Abstract
Background:
No established pharmacological treatment is available for the core symptoms of autism spectrum disorder (ASD). This study aimed at investigating the efficacy of antidepressants for the core and associated symptoms of ASD.
Methods:
We searched PubMed, Embase, ClinicalKey, Cochrane CENTRAL, ScienceDirect, Web of Science and ClinicalTrials.gov using the keywords “ASD” and “antidepressants.” We searched from database inception to June 2021 for randomized controlled trials of antidepressant use in patients with ASD. We calculated pooled effect sizes based on a random-effects model.
Results:
Analysis of 16 studies with 899 participants showed improvements in restricted and repetitive behaviours (effect size = 0.27) and global symptoms (effect size = 1.0) in patients with ASD taking antidepressants versus those taking placebos (p ≤ 0.01). We found no differences between the 2 groups (p ≥ 0.36) in terms of dropout rate (odds ratio [OR] = 1.17) or rate of study discontinuation because of adverse events (OR = 1.05). We also noted improvements in irritability and hyperactivity in the antidepressant group (Hedges g = 0.33 and 0.22, respectively, both p < 0.03). Subgroup analyses showed significant effects of medication type (i.e., clomipramine was better than SSRIs) and age (antidepressants were more effective in adults than in children or adolescents) on both restricted and repetitive behaviours and global improvement (p < 0.05). Meta-regression demonstrated that better therapeutic effects were associated with lower symptom severity and older age.
Limitations:
The small effect sizes and variations in treatment response that we found warrant further study.
Conclusion:
Our results supported the effectiveness of antidepressants for global symptoms and symptom subdomains of ASD, with tolerable adverse effects. Low symptom severity and adulthood were associated with better outcomes.
Introduction
Autism spectrum disorder (ASD) comprises a range of neurodevelopmental conditions that have their onset in childhood; ASD affects about 1% of the population and is a health burden for families and society.1 Although most people with ASD experience a variety of disabling symptoms — including irritability, hyperactivity, inappropriate speech, social withdrawal and mood symptoms such as anxiety2 —no established pharmacological treatment is available for the core symptoms of ASD. The US Food and Drug Administration has approved only 2 agents, aripiprazole and risperidone, for the treatment of irritability in ASD;3 the use of most other medications is off-label, with uncertain therapeutic efficacy. Identifying other pharmacological agents that are effective against the core and associated symptoms of ASD remains a critical clinical issue.
Antidepressants have been accepted as a standard treatment for obsessive–compulsive disorder (OCD), which is characterized by compulsive and repetitive behaviours.4,5 Because OCD and ASD share some symptoms,6–8 genetic risks9 and neurobiological pathologies,10 most previous meta-analyses have focused on the effects of antidepressants on restricted and repetitive behaviours in patients with ASD.11–13 In contrast, only 1 meta-analysis has investigated the effects of antidepressants on global improvement in ASD symptoms.14 The numbers of trials were also limited in these meta-analyses (all less than 10).11–14 Moreover, although the findings of 3 of the meta-analyses supported the use of antidepressant treatment for restricted and repetitive behaviours,11–13 the effect sizes were small (0.22 to 0.24) and none of the indicators of improvement achieved statistical significance. Finally, although the influences of dosage and age on the therapeutic effects of antidepressants in OCD have been well studied in previous meta-analyses,5 the effect of dosage on patients with ASD has not been adequately addressed.
The rationale for using antidepressants to treat ASD is based on the fact that abnormal levels of serotonin have been found in certain brain regions — particularly the frontal cortex — in patients with ASD.15,16 As well, serotonin-mediated neuroprotective effects17 and evidence of serotonin dysregulation such as the impaired availability of brain 5-hydroxytryptamine in people with ASD15,16,18 further support a physiologic basis for antidepressant treatment for patients with ASD in general — not only for restricted and repetitive behaviours. However, the effects of antidepressants on other symptom subdomains of ASD (e.g., inappropriate speech) have not been addressed in previous meta-analyses.
The present meta-analysis was aimed at investigating the efficacy of antidepressants for restricted and repetitive behaviours in patients with ASD, as well as their effects on global impairment and other symptom subdomains. We also evaluated the influence of different variables (e.g., age and dosage) on therapeutic outcomes with antidepressants and analyzed their potential adverse effects, tolerability and acceptability in patients with ASD.
Methods
Electronic searches and registration
Using the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines,19,20 we systematically searched for randomized controlled trials in the following databases from inception to June 2021: PubMed, Embase, ClinicalKey, Cochrane CENTRAL, ScienceDirect, Web of Science and ClinicalTrials.gov. The keywords we used in the various databases are listed in Appendix 1, Table S1, available at www.jpn.ca/lookup/doi/10.1503/jpn.210191/tab-related-content.
We registered the present study with the International Prospective Register of Systematic Reviews (PROSPERO CRD42021256770).
Study eligibility and definitions
Study eligibility criteria were as follows: studies were randomized controlled trials in patients with ASD; interventions included antidepressants and comparators; and outcome assessment used behavioural rating scales for primary or associated symptoms in patients with ASD. We excluded studies that were not clinical trials and studies that were unrelated to antidepressants in ASD.
Data extraction and management
Two reviewers (Y.C. and P.Y.) were responsible for data extraction and management; they determined the choice of databases and keywords by discussion.
After potentially eligible articles had been retrieved from the databases, all references were imported into EndNote (version X8) to identify and remove duplicates. Then, the 2 reviewers examined the titles and abstracts of the remaining records to select articles that met the inclusion criteria. Finally, the 2 reviewers read the full manuscripts of the selected studies independently to determine each study’s appropriateness for inclusion. We used the κ coefficient to evaluate inter-rater reliability.21 Disagreements about study eligibility were resolved via discussion. When data were missing, we contacted the corresponding authors for their original data. In cases of duplicate data, we selected the article with the largest sample size or the latest publication.
To assess possible sources of bias, we used the risk-of-bias assessment tool developed by the Cochrane Collaboration.22
Data synthesis and sensitivity analysis
Changes in outcomes were expressed as effect sizes using Hedges g and odds ratios. Primary outcomes were improvement in restricted and repetitive behaviours, improvement in global symptoms, and differences in dropout rates and incidence of adverse effects. Secondary outcomes were changes in the severity of anxiety and problem behaviours, including irritability, social withdrawal, hyperactivity and inappropriate speech. We used Comprehensive Meta-Analysis (version 2.2.064) to calculate effect sizes. Positive effect sizes indicated improvements in outcomes among participants who received antidepressants.
Results were standardized and averaged to produce a single effect size for studies with similar interventions (e.g., same medication with different doses). However, if a study had 2 or more treatment arms with distinctly different pharmacologic actions (e.g., binding to different receptors that might substantially affect treatment efficacy), we subdivided shared control (i.e., placebo) groups by the number of treatment arms.23 To prevent a reduction in statistical power from small sample sizes, we used a random-effects model to evaluate effect sizes, based on the assumption that true effect sizes were the same in all studies.24,25 In other words, this model adjusted for sample-size bias by averaging the distribution of effects across the eligible studies25 so that they carried similar weights for comparison.24
We also conducted subgroup analyses using a random-effects model.24 For continuous variables (e.g., age, percent female and IQ), we used mixed-effects metaregression to investigate their effects on outcomes in patients with ASD. We also calculated Q statistics and used the corresponding p values to assess the heterogeneity of effect sizes.
