Abstract
Objective:
To determine if the Transdiagnostic Intervention for Sleep and Circadian Dysfunction (TranS-C) improves functional impairment, psychiatric symptoms and sleep and circadian functioning.
Method:
Adults diagnosed with serious mental illness (SMI) and sleep and circadian dysfunction (N=121) were randomly allocated to TranS-C plus usual care (TranS-C+UC; n=61; 8 individual weekly sessions) or 6-months of Usual Care followed by Delayed Treatment with TranS-C (UC-DT; n=60). Schizophrenia (45%) and anxiety (47%) disorders were common. Blind assessments were conducted pre-treatment, post-treatment and 6 months later (6FU). The latter two were the post-randomization points of interest. The location was Alameda County Behavioral Health Care Services (ACBHCS), a Community Mental Health Center (CMHC) in California.
Results:
For the primary outcomes, relative to UC-DT, TranS-C+UC was associated with reduction in functional impairment (b=−3.18, p=0.025, d=−0.58), general psychiatric symptoms (b=−5.88, p=0.001, d=−0.64), sleep disturbance (b=−5.55, p<0.0001, d=−0.96) and sleep-related impairment (b=−9.14, p<0.0001, d=−0.81) from pre-treatment to post-treatment. These effects were maintained to 6-month follow-up (6FU) (d=−0.42–0.82), except functional impairment (d=−0.37). For the secondary outcomes, relative to UC-DT, TranS-C+UC was associated with improvement in sleep efficiency and on the Sleep Health Composite score from pre-treatment to 6FU. TranS-C+UC was also associated with reduced total wake time and waketime variability from pre-treatment to post-treatment, as well as reduced hallucinations and delusions, bedtime variability, and actigraphy measured waking activity count variability from pre-treatment to 6FU.
Conclusions:
A novel transdiagnostic treatment, delivered within a CMHC setting, improves selected measures of functioning, symptoms of comorbid disorders, and sleep and circadian outcomes.
Keywords: Transdiagnostic, sleep, circadian, serious mental illness, dissemination, community mental health
Sleep and circadian dysfunction contribute to vicious cycles of escalating symptoms and functional deficits in persons diagnosed with a serious mental illness (SMI). SMI is operationalized according to Public Law 102–321 and previous research (Kessler et al., 2003; Wang, Demler, & Kessler, 2002) as the presence, for 12-months, of at least one DSM mental disorder that leads to substantial interference with one or more major life activities. A range of sleep and circadian problems, including insomnia, hypersomnia, advanced and delayed phase, sleep continuity problems and irregular sleep-wake schedules are commonly comorbid with SMI (Baglioni et al., 2016). These problems often persist even when the SMI is treated (López, Lancaster, Gros, & Acierno, 2017) and they predict and predate SMI symptom onset and escalation (Hertenstein et al., 2018). Moreover, insufficient sleep exacerbates emotion regulation difficulty, poor problem solving, difficulty with cognitive functioning, and behaviors like impulsivity (Krause et al., 2017). Taken together, there is a need for treatment approaches that address the complexity of real-life sleep and circadian problems in mental illness. The Transdiagnostic Sleep and Circadian Intervention (TranS-C) (Harvey & Buysse, 2017) has been proposed to address the need for one short protocol to address the broader range of sleep and circadian dysfunction experienced by SMI patients. TranS-C is grounded in basic science and draws on cognitive behavior therapy for insomnia (CBT-I)—the first-line treatment for insomnia (Qaseem, Kansagara, Forciea, Cooke, & Denberg, 2016), which effectively treats insomnia across psychiatric disorders and often the comorbid disorder (Geiger-Brown et al., 2015)—along with Interpersonal and Social Rhythm Therapy (Frank et al., 2005), Chronotherapy (Wirz-Justice, Benedetti, & Terman, 2009) and Motivational Interviewing (Miller & Rollnick, 2002). TranS-C is transdiagnostic in two ways: It addresses a range of sleep and circadian problems across a range of SMI.
TranS-C is likely to be highly disseminable due to the substantial cost advantage to training providers in one treatment protocol that covers multiple problems (McHugh & Barlow, 2010). Further, an important gap in knowledge is the performance of treatments in routine practice settings as most treatment research is conducted in research settings. CBT-I has been effectively delivered in a range of real world settings (Espie et al., 2008; Karlin, Trockel, Taylor, Gimeno, & Manber, 2013). The present study was conducted to extend these findings by delivering TranS-C in Community Mental Health Centers (CMHCs). CMHCs are critical settings as they are major, publicly funded providers of treatment for SMI. They provide for the poorest and most underserved members of the community who experience high rates of comorbidity and complexity (Drake et al., 2001). As such, transdiagnostic treatments, like TranS-C, are appropriate as they target processes that underpin multiple disorders and afford treatment for a greater heterogeneity of clinical presentations.
The Sleep Health Framework (Buysse, 2014) underpins and guides TranS-C. This approach encourages sleep improvement along six dimensions that have been linked to mental and physical health outcomes (Buysse, 2014). The dimensions are: regularity of sleep and waking up; satisfaction with sleep or sleep quality; alertness during waking hours or daytime sleepiness; appropriate timing of the patient’s sleep within the 24-hour day; sleep efficiency, i.e., the ability to sleep for a large percentage of the time in bed, as indicated by ease of falling asleep at the beginning of the night and the ease of returning to sleep after awakenings across the night; and sleep duration which is the total amount of sleep obtained by the patient per 24 hours. (note the acronym RUSATED, i.e., Has your sleep “filled up” your emotional, cognitive, and physical need to sleep?). TranS-C aims to promote sleep health along these six dimensions.
As described in the study protocol (Harvey et al., 2016), the aim was to evaluate the effects of TranS-C plus usual care (TranS-C+UC) vs. Usual Care followed by Delayed Treatment with TranS-C (UC-DT) on functional impairment, general psychiatric symptoms and sleep and circadian function in participants receiving treatment for SMI in a CMHC. The hypothesis tested is that TranS-C+UC will be superior to UC-DT at post-treatment and 6-month follow-up (6FU).
Method
Study Design
Adults (N = 121) were randomly assigned, in a 1:1 parallel group design, to TranS-C plus Usual Care (TranS-C + UC; n=61) or Delayed Treatment with TranS-C following 6-months of Usual Care (UC-DT; n=60) (see Figure 1 for the CONSORT flowchart). Randomization was stratified by lifetime presence of a psychotic disorder (yes, no) and age (49 and under, 50+). Participants completed a battery of measures at pre-treatment, post-treatment (9–14 weeks later) and 6FU. Assessors were blinded to treatment allocation. A project coordinator conducted randomization after each eligibility assessment was completed. The Committee for the Protection of Human Subjects approved the study. A Data Safety and Management Committee reviewed the progress and safety of study procedures twice per year. No adverse events were reported.
Figure 1. CONSORT Diagram Illustrating the Flow of Participants Through the Study.
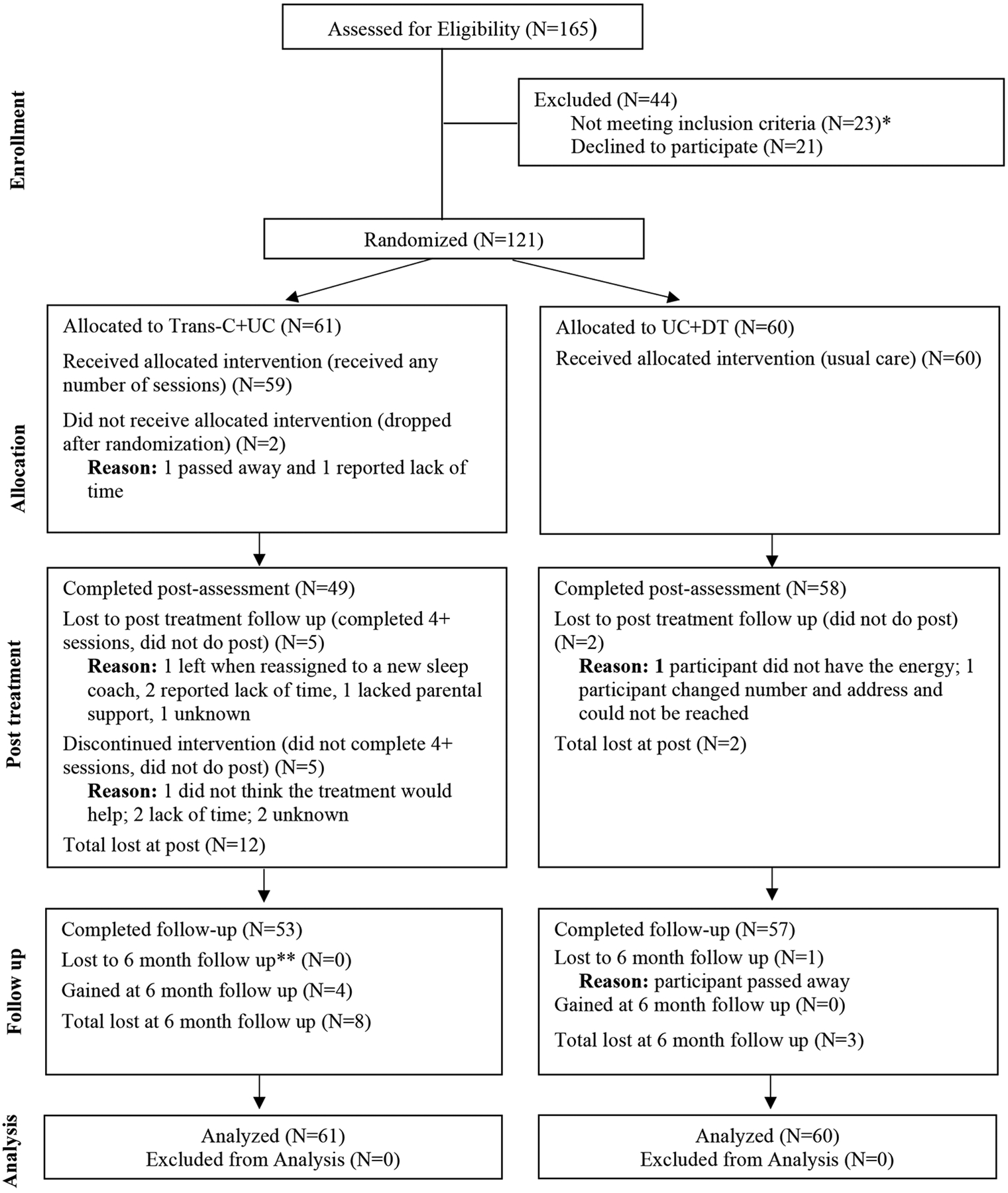
*Out of 44 who were ineligible, 23 did not meet one of more of the inclusion criteria or met for one or more of the exclusion criteria, and 21 either decided they did not want to participate or stopped contacting the researchers. **6-month follow-up was 6 months from the start of treatment.
