebrovascular accident including transient ischaemic attacks), or clinically significant ventricular arrhythmias within 6 months prior to randomisation or New York Heart Association Class II to IV heart disease; or any condition that, in the opinion of the investigator, would preclude participation in this study. Current evidence of any of the following was not permitted: uncontrolled hypertension (systolic blood pressure ≥160 mmHg or diastolic blood pressure ≥90 mmHg); gastrointestinal disorder affecting absorption; active infection or other medical condition that would make prednisone/prednisolone use contraindicated; or any chronic medical condition requiring a higher dose of corticosteroid than 10 mg prednisone/prednisolone once daily.
Randomisation and masking
Patients were randomly assigned by centralised interactive web response system (IWRS) (permuted block randomisation scheme; block size 4) in a 1:1 ratio to apalutamide plus abiraterone-prednisone or matching placebo plus abiraterone-prednisone. The treatment codes were maintained within the IWRS, and blinded to subjects, investigators, and study team. At randomisation, the IWRS assigned a unique subject identification number to each patient that was used on all study-related documents, including eCRFs. A treatment number was also assigned to each patient that linked a patient’s eCRF and blinded treatment group assignment. Randomisation was blinded to all patients, staff of investigators, and sponsor. Data that may potentially unblind the treatment assignment were handled with special care to ensure that the integrity of the blind was maintained and the potential for bias was minimised (strategies could include special provisions, such as segregating the data in question from investigators, clinical team, or others as appropriate until database lock and unblinding). To evaluate the success of masking, patients were asked the question: “Do you think you received the experimental drug or placebo?” The analysis showed the masking was maintained well.
Stratification factors for randomisation (based on IWRS) included presence or absence of visceral metastases, ECOG PS, and geographic region (Europe/United Kingdom, North America, rest of world).
Procedures
Patients in the apalutamide plus abiraterone-prednisone group received apalutamide 240 mg once daily as four 60-mg tablets, with or without food, and abiraterone acetate 1000 mg daily as four 250-mg tablets on an empty stomach, plus prednisone 10 mg as a 5-mg tablet twice daily administered orally (28-day treatment cycles) until unequivocal clinical disease progression, unacceptable toxicity, death, or withdrawal of consent. Patients in the abiraterone-prednisone group received placebo as four tablets, with or without food, and dose of abiraterone acetate and prednisone matched to that received by the apalutamide plus abiraterone-prednisone group. Patients continued background therapy with ADT.
The general principles on dose modifications were as follows: 1) grade 1 or grade 2 toxicities should be managed symptomatically without dose adjustments. Appropriate medical treatment should be used; 2) in the event of a grade 3 or higher toxicity that cannot definitively be attributed to abiraterone acetate or apalutamide/placebo, both abiraterone acetate and apalutamide/placebo should be held until toxicity has resolved to grade 1 or baseline; 3) if grade 3 or higher toxicity does not resolve to grade 1 or baseline within two cycles, the patient should be discontinued from treatment or the investigator’s rationale to continue treatment must be discussed with the sponsor; 4) a patient may have up to two dose adjustments for the same toxicity and if the same toxicity recurs at grade 3 or higher after two dose adjustments, the patient should discontinue study treatment(s); 5) dose modifications are provided as guidance and should not replace the investigator’s own clinical judgement; 6) the dose of prednisone can remain unchanged with dose modifications of abiraterone acetate or apalutamide/placebo. Abiraterone acetate dose modifications are per the local abiraterone acetate brand name label8,9; and 7) the investigator’s rationale to re-escalate treatment must be discussed with the sponsor’s medical monitor on an individual basis prior to implementation. See appendix p 2 for dose modifications for toxicity attributed to apalutamide/placebo.
Scans from all patients were reviewed by blinded independent central review (BICR) based on the primary analysis (cutoff March 19, 2018). Correlation coefficients between investigator-determined and blinded independent reviewer–assessed time-to-event endpoints with censoring were estimated with associated confidence intervals using the iterative multiple imputation approach.24
Investigator-assessed rPFS based on conventional radiographic imaging (CT or MRI [to be used consistently throughout the study] and technetium-99m bone scans) were performed at baseline, cycles 3 and 5, and then, beginning at cycle 7, every 3 months until end of treatment. CT/MRI scans must have included chest, abdomen, and pelvis. Unscheduled assessments by CT, MRI, or technetium-99m bone scans could be performed if signs of disease progression were observed. Serum chemistry, PSA, and testosterone were measured at screening. During treatment, serum chemistry, haematology samples, and liver function were measured at every cycle to cycle 6, then every second cycle up to cycle 24, and then every third cycle and end of treatment; fasting lipids and glucose samples were measured for cycle 1 and then every 12 cycles starting on cycle 12. PSA was measured at every cycle to cycle 12, every second cycle up to cycle 24, and then every third cycle and end of treatment. Thyroid-stimulating hormone was measured on cycle 1, cycles 3 and 4 (if abnormal) and then every second cycle up to cycle 24, then every third cycle and at the end of treatment. For patients participating in biomarker analysis, archived formalin-fixed paraffin-embedded primary tumour blocks/slides were collected, plasma and whole blood samples were collected on cycles 1 and 12, and serum samples were collected on cycles 1, 3 and 12.
Adverse events (AEs; coded using Medical Dictionary for Regulatory Activities Version 23.0), including type, incidence, severity (per the National Cancer Institute Common Terminology Criteria for Adverse Events [NCI CTCAE], version 4.03), seriousness, and action taken, as well as special reporting situations, whether serious or non-serious, were assessed continuously from the time a signed and dated informed consent form was obtained until 30 days after the last dose of study drug. Monthly visits were required for 24 months and then patients had visits every 3 months.
Study treatment must be discontinued if the following scenarios occur: 1) unequivocal clinical progression, defined as a) deterioration in ECOG PS to grade 3 or higher, or b) need to initiate any of the following because of tumour progression (even in the absence of radiographic evidence of disease): alternative anticancer therapy for prostate cancer; use of external beam radiation therapy to relieve skeletal symptoms; tumour-related orthopaedic surgical intervention; chronic opioid analgesics, defined as administration of additional opioid analgesics lasting for ≥3 weeks for oral, or ≥7 days for non-oral formulation (administration of as-needed, eg, not fixed or scheduled dosage, use of opioid analgesics or extended opioid use for treatment other than the patient’s prostate cancer did not require discontinuation from study treatment); 2) withdrawal of consent for continued treatment; 3) patients who had their treatment assignment unblinded (exception: independent data-monitoring committee recommendation to unblind the study) should discontinue treatment and enter the follow-up phase; or 4) the investigator believed that for safety reasons (eg, AE) it was in the best interest of the patient to discontinue study treatment.
A patient was considered withdrawn from the study for loss to follow-up or withdrawal of consent for subsequent data collection.
Outcomes
Efficacy was analysed in the intention-to-treat population. The primary endpoint, rPFS assessed by the investigator (local review; analysed at the primary endpoint of rPFS), was defined as the time from date of randomisation to date of radiographic progression (soft tissue lesion by CT/MRI per modified RECIST v1·123 or bone lesion progression per adaptation of PCWG2) or death, whichever occurred first. We also analysed rPFS at the final analysis for OS. rPFS was considered the most appropriate endpoint to demonstrate effectiveness when comparing with the active comparator. A patient was considered to have progressed if a bone scan with two or more new lesions compared with baseline was observed <12 weeks from randomisation and a second bone scan ≥6 weeks later showed two or more additional lesions (ie, four or more new lesions compared with baseline), or if the first bone scan with two or more new lesions compared with baseline occurred ≥12 weeks from randomisation and was verified on the next bone scan ≥6 weeks later (ie, two or more new lesions compared with baseline). Additional progression criteria included soft tissue lesions measured by CT or MRI as defined in modified RECIST 1·1 criteria.23
Secondary endpoints were OS, time to initiation of cytotoxic chemotherapy, time to chronic opioid use, and time to pain progression (analysed at the final analysis for OS). OS was defined as the time from date of randomisation to date of death from any cause; time to initiation of cytotoxic chemotherapy was defined as the time from date of randomisation to the date of initiation of cytotoxic chemotherapy; time to pain progression was defined as the time from randomisation to the first date a patient experienced an increase by 2 points from baseline in the Brief Pain Inventory-Short Form worst pain intensity item 3 or pain observed and reported on electronic case report form at two consecutive evaluations ≥4 weeks apart, or initiation of chronic opioids, whichever occurs first; time to chronic opioid use (oral opioid use for ≥3 weeks; parenteral opioid use for ≥7 days) was defined as the time from date of randomisation to the first date of opioid use.
Exploratory endpoints included confirmed decline of ≥50% in PSA level, time to PSA progression, time to clinical progression, time to second progression-free survival, objective response rate, health-related quality of life (Functional Assessment of Cancer Therapy-Prostate [FACT-P]; least squares mean change from baseline and time to degradation in FACT-P [total score]), and biomarker subtype associated with response to treatment group compared with control group.
Time to PSA progression was based on PCWG2 criteria (≥25% increase and absolute increase of ≥2 ng/mL [≥ 2 μg/L] from nadir [confirmed by second value obtained ≥3 weeks later]25). Clinical progression is a composite endpoint defined as time from randomisation to first occurrence of one of the following: 1) deterioration in ECOG PS to grade 3, or 2) need to initiate: alternative anticancer therapy (systemic), external beam radiation therapy for tumour-related symptoms, tumour-related surgical intervention/procedure, or chronic opioid analgesics (per protocol definition), or the occurrence of cancer-related symptomatic events of clear clinical significance. If no event was observed, patients were censored at the last known date alive. Time to second progression-free survival was defined as time from randomisation to the date of first progression (radiographic, clinical, or PSA progression) on the first subsequent therapy or death from any cause, whichever occurred first. Objective response rate was defined as the proportion of patients with measurable disease achieving a complete or partial response according to modified RECIST 1·1.23 FACT-P questionnaire was completed at every cycle to cycle 6 and then every third cycle for up to 12 months after treatment discontinuation. Degradation in FACT-P (total score) was defined as a decrease from the baseline score of at least 10 points. The biomarker analysis population included patients who consented to tumour collections and provided samples. Prespecified biomarker subgroups, PAM50-luminal subtype, and AR signalling activity were analysed based on molecular signatures in archived tissue biopsies. Hormone-responsive molecular signature refers to PAM50-luminal and AR high.26-28
Treatment-emergent AEs (TEAEs), defined as those events that occur or worsen on or after first dose of study drug through 30 days after the last dose of study drug, are reported for the safety population (all patients who received at least one dose of study drug). Serious AEs included those spontaneously reported to the investigator within 30 days after the last dose of study drug. A TEAE was categorised as drug-related if assessed by the investigator as possibly, probably, and very likely related to study drug. TEAEs of cardiac disorders were analysed according to Framingham Risk Score. Framingham Risk Score was based on baseline data available for all patients. By default, score for smoking was set at 0 points as smoking history was not captured. The total score corresponds to a 10-year risk for ischaemic heart disease as follows: 0 points: <1%; 1–4 points: 1%; 5–6 points: 2%; 7 points: 3%; 8 points: 4%; 9 points: 5%; 10 points: 6%; 11 points: 8%; 12 points: 10%; 13 points: 12%; 14 points: 16%; 15 points: 20%; 16 points: 25%; 17 or more points: over 30%.29,30 Framingham Risk Score was grouped according to low risk (≤10%), intermediate risk (>10%–<20%), and high risk (≥20%).
