Key Points
Question
What were the contemporary, real-world patterns of use and outcomes of multivessel vs culprit vessel–only percutaneous coronary intervention for acute myocardial infarction and cardiogenic shock in the US from 2009 to 2018?
Findings
In this cohort study of 64 301 patients, an increasing proportion of US patients underwent multivessel percutaneous coronary intervention for acute myocardial infarction and cardiogenic shock, with substantial variation in care practices across hospitals. The strategy of multivessel percutaneous coronary intervention was associated with worse periprocedural outcomes and excess in-hospital mortality, particularly in patients with ST-elevation myocardial infarction, without evidence of improved long-term outcomes.
Meaning
This study suggests that the use of multivessel percutaneous coronary intervention to treat patients with acute myocardial infarction and cardiogenic shock should be studied more extensively to potentially improve outcomes in this high-risk patient group.
This cohort study evaluates patterns in the use of multivessel vs culprit-vessel percutaneous coronary intervention in acute myocardial infarction and cardiogenic shock in the US from 2009 to 2018.
Abstract
Importance
Cardiogenic shock after acute myocardial infarction (AMI) is associated with high mortality, particularly among patients with multivessel coronary artery disease. Recent evidence suggests that use of multivessel percutaneous coronary intervention (PCI) may be associated with harm. However, little is known about recent patterns of care and outcomes for this patient population.
Objective
To evaluate patterns in the use of multivessel PCI vs culprit-vessel PCI in AMI and cardiogenic shock and outcomes in the US from 2009 to 2018.
Design, Setting, and Participants
This cohort study identified all patients in the CathPCI Registry) with AMI and cardiogenic shock who had multivessel coronary artery disease and underwent PCI between July 1, 2009, and March 31, 2018.
Exposures
Multivessel or culprit-vessel PCI for AMI and shock.
Primary Outcomes and Measures
The primary outcome was in-hospital mortality. Temporal trends and hospital variation in PCI strategies were evaluated, while accounting for differences in case mix using hierarchical models. As a secondary outcome, the association of PCI strategy with postdischarge outcomes was evaluated in the subset of patients who were Medicare beneficiaries.
Results
Of 64 301 patients (mean [SD] age, 66.4 [12.5] years; 20 366 [31.7%] female; 54 538 [84.8%] White) with AMI and shock at 1649 US hospitals, 34.9% had primary multivessel PCI. In the subgroup of 48 943 patients with ST-segment elevation myocardial infarction (STEMI), 31.5% underwent multivessel PCI. Between 2009 and 2018, this percentage increased by 6.7% per year for AMI and 5.8% for STEMI. Overall, multivessel PCI was associated with a greater adjusted risk of in-hospital complications (odds ratio [OR], 1.18; 95% CI, 1.14-1.23) and with greater in-hospital mortality in patients with STEMI (OR, 1.11; 95% CI, 1.06-1.16). Among Medicare beneficiaries, multivessel PCI use was not associated with postdischarge 1-year mortality (51.5% vs 49.8%; risk-adjusted OR, 0.97; 95% CI, 0.90-1.04; P = .37). Significant hospital variation was found in the use of multivessel PCI, with a higher multivessel PCI rate for similar patients across hospitals (median OR, 1.37; 95% CI, 1.33-1.41). Patients at hospitals with high rates of PCI in STEMI use had higher risk-adjusted in-hospital mortality (highest vs lowest hospital multivessel PCI quartile: OR, 1.10; 95% CI, 1.02-1.19).
Conclusions and Relevance
This cohort study found that multivessel PCI was increasingly used as the revascularization strategy in AMI and shock and that hospitals that used multivessel PCI more, especially among patients with STEMI, had worse outcomes. With recent evidence suggesting harm with this strategy, there appears to be an urgent need to change practice and improve outcomes in this high-risk population.
Introduction
One in 3 patients experiencing an acute myocardial infarction (AMI) complicated by cardiogenic shock does not survive to hospital discharge.1,2,3 Early revascularization is associated with improved long-term patient outcomes,4,5 but the optimal revascularization approach among patients with AMI and cardiogenic shock with multivessel obstructive coronary artery disease (CAD) has remained poorly understood. In these conditions, treatment options include revascularizing the culprit vessel alone or pursuing a more complete revascularization of both the culprit and other vessels with significant stenoses. Guidelines have recommended primary multivessel percutaneous coronary intervention (PCI) in patients with cardiogenic shock based on expert consensus.6 However, a large randomized clinical trial, Culprit Lesion Only PCI Vs Multi-vessel PCI in Cardiogenic Shock (CULPRIT-SHOCK), found that multivessel PCI during the index procedure in cardiogenic shock and AMI led to a lower rate of survival at 30 days7 and at 1-year follow-up compared with culprit vessel–only PCI.8
In light of prior guidelines and the recent findings of CULPRIT-SHOCK, it is crucial to understand recent real-world practice patterns regarding pursuit of multivessel PCI vs a culprit vessel–only approach for cardiogenic shock because it may guide interventions aimed at improving care for these high-risk patients. Moreover, such an assessment evaluates whether the harms of multivessel PCI demonstrated in a randomized clinical trial were observable in real-world practice. As a part of the American College of Cardiology’s Research to Practice initiative, we used the National Cardiovascular Data Registry (NCDR) CathPCI Registry to evaluate real-world patterns and variation in revascularization practices across US hospitals in PCI admissions for patients with multivessel CAD presenting with AMI and cardiogenic shock and the association of variation in revascularization practices with patient outcomes.
Methods
Data Sources
In this cohort study, we used data from the NCDR CathPCI Registry from July 1, 2009, to March 31, 2018. The CathPCI Registry includes all patients undergoing PCIs at participating US hospitals nationwide and captures information on patient and procedural characteristics and patient outcomes. In a subset of patients who were fee-for-service Medicare beneficiaries 65 years or older, we also obtained information on longitudinal outcomes after the index hospitalization from corresponding Medicare claims data linked to CathPCI Registry data using direct patient identifiers.9 Because this was an analysis of existing deidentified data, the study was nonhuman subject research under 45 CFR §46, with waiver of informed consent for secondary analysis of deidentified data.