Participants and Setting
Adults who met criteria for SMI and sleep and circadian dysfunction were recruited from multiple sites within Alameda County Behavioral Health Care Services (ACBHCS), the CMHC for Alameda County, California. Participants were referred via case managers and psychiatrists, and recruited via advertising in clinic waiting rooms and giving presentations. SMI was operationalized according to Public Law 102–321 and previous research (Wang et al., 2002) as the presence, for 12 months, of at least one Diagnostic and Statistical Manual-defined mental disorder that leads to substantial interference with one or more major life activities.
To enhance representativeness and generalizability, inclusion and exclusion criteria were kept to a minimum. Inclusion criteria: 1) Age 18+ years; 2) English language fluency; 3) presence of at least one DSM-5 mental disorder for 12 months; 4) one or more sleep or circadian problems for 3 months assessed with the Sleep and Circadian Problems Interview; 5) having a guaranteed bed to sleep in for 3 months; 6) receiving care for SMI at ACBHCS; and 6) consenting to regular communications between research team and psychiatrist and/or case manager. Sleep and circadian problems included: taking ≥30 mins to get to sleep 3 or more nights per week, waking in the middle of the night for ≥30 minutes 3 or more nights per week, obtaining <6 hours of sleep per night 3 or more nights per week, obtaining >9 hours of sleep per 24 hour period (i.e., nighttime sleep plus daytime napping) 3 or more nights per week, having more than 2.78 hours of variability in sleep-wake schedule across one week which was selected based on mean variability in total sleep time across a week in prior research (Gruber et al., 2009) and sleeping at a bedtime later than 2 am on 3 or more nights per week.
Exclusion criteria: 1) presence of an active and progressive physical illness or neurological degenerative disease and/or substance abuse/dependence making participation in the study infeasible; 2) current serious suicide or homicide risk (assessed by research staff, a case manager, or psychiatrist); 3) night shift work >2 nights per week in the past 3 months; 4) pregnancy or breast-feeding; 5) not able/willing to complete the pre-treatment assessments. As individuals with sleep apnea and periodic limb movement disorder often have comorbid insomnia and poor sleep habits, and can benefit from CBT-I (Edinger et al., 1996; Sweetman et al., 2019), they were included. Participants’ SMI medications often need to be changed. Excluding on this basis is neither feasible nor representative of clinical practice. Medication use and changes were recorded.
Measures
The assessors were blind to treatment allocation. The process of ensuring reliability of the assessments was carefully managed. First, a multi-step training process was required for each assessor including a classroom training session, mock interviews, scoring of tapes of real participant interviews and then shadowing and being shadowed by a certified assessor who fully scores the assessment. For the latter two steps the trainee has to achieve an 80% match on symptoms and 100% on diagnoses. Also, senior team members were closely involved in solving diagnostic dilemmas.
In addition to demographics, the following outcomes were assessed.
Primary Outcomes.
Functional Impairment was assessed with the Sheehan Disability Scale (SDS) (Sheehan, Harnett-Sheehan, & Raj, 1996a). The SDS assesses mood- and sleep-related impairment (Sheehan et al., 1998). The SDS evaluates the extent to which work/school, social life, and home/family responsibilities are impaired on a 0–10 (‘not at all’ to ‘extremely’) scale. The SDS has been found to have adequate reliability and validity in multiple studies (Leon, Olfson, Portera, Farber, & Sheehan, 1997; Leon, Shear, Portera, & Klerman, 1992; Sheehan, Harnett-Sheehan, & Raj, 1996b). The 3 items were averaged to assess global functional impairment (0 not impaired to 10 highly impaired).
The DSM-5 Cross-Cutting Measure (DSM-5) was included as a measure of general psychiatric symptoms (Narrow et al., 2013). This measure includes 23-items to assess mental health domains that are critical across psychiatric diagnoses (Clarke & Kuhl, 2014; Narrow et al., 2013). It measures impairment in the following domains: depression, anger, mania, somatic symptoms, suicidal ideation, psychosis, sleep problems, memory, repetitive thoughts and behaviors, dissociation, personality functioning, and substance use. Individuals report how much each domain has bothered them in the last two weeks on a 0–4 scale (‘not at all’ to ‘nearly every day’). Each item is rated on a 5-point scale, with higher scores indicating more severe impairment. The measure has demonstrated good test-retest reliability and clinical usefulness (Clarke & Kuhl, 2014; Narrow et al., 2013).
Sleep and circadian function was assessed with the PROMIS–Sleep Disturbance (PROMIS–SD) (Yu, Buysse, Germain, & Moul, 2012) and the PROMIS–Sleep-Related Impairment (PROMIS–SRI) (Yu et al., 2012). The PROMIS–Sleep Disturbance (PROMIS–SD) (Yu et al., 2012) was developed based on the PROMIS-Sleep Disturbance item bank. The 8-item short version assesses sleep disturbance over the past seven days, including restlessness, sleep quality, ability to fall and stay asleep, and refreshment following sleep using a 1–5 scale (‘not at all’ or ‘never’ to ‘very much’ or ‘always’). Scores range from 8 to 40, with higher scores indicating increased disturbance (Yu et al., 2012). The PROMIS-SD has demonstrated reliability and validity (Yu et al., 2012).
We administered the 16-item version of the PROMIS–Sleep Related Impairment (PROMIS-SRI) questionnaire in which participants rate difficulties during wakeful hours associated with sleep problems over the last week on a 5-point scale (Yu et al., 2012). The PROMIS-SRI measures perceptions of alertness, sleepiness and tiredness within the overall context of sleep-wake function, but does not directly assess cognitive, affective, or performance impairments. Scores range from 16 to 80, with higher scores indicating increased disturbance (Yu et al., 2012). The PROMIS-SRI has also demonstrated adequate validity when compared to other measures of daytime symptoms such as the Epworth Sleepiness Scale (Yu et al., 2012).
Secondary Outcomes.
General psychiatric symptoms were more specifically assessed by the Quick Inventory of Depressive Symptoms (QIDS) (Rush et al., 2003), Alcohol, Smoking and Substance Involvement Screening Test (ASSIST) (Group, 2002) and/or the Psychotic Symptoms Rating Scales (PSYRATS) (Haddock, McCarron, Tarrier, & Faragher, 1999).
The self-report version of the QIDS is a widely-used 16-item instrument assessing depressive symptoms. Each item is rated on a four-point scale from 0–3, with higher scores indicating greater symptom severity. The measure has demonstrated good reliability and validity (Rush et al., 2003).
The ASSIST evaluates psychoactive substance use and measures related impairment (Group, 2002). ASSIST measures lifetime substance use as well as frequency of use and related problems within the past three months. Frequency of use, substance dependence, and related health, social, legal, financial, and employment problems in the past three months are rated on a 5-point scale (‘never’ to ‘daily of almost daily’). Problems with family and friends caused by substance use and failed attempts to cut down or quit substance use are measured on a 3-point scale (‘no, never’, ‘yes, in the past 3 months’, ‘yes, but not in the past 3 months’). ASSIST has good reliability and clinical feasibility (Group, 2002).
The PSYRATS is a semi-structured interview designed to assess the subjective characteristics of hallucinations and delusions (Haddock et al., 1999). Each of the 17-items is rated on a 5-point scale (0–4). The PSYRATS has good reliability and validity in first episode samples and complimentary existing measures (Drake, Haddock, Tarrier, Bentall, & Lewis, 2007).
Functional impairment was further assessed with the self-administered version of the World Health Organization Disability Assessment Schedule (WHODAS) 2.0 and the 4-question ‘Healthy Days’ core module (Moriarty, Zack, & Kobau, 2003).
The World Health Organization Disability Assessment Schedule (WHODAS) 2.0 is a 36-item measure that assesses disability in adults ages 18 years and older. It assesses disability across six domains on a scale from 1–5 (‘none’ to ‘extreme or cannot do’). Each item on the self-administered version of the WHODAS-2.0 asks the individual to rate how much difficulty he or she has had in specific areas of functioning during the past 30 days (World Health Organization, 2012). The WHODAS 2.0 possesses strong psychometric properties and provides a global disability score as well as six domain scores: cognition, mobility, self-care, getting along with others, participation in society, and life activities (Konecky, Meyer, Marx, Kimbrel, & Morissette, 2014).
The ‘Healthy Days’ core module was developed by the Centers for Disease Control and Prevention to assess health related quality of life and is a 4-item measure. Health related quality of life was defined as “perceived physical and mental health over time.” This measure asks about self-rated general health and the number of recent days when a person was physically unhealthy, mentally unhealthy, or limited in usual activities. A summary measure combines physically and mentally unhealthy days. An “unhealthy days” summary measure based on the second and third questions and estimates the overall number of recent days when physical or mental health was not good. This measure is the brief, validated and is based on a clear and explicit definition (Moriarty et al., 2003).