Statistical analysis
Efficacy was analysed in the intention-to-treat population. We designed the study to enrol approximately 960 patients and observe 450 rPFS events to provide ≥90% power to detect a hazard ratio (HR) of 0·67 (median rPFS, 24 vs 16 months for apalutamide plus abiraterone-prednisone vs abiraterone-prednisone) at a two-sided level of significance of 0·05. For OS, the study was designed to provide 80% power (at a two-sided significance level of 0·05 with two interim analyses and one final analysis) to detect an HR of 0·8 assuming median OS of 43·75 months with apalutamide plus abiraterone-prednisone and 35 months with abiraterone-prednisone. To maintain an overall family-wise type I error at the 0·05 level, the multistage gatekeeping procedure was applied to test the secondary endpoints. Each secondary endpoint, except OS, was evaluated at the significance level of 0·00330 at the final analysis after rPFS reached statistical significance. The overall significance level of OS was 0·04–0·05 depending on the number of secondary endpoints that are statistically significant.
No interim analysis was planned to stop the study for superiority for the primary endpoint, rPFS, or the three secondary endpoints, time to initiation of chemotherapy, time to opioid use, and time to pain progression. Two interim analyses and one final analysis were planned for the OS endpoint, with the two interim analyses to occur when 45% and 62% of the required 659 deaths were reached.
Post hoc analyses were performed to further understand treatment effects on the level of PSA decline and the time to first subsequent anticancer therapy and to further investigate clinical subgroups of interest. Post hoc endpoints included undetectable PSA (<0·2 ng/mL at any time during treatment), decline of ≥90% in PSA level, and time to first subsequent anticancer therapy. Additionally, post hoc analyses were performed among important clinical subgroups for the endpoints of time to initiation of cytotoxic chemotherapy, time to chronic opioid use, and time to pain progression.
Time-to-event endpoints include rPFS (including baseline patient characteristics subgroups and exploratory biomarker subgroups), OS (including baseline patient characteristics and exploratory biomarker subgroups), all other secondary endpoints (including post hoc clinical subgroups), exploratory endpoints, time to PSA progression, time to clinical progression, time to second progression-free survival, and time to degradation in FACT-P total score, and the post hoc endpoint, time to first subsequent anticancer therapy. Distribution of the time-to-event endpoints were estimated by Kaplan-Meier methods, determined HRs and 95% confidence intervals by stratified Cox proportional-hazard models, and used stratified log-rank test to test for the treatment effect. We used the stratification factors used for randomisation (based on IWRS) for the stratified analyses. We censored patients without any postrandomisation data at randomisation date.
Relative response (95% CI) was calculated for the exploratory endpoints confirmed decline of ≥50% in PSA level and post hoc endpoints of undetectable PSA (<0·2 ng/mL at any time during treatment) and decline of ≥90% in PSA level; the Cochran-Mantel-Haenszel test was used to test for significance. For the exploratory endpoint of objective response rate, relative response (95% CI) was calculated and the chi-square test was used to test for significance.
Least squares mean change from baseline was calculated for the exploratory endpoint FACT-P total score. Least squares mean change was derived based on the mixed effects model with baseline, visit, treatment, and visit by treatment interaction as fixed effects and individual patient as random effect. Least squares mean difference between treatment was calculated as apalutamide + abiraterone-prednisone versus abiraterone-prednisone. Significance was determined based on the F-test comparing apalutamide + abiraterone-prednisone versus abiraterone-prednisone.
TEAEs are reported for the safety population (all patients who received at least one dose of study drug).
An exploratory sensitivity analysis for the BICR assessment of rPFS was performed where events identified by the investigators as an rPFS event but not by the BICR assessment because of lack of scans beyond 60 days of investigator-assessed rPFS event and before subsequent therapy (informative censoring in BICR analysis) were included.
SAS 9.4 statistical software was used.
The sponsor, Janssen Research & Development, commissioned an independent data-monitoring committee (IDMC) to monitor data on an ongoing basis, ensure continuing patient safety, and review efficacy data. ACIS was registered with ClinicalTrials.gov (number, NCT02257736).
Role of the funding source
Employees of the sponsor, academic authors, and the protocol steering committee designed this study; the sponsor funded data collection; and sponsor-employed statisticians analysed the data. All authors, academic and those employed by the sponsor, had full access to the data, participated in the interpretation of the data and development of the manuscript, approved the manuscript for submission, and assume responsibility for the completeness and integrity of the data and for the fidelity of the trial to the protocol. The sponsor provided funding for medical writing assistance. The corresponding author had full access to all of the data and the final responsibility to submit for publication.
Results
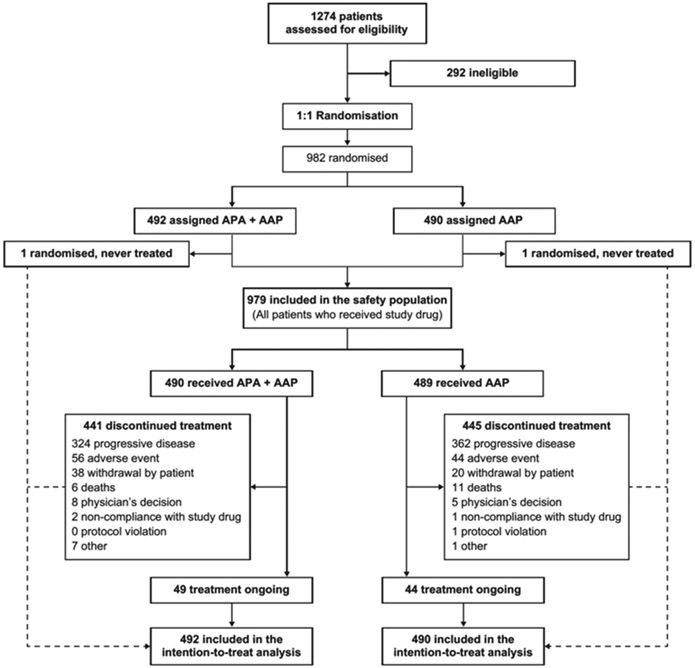
Nine hundred eighty-two patients (492, apalutamide plus abiraterone-prednisone; 490, abiraterone-prednisone) were randomised from December 10, 2014, to August 30, 2016 (figure 1). All 982 randomised patients were included in the analyses per intention-to-treat population. Of these, there were 65 patients (30 [6%] in apalutamide plus abiraterone-prednisone group and 35 [7%] in abiraterone-prednisone group) who were found to not meet all of the study entry criteria. The entry criteria that were most frequently not met were “A score of >3 on the Brief Pain Inventory-Short Form (BPI-SF) Question #3,” and “at study entry, need for parenteral or oral opioid analgesics.” However, all 65 patients were included in the analyses as part of the intention-to-treat population. 441 patients receiving apalutamide plus abiraterone-prednisone and 445 receiving abiraterone-prednisone discontinued treatment; 49 and 44 patients were receiving ongoing treatment with apalutamide plus abiraterone-prednisone and abiraterone-prednisone, respectively.
Figure 1: Trial profile.
APA=apalutamide. AAP=abiraterone acetate plus prednisone.
We report the primary endpoint analysis of rPFS (clinical cutoff, March 19, 2018; median follow-up, 25·7 months, interquartile range [IQR] 23·0–28·9). While treatment with apalutamide plus abiraterone-prednisone provided significant improvement in rPFS versus abiraterone-prednisone at the primary endpoint analysis of rPFS, the IDMC recommended maintaining the study blind until the planned final analysis for OS. We also report the final analysis of OS and updated rPFS (clinical cutoff, September 15, 2020; median follow-up, 54·8 months, IQR 51·5–58·4).
Baseline patient characteristics were well balanced between apalutamide plus abiraterone-prednisone (n=492) and abiraterone-prednisone (n=490) treatment groups; overall, the median age was 71 years for both apalutamide plus abiraterone-prednisone (IQR 66–78) and abiraterone-prednisone (IQR 65–77), and 36% (353/982) of patients were ≥75 years (table 1). Most patients (85%; 829/975) had bone metastasis, and 15% (143/975) had visceral disease at study entry. 437 of 492 (89%) patients receiving apalutamide plus abiraterone-prednisone and 435 of 490 (89%) patients receiving abiraterone-prednisone were alive at treatment discontinuation (see appendix p 3). Of these, 62% (273/437) treated with the combination and 65% (282/435) treated with abiraterone-prednisone received life-prolonging subsequent therapy for prostate cancer after discontinuing study treatment, including chemotherapy (docetaxel and cabazitaxel), hormonal therapy (darolutamide and enzalutamide), radium, and/or sipuleucel-T.
