Abstract
Background and Aims:
Medications for opioid use disorder (MOUD) are shown to reduce opioid use and the risk of overdose. People with opioid use disorder (OUD) who exit inpatient medically managed withdrawal programs (detox) without initiating MOUD and linking to outpatient care have high rates of overdose. While detox encounters provide a theoretical opportunity for MOUD initiation, this is not ubiquitous in the United States (US). We used simulation modeling to estimate the population-level health effects and cost-effectiveness of a policy encouraging MOUD initiation during inpatient detox encounters.
Design, setting, participants:
We employed a dynamic population state-transition model to evaluate the effectiveness and cost-effectiveness of using detox programs as venues for initiating MOUD in Massachusetts, USA. We compared standard of care, where no detox patients initiate MOUD or link to outpatient MOUD providers, to strategies of offering MOUD to detox patients and linking those patients to outpatient MOUD.
Measures:
budgetary impact to the Massachusetts healthcare sector, incremental cost-effectiveness ratios (ICER), and total counts and percent differences of fatal overdoses prevented.
Findings:
Initiating MOUD in detox with perfect linkage to outpatient MOUD would reduce fatal overdoses by 4.1% (95% confidence interval: 2.3-5.9%), at an ICER of $55,576 per quality-adjusted life-year (QALY) gained, compared with the standard of care. With moderate linkage, fatal overdoses would be reduced by 2.1% (95% confidence interval 1.2-3.1%) with an ICER of $78,526 per QALY gained, compared with standard of care. Budgetary increase to Massachusetts healthcare spending ranged from 0.5-1%.
Conclusion:
A simulation model indicates that initiation of medications for opioid use disorder and linkage policies among detox patients in Massachusetts, USA could prevent fatal opioid overdoses in the opioid use disorder population and would be cost-effective from a healthcare sector perspective.
Keywords: simulation modeling, injection drug use, opioids, mortality, detox, MOUD
INTRODUCTION
Worldwide, opioid dependence affects over 500 of every 100,000 people, and 70% of all overdose deaths are caused by opioids (1, 2). The United States (US) has the highest rate of opioid dependence in the world, with approximately 128 people dying of an opioid-related overdose daily (3). Between 2015 and 2018, the opioid epidemic cost the US economy over $600 billion (4). Those with opioid use disorder (OUD) face barriers when seeking treatment, including limited capacity for treatment due to a lack of clinicians and addiction treatment centers (5, 6). The scale-up of medications for opioid use disorder in the U.S. has not kept pace with the growth of the OUD epidemic (7). Policymakers, therefore, seek high-value venues at which to expand treatment capacity, reach new populations who have not yet engaged with treatment, and accelerate the pace of response (8).
Individuals with OUD often seek treatment through inpatient medically managed withdrawal programs, also known as “detox” programs that offer either an opioid agonist, such as methadone or buprenorphine, or a combination of non-opioid alternatives to treat the symptoms of withdrawal. Optimally, detox is followed by further treatment with additional inpatient treatment, psychotherapy, and/or medications for opioid use disorders (MOUD). However, linkage to further treatment often does not occur, even though most individuals are interested (9-12). Further, detox has been shown to be ineffective in preventing relapse and overdose (13). Studies show that as many as 65% of people return to active opioid use within one month of detox treatment (14). In addition, only 3% of detox visits are followed-up in the short-term by an admission to substance use treatment (7), and there is an increase in opioid-related overdoses following detox due to a decline in tolerance (15, 16). Nevertheless, many people choose to initiate treatment for their OUD by admitting themselves to detox (17), with over 401,743 individuals with OUD admitted in 2015 for heroin alone (18). Thus, the traditional detox episode presents an irony to patients and providers – while the intention is to engage individuals into treatment, it results in a high risk for relapse and overdose.
MOUD, in contrast, are clinically effective and cost-effective treatments for OUD (19-24). Yet, despite evidence for their effectiveness, only a small fraction of detox centers report offering MOUD to any patients in 2019, and still fewer offer agonists such as buprenorphine and methadone (25).
Given the frequency with which this population utilizes detox, and their potential interest in MOUD (26), detox centers could represent an important touchpoint to initiate MOUD treatment with linkage to treatment post-detox (27). In addition, detox could be a very cost-effective touch-point for MOUD initiation as no additional investment in outreach to the population at risk would be necessary.
The population-level effects and costs of transforming detox into a venue for MOUD initiation are unknown. This study employed dynamic state-transition simulation modeling to estimate the state population-level impact of offering MOUD initiation at detox centers, as well as to estimate the costs and cost-effectiveness of such an initiative from the healthcare sector perspective in Massachusetts.
METHODS
RESPOND model structure
We utilized the Researching Effective Strategies to Prevent Opioid Death (RESPOND) model, a dynamic population, state-transition simulation model of OUD in Massachusetts, to simulate the cost-effectiveness of using detox centers as a venue for initiating MOUD treatment.
The RESPOND model, which we describe in detail in previous work (28) and in the supplemental materials, simulates the progression of a state-level population with OUD as a series of transitions between health states and is stratified by age and sex. (Pseudo code for the simulation is available online at: www.syndemicslab.org/respond). The model can simulate a closed cohort as it progresses over a lifetime (similar to a traditional longitudinal cohort study), or it can simulate a dynamic population, representing the development of new OUD and/or movement of people with existing OUD into MA. We calibrate new arrivals and exits to the model to match the estimated population size of current and non-active individuals with OUD who are in and out of care (29). The model was validated to fatal opioid overdose counts in Massachusetts (Supplemental Materials Section E. Model Validation).
The model characterizes the relapsing-remitting nature of OUD with four health states: 1) Active non-injection use, 2) Non-active non-injection use (remission from non-injection use), 3) Active injection use, and 4) Non-active injection use (remission from injection use). Active use states are characterized by a lower quality of life and higher healthcare utilization than non-active states. Active use states also carry a risk of overdose, which is highest with injection use. Among those who overdose, a proportion experience a fatal overdose. Those who survive an overdose continue in the simulation in their previous state. Non-active use is stratified by injection and non-injection use to inform differing levels of movement into active and injection and non-injection use states (Supplemental Materials Section C. Model Visualization).