Sleep and circadian function was further assessed with the daily sleep diary and actigraphy (GT9X Link, ActiGraph), collected for 7 days at each assessment point. The daily sleep diary outcomes were mean and variability in sleep efficiency (total sleep time/time in bed × 100), total sleep time, total wake time, bedtime and waketime. Trained research assistants collected the sleep diary by phone each day. Times were selected after, but as close as possible to, rise time. Outcomes analyzed from actigraphy include the mean and variability for total sleep time and total wake time, as well as waking activity count. See the Supplement for further information on scoring.
To capture the complexity of the sleep problems in SMI, we calculated a Sleep Health Composite score (Dong, Martinez, Buysse, & Harvey, 2019), defined as the sum of scores on 6 sleep health dimensions (Buysse, 2014) (each dimension was dichotomized such that 1 = good; 0 = poor): Regularity (Midpoint fluctuation via sleep diary), Satisfaction (Sleep quality question on PROMIS-SD), Alertness (Daytime sleepiness question on PROMIS-SRI), Timing (Mean midpoint via sleep diary), Efficiency (Sleep efficiency via 7-day sleep diary) and Duration (total sleep time via 7-day sleep diary). Higher scores indicate better sleep health. This measure is proposed to capture the complexity of the sleep problems covered by TranS-C. Initial validity of this measure has been established (Buysse, 2014).
Measures included at pre-treatment assessment only.
The Mini-International Neuropsychiatric Interview (MINI) (DSM-5, Version 7.0.0 including Schizophrenia and Psychotic Disorders) was included as an evaluation of the presence of current and past SMI. The MINI was developed to meet the need for a simple, short and accurate structured psychiatric interview. Screening questions are used to initially rule out the presence of mental health disorders. Positive responses to screening questions prompt further probing into severity and disability caused by the disorder. The MINI has demonstrated good test retest reliability (kappa > .88) and validity (Lecrubier et al., 1997; Sheehan et al., 1998).
The diagnostic measure for sleep disorders was the Duke Structured Interview for Sleep Disorders (DUKE) (Edinger et al., 2009). The DSISD is a clinical semi-structured interview designed to detect the presence of a range of sleep and circadian disorders according to both the International Classification of Sleep Disorders (ICSD) and the Diagnostic and Statistical Manual of Mental Disorders criteria. Disorders assessed include insomnia, hypersomnia, circadian phase disorders, and multiple parasomnias. Each diagnostic criteria is represented as an independent question. At the end of the interview, participant reported symptomatology for each diagnostic criteria is used to determine a final diagnosis for that disorder: absent, subthreshold, or threshold. The DSISD has demonstrated discriminant validity and high reliability (kappa values range from .71 to .86). Diagnoses were clarified via a review of a 7-day daily sleep diary.
Sleep/insomnia history was obtained with the Sleep and Circadian Problems Interview (Morin, 1993), to assess inclusion/exclusion criteria. To improve our ability to identify obstructive sleep apnea, we supplemented the DUKE with STOP-BANG (Farney, Walker, Farney, Snow, & Walker, 2011), which is an 8-item screen. Both measures are well validated and widely used. Those suspected to have another sleep disorder were referred for non-study evaluation/treatment and were not excluded.
A Medication Tracking Log was administered at each assessment. Treatment credibility/expectancies were assessed after Session 2 via the Credibility/Expectancy Questionnaire (Devilly & Borkovec, 2000).
Treatments.
TranS-C
TranS-C was administered by masters’ level therapists, hired within the University of California, Berkeley, who traveled between the ACBHCS clinic sites. Clinicians attended a one-day workshop, used a manual, and received weekly supervision. Usual care was administered by CMHC providers.
The average number of 50-minute sessions attended was 8. Up to 12 sessions could be provided but this was rarely needed. TranS-C includes four cross-cutting modules featured in every session (functional analysis, education, behavior change and motivation, and goal-setting), four core modules that apply to the vast majority of participants (establishing regular sleep-wake times including learning a wind-down and wake-up routine, improving daytime functioning, correcting unhelpful sleep-related beliefs, and maintaining behavior change), and seven optional modules used less commonly, depending on the needs of each participant (improving sleep efficiency, reducing time in bed, dealing with delayed or advanced phase, reducing sleep-related worry/vigilance, promoting compliance with CPAP/exposure therapy for claustrophobic reactions to CPAP, negotiating sleep in a complicated environment, and reducing nightmares). Core and optional modules can be delivered in any sequence and are customized to the participant based on their presentation and goals for treatment (see Table 4 in Supplement for a summary).
Sessions were audio recorded. TranS-C treatment integrity was evaluated with the Cognitive Therapy Rating Scale (CTRS); a score of ≥40 is generally regarded as competent delivery (Young & Beck, 1980). A checklist of TranS-C elements was used to rate the presence/absence of treatment elements for a random subset of tapes (Gumport, Yu, Mullin, Mirzadegan, & Harvey, 2020).
How do TranS-C and CBT-I compare? TranS-C incorporates the key tenets and procedures of CBT-I. However, it also incorporates advances in knowledge from other sleep medicine approaches to address the broader range of sleep and circadian dysfunctions commonly experienced in SMI. Relatedly, poor sleep efficiency is often a treatment target for SMI patients. This calls for stimulus control and sleep restriction. However, a notable proportion of SMI patients exhibit sleep efficiency >85%, or their sleep efficiency is corrected by regularizing bed and wake times (Kaplan & Harvey, 2013). An additional issue is that stimulus control and sleep restriction can involve partial sleep deprivation. As sleep deprivation can trigger SMI relapse (Colombo, Benedetti, Barbini, Campori, & Smeraldi, 1999), in certain cases there are safety concerns.
UC-DT.
We carefully considered the choice of comparison condition before deciding on UC-DT. Our choice to compare TranS-C to UC-DT was preferred by our community partners and aimed to strike a balance between (a) including a comparison group to demonstrate the effectiveness of TranS-C in community settings; this information is critical to determining the potential of TranS-C for broader dissemination; (b) ensuring that all participants receive what we hypothesize to be an active treatment (TranS-C); and (c) maximizing efficiency in terms of study duration, budget, and participants’ time investment. Notably, usual care has been used as the comparison group in several influential studies (Craske et al., 2011; Daumit et al., 2013; Weisz et al., 2012).
UC-DT was conducted within ACBHCS, involved a case manager coordinating care and referring each client for a medication review and to various programs (e.g., health care, housing, vocational, ‘hearing voices’ group). After 8 months in UC-DT, the participants received 8 sessions of TranS-C.
Trial registration
We report the primary and secondary outcomes listed in the ClinicalTrials.gov protocol (NCT02469233). This was updated on December 19, 2019 to remove nap timing and duration via sleep diary because naps were inconsistently reported and the data quality was poor.
Data Analysis
Sample size (N=120) was determined assuming a medium effect size across outcomes based on prior literature (Espie et al., 2008; Harvey et al., 2015), with significance of 0.05, and power of 80%. The final sample was 121 because one eligible participant was already in the ‘assessment pipeline’ at the end of the study. One prior analysis was conducted for the purpose of a grant application and conference presentation in July 2018 (75% of the data had been collected and entered).
Data were analyzed using Stata 15. Analyses were adjusted for the stratification factors (age, lifetime presence of a psychotic disorder). Intent-to-treat analysis was performed. The endpoints (treatment effects of interest) are changes comparing TranS-C vs. UC-DT on the primary and secondary outcomes from pre-treatment to post-treatment and from pre-treatment to 6FU. Multilevel modeling, with maximum likelihood estimation and the assumption of missing at random, was used to examine the outcome variables, all modeled as continuous outcome variables. The fixed component included stratification factors, dummy-coded indicator for time (0=pre, 1=post, 2=6FU), an indicator for treatment condition (0=UC-DT, 1=Immediate TranS-C+UC), and a time-by-treatment interaction term. The random part included a subject-specific random intercept and a time- and subject-specific error term. A significant treatment-by-time interaction is the treatment effect of interest, and is interpreted as the difference between TranS-C+UC vs. UT-DT in mean change (for a given outcome variable) from pre-treatment to post-treatment and to 6FU. The Benjamini-Hochberg procedure (Benjamini & Hochberg, 1995) was used to correct for multiple testing for confirmatory analyses on the primary outcomes (Moyé, 2008) (i.e., TranS-C+UC effects on each of the 4 primary outcomes from pre-treatment to post-treatment and to 6FU). Assuming a 5% false discovery rate, all p values remain significant compared to the corresponding Benjamini-Hochberg critical values.
Results
Participant Characteristics
Dropout rates were 1.7% (2 participants) before session 1, 9.9% (12 participants) between session 1 and post-treatment assessment, and 0.8% (1 participant) dropped out between post and 6FU. Attrition rates were higher in TranS-C than UC-DT during treatment phase (16.9% in TranS-C; 3.3% in UC-DT; X2=6.04, df=1, p=0.001), but not significantly different prior to Session 1 (3.3% in TranS-C; 0% in UC-DT; X2=2.00, df=1, p=0.16) or at 6FU (13.1% in TranS-C; 5.0% in UC-DT; X2=2.38, df=1, p=0.12). Relative to completers, participants who did not begin treatment or who dropped out were not significantly different on gender (X2=0.09, df=1, p=0.76), age group (above or below 50 years; X2=2.15, df=1, p=0.14) or psychosis status (X2=0.01, df=1, p=0.94). Table 1 indicates the two treatment conditions did not differ on baseline variables.
Table 1.
