Abstract
Tn5 transposase (Tnp) overproduction is lethal to Escherichia coli. Genetic evidence suggested that this killing involves titration of E. coli topoisomerase I (Topo I). Here, we present biochemical evidence that supports this model. Tn5 Tnp copurifies with Topo I while nonkilling derivatives of Tnp, Δ37Tnp and Δ55Tnp (Inhibitor [Inh]), show reduced affinity or no affinity, respectively, for Topo I. In agreement with these results, the presence of Tnp, but not Δ37 or Inh derivatives of Tnp, inhibits the DNA relaxation activity of Topo I in vivo as well as in vitro. Other proteins, including RNA polymerase, are also found to copurify with Tnp. For RNA polymerase, reduced copurification with Tnp is observed in extracts from a topA mutant strain, suggesting that RNA polymerase interacts with Topo I and not Tnp.
Genome rearrangements occur frequently in many organisms. These events involve specific DNA sequences called mobile genetic elements. Transposons are a major class of mobile genetic elements. They are found in all organisms examined to date (1, 9, 15, 18). Tn5 is a composite transposon found in gram-negative bacteria (1). Tn5 contains two insertion sequences in an inverted orientation, IS50R and IS50L. Resistances to kanamycin, streptomycin, and bleomycin are encoded between the two insertion sequences (18). IS50R encodes two proteins involved in Tn5 transposition; a cis-acting 476-amino-acid transposase (Tnp) and a 421-amino-acid trans-acting inhibitor (Inh) (18). Tnp and Inh have the same amino acid sequences except that Inh lacks 55 N-terminal residues (2, 18).
In most cases Tnp and specific DNA sequences defining the ends of the transposon are thought to be sufficient for transposition (1). This is also true for Tn5 transposition; an in vitro transposition system that involves a hyperactive derivative of Tnp and specific 19-bp DNA sequences recognized by Tnp, termed OE, has been developed for Tn5 (8). However, in vivo studies have suggested that host factors could affect the frequency of Tn5 transposition. This host factor participation might ensure a successful relationship between the transposable element and its host (1, 15). In this relationship, a minimal level of transposition exists to maintain the element while a high frequency of transposition is prevented in order to block deleterious events. Therefore, the frequency of transposition events is very tightly regulated.
Various types of Tnp have been demonstrated to participate in protein-protein interactions. Tnp should homodimerize at least in synaptic complex formation. For Tn5 transposition this has been shown (1a). Tn5 Inh is also capable of homodimerization in solution as well as heterodimerization with Tnp (3). For the Mu system, it has been shown that ClpX, an ATP-dependent protease, and MuB interact with MuA transposase (12).
Previously, we used the Tnp overproduction phenotype to determine which host factors might directly interact with Tnp. Tnp overproduction is lethal to Escherichia coli. This killing does not require the presence of the specific end DNA sequences, but it has been shown to be closely correlated with the presence of an intact Tnp N terminus (22, 23). Tnp overproduction also causes cell filamentation and aberrant DNA segregation (22, 24). A similar phenomenon was also observed for bacteriophage MuB protein (1b). Thus, this could be a common property of transposases. We have shown that the killing is related to the level of topoisomerase I (Topo I), because an increase in Topo I levels suppresses Tnp-induced killing (25). In vivo studies indicated that Topo I stimulates Tn5 transposition 10- to 30-fold (25). These results also suggested that there might be a protein-protein interaction between Topo I and Tnp.
Here, we report the use of affinity chromatography to determine whether Tnp and Topo I interact. Tnp and Δ37Tnp (an N-terminal deletion of Tnp) were tagged at their C termini with His6. His6-fusion Tnp and His6-fusion Δ37Tnp were purified from crude cell extracts by using Ni column chromatography, and then the fractions containing Tnp or Δ37Tnp were examined for the presence of host proteins. The results demonstrated that Topo I copurifies with Tnp and that Δ37Tnp has very low affinity for Topo I. When N-terminally His6-tagged Tnp and Inh (Δ55Tnp) were used, identical results were observed. Finally, we have shown that the presence of Tnp but not Δ37Tnp or Δ55Tnp inhibits Topo I DNA relaxation activity. Taken together, these results suggest that Topo I is involved in Tnp overproduction killing and that this is due to the titration of Topo I by Tnp by means of a Tnp-Topo I direct interaction. The Tnp-Topo I interaction may also be stimulatory in Tn5 transposition.
MATERIALS AND METHODS
Strains and media.
The E. coli K-12 strains (BL21, DH5α, and MC1061 [20]) were grown in Luria broth (20). Luria broth plates contained 15 g of Bacto-agar per liter. Antibiotic concentrations were as follows: chloramphenicol, 20 μg/ml; ampicillin, 100 μg/ml; kanamycin, 40 μg/ml; streptomycin, 100 μg/ml; nalidixic acid, 5 μg/ml; and tetracycline, 15 μg/ml.
Plasmids.
pRZ4775 (encoding Tnp under λ pR control) was described by Weinreich et al. (22). pRZ4824 (encoding Tnp under Ptac control) was described by Weinreich (23). pJW312-SalI, encoding topA (Topo I), was obtained from J. Wang (27). Construction of pRZ10100 and pRZ10200 was described (2). pRZ10100 expresses a Tnp fusion containing 41 amino acids that consists of a His6 tag and a protein kinase recognition site at the Tnp N terminus. pRZ10200 expresses an Inh (Δ55Tnp) fusion identical to the Tnp fusion in pRZ10100.
pRZ8865 was constructed by ligating the BglII-SphI fragment from pRZ4759 into the same sites of pRZ4775. pRZ8866 was generated by cloning the BglII-SphI fragment from pRZ4759 (23) into the same sites of pRZ4773 (23). pRZ8865 and pRZ8866 encode Tnp and Δ37Tnp with His6 tags at their C termini. pRZ8867 was constructed by inserting an oligonucleotide encoding six histidines, arginine, glycine, and serine into the BglII-SphI site of pRZ4824.
Protein assays.
Protein concentrations in cell extracts were determined by the Bradford protein assay (19). For trichloroacetic acid (TCA)-precipitated samples, the Lowry assay was used (20). Bovine serum albumin (BSA) (Sigma) was used as a protein standard.
Measurement of plasmid supercoiling.