We assessed publication bias by inspecting funnel plots in cases of fewer than 10 data sets26 and by performing Egger tests in cases of 10 or more data sets.27 When we encountered funnel-plot asymmetry, we imputed the results of potentially missing studies with Duval and Tweedie’s trim-and-fill method.28 We conducted sensitivity tests using the leave-one-out approach (i.e., removing one study each time and repeating the step) to estimate the effect of each study on the overall effect size.26
Results
Study characteristics
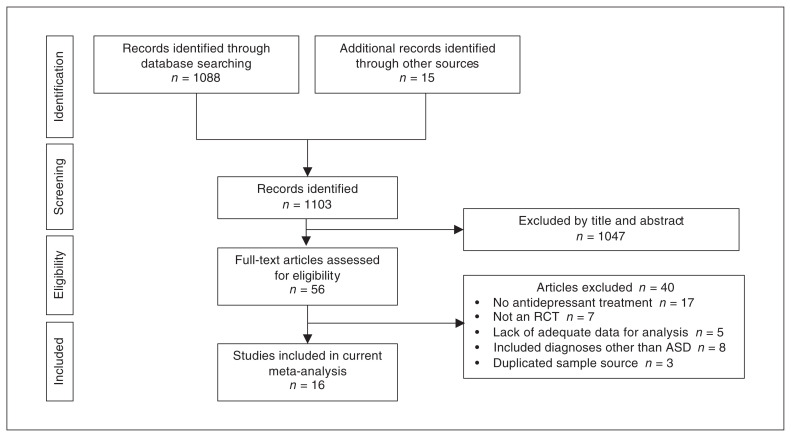
Figure 1 summarizes our identification of eligible studies for the meta-analysis. From the 1103 articles initially retrieved from the databases, 1047 were excluded after reviewing their titles and abstracts. Of the 56 full-text articles assessed for eligibility, 40 were excluded because they did not meet the inclusion criteria (Appendix 1, Table S2). A total of 16 studies were included in the meta-analysis,29–44 of which 2 were unpublished studies from ClinicalTrials.gov (Table 1).43,44 The κ coefficient for interrater reliability was 1.0.
Figure 1.
PRISMA flow diagram for study selection. ASD = autism spectrum disorder; PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-analyses; RCT = randomized controlled trial.
Table 1.
Characteristics of studies in the meta-analysis
| Study | Diagnosis (criteria) | Exclusion criteria | Design | Intervention and comparator | Duration, wk | Outcome | IQ | CGI-S | Mean age, yr (range) | Female, % | Country | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| Medication | DDD | n | |||||||||||
| SSRI versus placebo | |||||||||||||
| Herscu et al.30 (2020) | ASD (DSM-IV-TR) | AS, PDD-NOS, Rett syndrome, childhood disintegrative disorder, active seizure disorder; taking psychotropic medication; high levels of aggression or self-injurious behaviour | RCT | Fluoxetine 2–18 mg/d | NA | 78 | 14 | CY-BOCS-PDD | NA | 4.87 | 9 (5–17) | 14.55 | United States |
| Placebo | None | 80 | |||||||||||
| Potter et al.29 (2019) | ASD (DSM-5) | Fragile X syndrome full mutation; serious comorbid medical disorder affecting brain function | RCT | Sertraline 2.5–5 mg/d | NA | 32 | 24 | VAS (anxiety/ obsessive–compulsive behaviour, aggression/ hyperarousal/ hyperactivity, language/ communication); CGI-I; SRS; PARS-R | 49.6 | NA | 4.03 (2–6) | 20.7 | United States |
| Placebo | None | 26 | |||||||||||
| Reddihough et al.41 (2019) | ASD (DSM-IV-TR) | Rett syndrome, childhood disintegrative disorder, schizophrenia or major depression; taking psychotropic medications; comorbid medical conditions | RCT | Fluoxetine 4–30 mg/d | NA | 75 | 16 | CY-BOCS-PDD; CGI-I; ABC (irritability, hyperactivity, social withdrawal, inappropriate speech); Spence children anxiety | NA | NA | 11.2 (7.5–18) | 15 | Australia |
| Placebo | None | 71 | |||||||||||
| Sikich et al.44 (2014)* | Autism (DSM-III-R) | AS, PDD-NOS, Rett syndrome, childhood disintegrative disorder | RCT | Fluoxetine 2–20 mg/d | NA | 8 | 48 | ABC (irritability) | NA | NA | 3.62 (2.5–4.8) | 0 | United States |
| Placebo | None | 10 | |||||||||||
| Hollander et al.39 (2012) | ASD (DSM-IV); CGI-S ≥ 4 | None | RCT | Fluoxetine 20–80 mg/d | 3.24 | 22 | 12 | Y-BOCS; CGI-I | 103 | 4.39 | 34.31 (18–60) | 31 | United States |
| Placebo | None | 15 | |||||||||||
| King et al.38 (2009) | AD, AS, PDD-NOS (DSM-IV-TR); CGI-S ≥ 4 | Rett syndrome or childhood disintegrative disorder; seizure within the past 6 mo, weight < 15 kg; bipolar disorder or manic episode | RCT | Citalopram 10–20 mg/d | NA | 73 | 12 | CY-BOCS-PDD; ABC (irritability, hyperactivity, social withdrawal, inappropriate speech) | NA | 4.94 | 9.36 (5–17) | 14.1 | United States |
| Placebo | None | 76 | |||||||||||
| Hollander et al.36 (2005) | ASD (DSM-IV-TR) | Psychotic disorders, seizures; clinically significant medical illness | RCT/ cross-over | Fluoxetine 4.8–20 mg/d | NA | 19 | 8 | CY-BOCS; CGI-I | 63.7 | 4.61 | 8.18 (5–17) | 23.1 | United States |
| Placebo | None | 20 | |||||||||||
| Sugie et al.37 (2005) | Autism (DSM-IV) | Underlying diseases, such as chromosomal aberration; congenital rubella syndrome, apparent neurologic deficits | RCT/ cross-over | Fluvoxamine 1–3 mg/kg/d | NA | 18 | 12 | BAS (emotional instability, hyperactivity, social withdrawal, inappropriate speech) | NA | NA | 5.33 (3–8.5) | 26.7 | Japan |
| Placebo | None | 18 | |||||||||||
| McDougle et al.34 (1996) | AD (DSM-III-R); CGI-S ≥ 4 | Schizophrenia, psychotic symptoms, illicit substances within the previous 6 mo; notable medical condition, including seizure disorder; pregnancy | RCT | Fluvoxamine 50–300 mg/d | 2.77 | 15 | 12 | Y-BOCS; CGI-I; Brown Aggression Scale | 79.9 | 4.12 | 30.1 (18–53) | 10 | United States |
| Placebo | None | 15 | |||||||||||
| SNRI versus placebo | |||||||||||||
| Carminati et al.40 (2016) | PDD (ICD-10); mild to profound ID | Epilepsy or any indication against somatic–psychotropic treatments; pregnancy | RCT | Venlafaxine 18.75 mg/d | 0.185 | 6 | 8 | ABC (stereotype); CGI-I; ABC (irritability, hyperactivity, social withdrawal, inappropriate speech) | NA | NA | 22 (18–30) | 15.4 | Switzerland |
| Placebo | None | 7 | Median: 19 (19–31) | ||||||||||
| Tricyclic antidepressant versus placebo | |||||||||||||