Baseline Demographic and Clinical Characteristics of Patients in Both Treatment Conditions
| Characteristic | UC-DT (n = 60) | TranS-C+UC (n = 61) | ||
|---|---|---|---|---|
| n | % | n | % | |
| Female | 33 | 55.00 | 30 | 49.18 |
| Ethnicity | ||||
| Hispanic or Latino | 9 | 15.00 | 10 | 16.39 |
| Not Hispanic or Latino | 51 | 85.00 | 50 | 81.97 |
| Missing | 1 | 1.64 | ||
| Race | ||||
| White | 21 | 35.00 | 25 | 40.98 |
| African American/Black | 26 | 43.33 | 26 | 42.62 |
| American Indian or Alaskan Native | 4 | 6.67 | 4 | 6.56 |
| Asian | 5 | 8.33 | 2 | 3.28 |
| Native Hawaiian/Other Pacific Islander | 2 | 3.33 | 1 | 1.64 |
| Missing | 2 | 3.33 | 3 | 4.92 |
| Civil status | ||||
| Single | 42 | 70.00 | 38 | 62.3 |
| Married/common law partner | 4 | 6.67 | 5 | 8.2 |
| Separated/divorced/widowed | 14 | 23.33 | 18 | 29.51 |
| Education | ||||
| High school or below | 22 | 36.67 | 19 | 31.14 |
| Vocational school | 2 | 3.34 | 9 | 14.76 |
| Some college or completed college | 34 | 56.67 | 30 | 49.18 |
| Graduate school | 2 | 3.34 | 3 | 4.92 |
| Employment | ||||
| Full-time | 1 | 1.67 | 1 | 1.64 |
| Part-time | 6 | 10.00 | 9 | 14.75 |
| Unemployed | 49 | 81.66 | 49 | 80.33 |
| Other | 4 | 6.67 | 1 | 1.64 |
| Missing | 1 | 1.64 | ||
| Living arrangement | ||||
| Alone | 12 | 20.00 | 8 | 13.11 |
| With family (spouse or children) | 8 | 13.33 | 6 | 9.84 |
| With friend or roommate or pet | 11 | 18.34 | 11 | 18.03 |
| Supported housinga | 29 | 48.33 | 35 | 57.38 |
| Missing | 1 | 1.64 | ||
| MINI Diagnosis at pre-treatment (current or past)b | ||||
| Schizophrenia spectrum disorder | 29 | 49.15 | 26 | 43.33 |
| Bipolar disorder | 13 | 22.03 | 21 | 35.00 |
| Major depressive disorder | 17 | 28.81 | 11 | 18.33 |
| Any anxiety disorder | 27 | 45.76 | 30 | 50.00 |
| Obsessive compulsive disorder | 13 | 22.03 | 9 | 15.00 |
| Post-traumatic stress disorder | 12 | 20.34 | 6 | 10.00 |
| Substance Use Disorder | 20 | 33.90 | 19 | 31.67 |
| Psychotic symptoms/features | 42 | 71.19 | 39 | 65.00 |
| DUKE diagnoses at pre-treatment (current)b | ||||
| Insomnia disorder | 49 | 81.67 | 52 | 85.25 |
| Hypersomnolence disorder | 14 | 23.33 | 17 | 27.87 |
| Circadian Rhythm Disorder | ||||
| Delayed sleep phase type | 5 | 8.33 | 3 | 4.92 |
| Advanced sleep phase type | 0 | 0.00 | 2 | 3.28 |
| Irregular sleep-wake type | 0 | 0.00 | 1 | 1.64 |
| Restless leg syndrome | 3 | 5.00 | 2 | 3.28 |
| Periodic limb movement disorder | 4 | 6.67 | 1 | 1.64 |
| M | SD | M | SD | |
| Age (in years) | 45.45 | 13.25 | 47.97 | 11.51 |
| Education (in years) | 13.38 | 3.89 | 13.80 | 3.05 |
| Annual personal income | $12,429 | $15,317 | $12,636 | $9,850 |
| Annual household income | $24,091 | $27,507 | $26,537 | $23,576 |
Note.
Supported housing includes living in board & care homes, senior housing, transitional housing, and homeless shelter. Baseline variables did not differ between treatment conditions.
Comorbidity was common
Primary Outcomes
Descriptives are in Table 2. Multilevel modeling results are in Table 3. See Figure 2 for graphical presentation. There was no group difference between TranS-C+UC and UC-DT on any of the outcome variables at pre-treatment.
Table 2.
Descriptive statistics of outcome variables.
| Variable | Pre | Post | FU | d pre-post | d pre-FU | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UC-DT | TranS-C+UC | UC-DT | TranS-C+UC | UC-DT | TranS-C+UC | |||||||||
| M | SD | M | SD | M | SD | M | SD | M | SD | M | SD | |||
| Primary Outcomes | ||||||||||||||
| SDS | 14.30 | 8.51 | 12.03 | 6.20 | 11.76 | 7.57 | 6.57 | 7.07 | 10.51 | 8.36 | 7.00 | 6.41 | −0.58 | −0.37 |
| DSM5 Cross-Cutting Measure | 25.57 | 10.72 | 25.05 | 9.75 | 23.53 | 10.59 | 17.00 | 11.23 | 23.07 | 9.78 | 18.67 | 11.38 | −0.64 | −0.42 |
| PROMIS-SD | 29.02 | 6.57 | 28.26 | 6.06 | 27.33 | 7.22 | 20.88 | 8.91 | 26.40 | 8.00 | 20.87 | 8.50 | −0.96 | −0.82 |
| PROMIS-SRI | 51.02 | 14.09 | 47.93 | 11.80 | 47.22 | 14.14 | 35.16 | 14.01 | 44.30 | 15.22 | 35.65 | 13.83 | −0.81 | −0.56 |
| Secondary Outcomes | ||||||||||||||
| QIDS | 12.50 | 4.71 | 12.03 | 5.15 | 10.55 | 5.54 | 8.88 | 5.70 | 10.67 | 4.58 | 8.46 | 5.03 | −0.20 | −0.30 |
| ASSIST | 33.68 | 25.87 | 39.70 | 26.68 | 14.62 | 17.15 | 15.35 | 17.13 | 32.58 | 25.25 | 34.60 | 24.10 | −0.18 | −0.15 |
| PSYRATS | 36.60 | 5.13 | 39.60 | 14.29 | 41.73 | 11.39 | 36.00 | 14.82 | 42.58 | 9.44 | 25.80 | 11.23 | −1.25 | −2.13 |
| WHODAS | 81.53 | 25.56 | 76.48 | 21.38 | 81.48 | 26.80 | 68.93 | 25.53 | 73.45 | 26.14 | 66.63 | 23.17 | −0.35 | −0.14 |
| Healthy Days Overall Health | 3.57 | 1.17 | 3.44 | 1.03 | 3.40 | 1.26 | 3.37 | 1.27 | 3.40 | 1.26 | 3.37 | 1.27 | 0.08 | 0.08 |
| Sleep Diary | ||||||||||||||
| SE mean | 77.61 | 11.89 | 77.39 | 13.52 | 78.93 | 14.50 | 84.31 | 10.07 | 79.54 | 12.88 | 84.78 | 10.86 | 0.40 | 0.38 |
| SE variability | 12.52 | 7.63 | 11.92 | 6.71 | 11.86 | 8.49 | 10.01 | 7.95 | 11.25 | 6.71 | 9.24 | 7.87 | −0.20 | −0.23 |
| TST mean | 452.42 | 101.37 | 431.06 | 111.47 | 459.09 | 100.16 | 450.71 | 92.77 | 465.23 | 115.13 | 464.90 | 106.54 | 0.11 | 0.18 |
| TST variability | 109.59 | 55.14 | 103.96 | 46.37 | 123.59 | 79.11 | 96.50 | 97.84 | 99.30 | 52.38 | 90.87 | 58.77 | −0.41 | −0.10 |
| TWT mean | 131.89 | 80.47 | 124.84 | 75.03 | 131.29 | 116.57 | 84.37 | 49.45 | 118.92 | 77.55 | 83.55 | 65.53 | −0.53 | −0.39 |
| TWT variability | 77.85 | 54.92 | 74.04 | 46.48 | 82.07 | 77.64 | 61.33 | 62.27 | 73.27 | 47.70 | 52.69 | 52.56 | −0.35 | −0.38 |
| Bedtime mean | 22.20 | 1.95 | 22.17 | 2.36 | 22.39 | 1.56 | 22.00 | 1.65 | 22.41 | 1.88 | 21.96 | 2.13 | −0.17 | −0.20 |
| Bedtime variability | 1.39 | 1.06 | 1.28 | 0.79 | 1.41 | 1.07 | 1.01 | 0.93 | 1.55 | 1.32 | 1.00 | 0.68 | −0.36 | −0.51 |
| Waketime mean | 7.90 | 1.83 | 7.31 | 2.02 | 8.26 | 2.25 | 7.31 | 1.82 | 7.95 | 1.83 | 7.33 | 1.74 | −0.20 | −0.02 |
| Waketime variability | 1.42 | 0.88 | 1.34 | 1.00 | 1.61 | 1.11 | 1.10 | 1.08 | 1.22 | 0.69 | 1.26 | 0.95 | −0.46 | 0.15 |
| Actigraphy | ||||||||||||||
| TST mean | 421.35 | 126.92 | 453.65 | 147.28 | 411.82 | 108.28 | 427.23 | 135.85 | 443.24 | 119.47 | 437.69 | 137.88 | −0.10 | −0.28 |
| TST variability | 132.08 | 75.83 | 132.34 | 79.71 | 135.38 | 75.05 | 140.06 | 95.02 | 144.86 | 88.72 | 134.43 | 96.75 | 0.05 | −0.14 |
| TWT mean | 91.06 | 41.52 | 94.95 | 55.18 | 91.39 | 48.65 | 90.80 | 44.85 | 92.73 | 41.87 | 84.51 | 45.84 | −0.08 | −0.23 |
| TWT variability | 55.65 | 51.16 | 54.46 | 48.26 | 60.36 | 68.61 | 51.72 | 41.86 | 56.22 | 48.70 | 41.41 | 30.65 | −0.15 | −0.28 |
| Waking activity count mean | 1309.74 | 532.79 | 1280.09 | 607.46 | 1361.19 | 532.36 | 1257.82 | 680.06 | 1362.37 | 598.01 | 1281.35 | 667.83 | −0.13 | −0.10 |
| Waking activity count variability | 379.69 | 211.65 | 394.04 | 256.07 | 393.58 | 223.01 | 371.08 | 310.24 | 447.29 | 257.81 | 354.96 | 212.71 | −0.16 | −0.47 |
| Sleep health composite | 2.28 | 1.29 | 2.54 | 1.60 | 2.34 | 1.33 | 3.45 | 1.39 | 2.53 | 1.39 | 3.38 | 1.64 | 0.52 | 0.33 |
Note. FU = 6-month follow-up. SDS = Sheehan Disability Scale. PROMIS-SD = Patient-Reported Outcomes Measurement Information System–Sleep Disturbance. PROMIS-SRI = Patient-Reported Outcomes Measurement Information System– Sleep-Related Impairment. QIDS = Quick Inventory of Depressive Symptoms. ASSIST = Alcohol, Smoking and Substance Involvement Screening Test. PSYRATS = Psychotic Symptoms Rating Scales. WHODAS = World Health Organization Disability Assessment Schedule 2.0. CDC Healthy Days= overall health question from the 4-question healthy days core module developed by the Centers for Disease Control and Prevention. SE = sleep efficiency (total sleep time/time in bed × 100). TST = total sleep time. TWT = total wake time. BT = bedtime, WT = wake time. d pre-post = effect size for treatment effects from pre to post; d pre-FU = effect size for treatment effects from pre to 6-month follow-up; both ds are calculated using mean change scores and pretreatment raw SDs from each treatment condition, based on Feingold equation 5.