Table 1:
Demographics and baseline disease characteristics
| Apalutamide + abiraterone-prednisone (n=492) |
Abiraterone-prednisone (n=490) |
|
|---|---|---|
| Median age, years (IQR) | 71 (66–78) | 71 (65–77) |
| Age, years | ||
| <65 | 96 (20%) | 110 (22%) |
| 65–69 | 112 (23%) | 103 (21%) |
| 70–74 | 96 (20%) | 112 (23%) |
| ≥75 | 188 (38%) | 165 (34%) |
| Race | ||
| White | 365 (74%) | 373 (76%) |
| Asian | 58 (12%) | 53 (11%) |
| Other | 21 (4%) | 18 (4%) |
| Black or African American | 19 (4%) | 18 (4%) |
| American Indian or Alaska Native | 9 (2%) | 8 (2%) |
| Not reported/Unknown | 20 (4%) | 20 (4%) |
| Ethnicity | ||
| Hispanic or Latino | 50 (10%) | 54 (11%) |
| Not Hispanic or Latino | 422 (86%) | 411 (84%) |
| Not reported/Unknown | 20 (4%) | 25 (5%) |
| Region at stratification | 142 (29%) | 140 (29%) |
| North America (United States, Canada) | 156 (32%) | 157 (32%) |
| Europe (including UK) | 194 (39%) | 193 (39%) |
| Rest of world | ||
| Gleason score at initial diagnosis | n=491 | n=489 |
| <7 | 47 (10%) | 42 (9%) |
| 7 | 162 (33%) | 161 (33%) |
| >7 | 260 (53%) | 258 (53%) |
| Unknown | 22 (4%) | 28 (6%) |
| ECOG PS at stratification (IWRS) | 340 (69%) | 338 (69%) |
| 0 | 152 (31%) | 152 (31%) |
| 1 | ||
| ECOG PS at baseline | 336 (68%) | 333 (68%) |
| 0 | 156 (32%) | 157 (32%) |
| 1 | ||
| Previous prostate cancer therapy | n=492 | n=489 |
| Prostatectomy | 127 (26%) | 149 (30%) |
| Radiotherapy | 265 (54%) | 238 (49%) |
| Hormonal | 491 (100%) | 488 (100%) |
| Adjuvant/neoadjuvant chemotherapy | 8 (2%) | 11 (2%) |
| Other | 68 (14%) | 78 (16%) |
| Prior bone protective agent | 155 (32%) | 153 (31%) |
| Presence of visceral metastasis at stratification (eCRF) | 74 (15%) | 69 (14%) |
| Site of disease at baseline | n=488 | n=487 |
| Bone | 406 (83%) | 423 (87%) |
| Bone only | 207 (42%) | 205 (42%) |
| Lymph node | 235 (48%) | 230 (47%) |
| Soft tissue | 60 (12%) | 66 (14%) |
| Visceral | 74 (15%) | 69 (14%) |
| Adrenal gland | 6 (1%) | 5 (1%) |
| Liver | 21 (4%) | 20 (4%) |
| Lung | 53 (11%) | 50 (10%) |
| Evidence of disease progression at baseline | n=491 | n=486 |
| PSA | 431 (88%) | 438 (90%) |
| Radiographic bone | 169 (34%) | 176 (36%) |
| Radiographic soft tissue/lymph node | 126 (26%) | 127 (26%) |
| Tumour stage at diagnosis | n=490 | n=489 |
| T0–2 | 143 (29%) | 125 (26%) |
| T3–4 | 245 (50%) | 257 (53%) |
| Unknown | 102 (21%) | 107 (22%) |
| Lymph node stage at diagnosis | n=487 | n=486 |
| N0 | 225 (46%) | 207 (43%) |
| N1 | 106 (22%) | 117 (24%) |
| Unknown | 156 (32%) | 162 (33%) |
| Metastasis stage at diagnosis | n=490 | n=487 |
| M0 | 229 (47%) | 204 (42%) |
| M1 | 164 (33%) | 171 (35%) |
| Unknown | 97 (20%) | 112 (23%) |
| Bone lesions at baseline | n=490 | n=489 |
| ≤10* | 339 (69%) | 323 (66%) |
| >10 | 151 (31%) | 166 (34%) |
| BPI-SF pain score at baseline (worst pain over last 24 hours)† | n=485 | n=482 |
| 0–1 | 328 (68%) | 308 (64%) |
| 2–3 | 134 (28%) | 155 (32%) |
| >3 | 23 (5%) | 19 (4%) |
| Median (IQR) | 0·4 (0·0–1·4) | 0·5 (0·0–1·7) |
| PSA at baseline, ng/mL | 32·3 (11·5–91·4) | 31·2 (12·2–106·5) |
| Median (IQR) | ||
| Alkaline phosphatase at baseline, IU/L | n=490 | n=487 |
| Median (IQR) | 92·0 (69·0–139·0) | 91·0 (68·0–138·0) |
| Lactate dehydrogenase at baseline, IU/L | n=471 | n=469 |
| Median (IQR) | 186·0 (167·0–215·0) | 188·0 (167·0–221·0) |
| Time from initial diagnosis to randomisation, years | n=492 | n=490 |
| Median (IQR) | 4·9 (2.7–9.6) | 4·0 (2.1–8.4) |
| Time from ADT or orchiectomy (whichever first) to randomisation, years | n=487 | n=476 |
| Median (IQR) | 3·6 (1.8–6.2) | 2·8 (1.4–5.6) |
Data are n (%) and median (IQR). Intent-to-treat population. eCRF=electronic case report form. IQR=interquartile range. IWRS=interactive web response system.
Includes patients with no bone lesion.
Averaged over all available scores from day –6 to cycle 1 day 1, based on patients with at least 1-day assessments.
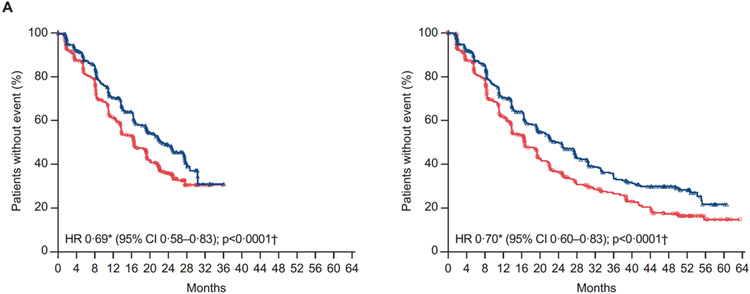
The primary analysis of rPFS by investigator review was performed after 484 rPFS events (212/492 [44%], apalutamide plus abiraterone-prednisone; 272/490 [56%], abiraterone-prednisone). Median rPFS was extended by 6 months with apalutamide plus abiraterone-prednisone versus abiraterone-prednisone (22·6 (95% CI 19·5–27·4) vs 16·6 (95% CI 13·9–19·3) months; HR 0·69 [95% CI 0·58–0·83]; p<0·0001) (figure 2A). The proportional hazards assumption was clearly met for the primary endpoint of rPFS through visual inspection. With long-term follow-up after 587 rPFS events (257/492 [52%], apalutamide plus abiraterone-prednisone; 330/490 [67%], abiraterone-prednisone), results were consistent, with a 7·4-month difference in median rPFS between treatments (24·0 (95% CI 19·7–27·5) vs 16·6 (95% CI 13·9–19·3) months; HR 0·70 [95% CI 0·60–0·83]; p<0·0001) (figure 2A).
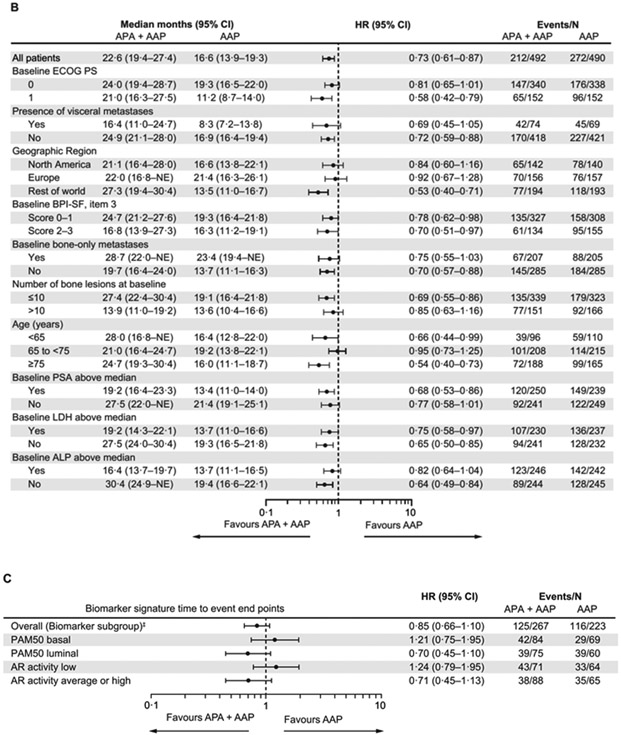
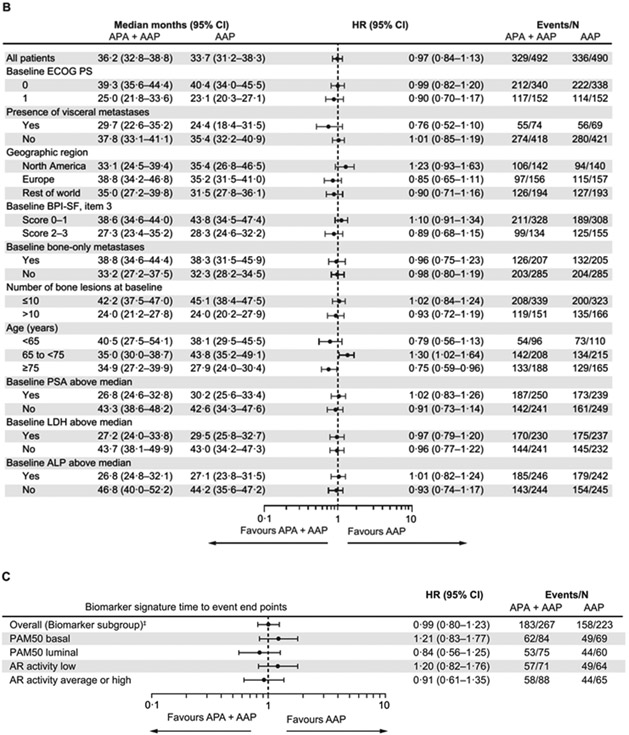
Figure 2: (A) Kaplan-Meier estimates of rPFS (primary end point analysis and updated rPFS at final analysis of OS; intention-to-treat population), (B) Forest plot of rPFS by baseline patient characteristics for primary end point analysis (intention-to-treat population), and (C) rPFS by biomarkers for primary end point analysis (biomarker population).
A) At left, rPFS by investigator review (primary endpoint analysis per protocol, March 19, 2018 cutoff). Median duration of follow-up of 25·7 months (IQR 23·0–28·9) at the final analysis of primary endpoint. At right, updated rPFS by investigator review (at final analysis of overall survival, September 15, 2020 cutoff). Median duration of follow-up of 54·8 months (IQR 51·5–58·4) at the final analysis.
*Stratified proportional hazards model (hazard ratio <1 favours apalutamide + abiraterone-prednisone). †Log-rank test stratified by the presence or absence of visceral metastases at screening, ECOG PS grade of 0 or 1 at screening, and region (Europe/United Kingdom, North America, and rest of world). ‡Total biomarker population based on protocol definition (at least one biomarker sample available).