In order to determine the extent of plasmid DNA supercoiling in the presence and absence of Tnp, a pRZ4824-containing strain was used. Overnight cultures were diluted 1:100 and grown at 37°C until they reached an optical density at 600 nm (OD600) of 0.6. At this time isopropyl-β-d-thiogalactopyranoside was added to a final concentration of 0.1 mM in order to induce Tnp production, and growth was continued for 1.25 h. Cell growth was then stopped by adding chloramphenicol (final concentration, 200 μg/ml), and plasmid DNA was isolated by the alkaline method described by Sambrook et al. (20). Plasmid DNAs were analyzed by 1% agarose gel electrophoresis in the presence of various concentrations of chloroquine to compare the levels of negative supercoiling of different plasmid preparations. For simplicity only the gels containing chloroquine at 24 μg/ml are shown. Electrophoresis was carried out as described previously (25). The gels were stained with Syber Green II (Molecular Probe) and analyzed by using a FluorImager (Molecular Dynamics).
Inhibition of Topo I relaxation activity by Tnp.
Fusion Tnp (N- and C-terminal His6 tagged), Δ37Tnp (C-terminal His6 tagged), and Δ55Tnp (Inh, N-terminal His6 tagged) were purified to >95% purity as described below. Purified Topo I was kindly supplied by R. J. DiGate (University of Maryland, Baltimore). The Topo I relaxation assay was carried out in 30-μl assays as described by Zumstein and Wang (27), except that purified Topo I was used and the gels were stained with Syber Green II (Molecular Probe) and were examined by using a FluorImager (Molecular Dynamics). Tnp or its derivatives were added to reaction buffer containing 0.6 μg of CsCl-purified pUC19 DNA (20), followed immediately by addition of the indicated amount of Topo I. The molar ratios of Tnp to Topo I and protein concentrations are shown on related figures.
Protein purification.
In order to purify His6-tagged Tnp and derivatives, cells were grown overnight, diluted 1:100, and then grown at 32°C to an OD600 of 0.8 and shifted to 42°C for 90 min to induce Tnp or Δ37Tnp synthesis. For purification of N-terminally His6-tagged Tnp and Inh, cells were grown at 37°C as described above and induced with 0.4 mM isopropyl-β-d-thiogalactopyranoside for 2 h.
Cells were harvested and resuspended in 0.4 M KCl–buffer A (50 mM Tris-HCl [pH 8.0], 10% glycerol, 1 mM 1,4-dithiothreitol [DTT], 20 mM imidazole, 0.3% Triton X-100) containing 10 mM MgCl2, 1 mM phenylmethylsulfonyl fluoride (PMSF), 135 μM N-α-tosyl-l-lysine chloromethyl ketone (TLCK), and 4 mM Pefabloc (Boehringer Mannheim). The cells were sonicated, and then RNase A (10 μg/ml) and DNase I (5 μg/ml) (Sigma) were added; incubation on ice was continued for 30 min. The resulting sonicate was cleared by centrifugation at 72,000 × g for 30 min. The cleared lysate (total protein, 1 g) was loaded onto a 10-ml Super flow Ni-nitriloacetic acid (NTA) (Qiagen) column at 0.5 ml/min with 0.4 M KCl–buffer A. The column was washed with 60 ml of 0.4 M KCl–buffer A at 3 ml/min. Then the column was washed with 60 ml of buffer A containing 1.2 M KCl and 5 mM β-mercaptoethanol at 2 ml/min to eliminate copurifying proteins. The column was further washed with 60 ml of 0.4 M KCl–buffer A and then washed with 60 ml of 40 mM imidazole in 0.4 M KCl–buffer A at 3 ml/min. The protein was then eluted with 120 ml of a 40 mM to 1 M imidazole linear gradient in 0.4 M KCl–buffer A, pH 7.0, at 3 ml/min. The relevant fractions were dialyzed against 0.3 M NaCl–buffer B (20 mM Tris-HCl [pH 7.5], 10% glycerol, 1 mM DTT, 1 mM EDTA, 0.1% Triton X-100), loaded onto a 20-ml Affi-Gel Heparin (Bio-Rad) column equilibrated with 0.2 M NaCl–buffer B, and washed with 60 ml of 0.2 M NaCl–buffer B at 0.7 ml/min. The proteins were eluted with 120 ml of 0.2 to 2 M NaCl–buffer B linear gradient at 0.7 ml/min. The pure fractions were combined and dialyzed against buffer C (0.4 M KCl, 50 mM Tris-HCl [pH 8.0], 10% glycerol) and stored at −70°C.
Copurification of host proteins with His6-tagged Tnp. (i) Copurification from the crude cell lysates.
Cells were grown and induced as described above. The cells were harvested and resuspended in buffer C containing 10 mM MgCl2, 10 mM imidazole, 0.1 mM PMSF, 135 μM TLCK, and 4 mM Pefabloc. The cells were lysed by addition of 1 mg of lysozyme (Sigma) per ml and incubated on ice for 30 min, followed by sonication on ice for 2 min (four 30-s 300-W bursts/5-min cooling). Then RNase A (10 μg/ml) and DNase I (5 μg/ml) (Sigma) were added to the lysate, and incubation on ice was continued for 30 min. The lysate was then cleared by centrifugation at 72,000 × g for 30 min at 2°C. The lysate was loaded onto a 5- to 10-ml Ni-NTA agarose column (Qiagen) with buffer C at 0.1 ml/min. The column was washed with six volumes of buffer C followed by six volumes of 35 mM imidazole in buffer C at 0.4 ml/min. The proteins were eluted with 120 ml of a 35 mM to 1 M imidazole (in 0.4 M KCl, 10% glycerol, 50 mM Tris-HCl [pH 7.0]) linear gradient at 0.4 ml/min. The fractions were examined by Tris-Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (10% polyacrylamide). The proteins were visualized by SYPRO Orange (Molecular Probes) or by Coomassie staining (Fisher). Molecular weights were calculated based on standard relative mobilities of mid-range and high-range molecular weight markers (Promega).
(ii) Copurification on Tnp or Inh affinity column.
The Tnp and Inh affinity columns were generated by preloading a 4-ml volume of a Ni-NTA agarose column with highly purified N-His6-Tnp or N-His6-Inh at 1 mg/ml in buffer C. A crude cell extract prepared from JM109/pJW312-SalI overproducing Topo I as described above was loaded onto the Tnp and Inh affinity columns (100 mg of protein per ml of column) and processed as described above.
Western blot analysis of Topo I.
The copurification fractions were used as is or after TCA precipitation in the presence of 2 mg of BSA/ml. Equal amounts of proteins from each sample were separated by Tris-Tricine SDS-PAGE (10% polyacrylamide) and transferred onto nitrocellulose filters. Western blottings were performed according to the manufacturer’s instructions (DuPont NEN). The antibody for E. coli DNA Topo I was kindly supplied by J. Wang (Harvard University). The antibodies for RNA polymerase subunits were kindly supplied by R. Burgess (University of Wisconsin—Madison). The Western blots were analyzed by densitometry and quantitated by using Imagequant and Excel programs.
