Abstract
Background:
Among dermatologic adverse events induced by immune checkpoint inhibitors (ICI), bullous life-threatening reactions are rare.
Objectives:
To better define the clinical and histological features, treatment and prognosis of ICI-related severe blistering cutaneous eruptions.
Methods:
This retrospective case series was conducted between 2014/05/15 to 2021/04/15 by the dermatology departments of four international registries involved in drug reactions. Inclusion criteria were age ≥ 18 years-old, skin eruption with blisters and/or detachment covering ≥ 1% body surface area and at least one mucous membrane involved, available pictures, and ICI as suspect drug. Autoimmune bullous disorders were excluded. Each participant medical team gave his own diagnosis conclusion: epidermal necrolysis (EN), severe lichenoid dermatosis (LD) or unclassified dermatosis (UD). After a standardized review of pictures, cases were reclassified by 4 experts in EN or LD/UD. Skin biopsies were blindly reviewed.
Results:
Thirty-two patients were included. Median time to onset was 52 days [3–420]. Cases were originally diagnosed as EN in 21 cases and LD/UD in 11 cases. After review by experts, 10/21 EN were reclassified as LD/UD. The following manifestations were more frequent or severe in EN: fever, purpuric macules, blisters, ocular involvement, maximal detachment. Most patients were treated with topical and/or systemic corticosteroids. Eight patients (25%) died in the acute phase. The culprit ICI was not resumed in 92% of cases. In three patients, another ICI was given with a good tolerance. Histology did not reveal significant differences between groups.
Conclusions:
Severe blistering cutaneous drug reactions induced by ICI are often overdiagnosed as EN. Consensus for management is pending.
Keywords: immune checkpoint inhibitor, drug reaction, epidermal necrolysis, lichenoid eruption, nivolumab, pembrolizumab
Background
Immune checkpoint inhibitors (ICI, anti-CTLA-4, -PD-1, -PD-L1) are responsible for dermatologic immune-related adverse events, mostly maculopapular rash and lichenoid eruptions. Since these reactions are usually self-limited, responsible drugs can be continued and patients can be managed by skin directed therapy [1,2].
Life-threatening cutaneous eruptions, including blistering dermatoses with epidermal detachment mimicking epidermal necrolysis (EN, including Stevens-Johnson syndrome, SJS, and toxic epidermal necrolysis, TEN), have been rarely reported, with less than 30 cases described to date [3–7]. However, it is still not established whether these blistering reactions are strictly EN or in some cases correspond to immune-mediated bullous lichenoid eruptions [8]. Histology may not be discriminant between severe lichenoid eruptions and “true” EN. Furthermore, there is no consensus regarding the management of such severe eruptions in this oncologic context. Systemic steroid therapy raises the question of increased infectious risk due to epidermal detachment. The use of cyclosporine or intravenous immunoglobulins (IVIG) has been also sporadically reported. Moreover, the effect of such treatments on the antitumor efficacy of ICI is poorly characterised and the safety of switching for another ICI is unknown [9,10].
The aim of our study was to better describe the clinical and histological features, treatment and prognosis of ICI-related severe blistering cutaneous eruptions.
Methods
This retrospective study was conducted between 2014/05/15 to 2021/04/15 by the dermatology departments that are members of four registries involved in drug reactions, namely the Task Force “dermatology for cancer patients” of EADV [1], ToxiTEN subgroup of ERN-skin, TOXIBUL French reference center (toxic bullous diseases and severe drug reactions) and FISARD (French study group for drug reactions).
Cases were extracted from local databases. The study was approved by the institutional review board of each participating center according local rules. Inclusion criteria were age ≥ 18 years-old, skin eruption with blisters and/or detachment covering ≥ 1% body surface area (BSA) and at least one mucous membrane involved (may be limited to the lips), available clinical pictures of acute phase lesions, with at least one ICI treatment ongoing at the time of the reaction. Due to the well-established variability in time between first exposure to ICI treatment and onset of severe bullous ICI-related reactions[6], ICI could be suspected as trigger no matter what the time span between first drug-intake and of onset of the dermatosis was. Exclusion criteria (based on usual clinical, histological, direct immunofluorescence and biological results) included other severe adverse drug eruptions (DRESS, AGEP, generalized bullous fixed drug eruption), autoimmune bullous diseases (especially paraneoplastic pemphigus, negative direct immunofluorescence), staphylococcal scalded skin syndrome and bullous impetigo.
First, data were collected by each participating center on a standardized data sheet from medical charts: demographics, cancer history, suspected ICI, time to onset, clinical presentation, percentage of initial and maximal detachment, mucous membrane involvement, treatment, length of hospitalization stay, complications (i.e. need for mechanical ventilation, infections), healing at 6 weeks, death at 6 weeks and at the date of the study (May 2021). Each participant medical team was required to give his own initial diagnosis within the provided nosological framework including: EN, severe lichenoid dermatosis (LD) or unclassified dermatosis (UD).
In a second step, pictures of the patients at the acute phase, provided by the participant medical team, were reviewed by the 4 members of the steering committee of the study (SIHO, BM, LF and VS), blinded with respect to the diagnosis provided by the participants; their task was to definitively reclassify the patients into two groups according to usual clinical diagnosis criteria: EN or LD/UD [1,2,11].
Histology of available skin biopsies (hematoxylin and eosin stained sections from formalin fixed and paraffin embedded material) were reviewed in a standardized manner, blindly with respect to the final reclassified diagnosis of the 4 above experts.
Comparison between EN and LD/UD (final diagnosis after review of pictures by experts) used Fisher and Mann-Whitney tests as appropriate (online Biostat TGV software).