| Remington et al.35 (2001) | Autism (DSM-IV) | NA | RCT/ cross-over | Clomipramine 100–150 mg/d | NA | 36 | 7 | ABC (repetitive behaviours, irritability, hyperactivity, social withdrawal, inappropriate speech); CARS | NA | NA | 16.3 (10–36) | 16.7 | Canada |
| Placebo | None | 36 | |||||||||||
| Gordon et al.33 (1993) | AD (DSM-III-R) | Significant problems, including seizures | RCT/ cross-over | Clomipramine 25–250 mg/d | NA | 12 | 10 | Modified CPRS OCD subscale; CPRS autism-relevant subscale | 57.1 | NA | 11.7 (6–23) | 33.3 | United States |
| Placebo | None | 12 | |||||||||||
| Gordon et al.31 (1992) | AD (DSM-III-R) | Significant medical problems, including seizures | RCT/ cross-over | Clomipramine 25–250 mg/d | NA | 7 | 5 | NIMH-GOCS; CPRS | NA | NA | 9.6 (6–18) | 28.6 | United States |
| Desipramine 25–250 mg/d | NA | 7 | |||||||||||
| Placebo | None | 7 | |||||||||||
| Other antidepressant versus placebo | |||||||||||||
| McDougle et al.43 (2018)* | AD, AS, PDD-NOS (DSM-IV) | Rett syndrome, childhood integrative disorder, OCD, post-traumatic stress disorder, major mood disorder, psychotic disorder, substance use disorder | RCT | Mirtazapine 7.5–45 mg/d | NA | 20 | 10 | PARS | NA | NA | 11 (5–17) | 20 | United States |
| Placebo | 10 | ||||||||||||
| Chugani et al.32 (2016) | ASD (DSM-IV-TR) | Neurologic disorders, phenylketonuria, tuberous sclerosis complex, Rett syndrome, Fragile X syndrome; Down syndrome; traumatic brain injury or other medical or behavioural problems | RCT | Buspirone 5 mg/d | NA | 54 | 24 | CY-BOCS; ADOS-CTS; ABC (social withdrawal, inappropriate speech); anxiety composite score (ABC irritability + Leiter emotion regulation) | 64.1 | NA | 3.62 (2–6) | 17.5 | United States |
| Buspirone 10 mg/d | NA | 55 | |||||||||||
| Placebo | None | 57 | |||||||||||
| Antidepressant versus antipsychotic | |||||||||||||
| Sanchez et al.42 (1995) | AD (DSM-III-R; infantile autism (DSM-III) | Identifiable causes of autism; seizures or other systemic disease | RCT/ cross-over | Clomipramine 2.8–4.4 mg/kg/d | NA | 8 | 4.5 | CGI-S; CPRS (hyperactivity, speech deviance) | NA | NA | 5.6 (2.3–7.8) | 12.5 | United States |
| Haloperidol 0.02–0.05 mg/kg/d | NA | 8 | |||||||||||
ABC = Aberrant Behavior Checklist; AD = autistic disorder; ADOS-CTS = Autism Diagnostic Observation Schedule Composite Total Score; AS = Asperger syndrome; ASD = autism spectrum disorder; BAS = Behavioural Assessment Scale; CARS = Childhood Autism Rating Scale; CGI-I = Clinical Global Impression–Improvement; CGI-S = Clinical Global Impression–Severity; CPRS = Children’s Psychiatric Rating Scale; CY-BOCS = Children’s Yale–Brown Obsessive–Compulsive Scale; CY-BOCS-PDD = Children’s Yale–Brown Obsessive–Compulsive Scale modified for pervasive developmental disorder; DDD = defined daily dose; DSM-III = Diagnostic and Statistical Manual of Mental Disorders, 3rd edition; DSM-III-R = Diagnostic and Statistical Manual of Mental Disorders, 3rd edition, revised; DSM-IV = Diagnostic and Statistical Manual of Mental Disorders, 4th edition; DSM-IV-TR = Diagnostic and Statistical Manual of Mental Disorders, 4th edition, text revision; DSM-5 = Diagnostic and Statistical Manual of Mental Disorders, 5th edition; ICD-10 = International Statistical Classification of Diseases and Related Health Problems, 10th revision; ID = intellectual disability; NA = not available; NIMH-GOCS = National Institute of Mental Health Global Obsessive–Compulsive Scale; OCD = obsessive–compulsive disorder; PARS = Pediatric Anxiety Rating Scale; PARS-R = Pediatric Anxiety Rating Scale revised; PDD = pervasive developmental disorder; PDD-NOS = pervasive developmental disorder, not otherwise specified; RCT = randomized controlled trial; SNRI = serotonin and norepinephrine reuptake inhibitor; SRS = Social Responsiveness Scale; SSRI = selective serotonin reuptake inhibitor; VAS = visual analogue scale; Y-BOCS = Yale–Brown Obsessive–Compulsive Scale.
Study identified from ClinicalTrials.gov.
The included studies involved a total of 899 participants with a mean age of 12.57 years (range 2.5–60 years); 18.62% (range 0%–33.3%) were female. Antidepressants were prescribed in 457 patients (50.8%), including selective serotonin reuptake inhibitors (SSRIs; e.g., fluoxetine, citalopram or sertraline), selective serotonin and norepinephrine reuptake inhibitors (e.g., venlafaxine), buspirone, mirtazapine and tricyclic antidepressants (e.g., clomipramine, desipramine). Of the 16 studies, 15 used placebos as controls and 1 used haloperidol.
Chugani and colleagues investigated the efficacy of 2 doses of a single agent (i.e., buspirone);32 for this study, we standardized the data from the 2 interventional groups and averaged them to produce a single effect size for comparison with the placebo group. For the study by Gordon and colleagues,31 we split the placebo group in half to compare it with 2 different pharmacological agents (clomipramine and desipramine) because of the reportedly more potent serotonergic effect of clomipramine.45
More than half of the included studies targeted children and adolescents; 4 (25%) also included an adult population. Seven data sets from 6 studies provided IQ data. The duration of treatment varied from 2 to 48 weeks. Most studies were conducted in the United States; 1 was conducted in Japan.
