Abstract

The synthesis, structure, photophysical properties, and electrochemistry of the first series of Pt(IV) tris-chelates bearing cyclometalated aryl-NHC ligands are reported. The complexes have the general formula [Pt(trz)2(C∧N)]+, combining two units of the cyclometalated, mesoionic aryl-NHC ligand 4-butyl-3-methyl-1-phenyl-1H-1,2,3-triazol-5-ylidene (trz) with a cyclometalated 2-arylpyridine [C∧N = 2-(2,4-difluorophenyl)pyridine (dfppy), 2-phenylpyridine (ppy), 2-(p-tolyl)pyridine (tpy), 2-(2-thienyl)pyridine (thpy), 2-(9,9-dimethylfluoren-2-yl)pyridine (flpy)], and presenting a mer arrangement or metalated aryls. They exhibit a significant photostability under UV irradiation and long-lived phosphorescence in the blue to yellow color range, arising from 3LC excited states involving the C∧N ligands, with quantum yields of up to 0.34 in fluid solution and 0.77 in the rigid matrix at 298 K. The time-dependent density functional theory (TD-DFT) calculations reveal that nonemissive, deactivating excited states of ligand-to-metal charge-transfer (LMCT) character are pushed to high energies as a consequence of the strong σ-donating ability of the carbenic moieties, making the Pt(trz)2 subunit an essential structural component that enables efficient emissions from the chromophoric C∧N ligands, with potential application for the development of different Pt(IV) emitters with tunable properties.
Short abstract
Tris-chelate Pt(IV) complexes that combine two cyclometalated mesoionic aryl-NHC ligands of the 1,2,3-triazolylidene subclass (C∧C*) with one cyclometalated 2-arylpyridine ligand (C∧N) are reported, which exhibit significant phosphorescent emissions that can be tuned by the variation of the C∧N ligand. The Pt(C∧C*)2 subunit is demonstrated as a useful platform for the development of efficient Pt(IV) emitters through the incorporation of different chromophoric ligands.
Introduction
The use of N-heterocyclic carbene ligands (NHCs) for the design of strongly luminescent transition-metal complexes has become widespread, mostly associated with the development of phosphors for organic light-emitting devices (OLEDs).1−4 Mesoionic NHCs have received special attention within this field because their exceptionally strong σ-donating ability makes them particularly well suited to induce large ligand-field splittings, raising the energies of dissociative, metal-centered (MC) excited states and reducing the nonradiative deactivation that takes place through the thermal population of such states.5−7 This effect has even been applied with remarkable success to extend the excited-state lifetimes of strongly deactivated first-row transition-metal complexes.8−11
Bidentate cyclometalated aryl-substituted NHC ligands (aryl-NHCs, C∧C*) have been extraordinarily successful with the Ir(III)12−21 and Pt(II)22−31 ions as a replacement of cyclometalated 2-arylpyridines (C∧N), enabling better photostabilities, wider color tunability, and higher emission efficiencies. These enhancements are brought about by the larger ligand-field splitting induced by the NHC moiety with respect to the pyridine and, consequently, the reduced thermal accessibility of MC states from the triplet, mixed ligand-centered/metal-to-ligand charge-transfer (3LC/MLCT) emissive state of Ir(III) and Pt(II) complexes. The most relevant tris-chelates bearing C∧C* ligands are homoleptic Ir(III) complexes mer/fac-[Ir(C∧C*)3]12,16,17,21 and mixed-carbene variations,19 which can achieve blue phosphorescent emissions, thanks to the large π–π* gap of the ligands. Heteroleptic tris-chelates of the type [Ir(C∧C*)(C∧N)2] have also been reported, in which the arylcarbene acts as a supporting, nonchromophoric ligand, whereas the emission is mostly determined by the C∧N ligands,13,18,32−34 except for a few cases that incorporate C∧C* ligands featuring low π–π* gaps.35−37 However, very few heteroleptic tris-chelates bearing two C∧C* ligands are known, which include complexes [Ir(C*∧C∧C∧C*)(C∧N)] bearing a bis-aryl-NHC38 and [Ir(C∧C*)2(N∧N)] or [Ir(C∧C*)2(N∧N)]+, where N∧N is a pyridylpyrazolate, pyridyltriazolate, pyridylbenzimidazolate,39−41 or bipyridyl.42 Such systems are interesting because the Ir(C∧C*)2 subunit functions as a robust platform for the development of efficient emitters whose properties can be tuned by incorporating different chromophoric C∧N or N∧N ligands.
When compared with other d6 metal ions, the photophysical properties of Pt(IV) complexes have received much less attention. In previous studies, we have shown that cyclometalated Pt(IV) complexes with 2-arylpyridine ligands may exhibit very efficient and long-lived phosphorescence,43−48 which makes them potentially useful as luminescence-based sensors, photosensitizers, or photocatalysts. Their emissive excited states are essentially 3LC in character, with very little metal orbital participation in the form of an MLCT admixture. Because of the high oxidation state of the metal, unoccupied dσ* orbitals have relatively low energies and, in some derivatives, electronic promotions to these orbitals, i.e., ligand-to-metal charge-transfer (LMCT) excited states, may become thermally accessible from the emissive state. Such states have dissociative character because dσ* orbitals are strongly antibonding, providing a pathway for nonradiative deactivation or photochemical reactivity.49,50 Therefore, an indispensable requirement for Pt(IV) complexes to reach high emission efficiencies is the presence of suitable strong σ-donor ligands, which induce a large ligand-field splitting and raise the energy of LMCT states.
Recently, we reported Pt(IV) complexes of the types [PtCl2(C∧C*)(C∧N)] and [PtCl(C∧C*)(C∧N∧C)], where C∧C* is a cyclometalated, mesoionic aryl-NHC ligand of the 1,2,3-triazolylidene subclass, which constituted the first examples of Pt(IV) emitters bearing a carbene ligand.51,52 Derivatives of the type [PtCl2(C∧C*)(C∧N)] exhibited strong 3LC emissions involving the C∧N ligand, with significantly higher quantum efficiencies with respect to the homologous C2-symmetrical [PtCl2(C∧N)2] complexes as a consequence of the electronic effects of the carbene moiety. However, their synthesis presented problems associated with the difficult cyclometalation of the aryl-NHC ligand, resulting in relatively low yields. Herein, we present a straightforward methodology involving two consecutive cyclometalations of aryl-NHC ligands that has allowed the synthesis of complexes of the type [Pt(C∧C*)2(C∧N)]+. These species are the first Pt(IV) tris-chelates bearing cyclometalated aryl-NHC ligands and show intense phosphorescent emissions that can be modulated through the variation of the C∧N ligand, demonstrating the usefulness of the Pt(C∧C*)2 subunit as a platform for the design of efficient emitters.
Results and Discussion
Synthesis and Characterization
The synthetic route to the target tris-cyclometalated Pt(IV) complexes is shown in Scheme 1. The reaction of the dimeric platinum precursor (Pr4N)2[Pt2Cl6] with 4 molar equiv of the in situ-prepared silver carbene intermediate “AgI(trzH)” (trzH = 4-butyl-3-methyl-1-phenyl-1H-1,2,3-triazol-5-ylidene) in 1,2-dichloroethane at 80 °C led to the selective formation of trans-[PtCl2(trzH)2] (1), which could be isolated in 85% yield. The trans geometry of 1 was established from an X-ray diffraction analysis (see below). The reason for the exclusive formation of this isomer is probably that, upon coordination of the first trzH ligand, the Pt–Cl bond trans to the carbenic carbon becomes highly labile and is rapidly abstracted by the silver ion, resulting in the coordination of a second trzH ligand in this position. Consistent with this, the attempts to obtain a complex with only one trzH ligand by employing a 1:2 molar ratio (dimeric platinum precursor to carbene) were unsuccessful, resulting always in the formation of 1. Similar results have been previously observed for the reactions of K2[PtCl4] with other silver carbenes.53−57
Scheme 1. Synthetic Route to the Target Tris-cyclometalated Pt(IV) Complexes.
(i) Ag2O, 1,2-dichloroethane (DCE), 50 °C; (ii) (Pr4N)2[Pt2Cl6], DCE, 80 °C; (iii) PhICl2, CH2Cl2; (iv) Na2CO3, 1,2-dichlorobenzene (DCB), 120 °C; and (v) 2 AgOTf, N∧CH, DCB, 120 °C.
The 1H and 13C NMR spectra of complex 1 show two sets of resonances for the trzH ligand in very similar proportions (Figure S1), pointing to the presence of two different conformational isomers that interconvert very slowly at room temperature as a consequence of restricted rotation about the Pt–C bond. This possibility was confirmed by a variable-temperature NMR study in DMSO-d6, which showed that the different pairs of aromatic and aliphatic signals coalesce in the range 35–60 °C (Figure S2). Using the Eyring equation,58 a free energy of activation of ΔG‡ = 15.0 kcal/mol was calculated for this process at the coalescence temperature of the NCH2 protons (60 °C). Several examples of restricted rotation of NHC ligands about the metal–C bond have been previously reported.53,59−61
The crystal structure of 1 is shown in Figure 1. The crystal analyzed corresponded to the conformer with an antiparallel orientation of the phenyl and butyl substituents of the trzH ligands. The Pt–Cl bonds lie along a crystallographic 2-fold axis and therefore the coordination around the metal is strictly planar. The mean plane of the triazolylidene ring is rotated by 68.85° with respect to the coordination plane. The Pt–C1 bond distance of 2.027(2) Å is typical of Pt(II) complexes with mutually trans NHC ligands.53−55,62−64
Figure 1.

Structure of complex 1 (thermal ellipsoids at 50% probability). Hydrogen atoms are omitted. Selected bond distances (Å) and angles (°): Pt–C1, 2.027(2); Pt–Cl1, 2.3253(8); Pt–C12, 2.3132(7); C1–Pt–Cl1, 90.35(6); C1–Pt–Cl2, 89.65(6); and Cl1–Pt–C12, 180.0.
Treatment of a CH2Cl2 solution of 1 with PhICl2 led to the oxidation to Pt(IV) and the electrophilic metalation of the pendant aryl group of one of the trzH ligands, resulting in the formation of the monocyclometalated species [PtCl3(trz)(trzH)] (2), which was isolated in 96% yield. The 1H NMR spectrum of 2 shows an aromatic resonance flanked by 195Pt satellites at δ = 6.99 ppm (JPtH = 37 Hz), arising from the proton ortho to the metalated carbon of a phenyl ring. The crystal structure (Figure 2) corroborated the presence of a cyclometalated trz and a coordinated trzH and revealed that the carbenic moieties remain mutually trans, resulting in a mer disposition of metalated carbons or chloride ligands.
Figure 2.