RESULTS
(i) Host proteins copurify with Tnp-C-His6 fusion.
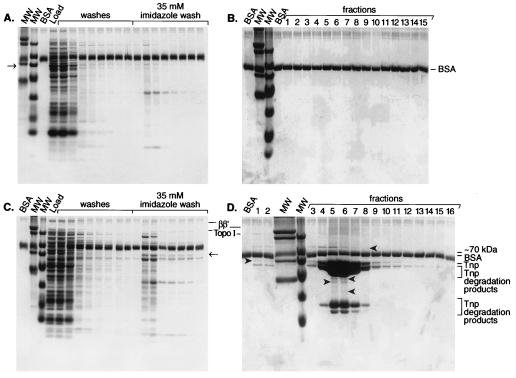
Tn5 Tnp overproduction is lethal to E. coli possibly because the Tnp titrates Topo I or other essential host proteins (22, 24). Thus, we examined the potential interaction of Tnp with host proteins by studying whether any specific proteins copurify with Tnp. In order to determine whether any host factors would bind to the Ni-NTA column in the absence of Tnp under the conditions in which copurification with Tnp-C-His6 is performed, the column was loaded with an equal amount of crude extract total protein subsequently used in copurification studies but lacking Tnp-C-His6. The copurification steps were performed, and 1.5-ml fractions of a linear (35 mM to 1 M) imidazole gradient were collected and TCA precipitated in the presence of BSA. An examination of these samples demonstrated that no host factors were retained on the column after the 35 mM imidazole washes (Fig. 1A and 1B). Under the same conditions, an equal amount of total protein prepared from cells overproducing Tnp-C-His6 tag was loaded onto the same column and purification was performed. Analysis of TCA-precipitated samples showed that some host proteins (indicated with arrowheads, Fig. 1C and D) copurified with Tnp-C-His6. The latter results also showed that some host factors and Tnp-C-His6 eluted in the 35 mM imidazole washes. These factors differed from the factors that bound to the column nonspecifically.
FIG. 1.
Host factors copurify with Tnp-C-His6 on a Ni-NTA column. The crude cell lysates were prepared from MC1061 in the presence and absence of Tnp-C-His6 overproduction. The lysates were loaded onto Ni-NTA and purifications were carried out as described in Materials and Methods. The fractions were TCA precipitated in the presence of BSA and analyzed by Tris-Tricine-SDS-PAGE. (A) Fractions from the loading buffer and 35 mM imidazole washes; (B) the linear imidazole gradient of the crude cell extract in the absence of Tnp-C-His6; (C) fractions from the loading buffer and 35 mM imidazole washes; (D) the linear imidazole gradient of the crude cell extract in the presence of Tnp-C-His6. This figure presents representative results from three experiments. MW, molecular weight markers; BSA, BSA control. The host proteins present are marked with arrowheads. Also evident in fractions 4 through 9 are Tnp and Tnp degradation products identified through Western blot analysis (data not shown).
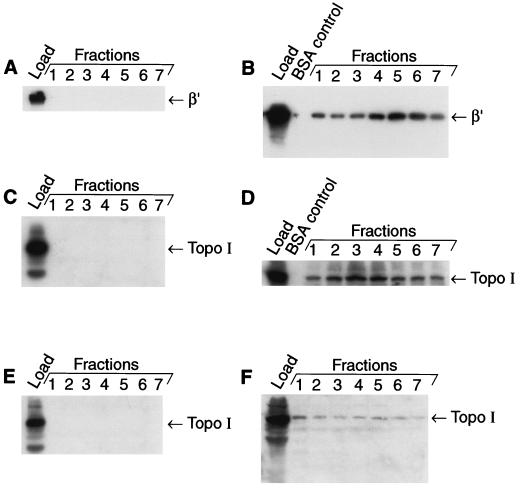
In our studies we focused on the host factors that stayed on the column beyond the 35 mM imidazole washes. In Fig. 1D it is shown that proteins very close in molecular weight to Topo I and RNA polymerase β and β′ are present. Western blot analyses of these fractions showed that both Topo I and the β′ subunit of RNA polymerase are present in the Tnp-C-His6-containing fractions (Fig. 2B and D). However, neither Topo I nor the β′ subunit of RNA polymerase was present in the fractions that were collected in the absence of Tnp-C-His6 (Fig. 2A and C). We estimate from the densitometric analysis of the Western blots shown in Fig. 2 that approximately 30% of the total Topo I and 35% of the total β′ present in the crude cell extracts copurify with Tnp-C-His6.
FIG. 2.
RNA polymerase and Topo I copurify with Tnp-C-His6; Topo I does not copurify with an N-terminal deletion variant of Tnp. Fractions shown in Fig. 1 were used for the Western blots shown in panels A through D, while fractions from a similar copurification experiment were used for the Western blot experiment yielding the fractions shown in panels E and F. Three milliliters of each fraction was concentrated 15-fold by TCA precipitation in the presence of 2 mg of BSA/ml and resuspended in 200 μl of SDS-PAGE loading buffer. Twenty microliters of each fraction (fractions 1 through 7 of the linear gradient) and a BSA control and 7 μl of the load were run on SDS-PAGE and transferred onto a polyvinylidene difluoride membrane. Then Western blot analysis was carried out by using antibody against β′ subunit of RNA polymerase, which was then stripped off and probed with anti-Topo I antibody (panels A through D). The gels shown in panels E and F were probed with only anti-Topo I antibody. Panels A and B show the anti-β′ antibody Western blots of crude cell extracts purified in the absence (A) and presence (B) of Tnp-C-His6. Panels C and D show the anti-Topo I antibody Western blots of the gels analyzed in panels A and B, respectively. Panels E and F present the anti-Topo I Western blot analysis of crude cell extract fractions purified in the presence of Δ37-Tnp-C-His6 (E) or in the presence of Tnp-C-His6 (F). This figure presents representative results from five experiments.
Having determined that a number of host factors copurify with Tnp, we examined some of these factors more closely. The copurification in the experiment analyzed in Fig. 3 was carried out exactly the same as that in Fig. 1 except that 3-ml fractions were collected in the linear imidazole gradient (35 mM to 1 M) and analyzed without TCA precipitation (this avoids contamination by proteins present in the BSA preparation). The 48-, 45-, 28-, 26-, and 24-kDa proteins present in fractions 5, 6, 7, and 8 are N-terminal degradation products of Tnp-C-His6 as determined by Western blot analysis (data not shown).
FIG. 3.