In the included studies, 2 categories of behavioural rating scales were adopted for outcome assessment. Some studies used domain-specific questionnaires mainly for restricted and repetitive behaviours, such as the Children’s Yale–Brown Obsessive–Compulsive Scale or the National Institute of Mental Health Global Obsessive–Compulsive Scale. Others used more general behavioural rating scales that contain different symptom subdomains, including stereotyped behaviours, irritability, social withdrawal, hyperactivity and inappropriate speech, such as the Aberrant Behavior Checklist, the Behavioural Assessment Scale, the Children’s Psychiatric Rating Scale and visual analogue scales.
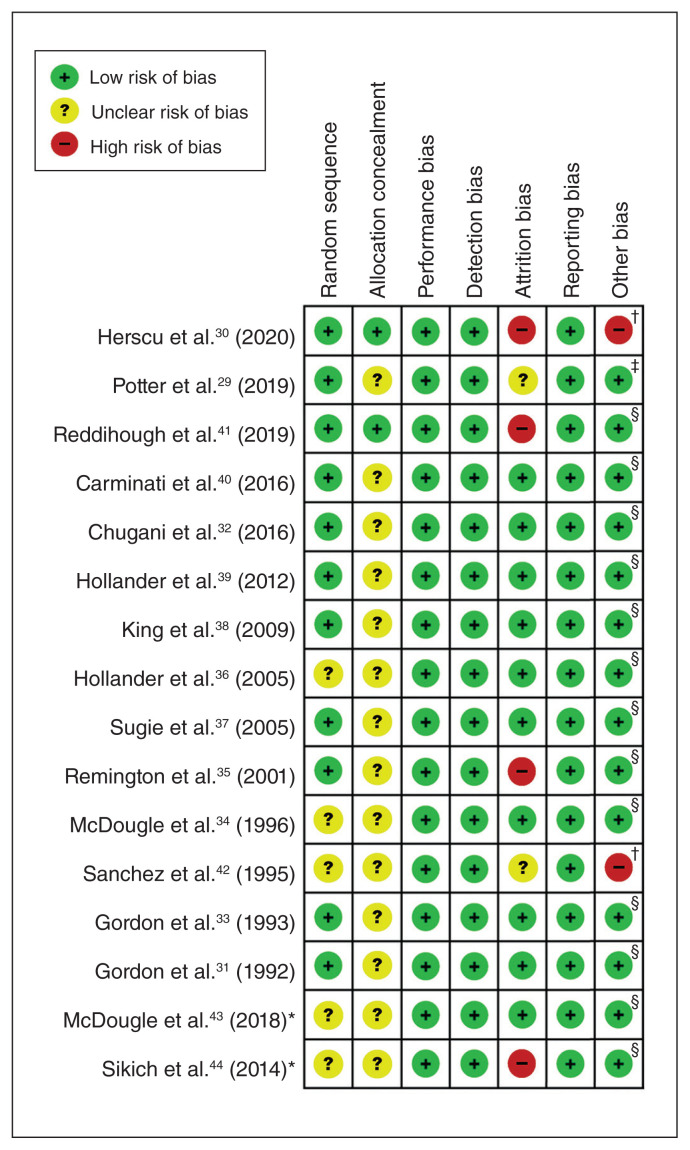
In terms of risk of bias, most studies were of fair quality. We found that 75.8% (81/112), 18.8% (21/112) and 5.4% (6/112) of the included studies had an overall low, unclear and high risk of bias, respectively (16 studies × 7 categories for the risk of bias tool = 112). Two studies received financial support from pharmaceutical companies (Figure 2).
Figure 2.
Risk of bias in included studies. *Study identified from ClinicalTrials.gov. †Study received financial support from pharmaceutical companies. ‡Authors received financial support from pharmaceutical companies (e.g., consultation). §Neither study nor authors received financial support from pharmaceutical companies.
Quantitative data synthesis
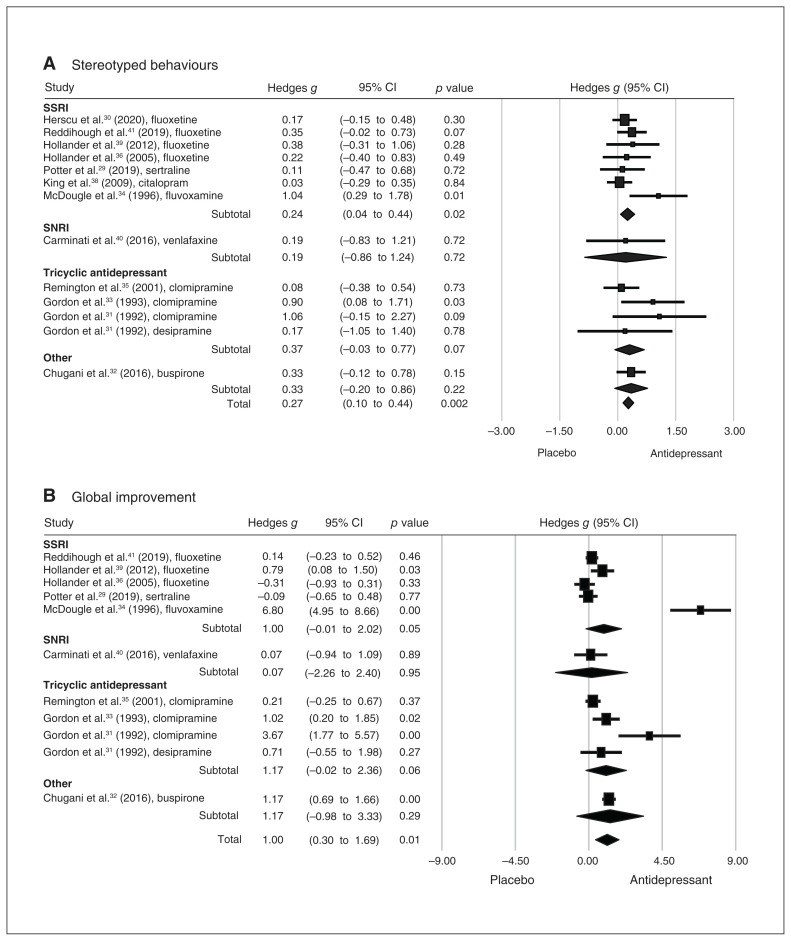
For restricted and repetitive behaviours, one of our primary outcomes, a meta-analysis of 13 data sets from 12 studies found significant improvement in patients with ASD who received antidepressants compared to controls (p < 0.01; Figure 3A). The effect size was strong in the leave-one-out sensitivity analysis (p < 0.01), indicating that no single study had a substantial influence on the overall effect size. The result of the Egger test was not significant (p = 0.053), suggesting a low risk of publication bias.
Figure 3.
Forest plots comparing differences in effect sizes of (A) stereotyped behaviours and (B) global improvement between antidepressant and control groups. CI = confidence interval; SNRI = serotonin and norepinephrine reuptake inhibitor; SSRI = selective serotonin reuptake inhibitor.