Structures of complexes 2 (a) and the cations of 4b (b) and 4d (c) (thermal ellipsoids at 50% probability). Hydrogen atoms are omitted. Selected bond distances (Å) and angles (°): 2: Pt–C1, 2.046(2); Pt–C9, 2.032(2); Pt–C14, 2.089(2); Pt–Cl1, 2.4509(5); Pt–C12, 2.3190(5); Pt–Cl3, 2.3395(5); C1–Pt–C9, 80.95(8); and C1–Pt–C14, 173.90(8). 4b: Pt–C1, 2.037(3); Pt–C9, 2.071(3); Pt–C14, 2.043(4), Pt–C22, 2.065(4); Pt–N7, 2.094(3); Pt–C31, 2.087(3); C1–Pt–C9, 80.22(14); C14–Pt–C22, 79.79(14); and N7–Pt–C31, 79.69(13). 4d: Pt–C1, 2.034(2); Pt–C9, 2.0414(19); Pt–C14, 2.0393(19), Pt–C22, 2.0837(19); Pt–N40, 2.1164(17); Pt–C31, 2.0790(19); C1–Pt–C9, 80.06(8); C14–Pt–C22, 80.05(8); and N–Pt–C31, 79.46(7).
The metalation of the pendant phenyl group of the remaining trzH ligand in 2 was achieved by heating at 120 °C in 1,2-dichlorobenzene in the presence of a base (Na2CO3), which resulted in the formation of the bis-cyclometalated complex [PtCl2(trz)2] (3, 78% yield). Its 1H NMR spectrum shows a single set of resonances arising from equivalent cyclometalated trz ligands. In addition, the proton ortho to the metalated carbon of the phenyl ring is strongly shielded (δ = 6.49 ppm, JPtH = 52 Hz), indicating that it is affected by the diamagnetic current of an orthogonal aromatic ring. These data demonstrate a C2-symmetrical configuration with mutually cis chloride ligands.
The introduction of different cyclometalated 2-arylpyridines (C∧N) as the third chelating ligand was accomplished by reacting 3 with 2.4 equiv of AgOTf (OTf– = trifluoromethanesulfonate) and an excess of the 2-arylpyridine in 1,2-dichlorobenzene at 120 °C, which afforded complexes [Pt(trz)2(C∧N)]OTf in 72–80% yields [C∧N = dfppy (4a), ppy (4b), tpy (4c), thpy (4d), flpy (4e)]. The 1H NMR spectra of these complexes show three distinctive aromatic resonances flanked by 195Pt satellites arising from the protons ortho to the metalated aryls, which are significantly shielded because they are directed toward orthogonal aromatic rings (δ = 7.04–6.29 ppm).
The crystal structures of complexes 4b and 4d were solved by X-ray diffraction analyses (Figure 2) and are completely analogous. The carbene moieties are mutually trans, and the coordinated nitrogen and metalated carbon of the C∧N ligand are trans to each of the metalated aryls of the trz ligands. Therefore, they retain the disposition of trz ligands found in their precursor, resulting in a mer configuration of metalated aryl rings. In the case of 4d, the positions of the thiophene and pyridine rings were found disordered, with one of the orientations presenting a much higher occupancy factor than the other (ca. 81:19). Hence, the Pt–C9 or Pt–C22 bonds in 4d can be considered as predominantly trans to the pyridyl or thienyl rings, respectively (Figure 2), the latter being significantly longer because of the stronger trans influence of the metalated carbon. However, in 4b the positions of the pyridyl and phenyl rings of the ppy ligand were not distinguished by the refinement model, resulting in very similar Pt–C9 and Pt–C22 bond distances.
Photophysical Properties
The electronic absorption spectra of 4a–e in CH2Cl2 solution (Table 1, Figure 3) show structured absorptions in the 250–400 nm range that can be ascribed to primarily 1LC transitions within the ligands. The shapes and energies of the observed bands are very similar to those of complexes [PtMe(Cl)(C∧N)2]44 and [PtCl2(trz)(C∧N)]52 with the respective 2-arylpyridine ligands, implying that the spectra are dominated by C∧N-centered absorptions, whereas those involving the trz ligands must be obscured. The lowest-energy absorption maximum shifts from 321 to 370 nm along the sequence 4a → 4e, as the expected energies of the lowest π–π* transition of the C∧N ligand decrease. As observed for [PtMe(Cl)(flpy)2],44 complex 4e presents a significantly higher molar absorptivity (25900 M–1 cm–1) with respect to the rest of the derivatives (8100–12100 M–1 cm–1). Intense absorptions are advantageous for applications such as photocatalysis or bioimaging.
Table 1. Electronic Absorption Data for the Studied Complexes in CH2Cl2 Solution (ca. 5 × 10–5 M) at 298 K.
| complex | λmax (nm) (ε × 10–2 (M–1 cm–1)) |
|---|---|
| 4a | 260 (237), 307 (169), 321 (121) |
| 4b | 265 (274), 307 (181), 320 (149), 332 (91) |
| 4c | 268 (262), 310 (173), 323 (154), 337 (106) |
| 4d | 258 (174), 268 (187), 286 (194), 346 (88), 356 (81) |
| 4e | 256 (226), 296 (194), 304 (198), 355 (219), 370 (259) |
Figure 3.
Electronic absorption spectra of complexes 4a–e in a CH2Cl2 solution at 298 K.
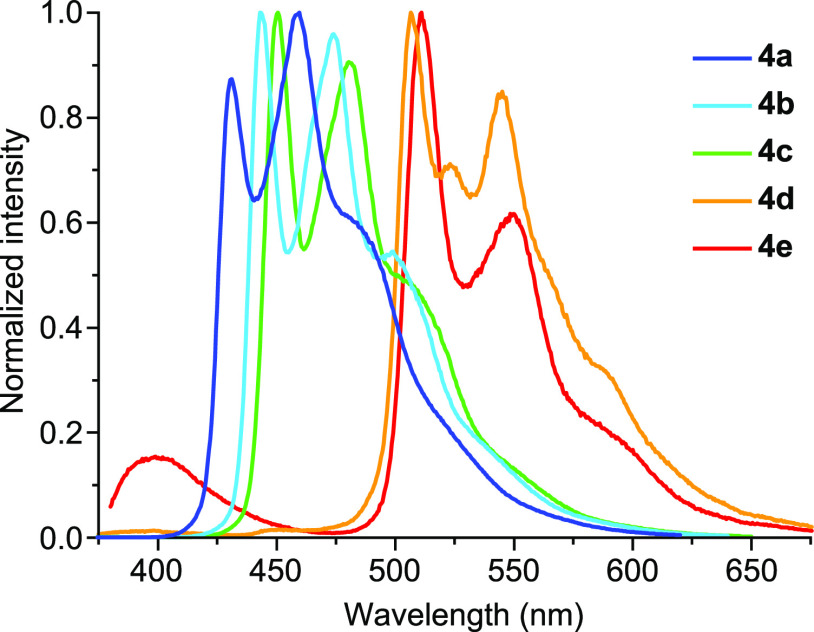
Before examining their luminescence, the photostability of 4a–e was checked by irradiating their solutions in CD3CN in quartz NMR tubes with UV light (310 nm) for 6 h at room temperature. Only in the cases of 4a–c were traces of decomposition products observed in the 1H NMR spectra (ca. 2% of the initial concentration; Figures S15–S19). This behavior is noteworthy because certain tris-chelate Pt(IV) complexes with a mer configuration of metalated aryl groups, mer-[Pt(C∧N)3]+ (C∧N = dfppy, ppy, tpy), isomerize to the fac complexes under iradiation with UV light as a consequence of the population of LMCT excited states.43,45 Instead, complexes 4a–e produce significant luminescent emissions, which were characterized from deaerated CH2Cl2 solutions and poly(methyl methacrylate) (PMMA) matrices (2 wt %) at 298 K and frozen butyronitrile (PrCN) glasses at 77 K. The emission data at 298 K are summarized in Table 2, and the emission spectra in CH2Cl2 solution are shown in Figure 4. The data at 77 K and the complete series of excitation and emission spectra are included in the Supporting Information. Vibronically structured emissions are observed in all cases, characterized by large Stokes Shifts and lifetimes in the hundreds of microseconds range, which demonstrate a 3LC emissive state. Given that emission energies decrease in the same order as the lowest-energy absorption, the involved ligand is clearly the cyclometalated 2-arylpyridine, and therefore the trz ligands play a supporting role. In the case of the flpy derivative 4e, a secondary emission band at a higher energy is assigned as fluorescence on the basis of its very short lifetime (<0.2 ns). This band represents a very small fraction of the emitted photons, with a quantum yield of ΦF ≈ 0.005 in both CH2Cl2 and PMMA. We have previously reported dual fluorescent/phosphorescent emissions from Pt(IV) complexes bearing flpy65 or other C∧N ligands with extended π systems,45,46 which are due to a relatively less efficient intersystem crossing to the triplet manifold as a consequence of a lower metal orbital contribution to the involved excited states and the reduced spin–orbit coupling effects induced by the metal. Excitation spectra monitored at the phosphorescent emission band correlate with the corresponding absorption profiles in all cases. The excitation spectrum of 4e monitored at the fluorescence band coincides with the one monitored at the phosphorescence band in the lower-energy region but shows some differences at higher energies that we tentatively attribute to relatively inefficient internal conversion between higher-lying 1LC(trz) states and the lowest 1LC(flpy) state (see Figure S21 for details). Compared with fac-[Pt(C∧N)3]+,43 [PtMe(Cl)(C∧N)2],44 and [PtCl2(trz)(C∧N)],52 the phosphorescent emissions of 4a–e are somewhat blue-shifted, probably as a consequence of the stronger π-acceptor character of the trz ligands relative to 2-arylpyridines,66 leading to a lower energy of metal dπ orbitals and hence a lower MLCT contribution to the emissive excited state.
Table 2. Emission Data of Complexes 4a–e at 298 K.
| complex | medium | λem (nm)a | ΦPb | τ (μs)c | kr × 10–3 (s–1)d | knr × 10–3 (s–1)e |
|---|---|---|---|---|---|---|
| 4a | CH2Cl2 | 431, 459, 486 | 0.34 | 118 | 2.9 | 5.6 |
| PMMA | 431, 458, 486 | 0.68 | 348 | 1.9 | 0.9 | |
| 4b | CH2Cl2 | 443, 474, 499 | 0.25 | 132 | 1.9 | 5.7 |
| PMMA | 443, 473, 500 | 0.77 | 341 | 2.3 | 0.7 | |
| 4c | CH2Cl2 | 450, 481, 507 | 0.24 | 169 | 1.4 | 4.5 |
| PMMA | 450, 480, 508 | 0.77 | 433 | 1.8 | 0.5 | |
| 4d | CH2Cl2 | 507, 523, 545, 588 | 0.15 | 148 | 1.0 | 5.7 |
| PMMA | 507, 523, 545, 589 | 0.44 | 514 | 0.9 | 1.1 | |
| 4e | CH2Cl2 | 400, 511, 550, 592 | 0.10 | 117 | 0.8 | 7.7 |
| PMMA | 395, 509, 547, 592 | 0.39 | 750 | 0.5 | 0.8 |
The most intense peak is italicized.