Table 3.
Multilevel Modeling Results for Primary and Secondary Outcomes
| Treatment Effect at Baseline | Treatment Effect on Change from Pre to Post | Treatment Effect on Change from Pre to FU6 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Coef. | SE | p | Coef. | SE | p | Coef. | SE | p | |
| Primary Outcomes | |||||||||
| SDS | −2.18 | 1.34 | 0.10 | −3.18 | 1.42 | 0.025 | −1.52 | 1.40 | 0.28 |
| DSM5 Cross-Cutting Measure | −0.25 | 1.89 | 0.89 | −5.88 | 1.81 | 0.001 | −3.90 | 1.79 | 0.03 |
| PROMIS-SD | −0.85 | 1.33 | 0.52 | −5.55 | 1.31 | <0.0001 | −4.92 | 1.30 | <0.0001 |
| PROMIS-SRI | −2.92 | 2.49 | 0.24 | −9.14 | 2.44 | <0.0001 | −5.37 | 2.42 | 0.027 |
| Secondary Outcomes | |||||||||
| QIDS | −0.62 | 0.92 | 0.50 | −0.95 | 0.95 | 0.32 | −1.67 | 0.94 | 0.08 |
| ASSIST | 0.26 | 0.24 | 0.26 | −0.09 | 0.24 | 0.72 | −0.08 | 0.23 | 0.74 |
| PSYRATS | 0.87 | 5.84 | 0.88 | −2.46 | 7.08 | 0.73 | −17.52 | 7.54 | 0.02 |
| WHODAS | −4.87 | 4.42 | 0.27 | −7.19 | 4.18 | 0.09 | −1.13 | 4.16 | 0.79 |
| CDC Healthy Days: Overall Health | −0.12 | 0.20 | 0.55 | 0.16 | 0.21 | 0.46 | −0.34 | 0.21 | 0.10 |
| Sleep Diary | |||||||||
| SE mean (min) | −0.46 | 2.35 | 0.85 | 5.68 | 2.67 | 0.03 | 5.89 | 2.64 | 0.03 |
| SE variability (min) | −0.33 | 1.45 | 0.82 | −1.45 | 1.86 | 0.43 | −1.55 | 1.84 | 0.40 |
| TST mean (min) | −24.98 | 19.33 | 0.20 | 24.85 | 20.60 | 0.23 | 34.31 | 20.36 | 0.09 |
| TST variability (min) | −3.08 | 12.89 | 0.81 | −19.90 | 15.23 | 0.19 | −1.76 | 15.07 | 0.91 |
| TWT mean (min) | −6.18 | 15.62 | 0.69 | −39.33 | 18.83 | 0.04 | −28.56 | 18.64 | 0.13 |
| TWT variability (min) | −2.20 | 11.18 | 0.84 | −17.57 | 14.11 | 0.21 | −16.05 | 13.97 | 0.25 |
| BT mean | 0.06 | 0.36 | 0.87 | −0.55 | 0.34 | 0.10 | −0.71 | 0.34 | 0.04 |
| BT variability | −0.08 | 0.19 | 0.68 | −0.31 | 0.25 | 0.20 | −0.45 | 0.25 | 0.07 |
| WT mean | −0.57 | 0.35 | 0.11 | −0.33 | 0.38 | 0.39 | −0.02 | 0.38 | 0.96 |
| WT variability | −0.10 | 0.18 | 0.59 | −0.39 | 0.20 | 0.047 | 0.20 | 0.20 | 0.30 |
| Actigraphy | |||||||||
| TST mean (min) | 36.92 | 23.14 | 0.11 | −12.68 | 23.42 | 0.59 | −28.33 | 23.30 | 0.22 |
| TST variability (min) | 0.15 | 15.44 | 0.99 | 4.65 | 18.72 | 0.80 | −7.57 | 18.63 | 0.68 |
| TWT mean (min) | 4.16 | 8.48 | 0.62 | −4.42 | 10.14 | 0.66 | −13.34 | 10.09 | 0.19 |
| TWT variability (min) | −0.005 | 0.13 | 0.97 | −0.05 | 0.17 | 0.77 | −0.22 | 0.17 | 0.20 |
| Waking activity count mean | −41.19 | 107.66 | 0.70 | −76.85 | 80.83 | 0.34 | −87.15 | 79.93 | 0.28 |
| Waking activity count variability | 0.01 | 0.10 | 0.95 | −0.15 | 0.11 | 0.18 | −0.27 | 0.11 | 0.02 |
| Sleep health composite | 0.26 | 0.26 | 0.32 | 0.91 | 0.30 | 0.002 | 0.64 | 0.30 | 0.03 |
Note. SDS = Sheehan Disability Scale. DSM 5 DSM-5 Cross-Cutting Measure. PROMIS-SD = Patient-Reported Outcomes Measurement Information System–Sleep Disturbance. PROMIS-SRI = Patient-Reported Outcomes Measurement Information System– Sleep-Related Impairment. QIDS = Quick Inventory of Depressive Symptoms. ASSIST = Alcohol, Smoking and Substance Involvement Screening Test. PSYRATS = Psychotic Symptoms Rating Scales. WHODAS = World Health Organization Disability Assessment Schedule 2.0. CDC Healthy Days: overall health question from the 4-question healthy days core module developed by the Centers for Disease Control and Prevention. SE = sleep efficiency (total sleep time/time in bed × 100). TST = total sleep time. TWT = total wake time. BT = bedtime, WT = wake time. All the p-values in bold are the exact p-values and remained significant after applying the Benjamini-Hochberg procedure with a 5% false discovery rate assumed.
Figure 2.
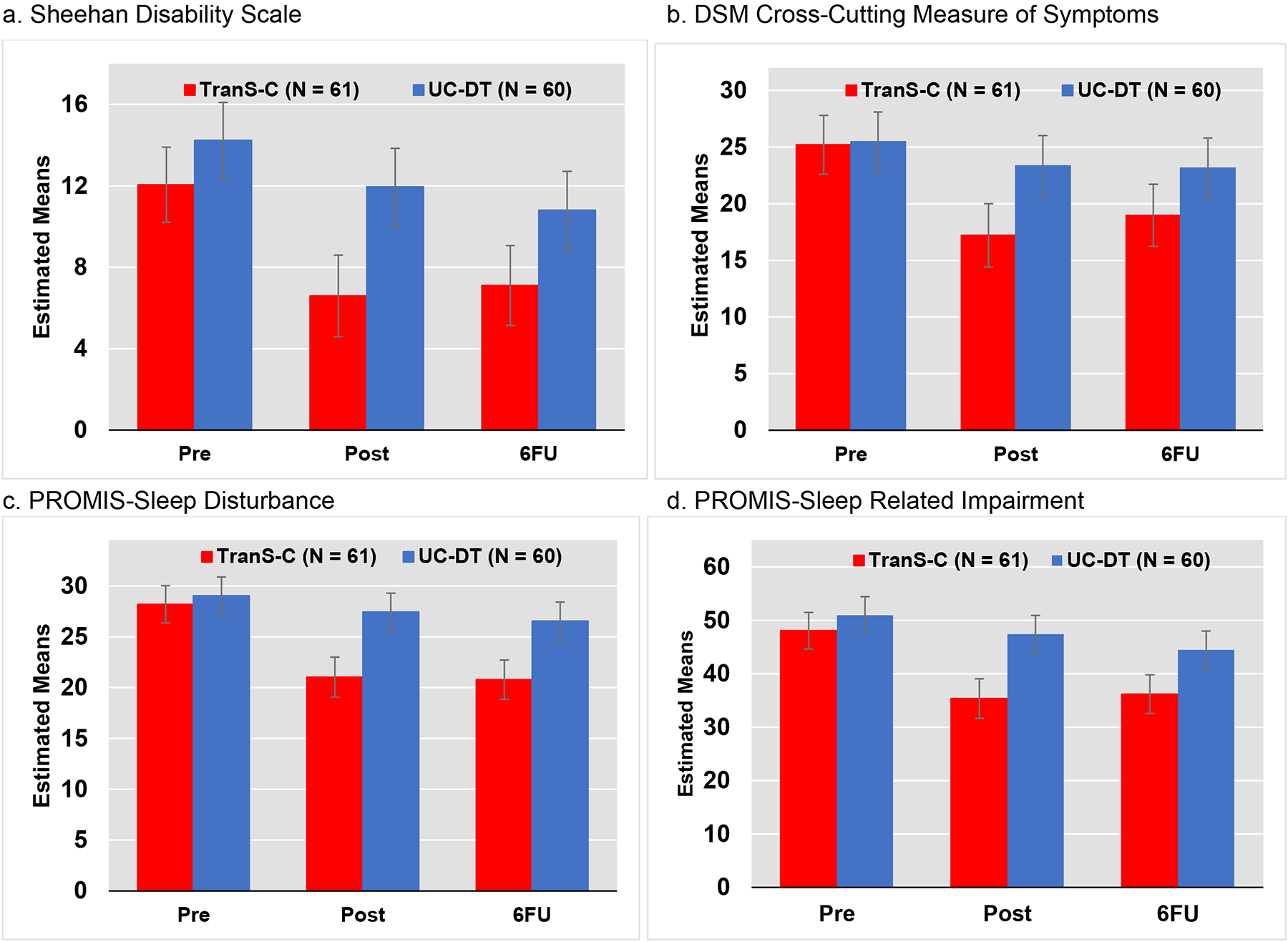
TranS-C+UC = Transdiagnostic Sleep and Circadian Intervention plus usual care, UC-DT = Usual care and delayed treatment, Pre = Pre-assessment, Post = Post-Assessment, 6FU = 6-month follow-up assessment, PROMIS = Patient-Reported Outcomes Measurement Information System. Error bars indicate 95% CI, plus or minus 2 standard errors.