Throughout the simulation, the population that is actively using opioids can transition to episodes of treatment, including detox and outpatient MOUD (buprenorphine-naloxone, methadone, or extended-release injection naltrexone (XR-NTX)). During the one-week course of a detox stay, we assume no drug use and no risk of overdose. Following discharge from detox without MOUD, there is a 4-week “post-treatment state,” which simulates the heightened risk of opioid relapse, including overdose, in the weeks following discontinuation of treatment (either MOUD or inpatient detox treatment) (14-16). The post-treatment state includes the same OUD health states (active and non-active, injection and non-injection) as both the simulation of no treatment and that of MOUD treatment, and all movement from post-treatment is into the no treatment state.
During outpatient treatment with MOUD, there is bi-directional movement between active and non-active drug-use states, with the balance of that movement tending to favor non-active drug use, which leads to both lower incidence of overdose, and lower all-cause mortality (30). In addition, each MOUD has an independent effect on the conditional risk of death among those who overdose (31, 32) (Table 1). In every time-step of the simulation, the population engaged with MOUD faces a probability of disengaging from care, which is based on data on retention and length of stay in MOUD (7, 33). Those engaged with an MOUD cannot transition to another MOUD without first transitioning through the post-treatment state and experiencing a heightened risk of overdose. For the population leaving outpatient treatment with MOUD, there is a 4-week period of “post-treatment state” that is analogous to the simulation of detox, during which the risk of overdose and death is increased.
Table 1.
Summary of impacts of MOUD on mortality in the RESPOND model. More detail can be found in the Supplemental Appendix
| Parameter Value |
Source | Supplemental Appendix Location | |
|---|---|---|---|
| Transition Probabilities, weekly | |||
| Active to Non-Active | |||
| No Treatment | 0.00058 | (50-52) |
C.2.2 Natural History of OUD
Table 4 |
| MOUD | 0.443-0.750 | (30, 35, 53) |
C.2.3.3 Transitions Between Active and Non-Active Opioid Use While Engaged With Treatment
Table 7 |
| Non-Active to Active | |||
| No Treatment | 0.00023-0.00307 | (50-52) |
C.2.2 Natural History of OUD
Table 4 |
| MOUD | 0.051-0.192 | (30, 35, 53) |
C.2.3.3 Transitions Between Active and Non-Active Opioid Use While Engaged With Treatment
Table 7 |
| Overdose multiplier for MOUD | 0.405-0.752 | (31, 32) |
C.2.4.2 Overdose While on Treatment
Table 11 |
| Standardized Mortality Ratio for drug use | |||
| Active use | 1.79-5.67 | (34) |
C.2.5.2 Competing risks of death (non-overdose mortality)
Table 14 |
| Non-active use | 1.83-5.62 | (34) |
C.2.5.2 Competing risks of death (non-overdose mortality)
Table 14 |
Data and parameter estimation
The primary data source for demography and treatment-seeking parameters in RESPOND is the Massachusetts Public Health Data Warehouse (MA PHD) (34). MA PHD is a longitudinally linked administrative records database that includes service encounter data from over 16 agencies in Massachusetts. To estimate the size of the population with OUD, including those with occult use who do not appear in national surveys or medical records, we employed capture-recapture analysis of MA PHD datasets (29). To estimate rates of treatment-seeking for both detox and MOUD treatment, we tabulated admissions over time as observed in MA PHD.
Data for the natural history of OUD come from cohort studies (Table 2). We estimated parameters on the efficacy of MOUD using urine toxicology data from the NIDA Clinical Trials Network XBOT trial (35)(Supplemental Materials Section C.1.2 NIDA Clinical Trial Network Protocols 0051 (CTN)). We employed a multi-state model to estimate bidirectional movement between active and non-active opioid use among trial participants who remained engaged in care and using an MOUD.
Table 2.
RESPOND parameters.
| Parameter | Baseline Value | Range | Source |
|---|---|---|---|
| Population Demographics and epidemiology | |||
| Proportion male | 0.58 | (43) | |
| Mean age, y | 47 | (43) | |
| Proportion in detox at baseline | 0.013 | (34) | |
| Proportion on MOUD at baseline | 0.16 | (43) | |
| Proportion with injection drug use at baseline | 0.25 | (54) | |
| Proportion actively using at baseline | 0.84 | (55, 56) | |
| Incidence of OUD, annual avg number of people | 17,950 | Range: 8,164-44,564 | (43) |
| Standardized mortality ratio for drug use | |||
| Injection drug users | 5.1 | 4.4-5.7 | 2010 Census, (43) |
| Non-injection drug users | 2.1 | 1.8-2.3 | 2010 Census, (43) |
| Movement into detox, monthly rate per 1000 people | 16.7 | 6.0-30.7 | (34) |
| Movement onto MOUD treatment, monthly rate per 1000 people | |||
| Buprenorphine | 21.1 | 11.4-41.8 | (43) |
| Methadone | 4.5 | 2.4-24.4 | (43) |
| Naltrexone 1 | 2.2 | 0.7-21.5 | (43) |
| Proportion retained on MOUD treatment at, 6-months | |||
| Buprenorphine | 0.34 | 0.17-0.51 | (7) |
| Methadone | 0.56 | 0.28-0.84 | (33) |
| Naltrexone 1 | 0.21 | 0.11-0.32 | (7) |
| Overdose monthly rate per 1,000 people | |||
| No treatment | 1.6 | 1.5-2.8 | (43) |
| Buprenorphine | 0.6 | 0.6-1.1 | (31) |
| Naltrexone 1 | 1.3 | 1.2-2.3 | (31) |
| Methadone | 1.2 | 1.1-2.1 | (32) |
| Post-treatment | 3.1 | 2.9-5.4 | |
| Fatal overdose proportion | 0.14 | 0.13-0.15 | (43) |
| Costs (Healthcare Sector) | |||
| Treatment Costs, Weekly (Visit and Medication costs) | |||
| Detox, no MOUD | $3,057 | $805-$8,204 | (37, 38) |
| Detox, with buprenorphine (16mg daily) | $3,259 | $1,007-$8,406 | (37, 38) |
| Detox, with methadone (80mg daily) | $3,215 | $963-$8,362 | (37, 38) |
| Detox, with naltrexone (Vivitrol injection, one monthly) | $4,421 | $2,169-$9568 | (37, 38) |
| Buprenorphine (16mg daily) | $114 | $75-$163 | Expert Opinion, Federal Supply Schedule |
| Methadone (80mg daily) | $126 | $116-$136 | https://www.drugabuse.gov/publications/research-reports/medications-to-treat-opioid-addiction/how-much-does-opioid-treatment-cost |
| Naltrexone (Vivitrol injection, one monthly) | $327 | $316-$338 | Expert Opinion, Federal Supply Schedule |
| Overdose costs 1 | |||
| Fatal overdose cost | $886 | $443-$1329 | (57, 58) |
| Non-fatal overdose cost | $4,557 | $2,279-$6,836 | (57, 58) |
Injectable naltrexone (Vivitrol).