Phosphorescence quantum yield.
Lifetime.
Radiative rate constant, kr = ΦP/τ.
Nonradiative rate constant, knr = (1 – ΦP)/τ.
Figure 4.
Emission spectra of complexes 4a–e in CH2Cl2 at 298 K.
Quantum yields vary in the range 0.10–0.34 in CH2Cl2 and 0.39–0.77 in PMMA matrix and reach the highest values for the derivatives bearing a ppy-based ligand (4a–c). The only previously reported luminescent Pt(IV) tris-chelates with a mer arrangement of metalated aryl rings contain at least one C∧N ligand of a relatively low energy for the π–π* transition, namely, mer-[Pt(flpy)3]+65 and the heteroleptic derivatives mer-[Pt(ppy)2(flpy)]+65 and mer-[Pt(C∧N)2(C′∧N′)]+ with C∧N = dfppy, ppy, C′∧N′ = thpy, 1-phenylisoquinoline (piq),45 and their quantum yields were in the range from 0.03 (for mer-[Pt(ppy)2(piq)]+) to 0.08 (for mer-[Pt(flpy)3]+).
The radiative and nonradiative rate constants (kr and knr, respectively) for the phosphorescent emissions were calculated assuming that the triplet emissive state is formed with unit efficiency. This assumption introduces a negligible error in the case of 4e because the fluorescence emission has a very low quantum yield.65 The kr values are similar to those of complexes fac-[Pt(C∧N)3]+ and [PtCl2(trz)(C∧N)] with the same C∧N ligands. The lower quantum yields of 4d and 4e are mainly attributable to their lower radiative rates, which are typically found for Pt(IV) complexes bearing thpy45,46,52 and flppy65 ligands and can be explained by a relatively poor metal-ligand orbital overlap, leading to decreased MLCT contributions to the emissive state. The knr values are drastically reduced in PMMA matrix in all cases, resulting in significantly higher quantum yields. This indicates that nonradiative deactivation in CH2Cl2 solution occurs mainly through molecular motion and collisions with solvent molecules.
Electrochemistry
The cyclic voltammograms of complexes 4a–e were registered in MeCN solution and are shown in Figure 5. The potentials of the observed redox processes and estimations of highest occupied/lowest unoccupied molecular orbital (HOMO/LUMO) energies are listed in Table 3. A single irreversible oxidation wave is observable within the accessible potential window for all complexes except 4e, which produces two irreversible waves. The anodic peak potentials decrease in the sequence 4a → 4e, corresponding to increasing HOMO energies as the C∧N ligand becomes more electron donating. Therefore, the HOMO is essentially a π orbital of the C∧N ligand in all cases, which agrees with the density functional theory (DFT) calculations on 4c (see below). The estimated HOMO energies are similar to those of other Pt(IV) complexes with the respective C∧N ligand in a similar coordination environment, i.e., homoleptic mer-[Pt(C∧N)3]+ or heteroleptic mer-[Pt(C∧N)2(C′∧N′)]+ complexes,45 whereas the fac isomers43,46,65 and the bis-cyclometalated complexes [PtCl2(C∧N)(trz)]52 show lower values.
Figure 5.
Cyclic voltammograms of complexes 4a–e in MeCN at 100 mV s–1.
Table 3. Electrochemical Dataa and HOMO/LUMO Energy Estimationsb for Complexes 4a–e.
| complex | Ep,ac | Ep,cd | E1/2e | EHOMO | ELUMO | ΔEHOMO-LUMO |
|---|---|---|---|---|---|---|
| 4a | 2.11 | –1.92, −2.17 | –2.04, −2.46 | –6.63 | –2.90 | 3.73 |
| 4b | 1.89 | –1.99, −2.23 | –2.08, −2.35, −2.52 | –6.46 | –2.82 | 3.64 |
| 4c | 1.79 | –1.99 | –2.16, −2.34, −2.55 | –6.37 | –2.81 | 3.56 |
| 4d | 1.68 | –1.92, −2.03, −2.29 | –2.48 | –6.28 | –2.86 | 3.42 |
| 4e | 1.58, 1.94 | –1.88, −2.07, −2.27, −2.39 | –6.18 | –2.88 | 3.30 |
In V vs SCE, registered in a 0.1 M solution of (Bu4N)PF6 in dry MeCN at 100 mV s–1.
In eV.
Irreversible anodic peak potentials.
Irreversible cathodic peak potentials.
For the reversible waves.
The first reduction wave is observed at very similar potentials for all complexes and is irreversible, except for 4e, which shows a quasi-reversible wave (Figure S24). Additional reversible processes are observed at more negative potentials, corresponding to the reduction and subsequent reoxidation of species arising from the first reduction. The very similar LUMO energies indicate that this orbital is the same for all complexes and is not affected by the C∧N ligand. On the basis of DFT calculations on complex 4c, the LUMO is composed of the combined lowest π* orbitals of the trz ligands.
Computational Study
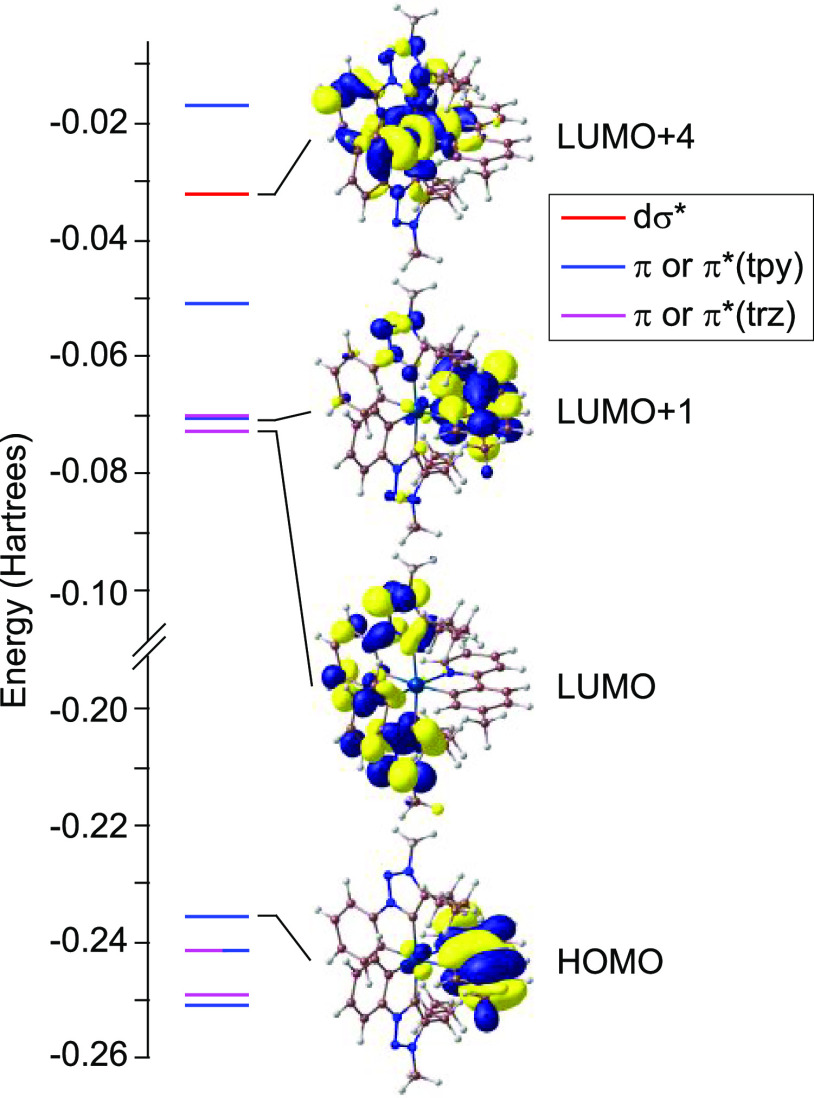
For a more precise understanding of the properties of complexes 4, DFT and time-dependent DFT (TD-DFT) calculations have been carried out for the tpy derivative 4c. Complete details are presented in the Supporting Information, including fragment contributions to the frontier orbitals (Table S2). Figure 6 shows an orbital energy diagram, including selected isosurfaces. The HOMO is mainly composed of the highest π orbital of the tpy ligand with some dπ orbital contribution from the metal (ca. 3%), whereas the LUMO is made of the lowest π* orbital of the trz ligands and is similarly distributed over them. The LUMO+1 is the lowest π* orbital of the tpy ligand. The lowest molecular orbital with dσ* character is LUMO+4.
Figure 6.
Molecular orbital energy diagram from DFT calculations and selected isosurfaces for complex 4c.
The TD-DFT results predict essentially LC transitions within the tpy ligand as the most intense, lowest-energy singlet excitations (Table S3), in agreement with the above interpretation of the experimental absorption spectrum. Analogous transitions involving the trz ligand are predicted at higher energies and have lower oscillator strengths. Ligand-to-ligand charge-transfer (LLCT) transitions between the tpy and trz ligands are predicted to occur at low energies, but their oscillator strengths are extremely low and therefore they cannot be identified in the experimental spectrum. The first three triplet excitations correspond to LC transitions within each of the cyclometalated ligands (Table S4), the lowest one involving the tpy, which is consistent with the assignment of the emissive state. The lowest triplet LMCT excitation involving an electronic promotion to LUMO+4 is 1.26 eV above the emissive state (T15, Table S4; cf. 0.64 or 0.78 eV for the lowest 3LMCT excitation in [PtCl2(tpy)2] or [PtCl2(trz)(tpy)], respectively),52 and is not expected to significantly contribute to nonradiative excited-state decay through thermal population.
A geometry optimization of the lowest triplet excited state of 4c was carried out for additional insight. The calculated spin density distribution (Figure 7) essentially corresponds to a π–π* transition within the tpy ligand, which agrees with the above assignment of the emissive state as a primarily 3LC(tpy) excited state. The calculated natural spin density of 0.010 on the metal atom is comparable to those found for heteroleptic complexes of the type mer-[Pt(C∧N)2(C′∧N′)]+ (range 0.004–0.009)45,65 and can be interpreted as a very small MLCT contribution to the excited state. The adiabatic T1–S0 energy difference is 2.68 eV (463 nm), in good agreement with the observed emission energy.
Figure 7.

Spin density distribution (0.0008 e bohr–3) of the optimized lowest triplet excited state of 4c.