Host factors other than Topo I and RNA polymerase β and β′ are present in copurification fractions in the presence of Tnp-C-His6. Purification of the crude cell lysate in the presence of Tnp-C-His6 was carried out as described for Fig. 1 except that 3-ml fractions were collected and the fractions were analyzed by Tris-Tricine-SDS-PAGE without added BSA or TCA precipitation. The figure shows the gel analysis of fractions of the linear imidazole gradient. This figure presents representative results from five experiments. The gel was stained with SYPRO Orange (Molecular Probes) and visualized with a FluorImager (Molecular Dynamics).
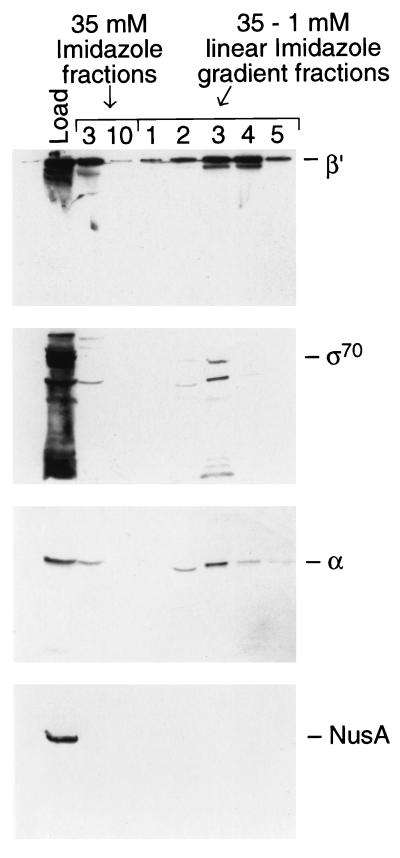
As discussed above, the 100-kDa band includes Topo I and the protein around 150 to 160 kDa is β′ (see Fig. 2B and D). The fractions also contain 70-, 42- to 40-, and 36-kDa proteins (Fig. 3). Because of the presence of β′ and also due to the presence of 70- and 36-kDa proteins, we examined for the presence of other RNA polymerase subunits by Western blot analysis. All of the subunits of RNA polymerase are present in the fractions (Fig. 4; note that we have not directly checked for the presence of β but we assume that it is present). The result shows that ς70 (8 to 10% of the total load) and α (23 to 30% of the total load) elute in the same fractions as β′ (27% of the total load). These results indicate that RNA polymerase is present in the purification fractions containing Tnp-C-His6. Below we will present evidence suggesting that this is due to an indirect interaction between RNA polymerase and Tnp.
FIG. 4.
RNA polymerase subunits but not NusA copurify with Tnp-C-His6. The fractions (fractions 1 through 5) shown in Fig. 3 and the peak and last fractions of the 35 mM imidazole wash were analyzed by Western blotting by using antibodies to β′, ς70 antibody, α, and NusA. This figure presents representative results from two experiments.
In order to determine whether the interaction with RNA polymerase was due to a simple acidic protein (RNA polymerase)-basic (Tnp) protein interaction, we examined the fractions for the presence of NusA, a very acidic protein present in E. coli. Figure 4 shows that NusA is not present in these fractions. Therefore, we conclude that the presence of RNA polymerase subunits in the copurification fractions might be due to a specific interaction between RNA polymerase and Tnp-C-His6 or to another protein in the preparation.
Other major proteins copurifying with Tnp-C-His6 are about 66, 56, 20, and 11 kDa. The 20- and 11-kDa proteins are present only in the peak fractions. All of the host proteins elute before the final Tnp-containing fractions.
(ii) RNA polymerase does not copurify with and Topo I has lower affinity for the N-terminal Tnp deletion Δ37.
Overproduction of N-terminal deletion variants of Tnp is not lethal (22); thus we examined whether one such mutant protein (Δ37Tnp) is defective in interacting with host proteins Topo I and RNA polymerase β′. Copurification experiments with Δ37Tnp were carried out as described above (except that BL21 was used as a host), and the results were compared by Western blot analysis to the results found with full-length Tnp-C-His6 (also produced in BL21) (Fig. 2E and F). RNA polymerase β′ does not copurify with Δ37Tnp (data not shown). From the Western blot results it is clear that Δ37Tnp fractions have little or no Topo I (Fig. 2E) as compared to the Tnp-C-His6 fractions (Fig. 2F).
(iii) Topo I copurifies with N-His6-Tnp but not with the N-His6-Inh.
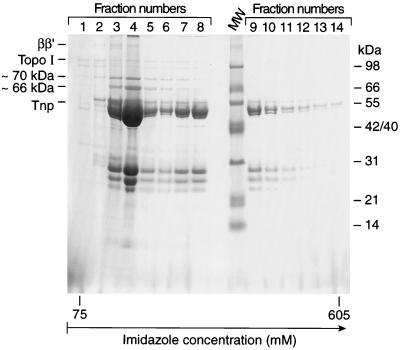
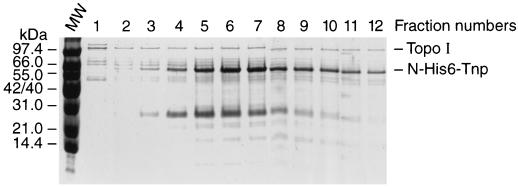
In order to study further the role of the Tnp N-terminal sequence in interacting with Topo I, we analyzed the binding of Topo I to affinity columns containing full-length Tnp or Inh (an N-terminal deletion derivative of Tnp whose overproduction is not lethal). In these experiments N-terminal fusions of Tnp and Inh were used to eliminate the complication of Tnp degradation products (2). The Tnp N-terminal His6 fusion was purified to >95% purity and loaded onto a Ni-NTA column (1 mg of pure protein per ml of the resin). A crude cell extract prepared from JM109 cells overproducing Topo I was loaded onto the Tnp affinity column (100 mg of total protein per 1-ml volume of Tnp affinity column). The column was washed, and the remaining proteins were eluted (Fig. 5). Topo I was retained on the Tnp affinity column. In the elution fractions, Tnp and Topo I are the major proteins; however, additional host factors were also retained on the column (Fig. 5). Topo I was present in all fractions (Fig. 5). Tnp eluted in later-eluted fractions. In these fractions, Topo I and Tnp were the major proteins present (Fig. 5). When the amounts of Topo I and Tnp were calculated from the relative intensities of the corresponding bands (Fig. 5), it was found that the Tnp/Topo I ratio was 4 to 5/1.
FIG. 5.