Overdose costs include ambulance costs, emergency room costs, and inpatient costs.
MOUD: medications for opioid use disorder
OUD: opioid use disorder
Costs
We conducted the analysis from the healthcare sector perspective. We denominate costs in 2019 U.S. dollars (36) and discount annually at 3%. RESPOND includes three cost components: 1) costs of healthcare utilization for all services other than OUD treatment, 2) costs of OUD treatment, and 3) costs related to overdose. We estimated the cost of all three health service types using econometric multi-variable models and person-level data from NIDA CTN clinical trial 0051 (37, 38).
Health state utilities
We used published estimates of health state utilities among persons who use drugs (38, 39). All quality of life measures are health state utilities collected using the standard gamble direct method of utility measurement (40). We estimated multi-state utility functions using the minimal utility approach (36). We employed the multiplicate utility approach in sensitivity analyses.
Analyses
Strategies considered
We employed the model to simulate two strategies:
Standard of care: no MOUD initiations in detox and no linkage to outpatient MOUD, with all people transitioning from detox to the post-treatment state.
- MOUD in detox: all patients with OUD entering detox are offered MOUD initiation with either buprenorphine, methadone, or naltrexone as deemed appropriate by the patient and provider. Overall, 78% accept the offer, with 28% choosing buprenorphine, 18% methadone, and 32% naltrexone injection) (41). A portion link to outpatient MOUD after discharge from detox. Those who do not link to outpatient MOUD after discharge from detox enter the post-treatment state, regardless of MOUD status on detox. We considered two linkage scenarios:
- Perfect-linkage scenario: 100% of those who initiate MOUD link from detox directly to outpatient MOUD, avoiding post-treatment heightened risk period (41).
- Moderate-linkage scenario: identical initiation to the perfect-linkage strategy, but only 50% of those initiated on MOUD in detox link to outpatient MOUD treatment
We chose to model the standard of care as being no MOUD initiation in detox and no direct linkage to community MOUD from detox, because few patients link directly to community MOUD care without first spending time in the high-risk “post-detox” period. In addition, we explored a sensitivity analysis in which 15% of patients admitted to detox link directly to community MOUD after their detox stay, reducing the risk of overdose in the vulnerable period immediately following detox (42).
We conducted the analyses using two simulation approaches: 1. a closed cohort of individuals in Massachusetts entering detox (detox cohort simulations), and 2. simulating a dynamic population of people with OUD in (Massachusetts OUD population simulations).
Detox cohort simulations
The detox cohort simulation simulated a closed cohort of 40,000 people admitted to detox, which is similar to the total number of people who enter detox annually in Massachusetts (43). We then followed the cohort for one year. This simulation approach provides data to inform what could be expected for people who are directly exposed to the intervention. Clinical outcomes include fatal overdoses prevented, person-time on outpatient MOUD gained, and person-time in active opioid use averted.
Massachusetts OUD population simulations
The Massachusetts OUD population simulations modeled a dynamic population of all people with OUD in Massachusetts over a 10-year time horizon (2021 to 2030). The population moved through the simulation, with some members accessing detox at various times and others never doing so. The population scenario provides estimates of state-level outcomes, such as total overdoses and budgetary impact. Outcomes from the Massachusetts population scenario include fatal overdoses prevented, person-time on outpatient MOUD gained, person-time in active opioid use averted, life-years saved, quality-adjusted life-years (QALYs) saved, and incremental cost-effectiveness ratios (ICERs).
We calculate ICERs using the standard approach, whereby the numerator is the difference in mean discounted lifetime cost between one strategy and the next less costly strategy, and the denominator consists of the difference in discounted QALY between the two strategies. We interpret ICERs using a societal willingness to pay threshold of $100,000 per QALY gained. All ICERs were calculated as compared to the standard of care. We estimated the total budgetary impact for the healthcare sector in the state of Massachusetts over a 10-year horizon. Budgetary impact estimates employ non-discounted costs. We report costs categorized as: 1) healthcare utilization costs, 2) overdose costs, and 3) OUD treatment and pharmaceutical costs (Table 2, Supplemental Materials Section D. Costs and Utilities).
Sensitivity analyses
We conducted a series of deterministic and probabilistic sensitivity analyses to analyze the impact of uncertainty in model parameters on the results of the analysis and to identify key parameters that influenced the results. For deterministic sensitivity analyses, we varied the value of a single model parameter through its feasible range. Sensitivity analyses of a priori significance focused on the rate of initiation on MOUD, as well as linkage and retention on outpatient MOUD.
For probabilistic sensitivity analyses, we defined probability density functions around model parameter values where ranges were estimated using prior information derived from data and literature sources. We then repeated the simulation 1,000 times, each time drawing the value of model parameters from its uncertainty distribution (Supplemental Materials). This was used to determine a confidence interval for all outcome estimates. We visualized the results of the probabilistic sensitivity analyses using Cost-Effectiveness Acceptability Curves (CEACs) (36).
This analysis was not pre-registered on a publicly available platform, and results should be considered exploratory.
RESULTS
Detox cohort simulations
In comparison to standard of care, the MOUD in detox strategy with perfect-linkage resulted in a 21.4% decrease in person-time in active opioid use, a 334% increase in person-time on outpatient MOUD, and a 25.1% reduction in fatal overdoses over the course of one year (Table 3; Supplemental Materials Section G. Supplemental Tables and Figures, Supplemental Figure 5).
Table 3.