AAP=abiraterone acetate plus prednisone. ALP=alkaline phosphatase. APA=apalutamide. AR=androgen receptor. BPI-SF=Brief Pain Inventory-Short Form. ECOG-PS=Eastern Cooperative Oncology Group performance status. IQR=interquatile range. LDH=lactate dehydrogenase. OS=overall survival. PSA=prostate-specific antigen. rPFS, radiographic progression-free survival.
A BICR, based on the primary analysis (cutoff March 19, 2018), showed 75% (741/982) concordance in radiographic progression determination with the investigator review, with high positive correlation coefficients in both treatment groups (r=0·839 for apalutamide plus abiraterone-prednisone and r=0·801 for abiraterone-prednisone) between the BICR- and investigator-assessed rPFS. The HR based on BICR-assessed rPFS of 0·86 (95% CI 0·72–1·0) favoured apalutamide plus abiraterone-prednisone versus abiraterone-prednisone. In the exploratory sensitivity analysis for the BICR assessment of rPFS, there were 45 patient cases in the abiraterone-prednisone group identified by the investigators as an rPFS event but not by the BICR assessment because of lack of scans beyond 60 days of investigator-assessed rPFS event and before subsequent therapy (informative censoring in BICR analysis). When these events were added to the abiraterone-prednisone arm of the central review results, the HR of 0·74 (95% CI 0·62–0·89) was consistent with the investigator-assessed rPFS results.
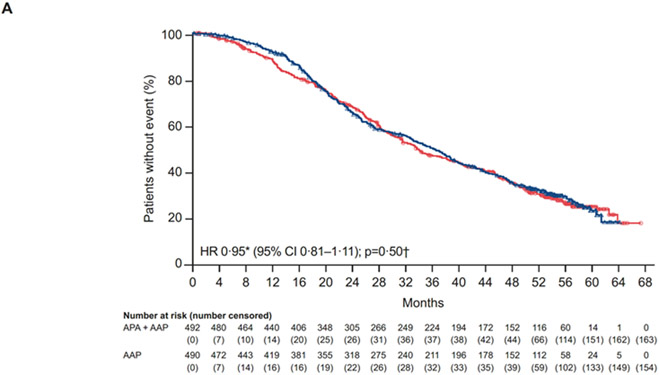
At the final analysis, after 665 deaths (329/492 [70%], apalutamide plus abiraterone-prednisone; 336/490 [69%], abiraterone-prednisone), the median OS was 36·2 (95% CI 32·8–38·8) months with apalutamide plus abiraterone-prednisone and 33·7 (95% CI 31·2–38·3) months with abiraterone-prednisone; this difference did not attain statistical significance (HR 0·95 [95% CI 0·81–1·11]; p=0·50 [figure 3A, table 2]). Time to initiation of cytotoxic chemotherapy, time to chronic opioid use, and time to pain progression were also not significantly different between treatments (table 2).
Figure 3: (A) Kaplan-Meier estimates of OS (intention-to-treat population), (B) Forest plot of OS by baseline patient characteristics (intention-to-treat population), (C) Forest plot of OS by biomarkers (biomarker population).
*Stratified proportional hazards model (hazard ratio <1 favours apalutamide + abiraterone-prednisone). †Log-rank test stratified by the presence or absence of visceral metastases at screening, ECOG PS grade of 0 or 1 at screening, and region (Europe/United Kingdom, North America, and rest of world). ‡Total biomarker population based on protocol definition (at least one biomarker sample available).
AAP=abiraterone acetate plus prednisone. ALP=alkaline phosphatase. APA=apalutamide. AR=androgen receptor. BPI-SF=Brief Pain Inventory-Short Form. ECOG-PS=Eastern Cooperative Oncology Group performance status. LDH=lactate dehydrogenase. OS=overall survival. PSA=prostate-specific antigen.
Table 2:
Prespecified secondary and exploratory efficacy endpoints
| Endpoint | Median months (95% CI) | HR or RR (95% CI) |
p Value | Events, n | ||
|---|---|---|---|---|---|---|
| Apalutamide + abiraterone- prednisone (n=492) |
Abiraterone- prednisone (n=490) |
Apalutamide + abiraterone- prednisone (n=492) |
Abiraterone- prednisone (n=490) |
|||
| Secondary endpoints | ||||||
| Overall survival | 36·2 (32·8–38·8) | 33·7 (31·2–38·3) | 0·95 (0·81–1·11)* | 0·50† | 329 | 336 |
| Time to initiation of cytotoxic chemotherapy | 36·1 (32·2–42·6) | 34·2 (29·5–39·2) | 0·94 (0·78–1·13)* | 0·51† | 231 | 239 |
| Time to chronic opioid use | 47·0 (39·2–NE) | 53·3 (42·0–NE) | 1·07 (0·87–1·32)* | 0·50† | 192 | 182 |
| Time to pain progression | 21·8 (18·0–25·7) | 26·5 (22·6–29·5) | 1·12 (0·95–1·33)* | 0·19† | 286 | 271 |
| Exploratory and post hoc endpoints | ||||||
| Time to PSA progression | 13·8 (12·0–15·6) | 12·0 (10·2–13·8) | 0·87 (0·74–1·02)* | 0·076† | 325 | 340 |
| Time to clinical progression | 16·0 (14·3–17·3) | 18·1 (16·4–19·8) | 1·10 (0·96–1·27)* | 0·18† | 398 | 389 |
| Time to first subsequent anticancer therapy | 25·6 (22·6–28·6) | 23·5 (20·4–27·3) | 0·96 (0·82–1·13)* | 0·63† | 308 | 310 |
| Time to second progression-free survival | 31·8 (28·4–36·9) | 30·2 (27·2–34·9) | 0·92 (0·78–1·08)* | 0·31† | 288 | 290 |
| Undetectable PSA (<0·2 ng/mL) at any time during treatment, n (%) | 121 (25%) | 94 (19% | 1·28 (1·01–1·62)‡ | 0·040§ | – | – |
| Confirmed decline of ≥50% in PSA level, n (%) | 391 (79% | 357 (73%) | 1·09 (1·02–1·17)‡ | 0·015§ | – | – |
| Decline of ≥90% in PSA level, n (%) | 262 (53%) | 238 (49% | 1·10 (0·97–1·24)‡ | 0·14§ | – | – |
| Objective response rate (RECIST 1·1), n (%) | n=187 | n=162 | ||||
| Number of patients with measurable disease at baseline | ||||||
| Responder (complete response or partial response)§§ | 109 (58%) | 86 (53%) | 1·10 (0·91–1·33)‡ | 0·33¶ | – | – |
| Non-responder | 78 (42%) | 76 (47%) | ||||
Intent-to-treat population. RECIST=Response Evaluation Criteria In Solid Tumors. NE=not estimable; RR=relative response.
Stratified proportional hazards model (HR <1 favours apalutamide + abiraterone-prednisone).
Log-rank test stratified by ECOG PS at screening (0, 1), presence of visceral metastases at screening (yes, no), and geographic region (North America, European Union, rest of world).
Relative response (>1 favours apalutamide + abiraterone-prednisone).
Stratified Cochran–Mantel–Haenszel test.
Complete response and partial response did not have to be confirmed.
Chi-square test.
A benefit was seen with apalutamide plus abiraterone-prednisone versus abiraterone-prednisone in the proportion of patients who had a confirmed decline in PSA of ≥50% (relative response [RR] 1·09 [95% CI 1·02–1·17]; p=0·015), a decline in PSA of ≥90% that was not statistically significant (RR 1·10 [95% CI 0·97–1·24]; p=0·14), and in the proportion who reached undetectable PSA level of <0·2 ng/mL nadir (RR 1·28 [95% CI 1·01–1·62]; p=0·040) (table 2). Median time to PSA progression was longer with the combination versus abiraterone-prednisone alone (13·8 (95% CI 12·0–15·6) vs 12.0 (95% CI 10·2–13·8) months; HR 0·87 [95% CI 0·74–1·02]; p=0·076) (table 2). Objective response rate was numerically higher with the combination versus abiraterone-prednisone (table 2).
Health-related quality of life (HRQoL) was maintained in patients in both treatment groups. Differences between treatment groups in least squares mean change from baseline FACT-P total scores were statistically significant in cycles 2–9 and 15. However, these differences were less than the smallest recommended minimally important difference (MID) threshold of 6 points and are therefore not considered clinically meaningful (appendix p 4 and 34). With the smallest recommended MID threshold of 6 points, both groups declined in FACT-P total scores starting in cycle 33 (with no differences between the groups). Median time to deterioration (95% CI) for the FACT-P total was 12·9 (9·0–15·7) months for apalutamide plus abiraterone-prednisone and 15·7 (12·8–18·4) months for abiraterone-prednisone (HR 1·16 [95% CI 0·94–1·42]; p=0·16) (appendix p 35).
For rPFS and OS, the treatment effects in the prespecified baseline characteristic subgroups are shown in figure 2B and figure 3B. Patients in the prespecified clinical subgroup of those aged ≥75 years experienced a consistent benefit in rPFS and OS with the combination versus abiraterone-prednisone (≥75 years subgroup: rPFS-HR 0·54 [95% CI 0·40–0·73], and OS-HR 0·75 [95% CI, 0·59–0·96]. In the prespecified clinical subgroup of patients with visceral metastases the HR was 0·69 [95% CI 0·45–1·05] for rPFS and 0·76 [95% CI 0·52–1·10] for OS [figures 2B, 3B, appendix p 5]). The interaction between age and treatment effect was significant for rPFS (p=0·020) and OS (p=0·0027); however, interaction between visceral disease and treatment effect for rPFS (p=0·83) and OS (p=0·17) was not, most likely due to the small sample and event size. Post hoc analyses of time to initiation of cytotoxic chemotherapy, time to chronic opioid use, and time to pain progression in these subgroups are shown on appendix p 5.
Analyses of prespecified biomarker subgroups based on molecular signatures in a subset of the overall biomarker population (n=490) that had archived tissue biopsies available (n=288) are shown in figures 2C and 3C. The PAM50 luminal and AR signalling activity average/high subgroups were 78% (105/135) mutually overlapping.
Overall incidence of any TEAE and serious TEAEs was similar between the combination and abiraterone-prednisone groups (table 3). All grade 1–2 events with an incidence of ≥10% in either treatment group and all grade 3–5 events with an incidence of ≥2% in either treatment group are shown in table 3; the full list of grade 3–5 events is on appendix p 6. Serious AEs and drug-related serious AEs are listed on appendix p 13 and 18.