Study Population
We identified all PCI admissions for patients who presented to a catheterization laboratory with AMI and cardiogenic shock with multivessel CAD. Patients with AMI included those with ST-segment elevation myocardial infarction (STEMI) or non-STEMI. Cardiogenic shock was defined as a sustained (>30-minute) episode of systolic blood pressure less than 90 mm Hg and/or a cardiac index less than 2.2 L/min/m2 determined to be secondary to cardiac dysfunction, and/or the requirement for parenteral inotropic or vasopressor agents or mechanical support to maintain blood pressure and cardiac index above those levels. Multivessel CAD was defined as a stenosis of 70% or greater in 2 or more epicardial coronary arteries other than the left main, where a stenosis of 50% or more was considered obstructive. The culprit vessel for AMI was defined as the coronary segment with the highest degree of stenosis and lesion complexity identified during diagnostic coronary angiography. Multivessel PCI was defined as PCI in 2 or more territories (left main, left anterior descending, left circumflex, or right coronary artery) during the index procedure. We excluded admissions in which the patient had a prior coronary artery bypass graft (CABG). In addition, hospital-level analyses were restricted to hospitals with 10 or more eligible admissions.
Study Covariates
We identified the following patient-level factors that are associated with outcomes based on published models for the CathPCI Registry10: (1) demographics (age, sex, and race/ethnicity), (2) body mass index, (3) medical history (hypertension, diabetes, heart failure, current or recent smoking, dyslipidemia, prior myocardial infarction, valve surgery, cerebrovascular disease, peripheral artery disease, chronic lung disease, prior PCI, or prior CABG), (4) family history of CAD, (5) pertinent laboratory values (glomerular filtration rate on presentation), and (6) presentation characteristics (AMI type [STEMI or non-STEMI], anginal symptoms within 2 weeks before presentation based on Canadian Cardiovascular Society angina grade [I to IV], antianginal medication use before presentation, heart failure symptoms on presentation according to New York Heart Association class [I to IV], cardiomyopathy or left ventricular systolic dysfunction, and intra-aortic balloon pump placement and its timing relative to revascularization).
The preangiographic characteristics have high discrimination for in-hospital mortality10 and, therefore, would represent the major confounders in all patients with cardiogenic and multivessel disease. To further account for the severity of CAD that could confound the association of PCI strategies with outcomes, we included an additional covariate for CAD severity based on the number of coronary vessels with obstructive epicardial disease (2-vessel, 3-vessel, or 4-vessel CAD). We used the above covariates in risk adjustment of all in-hospital outcomes (mortality and complications), given the substantial overlap in risk factors for these outcomes.10,11,12,13 We also identified characteristics for participating hospitals, including teaching status, urban or rural status, ownership, bed size, US census region, number of beds, and annual PCI volume.
Study Exposure and Outcomes
The study exposure was the use of multivessel PCI or culprit vessel–only PCI. The primary outcome was in-hospital mortality. We identified several secondary outcomes based on a prior study9 that used the CathPCI Registry. These secondary outcomes included in-hospital bleeding, vascular complications (pseudoaneurysm, dissection, and lower extremity ischemia), intraprocedure or postprocedure events (myocardial infarction and stroke), and acute kidney injury (AKI) that required initiation of dialysis. For the subgroup of patients 65 years or older linked with Medicare claims from July 1, 2009, to December 31, 2016, we examined 1-year major adverse coronary events—a composite of all-cause death, hospitalization for MI, or additional coronary revascularization occurring from primary hospitalization to 1-year follow-up.14 We also evaluated postdischarge heart failure hospitalizations. We identified outcomes of additional revascularization, readmission for MI, and heart failure in Medicare claims data, as previously described.9
Statistical Analysis
All analyses were conducted in the overall population with AMI and in the major subgroup of patients with STEMI. At the patient level, we compared differences in patient, angiographic, and procedural characteristics between these groups using the χ2 test for categorical variables and the t test for continuous variables. We also calculated standardized differences in characteristics, with values greater than 10% representing meaningful differences between groups.15,16 We evaluated temporal trends in multivessel PCI across calendar quarters in the cohort.
At the hospital level, we reported the frequency distribution of multivessel PCI across hospitals as a proportion of their eligible patients. We grouped hospitals into quartiles based on the proportion of multivessel PCI used during the study period and examined differences in hospital and PCI characteristics across quartiles.
To better quantify the site-level variation in the use of multivessel vs culprit vessel–only PCI, we constructed a patient-level hierarchical logistic regression model with use of multivessel vs culprit vessel–only PCI as the dependent variable, patient characteristics as fixed effects, and hospital site as the random effect. Using the model, we calculated the median odds ratio (OR), which represents the mean odds of 2 identical patients receiving multivessel revascularization at one randomly selected hospital compared with another.
Next, we compared differences in outcomes between multivessel and culprit vessel–only PCI. For this, we constructed a hierarchical logistic regression model with hospital-level random effects, patient-level fixed effects (described in study covariates above), and in-hospital mortality as the outcome. We then repeated these analyses for each of the secondary outcomes. Furthermore, we examined the risk-adjusted odds of these outcomes across quartiles of hospitals based on their rates of multivessel PCI.
Finally, in the subset of patients who were Medicare beneficiaries with a claim record, we repeated the above analyses for postdischarge outcomes, including mortality, bleeding, and rates of additional revascularization. We constructed Kaplan-Meier curves to assess rates of these outcomes across the period of follow-up. Using risk-adjustment strategies outlined above, we assessed risk-adjusted odds of mortality, bleeding, and revascularization in the year after the index intervention.
Analyses were performed using SAS statistical software, version 9.4 (SAS Institute Inc), and statistical significance was set at 2-sided P < .05.
Results
A total of 122 522 patients with AMI and cardiogenic shock underwent cardiac catheterization and revascularization. Of these, 11 146 patients had prior CABG and 47 075 had single-vessel CAD. The remaining 64 301 patients (57.7%; mean [SD] age, 66.4 [12.5] years; 20 366 [31.7%] female; 54 538 [84.8%] White) had multivessel CAD and underwent multivessel or culprit vessel–only PCI at 1 of 1649 hospitals (eFigure in the Supplement). A total of 48 943 patients (76.1%) presented with STEMI, and 15 358 (23.9%) presented with non-STEMI. On diagnostic angiography, 8962 patients (13.9%) had obstructive disease of the left main artery, 49 765 (77.4%) of the left anterior descending artery, 40 365 (62.8%) of the left circumflex artery, and 47 052 (73.2%) of the right coronary artery (Table).