Conclusions
A method to obtain the C2-symmetrical, bis-cyclometalated species [PtCl2(trz)2] (3) has been developed, involving an oxidation of the Pt(II) bis-carbene complex, trans-[PtCl2(trzH)2] (1), and the successive electrophilic metalations of the pendant phenyl groups of the trzH ligands. Complex 3 is an excellent precursor for the preparation of cationic tris-cyclometalated complexes [Pt(trz)2(C∧N)]OTf (4) via chloride abstraction with AgOTf in the presence of 2-arylpyridine ligands. These are the first reported Pt(IV) tris-chelates bearing cyclometalated aryl-NHC ligands. Despite presenting a mer configuration of metalated aryls, they are significantly photostable under irradiation with UV light, in sharp contrast with most of the homologous mer-[Pt(C∧N)3]OTf complexes with the same C∧N ligands, which undergo photoisomerization reactions. In addition, they show significant phosphorescent emissions in different media, arising from 3LC states involving the C∧N ligands. The computational results show that the energies of deactivating 3LMCT states are high enough not to have an adverse effect on the emissions via thermal population from the emissive state, which is consistent with the observed photostabilities and can be explained by the strong σ-donation from the NHC moieties. Hence, the Pt(trz)2 subunit is demonstrated as a suitable platform upon which phosphorescent Pt(IV) tris-chelates with tunable emission energies can be built by incorporating different cyclometalated 2-arylpyridines as chromophoric ligands, opening the way to the development of new classes of emissive Pt(IV) complexes by employing other chromophoric bidentate ligands.
Experimental Section
General Considerations and Instrumentation
Preparations were carried out under atmospheric conditions, except for those that required silver reagents, which were conducted in the dark under an N2 atmosphere. Synthesis grade solvents were employed in all cases. The triazolium iodide salt,67 (Pr4N)2[Pt2Cl6],68 and PhICl269 were synthesized according to reported procedures. All other reagents were obtained from commercial sources. Elemental analyses were carried out with a LECO CHNS-932 microanalyzer. Electrospray ionization high-resolution mass spectra (ESI-HRMS) were recorded on an Agilent 6220 Accurate-Mass time-of-flight (TOF) LC/MS. NMR spectra were registered on a 600 MHz Bruker Avance spectrometer at 298 K. The variable-temperature NMR spectra of 1 were registered on a 300 MHz Bruker Avance spectrometer. Chemical shifts (δ) were referenced to residual signals of nondeuterated solvent and are given in ppm downfield from tetramethylsilane. Abbreviations: s, singlet: d, doublet; t, triplet, q, quartet; p, pentet; sext, sextet; and m, multiplet.
Preparation of trans-[PtCl2(trzH)2] (1)
The triazolium salt (250 mg, 0.73 mmol) and Ag2O (110 mg, 0.47 mmol) were mixed in 1,2-dichloroethane (15 mL), and the resulting suspension was stirred at 50 °C for 14 h and then filtered through Celite. (NPr4)2[Pt2Cl6] (178 mg, 0.18 mmol) was added to the filtrate, and the mixture was stirred for 14 h at 80 °C and filtered through Celite. The filtrate was evaporated to dryness, and the residue was triturated with MeOH (3 × 4 mL) to give a white solid, which was recrystallized from CH2Cl2/Et2O and vacuum-dried to give 1. Yield: 217 mg (85%). 1H NMR (600 MHz, CD2Cl2): δ = 8.47 (d, JHH = 7.8 Hz, 2H, CH), 8.29 (d, JHH = 7.8 Hz, 2H, CH), 7.58–7.51 (m, 4H, CH), 7.43 (t, JHH = 7.7 Hz, 2H, CH), 4.05 (s, 3H, NCH3), 4.04 (s, 3H, NCH3), 3.22 (t, JHH = 8.0 Hz, 2H, CH2), 3.04 (m, JHH = 8.0 Hz, 2H, CH2), 2.01 (p, JHH = 7.7 Hz, 2H, CH2), 1.79 (p, JHH = 7.7 Hz, 2H, CH2), 1.57 (sext, JHH = 7.3 Hz, 2H, CH2), 1.45 (sext, JHH = 7.4 Hz, 2H, CH2), 1.04 (t, JHH = 7.4 Hz, 3H, CH3), 0.97 (t, JHH = 7.4 Hz, 2H, CH3). 13C APT NMR (150 MHz, CD2Cl2): δ = 157.3 (C), 157.0 (C), 147.2 (C), 147.0 (C), 140.7 (C), 140.5 (C), 129.6 (CH), 129.4 (CH), 129.3 (CH), 129.0 (CH), 125.5 (CH), 125.2 (CH), 36.6 (2 NCH3), 31.9 (CH2), 31.7 (CH2), 25.7 (CH2), 25.4 (CH2), 23.5 (CH2), 23.4 (CH2), 14.34 (CH3), 14.29 (CH3). Anal. Calcd for C26H34Cl2N6Pt: C, 44.83; H, 4.92; N, 12.06. Found: C, 44.71, H, 4.72, N, 12.12.
Preparation of [PtCl3(trz)(trzH)] (2)
To a solution of 1 (198 mg, 0.28 mmol) in CH2Cl2 (25 mL) was added PhICl2 (94 mg, 0.34 mmol), and the mixture was stirred at room temperature for 1 h. Partial evaporation of the resulting solution under reduced pressure (2 mL) and addition of Et2O (10 mL) led to the precipitation of a white solid, which was collected by filtration and vacuum-dried to give 2. Yield: 200 mg (96%). 1H NMR (600 MHz, CD2Cl2): δ = 7.81–7.79 (m, 2H, CH), 7.65 (dd, JHH = 7.8, 1.5 Hz, 1H, CH), 7.48–7.44 (m, 1H, CH), 7.39–7.35 (m, 2H, CH), 7.19 (td, JHH = 7.6, 1.2 Hz, 1H, CH), 7.14 (td, JHH = 7.6, 1.5 Hz, 1H, CH), 6.99 (dd with satellites, JHH = 7.8, 1.3 Hz, JPtH = 37 Hz, 1H, CH), 4.22 (s, 3H, NCH3), 4.10 (s, 3H, NCH3), 3.54 (ddd, JHH = 14.1, 11.0, 5.3 Hz, 1H, CH2), 3.35 (ddd, JHH = 14.6, 12.6, 4.5 Hz, 1H, CH2), 3.10 (ddd, JHH = 14.1, 11.0, 5.4 Hz, 1H, CH2), 2.74 (ddd, JHH = 14.6, 12.3, 4.3 Hz, 1H, CH2), 1.97–1.90 (m, 1H, CH2), 1.80–1.73 (m, 1H, CH2), 1.65–1.57 (m, 2H, CH2), 150–1.43 (m, 2H, CH2), 1.40–1.29 (m, 2H, CH2), 0.96 (t, JHH = 7.4 Hz, 3H, CH3), 0.89 (t, JHH = 7.4 Hz, 3H, CH3). 13C APT NMR (150 MHz, CD2Cl2): δ = 148.5 (JPtC = 44 Hz, C), 146.0 (C), 142.1 (C), 141.0 (C), 140.2 (C), 139.4 (C), 135.1 (CH), 130.5 (CH), 130.3 (CH), 129.5 (JPtC = 40 Hz, CH), 127.2 (CH), 126.4 (CH), 123.9 (C), 116.4 (CH), 37.2 (NCH3), 36.9 (NCH3), 32.0 (CH2), 31.7 (CH2), 27.1 (CH2), 23.7 (CH2), 23.2 (CH2), 23.1 (CH2), 14.13 (CH3), 14.06 (CH3). Anal. Calcd for C26H33Cl3N6Pt: C, 42.72; H, 4.55; N, 11.50. Found: C, 42.66, H, 4.47, N, 11.60.
Preparation of [PtCl2(trz)2] (3)
A Carius tube was charged with complex 2 (75 mg, 0.10 mmol), Na2CO3 (54 mg, 0.51 mmol), and 1,2-dichlorobenzene (3 mL), and the mixture was stirred at 120 °C for 14 h. After cooling down to room temperature, Et2O (10 mL) was added, and the precipitate was collected by filtration. The product was extracted with CH2Cl2 (5 × 5 mL). Partial evaporation of the combined extracts under reduced pressure (2 mL) and addition of Et2O (10 mL) led to the precipitation of a white solid, which was collected by filtration and vacuum-dried to give 3. Yield: 56 mg (78%). 1H NMR (600 MHz, CD2Cl2): δ = 7.62 (dd with satellites, JHH = 7.9, 1.4 Hz, JPtH = 9 Hz, 2H, CH), 7.09 (ddd, JHH = 7.8, 7.5, 1.2 Hz, 2H, CH), 6.86 (td, JHH = 7.7, 1.4 Hz, 2H, CH), 6.49 (dd with satellites, JHH = 7.8, 1.2 Hz, JPtH = 52 Hz, 2H, CH), 4.25 (s, 6H, NCH3), 3.48 (ddd, JHH = 14.2, 10.1, 5.8 Hz, 2H, CH2), 3.24 (ddd, JHH = 14.3, 10.2, 6.1 Hz, 2H, CH2), 1.86–1.74 (m, 4H, CH2), 1.50 (sext, JHH = 7.4 Hz, 4H, CH2), 0.97 (t, JHH = 7.4 Hz, 6H, CH3). 13C APT NMR (150 MHz, CD2Cl2): δ = 149.3 (JPtC = 802 Hz, C), 146.8 (JPtC = 58 Hz, C), 142.2 (C), 133.4 (JPtC = 25 Hz, CH), 129.8 (JPtC = 54 Hz, CH), 126.1 (JPtC = 814 Hz, C), 125.8 (CH), 116.1 (JPtC = 30 Hz, CH), 37.0 (NCH3), 32.0 (CH2), 24.0 (CH2), 23.2 (CH2), 14.1 (CH3). Anal. Calcd for C26H32Cl2N6Pt: C, 44.96; H, 4.64; N, 12.10. Found: C, 45.03; H, 4.74; N, 12.09.
General Procedure for the Preparation of [Pt(trz)2(C∧N)]OTf (4)
A Carius tube was charged with complex 3 (60 mg, 0.09 mmol), AgOTf (54 mg, 0.21 mmol), the N∧CH ligand (0.45 mmol), and 1,2-dichlorobenzene (2 mL), and the mixture was stirred at 120 °C for 14 h. After cooling down to room temperature, CH2Cl2 (10 mL) was added, and the mixture was filtered through Celite. An excess of NaOAc was then added, and the suspension was stirred for 30 min and filtered through Celite. Partial evaporation of the filtrate and addition to Et2O (10 mL) led to the precipitation of a white solid, which was collected by filtration and vacuum-dried to give the corresponding complex 4.