For global improvement, 11 data sets from 11 studies showed a significant improvement in global symptoms in patients with ASD who received antidepressants compared to controls (p = 0.01; Figure 3B). The effect size was strong in the leave-one-out sensitivity analysis (p < 0.01), indicating that no single study had a substantial influence on the overall effect size. The result of the Egger test was not significant (p = 0.10), suggesting a low risk of publication bias.
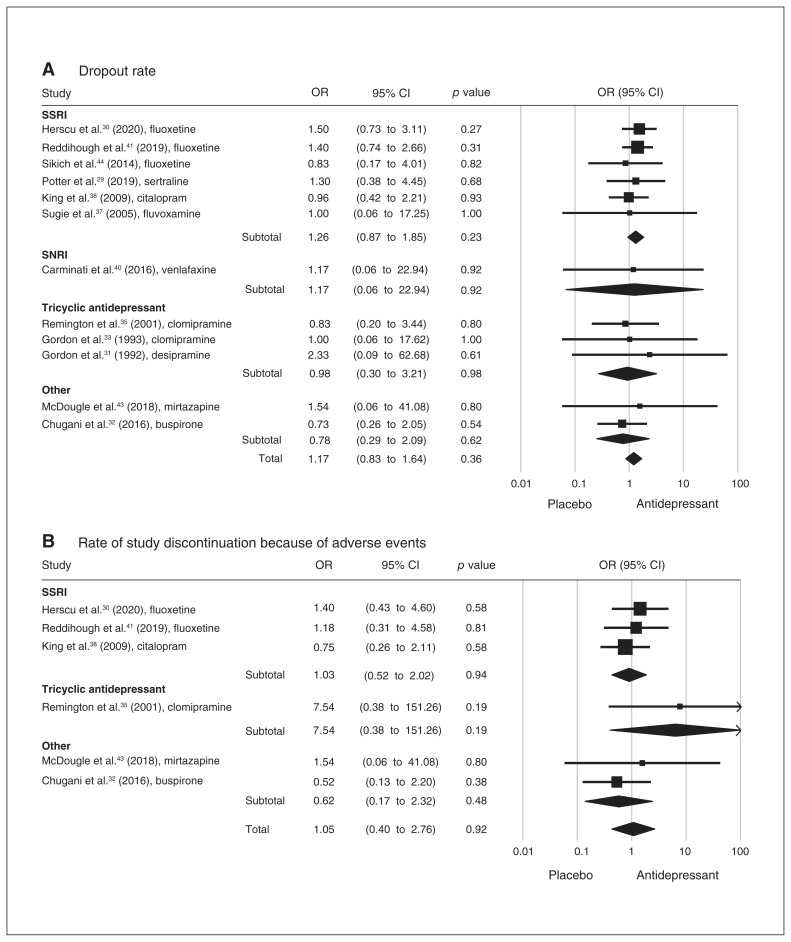
Based on 14 data sets from 13 studies, we found no significant differences between patients with ASD who received antidepressants and controls with respect to dropout rate or rate of study discontinuation because of adverse events (Figure 4). The leave-one-out sensitivity analysis of effect size for both dropout rate and study discontinuation demonstrated nonsignificant effects of individual studies on the overall outcomes. The results of the Egger tests were nonsignificant for dropout rate and study discontinuation, suggesting a low risk of publication bias.
Figure 4.
Forest plots comparing odds ratios for (A) dropout rate and (B) rate of study discontinuation because of adverse events between antidepressant and control groups. CI = confidence interval; OR = odds ratio; SNRI = serotonin and norepinephrine reuptake inhibitor; SSRI = selective serotonin reuptake inhibitor.
We also found no significant differences between the antidepressant and control groups in the incidence of overall and specific adverse events, including irritability (or activation), suicidal ideation, sedation (or lethargy), self-injury or insomnia (all p > 0.22).
With respect to secondary outcomes, 7 of the included studies provided data on irritability, 7 on social withdrawal, 7 on hyperactivity, 7 on inappropriate speech and 4 on anxiety. Among patients with ASD who received antidepressants, we found significantly better improvement in irritability (Hedges g = 0.33, p < 0.03) and hyperactivity (Hedges g = 0.22, p < 0.03), but not in inappropriate speech (Hedges g = 0.24, p = 0.1), social withdrawal (Hedges g = 0.03, p = 0.88) or anxiety (Hedges g = 0.44, p = 0.31). Leave-one-out sensitivity analysis of the effect sizes for the secondary outcomes found that the main effects were not driven by any single study. The risks of publication bias reflected by funnel plot asymmetry for these outcomes are shown in Appendix 1, Figure S1. With the random-effects model, the trim-and-fill method revealed potentially missing studies on the left side of the plot: 2 for irritability, yielding an adjusted effect size of 0.27 (0.03 to 0.50); 2 for social withdrawal, yielding an adjusted effect size of −0.1 (−0.42 to 0.22); 2 for hyperactivity, yielding an adjusted effect size of 0.19 (0.02 to 0.36); 0 for inappropriate speech, yielding an adjusted effect size of 0.24 (−0.05 to 0.54); and 0 for anxiety, yielding an adjusted effect size of 0.44 (−0.41 to 1.29).
Subgroup analysis and meta-regression
Subgroup analyses showed significant effects of type of medication (i.e., SSRI v. clomipramine) and age group (i.e., children and adolescents v. adults) on restricted and repetitive behaviours, as well as on global improvement (all p < 0.04; Table 2). Using a mixed-effects model, meta-regression demonstrated significant associations between the therapeutic effects of antidepressants for the symptoms of ASD and the Severity aspect of the Clinical Global Impression scale (regression coefficient −0.95 for restricted and repetitive behaviours, p = 0.02). We also detected a positive correlation between the effects of antidepressants and age for global improvement (regression coefficient 0.08, p = 0.02; Appendix 1, Table S3).
Table 2.
Difference in repeated behaviours and global improvement among participants with autism spectrum disorder taking antidepressants versus placebo — subgroup analysis
| Characteristic | No. of trials | Hedges g (95% CI)* | Z score | Cochran Q† | p value‡ |
|---|---|---|---|---|---|
| Stereotyped behaviours | |||||
| Drug intervention | |||||
| SSRI | 7 | 0.25 (0.03 to 0.48) | 2.21§ | 0.55 | 0.005 |
| Clomipramine (TCA) | 3 | 0.45 (−0.01 to 0.90) | 1.93 | ||
| Age group | |||||
| Children and adolescents | 8 | 0.21 (0.09 to 0.38) | 2.64§ | 1.94 | 0.001 |
| Adults | 3 | 0.58 (0.11 to 1.04) | 2.29§ | ||
| Global improvement | |||||
| Drug intervention | |||||
| SSRI | 5 | 1.04 (−0.04 to 2.12) | 1.90§ | 0.20 | 0.007 |
| Clomipramine (TCA) | 3 | 1.45 (0.02 to 2.89) | 1.98§ | ||
| Age group | |||||
| Children and adolescents | 6 | 0.59 (−0.09 to 1.27) | 1.69 | 1.49 | 0.040 |
| Adults | 3 | 2.41 (−0.44 to 5.25) | 1.66 | ||
CI = confidence interval; SSRI = selective serotonin reuptake inhibitor; TCA = tricyclic antidepressant.