Table 3:
Treatment-emergent adverse events
| Apalutamide + abiraterone-prednisone (n=490) |
Abiraterone-prednisone (n=489) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Graded TEAEs | Grade 1-2 | Grade 3 | Grade 4 | Grade 5 | Grade 1-2 | Grade 3 | Grade 4 | Grade 5 |
| Any TEAE | 173 (35%) | 268 (55%) | 26 (5%) | 17 (3%) | 187 (38%) | 214 (44%) | 36 (7%) | 37 (8%) |
| Drug-related TEAEs | 228 (47%) | 132 (27%) | 6 (1%) | 3 (1%) | 228 (47%) | 93 (19%) | 6 (1%) | 5 (1%) |
| Serious TEAEs | 9 (2%) | 151 (31%) | 18 (4%) | 17 (3%) | 11 (2%) | 110 (22%) | 22 (4%) | 37 (8%) |
| Drug-related serious TEAEs | 1 (<1%) | 23 (5%) | 3 (1%) | 3 (1%) | 5 (1%) | 18 (4%) | 3 (1%) | 5 (1%) |
| TEAEs leading to discontinuation of the trial regimen | 28 (6%) | 38 (8%) | 8 (2%) | 9 (2%) | 7 (1%) | 22 (4%) | 9 (2%) | 23 (5%) |
| Drug-related TEAE leading to discontinuation of the trial regimen | 21 (4%) | 19 (4%) | 1 (<1%) | 2 (<1%) | 2 (<1%) | 4 (1%) | 2 (<1%) | 3 (1%) |
| TEAEs associated with death | 17 (3%) | 37 (8%) | ||||||
| Any TEAE* | ||||||||
| Fatigue | 150 (31%) | 15 (3%) | 0 | 0 | 122 (25%) | 12 (2%) | 0 | 0 |
| Back pain | 140 (29%) | 16 (3%) | 0 | 0 | 117 (24%) | 17 (3%) | 0 | 0 |
| Hypertension | 62 (13%) | 82 (17%) | 0 | 0 | 73 (15%) | 49 (10%) | 0 | 0 |
| Weight decreased | 128 (26%) | 8 (2%) | 0 | 0 | 78 (16%) | 6 (1%) | 0 | 0 |
| Arthralgia | 114 (23%) | 14 (3%) | 0 | 0 | 115 (24%) | 6 (1%) | 0 | 0 |
| Fall | 90 (18%) | 16 (3%) | 0 | 0 | 90 (18%) | 3 (1%) | 0 | 0 |
| Constipation | 96 (20%) | 0 | 0 | 0 | 94 (19%) | 2 (< 1%) | 0 | 0 |
| Diarrhoea | 85 (17%) | 7 (1%) | 0 | 0 | 71 (15%) | 3 (1%) | 0 | 0 |
| Nausea | 76 (16%) | 8 (2%) | 0 | 0 | 72 (15%) | 5 (1%) | 0 | 0 |
| Pain in extremity | 72 (15%) | 9 (2%) | 0 | 0 | 56 (11%) | 1 (< 1%) | 0 | 0 |
| Headache | 75 (15%) | 4 (1%) | 0 | 0 | 61 (12%) | 2 (< 1%) | 0 | 0 |
| Hypokalemia | 62 (13%) | 14 (3%) | 3 (1%) | 0 | 54 (11%) | 17 (3%) | 3 (1%) | 0 |
| Oedema peripheral | 76 (16%) | 0 | 0 | 0 | 70 (14%) | 3 (1%) | 0 | 0 |
| Hot flush | 74 (15%) | 0 | 0 | 0 | 56 (11%) | 0 | 0 | 0 |
| Decreased appetite | 69 (14%) | 3 (1%) | 0 | 0 | 52 (11%) | 8 (2%) | 1 (<1%) | 0 |
| Anaemia | 53 (11%) | 17 (3%) | 0 | 0 | 55 (11%) | 24 (5%) | 0 | 0 |
| Asthaenia | 57 (12%) | 9 (2%) | 0 | 0 | 58 (12%) | 7 (1%) | 0 | 0 |
| Dizziness | 58 (12%) | 3 (1%) | 0 | 0 | 46 (9%) | 0 | 0 | 0 |
| Cough | 53 (11%) | 0 | 0 | 0 | 66 (13%) | 1 (<1%) | 0 | 0 |
| Nasopharyngitis | 51 (10%) | 0 | 0 | 0 | 57 (12%) | 0 | 0 | 0 |
| Musculoskeletal pain | 49 (10%) | 1 (<1%) | 0 | 0 | 43 (9%) | 3 (1%) | 0 | 0 |
| Musculoskeletal chest pain | 47 (10%) | 1 (<1%) | 0 | 0 | 50 (10%) | 0 | 0 | 0 |
| Haematuria | 42 (9%) | 4 (1%) | 1 (<1%) | 0 | 38 (8%) | 13 (3%) | 1 (<1%) | 0 |
| Rash | 36 (7%) | 9 (2%) | 0 | 0 | 23 (5%) | 1 (<1%) | 0 | 0 |
| Urinary tract infection | 28 (6%) | 15 (3%) | 0 | 0 | 43 (9%) | 10 (2%) | 1 (<1%) | 0 |
| Bone pain | 29 (6%) | 6 (1%) | 0 | 0 | 38 (8%) | 12 (2%) | 0 | 0 |
| Contusion | 34 (7%) | 0 | 0 | 0 | 49 (10%) | 0 | 0 | 0 |
| Pneumonia | 11 (2%) | 17 (3%) | 1 (<1%) | 2 (<1%) | 13 (3%) | 9 (2%) | 0 | 1 (<1%) |
| Hyperglycaemia | 19 (4%) | 9 (2%) | 1 (<1%) | 0 | 32 (7%) | 3 (1%) | 3 (1%) | 0 |
| Blood pressure increased | 5 (1%) | 21 (4%) | 0 | 0 | 0 | 17 (3%) | 0 | 0 |
| Rash maculo-papular | 14 (3%) | 9 (2%) | 0 | 0 | 3 (1%) | 0 | 0 | 0 |
| Aspartate aminotransferase increased | 19 (4%) | 2 (<1%) | 0 | 0 | 37 (8%) | 15 (3%) | 1 (<1%) | 0 |
| Alanine aminotransferase increased | 16 (3%) | 4 (1%) | 0 | 0 | 22 (4%) | 32 (7%) | 3 (1%) | 0 |
| Blood alkaline phosphatase increased | 19 (4%) | 0 | 0 | 0 | 26 (5%) | 8 (2%) | 0 | 0 |
| Cataract | 7 (1%) | 10 (2%) | 0 | 0 | 12 (2%) | 7 (1%) | 0 | 0 |
| Syncope | 0 | 17 (3%) | 0 | 0 | 0 | 12 (2%) | 0 | 0 |
| Neutropenia | 9 (2%) | 4 (1%) | 3 (1%) | 0 | 7 (1%) | 5 (1%) | 6 (1%) | 0 |
| Acute kidney injury | 5 (1%) | 6 (1%) | 0 | 1 (<1%) | 4 (1%) | 7 (1%) | 1 (<1%) | 1 (<1%) |
| Hydronephrosis | 1 (<1%) | 10 (2%) | 1 (<1%) | 0 | 2 (<1%) | 6 (1%) | 0 | 0 |
| Sepsis | 0 | 1 (<1%) | 5 (1%) | 1 (<1%) | 1 (1<%) | 0 | 9 (2%) | 0 |
| Hyponatraemia | 4 (1%) | 2 (<1%) | 0 | 0 | 8 (2%) | 7 (1%) | 2 (<1%) | 0 |
| Pulmonary embolism | 0 | 2 (<1%) | 0 | 2 (<1%) | 1 (<1%) | 7 (1%) | 2 (<1%) | 1 (<1%) |
| TEAEs of special interest (grouped term) | ||||||||
| Hypertension | 57 (12%) | 100 (20%) | 1 (<1%) | 0 | 69 (14%) | 61 (12%) | 0 | 0 |
| Fall | 90 (18%) | 16 (3%) | 0 | 0 | 90 (18%) | 3 (1%) | 0 | 0 |
| Skin rash | 79 (16%) | 21 (4%) | 1 (<1%) | 0 | 47 (10%) | 2 (<1%) | 0 | 0 |
| Cardiac disorders† | 43 (9%) | 38 (8%) | 6 (1%) | 6 (1%) | 48 (10%) | 26 (5%) | 2 (<1%) | 18 (4%) |
| Hypokalemia‡ | 62 (13%) | 14 (3%) | 3 (1%) | 0 | 54 (11%) | 17 (3%) | 3 (1%) | 0 |
| Peripheral oedema | 90 (18%) | 1 (<1%) | 0 | 0 | 89 (18%) | 4 (1%) | 0 | 0 |
| Fracture (excluding bone metastasis–related fractures %) and osteoporosis | 54 (11%) | 20 (4%) | 0 | 0 | 52 (11%) | 7 (1%) | 0 | 0 |
| Ischaemic cerebrovascular disorders | 5 (1%) | 3 (1%) | 0 | 1 (<1%) | 7 (1%) | 2 (<1%) | 4 (1%) | 1 (<1%) |
| Seizures | 2 (<1%) | 1 (<1%) | 0 | 0 | 0 | 0 | 0 | 1 (<1%) |
Safety population. Values are n (%). All grade 3–5 events are listed in the appendix (p 6). TEAEs are those that occurred between the date of first dose of study drug and date of last dose of study drug plus 30 days. An AE is categorized as drug-related if assessed by the investigator as possibly, probably, and very likely related to study drug. Patients are counted only once for any given event, regardless of the number of times they actually experienced the event. The event experienced by the patient with the worst toxicity grade is used. If a patient had all AEs with missing toxicity grades, the patient is not counted. AEs are coded using Medical Dictionary for Regulatory Activities Version 23.0. Toxicity grade is based on National Cancer Institute common toxicity criteria, version 4.03.
AE=adverse event. TEAE=treatment-emergent adverse event.
All grade 1–2 events with an incidence of ≥10% in either treatment group and all grade 3–5 events with an incidence of ≥2% in either treatment group are shown.
See appendix p 20.
Includes only one term.