From pre-treatment to post-treatment, TranS-C+UC had significant effects on all four primary outcomes, relative to UC-DT. Specifically, participants in TranS-C+UC, relative to UC-DT, exhibited a reduction in SDS (b=−3.18, p=0.025), DSM-5 cross-cutting symptoms (b=−5.88, p=0.001), PROMIS-SD (b=−5.55, p<0.0001), and PROMIS-SRI (b=−9.14, p<0.0001). Treatment gains for TranS-C+UC, relative to UC-DT, were maintained through 6FU for DSM-5 cross-cutting symptoms (b=−3.90, p=0.03), PROMIS-SD (b=−4.92, p<0.0001), and PROMIS-SRI (b=−5.37, p=0.027), but not for SDS. See Supplement for a discussion and presentation of PROMIS T scores.
Secondary Outcomes
Descriptive statistics are in Table 2. Multilevel modeling results are in Table 3. Relative to UC-DT, TranS-C+UC had reduced PSYRATS scores from pre-treatment to 6FU (b=−17.52, p=0.02). For sleep diary, TranS-C+UC had improved sleep efficiency from pre-treatment to post-treatment (b=5.68, p=0.03) and 6FU (b=5.89, p=0.03), relative to UC-DT. Relative to UC-DT, TranS-C+UC showed reduced total wake time mean (b=−39.33, p=0.04) and reduced wake time variability (b=−0.39, p=0.047) from pre-treatment to post-treatment, and earlier bedtime from pre-treatment to 6FU (b=−0.71, p=0.04). For actigraphy, TranS-C+UC showed significantly reduced variability in waking activity count (b=−0.27, p=0.02) from pre-treatment to 6FU. TranS-C+UC did not show advantage over UC-DT on other outcomes.
For the Sleep Health Composite, TranS-C+UC exhibited improved sleep health from pre-treatment to post-treatment (b=0.91, p=0.002) and to 6FU (b=0.64, p=0.03), relative to UC-DT.
Treatment Integrity, Credibility and Fidelity
CTRS (n=203 recordings, M=51.08, SD=5.66, 98.5% 40 or over) indicate that TranS-C+UC was delivered with fidelity. Coding of a random subset of patients with no missing recordings (n=19; 31.15% of TranS-C participants) indicated that all 8 sessions of recordings for each patient were coded (n=152 sessions). 94.74% received all 4 cross-cutting modules (100% received functional analysis, education, and motivational enhancement). 36.84% received all 4 core modules (100% Core Module 1, 68.42% Core Module 2, 57.89% Core Module 3, 100% Core Module 4). 100% received at least 1 optional module. No non-TranS-C elements were coded.
Medications
At study entry, 90/121 (74.4%) were taking prescription SMI medications and 16/121 (13.2%) were taking sleep medications. The mean±SD (median) number of medications per participant was 2.80±1.41.
When considering each medication for each participant separately, the doses of 51.2% of SMI medications and 71.4% of sleep medications remained stable across the treatment phase. When considering all medications for a particular participant, 22.8% remained on stable doses of all SMI medications and 68.4% remained on stable doses of all sleep medications across the treatment phase.
The percentage of TranS-C+UC compared to UC-DT participants taking SMI medications was statistically similar at baseline (75.4% vs. 73.3%), post-treatment (73.8% vs. 66.7%), and 6FU (73.8% vs. 66.7%). There was no significant difference in the percentage of participants discontinuing at least one SMI medication at some point during the treatment phase (0% vs. 0%) or during the 6FU (0% vs. 0.03%).
The percentage of TranS-C+UC compared to UC-DT taking sleep medications was statistically similar at baseline (11.5% vs. 15.0%), post-treatment (13.1% vs. 13.3%), and 6FU (16.4% vs. 13.3%). There was no significant difference in the percentage of participants discontinuing at least one sleep medication during the treatment phase (0% vs. 0%) or during the 6FU (0% vs. 0%).
Discussion
Relative to UC-DT, TranS-C was associated with improvement from pre-treatment to post-treatment for all primary outcomes. This confirms our hypothesis that, at post-treatment, TranS-C was superior to UC-DT for functional impairment, general psychiatric symptoms, and sleep and circadian function. These findings were retained at the 6FU for all outcomes except functional impairment, although the means were in the hypothesized direction. These findings replicate prior research showing that sleep treatments improve functioning, symptoms of comorbid mental health conditions as well as sleep and circadian functioning (Geiger-Brown et al., 2015; Taylor & Pruiksma, 2014). In addition, these findings extend prior research by testing a transdiagnostic treatment designed to address a range of sleep and circadian problems experienced by a mixed diagnosis SMI sample in a community setting. CMHC settings are critically important as they treat the most socioeconomically disadvantaged and underserved members of our community, as evident from the demographics in Table 1.
Secondary outcomes were included to index three general psychiatric symptoms: depression, substance use, and hallucinations and delusions. Reduced hallucinations and delusions were observed for TranS-C, relative to UC-DT, from pre-treatment to 6FU. This finding is consistent with prior research showing a tight coupling of psychotic symptoms and sleep (Freeman et al., 2015). However, note that the PSYRATS is only given to those experiencing active psychotic symptoms. Thus, the large effects should be interpreted with caution given the small sample for this analysis. While the total score for depression did not yield a significant difference between the treatments, an inspection of the means suggests a non-significant advantage on depression for TranS-C. Also, the mean QIDS score for both groups started in the ‘moderate depression range’, which may have limited potential treatment effects. The total score for the measure of alcohol, smoking and substance use also did not yield a significant difference between the treatments. Substance abuse/dependence was exclusionary if it made study participation infeasible. This may have contributed to restricted range. Importantly, the time frame for the ASSIST is the past 3 months or lifetime. At the post-treatment assessment, we assessed the past 2 weeks so the timeframe did not cover the pre-treatment timepoint. Thus, the mean ASSIST score is lower at the post-treatment assessment, relative to the pre-treatment or 6FU assessments; this may have contributed to the null results.
For sleep diary, relative to UC-DT, TranS-C+UC was associated with improved sleep efficiency at all timepoints. Also, relative to UC-DT, TranS-C+UC showed reduced total wake time mean and wake time variability from pre-treatment to post-treatment, and earlier bedtime from pre-treatment to 6FU. There was also a non-significant advantage to TranS-C+UC for total sleep time (which increased by 33 mins from pre-treatment to 6FU, relative to 13 mins for UC-DT) and total wake time (which decreased by 40 mins from pre-treatment to 6FU, relative to 13 mins for UC-DT). For actigraphy, TranS-C+UC showed reduced variability in waking activity count from pre-treatment to 6FU, but no other advantage over UC-DT on other outcomes. Given the problem of the wide transdiagnostic inclusion gates discussed above, we were not surprised that some sleep diary and actigraphy outcomes were not significant. Updated sleep diary and actigraphy reporting standards (Buysse, Ancoli-Israel, Edinger, Lichstein, & Morin, 2006) may be needed for transdiagnostic samples. There are two other possible contributors to the lack of treatment effects for actigraphy. The GT9X Link does not have an “event marker”, by which a participant indicates going to bed and getting out of bed. Actigraphy itself is not sensitive for these events, which may have reduced validity (Withrow, Roth, Koshorek, & Roehrs, 2019). Also, many participants reported spending substantial periods engaging in motionless behaviors (e.g., watching television), which may have been incorrectly scored as sleep.
For the sleep diary and actigraphy outcomes, it is important to note that these suffer from wide inclusion gates inherent to transdiagnostic research. For example, reducing total sleep time and increasing total sleep time can both be treatment goals within TranS-C+UC. Therefore, mean total sleep time does not accurately reflect treatment change. This is also the case for waketime. Within TranS-C+UC, the treatment goal for some participants is to wake earlier (e.g., a patient who sleeps until noon), while for others the goal is to wake later (e.g., a patient who wakes at 4:30am). Again, the mean value will not provide an index of the impact of treatment for a transdiagnostic sample. To address this, we developed a new measure called the Sleep Health Composite (Dong et al., 2019) which combines sleep diary and global indices of sleep for the six sleep health dimensions (see Table 3 in Supplement). On this novel metric, TranS-C+UC exhibited improved sleep health from pre-treatment to post-treatment to 6FU, relative to UC-DT.
As evidenced in Table 1, most participants were diagnosed with insomnia or hypersomnia, whereas only approximately 9.1% of the sample was diagnosed with circadian disturbance. This estimate is consistent with the prevalence of circadian disturbance for some diagnoses of serious mental illness (e.g., schizophrenia; Reeve et al., 2019) but inconsistent with the prevalence rates of circadian disturbance for other diagnoses (bipolar disorder; Bradley et al., 2017). Of note, in our sample, circadian disturbances were quite common at the subsyndromal level (25.6%). Overall, comorbidity between circadian disturbance and other sleep and circadian disorders was exceptionally common (34.7%) (Sarfan, Hilmoe, Gumport, Gasperetti, & Harvey, 2020). Together, more research is needed to clarify the prevalence of specific sleep and circadian disorders in patients with psychiatric disorders (Hombali et al., 2019).