Effectiveness outcomes of initiation of medications for opioid use disorder (MOUD) from inpatient medically managed withdrawal programs (detox), and subsequent linkage to outpatient MOUD, showing decrease in fatal overdoses and person-time in active opioid use, and increase in person-time in outpatient MOUD. Results shown for both detox cohort perspective of 40,000 detox patients followed for one year, and full MA OUD population followed for 10 years.
| Scenario | Fatal overdoses | Person-time in active opioid use (years) | Person-time on outpatient MOUD (years) | |||
|---|---|---|---|---|---|---|
| N (Confidence Interval) |
% decrease (from standard of care) |
N (Confidence Interval) |
% decrease (from standard of care) |
N (Confidence Interval) |
% increase (from standard of care) |
|
| Detox cohort simulation | ||||||
| Standard of Care | 550 (498-624) | - | 31,700 (29,000-33,700) | - | 3,800 (3,300-4,300) | - |
| Perfect-linkage | 412 (388-457) | 25.1% | 25,200 (23,800-26,100) | 21.4% | 16,300 (15,000-16,300) | 334% |
| Moderate-linkage | 478 (445-533) | 13.1% | 28,600 (26,400-29,700) | 11.0% | 10,201 (9,400-10,400) | 172% |
| Massachusetts OUD population simulation | ||||||
| Standard of Care | 22,200 (20,470-24,739) | - | 2,493,000 (2,282,000-2,604,000) | - | 473,000 (444,000-539,000) | - |
| Perfect-linkage | 21,208 (19,734-23,605) | 4.5% | 2,433,000 (2,249,000-2,526,000) | 2.4% | 664,000 (606,000-721,000) | 40.3% |
| Moderate-linkage | 21,689 (20,126-24,162) | 2.3% | 2,462,000 (2,268,000-2,562,000) | 1.2% | 571,000 (528,0000-632,000) | 20.7% |
In comparison, the MOUD in detox strategy with moderate-linkage resulted in an 11.0% decrease in person-time in active use, a 172% increase in person-time on MOUD, and a 13.1% reduction in fatal overdoses over the course of one year (Table 3; Supplemental Materials Section G. Supplemental Tables and Figures, Supplemental Figure 5).
Massachusetts OUD population simulations
In comparison to standard of care, the MOUD in detox strategy with perfect linkage resulted in a 2.4% decrease in person-time in active opioid use, a 26.0% increase in person-time on outpatient MOUD, and prevented 4.5% of fatal overdoses over the course of 10 years (Table 3, Figure 1). The undiscounted cost of the intervention was $629 million dollars at the population-level over 10 years. The discounted, incremental cost of the intervention compared to standard of care was $306 million (Table 4a). The incremental cost-effectiveness ratio of MOUD in detox with perfect linkage compared to standard of care was $67,600/LY saved and $55,600/QALY saved. In probabilistic sensitivity analyses that incorporated uncertainty, the perfect-linkage strategy was shown to be cost-effective 74.0% of the time at the $100,000 per QALY threshold (Supplemental Materials Section G. Supplemental Tables and Figures, Supplemental Figure 6).
Figure 1.
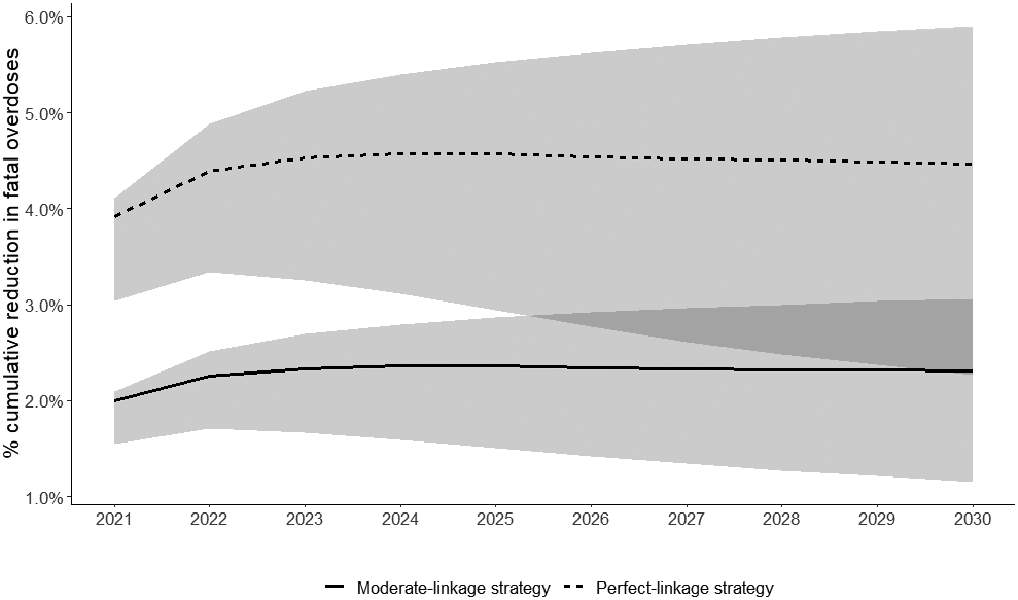
Cumulative percent reduction in fatal overdoses over 10 years in simulated Massachusetts population with opioid use disorder, with initiation of medications for opioid use disorder (MOUD) in medically managed opioid withdrawal (detox) and linkage to outpatient MOUD. The dashed line shows the perfect-linkage strategy (78% of all people in detox initiating MOUD (18% to methadone, 28% to buprenorphine, and 32% to naltrexone), and 100% linking to outpatient MOUD), as compared to the standard of care strategy where all people move from detox directly to post-treatment, with no linkage to outpatient MOUD. The solid line shows the moderate-linkage strategy (78% of all people in detox initiating MOUD (18% to methadone, 28% to buprenorphine, and 32% to naltrexone), and 50% linking to outpatient MOUD), as compared to the standard of care strategy where all people move from detox directly to post-treatment, with no linkage to outpatient MOUD.
Table 4.
Cost effectiveness analysis of initiation of medications for opioid use disorder (MOUD) from inpatient medically managed withdrawal programs (detox), and subsequent linkage to outpatient MOUD, showing cost, life years (LY) and quality-adjusted life-years (QALY) comparing the standard of care strategy to MOUD in detox scenarios with A) perfect linkage to outpatient MOUD care and B) moderate linkage to outpatient MOUD care.