Data for [Pt(trz)2(dfppy)]OTf (4a)
Yield: 61 mg (73%). 1H NMR (600 MHz, CD2Cl2): δ = 8.45 (ddd, J = 9.0, 2.9, 1.4 Hz, 1H, CH), 8.05–8.00 (m, 2H, CH), 7.82 (dd, J = 8.0, 1.3 Hz, 1H, CH), 7.75 (dd with satellites, J = 8.0, 1.4 Hz, JPtH ∼ 8 Hz, 1H, CH), 7.31 (ddd, J = 7.9, 7.5, 1.3 Hz, 1H, CH), 7.27 (ddd, J = 7.9, 7.5, 1.2 Hz, 1H, CH), 7.17–7.13 (m, 2H, CH), 7.04 (td with satellites, J = 7.6, 1.4 Hz, JPtH ∼ 7 Hz, 1H, CH), 6.87 (dd with satellites, J = 7.4, 1.3 Hz, JPtH = 26 Hz, 1H, CH), 6.72 (ddd, J = 12.7, 8.8, 2.4 Hz, 1H, CH), 6.57 (dd with satellites, J = 7.8, 1.2 Hz, JPtH = 49 Hz, 1H, CH), 6.29 (dd with satellites, J = 7.3, 2.4 Hz, JPtH = 32 Hz, 1H, CH), 4.13 (s, 3H, NCH3), 4.13 (s, 3H, NCH3), 2.09 (ddd, J = 14.8, 11.8, 4.6 Hz, 1H, CH2), 1.99–1.91 (m, 2H, CH2), 1.90–1.84 (m, 1H, CH2), 1.26–1.10 (m, 2H, CH2), 1.03–0.82 (m, 6H, CH2), 0.76 (t, JHH = 7.2 Hz, 3H, CH3), 0.73 (t, JHH = 7.0 Hz, 3H, CH3). 13C APT NMR (150 MHz, CD2Cl2): δ = 164.7 (dd, JFC = 246, 10 Hz, C), 163.4 (d, JFC = 7 Hz, C), 162.9 (dd, JFC = 250, 11 Hz, C), 158.2 (JPtC = 536 Hz, C), 150.4 (CH), 146.2 (C), 144.3 (C), 143.7 (C), 141.5 (C), 141.1 (CH), 140.6 (JPtC = 508 Hz, C), 135.5 (CH), 132.8 (JPtC = 22 Hz, CH), 131.2 (JPtC = 29 Hz, CH), 130.8 (JPtC = 52 Hz, CH), 128.6 (C), 126.9 (CH), 126.1 (CH), 125.2 (JPtC = 780 Hz, C), 125.1 (JFC = 22 Hz, CH), 124.3 (CH), 117.3 (d with satellites, JFC = 19 Hz, JPtC = 39 Hz, CH), 116.6 (CH), 116.5 (CH), 101.5 (t, JFC = 27 Hz, CH), 37.3 (NCH3), 37.2 (NCH3), 32.3 (CH2), 32.3 (CH2), 24.2 (CH2), 24.2 (CH2), 23.3 (CH2), 23.1 (CH2), 13.9 (CH3), 13.8 (CH3). HRMS (ESI+) m/z: [M+] Calcd for C37H38F2N7Pt 813.2807; Found 813.2813. Anal. Calcd for C38H38F5N7O3PtS: C, 47.40; H, 3.98; N, 10.18; S, 3.33. Found: C, 47.40; H, 3.89; N, 10.20; S, 3.34.
Data for [Pt(trz)2(ppy)]OTf (4b)
Yield: 61 mg (76%). 1H NMR (600 MHz, CD2Cl2): δ = 8.11 (d, JHH = 8.1 Hz, 1H, CH), 8.02–7.96 (m, 2H, CH), 7.88 (dd, JHH = 7.9, 0.9 Hz, 1H, CH), 7.81 (ddd, JHH = 7.9, 1.2, 0.5 Hz, 1H, CH), 7.74 (dd with satellites, JHH = 8.0, 1.4 Hz, JPtH ∼ 8 Hz, 1H, CH), 7.29 (ddd, JHH = 8.0, 7.4, 1.3 Hz, 1H, CH), 7.25 (ddd, JHH = 8.0, 7.4, 1.2 Hz, 1H, CH), 7.23 (ddd, JHH = 7.9, 7.3, 1.3 Hz, 1H, CH), 7.15 (td, JHH = 7.4, 1.2 Hz, 1H, CH), 7.11 (ddd, JHH = 7.4, 5.7, 1.3 Hz, 1H, CH), 7.07–7.00 (m, 2H, CH), 6.92 (ddd with satellites, JHH = 7.2, 1.3, 0.5 Hz, JPtH = 25 Hz, 1H, CH), 6.75 (dd with satellites, JHH = 7.5, 1.2 Hz, JPtH = 27 Hz, 1H, CH), 6.61 (dd with satellites, JHH = 7.8, 1.2 Hz, JPtH = 50 Hz, 1H, CH), 4.10 (s, 3H, NCH3), 4.09 (s, 3H, NCH3), 2.03–1.95 (m, 2H, CH2), 1.85–1.76 (m, 2H, CH2), 1.18–1.10 (m, 2H, CH2), 1.01–0.79 (m, 6H, CH2), 0.73 (t, JHH = 7.2 Hz, 3H, CH3), 0.71 (t, JHH = 7.1 Hz, 3H, CH3). 13C APT NMR (150 MHz, CD2Cl2): δ = 167.1 (JPtC = 29 Hz, C), 152.0 (JPtC = 533 Hz, C), 150.1 (CH), 146.3 (JPtC = 77 Hz, C), 144.9 (JPtC = 795 Hz, C), 144.6 (C), 144.5 (C), 144.0 (JPtC ∼ 60 Hz, C), 142.5 (JPtC ∼ 495 Hz, C), 141.6 (C), 140.5(CH), 135.8 (JPtC = 15 Hz, CH), 135.1 (JPtC = 42 Hz, CH), 133.0 (JPtC = 24 Hz, CH), 131.7 (JPtC = 35 Hz, CH), 130.9 (JPtC = 28 Hz, CH), 130.5 (JPtC = 53 Hz, CH), 126.6 (CH), 126.0 (JPtC = 794 Hz, C), 125.9 (CH), 125.54 (CH), 125.49 (CH), 124.2 (CH), 121.2 (CH), 116.4 (CH), 37.1 (NCH3), 37.0 (NCH3), 32.3 (CH2), 32.2 (CH2), 24.2 (CH2), 24.0 (CH2), 23.13 (CH2), 23.07 (CH2), 13.9 (2 CH3). HRMS (ESI+) m/z: [M+] Calcd for C37H40N7Pt 777.2996; Found: 777.3005. Anal. Calcd for C38H40F3N7O3PtS: C, 49.24; H, 4.35; N, 10.58; S, 3.46. Found: C, 49.30; H, 4.32; N, 10.50; S, 3.40.
Data for [Pt(trz)2(tpy)]OTf (4c)
Yield: 65 mg (80%). 1H NMR (600 MHz, CD2Cl2): δ = 8.04 (d, JHH = 8.2 Hz, 1H, CH), 7.97–7.91 (m, 2H, CH), 7.80 (d, JHH = 8.0 Hz, 1H, CH), 7.77 (d, JHH = 8.0 Hz, 1H, CH), 7.74 (dd with satellites, JHH = 8.0, 1.4 Hz, JPtH ∼ 8 Hz, 1H, CH), 7.29 (td, JHH = 7.7, 1.1 Hz, 1H, CH), 7.25 (td, JHH = 7.7, 1.0 Hz, 1H, CH), 7.15 (td, JHH = 7.4, 1.0 Hz, 1H, CH), 7.07–7.00 (m, 3H, CH), 6.92 (dd with satellites, JHH = 7.2, 1.3 Hz, JPtH = 25 Hz, 1H, CH), 6.60 (dd with satellites, JHH = 7.8, 1.2 Hz, JPtH = 50 Hz, 1H, CH), 6.54 (s with satellites, JPtH = 27 Hz, 1H, CH), 4.10 (s, 3H, NCH3), 4.09 (s, 3H, NCH3), 2.12 (s, 3H, CH3), 2.06–1.95 (m, 2H, CH2), 1.86–1.79 (m, 1H, CH2), 1.78–1.72 (m, 1H, CH2), 1.20–1.11 (m, 2H, CH2), 1.02–0.84 (m, 6H, CH2), 0.73 (t, JHH = 7.2 Hz, 3H, CH3), 0.73 (t, JHH = 7.1 Hz, 3H, CH3). 13C APT NMR (150 MHz, CD2Cl2): δ = 167.2 (C), 152.1 (JPtC = 532 Hz, C), 150.0 (CH), 146.2 (JPtC = 76 Hz, C), 145.0 (C), 144.5 (C), 144.1 (C), 144.0 (C), 142.8 (C), 142.2 (C), 142.0 (C), 141.6 (C), 140.3 (CH), 135.9 (CH), 135.8 (CH), 132.9 (JPtC = 24 Hz, CH), 130.9 (JPtC = 28 Hz, CH), 130.5 (JPtC = 52 Hz, CH), 126.5 (CH), 126.4 (CH), 126.0 (JPtC = 793 Hz, C), 125.8 (JPtC = 19 Hz, CH), 125.5 (CH), 123.6 (CH), 120.9 (CH), 116.4 (2 CH), 37.1 (NCH3), 37.0 (NCH3), 32.3 (CH2), 32.2 (CH2), 24.2 (CH2), 24.0 (CH2), 23.2 (2 CH2), 21.9 (CH3), 14.0 (CH3), 13.9 (CH3). HRMS (ESI+) m/z: [M+] Calcd for C38H43N7Pt 791.3152; Found 791.3153. Anal. Calcd for C39H42F3N7O3PtS: C, 49.78; H, 4.50; N, 10.42; S, 3.41. Found: C, 49.60; H, 4.60; N, 10.24; S, 2.90.