Tnp affinity column retains Topo I. An N-His6-Tnp affinity column was prepared as described in Materials and Methods. A crude cell lysate prepared from a wild-type strain overproducing Topo I was loaded onto the Ni-NTA column (100 mg of protein per ml of column matrix). An imidazole gradient fractionation was performed. Tris-Tricine-SDS-PAGE analysis of fractions 1 through 12 of a 35 mM to 1 M imidazole gradient is shown. This figure presents representative results from two experiments. The molar ratios of Tnp to Topo I calculated from the intensity of corresponding bands were four- to fivefold. MW, molecular weight marker.
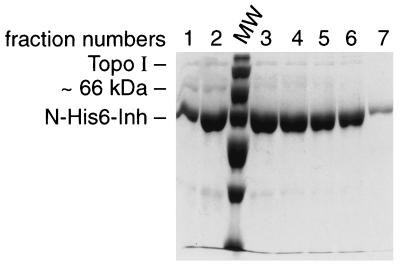
An Inh N-terminal His6 fusion was also used to generate an Inh affinity column as described above, and the same crude cell extract as used in the experiment described above was loaded onto the column, washed, and eluted similarly. Most of the Topo I was in the flowthrough fraction (data not shown). Some of the Inh was washed away by 35 mM imidazole (data not shown). Early-eluted fractions contained a 66-kDa protein and some Topo I, and the rest of the fractions were >95% pure Inh (Fig. 6). When the amounts of Topo I and Inh were calculated from the relative intensities of the corresponding bands (Fig. 6), it was found that the Inh/Topo I ratio was 50 to 60/1.
FIG. 6.
Inh affinity column retains reduced levels of Topo I. An N-His6-Inh affinity column was prepared as described in Materials and Methods. A crude cell lysate from a wild-type strain overproducing Topo I (as used in the Tnp experiment, Fig. 5) was loaded onto the Inh column. An imidazole gradient fractionation was performed. Tris-Tricine-SDS-PAGE analysis of fractions 1 through 7 of a 35 mM to 1 M imidazole gradient is shown. Inh was eluted from the column at lower imidazole concentrations than was Tnp (Fig. 5). A ∼66-kDa protein band and a very faint Topo I protein band are present in the first two fractions. This figure presents representative results from two experiments. The ratio of Inh to Topo I calculated from the intensity of corresponding bands was 50- to 60-fold. MW, molecular weight marker.
These results, in addition to the results for Δ37Tnp described above, suggest that Topo I and Tnp interact specifically and that the N terminus of Tnp is critical for this interaction.
(iv) Mutant Topo I from a topA suppressor of Tnp killing copurifies with Tnp-C-His6.
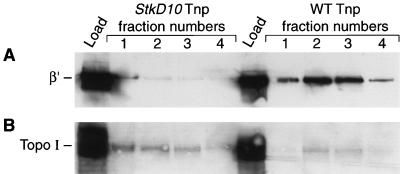
Tnp overproduction killing is suppressed in the Topo I mutant stkD10 (25); thus, we examined the copurification of the mutant Topo I and RNA polymerase β′ with Tnp-C-His6 from this strain. Equal amounts of protein from the stkD10 and wild-type strains producing Tnp-C-His6 were loaded onto Ni-NTA columns, and the linear imidazole gradient fractions were examined by Western blot analysis. The fractions known to contain Tnp-C-His6 from both strains also contained Topo I (Fig. 7B); however, the fractions from stkD10 contained two- to threefold more Topo I than the fractions from the wild-type strain. The stkD10 Topo I is approximately sixfold more abundant in the cells (25), so this likely explains its increased presence in the copurification fractions and suggests that the mutation has little effect on the affinity of Topo I for Tnp. Interestingly, the amount of RNA polymerase β′ was reduced 20- to 30-fold in the stkD10 copurification fractions (Fig. 7A) in comparison to that in the wild-type background. This observation suggests that RNA polymerase does not directly interact with Tnp but rather that Topo I is somehow involved.
FIG. 7.
Mutant Topo I and RNA polymerase copurification with Tnp-C-His6 from an stkD10 strain. The crude cell lysates from stkD10 Topo I and wild-type Topo I-containing strain overproducing Tnp-C-His6 were prepared and loaded onto Ni-NTA and purifications were carried out as described in Materials and Methods. Fractions 1 through 4 of the linear imidazole gradients were separated by SDS-PAGE and transferred onto polyvinylidene difluoride membranes. Western blot analyses were carried out by using antibody against the β′ subunit of RNA polymerase and then stripped off and probed with the Topo I antibody. Panel A shows the Western blot obtained with the β′ antibody, while panel B shows the Western blot obtained with the Topo I antibody. This figure presents representative results from three experiments.
(v) Tnp but not Δ37 or Inh inhibits Topo I relaxation activity. (a) Presence of Tnp results in increase in the amount of supercoiled DNA in vivo.
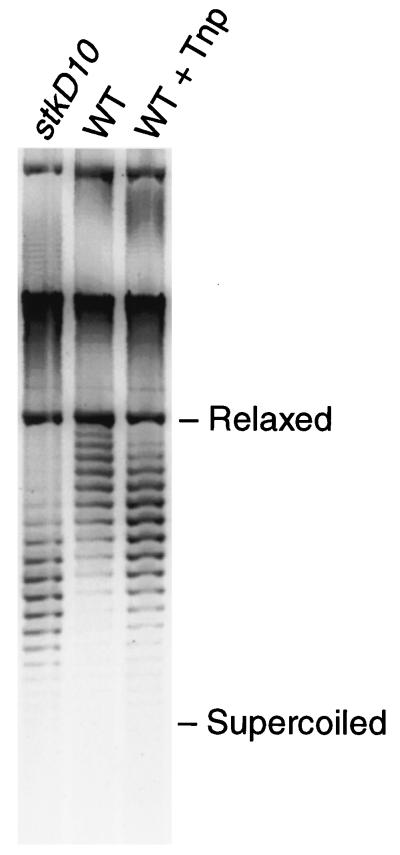
To determine whether there was a change in the level of supercoiled DNA upon Tnp overproduction, we examined the degree of plasmid DNA supercoiling in vivo in the presence and absence of Tnp overproduction (Fig. 8). pRZ4824 was used for these experiments. The results showed that wild-type cells accumulated more supercoiled DNA in the presence of Tnp than in the absence of Tnp (Figure 8).
FIG. 8.