Grade 3 or 4 TEAEs, driven mainly by hypertension, were reported in 60% (294/490) of patients receiving apalutamide plus abiraterone-prednisone versus 51% (250/489) receiving abiraterone-prednisone; grade 4 TEAEs accounted for 5% (26/490) and 7% (36/489), respectively (table 3 and appendix p 6). Prior to study entry, 61% (301/490) of patients receiving apalutamide plus abiraterone-prednisone and 58% (283/489) of patients receiving abiraterone-prednisone had a history of hypertension. Grade 3 or 4 fatigue occurred in 3% (15/490) and 2% (12/289) of patients in apalutamide plus abiraterone-prednisone and abiraterone-prednisone groups, respectively. TEAEs of special interest (grouped terms) for apalutamide plus abiraterone-prednisone and abiraterone-prednisone included hypertension, fall, skin rash, cardiac disorder, hypokalemia, peripheral oedema, and fracture and osteoporosis (table 3 and appendix p 20).
Cardiac disorders occurred in both arms with similar frequency, 19% (93/490) for apalutamide plus abiraterone-prednisone and 19% (94/489) for abiraterone-prednisone (table 3); cardiac events leading to death occurred in 1% (6/490) and 3% (13/489), respectively (appendix p 22). Of note, medical history of cardiac disorders (apalutamide plus abiraterone-prednisone, 29% [141/490]; abiraterone-prednisone 33% [159/489]) and the presence of a medical history of cardiac events, stroke, or pulmonary embolism or risk factors for cardiac disorders such as diabetes (apalutamide plus abiraterone-prednisone, 77% [379/490]; abiraterone-prednisone 78% [380/489]) were frequent in both treatment groups. With both treatments, TEAEs of cardiac disorders occurred more frequently in patients with an intermediate or high versus low Framingham Risk Score and were similar between treatments (see appendix p 23). Grade 3 or 4 TEAEs leading to treatment discontinuation (9% [46/490], apalutamide plus abiraterone-prednisone; 6% [31/489], abiraterone-prednisone) were driven primarily by grade 3 rash/rash-like disorders (skin and subcutaneous tissue disorders, 2% [8/490] vs <1% [1/489]) and hypertension (1% [5/490] vs 0% [0/489]; table 3 and appendix p 24). Drug-related TEAEs leading to treatment discontinuation, TEAEs leading to dose reduction/interruption, and dose adjustments are described on appendix p 27, 29, and 33, respectively.
TEAEs associated with death occurred in 3% (17/490) of patients treated with apalutamide plus abiraterone-prednisone and in 8% (37/489) treated with abiraterone-prednisone (table 3 and appendix p 22). Drug-related TEAEs associated with death occurred in 1% (3/490) of patients treated with apalutamide plus abiraterone-prednisone (n=2 pulmonary embolism, n=1 cardiac failure) and in 1% (5/489) treated with abiraterone-prednisone (n=1 cardiac failure, n=1 cardiac arrest, n=1 mesenteric arterial occlusion, n=1 seizure, n=1 sudden death; table 3 and appendix p 22).
Grade 3 or 4 laboratory abnormalities occurring in ≥5% of patients with apalutamide plus abiraterone-prednisone or abiraterone-prednisone were elevated alanine aminotransferase (1% [4/488] vs 8% [38/488]) and elevated alkaline phosphatase (7% [36/488] vs 10% [49/488]).
Discussion
This is the first phase 3 combination study of two active treatments to meet its primary endpoint in CRPC, to our knowledge. In the primary analysis (cutoff March 19, 2018), ACIS met its primary endpoint of rPFS; however, based on IDMC recommendation, the study blind was continued until final analysis for OS. Treatment with apalutamide plus abiraterone-prednisone led to a significant improvement in the primary endpoint of rPFS at the primary analysis (median follow-up, 25·7 months), extending median rPFS by 6 months compared with abiraterone-prednisone (HR 0·69 [95% CI 0·58–0·83]; p<0·0001). At the final analysis, with ~4·5 years of follow-up, the significant rPFS benefit observed with the combination over abiraterone-prednisone persisted, with median rPFS extended by 7·4 months versus abiraterone-prednisone (HR 0·70 [95% CI 0·60–0·83]; p<0·0001), while maintaining quality of life. The secondary endpoints of OS, time to initiation of cytotoxic chemotherapy, time to chronic opioid use, and time to pain progression did not attain statistical significance. Safety was consistent with the previously reported safety profile of the individual drugs.
The rPFS benefit was apparent and was attained versus an active comparator (ie, abiraterone-prednisone). The median rPFS observed with abiraterone-prednisone (16·6 months) is consistent with results for the abiraterone-prednisone arm in the COU-AA-302 (with ongoing ADT) and IPATential150 studies, which both investigated a similar patient population, chemotherapy-naive patients with mCRPC (16·5 months for both studies).7,31 Thus, the addition of abiraterone and prednisone to ADT in COU-AA-302 added 8.2 months’ rPFS,7 and the further addition of apalutamide to abiraterone-prednisone added an extra 6 months’ rPFS in ACIS. The addition of apalutamide to abiraterone-prednisone versus abiraterone-prednisone alone resulted in a significantly higher rate of confirmed ≥50% PSA decline and undetectable PSA nadir. Notably, this translated to only a marginal delay in time to PSA progression, possibly owing, at least in part, to the Prostate Cancer Working Group-2 definition of PSA progression used in ACIS for confirmation of PSA progression.
The concordance between BICR and investigator (local) review of radiographic progressive disease was >75%, with a high positive correlation coefficient for both treatments. The HR of 0·86 (95% CI 0·72–1·0) based on BICR-assessed rPFS favoured apalutamide plus abiraterone-prednisone versus abiraterone-prednisone. The discordance (~25%) can likely be explained by methodological aspects and limitations inherent to retrospective BICR, including but not limited to the lack of additional scans after investigator-assessed radiographic progressive disease and absence of clinical information for BICR assessment. For exploratory sensitivity analysis of the BICR assessment of rPFS where events in the abiraterone-prednisone group identified by the investigators as an rPFS event but not by the BICR assessment were added to the abiraterone-prednisone arm of the central review results, the HR was consistent with the investigator-assessed rPFS results.
The extended rPFS with apalutamide plus abiraterone-prednisone versus abiraterone-prednisone in ACIS is corroborated by previous findings with other combination therapies for mCRPC. In the Alliance phase 3 study (A031201) for progressive mCRPC, median rPFS was improved by 2·8 months with enzalutamide plus abiraterone acetate plus prednisone (hereafter, enzalutamide plus abiraterone-prednisone) versus enzalutamide alone (HR 0·88; p=0·05).32 In the phase 2 CHEIRON study, enzalutamide plus docetaxel significantly improved PFS in mCRPC by 1·0 month at a median follow-up of 24 months versus docetaxel alone (p=0·01).33
The median OS, a secondary endpoint in ACIS, surpassed 3 years with apalutamide plus abiraterone-prednisone versus abiraterone-prednisone (36·2 vs 33·7 months, respectively), which, however, did not attain statistical significance. Approximately two thirds of the patients who discontinued treatment received subsequent life-prolonging therapy, not including the study therapies, abiraterone-prednisone and apalutamide. Also, currently, the phase 3 studies that have shown a survival advantage have used a single active therapy plus ADT compared with ADT alone. No combination of two active therapies assessed in mCRPC clinical trials has shown a statistically significant OS benefit to date,32-35 and this may be due partly to the number of active life-prolonging treatments available to patients following first-line AR-targeted treatment for mCRPC and the uncertainties regarding optimal sequencing. This underscores the importance of intermediary endpoints such as rPFS, which potentially defer the need for subsequent interventions.
The apalutamide plus abiraterone-prednisone combination was well tolerated, and no new safety signals were reported. Frequencies of TEAEs leading to permanent treatment discontinuation were marginally different between treatment groups (17% vs 12%, apalutamide plus abiraterone-prednisone vs abiraterone-prednisone, respectively). In the aforementioned Alliance study, the rate of discontinuation due to AEs with enzalutamide plus abiraterone-prednisone was higher than with enzalutamide (12% vs 5%).32 Also, in ACIS, although the incidence of grade 3/4 cardiac disorders was higher in the combination arm, this did not translate into a greater number of cardiac-related deaths.
The delay in disease progression observed with apalutamide plus abiraterone was not at the expense of exacerbated AEs from either therapy and the safety profile was generally consistent with that reported for the individual drugs.5,14 Increased rates of treatment toxicities have been reported with combination treatments compared with their respective controls.32-36 Some elevated toxicities (all-grade and/or grade 3–4) noted with different combinations of drugs approved for mCRPC versus their study comparator include anxiety,34 decrease in neutrophil count,36 fatigue,34,36 hypertension,36 neutropenia,33 and syncope.36 In the current study, the TEAEs that were more common with combination treatment versus abiraterone-prednisone included the grade 3 or 4: rash (based on skin and subcutaneous tissue disorders), pain in extremity, and hypertension and the grade 1-2 TEAEs back pain, fatigue, weight loss.
Time to chronic opioid use and time to pain progression were shorter in the combination group. A similar observation regarding chronic opioid use was made in the PLATO study, in which patients with chemotherapy-naive mCRPC received open-label enzalutamide until PSA progression, followed by either enzalutamide plus abiraterone and prednisone or abiraterone and prednisone.37 In PLATO, the rates of chronic opioid use owing to unequivocal clinical progression were higher in the combination group (11·1% vs 4·8%) despite the rPFS advantage (10·0 months with the combination vs 7·0 months with abiraterone alone; HR 0·67; p=0·02).37 Prednisone when given together with abiraterone acetate, besides balancing the mineralocorticoid excess side effect of abiraterone, may have additional therapeutic benefit. The observations on time to chronic opioid use and time to pain progression reported in ACIS could be related, at least in part, to a potential lower exposure to prednisone upon addition of apalutamide to abiraterone as this combination previously demonstrated a drug-drug interaction that affected systemic prednisone exposure.22
The extent of individual patient response to mCRPC treatment varies greatly. One approach to optimise treatment is to identify patient subgroups that are more likely to benefit. rPFS and OS outcomes in a difficult to treat subgroup,38 patients aged ≥75 years, signified possible favourable results with apalutamide and abiraterone-prednisone versus abiraterone-prednisone alone. The clinical and molecular subgroups were analysed, with low patient numbers in the current study, and need further exploration to determine clinical benefit with the apalutamide plus abiraterone-prednisone combination.