Data for [Pt(trz)2(thpy)]OTf (4d)
Yield: 59 mg (73%). 1H NMR (600 MHz, CD2Cl2): δ = 7.88 (td, JHH = 7.7, 1.4 Hz, 1H, CH), 7.82–7.78 (m, 2H, CH), 7.72 (dd with satellites, JHH = 7.9, 1.4 Hz, JPtH ∼ 8 Hz, 1H, CH), 7.70 (dt, JHH = 8.1, 1.1 Hz, 1H, CH), 7.39 (d with satellites, JHH = 4.7 Hz, JPtH ∼ 5 Hz, 1H, CH), 7.30 (ddd, JHH = 7.9, 7.5, 1.3 Hz, 1H, CH), 7.23 (ddd, JHH = 8.0, 7.5, 1.2 Hz, 1H, CH), 7.14 (td, JHH = 7.4, 1.2 Hz, 1H, CH), 7.00 (td with satellites, JHH = 7.7, 1.4 Hz, JPtH ∼ 8 Hz, 1H, CH), 6.93 (ddd, JHH = 7.2, 5.6, 1.3 Hz, 1H, CH), 6.90 (dd with satellites, JHH = 7.3, 1.3 Hz, JPtH = 28 Hz, 1H, CH), 6.63 (d with satellites, JHH = 7.9 Hz, JPtH = 52 Hz, 1H, CH), 6.43 (d with satellites, JHH = 4.7 Hz, JPtH = 10 Hz, 1H, CH), 4.13 (s, 3H, NCH3), 4.12 (s, 3H, NCH3), 2.12 (ddd, JHH = 14.3, 11.7, 5.1 Hz, 1H, CH2), 2.06–1.91 (m, 3H, CH2), 1.24–1.15 (m, 2H, CH2), 1.10–0.84 (m, 6H, CH2), 0.78 (t, JHH = 7.2 Hz, 3H, CH3), 0.74 (t, JHH = 7.2 Hz, 3H, CH3). 13C APT NMR (150 MHz, CD2Cl2): δ = 162.3 (C), 156.6 (JPtC = 544 Hz, C), 150.0 (CH), 146.1 (JPtC = 74 Hz, C), 144.5 (C), 144.3 (C), 144.2 (C), 144.1 (C), 141.9 (JPtC = 46 Hz, C), 141.4 (C), 140.8 (CH), 140.3 (JPtC = 530 Hz, C), 135.7 (JPtC = 17 Hz, CH), 133.8 (JPtC = 60 Hz, CH), 133.5 (JPtC = 27 Hz, CH), 130.9 (JPtC = 30 Hz, CH), 130.8 (JPtC = 36 Hz, CH), 130.2 (JPtC = 53 Hz, CH), 126.7 (CH), 125.7 (CH), 122.2 (JPtC = 785 Hz, C), 121.8 (CH), 120.2 (CH), 116.4 (JPtC = 19 Hz, CH), 116.2 (JPtC = 30 Hz, CH), 37.2 (NCH3), 37.1 (NCH3), 32.3 (CH2), 32.2 CH2), 24.3 (CH2), 24.2 (CH2), 23.3 (CH2), 23.2 (CH2), 14.0 (2 CH3). HRMS (ESI+) m/z: [M+] Calcd for C35H38N7PtS 783.2559; Found 783.2550. Anal. Calcd for C36H38F3N7O3PtS2: C, 46.35; H, 4.11; N, 10.51; S, 6.87. Found: C, 46.19; H, 4.12; N, 10.30; S, 6.79.
Data for [Pt(trz)2(flpy)]OTf (4e)
Yield: 65 mg (72%). 1H NMR (600 MHz, CD2Cl2): δ = 8.19 (d, JHH = 8.2 Hz, 1H, CH), 8.03––7.97 (m, 2H, CH), 7.96 (s, 1H, CH), 7.83–7.81 (m, 1H, CH), 7.78 (dd with satellites, JHH = 8.0, 1.3 Hz, JPtH ∼ 8 Hz, 1H, CH), 7.43 (dt, JHH = 7.4, 0.9 Hz, 1H, CH), 7.33–7.27 (m, 4H, CH), 7.20 (td, JHH = 7.4, 1.1 Hz, 1H, CH), 7.16 (td, JHH = 7.4, 1.2 Hz, 1H, CH), 7.11–7.06 (m, 2H, CH), 7.04 (s with satellites, JPtH = 28 Hz, 1H, CH), 6.95 (ddd with satellites, JHH = 7.3, 1.3, 0.5 Hz, JPtH = 25 Hz, 1H, CH), 6.67 (dd with satellites, JHH = 7.8, 1.2 Hz, JPtH = 50 Hz, 1H, CH), 4.08 (s, 6H, NCH3), 2.07–1.95 (m, 2H, CH2), 1.93–1.80 (m, 2H, CH2), 1.53 (s, 3H, CH3), 1.48 (s, 3H, CH3), 1.17–1.09 (m, 2H, CH2), 1.02–0.80 (m, 4H, CH2), 0.72 (t, JHH = 7.1 Hz, 3H, CH3), 0.67–0.58 (m, 2H, CH2), 0.48 (t, JHH = 7.3 Hz, 3H, CH3). 13C APT NMR (150 MHz, CD2Cl2): δ = 167.1 (JPtC = 29 Hz, C), 155.1 (C), 151.3 (C), 151.1 (JPtC = 532 Hz, C), 150.2 (CH), 146.3 (C), 145.0 (C), 144.6 (C), 144.2 (C), 144.1 (C), 143.4 (C), 142.9 (JPtC = 37 Hz, C), 142.6 (C), 141.8 (C), 140.3 (CH), 138.7 (C), 135.9 (CH), 133.0 (JPtC = 22 Hz, CH), 131.0 (JPtC = 28 Hz, CH), 130.6 (JPtC = 52 Hz, CH), 128.6 (CH), 127.5 (CH), 126.5 (CH), 126.3 (JPtC = 790 Hz, C), 126.0 (JPtC = 42 Hz, CH), 125.7 (CH), 123.6 (CH), 123.3 (CH), 121.1 (CH), 120.7 (CH), 120.2 (JPtC = 20 Hz, CH), 116.5 (JPtC = 30 Hz, CH), 116.4 (JPtC ∼ 18 Hz, CH), 47.1 (C), 37.1 (NCH3), 37.0 (NCH3), 32.4 (CH2), 32.3 (CH2), 27.7 (CH3), 27.6 (CH3), 24.3 (CH2), 24.2 (CH2), 23.2 (CH2), 23.1 (CH2), 14.0 (CH3), 13.8 (CH3). HRMS (ESI+) m/z: [M+] Calcd for C46H48N7Pt 893.3617; Found: 893.3632. Anal. Calcd for C47H48F3N7O3PtS: C, 54.12; H, 4.64; N, 9.40; S, 3.07. Found: C, 54.11; H, 4.55; N, 9.33; S, 2.99.
Acknowledgments
This work was supported by grant PGC2018-100719-B-I00 funded by MCIN/AEI/10.13039/501100011033 and “ERDF A way of making Europe”, and grant IJC2019-039057-I funded by MCIN/AEI/10.13039/501100011033 and “ESF Investing in your future”.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.inorgchem.2c02039.
Photophysical characterization, X-ray structure determinations, crystallographic data, NMR spectra, electrochemical measurements, excitation and emission spectra, and computational methods and data (PDF)
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Mercs L.; Albrecht M. Beyond Catalysis: N-Heterocyclic Carbene Complexes as Components for Medicinal, Luminescent, and Functional Materials Applications. Chem. Soc. Rev. 2010, 39, 1903–1912. 10.1039/b902238b. [DOI] [PubMed] [Google Scholar]
- Visbal R.; Gimeno M. C. N-Heterocyclic Carbene Metal Complexes: Photoluminescence and Applications. Chem. Soc. Rev. 2014, 43, 3551–3574. 10.1039/C3CS60466G. [DOI] [PubMed] [Google Scholar]
- Strassner T. Phosphorescent Platinum(II) Complexes with C∧C* Cyclometalated NHC Ligands. Acc. Chem. Res. 2016, 49, 2680–2689. 10.1021/acs.accounts.6b00240. [DOI] [PubMed] [Google Scholar]
- Elie M.; Renaud J. L.; Gaillard S. N-Heterocyclic Carbene Transition Metal Complexes in Light Emitting Devices. Polyhedron 2018, 140, 158–168. 10.1016/j.poly.2017.11.045. [DOI] [Google Scholar]
- Suntrup L.; Stein F.; Hermann G.; Kleoff M.; Kuss-Petermann M.; Klein J.; Wenger O. S.; Tremblay J. C.; Sarkar B. Influence of Mesoionic Carbenes on Electro- and Photoactive Ru and Os Complexes: A Combined (Spectro-)Electrochemical, Photochemical, and Computational Study. Inorg. Chem. 2018, 57, 13973–13984. 10.1021/acs.inorgchem.8b02551. [DOI] [PubMed] [Google Scholar]
- Donnelly K. F.; Petronilho A.; Albrecht M. Application of 1,2,3-Triazolylidenes as Versatile NHC-Type Ligands: Synthesis, Properties, and Application in Catalysis and Beyond. Chem. Commun. 2013, 49, 1145–1159. 10.1039/C2CC37881G. [DOI] [PubMed] [Google Scholar]
- Karmis R. E.; Carrara S.; Baxter A. A.; Hogan C. F.; Hulett M. D.; Barnard P. J. Luminescent Iridium(Iii) Complexes of N-Heterocyclic Carbene Ligands Prepared Using the “Click Reaction”. Dalton Trans. 2019, 48, 9998–10010. 10.1039/C9DT01362H. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Kjær K. S.; Fredin L. A.; Chábera P.; Harlang T.; Canton S. E.; Lidin S.; Zhang J.; Lomoth R.; Bergquist K. E.; Persson P.; Wärnmark K.; Sundström V. A Heteroleptic Ferrous Complex with Mesoionic Bis(1,2,3-Triazol-5-Ylidene) Ligands: Taming the MLCT Excited State of Iron(II). Chem. - Eur. J. 2015, 21, 3628–3639. 10.1002/chem.201405184. [DOI] [PubMed] [Google Scholar]
- Chábera P.; Kjaer K. S.; Prakash O.; Honarfar A.; Liu Y.; Fredin L. A.; Harlang T. C. B.; Lidin S.; Uhlig J.; Sundström V.; Lomoth R.; Persson P.; Wärnmark K. FeII Hexa N-Heterocyclic Carbene Complex with a 528 ps Metal-To-Ligand Charge-Transfer Excited-State Lifetime. J. Phys. Chem. Lett. 2018, 9, 459–463. 10.1021/acs.jpclett.7b02962. [DOI] [PubMed] [Google Scholar]
- Wenger O. S. Is Iron the New Ruthenium?. Chem. - Eur. J. 2019, 25, 6043–6052. 10.1002/chem.201806148. [DOI] [PubMed] [Google Scholar]
- Wegeberg C.; Wenger O. S. Luminescent First-Row Transition Metal Complexes. JACS Au 2021, 1, 1860–1876. 10.1021/jacsau.1c00353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajoto T.; Djurovich P. I.; Tamayo A.; Yousufuddin M.; Bau R.; Thompson M. E.; Holmes R. J.; Forrest S. R. Blue and Near-UV Phosphorescence from Iridium Complexes with Cyclometalated Pyrazolyl or N-Heterocyclic Carbene Ligands. Inorg. Chem. 2005, 44, 7992–8003. 10.1021/ic051296i. [DOI] [PubMed] [Google Scholar]
- Stringer B. D.; Quan L. M.; Barnard P. J.; Wilson D. J. D.; Hogan C. F. Iridium Complexes of N-Heterocyclic Carbene Ligands: Investigation into the Energetic Requirements for Efficient Electrogenerated Chemiluminescence. Organometallics 2014, 33, 4860–4872. 10.1021/om500076w. [DOI] [Google Scholar]
- Monti F.; La Placa M. G. I.; Armaroli N.; Scopelliti R.; Grätzel M.; Nazeeruddin M. K.; Kessler F. Cationic Iridium(III) Complexes with Two Carbene-Based Cyclometalating Ligands: Cis Versus Trans Isomers. Inorg. Chem. 2015, 54, 3031–3042. 10.1021/acs.inorgchem.5b00148. [DOI] [PubMed] [Google Scholar]