The presence of Tnp causes an increase in plasmid DNA supercoiling in vivo. Plasmid DNA (2 μg of pRZ4824), prepared from a parental wild-type strain and an stkD10 strain in the presence and absence of Tnp overproduction and from an stkD10 strain in the absence of Tnp, was electrophoresed in the dark on a 1% agarose gel containing 24 μg of chloroquine/ml. The gel was stained with Syber Green II and analyzed with a FluorImager (Molecular Dynamics). From left to right, the lanes contain plasmid DNA prepared from the stkD10 strain in the absence of Tnp, plasmid DNA prepared from a wild-type (WT) strain in the absence of Tnp, and plasmid DNA prepared from a WT strain in the presence of Tnp overproduction. This figure presents representative results from four experiments. In another similar experiment rifampin (final concentration, 150 μg/ml) was added 15 min prior to harvesting plasmid DNA from one aliquot of the preparation with added Tnp. The addition of rifampin had no noticeable effect on the plasmid supercoil profile, suggesting that the effect of added Tnp was not due to mere transcription (data not shown) (7a). Relaxed, relaxed DNA; Supercoiled, supercoiled DNA.
(b) Purified Tnp but not Δ37Tnp or Inh inhibits purified Topo I in vitro.
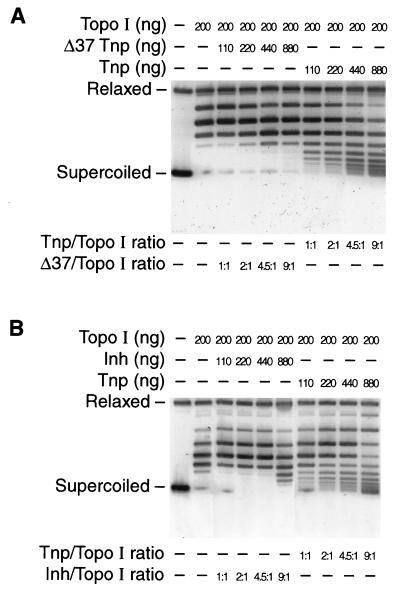
In order to determine whether Tnp inhibition of Topo I activity was dependent on the Tnp N terminus, we examined Topo I relaxation activity in vitro in the presence of various Tnp derivatives. Highly purified Topo I was used. Purified Tnp-C-His6, Δ37Tnp-C-His6, N-His6-Tnp, and N-His6-Inh were added to the reaction mixtures at a concentration that approximated that found in Tn5-containing cells (approximately 100 molecules per cell [11] or 0.15 μM). Tnp or its derivatives were added first to the reaction buffer containing the largely supercoiled pUC19 DNA, followed immediately by addition of Topo I. The molar ratios of the proteins were 1/1, 2/1, 4.5/1, and 9/1 (Tnp or Tnp derivatives/Topo I). The resulting topoisomers of pUC19 DNA were analyzed by agarose gel electrophoresis. The gels presented in Fig. 9A and B show that Tnp-C-His6 or N-His6-Tnp partially inhibited Topo I at a 1/1 molar ratio (the Tnp concentration was 0.067 μM). Conversely, Δ37Tnp-C-His6 or N-His6-Inh showed only a low level of inhibition of Topo I at a molar ratio of 9/1 (Fig. 9A and B).
FIG. 9.
Tnp but not Δ37Tnp or Inh inhibits Topo I in vitro. Tnp-His6 and its derivatives were purified to nearly 100% purity by Ni-NTA column chromatography followed by Affi-Gel Heparin (Bio-Rad) chromatography. Purified Topo I was kindly supplied by R. DiGate. Topo I relaxation activity was assayed by using a pUC19 substrate as described in Materials and Methods. Tnp or Δ37Tnp or Inh was added to the reaction mixtures (110 to 880 ng) in the presence of 200 ng of Topo I. (A) Tnp-C-His6 and Δ37Tnp-C-His6 Topo I inhibition experiments; (B) N-His6-Tnp and N-His6-Inh Topo I inhibition experiments. This figure presents representative results from four experiments. Relaxed, relaxed DNA; Supercoiled, supercoiled DNA.
These results support the copurification results in that Δ37Tnp-C-His6 and N-His6-Inh have reduced inhibitory activities on and therefore reduced affinities for Topo I compared to full-length Tnp. Moreover, Tnp inhibits Topo I at concentrations approximating those found in vivo.
DISCUSSION
Host proteins interact with Tnp.
Genetic studies suggested that some host factors may influence Tn5 transposition (11, 18, 21, 26). However, compensatory mutations and the lethal effects of certain alleles prevent a definitive in vivo examination of the role in transposition of various host factors, such as Topo I and gyrase. Alternative in vitro methods have been used for studying other transposition systems (17). These studies determined that transposase or retroviral integrase can interact with host factors and that these host factors can affect various steps in transposition. For example, Levchenko et al. (12) have shown that ClpX interacts with MuA, the Mu transposase. This interaction is important in removing MuA from the transposed complex. Therefore, in these studies we used biochemical approaches, based primarily on affinity chromatography, to determine whether any host factors interact with Tn5 Tnp.
Topo I and RNA polymerase are found to copurify with Tnp.
Since our genetic studies strongly suggested that Topo I stimulates Tn5 transposition and is involved in Tnp overproduction killing, we investigated whether Topo I is present in purification fractions following Ni-NTA column chromatography of His6-Tnp fusions from crude cell extracts. The results show that Topo I copurifies with Tnp but not with Δ37Tnp and Inh (Δ55Tnp) His6 fusions (Fig. 1 to 3, 5, and 6). The latter two have approximately 15- to 20-fold (Δ37Tnp)- and 50- to 60-fold [Inh (Δ55Tnp)]-lower levels of Topo I in the relevant Ni-NTA fractions.
These results were supported by the discovery of Topo I inhibition by Tnp but not by Δ37Tnp or Inh (Δ55Tnp) (Fig. 9). Full-length purified Tnp was found to significantly inhibit purified Topo I relaxation activity when present at a molar ratio of 1/1, whereas Δ37Tnp only modestly inhibits Topo I at a molar ratio of 9/1 (Δ37Tnp/Topo I) (Fig. 9). Moreover, full-length Tnp inhibits Topo I activity in vitro at concentrations that approximate Tnp’s in vivo abundance. The results for Topo I inhibition in vitro observed for Inh (Δ55Tnp) are very similar to those observed for Δ37Tnp (Fig. 9).
Recently a partial proteolytic analysis of Inh (Δ55Tnp) and Tnp demonstrated that these two proteins fold similarly (2). Thus, the only difference between Inh (Δ55Tnp) or Δ37Tnp with Tnp is likely to be the functions encoded by the first 37 to 55 amino acid residues. This suggests that the N terminus of Tnp plays a specific role in the interaction with and inhibition of Topo I.