Table. Characteristics of Patients Presenting With AMI and Cardiogenic Shock With Multivessel Coronary Artery Diseasea.
| Characteristic | Total (N = 64 301) | Multivessel PCI | P value | |
|---|---|---|---|---|
| No (n = 41 883) | Yes (n = 22 418) | |||
| Demographics | ||||
| Patient age, mean (SD), y | 66.4 (12.5) | 66.6 (12.4) | 65.9 (12.6) | <.001 |
| Age >70 y | 23 879 (37.1) | 15 832 (37.8) | 8047 (35.9) | <.001 |
| Female | 20 366 (31.7) | 13 297 (31.8) | 7069 (31.5) | .58 |
| Race/ethnicity | ||||
| White | 54 538 (84.8) | 35 671 (85.2) | 18 867 (84.2) | <.001 |
| Black | 5388 (8.4) | 3542 (8.5) | 1846 (8.2) | |
| Other | 4375 (6.8) | 2670 (6.4) | 1705 (7.6) | |
| BMI, mean (SD) | 28.65 (6.4) | 28.53 (6.4) | 28.87 (6.5) | <.001 |
| History | ||||
| Hypertension | 46 027 (71.6) | 29 916 (71.4) | 16 111 (71.9) | .24 |
| Diabetes | 21 126 (32.9) | 13 279 (31.7) | 7847 (35.0) | <.001 |
| Non–insulin-dependent diabetes | 11 553 (18.0) | 7510 (17.9) | 4043 (18.0) | |
| Insulin-dependent diabetes | 9573 (14.9) | 5769 (13.8) | 3804 (17.0) | |
| GFR, mean (SD), mL/min/1.73 m2 | 60.5 (31.7) | 60.4 (32.5) | 60.6 (30.3) | .42 |
| Hemodialysis at presentation | 2621 (4.1) | 1456 (3.5) | 1165 (5.2) | <.001 |
| Cerebrovascular disease | 8110 (12.6) | 5270 (12.6) | 2840 (12.7) | .76 |
| Peripheral arterial disease | 7700 (12.0) | 4909 (11.7) | 2791 (12.5) | .001 |
| Chronic lung disease | 10 056 (15.6) | 6590 (15.7) | 3466 (15.5) | .36 |
| Active tobacco use | 21 466 (33.4) | 14 435 (34.5) | 7031 (31.4) | <.001 |
| Dyslipidemia | 37 688 (58.6) | 24 276 (58.0) | 13 412 (59.8) | <.001 |
| Family history of premature CAD | 8650 (13.5) | 5711 (13.6) | 2939 (13.1) | .06 |
| MI | 14 583 (22.7) | 9240 (22.1) | 5343 (23.8) | <.001 |
| Heart failure | 9263 (14.4) | 5549 (13.3) | 3714 (16.6) | <.001 |
| Valve surgery or procedure | 479 (0.7) | 304 (0.7) | 175 (0.8) | .44 |
| PCI | 14 258 (22.2) | 9036 (21.6) | 5222 (23.3) | <.001 |
| Catheterization laboratory visit | ||||
| CAD presentation | <.001 | |||
| STEMIb | 48 943 (76.1) | 33 549 (80.1) | 15 394 (68.7) | |
| Anginal classification within 2 wk | ||||
| No symptoms | 7945 (12.4) | 5389 (12.9) | 2556 (11.4) | <.001 |
| CCS I | 470 (0.7) | 325 (0.8) | 145 (0.7) | |
| CCS II | 1339 (2.1) | 909 (2.2) | 430 (1.9) | |
| CCS III | 6481 (10.1) | 4146 (9.9) | 2335 (10.4) | |
| CCS IV | 47 897 (74.5) | 30 998 (74.0) | 16 899 (75.4) | |
| NA or missing | 169 (0.3) | 116 (0.3) | 53 (0.2) | |
| Antianginal medication within 2 wk | 28 649 (44.6) | 18 193 (43.4) | 10 456 (46.6) | <.001 |
| Heart failure within 2 wkb | 20 382 (31.7) | 11 787 (28.1) | 8595 (38.3) | <.001 |
| NYHA class within 2 wk | ||||
| Class I | 634 (1.0) | 413 (1.0) | 221 (1.0) | <.001 |
| Class II | 1835 (2.9) | 1162 (2.8) | 673 (3.0) | |
| Class III | 4084 (6.4) | 2487 (5.9) | 1597 (7.1) | |
| Class IV | 13 742 (21.4) | 7675 (18.3) | 6067 (27.1) | |
| NA or missing | 44 006 (68.4) | 30 146 (72.0) | 13 860 (61.8) | |
| Cardiomyopathy or left ventricular systolic dysfunctionb | 14 011 (21.8) | 8079 (19.3) | 5932 (26.5) | <.001 |
| Intra-aortic balloon pumpb | 31 814 (49.5) | 19 923 (47.6) | 11 891 (53.0) | <.001 |
| Other mechanical circulatory support devicesb | 1770 (9.8) | 933 (7.5) | 837 (14.6) | <.001 |
| Coronary anatomyc | ||||
| Left main | 8962 (13.9) | 4098 (9.8) | 4864 (21.7) | <.001 |
| LAD | 49 765 (77.4) | 30 787 (73.5) | 18 978 (84.7) | <.001 |
| LCX | 40 365 (62.8) | 24 304 (58.0) | 16 061 (71.6) | <.001 |
| RCA | 47 052 (73.2) | 31 675 (75.6) | 15 377 (68.6) | <.001 |
| PCI procedure | ||||
| Pre-PCI left ventricular ejection fraction, mean (SD), % | 34.87 (15.5) | 36.18 (15.6) | 32.73 (15.1) | <.001 |
| NA or missing | 36 585 (56.9) | 24 703 (59.0) | 11 882 (53.0) | <.001 |
| Highest-risk lesion or culprit vesseld | ||||
| Proximal RCA, mid LAD, and proximal LCX | 24 611 (38.3) | 17 412 (41.6) | 7199 (32.1) | <.001 |
| Proximal LAD | 19 522 (30.4) | 11 226 (26.8) | 8296 (37.0) | |
| Left main | 6180 (9.6) | 1375 (3.3) | 4805 (21.4) | |
| Other | 13 833 (21.5) | 11 715 (28.0) | 2118 (9.5) | |
| Unknown | 155 (0.2) | 155 (0.4) | 0 (0.0) | |
| Additional revascularization | ||||
| Subsequent PCI during hospitalization | 5150 (8.0) | 1206 (2.9) | 3944 (17.6) | <.001 |
| CABG during hospitalization | 4443 (6.9) | 3733 (8.9) | 710 (3.2) | <.001 |
| Hospital disposition | ||||
| In-hospital death | 23 159 (36.0) | 15 049 (35.9) | 8110 (36.2) | <.001 |
| Home | 26 356 (41.0) | 17 241 (41.2) | 9115 (40.7) | |
| Extended care and rehabilitation | 6310 (9.8) | 3939 (9.4) | 2371 (10.6) | |
| Other acute care hospital | 4902 (7.6) | 3339 (8.0) | 1563 (7.0) | |
| Nursing home | 1968 (3.1) | 1239 (3.0) | 729 (3.3) | |
| Hospice | 1216 (1.9) | 825 (2.0) | 391 (1.7) | |
| Other | 204 (0.3) | 123 (0.3) | 81 (0.4) | |
| Left against medical advice | 186 (0.3) | 128 (0.3) | 58 (0.3) | |
Abbreviations: AMI, acute myocardial infarction; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CABG, coronary artery bypass graft; CAD, coronary artery disease; CCS, Canadian Cardiovascular Society; GFR, glomerular filtration rate; LAD, left anterior descending artery; LCX, left circumflex artery; MI, myocardial infarction; NA, not applicable; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; RCA, right coronary artery; SCAI, Society for Cardiovascular Angiography and Interventions; STEMI, ST-segment elevation MI.