| A. Perfect Linkage | ||||||||
|---|---|---|---|---|---|---|---|---|
| Intervention | Discounted Cost (billions) |
Incremental Discounted Cost (millions) |
Discounted LY (millions) |
Discounted QALY (millions) |
Incremental Discounted LY |
Incremental Discounted QALY |
ICER ($/LY) |
ICER ($/QALY) |
| Standard of care strategy (reference) | 68.79 (36.33-101.26) | - | 3.09 (3.02-3.16) | 2.06 (1.94-2.18) | - | - | - | |
| Perfect-linkage strategy | 69.10 (37.04-101.16) | 306 | 3.09 (3.02-3.17) | 2.07 (1.95-2.19) | 4,530 | 5,512 | $67,600 | $55,600 |
| B. Moderate Linkage | ||||||||
| Intervention | Discounted Cost (billions) |
Incremental Discounted Cost (millions) |
Discounted LY (millions) |
Discounted QALY (millions) |
Incremental Discounted LY |
Incremental Discounted QALY |
ICER ($/LY) |
ICER ($/QALY) |
| Standard of care strategy (reference) | 68.79 (36.33-101.26) | - | 3.09 (3.02-3.16) | 2.06 (1.94-2.18) | - | - | - | |
| Moderate-linkage strategy | 69.02 (36.77-101.27) | 225 | 3.09 (3.02-3.17) | 2.07 (1.94-2.19) | 2,315 | 2,869 | $97,300 | $78,500 |
In comparison to standard of care, the MOUD in detox strategy with moderate linkage resulted in a 1.2% decrease in person-time in active opioid use, a 15.2% increase in person-time on MOUD, and a 2.3% decrease in fatal overdoses over a 10-year period (Table 3, Figure 1). The undiscounted cost of the intervention was $323 million dollars at the population-level over 10 years. The discounted, incremental cost of the intervention compared to standard of care was $225 million (Table 4b). The incremental cost-effectiveness ratio of MOUD in detox wither moderate linkage compared to standard of care was $97,300/LY saved and $78,500/QALY saved. In probabilistic sensitivity analyses that incorporated uncertainty, the moderate-linkage strategy was shown to be cost-effective 60.2% of the time at the $100,000 per QALY threshold (Supplemental Materials Section G. Supplemental Tables and Figures, Supplemental Figure 6).
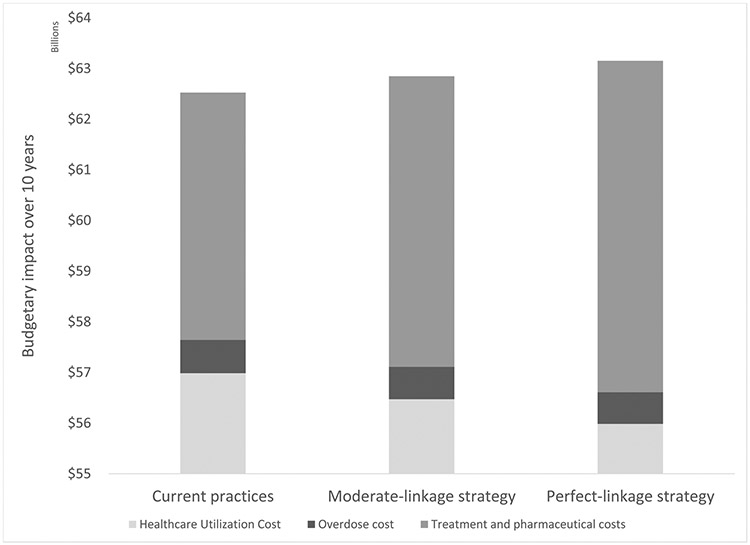
Assuming the standard of care, the Massachusetts healthcare sector can expect to spend a total of $62.5 billion: $57.0 billion dollars on healthcare utilization by the population with OUD, $655 million on opioid overdose costs, and $4.9 billion on treatment visits and pharmaceuticals for OUD ((Supplemental Materials Section G. Supplemental Tables and Figures, Table 17). Implementing MOUD in detox comes with net cost. The state-level healthcare sector budgetary impact of implementing MOUD in detox centers with perfect linkage to care was $63.2 billion over 10 years ($628 million increase compared to standard of care) and $62.8 billion assuming moderate linkage to care ($323 million increase compared to standard of care) (Figure 2).
Figure 2.
Undiscounted budgetary impact for Massachusetts over 10 years of intervention initiating detox patients in medications for opioid use disorder (MOUD), and linking to outpatient MOUD treatment. Results shown for total costs for standard of care strategy, scenario simulating 78% of all people in detox initiating MOUD (18% to methadone, 28% to buprenorphine, and 32% to naltrexone), and 100% linking to outpatient MOUD, and scenario simulating 78% of all people in detox initiating MOUD (18% to methadone, 28% to buprenorphine, and 32% to naltrexone), and 50% linking to outpatient MOUD. Column with vertical lines show total treatment and pharmaceutical costs, solid grey shows overdose costs, and diagonal lines show healthcare utilization costs.
The primary driver of those higher costs was spending on OUD treatment visits and MOUD ($6.5 billion for perfect linkage and $5.7 billion for moderate linkage). Decreases in spending on the medical sequelae of drug use, however, substantially offset pharmaceutical costs. Healthcare utilization costs were reduced by $1.0 billion assuming perfect linkage, and $515 million assuming moderate linkage. Similarly, overdose costs were reduced by $29 million with perfect linkage, and $15 million for moderate linkage.
Population-level sensitivity analyses showed that parameters of greatest significance on the results included retention on MOUD and percent initiation and linkage to MOUD. Improvements in retention on MOUD led to greater efficacy of the intervention, with a 6-month retention on MOUD of 66%, leading to an 8.1% reduction in fatal overdoses over 10 years in the perfect-linkage scenario. Improvements in linkage from detox to outpatient MOUD led to a larger decrease in fatal overdoses and a more cost-effective intervention, whereas increased initiation without linkage to care did not lead to increased effectiveness or cost-effectiveness. Results were robust when other parameters were varied (Supplemental Materials Section H. Additional Sensitivity Analyses).
DISCUSSION
We employed simulation modeling to estimate the effectiveness and cost of transforming detox centers into venues for initiating MOUD and linking to outpatient MOUD treatment. We found that initiating MOUD in detox and linking to outpatient treatment led to a substantial decrease in overdose deaths in a cohort of people in detox, with 13-25% of fatal overdoses prevented in one year. In addition, this intervention could have substantial impact at the population level, preventing 2-4% of fatal overdoses over the course of a 10-year period in Massachusetts. While this absolute reduction in overdose will not resolve the opioid overdose crisis on its own, it represents a substantial improvement in mortality for a known high-risk population. Transforming detox into a venue for MOUD initiation would likely be one effective component of a larger overdose prevention strategy.