Random-effects model.
For heterogeneity assessment based on random-effects analysis.
Significance of the difference between effect sizes in the subgroups.
p < 0.05.
Discussion
To the best of our knowledge, this was the first meta-analysis to investigate the efficacy of antidepressants in patients with ASD to identify important variables that might influence therapeutic outcomes.
Only a few meta-analyses with limited numbers of trials (all less than 10) have studied the effects of antidepressants for symptoms of ASD,11–14 and they did not address important factors that might affect treatment outcomes. As well, most previous meta-analyses have focused on the effects of antidepressants on restricted and repetitive behaviours in patients with ASD, and have failed to show significant therapeutic benefits in this setting.11,13 One meta-analysis showed minimal effectiveness of antidepressants (effect size 0.22, p < 0.05), and this finding became nonsignificant after adjustment for publication bias.12
Our study demonstrated that antidepressants had significant effectiveness for the treatment of restricted and repetitive behaviours in patients with ASD compared to placebo, without evidence of publication bias. Still, our small effect size (0.27) was similar to that reported in previous meta-analyses (0.22 to 0.24). One reason that our findings achieved significance may have been that we included more studies (n = 16), together with narrower confidence intervals compared to those previously reported.12 As well, although a previous meta-analysis46 found a superior treatment effect with clomipramine compared to SSRIs for pediatric OCD, none of the previous meta-analyses in patients with ASD included trials for clomipramine.11–13 One of the interesting findings of our subgroup analysis was the higher efficacy of clomipramine compared to SSRIs, highlighting a possible contribution of clomipramine to the higher efficacy of antidepressants in the present study compared to previous meta-analyses.
Despite our positive findings for antidepressants to treat restricted and repetitive behaviours in patients with ASD, the effect size was still very small. Previous studies have reported that antidepressant treatments were less effective in adolescents or children with ASD than in adults.3,47 This finding is further supported by the results of our subgroup analyses, which showed that adults had a better response to antidepressants than adolescents and children. Together with evidence demonstrating that drug susceptibility in the central nervous system varies with age,48 our results support the idea that age might be an important factor influencing the efficacy of antidepressants for restricted and repetitive behaviours in patients with ASD.
In addition to age, our finding of a negative association between symptom severity and antidepressant efficacy implies that antidepressants may be more effective for patients with less severe ASD. Experts have consistently suggested that more severe forms of ASD may be less responsive to SSRI treatment and more susceptible to the adverse effects of medications.49 Our study is the first meta-analysis to support this concept. This idea is also reinforced by the findings of a study that showed a good response to SSRIs in a subgroup of patients with high-functioning ASD and familial major affective disorders who demonstrated a relatively high level of anxiety.50 Together with evidence supporting the use of anti-psychotics for severe behavioural symptoms in patients with ASD,51–53 our finding might suggest the need to tailor medication treatments for different subgroups of patients with ASD.48,49 Further studies are needed to investigate the correlation between severity of ASD and response to SSRIs.
For the therapeutic effect of antidepressants on global improvement of the symptoms of autism, the present study found that antidepressant treatment was significantly better than placebo, with a large effect size (1.0) in 10 trials, similar to a study published by the Cochrane Collaboration (Hedges g = 0.89, p < 0.01).14 In the studies included in our meta-analysis, the efficacy of antidepressants for global improvement correlated well with improvements in restricted and repetitive behaviours in patients with ASD, highlighting an overall improvement attributable to the alleviation of ASD symptom subdomains. Moreover, our secondary analyses showed that antidepressants were effective against irritability and hyperactivity in patients with ASD, suggesting that the overall therapeutic benefits of antidepressants might come from collective improvements in different symptom subdomains. Similar to findings for restricted and repetitive behaviours, the results of our subgroup analysis of global improvement of ASD symptoms demonstrated positive associations with older age (v. younger age) and clomipramine (v. SSRIs). Moreover, our metaregression analysis also supported the finding that older age was correlated with better global effects of antidepressants in patients with ASD.
Previous evidence supporting the effectiveness of antidepressants to treat irritability and hyperactivity remains controversial, because adverse effects (e.g., agitation) have been reported more frequently in trials targeting adolescents and children with ASD.54,55 The results of our secondary analyses showed equal effectiveness for antidepressants in all 3 behavioural subdomains of ASD (i.e., irritability, hyperactivity and restricted and repetitive behaviours). Although antidepressants were originally designed to improve mood, none of the studies included in the present meta-analysis investigated their therapeutic effects on depressive symptoms, and only 4 included symptoms of anxiety. Our results showed that antidepressants might be more effective than placebos for improving anxiety, despite their failure to reach statistical significance. Given the small sample size in our analysis for anxiety, further studies are needed to address this issue in patients with ASD.
The findings of the present study demonstrated that the most common adverse effects on the central nervous system were lethargy, irritability, activation and insomnia. Insomnia was more commonly associated with fluoxetine, citalopram, sertraline and buspirone; sedation occurred more frequently in patients taking mirtazapine (Appendix 1, Table S4). Nevertheless, we found no significant differences between the antidepressant and placebo groups in terms of percentage of overall adverse effects or incidence of irritability, insomnia, lethargy, suicidal ideation or self-injurious behaviours. We also found no significant differences between the 2 groups in terms of dropout rate or rate of study discontinuation because of adverse events. One study using clomipramine (a tricyclic antidepressant) reported severe tachycardia and grand mal seizure in a 7-year-old girl.33 Furthermore, a previous review highlighted the possibility of sudden death related to the use of tricyclic antidepressants in children,56 raising serious concerns about the safety of prescribing them for children. Although our study showed satisfactory tolerability of adverse effects related to antidepressant use compared to placebo, treatment strategies should be individualized, taking into account the potential adverse effects of different agents.
Limitations
The present meta-analysis had some limitations. First, although we included more trials than previous meta-analyses, our total sample size (n = 899) and effect sizes were still too small to reach robust conclusions for most of our outcome measures. In particular, compared to our primary outcomes, the sample sizes were much smaller for our secondary outcomes, and we found evidence of publication bias; these findings should be interpreted with caution. Second, the heterogeneity of the included studies — including differences in choice of antidepressants, dosage and age groups — might limit the generalizability of our results. Still, we performed subgroup analyses and meta-regression analyses to help identify important factors that might have influenced treatment response. Third, we could provide only effect sizes because of limited availability of data; we were unable to provide odds ratios or numbers needed to treat, which would have offered clearer information for reference in clinical practice. Fourth, 1 study allowed the use of other psychotropic medications (e.g., zuclopenthixol and clonazepam) in the study and control groups.40 Because the doses of those psychotropics in the placebo arm were higher than those in the study arm by the end of the 56-day trial, it was difficult to determine the effectiveness of the antidepressants versus placebo based on the results of that study. Nevertheless, our sensitivity test did not reveal a substantial influence of any single study, including that particular trial,40 on the overall effect size. Fifth, because information was limited about defined daily doses for children and adolescents, we were unable to investigate the effects of dosage on the efficacy of antidepressants for the symptoms of ASD in this age group. Finally, the pooling of results from different assessment tools for behavioural outcomes is an inherent down side of meta-analyses in the psychiatric field. Nevertheless, most of our included studies used similar tools for outcome measurements (e.g., Children’s Yale–Brown Obsessive–Compulsive Scale or Aberrant Behavior Checklist), so that the confounding effect was relatively minor.