- Li T.-Y.; Liang X.; Zhou L.; Wu C.; Zhang S.; Liu X.; Lu G.-Z.; Xue L.-S.; Zheng Y.-X.; Zuo J.-L. N-Heterocyclic Carbenes: Versatile Second Cyclometalated Ligands for Neutral Iridium(III) Heteroleptic Complexes. Inorg. Chem. 2015, 54, 161–173. 10.1021/ic501949h. [DOI] [PubMed] [Google Scholar]
- Lee J.; Chen H.-F.; Batagoda T.; Coburn C.; Djurovich P. I.; Thompson M. E.; Forrest S. R. Deep Blue Phosphorescent Organic Light-Emitting Diodes with Very High Brightness and Efficiency. Nat. Mater. 2016, 15, 92–98. 10.1038/nmat4446. [DOI] [PubMed] [Google Scholar]
- Lee J.; Jeong C.; Batagoda T.; Coburn C.; Thompson M. E.; Forrest S. R. Hot Excited State Management for Long-Lived Blue Phosphorescent Organic Light-Emitting Diodes. Nat. Commun. 2017, 8, 15566 10.1038/ncomms15566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamovich V.; Bajo S.; Boudreault P.-L. T.; Esteruelas M. A.; López A. M.; Martín J.; Oliván M.; Oñate E.; Palacios A. U.; San-Torcuato A.; Tsai J.-Y.; Xia C. Preparation of Tris-Heteroleptic Iridium(III) Complexes Containing a Cyclometalated Aryl-N-Heterocyclic Carbene Ligand. Inorg. Chem. 2018, 57, 10744–10760. 10.1021/acs.inorgchem.8b01367. [DOI] [PubMed] [Google Scholar]
- Na H.; Cañada L. M.; Wen Z.; I-Chia Wu J.; Teets T. S. Mixed-Carbene Cyclometalated Iridium Complexes with Saturated Blue Luminescence. Chem. Sci. 2019, 10, 6254–6260. 10.1039/C9SC01386E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cañada L. M.; Kölling J.; Wen Z.; Wu J. I. C.; Teets T. S. Cyano-Isocyanide Iridium(III) Complexes with Pure Blue Phosphorescence. Inorg. Chem. 2021, 60, 6391–6402. 10.1021/acs.inorgchem.1c00103. [DOI] [PubMed] [Google Scholar]
- Yan J.; Xue Q.; Yang H.; Yiu S.; Zhang Y.; Xie G.; Chi Y. Regioselective Syntheses of Imidazo[4,5-b]Pyrazin-2-Ylidene-Based Chelates and Blue Emissive Iridium(III) Phosphors for Solution-Processed OLEDs. Inorg. Chem. 2022, 61, 8797. 10.1021/acs.inorgchem.2c00750. [DOI] [PubMed] [Google Scholar]
- Unger Y.; Meyer D.; Molt O.; Schildknecht C.; Münster I.; Wagenblast G.; Strassner T. Green-Blue Emitters: NHC-Based Cyclometalated [Pt(C∧C)(Acac)] Complexes. Angew. Chem., Int. Ed. 2010, 49, 10214–10216. 10.1002/anie.201001316. [DOI] [PubMed] [Google Scholar]
- Tronnier A.; Heinemeyer U.; Metz S.; Wagenblast G.; Muenster I.; Strassner T. Heteroleptic Platinum(II) NHC Complexes with a C∧C* Cyclometalated Ligand – Synthesis, Structure and Photophysics. J. Mater. Chem. C 2015, 3, 1680–1693. 10.1039/C4TC02575J. [DOI] [Google Scholar]
- Soellner J.; Tenne M.; Wagenblast G.; Strassner T. Phosphorescent Platinum(II) Complexes with Mesoionic 1H-1,2,3-Triazolylidene Ligands. Chem. - Eur. J. 2016, 22, 9914–9918. 10.1002/chem.201601060. [DOI] [PubMed] [Google Scholar]
- Fuertes S.; Chueca A. J.; Perálvarez M.; Borja P.; Torrell M.; Carreras J.; Sicilia V. White Light Emission from Planar Remote Phosphor Based on NHC Cycloplatinated Complexes. ACS Appl. Mater. Interfaces 2016, 8, 16160–16169. 10.1021/acsami.6b03288. [DOI] [PubMed] [Google Scholar]
- Fuertes S.; Chueca A. J.; Arnal L.; Martín A.; Giovanella U.; Botta C.; Sicilia V. Heteroleptic Cycloplatinated N-Heterocyclic Carbene Complexes: A New Approach to Highly Efficient Blue-Light Emitters. Inorg. Chem. 2017, 56, 4829–4839. 10.1021/acs.inorgchem.6b02826. [DOI] [PubMed] [Google Scholar]
- Soellner J.; Strassner T. Phosphorescent Cyclometalated Platinum(II) ANHC Complexes. Chem. - Eur. J. 2018, 24, 15603–15612. 10.1002/chem.201802725. [DOI] [PubMed] [Google Scholar]
- Sicilia V.; Arnal L.; Chueca A. J.; Fuertes S.; Babaei A.; Muñoz A. M. I.; Sessolo M.; Bolink H. J. Highly Photoluminescent Blue Ionic Platinum-Based Emitters. Inorg. Chem. 2020, 59, 1145–1152. 10.1021/acs.inorgchem.9b02782. [DOI] [PubMed] [Google Scholar]
- Jaime S.; Arnal L.; Sicilia V.; Fuertes S. Cyclometalated NHCs Pt(II) Compounds with Chelating P∧P and S∧S Ligands: From Blue to White Luminescence. Organometallics 2020, 39, 3695–3704. 10.1021/acs.organomet.0c00510. [DOI] [Google Scholar]
- Sicilia V.; Arnal L.; Escudero D.; Fuertes S.; Martín A. Chameleonic Photo- And Mechanoluminescence in Pyrazolate-Bridged NHC Cyclometalated Platinum Complexes. Inorg. Chem. 2021, 60, 12274–12284. 10.1021/acs.inorgchem.1c01470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stipurin S.; Wurl F.; Strassner T. CC* Platinum(II) Complexes with PtXPX Metallacycle Forming (X = N and S) Auxiliary Ligands: Synthesis, Crystal Structures, and Properties. Organometallics 2022, 41, 313–320. 10.1021/acs.organomet.1c00655. [DOI] [Google Scholar]
- Tennyson A. G.; Rosen E. L.; Collins M. S.; Lynch V. M.; Bielawski C. W. Bimetallic N-Heterocyclic Carbene-Lridium Complexes: Investigating Metal-Metal and Metal-Ligand Communication via Electrochemistry and Phosphorescence Spectroscopy. Inorg. Chem. 2009, 48, 6924–6933. 10.1021/ic900391q. [DOI] [PubMed] [Google Scholar]
- Aghazada S.; Huckaba A. J.; Pertegas A.; Babaei A.; Grancini G.; Zimmermann I.; Bolink H.; Nazeeruddin M. K. Molecular Engineering of Iridium Blue Emitters Using Aryl N-Heterocyclic Carbene Ligands. Eur. J. Inorg. Chem. 2016, 2016, 5089–5097. 10.1002/ejic.201600971. [DOI] [Google Scholar]
- Quan L. M.; Stringer B. D.; Haghighatbin M. A.; Agugiaro J.; Barbante G. J.; Wilson D. J. D.; Hogan C. F.; Barnard P. J. Tuning the Electrochemiluminescent Properties of Iridium Complexes of N-Heterocyclic Carbene Ligands. Dalton Trans. 2019, 48, 653–663. 10.1039/C8DT04433C. [DOI] [PubMed] [Google Scholar]
- Lanoë P.-H.; Chan J.; Groué A.; Gontard G.; Jutand A.; Rager M.-N.; Armaroli N.; Monti F.; Barbieri A.; Amouri H. Cyclometalated N-Heterocyclic Carbene Iridium(III) Complexes with Naphthalimide Chromophores: A Novel Class of Phosphorescent Heteroleptic Compounds. Dalton Trans. 2018, 47, 3440–3451. 10.1039/C7DT04369D. [DOI] [PubMed] [Google Scholar]
- Macé A.; Hellou N.; Hammoud J.; Martin C.; Gauthier E. S.; Favereau L.; Roisnel T.; Caytan E.; Nasser G.; Vanthuyne N.; Williams J. A. G.; Berrée F.; Carboni B.; Crassous J. An Enantiopure Cyclometallated Iridium Complex Displaying Long-Lived Phosphorescence Both in Solution and in the Solid State. Helv. Chim. Acta 2019, 102, e1900044 10.1002/hlca.201900044. [DOI] [Google Scholar]
- Groué A.; Montier-Sorkine E.; Cheng Y.; Rager M. N.; Jean M.; Vanthuyne N.; Crassous J.; Lopez A. C.; Saavedra Moncada A.; Barbieri A.; Cooksy A. L.; Amouri H. Enantiopure, Luminescent, Cyclometalated Ir(III) Complexes with N-Heterocyclic Carbene-Naphthalimide Chromophore: Design, Vibrational Circular Dichroism and TD-DFT Calculations. Dalton Trans. 2022, 51, 2750–2759. 10.1039/D1DT04006E. [DOI] [PubMed] [Google Scholar]
- Esteruelas M. A.; López A. M.; Oñate E.; San-Torcuato A.; Tsai J.-Y.; Xia C. Preparation of Phosphorescent Iridium(III) Complexes with a Dianionic C,C,C,C-Tetradentate Ligand. Inorg. Chem. 2018, 57, 3720–3730. 10.1021/acs.inorgchem.7b02993. [DOI] [PubMed] [Google Scholar]
- Hsieh C.-H.; Wu F.-I.; Fan C.-H.; Huang M.-J.; Lu K.-Y.; Chou P.-Y.; Yang Y.-H. O.; Wu S.-H.; Chen I.-C.; Chou S.-H.; Wong K.-T.; Cheng C.-H. Design and Synthesis of Iridium Bis(Carbene) Complexes for Efficient Blue Electrophosphorescence. Chem. - Eur. J. 2011, 17, 9180–9187. 10.1002/chem.201100317. [DOI] [PubMed] [Google Scholar]
- Lu K. Y.; Chou H. H.; Hsieh C. H.; Yang Y. H. O.; Tsai H. R.; Tsai H. Y.; Hsu L. C.; Chen C. Y.; Chen I. C.; Cheng C. H. Wide-Range Color Tuning of Iridium Biscarbene Complexes from Blue to Red by Different N∧N Ligands: An Alternative Route for Adjusting the Emission Colors. Adv. Mater. 2011, 23, 4933–4937. 10.1002/adma.201102886. [DOI] [PubMed] [Google Scholar]