Data are presented as number (percentage) of patients unless otherwise indicated.
Characteristics with standardized differences exceeding 10%.
Lesion in coronary artery was classified as obstructive at 50% stenosis or more for left main artery and 70% stenosis or more of other vessels.
Highest degree of stenosis and lesion complexity.
Patient-Level Use of PCI Strategies in AMI and Shock
A total of 22 418 patients (34.9%) underwent multivessel PCI; the remaining 41 883 (65.1%) underwent culprit vessel–only PCI. The rates of multivessel PCI were similar in STEMI (15 394 of 48 943 [31.5%]). The proportion of admissions with multivessel PCI in this context increased over time for all patients with AMI and cardiogenic shock, from 28.8% in quarter 3 of 2009 to 44.1% in quarter 1 of 2018 (mean relative annual increase of 6.7%), and a corresponding increase of 25.7% to 39.9% was seen in those with STEMI and cardiogenic shock (mean relative annual increase of 5.8%) (Figure 1). No changes occurred in rates of multivessel PCI in the months spanning the publication of the CULPRIT-SHOCK trial in October 2017 (Figure 1). Characteristics of patients undergoing multivessel PCI and culprit vessel–only PCI are compared in the Table.
Figure 1. Temporal Trends in Multivessel Percutaneous Coronary Intervention (PCI).
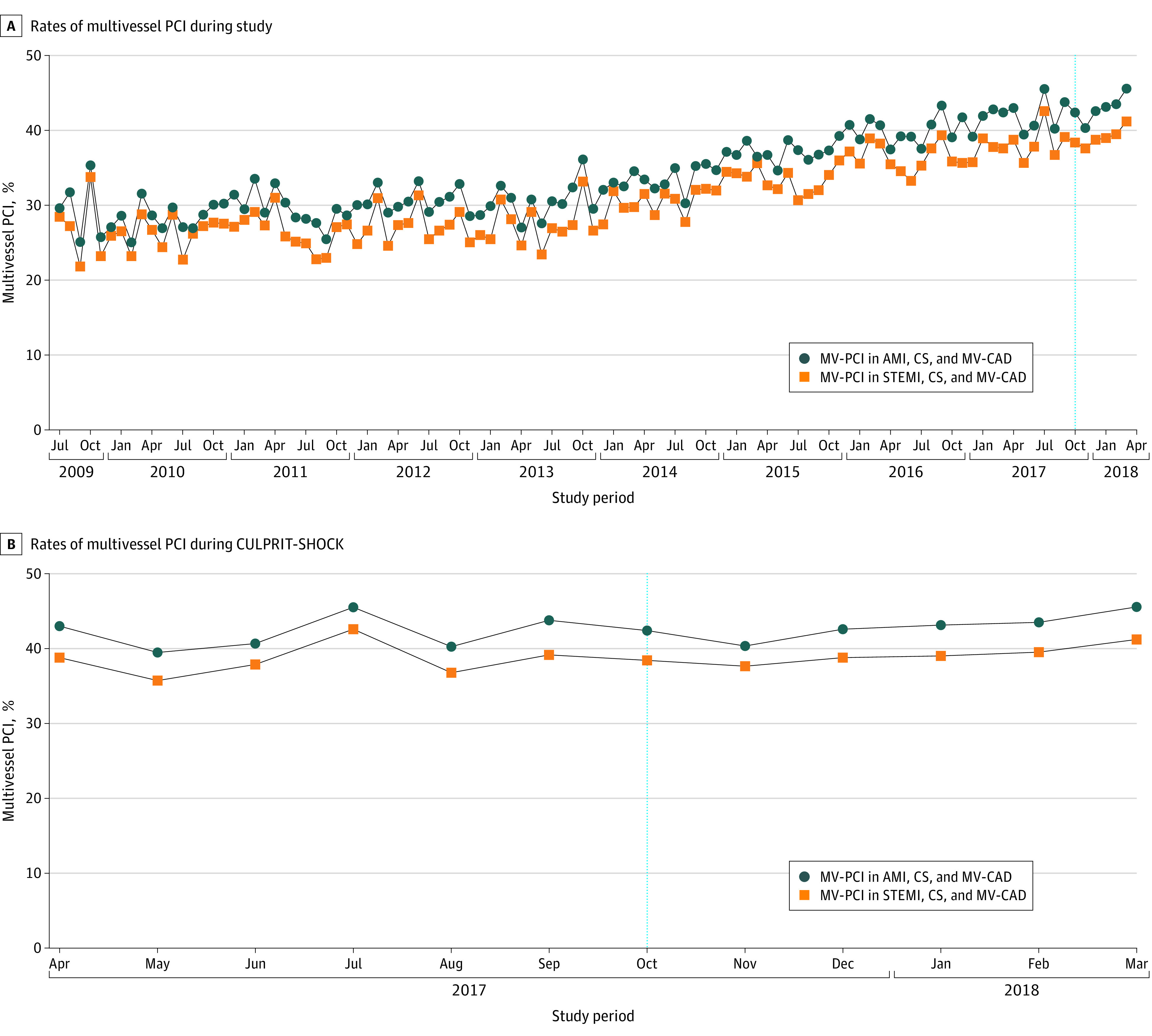
Trends are presented as monthly rates of multivessel PCI across the study period (July 2009-March 2018) (A) and across the 12-month period spanning the publication of the Culprit Lesion Only PCI vs Multi-vessel PCI in Cardiogenic Shock (CULPRIT-SHOCK) trial in October 2017 (dotted vertical line) (B). AMI indicates acute myocardial infarction; CS, cardiogenic shock; MV-CAD, multivessel coronary artery disease; and STEMI, ST-segment elevation myocardial infarction.