In addition, we demonstrate that investment in the transformation and maintenance of detox units as venues for MOUD initiation provides good economic value. We estimate an ICER of $67,000-$97,000 per non-discounted life-year saved and $56,000-$79,000 per QALY saved. Previous cost-effectiveness analyses that have compared MOUD to non-MOUD treatment strategies have typically either found the MOUD strategy to dominate the non-MOUD alternative (i.e., lower mean costs and greater mean QALYs), or have estimated cost-per-QALY ICERs comparable in magnitude to what we found here, with similarly high levels of confidence at traditional value thresholds (23, 24). The cost-effectiveness analysis reveals the critical importance of the cascade of MOUD care to both effectiveness and cost-effectiveness. When linkage to outpatient care is poor, or when retention on MOUD decreases, then the costs of MOUD initiation in detox become uncoupled from the benefits, and the ICER rises. Similarly, in communities that already link some patients from detox directly to community care, it is important to ensure that efforts to expand MOUD in detox sustain similar linkage to care rates, because the cost-effectiveness of MOUD in detox decreases when linkage from detox falls. Plans to offer MOUD in detox centers must include careful thought about linking patients to outpatient care (44).
Our results demonstrate that transforming Massachusetts detox centers into venues for MOUD initiation would cost approximately $32.3-$62.9 million to the healthcare sector annually, or approximately $100-400 per person with OUD in Massachusetts, assuming our population estimates (29). It would require approximately a 0.5-1% budgetary increase from the current spending. For perspective, the fiscal year 2019 budget for the Massachusetts Department of Public Health Bureau of Substance Addiction Services (BSAS) was approximately $142 million (45). Additionally, in 2017 Massachusetts Medicaid (MassHealth) spent $1.5 billion dollars on its OUD population, with 5% (N=90,000) of MassHealth members being diagnosed with OUD (46). The cost of implementing MOUD in detox would be shared by multiple payer types, including both public and commercial health insurance payers, but the juxtaposition of BSAS total budget, MassHealth spending on OUD, and the cost of offering MOUD in detox provides perspective on the scope of the commitment required.
It is notable that although we find that linking to outpatient MOUD from detox is a cost-effective way of preventing a substantial number of overdoses, few jurisdictions are doing this at the current time. Randomized trials (37, 38, 47) demonstrate the safety and clinical effectiveness of MOUD in detox, and this simulation modeling study now additionally shows the effectiveness and economic value of this intervention at the Massachusetts population level. At this time, the evidence base is solid, but the policies have yet to follow. Policymakers at the state and federal level should consider approaches to promote the availability of MOUD in detox and MOUD linkage from detox as a more standard occurrence, whether through reimbursement programs encouraging this practice or regulatory oversight requiring clinicians working detox be licensed to prescribe MOUD.
It is also important to note that our model estimated a conservative retention on outpatient MOUD (6-month retention of 34% with buprenorphine, 56% on methadone, and 21% on naltrexone). Sensitivity analyses showed that raising average 6-month retention on all MOUD to 66% almost doubled the efficacy of offering MOUD in the detox setting, to a fatal overdose reduction of over 5% for the moderate linkage scenario and over 8.5% in the perfect-linkage scenario. Recent studies have shown that retention on MOUD is incredibly important in reducing population-level mortality due to opioid overdose (28, 48, 49), and regardless of method of MOUD initiation, we should work to improve retention rates whenever possible.
There are limitations to this work. This analysis was conducted using a simulation model calibrated to Massachusetts data, but it is not expected to provide exact answers. Any drastic change in trends of drug use or treatment will affect accuracy of future predictions. Some model parameters rely on older data, are challenging estimates to obtain, and may be underestimated in this analysis. In addition, we lacked data to inform transitions directly between different MOUD without entering the high-risk post-treatment state. However, we know from our parameter estimation work that as a proportion of total MOUD interactions, such direct transitions are a tiny fraction. The model does not account for time-updating in loss to follow-up or mortality, though we expect the lower effectiveness of MOUD in the first month of treatment to be represented in the uncertainty around our MOUD mortality effect estimates. In addition, the first week of treatment in the intervention scenario is spent under supervision at the medically managed withdrawal program, limiting the risk of overdose during this time and likely helping to stabilize the patient thereafter. In addition, proportion of all overdoses that are fatal is constant between injection and non-injection drug use, though it is probable that fatality of overdose may vary between the two methods of drug use. The model scenarios assumed a relatively high interest in MOUD in detox patients, which may not be the case. We attempted to remedy this limitation with sensitivity analyses looking at uptake of MOUD in detox and by varying the number of people who are linked to outpatient MOUD treatment from inpatient induction. Additionally, our analysis investigates primarily overdose deaths prevented. While movements out of active drug use do tend to reduce the risk of non-overdose mortality among persons who use drugs, those effects are more challenging to quantify and hence, they are less-well specified in the model. Similarly, we did not consider an independent effect of MOUD on non-overdose mortality beyond MOUD direct effect on drug use. Both of these factors may lead to an underestimation of the effectiveness and cost-effectiveness of offering MOUD in detox settings, which suggests this intervention may be even more favorable than what we have shown here. Finally, our analysis does not include the criminal justice perspective, which may benefit from some of the biggest cost-benefits from MOUD. However, it is important to note that even when not evaluating the biggest cost savings areas, we still found the intervention to be cost-effective.
Conclusion
Our model indicates that incorporating MOUD initiation into detox centers, followed by linkage to community outpatient MOUD treatment, could likely be a cost-effective way to increase life-years and QALYs. When implementing such a strategy, it is essential to think carefully about linkage to outpatient treatment after discharge, because the benefits of MOUD initiation in detox cannot sustain without successful linkage to longitudinal care in the community. With thoughtful planning and sustained investment, detox centers have potential to play a major role in ending the opioid overdose epidemic by incorporating MOUD initiation and linkage as part of standard practice, as opposed to focusing on detoxification alone. Future research should evaluate additional real-world barriers to MOUD initiation in detox and linkage to outpatient care, including interest in MOUD initiation and costs of linkage interventions.