Several limitations warrant consideration. We acknowledge that the UC-DT design may inflate the effect size differences (Cunningham, Kypri, & McCambridge, 2013) and that corrections for multiple secondary outcomes were not conducted. The secondary outcomes are not considered confirmatory, replication is needed. Fidelity to the core modules was lower than expected. While there was 100% fidelity to Core Modules 1 and 4, only 68% of cases received Core Module 2 (Daytime impairment) and 58% received Core Module 3 (Unhelpful beliefs about sleep). Due to the severity of impairment present for some individuals, the provider may have focused on the basics of sleep health (i.e., Core Modules 1 and 4). As SMI medications are used to address multiple symptoms including sleep, 13.2% likely under-estimates the use of sleep medications. We had hoped to measure usual care. However, adding to case managers already large and complex workload was not feasible. Assessors were blind to treatment condition, but participants were not. The self-report retrospective primary outcome measures may share method variance. A simplified version of the consensus sleep diary (Carney, 2012) was collected over 7-days as the full version over 14-days was not feasible in the SMI/CMHC context. The inclusion criteria relied on retrospective self-reported estimates of sleep parameters. UC-DT does not control for attention from a therapist. Importantly, while the present study monitored adverse events, adverse events in psychological therapies should be pre-specified, pre-defined and measured (Condon, Maurer, & Kyle, 2020). This is particularly important in sleep treatments as adverse events have been documented (Condon et al., 2020).
In summary, this study adds to the evidence that dysregulated sleep and circadian rhythms in SMI are important and understudied maintaining mechanisms. The findings are consistent with the conceptual model that sleep and circadian dysfunction contribute to vicious cycles of escalating symptoms and dysfunction in SMI (Harvey, 2008). The findings underscore the potential of a transdiagnostic treatment designed to treat a wide range of sleep and circadian problems experienced by adults with a wide range of SMI and support the utility of a novel outcome measure: the Sleep Health Composite. Importantly, this study was conducted in a community mental health setting. This represents a step toward bridging the large gap between research and routine practice. The providers were employed, trained and supervised within a university setting. Hence, it will be important to determine if similar results are obtained by community-based providers. Encouragingly, we compared the demographics of the therapists who delivered the treatment for this study (Gumport, Yu, Mullin, et al., 2020) with those of a small sample of CMHC providers (Gumport, Yu, & Harvey, 2020). While the former were younger (Mean age 31 vs. 47), the two groups were broadly similar for education. Finally, we have discussed our approach to retention and barriers to implementation elsewhere (Gumport, Yu, & Harvey, 2020; Yu et al., 2020).
Supplementary Material
Public Health Significance.
This study suggests that the Transdiagnostic Intervention for Sleep and Circadian Dysfunction is an effective treatment for a range of sleep and circadian problems across a range of mental illnesses.
This study confirms prior research indicating that improving sleep is associated with improvement in mental health symptoms, including psychotic symptoms.
Acknowledgments.
This study was funded by the National Institute of Mental Health (MH105513). We are deeply grateful to Faith Fuller, and the staff, case managers and physicians at ACBHCS including Dr. Amanda Bachuss, Erin Bliss, Algernol Boozer, Dr. Floyd Brown, Andrea Christian, Dr. Alan Cohen, Gil Cortes, Maureen Costello, Breton Courtney, Carla Danby, Sam Davis, Marc Diamond, Kim Flores, Dr. Lori Glassie, Dr. Shana Green, Mark Gross, Karen Hamayadan, Beau Heath, Nandita Hegde, Vermeille Hill, Dr. James Hinson, Manton Hurd, Madilyn Johnson, Denise Kennedy, Lorraine Lilley, Tony Limperopulos, Dr. Nia Lozano, Susannah MacKaye, Michelle Nelson-Lewis, Corani Robles, Dr. Luisito Roxas, Susie Saechao, Courtney Sage, Tiffany Sarrach, Sandra Smith, Todd Stephenson, Kenya Sullivan, Dr. Sui-Kwong Sung, Colleen Timpane, Remy Wax, Shawan Worsley and Sadaya Zimmerle. The DSMB was composed of three members: Descartes Li MD, Rachel Loewy PhD and Philip Gehrman PhD.
Disclosures.
AGH has received research support from the National Institutes of Health and book royalties from American Psychological Association, Guilford Press, and Oxford University Press. DJB reports personal fees from BeHealth, Emmi Solutions, American Academy of Physician Assistants, Bayer, CME Institute, Ebb Therapeutics, Weight Watchers International, Eisai, Pear Therapeutics, as well as grants from NIH and Weight Watchers International. In addition, Dr. Buysse has a patent Copyright issued. The views expressed in this article do not represent those of the U.S. Department of Veterans Affairs or other public entity. All other authors declare that they have no competing interests.
Appendix
Related publications
One manuscript has been published on a subset of participants from the study described in this submission. The focus of the published paper was on different outcomes to those presented in this paper. Thus, there is minimal overlap between the submitted and published papers.
Footnotes
Trial Registration: ClinicalTrials.gov Identifier: NCT02469233, registered 9 June 2015.
References
- Baglioni C, Nanovska S, Regen W, Spiegelhalder K, Feige B, Nissen C, et al. (2016). Sleep and mental disorders: A meta-analysis of polysomnographic research. Psychological bulletin, 142(9), 969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, & Hochberg Y (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal statistical society: series B (Methodological), 57(1), 289–300. [Google Scholar]
- Bradley A, Webb-Mitchell R, Hazu A, Slater N, Middleton B, Gallagher P, et al. (2017). Sleep and circadian rhythm disturbance in bipolar disorder. Psychological Medicine, 47(9), 1678–1689. [DOI] [PubMed] [Google Scholar]
- Buysse DJ (2014). Sleep health: can we define it? Does it matter. Sleep, 37(1), 9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Ancoli-Israel S, Edinger JD, Lichstein KL, & Morin CM (2006). Recommendations for a standard research assessment of insomnia. Sleep, 29, 1155–1173. [DOI] [PubMed] [Google Scholar]
- Carney CE, Buysse DJ, Ancoli-Israel S, Edinger JD, Krystal AD, Lichstein KL, & Morin CM (2012). The consensus sleep diary: Standardizing prospective sleep self-monitoring. Sleep, 35, 287–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke DE, & Kuhl EA (2014). DSM-5 cross-cutting symptom measures: a step towards the future of psychiatric care? World Psychiatry, 13(3), 314–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo C, Benedetti F, Barbini B, Campori E, & Smeraldi E (1999). Rate of switch from depression into mania after therapeutic sleep deprivation in bipolar depression. Psychiatry Research, 86, 267–270. [DOI] [PubMed] [Google Scholar]
- Condon HE, Maurer LF, & Kyle SD (2020). Reporting of adverse events in cognitive behavioural therapy for insomnia: A systematic examination of randomised controlled trials. Sleep Medicine Reviews, 101412. [DOI] [PubMed] [Google Scholar]
- Craske MG, Stein MB, Sullivan G, Sherbourne C, Bystritsky A, Rose RD, et al. (2011). Disorder-specific impact of coordinated anxiety learning and management treatment for anxiety disorders in primary care. Archives of General Psychiatry, 68(4), 378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham JA, Kypri K, & McCambridge J (2013). Exploratory randomized controlled trial evaluating the impact of a waiting list control design. BMC medical research methodology, 13(1), 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daumit GL, Dickerson FB, Wang N-Y, Dalcin A, Jerome GJ, Anderson CA, et al. (2013). A behavioral weight-loss intervention in persons with serious mental illness. New England Journal of Medicine, 368(17), 1594–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devilly GJ, & Borkovec TD (2000). Psychometric properties of the credibility/expectancy questionnaire. Journal of Behavior Therapy and Experimental Psychiatry, 31, 73–86. [DOI] [PubMed] [Google Scholar]
- Dong L, Martinez AJ, Buysse DJ, & Harvey AG (2019). A composite measure of sleep health predicts concurrent mental and physical health outcomes in adolescents prone to eveningness. Sleep health, 5(2), 166–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake R, Haddock G, Tarrier N, Bentall R, & Lewis S (2007). The Psychotic Symptom Rating Scales (PSYRATS): their usefulness and properties in first episode psychosis. Schizophr Res, 89(1–3), 119–122. [DOI] [PubMed] [Google Scholar]
- Drake RE, Goldman HH, Leff HS, Lehman AF, Dixon L, Mueser KT, et al. (2001). Implementing evidence-based practices in routine mental health service settings. Psychiatric Services, 52(2), 179–182. [DOI] [PubMed] [Google Scholar]
- Edinger JD, Fins AI, Sullivan RJ, Marsh GR, Dailey DS, & Young M (1996). Comparison of cognitive-behavioral therapy and clonazepam for treating periodic limb movement disorder. Sleep: Journal of Sleep Research and Sleep Medicine. [PubMed] [Google Scholar]
- Edinger JD, Wyatt JK, Olsen MK, Stechuchak KM, Carney CE, Chiang A, et al. (2009). Reliability and validity of the Duke Structured Interview for Sleep Disorders for insomnia screening. Paper presented at the 23rd Annual Meeting of the Associated Professional Sleep Societies, LLC. [Google Scholar]
- Espie CA, Fleming L, Cassidy J, Samuel L, Taylor LM, White CA, et al. (2008). Randomized controlled clinical effectiveness trial of cognitive behavior therapy compared with treatment as usual for persistent insomnia in patients with cancer. Journal of Clinical Oncology, 26(28), 4651–4658. [DOI] [PubMed] [Google Scholar]