Hospital-Level Variation of PCI Strategies in AMI and Shock
Of the 1336 hospitals in the hospital-level analysis, the median rate of multivessel PCI use (as a proportion of the number of eligible patients) was 32.7%, with a wide variation across hospitals (interquartile range [IQR], 25.0%-41.7%) (Figure 2). Across hospitals, those in the higher quartiles of multivessel PCI use were more frequently urban teaching hospitals, had larger bed sizes, and had higher annual PCI volumes compared with hospitals in lower quartiles (eTable 1 in the Supplement). No significant differences were found in the distribution of these hospitals across US census regions (eTable 1 in the Supplement). Differences in characteristics of patients across quartiles of hospitals, based on their rate of multivessel PCI in the overall AMI population and among patients with STEMI are included in eTables 2 and 3 in the Supplement, respectively. After accounting for differences in case mix across hospitals, a patient was more likely to receive multivessel PCI with a similar presentation and coronary anatomy at one randomly selected hospital compared with another (median OR, 1.37; 95% CI, 1.33-1.41). There was a similar variation in the use of multivessel PCI across hospitals with similar structural characteristics and case mix (median OR, 1.34; 95% CI, 1.30-1.38).
Figure 2. Variation in the Use of Multivessel Percutaneous Coronary Intervention (PCI) Across Hospitals.

The panels represent the frequency distribution plots for hospitals based on their multivessel PCI use to treat all AMI (A) and AMI with STEMI (B), shown as the percentage of procedures among all eligible patients. AMI indicates acute myocardial infarction; STEMI, ST-segment elevation myocardial infarction.
In-Hospital Outcomes
Among patients with AMI and cardiogenic shock undergoing PCI for multivessel CAD, those undergoing multivessel PCI had higher unadjusted rates of hemorrhage at access site and internal bleeding, vascular complications, new-onset hemodialysis, and stroke compared with a culprit vessel–only PCI strategy (eTables 4 and 5 in the Supplement). After adjustment for pre-PCI characteristics, including patient demographics, medical comorbidities, clinical presentation to the catheterization laboratory, and the angiographic severity of disease, the use of multivessel PCI strategy remained associated with higher odds of 1 or more complications compared with a culprit vessel–only PCI strategy in the overall AMI population (risk-adjusted OR, 1.18, 95% CI, 1.14-1.23; P < .001) and higher odds of complications in the subgroup with STEMI (risk-adjusted OR, 1.19, 95% CI, 1.17-1.26; P < .001). These observations were consistent in analyses that excluded patients with STEMI with a left main culprit, with higher risk-adjusted odds of complications in the multivessel PCI compared with culprit vessel–only PCI (risk-adjusted OR, 1.20; 95% CI, 1.15-1.25; P < .001).
Multivessel PCI was also associated with higher risk-adjusted rates of complications individually (eTable 4 in the Supplement). In risk-adjusted analyses, the odds of AKI requiring dialysis (OR, 1.20; 95% CI, 1.11-1.30) and vascular complications were higher with multivessel PCI compared with culprit vessel–only PCI (OR, 1.43, 95% CI, 1.36-1.50) in the overall population, with similar excess risk-adjusted odds for these complications in the group with STEMI (eTable 5 in the Supplement).
In the patients with STEMI and cardiogenic shock, multivessel PCI was associated with higher in-hospital mortality (risk-adjusted OR, 1.11; 95% CI, 1.06-1.16; P < .001). However, in the overall AMI population that included both patients with STEMI and those with non-STEMI, multivessel PCI was associated with lower risk-adjusted odds of mortality (risk-adjusted OR, 0.96; 95% CI, 0.92-0.99; P = .02). In the sensitivity analysis of STEMI without a left main culprit, risk-adjusted odds of in-hospital mortality did not differ significantly between multivessel and culprit vessel–only PCI (risk-adjusted OR, 0.97; 95% CI, 0.92-1.01; P = .12).
Hospitals in the highest quartile of multivessel PCI use more frequently had vascular, bleeding, and kidney complications and had a higher rate of death compared with hospitals in the lowest quartile of multivessel PCI use (eTable 6 in the Supplement). After accounting for differences in case mix across hospitals in the overall AMI population, no significant differences were found in in-hospital mortality at hospitals with more frequent use of multivessel PCI. However, patients at hospitals in the highest quartile for multivessel PCI in STEMI had higher odds of in-hospital mortality compared with hospitals in the lowest quartile (risk-adjusted OR, 1.10; 95% CI, 1.02-1.19; P = .02) (Figure 3).
Figure 3. Risk-Adjusted Odds of In-Hospital Mortality and Any Procedural Complication Across Hospital Quartiles of Multivessel Percutaneous Coronary Intervention (PCI) Use.
The panels represent) mortality in all patients with shock who had acute myocardial infarction (AMI) (A), mortality in patients with shock who had ST-segment elevation myocardial infarction (STEMI), any complication in all patients with shock who had AMI (C), and any complication in patients with shock who had STEMI (D). The reference group is hospitals in the lowest quartile (quartile 1) for multivessel PCI use in myocardial infarction and shock.