- Chang C.-F.; Cheng Y.-M.; Chi Y.; Chiu Y.-C.; Lin C.-C.; Lee G.-H.; Chou P.-T.; Chen C.-C.; Chang C.-H.; Wu C.-C. Highly Efficient Blue-Emitting Iridium(III) Carbene Complexes and Phosphorescent OLEDs. Angew. Chem., Int. Ed. 2008, 47, 4542–4545. 10.1002/anie.200800748. [DOI] [PubMed] [Google Scholar]
- Di Girolamo A.; Monti F.; Mazzanti A.; Matteucci E.; Armaroli N.; Sambri L.; Baschieri A. 4-Phenyl-1,2,3-Triazoles as Versatile Ligands for Cationic Cyclometalated Iridium(III) Complexes. Inorg. Chem. 2022, 61, 8509. 10.1021/acs.inorgchem.2c00567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliá F.; Bautista D.; Fernández-Hernández J. M.; González-Herrero P. Homoleptic Tris-Cyclometalated Platinum(IV) Complexes: A New Class of Long-Lived, Highly Efficient 3LC Emitters. Chem. Sci. 2014, 5, 1875–1880. 10.1039/C3SC53187B. [DOI] [Google Scholar]
- Juliá F.; Bautista D.; González-Herrero P. Developing Strongly Luminescent Platinum(IV) Complexes: Facile Synthesis of Bis-Cyclometalated Neutral Emitters. Chem. Commun. 2016, 52, 1657–1660. 10.1039/C5CC09566B. [DOI] [PubMed] [Google Scholar]
- Juliá F.; Aullón G.; Bautista D.; González-Herrero P. Exploring Excited-State Tunability in Luminescent Tris-Cyclometalated Platinum(IV) Complexes: Synthesis of Heteroleptic Derivatives and Computational Calculations. Chem. - Eur. J. 2014, 20, 17346–17359. 10.1002/chem.201404583. [DOI] [PubMed] [Google Scholar]
- López-López J. C.; Bautista D.; González-Herrero P. Stereoselective Formation of Facial Tris-Cyclometalated Pt(IV) Complexes: Dual Phosphorescence from Heteroleptic Derivatives. Chem. - Eur. J. 2020, 26, 11307–11315. 10.1002/chem.202001164. [DOI] [PubMed] [Google Scholar]
- López-López J.-C.; Bautista D.; González-Herrero P. Luminescent Halido(Aryl) Pt(IV) Complexes Obtained via Oxidative Addition of Iodobenzene or Diaryliodonium Salts to Bis-Cyclometalated Pt(II) Precursors. Dalton Trans. 2021, 50, 13294–13305. 10.1039/D1DT02349G. [DOI] [PubMed] [Google Scholar]
- López-López J.-C.; Bautista D.; González-Herrero P. Phosphorescent Biaryl Platinum(IV) Complexes Obtained through Double Metalation of Dibenzoiodolium Ions. Chem. Commun. 2022, 58, 4532–4535. 10.1039/D2CC01018F. [DOI] [PubMed] [Google Scholar]
- Juliá F.; García-Legaz M.-D.; Bautista D.; González-Herrero P. Influence of Ancillary Ligands and Isomerism on the Luminescence of Bis-Cyclometalated Platinum(IV) Complexes. Inorg. Chem. 2016, 55, 7647–7660. 10.1021/acs.inorgchem.6b01100. [DOI] [PubMed] [Google Scholar]
- Vivancos Á.; Poveda D.; Muñoz A.; Moreno J.; Bautista D.; González-Herrero P. Selective Synthesis, Reactivity and Luminescence of Unsymmetrical Bis-Cyclometalated Pt(IV) Complexes. Dalton Trans. 2019, 48, 14367–14382. 10.1039/C9DT02431J. [DOI] [PubMed] [Google Scholar]
- Vivancos Á.; Bautista D.; González-Herrero P. Luminescent Platinum(IV) Complexes Bearing Cyclometalated 1,2,3-Triazolylidene and Bi- or Terdentate 2,6-Diarylpyridine Ligands. Chem. - Eur. J. 2019, 25, 6014–6025. 10.1002/chem.201900489. [DOI] [PubMed] [Google Scholar]
- Vivancos Á.; Jiménez-García A.; Bautista D.; González-Herrero P. Strongly Luminescent Pt(IV) Complexes with a Mesoionic N-Heterocyclic Carbene Ligand: Tuning Their Photophysical Properties. Inorg. Chem. 2021, 60, 7900–7913. 10.1021/acs.inorgchem.1c00410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman C. P.; Deeth R. J.; Clarkson G. J.; Rourke J. P. Synthesis of Mixed NHC/L Platinum(II) Complexes: Restricted Rotation of the NHC Group. Organometallics 2007, 26, 6225–6233. 10.1021/om700671y. [DOI] [Google Scholar]
- Winkel R. W.; Dubinina G. G.; Abboud K. A.; Schanze K. S. Photophysical Properties of Trans-Platinum Acetylide Complexes Featuring N-Heterocyclic Carbene Ligands. Dalton Trans. 2014, 43, 17712–17720. 10.1039/C4DT01520G. [DOI] [PubMed] [Google Scholar]
- Bullock J. D.; Salehi A.; Zeman C. J.; Abboud K. A.; So F.; Schanze K. S. In Search of Deeper Blues: Trans-N-Heterocyclic Carbene Platinum Phenylacetylide as a Dopant for Phosphorescent OLEDs. ACS Appl. Mater. Interfaces 2017, 9, 41111–41114. 10.1021/acsami.7b12107. [DOI] [PubMed] [Google Scholar]
- Miguel-Coello A. B.; Bardají M.; Coco S.; Donnio B.; Heinrich B.; Espinet P. Triphenylene-Imidazolium Salts and Their NHC Metal Complexes, Materials with Segregated Multicolumnar Mesophases. Inorg. Chem. 2018, 57, 4359–4369. 10.1021/acs.inorgchem.7b03178. [DOI] [PubMed] [Google Scholar]
- Rehm T.; Rothemund M.; Dietel T.; Kempe R.; Schobert R. Synthesis, Structures and Cytotoxic Effects: In Vitro of Cis-and Trans-[PtIVCl4(NHC)2] Complexes and Their PtII Precursors. Dalton Trans. 2019, 48, 16358–16365. 10.1039/C9DT02438G. [DOI] [PubMed] [Google Scholar]
- Friebolin H.Basic One- and Two-Dimensional NMR Spectroscopy, 5th ed.; Wiley-VCH: Weinheim, Germany, 2010. [Google Scholar]
- Burling S.; Douglas S.; Mahon M. F.; Nama D.; Pregosin P. S.; Whittlesey M. K. Cationic Tris N-Heterocyclic Carbene Rhodium Carbonyl Complexes: Molecular Structures and Solution NMR Studies. Organometallics 2006, 25, 2642–2648. 10.1021/om060202p. [DOI] [Google Scholar]
- Shikata Y.; Yasue R.; Yoshida K. Coordination Behavior of a Planar Chiral Cyclic (Amino)(Ferrocenyl)Carbene Ligand in Iridium Complexes. Chem. - Eur. J. 2017, 23, 16806–16812. 10.1002/chem.201703467. [DOI] [PubMed] [Google Scholar]
- Nguyen V. H.; Dang T. T.; Nguyen H. H.; Huynh H. V. Platinum(II) 1,2,4-Triazolin-5-Ylidene Complexes: Stereoelectronic Influences on Their Catalytic Activity in Hydroelementation Reactions. Organometallics 2020, 39, 2309–2319. 10.1021/acs.organomet.0c00260. [DOI] [Google Scholar]
- Lewis-Alleyne L. C.; Bassil B. S.; Böttcher T.; Röschenthaler G. V. Selective Synthesis of Cis- and Trans-[(NHCMe)2PtCl2] and [NHCMePt(COD)Cl][NHCMePtCl3] Using NHCMeSiCl4. Dalton Trans. 2014, 43, 15700–15703. 10.1039/C4DT02214A. [DOI] [PubMed] [Google Scholar]
- Valandro S. R.; He R.; Bullock J. D.; Arman H.; Schanze K. S. Ultrafast Excited-State Dynamics in Trans-(N-Heterocyclic Carbene)Platinum(II) Acetylide Complexes. Inorg. Chem. 2021, 60, 10065–10074. 10.1021/acs.inorgchem.1c00288. [DOI] [PubMed] [Google Scholar]
- He R.; Xu Z.; Valandro S.; Arman H. D.; Xue J.; Schanze K. S. High-Purity and Saturated Deep-Blue Luminescence from Trans-NHC Platinum(II) Butadiyne Complexes: Properties and Organic Light Emitting Diode Application. ACS Appl. Mater. Interfaces 2021, 13, 5327–5337. 10.1021/acsami.0c21193. [DOI] [PubMed] [Google Scholar]
- Juliá F.; González-Herrero P. Spotlight on the Ligand: Luminescent Cyclometalated Pt(IV) Complexes Containing a Fluorenyl Moiety. Dalton Trans. 2016, 45, 10599–10608. 10.1039/C6DT01722C. [DOI] [PubMed] [Google Scholar]
- Munz D. Pushing Electrons—Which Carbene Ligand for Which Application?. Organometallics 2018, 37, 275–289. 10.1021/acs.organomet.7b00720. [DOI] [Google Scholar]
- Poulain A.; Canseco-Gonzalez D.; Hynes-Roche R.; Müller-Bunz H.; Schuster O.; Stoeckli-Evans H.; Neels A.; Albrecht M. Synthesis and Tunability of Abnormal 1,2,3-Triazolylidene Palladium and Rhodium Complexes. Organometallics 2011, 30, 1021–1029. 10.1021/om101076u. [DOI] [Google Scholar]
- Goggin P. L.; Goodfellow R. J.; Reed F. J. S. Trimethylamine Complexes of Platinum(II) and Palladium(II) and Their Vibrational and Proton Nuclear Magnetic Resonance Spectra. J. Chem. Soc., Dalton Trans. 1972, 1298–1303. 10.1039/dt9720001298. [DOI] [Google Scholar]
- Powers D. C.; Benitez D.; Tkatchouk E.; Goddard W. A.; Ritter T. Bimetallic Reductive Elimination from Dinuclear Pd(III) Complexes. J. Am. Chem. Soc. 2010, 132, 14092–14103. 10.1021/ja1036644. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.