- Farney RJ, Walker BS, Farney RM, Snow GL, & Walker JM (2011). The STOP-Bang equivalent model and prediction of severity of obstructive sleep apnea: relation to polysomnographic measurements of the apnea/hypopnea index. Journal of Clinical Sleep Medicine, 7(5), 459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank E, Kupfer DJ, Thase ME, Mallinger A, Swartz H, Fagioli A, et al. (2005). Two Year Outcomes for interpersonal and social rhythm therapy in individuals with bipolar I disorder. Archives of General Psychiatry, 62, 996–1004. [DOI] [PubMed] [Google Scholar]
- Freeman D, Dunn G, Startup H, Pugh K, Cordwell J, Mander H, et al. (2015). Effects of cognitive behaviour therapy for worry on persecutory delusions in patients with psychosis (WIT): a parallel, single-blind, randomised controlled trial with a mediation analysis. The Lancet Psychiatry, 2(4), 305–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger-Brown JM, Rogers VE, Liu W, Ludeman EM, Downton KD, & Diaz-Abad M (2015). Cognitive behavioral therapy in persons with comorbid insomnia: a meta-analysis. Sleep medicine reviews, 23, 54–67. [DOI] [PubMed] [Google Scholar]
- Group W (2002). The alcohol, smoking and substance involvement screening test (ASSIST): development, reliability and feasibility. Addiction, 97(9), 1183–1194. [DOI] [PubMed] [Google Scholar]
- Gruber J, Harvey AG, Wang PW, Brooks III JO, Thase ME, Sachs GS, et al. (2009). Sleep functioning in relation to mood, function, and quality of life at entry to the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD). Journal of affective disorders, 114(1–3), 41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumport NB, Yu SH, & Harvey AG (2020). Implementing a transdiagnostic sleep and circadian intervention in a community mental health setting: A qualitative process evaluation with community stakeholders. Psychiatry Research, 293, 113443. [DOI] [PubMed] [Google Scholar]
- Gumport NB, Yu SH, Mullin AC, Mirzadegan IA, & Harvey AG (2020). The Validation of a Provider-Reported Fidelity Measure for the Transdiagnostic Sleep and Circadian Intervention in a Community Mental Health Setting. Behavior Therapy, 51(5), 800–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddock G, McCarron J, Tarrier N, & Faragher E (1999). Scales to measure dimensions of hallucinations and delusions: the psychotic symptom rating scales (PSYRATS) Psychological Medicine, 29, 879. [DOI] [PubMed] [Google Scholar]
- Harvey AG (2008). Insomnia, Psychiatric Disorders, and the Transdiagnostic Perspective. Current Directions in Psychological Science, 17, 299–303. [Google Scholar]
- Harvey AG, & Buysse DJ (2017). Treating Sleep Problems: A Transdiagnostic Approach. New York: Guilford. [Google Scholar]
- Harvey AG, Hein K, Dong L, Smith FL, Lisman M, Yu S, et al. (2016). A transdiagnostic sleep and circadian treatment to improve severe mental illness outcomes in a community setting: study protocol for a randomized controlled trial. Trials, 17(1), 606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey AG, Soehner AM, Kaplan KA, Hein K, Lee J, Kanady J, et al. (2015). Treating insomnia improves sleep, mood and functioning in bipolar disorder: A pilot randomized controlled trial. Journal of Consulting and Clinical Psychology, 83(3), 564–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertenstein E, Feige B, Gmeiner T, Kienzler C, Spiegelhalder K, Johann A, et al. (2018). Insomnia as a predictor of mental disorders: a systematic review and meta-analysis. Sleep medicine reviews. [DOI] [PubMed] [Google Scholar]
- Hombali A, Seow E, Yuan Q, Chang SHS, Satghare P, Kumar S, et al. (2019). Prevalence and correlates of sleep disorder symptoms in psychiatric disorders. Psychiatry research, 279, 116–122. [DOI] [PubMed] [Google Scholar]
- Kaplan KA, & Harvey AG (2013). Behavioral treatment of insomnia in bipolar disorder. American Journal of Psychiatry, 170(7), 716–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlin BE, Trockel M, Taylor CB, Gimeno J, & Manber R (2013). National dissemination of cognitive behavioral therapy for insomnia in veterans: Therapist-and patient-level outcomes. [DOI] [PubMed]
- Kessler RC, Barker PR, Colpe LJ, Epstein JF, Gfroerer JC, Hiripi E, et al. (2003). Screening for serious mental illness in the general population. Archives of General Psychiatry, 60, 184. [DOI] [PubMed] [Google Scholar]
- Konecky B, Meyer EC, Marx BP, Kimbrel NA, & Morissette SB (2014). Using the WHODAS 2.0 to assess functional disability associated with DSM-5 mental disorders. Am J Psychiatry, 171(8), 818–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause AJ, Simon EB, Mander BA, Greer SM, Saletin JM, Goldstein-Piekarski AN, et al. (2017). The sleep-deprived human brain. Nature Reviews Neuroscience, 18(7), 404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecrubier Y, Sheehan DV, Weiller E, Amorim P, Bonora I, Sheehan KH, et al. (1997). The Mini International Neuropsychiatric Interview (MINI). A short diagnostic structured interview: reliability and validity according to the CIDI. European psychiatry, 12(5), 224–231. [Google Scholar]
- Leon AC, Olfson M, Portera L, Farber L, & Sheehan DV (1997). Assessing psychiatric impairment in primary care with the Sheehan Disability Scale. Int J Psychiatry Med, 27(2), 93–105. [DOI] [PubMed] [Google Scholar]
- Leon AC, Shear MK, Portera L, & Klerman GL (1992). Assessing impairment in patients with panic disorder: the Sheehan Disability Scale. Soc Psychiatry Psychiatr Epidemiol, 27(2), 78–82. [DOI] [PubMed] [Google Scholar]
- López CM, Lancaster CL, Gros DF, & Acierno R (2017). Residual sleep problems predict reduced response to prolonged exposure among veterans with PTSD. Journal of psychopathology and behavioral assessment, 39(4), 755–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh RK, & Barlow DH (2010). The dissemination and implementation of evidence-based psychological treatments: a review of current efforts. American Psychologist, 65(2), 73. [DOI] [PubMed] [Google Scholar]
- Miller WR, & Rollnick S (2002). Motivational interviewing: Preparing people for change. New York: Guilford Press. [Google Scholar]
- Moriarty D, Zack M, & Kobau R (2003). The Centers for Disease Control and Prevention’s Healthy Days Measures: Population tracking of perceived physical and mental health over time. Health and Quality of Life Outcomes, 1(1), 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin CM (1993). Insomnia: Psychological assessment and management. New York: Guilford Press. [Google Scholar]
- Moyé LA (2008). Disciplined analyses in clinical trials: The dark heart of the matter. Statistical methods in medical research, 17(3), 253–264. [DOI] [PubMed] [Google Scholar]
- Narrow WE, Clarke DE, Kuramoto SJ, Kraemer HC, Kupfer DJ, Greiner L, et al. (2013). DSM-5 field trials in the United States and Canada, Part III: development and reliability testing of a cross-cutting symptom assessment for DSM-5. American Journal of Psychiatry, 170(1), 71–82. [DOI] [PubMed] [Google Scholar]
- Qaseem A, Kansagara D, Forciea MA, Cooke M, & Denberg TD (2016). Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Annals of internal medicine, 165(2), 125–133. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, et al. (2003). The 16-item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): A psychometric evaluation in patients with chronic major depression. Biological Psychiatry, 54, 573–583. [DOI] [PubMed] [Google Scholar]
- Sarfan LD, Hilmoe HE, Gumport NB, Gasperetti C, & Harvey AG (2020). Outcomes of Transdiagnostic Intervention for Sleep and Circadian Dysfunction (TranS-C) in a community setting: Unpacking comorbidity. . Manuscript in preparation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Harnett-Sheehan K, & Raj BA (1996a). The measurement of disability. International Clinical Psychopharmacology, 11(suppl 3), 89–95. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Harnett-Sheehan K, & Raj BA (1996b). The measurement of disability. Int Clin Psychopharmacol, 11 Suppl 3, 89–95. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. (1998). The Mini-International Neuropsychiatric Interview (M.I.N.I): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry, 59, 22–33. [PubMed] [Google Scholar]
- Sweetman A, Lack L, Catcheside PG, Antic NA, Smith S, Chai-Coetzer CL, et al. (2019). Cognitive and behavioral therapy for insomnia increases the use of continuous positive airway pressure therapy in obstructive sleep apnea participants with co-morbid insomnia: A randomized clinical trial. Sleep. [DOI] [PubMed] [Google Scholar]
- Taylor DJ, & Pruiksma KE (2014). Cognitive and behavioural therapy for insomnia (CBT-I) in psychiatric populations: a systematic review. International review of psychiatry, 26(2), 205–213. [DOI] [PubMed] [Google Scholar]
- Wang PS, Demler O, & Kessler RC (2002). Adequacy of treatment for serious mental illness in the United States. Journal Information, 92(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisz JR, Chorpita BF, Palinkas LA, Schoenwald SK, Miranda J, Bearman SK, et al. (2012). Testing standard and modular designs for psychotherapy treating depression, anxiety, and conduct problems in youth: A randomized effectiveness trial. Archives of general psychiatry, 69(3), 274–282. [DOI] [PubMed] [Google Scholar]
- Wirz-Justice A, Benedetti F, & Terman M (2009). Chronotherapeutics for Affective Disorders: A Clinician’s Manual for Light & Wake Therapy (1 ed.). Basel: Karger. [Google Scholar]
- Withrow D, Roth T, Koshorek G, & Roehrs T (2019). Relation between ambulatory actigraphy and laboratory polysomnography in insomnia practice and research. Journal of sleep research, e12854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J, & Beck A (1980). Cognitive Therapy Scale: Rating Manual. University of Pennsylavania, Philadelphia. [Google Scholar]
- Yu L, Buysse DJ, Germain A, & Moul D (2012). Development of short forms from the PROMIS sleep disturbance and sleep-related impairment item banks. Behavioral Sleep Medicine, 10, 6–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu SH, Gumport NB, Mirzadegan IA, Mei Y, Hein K, & Harvey AG (2020). Addressing the challenges of recruitment and retention in sleep and circadian clinical trials. Behavioral Sleep Medicine, 18, 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