The other host factor that was easy to identify in the copurification fractions was RNA polymerase, due to the characteristic mobility of β and β′ on SDS-PAGE. Interestingly, the results have shown that all subunits of RNA polymerase are present in the copurification fractions of Tnp (Fig. 4). A Western blot analysis for the presence of NusA was used to determine whether the presence of RNA polymerase might be due to a simple nonspecific acidic protein (RNA polymerase)-basic protein (Tnp) interaction. This is not likely to be the case since NusA was not present. Additionally, RNA polymerase does not copurify to detectable levels with either Δ37Tnp or Inh (Δ55Tnp), both of which are basic proteins.
Does the presence of RNA polymerase indicate a direct Tnp-RNA polymerase interaction? The determination that there is a reduced level, 20- to 30-fold, of RNA polymerase in the copurification fractions from the stkD10 strain indicates that a Topo I mutant can decrease apparent association of RNA polymerase with Tnp. This observation suggests that RNA polymerase is not associated directly with Tnp but rather with Topo I.
A possible concern is that RNA polymerase and Topo I may appear to copurify with Tnp by virtue of the fact that they are all DNA binding proteins; that is, it is DNA and not each other that they are bound to. We think that this is very unlikely for the following reasons. The protein preparations were extensively treated with DNase I (and RNase) at early steps in purification. In addition to the DNase treatment, in some experiments protein samples were loaded onto the Ni-NTA column after precipitation of nucleic acids with 0.25% polyethyleneimine and similar results were observed (data not shown). The quantities of Topo I and RNA polymerase copurifying with Tnp were quite high (concentrations approaching 30% of available protein are found in Tnp-containing gradient fractions). At least for RNA polymerase, the amount of copurifying RNA polymerase is greatly reduced in the presence of Topo I from stkD10, indicating that DNA bound to Tnp cannot bring along the RNA polymerase. Finally, highly purified Tnp inhibits Topo I in a stoichiometric fashion.
Role of Tnp-host factor interactions in Tnp overproduction killing.
Topo I is essential for cell survival (5–7, 13). Tnp overproduction killing was shown to be not only associated with defective nucleoid segregation but also inversely associated with the level of Topo I (22, 25). A detailed study of two suppressors of Tnp-associated killing suggested that these mutants have an increased abundance of Topo I caused by the mutation that suppressed the killing (25). Therefore, we hypothesized that Tnp killing is due to a titration of Topo I (25).
The results presented in this report support this proposal. The copurification and inhibition results suggest that there is a specific interaction between Tnp and Topo I and that this interaction is detrimental for Topo I activity. Thus, the following scenario could explain the deleterious results in cells overproducing Tnp; Tnp titrates out and inhibits Topo I, and cell death occurs. Neither Δ37Tnp nor Inh (Δ55Tnp) overproduction is lethal to E. coli. This also supports the idea that cell killing is due to a titration of Topo I, since these Tnp deletions have a very low affinity for Topo I and do not inhibit Topo I activity. The mutant Topo I in the stkD10 strain also copurifies with Tnp. Although the affinity of Tnp for this mutant Topo I may be somewhat reduced, the mechanism of suppression in this background may be due to the high level of Topo I (approximately sixfold elevated) found in this strain. Note that a high level of the Topo I protein is also found in a second strain (a strain containing the rpoH mutation, stkA14) that suppresses Tnp overproduction killing (25).
The possible role of host factors in Tn5 transposition.
It is not entirely surprising to find that Tn5 Tnp can interact with host factors because an interaction between Tnp and host proteins has been shown in other systems (2, 4, 12). Some host factors have been suggested to be involved in various steps of different transposition systems (4, 10, 12, 21). For instance, one critical step in transposition that may be affected by host factors is target recognition. Target site selection is carried out by Tnp; however, in many cases accessory proteins encoded by the element or the host can direct insertions to certain regions or enhance target recognition (4). Bacteriophage Mu transposase can capture target DNA, but MuB, an accessory protein encoded by Mu, stimulates target capture by forming a MuB-target complex (14, 16). In yeast, Ty3 insertion into RNA polymerase III promoters is regulated by an interaction between the Tnp and one of the transcription factors, TFIIIB or TFIIIC (4). Human immunodeficiency virus type 1 integration is also stimulated by a specific interaction with the transcription factor Ini1 (4).
It has recently been shown that Tn5 Tnp alone is sufficient for target recognition (8). Here, we hypothesize that the presence of some host factors may be stimulatory in this step. We specifically focus our discussion on Topo I based upon our in vivo studies showing that Topo I stimulates Tn5 transposition 10- to 30-fold (25). Since there is no genetic or biochemical evidence that RNA polymerase is involved in Tn5 transposition, we will not consider its possible role. The fact that Topo I is a DNA binding protein suggests a model involving target recognition in Tn5 transposition. An interaction between Tnp and Topo I could stimulate insertions into supercoiled DNA. This hypothesis is consistent with previously acquired in vivo data that demonstrate that Tn5 transposition prefers supercoiled target DNA (10). Biasing insertions into supercoiled DNA would reduce the interruption of ongoing cellular processes involving relaxed DNA (10).
Other steps in transposition could be also be affected by host factors. Determining these interactions and the roles played by these factors in transposition is critical to achieving an understanding of cellular processes regulating transposition.
Usefulness of the in vitro affinity techniques.
The copurification method is a very powerful tool in determining protein-protein interactions. This kind of approach becomes very useful when genetic studies are complicated due to the lethal effects of mutations in the gene of interest. This technique could be used in other transposition systems to determine the differences and similarities regarding the involvement of host factors in transposition.
ACKNOWLEDGMENTS
This work was supported by NIH grant GM50692. H.Y. is a recipient of a fellowship from the Turkish Ministry of National Education. W.S.R. is the Evelyn Mercer Professor of Biochemistry and Molecular Biology.
J. Wang is thanked for supplying the Topo I antibody and pJW312-SalI, R. DiGate is thanked for supplying the purified Topo I, and R. Burgess is thanked for supplying the antibodies against RNA polymerase subunits. M. Cox is thanked for his very helpful suggestions regarding experimental design. L. A. Braam is thanked for her suggestions made throughout these studies. We thank L. Barlow, R. Burgess, T. Donohue, N. Gray, P. Kiley, T. Naumann, G. Roberts, and M. Weinreich for very helpful discussions and comments on the manuscript.
REFERENCES
- 1.Berg D E, Howe M M, editors. Mobile DNA. Washington, D.C: American Society for Microbiology; 1989. [Google Scholar]
- 1a.Bhasin, A., I. Y. Goryshin, and W. S. Reznikoff. Unpublished data.