The tolerability of the combination treatment was also reflected in the maintained HRQoL, evidenced by no clinically meaningful change in FACT-P total score from baseline and no clinically meaningful differences between treatment groups.39 Maintenance of HRQoL with the addition of apalutamide is especially relevant in those subgroups that derived the greatest benefit from the combination therapy and are more likely to be vulnerable (aged ≥75 years) or have significant disease burden (visceral disease).
Study limitations include interpreting OS benefit given subsequent use of multiple lines of treatment with overlapping mechanisms of action. The patient size for biomarker subgroup analyses was limited due to additional patient consent requirements, tumour tissue availability, and sample quality; biomarker studies with a larger patient population are needed to confirm the findings. Additionally, the study attempted to recruit a diverse population (~75% White, ~11% Asian, ~14% other races or not reported/unknown) across different geographic regions and was well matched across the treatment groups. Although disease disparity occurs, outcomes were not analysed according to race due to limited patient numbers. Lastly, the study design did not allow for the evaluation of treatment sequences such as upfront treatment with the combination of apalutamide plus abiraterone-prednisone versus their sequential use. However, based on prior literature,40,41 resistance has been hypothesized to occur when androgen signalling inhibitors are used in sequence for mCRPC treatment, based on mutations and AR splice variants induced by first-line treatment with the androgen signalling inhibitor enzalutamide. In consideration of such potential concern of sequencing of two androgen signalling inhibitors, the results of the rPFS advantage shown with the combination of apalutamide plus abiraterone-prednisone may be informative for therapeutic strategies in mCRPC. This requires further exploration in future research.
In conclusion, ACIS is the first study with a combination of two active therapies in mCRPC to meet its primary endpoint, to our knowledge. Despite comparison against an active therapy and the use as first-line treatment, the apalutamide plus abiraterone-prednisone combination consistently improved rPFS in chemotherapy-naive mCRPC patients versus abiraterone-prednisone while maintaining quality of life. As survival benefit is limited with non-targeted therapies in mCRPC, we aimed to identify subgroups of patients who might benefit from therapy, such as those at an older age. Future studies will further refine the treatment of men with advanced prostate cancer, especially in view of use of these agents in mCSPC and non-metastatic CRPC.
Supplementary Material
RESEARCH IN CONTEXT.
Evidence before this study
Despite recent progress with newer therapies, the majority of patients with metastatic castration-resistant prostate cancer (mCRPC) will experience disease progression of a uniformly fatal disease. Current standard of care for mCRPC comprises therapies that target a single androgen signalling mechanism with ongoing androgen deprivation therapy.
We searched PubMed for published clinical trials of combination therapies that both suppress androgen signalling, without search restrictions, from inception until May 6, 2021, using the search string ("metastatic castration-resistant prostate cancer" OR "mCRPC" OR "metastatic CRPC") AND (("androgen") AND ("receptor" OR "signalling" OR "hormon*") AND ("inhibitor" OR "antagonist")) AND (combination). Preliminary evidence suggests that combining therapies that suppress the androgen signalling axis in different ways may delay resistance and improve outcomes in patients with mCRPC. The combination of apalutamide, an androgen receptor inhibitor, and abiraterone acetate plus prednisone, which suppresses androgen biosynthesis, has not been studied extensively in mCRPC except for a phase 1b study which demonstrated antitumour activity with a good tolerability profile. The combination of abiraterone acetate plus prednisone and dutasteride, which also suppresses androgen receptor signalling, was studied in a phase 2 study in which complete suppression of androgen signalling axis was not achieved; however, the comparative efficacy of the dual therapy could not be evaluated due to the single-arm design. Early, single-centre, combination studies of enzalutamide, an androgen receptor inhibitor, and abiraterone acetate plus prednisone/prednisolone suggested improvement in prostate-specific antigen response compared with individual therapies; however, dissemination of these studies has been limited to congress abstracts. The simultaneous multi-modal blockage of androgen axis signalling through the combination of apalutamide and abiraterone acetate (with prednisone and androgen deprivation therapy) represents the first androgen annihilation therapy for prostate cancer, to our knowledge.
Added value of this study
We present the primary and final analyses results from the ACIS study on the combination of apalutamide and abiraterone acetate plus prednisone versus standard of care (abiraterone acetate plus prednisone) for mCRPC. This is the first phase 3 combination study of two active treatments to meet its primary endpoint in mCRPC, to our knowledge. Safety was consistent with the previously reported safety profile of the individual drugs and patient-reported health-related quality of life was maintained.
Implications of all the available evidence
This analysis of the ACIS study demonstrates that apalutamide in combination with abiraterone acetate plus prednisone significantly extended rPFS versus abiraterone-prednisone, while maintaining quality of life in chemotherapy-naive mCRPC patients. The ACIS study additionally highlighted potential long-term clinical benefits associated with the combination of apalutamide and abiraterone acetate plus prednisone in clinical subgroups of patients aged ≥75 years, those with visceral disease, and those with specific hormone-responsive molecular signatures at baseline. However, subgroup results signify a possible trend and require further exploration.
Acknowledgements
This study was funded by Janssen Research & Development, LLC. We would like to thank the patients who participated in this study and their families, as well as the study teams at each participating institution. Editorial assistance was provided by Breanne Landry, PhD, of PAREXEL, and was funded by Janssen Global Services, LLC.
Footnotes
Declaration of interests
FS has received support for the present manuscript from Janssen; grants/contracts, consulting fees, and payment/honoraria for lectures, presentations, speakers bureaus, and manuscript writing or educational events from Astellas, AstraZeneca, Bayer, BMS, Janssen, Myovant, Novartis, and Sanofi.
EE has received support for the present manuscript from Janssen; grants/contracts from Astellas, AstraZeneca, Janssen, Merck, Oric, and Sanofi; consulting fees from Astellas, AstraZeneca, Bayer, Janssen, Merck, Pfizer, and Sanofi; payment/honoraria for lectures, presentations, speakers bureaus, and manuscript writing or educational events from Bayer, Janssen, and Sanofi; and support for attending meetings and/or travel from Astellas, Janssen, and Sanofi. EE has participated in Data Safety Monitoring Board or Advisory Board meetings for AstraZeneca, Janssen, Merck, Pfizer, and Sanofi. EE reports leadership or fiduciary role in other board, society, committee, or advocacy group, paid or unpaid, for the European Society of Medical Oncology (ESMO).
GA has received support for the present manuscript from Janssen; grants/contracts from Astellas and Janssen; consulting fees from Astellas, Bayer, Beigene, Clovis, Janssen, Novartis, Pfizer, and Sanofi; payment/honoraria for lectures, presentations, speakers bureaus, and manuscript writing or educational events from Astellas, Bayer, Janssen, Novartis, and Sanofi; and support for attending meetings and/or travel from Astellas, Bayer, Janssen, Novartis, Pfizer, and Sanofi. GA has participated in Data Safety Monitoring Board or Advisory Board meetings for Astellas, Bayer, Janssen, Novartis, Pfizer, and Sanofi. GA has received royalties from Janssen to the Institute of Cancer Research and is included on the list of rewards to discoverers of abiraterone.
TWF has received grants/contracts from Agensys, Aragon Pharmaceuticals, Astellas Pharma, AstraZeneca/MedImmune, Bristol Myers Squibb, Bavarian Nordic, Dendreon, Exelixis, GTx, Janssen Oncology, La Roche-Posay, Lilly, Medivation, Merck, Novartis, Pfizer, Roche/Genentech, Sanofi, Sotio, Seagen, Seattle Genetics, and Tokai Pharmaceuticals; and consulting fees from Aurora Oncology, Janssen Oncology, and Seattle Genetics. TWF (The University of Colorado) has filed two related patents for which he is an inventor. These patents are related to early-stage bladder cancer treatment and detection. Neither are commercialized or in clinical trials at this time. TWF is the founder and a stockholder for Aurora Oncology.
OBG has received consulting fees from Bayer and Janssen; and payment/honoraria for lectures, presentations, speakers bureaus, and manuscript writing or educational events from Janssen.
SO has received consulting fees, payment/honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events, and support for attending meetings and/or travel from Astellas, AstraZeneca, Bayer, Bristol Myers Squibb, Ipsen, Janssen, Merck, Novartis, Pfizer, and Sanofi. SO participated in Data Safety Monitoring Board or Advisory Board meetings for Astellas, Janssen, Roche, and Sanofi.
TS has received consulting fees and payment/honoraria for lectures, presentations, speakers bureaus, and manuscript writing or educational events from Astellas Oncology, Bayer Health, Janssen, and Sanofi.
HS has received support for the present manuscript from Janssen; grants/contracts from Asahi Kasei, Bayer, Daiichi Sankyo, Kissei, Nippon Kayaku, Nippon Shinyaku, Ono, Sanofi, Taiho, and Takeda; consulting fees from Astellas, AstraZeneca, Bayer, Eli Lilly, Janssen, MSD, Nihon Medi-Physics, Roche/Chugai, and Sanofi; payment/honoraria for lectures, presentations, speakers bureaus, and manuscript writing or educational events from Astellas, AstraZeneca, Bayer, Janssen, Merck Biopharma, MSD, Nippon Shinyaku, Ono, Otsuka, Pfizer, Sanofi, and Takeda; and support for attending meetings and/or travel from Astellas, Bayer, Eli Lilly, Janssen, and Sanofi. HS participated in Advisory Board meetings for Astellas, Bayer, Janssen, Roche/Chugai, and Sanofi.
DW, KY, PDP, SB-M, SL, JL, ST, KBB, SDM, and SAM are employed by Janssen Research & Development, and hold stock in Johnson & Johnson.
DER has received support for the present manuscript from Janssen. DER reports uncompensated participation in advisory boards for AstraZeneca, Bayer, Genentech, Janssen, and Myovant.
FF declares no competing interests.
Prior Submission: Accepted for Submission as a Late-Breaking Abstract to Genitourinary Cancer Symposium 2021 as an Oral Abstract Session Presentation (Prostate Cancer, February 11, 2021). A post hoc analysis of the ACIS study was submitted to ASCO and was accepted for presentation as a Poster Session (June 4–8, 2021).
A complete list of investigators in the ACIS trial is provided on appendix p 36.
Contributor Information
Prof Fred Saad, Centre Hospitalier de l'Université de Montréal, Université de Montréal, Montréal, Québec, Canada.
Prof Eleni Efstathiou, Athens Medical Center, Dept of GU Oncology, Athens, Greece.
Prof Gerhardt Attard, University College London, London, UK.
Thomas W Flaig, University of Colorado Cancer Center, Aurora, Colorado, USA.