Conclusion
Our results support the effectiveness of antidepressants in treating the global symptoms of ASD, as well as symptom subdomains, including irritability and hyperactivity, with tolerable adverse effect profiles. Treatment response might be better achieved in patients with less severe overall ASD symptoms and in the adult population. However, given the small effect sizes we found for each symptom subdomain, wide variations in treatment response in different subgroups and publication bias associated with outcomes for irritability and hyperactivity, we suggest judicious and individually tailored use of antidepressants for patients with ASD.
Supplementary Material
Footnotes
Competing interests: None declared.
Contributors: S. Lian, H. Fan, W. Chung, R. Tzang, K. Hung, H. Chiu and Y. Cheng designed the study. C. Sun and P. Yeh analyzed the data. S. Liang, C. Sun, Y. Cheng and P. Yeh wrote the article, which H. Fan, W. Chung, R. Tzang, K. Hung and H. Chiu reviewed. All authors approved the final version to be published, agree to be accountable for all aspects of the work and can certify that no other individuals not listed as authors have made substantial contributions to the paper.
Disclaimer: The contents of this article are the authors’ sole responsibility and do not necessarily represent the views of their institutions.
References
- 1.Lyall K, Croen L, Daniels J, et al. The changing epidemiology of autism spectrum disorders. Annu Rev Public Health 2017;38:81–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maneeton N, Maneeton B, Putthisri S, et al. Aripiprazole in acute treatment of children and adolescents with autism spectrum disorder: a systematic review and meta-analysis. Neuropsychiatr Dis Treat 2018;14:3063–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goel R, Hong JS, Findling RL, et al. An update on pharmacotherapy of autism spectrum disorder in children and adolescents. Int Rev Psychiatry 2018;30:78–95. [DOI] [PubMed] [Google Scholar]
- 4.Soomro GM, Altman D, Rajagopal S, et al. Selective serotonin reuptake inhibitors (SSRIs) versus placebo for obsessive compulsive disorder (OCD). Cochrane Database Syst Rev 2008;2008:CD001765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bloch MH, McGuire J, Landeros-Weisenberger A, et al. Meta-analysis of the dose-response relationship of SSRI in obsessive-compulsive disorder. Mol Psychiatry 2010;15:850–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zandt F, Prior M, Kyrios M. Similarities and differences between children and adolescents with autism spectrum disorder and those with obsessive compulsive disorder: executive functioning and repetitive behaviour. Autism 2009;13:43–57. [DOI] [PubMed] [Google Scholar]
- 7.Zandt F, Prior M, Kyrios M. Repetitive behaviour in children with high functioning autism and obsessive compulsive disorder. J Autism Dev Disord 2007;37:251–9. [DOI] [PubMed] [Google Scholar]
- 8.McDougle CJ, Kresch LE, Goodman WK, et al. A case-controlled study of repetitive thoughts and behavior in adults with autistic disorder and obsessive-compulsive disorder. Am J Psychiatry 1995; 152:772–7. [DOI] [PubMed] [Google Scholar]
- 9.Micali N, Chakrabarti S, Fombonne E. The broad autism phenotype: findings from an epidemiological survey. Autism 2004;8:21–37. [DOI] [PubMed] [Google Scholar]
- 10.Hollander E, Kim S, Braun A, et al. Cross-cutting issues and future directions for the OCD spectrum. Psychiatry Res 2009;170:3–6. [DOI] [PubMed] [Google Scholar]
- 11.Zhou MS, Nasir M, Farhat LC, et al. Meta-analysis: pharmacologic treatment of restricted and repetitive behaviors in autism spectrum disorders. J Am Acad Child Adolesc Psychiatry 2021;60:35–45. [DOI] [PubMed] [Google Scholar]
- 12.Carrasco M, Volkmar FR, Bloch MH. Pharmacologic treatment of repetitive behaviors in autism spectrum disorders: evidence of publication bias. Pediatrics 2012;129:e1301–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu Y, Chaulagain A, Pedersen SA, et al. Pharmacotherapy of restricted/repetitive behavior in autism spectrum disorder: a systematic review and meta-analysis. BMC Psychiatry 2020;20:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williams K, Brignell A, Randall M, et al. Selective serotonin reuptake inhibitors (SSRIs) for autism spectrum disorders (ASD). Cochrane Database Syst Rev 2013;CD004677. [DOI] [PubMed] [Google Scholar]
- 15.Boccuto L, Chen CF, Pittman AR, et al. Decreased tryptophan metabolism in patients with autism spectrum disorders. Mol Autism 2013;4:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chandana SR, Behen ME, Juhász C, et al. Significance of abnormalities in developmental trajectory and asymmetry of cortical serotonin synthesis in autism. Int J Dev Neurosci 2005;23:171–82. [DOI] [PubMed] [Google Scholar]
- 17.Liu MT, Kuan Y-H, Wang J, et al. 5-HT4 receptor-mediated neuro-protection and neurogenesis in the enteric nervous system of adult mice. J Neurosci 2009;29:9683–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Makkonen I, Riikonen R, Kokki H, et al. Serotonin and dopamine transporter binding in children with autism determined by SPECT. Dev Med Child Neurol 2008;50:593–7. [DOI] [PubMed] [Google Scholar]
- 19.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009;62:1006–12. [DOI] [PubMed] [Google Scholar]
- 20.Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McHugh ML. Interrater reliability: the kappa statistic. Biochem Med (Zagreb) 2012;22:276–82. [PMC free article] [PubMed] [Google Scholar]
- 22.Higgins JP, Thomas J, Chandler J, et al. Cochrane handbook for systematic reviews of interventions. Hoboken (NJ): John Wiley & Sons; 2019. [Google Scholar]
- 23.Higgins JPT, Eldridge S, Li T. Including variants on randomized trials. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane handbook for systematic reviews of interventions version 6.3 (updated February 2022). London: Cochrane Collaboration; 2022: chapter 23. Available: www.training.cochrane.org/handbook (accessed 2022 May 28). [Google Scholar]
- 24.Borenstein M, Hedges LV, Higgins JP, et al. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods 2010;1:97–111. [DOI] [PubMed] [Google Scholar]
- 25.Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta-analyses. BMJ 2011;342:d549. [DOI] [PubMed] [Google Scholar]
- 26.Higgins J, Green S, editors. Cochrane handbook for systematic reviews of interventions. London: Cochrane Collaboration; 2009. [Google Scholar]
- 27.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000;56:455–63. [DOI] [PubMed] [Google Scholar]