Long-term Outcomes
A total of 18 142 patients at 1332 hospitals were matched to Medicare claims from July 1, 2009, to December 31, 2016, with a median 4.6 years (IQR, 2.8-6.5 years) of follow-up. No significant differences were found in 1-year mortality in the multivessel and culprit vessel-only PCI groups (51.5% vs 49.8%, risk-adjusted OR, 0.97; 95% CI, 0.90-1.04; P = .37). There were similarly no differences in rates of readmissions, both overall and for recurrent AMI, within a year from discharge. Notably, patients in the culprit vessel–only PCI strategy had higher rates of revascularization in the year after the index hospitalization (5.6% vs 3.0%, P < .001). Overall, the combination of postdischarge mortality, recurrent AMI, and additional revascularization were not different between groups based on multivessel PCI during their index presentation with cardiogenic shock (Figure 4 and eTable 6 in the Supplement). There were also no differences in rates of heart failure hospitalization in the 1-year period after index hospitalization across groups based on the PCI strategy (eTable 6 in the Supplement).
Figure 4. Postdischarge Long-term Outcomes in Acute Myocardial Infarction (AMI) Complicated With Cardiogenic Shock.
Data are based on multivessel percutaneous coronary intervention (PCI) during index catheterization. AMI indicates acute myocardial infarction; MACE, major adverse cardiovascular event (including postdischarge death, recurrent AMI, or additional revascularization).
Discussion
In this cohort study of PCI strategies in AMI and cardiogenic shock, one-third of patients identified in the cathPCI Registry underwent multivessel PCI during the index procedure, with increasing use of multivessel PCI between 2009 and 2018. There was no change in multivessel PCI use in AMI and cardiogenic shock in the months after the publication of the CULPRIT-SHOCK trial, which demonstrated harm with this strategy. Multivessel PCI was also associated with higher rates of periprocedural bleeding and vascular and kidney complications, without evidence of improvement in long-term survival or recurrent AMI. Moreover, there was significant variation in practices across US hospitals, and patients treated at hospitals with greater use of multivessel PCI had more procedural complications. There was evidence of higher mortality in patients with STEMI who were treated using a multivessel PCI strategy and in patients at hospitals that more frequently used this strategy for STEMI and shock.
The practice patterns observed in the current study may reflect the evolving evidence supporting revascularization in patients with multivessel CAD presenting with AMI and cardiogenic shock. The Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock (SHOCK) trial was the most prominent randomized clinical trial that supported early revascularization in AMI and cardiogenic shock to improve mortality.5 Although clinical guidelines for STEMI have recommended against multivessel revascularization for STEMI, they have historically provided an exception that supports multivessel PCI in patients with cardiogenic shock despite a lack of evidence in this area.17 The increasing use of multivessel PCI during the index PCI in the past 5 years likely reflects increasing evidence from randomized clinical trials in patients without shock in whom early multivessel revascularization was noted to be beneficial.18,19,20 Our findings that suggest harm associated with multivessel PCI in the context of shock are concordant with evidence from CULPRIT-SHOCK,7,8 with evidence of increased complications during hospitalization and higher mortality among patients with STEMI and shock who were treated with multivessel PCI. Moreover, hospitals that more frequently performed multivessel PCI for STEMI and shock had higher risk-adjusted mortality. These observations suggest that a reversal of prevailing practice patterns is needed.
We found that although there were higher rates of complications with multivessel PCI in the overall population, which included patients with STEMI and those with non-STEMI, with a higher rate of AKI requiring initiation of hemodialysis, the association of multivessel PCI with higher mortality was restricted to the STEMI group, with slightly lower risk-adjusted mortality when we also included the non-STEMI group. These results are not directly explained by treatment effects in randomized clinical trials because there was no interaction between type of AMI and revascularization strategies for the primary composite outcome of in-hospital mortality and the requirement of hemodialysis in the CULPRIT-SHOCK trial.7 Notably, the outcome of in-hospital mortality in patients with non-STEMI was not reported separately in CULPRIT-SHOCK. Nevertheless, we suspect that the lower mortality in patients with non-STEMI undergoing multivessel PCI may reflect the lower acuity of the ischemic event compared with that in patients with STEMI and the possible selection of lower-risk individuals for multivessel PCI. This finding is similar to a prior analysis21 of older individuals with AMI with and without shock in which primary PCI of the nonculprit artery was associated with adverse outcomes in patients with STEMI but was not observed in patients with non-STEMI.
This study has important implications for the management of AMI and cardiogenic shock. Despite this more contemporary assessment, 1 in 3 patients with AMI and shock did not survive hospitalization. Many strategies to support patient recovery have been found to be ineffective in clinical practice.22,23,24 Therefore, it is important to deadopt practices that are not supported by evidence and could be associated with harm. This study highlights opportunities to improve care practices for these patients nationally. Furthermore, the temporal trends that suggest increasing adoption of multivessel revascularization for patients with shock based on evidence in patients without shock also highlight the need to ensure awareness among physicians about distinguishing the clinical treatment of this high-risk patient group and the harms associated with extrapolating evidence to unstudied populations. This finding is especially pertinent in an era when complete revascularization for AMI in the absence of shock is associated with improved outcomes and will likely become the standard of care.25,26 Although the pathophysiologic derangements in cardiogenic shock that are associated with primary multivessel PCI are poorly understood, it is possible that prolonged procedures with their associated blood loss and the higher load of iodinated contrast used in multivessel PCI are associated with a poorer outcome in patients who already have metabolic and hemodynamic derangements. Exposing nonculprit regions to potential procedure-related complications or myocardial injury may also more than offset the any short-term benefit associated with additional revascularization. Finally, an association between greater use of mechanical circulatory support devices to support these complex procedures and excess mortality has recently been observed.15,27,28,29
Limitations
This study has limitations. First, we could not determine whether some multivessel revascularization procedures were performed in the context of thrombotic coronary lesions in multiple coronary vessels. However, these are rare in AMI30 and likely do not explain the large variation in care practices in otherwise similar patients. Moreover, we also found high use of and adverse outcomes with multivessel PCI in patients with STEMI who frequently have an identifiable culprit vessel on presentation. Second, although we found evidence suggesting excess in-hospital mortality, bleeding, stroke, recurrent AMI, and revascularization in some of our analyses, we could not prove a causal effect of multivessel PCI on these outcomes. Nevertheless, our findings are directionally consistent in terms of both the mortality and secondary outcomes. It is possible that unmeasured confounding underlies the observed excess risk-adjusted mortality in patients undergoing multivessel PCI and at hospitals with high rates of multivessel PCI use in AMI and shock. However, the risk-adjustment strategy captures a large number of clinical as well as angiographic features at the point of care and comports well with reported risks of death and adverse outcomes in patients undergoing PCI.10 In addition, the consistency in direction and magnitude of the association observed in the patient-level and hospital-level analyses, as well their consistency with results from the CULPRIT-SHOCK trial, suggest that the results are unbiased. Third, the study did not include an assessment of AMI without shock; therefore, our observations of multivessel revascularization and worse patient outcomes do not generalize to patients with AMI without shock.