- 1b.Boeckh C, Bade E G, Delius H, Reeve J N. Inhibition of bacterial segregation by early functions of phage Mu and association of replication protein B with the inner cell membrane. Mol Gen Genet. 1986;202:461–466. doi: 10.1007/BF00333277. [DOI] [PubMed] [Google Scholar]
- 2.Braam L A M, Reznikoff W S. Functional characterization of the Tn5 transposase by limited proteolysis. J Biol Chem. 1998;273:10908–10913. doi: 10.1074/jbc.273.18.10908. [DOI] [PubMed] [Google Scholar]
- 3.Braam L A M, Goryshin I Y, Reznikoff W S. A mechanism for Tn5 inhibition. J Biol Chem. 1999;274:86–92. doi: 10.1074/jbc.274.1.86. [DOI] [PubMed] [Google Scholar]
- 4.Craig N L. Target site selection in transposition. Annu Rev Biochem. 1997;66:437–474. doi: 10.1146/annurev.biochem.66.1.437. [DOI] [PubMed] [Google Scholar]
- 5.DiGate R J, Marians K J. Identification of a potent decatenating enzyme from E. coli. J Biol Chem. 1988;263:13366–13373. [PubMed] [Google Scholar]
- 6.DiNardo S, Voelkel K A, Sternglanz R. E. coli DNA topoisomerase I mutants have compensatory mutations in DNA gyrase genes. Cell. 1982;31:43–51. doi: 10.1016/0092-8674(82)90403-2. [DOI] [PubMed] [Google Scholar]
- 7.Donachie W D. The cell cycle of E. coli. Annu Rev Microbiol. 1993;47:199–230. doi: 10.1146/annurev.mi.47.100193.001215. [DOI] [PubMed] [Google Scholar]
- 7a.Drlica K, Franco R J, Steck T R. Rifampin and rpoB mutations can alter DNA supercoiling in Escherichia coli. J Bacteriol. 1988;170:4983–4985. doi: 10.1128/jb.170.10.4983-4985.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goryshin I Y, Reznikoff W S. Tn5 in vitro transposition. J Biol Chem. 1998;273:7367–7374. doi: 10.1074/jbc.273.13.7367. [DOI] [PubMed] [Google Scholar]
- 9.Grindley N D F, Leschziner A E. DNA transposition: from a black box to a color monitor. Cell. 1996;83:1063–1066. doi: 10.1016/0092-8674(95)90132-9. [DOI] [PubMed] [Google Scholar]
- 10.Isberg R, Syvanen M. DNA gyrase is a host factor required for transposition of Tn5. Cell. 1982;30:9–18. doi: 10.1016/0092-8674(82)90006-x. [DOI] [PubMed] [Google Scholar]
- 11.Johnson R C, Reznikoff W S. Role of the IS50R proteins in the promotion and control of Tn5 transposition. J Mol Biol. 1984;177:645–661. doi: 10.1016/0022-2836(84)90042-1. [DOI] [PubMed] [Google Scholar]
- 12.Levchenko I, Yamauchi M, Baker T. ClpX and MuB interact with overlapping regions of Mu transposase: implications for control of the transposition pathway. Genes Dev. 1997;11:1561–1572. doi: 10.1101/gad.11.12.1561. [DOI] [PubMed] [Google Scholar]
- 13.Menzel R, Gellert M. Regulation of the genes for E. coli: homeostatic control of DNA supercoiling. Cell. 1983;34:105–113. doi: 10.1016/0092-8674(83)90140-x. [DOI] [PubMed] [Google Scholar]
- 14.Millner A, Choconas G. Disruption of target DNA binding in Mu DNA transposition by alteration of position 99 in the MuB protein. J Mol Biol. 1998;275:233–243. doi: 10.1006/jmbi.1997.1446. [DOI] [PubMed] [Google Scholar]
- 15.Mizuuchi K. Transpositional recombination: mechanistic insights from studies of Mu and other elements. Annu Rev Biochem. 1992;61:1011–1051. doi: 10.1146/annurev.bi.61.070192.005051. [DOI] [PubMed] [Google Scholar]
- 16.Naigamwalla D Z, Chaconas G. A new step of Mu DNA transposition intermediates: alternate pathways of target capture preceding strand transfer. EMBO J. 1997;16:5227–5234. doi: 10.1093/emboj/16.17.5227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Phizicky E M, Fields S. Protein-protein interactions: methods for detection and analysis. Microbiol Rev. 1995;59:94–123. doi: 10.1128/mr.59.1.94-123.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reznikoff W S. The Tn5 transposon. Annu Rev Microbiol. 1993;47:945–963. doi: 10.1146/annurev.mi.47.100193.004501. [DOI] [PubMed] [Google Scholar]
- 19.Rossomando E F. Measurement of enzyme activity. Methods Enzymol. 1990;182:38–68. doi: 10.1016/0076-6879(90)82007-o. [DOI] [PubMed] [Google Scholar]
- 20.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 21.Sternglanz R, DiNardo S, Voelker K A, Nishimura Y, Hirota Y, Becherer K, Zumstein L, Wang J C. Mutations in the gene coding for E. coli DNA topoisomerase I affect transcription and transposition. Proc Natl Acad Sci USA. 1981;78:2747–2751. doi: 10.1073/pnas.78.5.2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weinreich M D, Yigit H, Reznikoff W S. Overexpression of the Tn5 transposase in Escherichia coli results in filamentation, aberrant nucleoid segregation, and cell death: analysis of E. coli and transposase suppressor mutations. J Bacteriol. 1994;176:5494–5504. doi: 10.1128/jb.176.17.5494-5504.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weinreich M D. Ph.D. thesis. University of Wisconsin—Madison; 1993. [Google Scholar]
- 24.Yigit H, Reznikoff W S. Examination of the Tn5 transposase overproduction phenotype in Escherichia coli and localization of a suppressor of transposase overproduction killing that is an allele of rpoH. J Bacteriol. 1997;179:1704–1713. doi: 10.1128/jb.179.5.1704-1713.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yigit H, Reznikoff W S. Escherichia coli DNA topoisomerase I and suppression of killing by Tn5 transposase overproduction: topoisomerase I modulates Tn5 transposition. J Bacteriol. 1998;180:5866–5874. doi: 10.1128/jb.180.22.5866-5874.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yin J C P, Reznikoff W S. dnaA, an essential host gene, and Tn5 transposition. J Bacteriol. 1987;169:4637–4645. doi: 10.1128/jb.169.10.4637-4645.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zumstein L, Wang J C. Probing the structural domains and function in vivo of E. coli DNA topoisomerase I by mutagenesis. J Mol Biol. 1986;191:333–340. doi: 10.1016/0022-2836(86)90130-0. [DOI] [PubMed] [Google Scholar]