- 29.Potter LA, Scholze DA, Biag HMB, et al. A randomized controlled trial of sertraline in young children with autism spectrum disorder. Front Psychiatry 2019;10:810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herscu P, Handen BL, Arnold LE, et al. The SOFIA study: negative multi-center study of low dose fluoxetine on repetitive behaviors in children and adolescents with autistic disorder. J Autism Dev Disord 2020;50:3233–44. [DOI] [PubMed] [Google Scholar]
- 31.Gordon CT, Rapoport JL, Hamburger SD, et al. Differential response of seven subjects with autistic disorder to clomipramine and desipramine. Am J Psychiatry 1992;149:363–6. [DOI] [PubMed] [Google Scholar]
- 32.Chugani DC, Chugani HT, Wiznitzer M, et al. Efficacy of low-dose buspirone for restricted and repetitive behavior in young children with autism spectrum disorder: a randomized trial. J Pediatr 2016; 170: 45–53.e1–4. [DOI] [PubMed] [Google Scholar]
- 33.Gordon CT, State RC, Nelson JE, et al. A double-blind comparison of clomipramine, desipramine, and placebo in the treatment of autistic disorder. Arch Gen Psychiatry 1993;50:441–7. [DOI] [PubMed] [Google Scholar]
- 34.McDougle CJ, Naylor ST, Cohen DJ, et al. A double-blind, placebo-controlled study of fluvoxamine in adults with autistic disorder. Arch Gen Psychiatry 1996;53:1001–8. [DOI] [PubMed] [Google Scholar]
- 35.Remington G, Sloman L, Konstantareas M, et al. Clomipramine versus haloperidol in the treatment of autistic disorder: a double-blind, placebo-controlled, crossover study. J Clin Psychopharmacol 2001;21: 440–4. [DOI] [PubMed] [Google Scholar]
- 36.Hollander E, Phillips A, Chaplin W, et al. A placebo controlled crossover trial of liquid fluoxetine on repetitive behaviors in childhood and adolescent autism. Neuropsychopharmacology 2005; 30:582–9. [DOI] [PubMed] [Google Scholar]
- 37.Sugie Y, Sugie H, Fukuda T, et al. Clinical efficacy of fluvoxamine and functional polymorphism in a serotonin transporter gene on childhood autism. J Autism Dev Disord 2005;35:377–85. [DOI] [PubMed] [Google Scholar]
- 38.King BH, Hollander E, Sikich L, et al. Lack of efficacy of citalopram in children with autism spectrum disorders and high levels of repetitive behavior: citalopram ineffective in children with autism. Arch Gen Psychiatry 2009;66:583–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hollander E, Soorya L, Chaplin W, et al. A double-blind placebo-controlled trial of fluoxetine for repetitive behaviors and global severity in adult autism spectrum disorders. Am J Psychiatry 2012;169:292–9. [DOI] [PubMed] [Google Scholar]
- 40.Carminati GG, Gerber F, Darbellay B, et al. Using venlafaxine to treat behavioral disorders in patients with autism spectrum disorder. Prog Neuropsychopharmacol Biol Psychiatry 2016;65:85–95. [DOI] [PubMed] [Google Scholar]
- 41.Reddihough DS, Marraffa C, Mouti A, et al. Effect of fluoxetine on obsessive-compulsive behaviors in children and adolescents with autism spectrum disorders: a randomized clinical trial. JAMA 2019;322:1561–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sanchez LE, Adams PB, Uysal S, et al. A comparison of live and videotape ratings: clomipramine and haloperidol in autism. Psychopharmacol Bull 1995;31:371–8. [PubMed] [Google Scholar]
- 43.Mirtazapine treatment of anxiety in children and adolescents with pervasive developmental disorders. ClinicalTrials.gov: NCT01302964; 2018. Available: www.clinicaltrials.gov/show/NCT01302964 (accessed 2021 Jul. 23). [Google Scholar]
- 44.Early intervention with fluoxetine in autism. ClinicalTrials.gov: NCT00183339; 2014. Available: www.ClinicalTrials.gov/show/NCT00183339 (accessed 2021 Jul. 23). [Google Scholar]
- 45.Leonard HL, Swedo SE, Rapoport JL, et al. Treatment of obsessive-compulsive disorder with clomipramine and desipramine in children and adolescents. A double-blind crossover comparison. Arch Gen Psychiatry 1989;46:1088–92. [DOI] [PubMed] [Google Scholar]
- 46.Geller DA, Biederman J, Stewart SE, et al. Which SSRI? A meta-analysis of pharmacotherapy trials in pediatric obsessive-compulsive disorder. Am J Psychiatry 2003;160:1919–28. [DOI] [PubMed] [Google Scholar]
- 47.Doyle CA, McDougle CJ. Pharmacologic treatments for the behavioral symptoms associated with autism spectrum disorders across the lifespan. Dialogues Clin Neurosci 2012;14:263–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brandenburg C, Blatt GJ. Differential serotonin transporter (5-HTT) and 5-HT(2) receptor density in limbic and neocortical areas of adults and children with autism spectrum disorders: implications for selective serotonin reuptake inhibitor efficacy. J Neurochem 2019;151:642–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reiersen AM, Handen B. Commentary on “Selective serotonin reuptake inhibitors (SSRIs) for autism spectrum disorders (ASD)”. Evid Based Child Health 2011;6:1082–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.DeLong GR, Ritch CR, Burch S. Fluoxetine response in children with autistic spectrum disorders: correlation with familial major affective disorder and intellectual achievement. Dev Med Child Neurol 2002;44:652–9. [DOI] [PubMed] [Google Scholar]
- 51.Downs J, Hotopf M, Ford T, et al. Clinical predictors of antipsychotic use in children and adolescents with autism spectrum disorders: a historical open cohort study using electronic health records. Eur Child Adolesc Psychiatry 2016;25:649–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Posey DJ, Stigler KA, Erickson CA, et al. Antipsychotics in the treatment of autism. J Clin Invest 2008;118:6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Perry R, Campbell M, Adams P, et al. Long-term efficacy of haloperidol in autistic children: continuous versus discontinuous drug administration. J Am Acad Child Adolesc Psychiatry 1989;28:87–92. [DOI] [PubMed] [Google Scholar]
- 54.McDougle CJ, Kresch LE, Posey DJ. Repetitive thoughts and behavior in pervasive developmental disorders: treatment with serotonin reuptake inhibitors. J Autism Dev Disord 2000;30:427–35. [DOI] [PubMed] [Google Scholar]
- 55.Reinblatt SP, DosReis S, Walkup JT, et al. Activation adverse events induced by the selective serotonin reuptake inhibitor fluvoxamine in children and adolescents. J Child Adolesc Psychopharmacol 2009;19:119–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Varley CK. Sudden death related to selected tricyclic antidepressants in children: epidemiology, mechanisms and clinical implications. Paediatr Drugs 2001;3:613–27. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.