Conclusions
This cohort study found that an increasing proportion of US patients underwent multivessel PCI for AMI and cardiogenic shock between 2009 and 2018, with substantial variation in care practices across hospitals. In contrast to a strategy focusing on culprit vessel–only revascularization, multivessel revascularization was associated with worse periprocedural outcomes and excess in-hospital mortality, particularly in patients with STEMI, without any evidence of improved long-term outcomes.
Figure. Study Flow Sheet
eTable 1. Characteristics of Hospitals Across Quartiles Based on Rate of Multi-Vessel PCI in the Overall AMI Population
eTable 2. Patients Characteristics Stratified by Percentage of Hospital Multi-Vessel PCI in the Overall AMI Population
eTable 3. Patients Characteristics Stratified by Percentage of Hospital Multi-Vessel PCI in the STEMI Population
eTable 4. Rates of In-hospital and Long-term Outcomes of Patients With AMI and Cardiogenic Shock Undergoing Multivessel PCI
eTable 5. Risk-adjusted In-hospital Complications With AMI and Cardiogenic Shock With Multivessel PCI Compared With Culprit-Only PCI
eTable 6. Rates of In-hospital Outcomes of Patients With AMI and Cardiogenic Shock Undergoing Multivessel PCI Across Hospital Quartiles of Multivessel PCI
References
- 1.Kolte D, Khera S, Aronow WS, et al. Trends in incidence, management, and outcomes of cardiogenic shock complicating ST-elevation myocardial infarction in the United States. J Am Heart Assoc. 2014;3(1):e000590. doi: 10.1161/JAHA.113.000590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kolte D, Khera S, Dabhadkar KC, et al. Trends in coronary angiography, revascularization, and outcomes of cardiogenic shock complicating non–ST-elevation myocardial infarction. Am J Cardiol. 2016;117(1):1-9. doi: 10.1016/j.amjcard.2015.10.006 [DOI] [PubMed] [Google Scholar]
- 3.Wayangankar SA, Bangalore S, McCoy LA, et al. Temporal trends and outcomes of patients undergoing percutaneous coronary interventions for cardiogenic shock in the setting of acute myocardial infarction: a report from the CathPCI Registry. JACC Cardiovasc Interv. 2016;9(4):341-351. doi: 10.1016/j.jcin.2015.10.039 [DOI] [PubMed] [Google Scholar]
- 4.Spence N, Abbott JD. Coronary revascularization in cardiogenic shock. Curr Treat Options Cardiovasc Med. 2016;18(1):1. doi: 10.1007/s11936-015-0423-9 [DOI] [PubMed] [Google Scholar]
- 5.Hochman JS, Sleeper LA, Webb JG, et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock: should we emergently revascularize occluded coronaries for cardiogenic shock? N Engl J Med. 1999;341(9):625-634. doi: 10.1056/NEJM199908263410901 [DOI] [PubMed] [Google Scholar]
- 6.O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61(4):e78-e140. doi: 10.1016/j.jacc.2012.11.019 [DOI] [PubMed] [Google Scholar]
- 7.Thiele H, Akin I, Sandri M, et al. ; CULPRIT-SHOCK Investigators . PCI strategies in patients with acute myocardial infarction and cardiogenic shock. N Engl J Med. 2017;377(25):2419-2432. doi: 10.1056/NEJMoa1710261 [DOI] [PubMed] [Google Scholar]
- 8.Thiele H, Akin I, Sandri M, et al. ; CULPRIT-SHOCK Investigators . One-year outcomes after PCI strategies in cardiogenic shock. N Engl J Med. 2018;379(18):1699-1710. doi: 10.1056/NEJMoa1808788 [DOI] [PubMed] [Google Scholar]
- 9.Fanaroff AC, Zakroysky P, Wojdyla D, et al. Relationship between operator volume and long-term outcomes after percutaneous coronary intervention. Circulation. 2019;139(4):458-472. doi: 10.1161/CIRCULATIONAHA.117.033325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brennan JM, Curtis JP, Dai D, et al. ; National Cardiovascular Data Registry . Enhanced mortality risk prediction with a focus on high-risk percutaneous coronary intervention: results from 1,208,137 procedures in the NCDR (National Cardiovascular Data Registry). JACC Cardiovasc Interv. 2013;6(8):790-799. doi: 10.1016/j.jcin.2013.03.020 [DOI] [PubMed] [Google Scholar]
- 11.Peterson ED, Dai D, DeLong ER, et al. ; NCDR Registry Participants . Contemporary mortality risk prediction for percutaneous coronary intervention: results from 588,398 procedures in the National Cardiovascular Data Registry. J Am Coll Cardiol. 2010;55(18):1923-1932. doi: 10.1016/j.jacc.2010.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rao SV, McCoy LA, Spertus JA, et al. An updated bleeding model to predict the risk of post-procedure bleeding among patients undergoing percutaneous coronary intervention: a report using an expanded bleeding definition from the National Cardiovascular Data Registry CathPCI Registry. JACC Cardiovasc Interv. 2013;6(9):897-904. doi: 10.1016/j.jcin.2013.04.016 [DOI] [PubMed] [Google Scholar]