Supplementary Material
Funding:
R01DA046527
Footnotes
Conflicts of interest: none
REFERENCES
- 1.Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilson N, Kariisa M, Seth P, Smith HI, Davis N. Drug and Opioid-Involved Overdose Deaths — United States, 2017–2018. MMWR Morb Mortal Wkly Rep 2020;69:290–297. DOI: 10.15585/mmwr.mm6911a4external icon. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.CDC/NCHS NVSS, Mortality. CDC WONDER, Atlanta, GA: US Department of Health and Human Services, CDC; 2018. https://wonder.cdc.gov. 2018. [Google Scholar]
- 4.Davenport SW A; Caverly M Economic Impact of Non-Medical Opioid Use in the United States; Annual Estimates and Projections for 2015 through 2019. 2019. [Google Scholar]
- 5.Abraham AJ, Adams GB, Bradford AC, Bradford WD. County-level access to opioid use disorder medications in medicare Part D (2010-2015). Health Serv Res. 2019;54(2):390–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones CM, Campopiano M, Baldwin G, McCance-Katz E. National and State Treatment Need and Capacity for Opioid Agonist Medication-Assisted Treatment. Am J Public Health. 2015;105(8):e55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morgan JR, Schackman BR, Leff JA, Linas BP, Walley AY. Injectable naltrexone, oral naltrexone, and buprenorphine utilization and discontinuation among individuals treated for opioid use disorder in a United States commercially insured population. J Subst Abuse Treat. 2018;85:90–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Volkow ND, Collins FS. The Role of Science in Addressing the Opioid Crisis. N Engl J Med. 2017;377(4):391–4. [DOI] [PubMed] [Google Scholar]
- 9.Stein MD, Anderson BJ, Bailey GL. Preferences for Aftercare Among Persons Seeking Short-Term Opioid Detoxification. J Subst Abuse Treat. 2015;59:99–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stein MD, Flori JN, Risi MM, Conti MT, Anderson BJ, Bailey GL. Overdose history is associated with postdetoxification treatment preference for persons with opioid use disorder. Subst Abus. 2017;38(4):389–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spear SE. Reducing readmissions to detoxification: an interorganizational network perspective. Drug Alcohol Depend. 2014;137:76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mark TL, Vandivort-Warren R, Montejano LB. Factors affecting detoxification readmission: analysis of public sector data from three states. J Subst Abuse Treat. 2006;31(4):439–45. [DOI] [PubMed] [Google Scholar]
- 13.Wakeman SE, Larochelle MR, Ameli O, Chaisson CE, McPheeters JT, Crown WH, et al. Comparative Effectiveness of Different Treatment Pathways for Opioid Use Disorder. JAMA Netw Open. 2020;3(2):e1920622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Broers B, Giner F, Dumont P, Mino A. Inpatient opiate detoxification in Geneva: follow-up at 1 and 6 months. Drug Alcohol Depend. 2000;58(1-2):85–92. [DOI] [PubMed] [Google Scholar]
- 15.Strang J, McCambridge J, Best D, Beswick T, Bearn J, Rees S, et al. Loss of tolerance and overdose mortality after inpatient opiate detoxification: follow up study. Bmj. 2003;326(7396):959–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Binswanger IA, Stern MF, Deyo RA, Heagerty PJ, Cheadle A, Elmore JG, et al. Release from prison--a high risk of death for former inmates. N Engl J Med. 2007;356(2):157–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huhn AS, Tompkins DA, Dunn KE. The relationship between treatment accessibility and preference amongst out-of-treatment individuals who engage in non-medical prescription opioid use. Drug Alcohol Depend. 2017;180:279–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Administration SAaMHS. Treatment Episode Data Set (TEDS) 2005-2015: State Admissions to Substance Abuse Treatment Services. In: SERVICES DOHAH, editor. 2017. [Google Scholar]
- 19.Larochelle MR, Bernson D, Land T, Stopka TJ, Wang N, Xuan Z, et al. Medication for Opioid Use Disorder After Nonfatal Opioid Overdose and Association With Mortality: A Cohort Study. Ann Intern Med. 2018;169(3):137–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krupitsky E, Nunes EV, Ling W, Illeperuma A, Gastfriend DR, Silverman BL. Injectable extended-release naltrexone for opioid dependence: a double-blind, placebo-controlled, multicentre randomised trial. Lancet. 2011;377(9776):1506–13. [DOI] [PubMed] [Google Scholar]
- 21.Mattick RP, Breen C, Kimber J, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2014(2):Cd002207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mattick RP, Breen C, Kimber J, Davoli M. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst Rev. 2009(3):Cd002209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murphy SM, Polsky D. Economic Evaluations of Opioid Use Disorder Interventions. Pharmacoeconomics. 2016;34(9):863–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Onuoha EO LJ, Schackman BR, McCollister KE, Polsky D, Murphy SM. . Economic evaluations of pharmacological treatment for opioid use disorder: A systematic literature review. . Value Health. (In press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Substance Abuse and Mental Health Service Administration (SAMHSA): 2019 DoSATF. National Survet of Substance AbUse Treatment Service (N-SSATS). 2020. [Google Scholar]
- 26.Morgan JR, Wang J, Barocas JA, Jaeger JL, Durham NN, Babakhanlou-Chase H, et al. Opioid overdose and inpatient care for substance use disorder care in Massachusetts. J Subst Abuse Treat. 2020;112:42–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Friedmann PD, Suzuki J. More beds are not the answer: transforming detoxification units into medication induction centers to address the opioid epidemic. Addict Sci Clin Pract. 2017;12(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Linas BP, Savinkina A, Madushani RWMA, Wang J, Eftekhari Yazdi G, Chatterjee A, et al. Projected Estimates of Opioid Mortality After Community-Level Interventions. JAMA Network Open. 2021;4(2):e2037259–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barocas JA, White LF, Wang J, Walley AY, LaRochelle MR, Bernson D, et al. Estimated Prevalence of Opioid Use Disorder in Massachusetts, 2011-2015: A Capture-Recapture Analysis. Am J Public Health. 2018;108(12):1675–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee JD, Nunes EV Jr., Novo P, Bachrach K, Bailey GL, Bhatt S, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391(10118):309–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morgan JR, Schackman BR, Weinstein ZM, Walley AY, Linas BP. Overdose following initiation of naltrexone and buprenorphine medication treatment for opioid use disorder in a United States commercially insured cohort. Drug Alcohol Depend. 2019;200:34–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sordo L, Barrio G, Bravo MJ, Indave BI, Degenhardt L, Wiessing L, et al. Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. Bmj. 2017;357:j1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soyka M, Zingg C, Koller G, Kuefner H. Retention rate and substance use in methadone and buprenorphine maintenance therapy and predictors of outcome: results from a randomized study. Int J Neuropsychopharmacol. 2008;11(5):641–53. [DOI] [PubMed] [Google Scholar]
- 34.Mass.gov. Massachusetts Public Health Data Warehouse (PHD) 2020. [Available from: https://www.mass.gov/public-health-data-warehouse-phd.