Fabio Franke, Oncosite/Hospital Unimed Noroeste - Ijuí, Brazil.
Oscar B Goodman, Jr, Comprehensive Cancer Centers of Nevada, US Oncology Network, Las Vegas, Nevada, USA.
Prof Stéphane Oudard, Georges Pompidou Hospital, University of Paris, Paris, France.
Prof Thomas Steuber, Martini-Klinik Prostate Cancer Center, University Hospital Hamburg-Eppendorf, Hamburg, Germany.
Prof Hiroyoshi Suzuki, Toho University Sakura Medical Center, Chiba, Japan.
Daphne Wu, Janssen Research & Development, Los Angeles, California, USA.
Kesav Yeruva, Janssen Research & Development, Los Angeles, California, USA.
Peter De Porre, Janssen Research & Development, Beerse, Belgium.
Prof Sabine Brookman-May, Janssen Research & Development, Los Angeles, California, USA; Ludwig Maximilians University, Munich, Germany.
Susan Li, Janssen Research & Development, Spring House, Pennsylvania, USA.
Jinhui Li, Janssen Research & Development, San Diego, California, USA.
Shibu Thomas, Janssen Research & Development, Spring House, Pennsylvania, USA.
Katherine B Bevans, Janssen Global Services LLC, Horsham, Pennsylvania.
Suneel D Mundle, Janssen Research & Development, Raritan, New Jersey, USA.
Sharon A McCarthy, Janssen Research & Development, Raritan, New Jersey, USA.
Dana E Rathkopf, Memorial Sloan Kettering Cancer Center and Weill Cornell Medicine, New York, New York, USA.
Data Sharing
The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparency. As noted on this site, requests for access to the study data can be submitted through Yale Open Data Access (YODA) Project site at http://yoda.yale.edu.
References
- 1.James ND, Spears MR, Clarke NW, et al. Survival with newly diagnosed metastatic prostate cancer in the "docetaxel era": data from 917 patients in the control arm of the STAMPEDE trial (MRC PR08, CRUK/06/019). Eur Urol 2015; 67: 1028–38. [DOI] [PubMed] [Google Scholar]
- 2.Schalken J, Fitzpatrick JM. Enzalutamide: targeting the androgen signalling pathway in metastatic castration-resistant prostate cancer. BJU Int 2016; 117: 215–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamaoka M, Hara T, Kusaka M. Overcoming persistent dependency on androgen signaling after progression to castration-resistant prostate cancer. Clin Cancer Res 2010; 16: 4319–24. [DOI] [PubMed] [Google Scholar]
- 4.Efstathiou E, Titus M, Tsavachidou D, et al. Effects of abiraterone acetate on androgen signaling in castrate-resistant prostate cancer in bone. J Clin Oncol 2012; 30: 637–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ryan CJ, Smith MR, Fizazi K, et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol 2015; 16: 152–60. [DOI] [PubMed] [Google Scholar]
- 6.de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med 2011; 364: 1995–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med 2013; 368: 138–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.ZYTIGA (aberaterone acetate) [prescribing information]. Janssen Pharmaceutical Companies, Horsham, PA; 2020. [Google Scholar]
- 9.European Medicines Agency. Summary of product characteristics: Zytiga (abiraterone). https://www.ema.europa.eu/en/medicines/human/EPAR/zytiga#authorisation-details-section (accessed June 13, 2021).
- 10.Fizazi K, Tran N, Fein L, et al. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med 2017; 377: 352–60. [DOI] [PubMed] [Google Scholar]
- 11.Romanel A, Gasi Tandefelt D, Conteduca V, et al. Plasma AR and abiraterone-resistant prostate cancer. Sci Transl Med 2015; 7: 312re10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jayaram A, Wingate A, Wetterskog D, et al. Plasma androgen receptor copy number status at emergence of metastatic castration-resistant prostate cancer: a pooled multicohort analysis. JCO Precis Oncol 2019; 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clegg NJ, Wongvipat J, Joseph JD, et al. ARN-509: a novel antiandrogen for prostate cancer treatment. Cancer Res 2012; 72: 1494–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith MR, Saad F, Chowdhury S, et al. Apalutamide and overall survival in prostate cancer. Eur Urol 2021; 79: 150–8. [DOI] [PubMed] [Google Scholar]
- 15.Smith MR, Saad F, Chowdhury S, et al. Apalutamide treatment and metastasis-free survival in prostate cancer. N Engl J Med 2018; 378: 1408–18. [DOI] [PubMed] [Google Scholar]
- 16.Chi KN, Agarwal N, Bjartell A, et al. Apalutamide for metastatic, castration-sensitive prostate cancer. N Engl J Med 2019; 381: 13–24. [DOI] [PubMed] [Google Scholar]
- 17.ERLEADA (apalutamide) [prescribing information]. Janssen Pharmaceutical Companies, Horsham, PA; 2019. [Google Scholar]
- 18.European Medicines Agency. Summary of product characteristics: Erleada 60 mg film-coated tablets. https://www.ema.europa.eu/en/medicines/human/EPAR/erleada (accessed June 13, 2021).
- 19.McKay RR, Werner L, Mostaghel EA, et al. A phase II trial of abiraterone combined with dutasteride for men with metastatic castration-resistant prostate cancer. Clin Cancer Res 2017; 23: 935–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Efstathiou E, Titus M, Wen S, et al. Enzalutamide in combination with abiraterone acetate in bone metastatic castration-resistant prostate cancer patients. Eur Urol Oncol 2020; 3: 119–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Efstathiou E, Titus M, Wen S, et al. Molecular characterization of enzalutamide-treated bone metastatic castration-resistant prostate cancer. Eur Urol 2015; 67: 53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Posadas EM, Chi KN, de Wit R, et al. Pharmacokinetics, safety, and antitumor effect of apalutamide with abiraterone acetate plus prednisone in metastatic castration-resistant prostate cancer: phase Ib study. Clin Cancer Res 2020; 26: 3517–24. [DOI] [PubMed] [Google Scholar]
- 23.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–47. [DOI] [PubMed] [Google Scholar]
- 24.Schemper M, Kaider A, Wakounig S, Heinze G. Estimating the correlation of bivariate failure times under censoring. Stat Med 2013; 32: 4781–90. [DOI] [PubMed] [Google Scholar]
- 25.Scher HI, Halabi S, Tannock I, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol 2008; 26: 1148–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spratt DE, Alshalalfa M, Fishbane N, et al. Transcriptomic heterogeneity of androgen receptor activity defines a de novo low AR-active subclass in treatment naive primary prostate cancer. Clin Cancer Res 2019; 25: 6721–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao SG, Chang SL, Erho N, et al. Associations of luminal and basal subtyping of prostate cancer with prognosis and response to androgen deprivation therapy. JAMA Oncol 2017; 3: 1663–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang D, Park D, Zhong Y, et al. Stem cell and neurogenic gene-expression profiles link prostate basal cells to aggressive prostate cancer. Nat Commun 2016; 7: 10798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.D'Agostino RB Sr., Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation 2008; 117: 743–53. [DOI] [PubMed] [Google Scholar]
- 30.Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation 1998; 97: 1837–47. [DOI] [PubMed] [Google Scholar]
- 31.de Bono JS, Bracarda S, Sternberg CN, et al. IPATential150: phase III study of ipatasertib (ipat) plus abiraterone (abi) vs placebo (pbo) plus abi in metastatic castration-resistant prostate cancer (mCRPC). Ann Oncol 2020; 31: S1142–S215. [Google Scholar]
- 32.Morris MJ, Heller G, Bryce AH, et al. Alliance A031201: a phase III trial of enzalutamide (ENZ) versus enzalutamide, abiraterone, and prednisone (ENZ/APP) for metastatic castration resistant prostate cancer (mCRPC). Presented at: ASCO Annual Meeting, May 31 – June 4, 2019; Chicago, IL. [Google Scholar]
- 33.Caffo O, Palesandro E, Nole F, et al. A multicentric phase II randomized trial of docetaxel (D) plus enzalutamide (E) versus docetaxel (D) as first-line chemotherapy for patients (pts) with metastatic castration-resistant prostate cancer (mCRPC): CHEIRON study. J Clin Oncol 2019; 37: 5050. [Google Scholar]
- 34.Armstrong AJ, Halabi S, Healy P, et al. Phase II trial of the PI3 kinase inhibitor buparlisib (BKM-120) with or without enzalutamide in men with metastatic castration resistant prostate cancer. Eur J Cancer 2017; 81: 228–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sweeney CJ, Gillessen S, Rathkopf D, et al. IMbassador250: a phase III trial comparing atezolizumab with enzalutamide vs enzalutamide alone in patients with metastatic castration-resistant prostate cancer (mCRPC). Presented at: AACR Annual Meeting 2020, June 22-24, 2020; Virtual [Google Scholar]
- 36.Davis ID, Martin AJ, Stockler MR, et al. Enzalutamide with standard first-line therapy in metastatic prostate cancer. N Engl J Med 2019; 381: 121–31. [DOI] [PubMed] [Google Scholar]
- 37.Attard G, Borre M, Gurney H, et al. Abiraterone alone or in combination with enzalutamide in metastatic castration-resistant prostate cancer with rising prostate-specific antigen during enzalutamide treatment. J Clin Oncol 2018; 36: 2639–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith MR, Rathkopf DE, Mulders PF, et al. Efficacy and safety of abiraterone acetate in elderly (75 years or older) chemotherapy naive patients with metastatic castration resistant prostate cancer. J Urol 2015; 194: 1277–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cella D, Nichol MB, Eton D, Nelson JB, Mulani P. Estimating clinically meaningful changes for the Functional Assessment of Cancer Therapy--Prostate: results from a clinical trial of patients with metastatic hormone-refractory prostate cancer. Value Health 2009; 12: 124–9. [DOI] [PubMed] [Google Scholar]
- 40.de Wit R, de Bono J, Sternberg CN, et al. Cabazitaxel versus abiraterone or enzalutamide in metastatic prostate cancer. N Engl J Med 2019; 381: 2506–18. [DOI] [PubMed] [Google Scholar]
- 41.Khalaf DJ, Annala M, Taavitsainen S, et al. Optimal sequencing of enzalutamide and abiraterone acetate plus prednisone in metastatic castration-resistant prostate cancer: a multicentre, randomised, open-label, phase 2, crossover trial. Lancet Oncol 2019; 20: 1730–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparency. As noted on this site, requests for access to the study data can be submitted through Yale Open Data Access (YODA) Project site at http://yoda.yale.edu.