- 13.Tsai TT, Patel UD, Chang TI, et al. Validated contemporary risk model of acute kidney injury in patients undergoing percutaneous coronary interventions: insights from the National Cardiovascular Data Registry Cath-PCI Registry. J Am Heart Assoc. 2014;3(6):e001380. doi: 10.1161/JAHA.114.001380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fanaroff AC, Zakroysky P, Wojdyla D, et al. Relationship between operator volume and long-term outcomes after percutaneous coronary intervention: a report from the NCDR CathPCI registry. Circulation. 2019;139(4):458-472. doi: 10.1161/CIRCULATIONAHA.117.033325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khera R, Cram P, Lu X, et al. Trends in the use of percutaneous ventricular assist devices: analysis of national inpatient sample data, 2007 through 2012. JAMA Intern Med. 2015;175(6):941-950. doi: 10.1001/jamainternmed.2014.7856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khera R, Pandey A, Kumar N, et al. Variation in hospital use and outcomes associated with pulmonary artery catheterization in heart failure in the United States. Circ Heart Fail. 2016;9(11):e003226. doi: 10.1161/CIRCHEARTFAILURE.116.003226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Antman EM, Anbe DT, Armstrong PW, et al. ; American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1999 Guidelines for the Management of Patients With Acute Myocardial Infarction) . ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction—executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1999 Guidelines for the Management of Patients With Acute Myocardial Infarction). Circulation. 2004;110(5):588-636. doi: 10.1161/01.CIR.0000134791.68010.FA [DOI] [PubMed] [Google Scholar]
- 18.Gershlick AH, Khan JN, Kelly DJ, et al. Randomized trial of complete versus lesion-only revascularization in patients undergoing primary percutaneous coronary intervention for STEMI and multivessel disease: the CvLPRIT trial. J Am Coll Cardiol. 2015;65(10):963-972. doi: 10.1016/j.jacc.2014.12.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Engstrøm T, Kelbæk H, Helqvist S, et al. ; DANAMI-3—PRIMULTI Investigators . Complete revascularisation versus treatment of the culprit lesion only in patients with ST-segment elevation myocardial infarction and multivessel disease (DANAMI-3—PRIMULTI): an open-label, randomised controlled trial. Lancet. 2015;386(9994):665-671. doi: 10.1016/S0140-6736(15)60648-1 [DOI] [PubMed] [Google Scholar]
- 20.Wald DS, Morris JK, Wald NJ, et al. ; PRAMI Investigators . Randomized trial of preventive angioplasty in myocardial infarction. N Engl J Med. 2013;369(12):1115-1123. doi: 10.1056/NEJMoa1305520 [DOI] [PubMed] [Google Scholar]
- 21.Wang TY, McCoy LA, Bhatt DL, et al. Multivessel vs culprit-only percutaneous coronary intervention among patients 65 years or older with acute myocardial infarction. Am Heart J. 2016;172:9-18. doi: 10.1016/j.ahj.2015.10.017 [DOI] [PubMed] [Google Scholar]
- 22.Thiele H, Ohman EM, Desch S, Eitel I, de Waha S. Management of cardiogenic shock. Eur Heart J. 2015;36(20):1223-1230. doi: 10.1093/eurheartj/ehv051 [DOI] [PubMed] [Google Scholar]
- 23.Thiele H, Zeymer U, Neumann FJ, et al. ; IABP-SHOCK II Trial Investigators . Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med. 2012;367(14):1287-1296. doi: 10.1056/NEJMoa1208410 [DOI] [PubMed] [Google Scholar]
- 24.Ouweneel DM, Eriksen E, Sjauw KD, et al. Percutaneous mechanical circulatory support versus intra-aortic balloon pump in cardiogenic shock after acute myocardial infarction. J Am Coll Cardiol. 2017;69(3):278-287. doi: 10.1016/j.jacc.2016.10.022 [DOI] [PubMed] [Google Scholar]
- 25.Mehta SR, Wood DA, Storey RF, et al. ; COMPLETE Trial Steering Committee and Investigators . Complete revascularization with multivessel PCI for myocardial infarction. N Engl J Med. 2019;381(15):1411-1421. doi: 10.1056/NEJMoa1907775 [DOI] [PubMed] [Google Scholar]
- 26.Køber L, Engstrøm T. A more COMPLETE picture of revascularization in STEMI. N Engl J Med. 2019;381(15):1472-1474. doi: 10.1056/NEJMe1910898 [DOI] [PubMed] [Google Scholar]
- 27.Dhruva SS, Ross JS, Mortazavi BJ, et al. Association of use of an intravascular microaxial left ventricular assist device vs intra-aortic balloon pump with in-hospital mortality and major bleeding among patients with acute myocardial infarction complicated by cardiogenic shock. JAMA. Published online February 10, 2020. doi: 10.1001/jama.2020.0254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amin AP, Spertus JA, Curtis JP, et al. The evolving landscape of impella use in the United States among patients undergoing percutaneous coronary intervention with mechanical circulatory support. Circulation. 2020;141(4):273-284. doi: 10.1161/CIRCULATIONAHA.119.044007 [DOI] [PubMed] [Google Scholar]
- 29.Khera R, Cram P, Vaughan-Sarrazin M, Horwitz PA, Girotra S. Use of mechanical circulatory support in percutaneous coronary intervention in the United States. Am J Cardiol. 2016;117(1):10-16. doi: 10.1016/j.amjcard.2015.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pollak PM, Parikh SV, Kizilgul M, Keeley EC. Multiple culprit arteries in patients with ST segment elevation myocardial infarction referred for primary percutaneous coronary intervention. Am J Cardiol. 2009;104(5):619-623. doi: 10.1016/j.amjcard.2009.04.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure. Study Flow Sheet
eTable 1. Characteristics of Hospitals Across Quartiles Based on Rate of Multi-Vessel PCI in the Overall AMI Population
eTable 2. Patients Characteristics Stratified by Percentage of Hospital Multi-Vessel PCI in the Overall AMI Population
eTable 3. Patients Characteristics Stratified by Percentage of Hospital Multi-Vessel PCI in the STEMI Population
eTable 4. Rates of In-hospital and Long-term Outcomes of Patients With AMI and Cardiogenic Shock Undergoing Multivessel PCI
eTable 5. Risk-adjusted In-hospital Complications With AMI and Cardiogenic Shock With Multivessel PCI Compared With Culprit-Only PCI
eTable 6. Rates of In-hospital Outcomes of Patients With AMI and Cardiogenic Shock Undergoing Multivessel PCI Across Hospital Quartiles of Multivessel PCI