- 35.Lee JD, Nunes EV, Mpa PN, Bailey GL, Brigham GS, Cohen AJ, et al. NIDA Clinical Trials Network CTN-0051, Extended-Release Naltrexone vs. Buprenorphine for Opioid Treatment (X:BOT): Study design and rationale. Contemp Clin Trials. 2016;50:253–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neumann PJ SG, Russell LB, Siegel JE, Ganiats TG. Cost-Effectiveness in Health and Medicine. 2nd ed. New York: Oxford University Press; 2017. [Google Scholar]
- 37.McCollister KE, Leff JA, Yang X, Lee JD, Nunes EV, Novo P, et al. Cost of pharmacotherapy for opioid use disorders following inpatient detoxification. Am J Manag Care. 2018;24(11):526–31. [PMC free article] [PubMed] [Google Scholar]
- 38.Murphy SM, McCollister KE, Leff JA, Yang X, Jeng PJ, Lee JD, et al. Cost-Effectiveness of Buprenorphine-Naloxone Versus Extended-Release Naltrexone to Prevent Opioid Relapse. Ann Intern Med. 2019;170(2):90–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wittenberg E, Bray JW, Aden B, Gebremariam A, Nosyk B, Schackman BR. Measuring benefits of opioid misuse treatment for economic evaluation: health-related quality of life of opioid-dependent individuals and their spouses as assessed by a sample of the US population. Addiction. 2016;111(4):675–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hunink MGM, Weinstein MC, Wittenberg E, Drummond MF, Pliskin JS, Wong JB, Wong JB, & Glasziou PP . Decision making in health and medicine: Integrating evidence and values. : Cambridge University Press; 2014. [Google Scholar]
- 41.Uebelacker LA, Bailey G, Herman D, Anderson B, Stein M. Patients' Beliefs About Medications are Associated with Stated Preference for Methadone, Buprenorphine, Naltrexone, or no Medication-Assisted Therapy Following Inpatient Opioid Detoxification. J Subst Abuse Treat. 2016;66:48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walley AY, Lodi S, Li Y, Bernson D, Babakhanlou-Chase H, Land T, et al. Association between mortality rates and medication and residential treatment after in-patient medically managed opioid withdrawal: a cohort analysis. Addiction. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Health. MDoP. An assessment of fatal and nonfatal opioid overdoses in Massachusetts (2011–2015). [Available from: https://www.mass.gov/files/documents/2017/08/31/legislative-report-chapter-55-aug-2017.pdf. .
- 44.Morgan JR, Barocas JA, Murphy SM, Epstein RL, Stein MD, Schackman BR, et al. Comparison of Rates of Overdose and Hospitalization After Initiation of Medication for Opioid Use Disorder in the Inpatient vs Outpatient Setting. JAMA Network Open. 2020;3(12):e2029676–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Massachusetts Co. Budget Summary FY2019- Department of Public Health. In: Massachusetts Co, editor. 2018. [Google Scholar]
- 46.Harvey P SA. MassHealth Medication Assisted Treatment Analysis 2019. [Available from: https://www.mass.gov/doc/masshealth-presentation-on-mat-initiation-among-members-with-opioid-use-disorder/download.
- 47.Stein M, Herman D, Conti M, Anderson B, Bailey G. Initiating buprenorphine treatment for opioid use disorder during short-term in-patient 'detoxification': a randomized clinical trial. Addiction. 2020;115(1):82–94. [DOI] [PubMed] [Google Scholar]
- 48.Ching JH, Owens DK, Trafton JA, Goldhaber-Fiebert JD, Salomon JA. Impact of treatment duration on mortality among Veterans with opioid use disorder in the United States Veterans Health Administration. Addiction. 2021;116(12):3494–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hickman M, Steer C, Tilling K, Lim AG, Marsden J, Millar T, et al. The impact of buprenorphine and methadone on mortality: a primary care cohort study in the United Kingdom. Addiction. 2018;113(8):1461–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nosyk B, Li L, Evans E, Huang D, Min J, Kerr T, et al. Characterizing longitudinal health state transitions among heroin, cocaine, and methamphetamine users. Drug Alcohol Depend. 2014;140:69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shah NG, Galai N, Celentano DD, Vlahov D, Strathdee SA. Longitudinal predictors of injection cessation and subsequent relapse among a cohort of injection drug users in Baltimore, MD, 1988-2000. Drug Alcohol Depend. 2006;83(2):147–56. [DOI] [PubMed] [Google Scholar]
- 52.Neaigus A, Gyarmathy VA, Miller M, Frajzyngier VM, Friedman SR, Des Jarlais DC. Transitions to injecting drug use among noninjecting heroin users: social network influence and individual susceptibility. J Acquir Immune Defic Syndr. 2006;41(4):493–503. [DOI] [PubMed] [Google Scholar]
- 53.Nunes EV, Lee JD, Sisti D, Segal A, Caplan A, Fishman M, et al. Ethical and clinical safety considerations in the design of an effectiveness trial: A comparison of buprenorphine versus naltrexone treatment for opioid dependence. Contemp Clin Trials. 2016;51:34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Center for Behavioral Health Statistics and Quality. (2014). 2013 National Survey on Drug Use and Health: Detailed Tables. Substance Abuse and Mental Health Services Administration, Rockville, MD. [Google Scholar]
- 55.Centers for Disease Control and Prevention. HIV Infection Risk, Prevention, and Testing Behaviors among Persons Who Inject Drugs—National HIV Behavioral Surveillance: Injection Drug Use, 23 U.S. Cities, 2018. HIV Surveillance Special Report 24. http://www.cdc.gov/hiv/library/reports/hivsurveillance.html. Published February 2020.
- 56.Cedarbaum ER, Banta-Green CJ. Health behaviors of young adult heroin injectors in the Seattle area. Drug Alcohol Depend. 2016;158:102–9. [DOI] [PubMed] [Google Scholar]
- 57.Coffin PO, Sullivan SD. Cost-effectiveness of distributing naloxone to heroin users for lay overdose reversal. Ann Intern Med. 2013;158(1):1–9. [DOI] [PubMed] [Google Scholar]
- 58.Jiang Y, McDonald JV, Koziol J, McCormick M, Viner-Brown S, Alexander-Scott N. Can Emergency Department, Hospital Discharge, and Death Data Be Used to Monitor Burden of Drug Overdose in Rhode Island? J Public Health Manag Pract. 2017;23(5):499–506. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.