Results
Thirty-six cases of ICI-related severe blistering cutaneous eruptions were initially collected from 15 European and American centers (8 countries), of which 4 were excluded due to lack of- or insufficiently informative clinical photographs. Finally, 32 patients from 14 centers (7 countries) were analysed (17 male, 15 female, median age 60 years [21–85]). The most frequent types of cancer were lung cancer (n=13) and melanoma (n=11). Previous antineoplastic treatments included chemotherapy (n=6), chemoembolization + radiofrequency (n=1), targeted therapy (n=6), surgery (n=6), other ICI (n=1) or none (n=13). The suspect ICI was pembrolizumab (n=14), nivolumab (n=8), ipilimumab (n=2), anti-CTLA-4 + anti-PD-1/PD-L1 in combination (n=7), or anti-PD-L1 cemiplimab (n=1). ICI was administered alone (without other antineoplastic drug) in 20 cases, in association with chemotherapy in 10 cases, and radiotherapy in 2. Median time from the first ICI cycle to onset of cutaneous lesions was 52 days [3–420]. The ICI was considered as the most likely causal drug in all cases, as no patient had evidence of intake of other drugs within the past 28 days, especially high-risk drugs for EN [12].
Cases were originally diagnosed as EN in 21 cases and LD/UD in 11 cases. Based on the clinical review of photographic material by the steering committee, 10 of the above EN were reclassified as LD/UD. Finally, a diagnosis of EN was retained in 11 patients, and LD/UD in 21 patients (Table 1, Fig 1). Several clinical manifestations were noted with a higher incidence, or were more severe in patients with EN: fever, purpuric macules, blisters, ocular involvement, maximal detachment (35% versus 8%, p=0.01) (Table 1). Median time to onset after ICI introduction was shorter in EN (34 days) than in LD/UD (53 days, p=0.06).
Table 1 :
Clinical characteristics of the 32 patients included in the study, with comparison between epidermal necrolysis and lichenoid/unclassified dermatoses
| All patients N=32 | Epidermal necrolysis spectrum N=11 | Lichenoid/unclassified dermatoses N=21 | p-value | |
|---|---|---|---|---|
|
| ||||
| Female sex, n (%) | 15 (47) | 5 (45) | 10 (48) | 1 |
|
| ||||
| Median age (range), years | 60 (21–85) | 63 (57–72) | 59 (21–85) | 0.53 |
|
| ||||
| Type of cancer | Lung n=13 Melanoma n=11 Liver n=4 Kidney n=1 Thyroid n=1 Thymus n=1 Unknown n=1 |
Lung n=5 Melanoma n=2 Liver n=1 Kidney n=1 Thymus n=1 Unknown n=1 |
Lung n=8 Melanoma n=9 Liver n=3 Thyroid n=1 |
0.72 0.25 |
|
| ||||
| Median time to onset (range), days | 52 (3–420) | 34 (5–90) | 53 (3–420) | 0.06 |
|
| ||||
| Fever, n (%) | 19 (59) | 9 (82) | 10 (48) | 0.13 |
|
| ||||
| Maculopapular exanthema, n (%) | 25 (78) | 9 (82) | 16 (76) | 1 |
|
| ||||
| Lichenoid lesions, n (%) | 14 (44) | 4 (36) | 10 (48) | 0.71 |
|
| ||||
| Purpuric macules, n (%) | 21 (66) | 10 (91) | 11 (52) | 0.05 |
|
| ||||
| Blisters, n (%) | 27 (84) | 11 (100) | 16 (76) | 0.14 |
|
| ||||
| Nikolsky and or detachment > 5 cm, n (%) | 21 (66) | 9 (82) | 12 (57) | 0.25 |
|
| ||||
| Initial detached-detachable BSA, % (range) | 5 (0–45) | 10 (0–35) | 2 (0–45) | 0.25 |
|
| ||||
| Maximal detached-detachable BSA, % (range) | 18 (1–100) | 35 (2–100) | 8 (1–74) | 0.01 |
|
| ||||
| Mucous membrane involvement, n (%) | ||||
| Oral | 29 (91) | 10 (91) | 19 (90) | 1 |
| Laryngeal | 8 (25) | 3 (27) | 5 (24) | 1 |
| Genital-anal | 9 (28) | 2 (18) | 7 (33) | 0.44 |
| Ocular | 15 (47) | 8 (73) | 7 (33) | 0.06 |
|
| ||||
| Median baseline SCORTEN (ext) | 3 (1–5) | 3 (2–5) | 3 (1–5) | 0.29 |
|
| ||||
| Median lenght of hospitalization (range), days | 14 (0–121) | 17 (3–121) | 14 (0–45) | 0.59 |
|
| ||||
| Treatment, n | ||||
| Supportive care only | 3 (9) | 2 (18) | 1 (5) | 0.54 |
| Topical steroids | 16 (50) | 1 (9) | 15 (71) | 0.002 |
| Systemic steroids | 26 (81) | 8 (73) | 18 (86) | 0.39 |
| And/or others (IgIV, G-CSF, ciclo, MMF, phototherapy palms/soles | 10 (31) | 5 (45) | 5 (24) | 0.25 |
|
| ||||
| Median duration of steroids (range), days | 30 (3–120) | 30 (25–60) | 25 (3–120) | 0.29 |
|
| ||||
| Healed at 6 weeks, n (% among alive patients) | 18 (56) | 5 (71) | 13 (62) | 0.46 |
|
| ||||
| Death at 6 weeks, n (%) | 8 (25) | 4 (36) | 4 (19) | 0.40 |
|
| ||||
| Death at last news, n (%) | 20 (62) | 9 (82) | 11 (52) | 0.14 |
Figure 1:
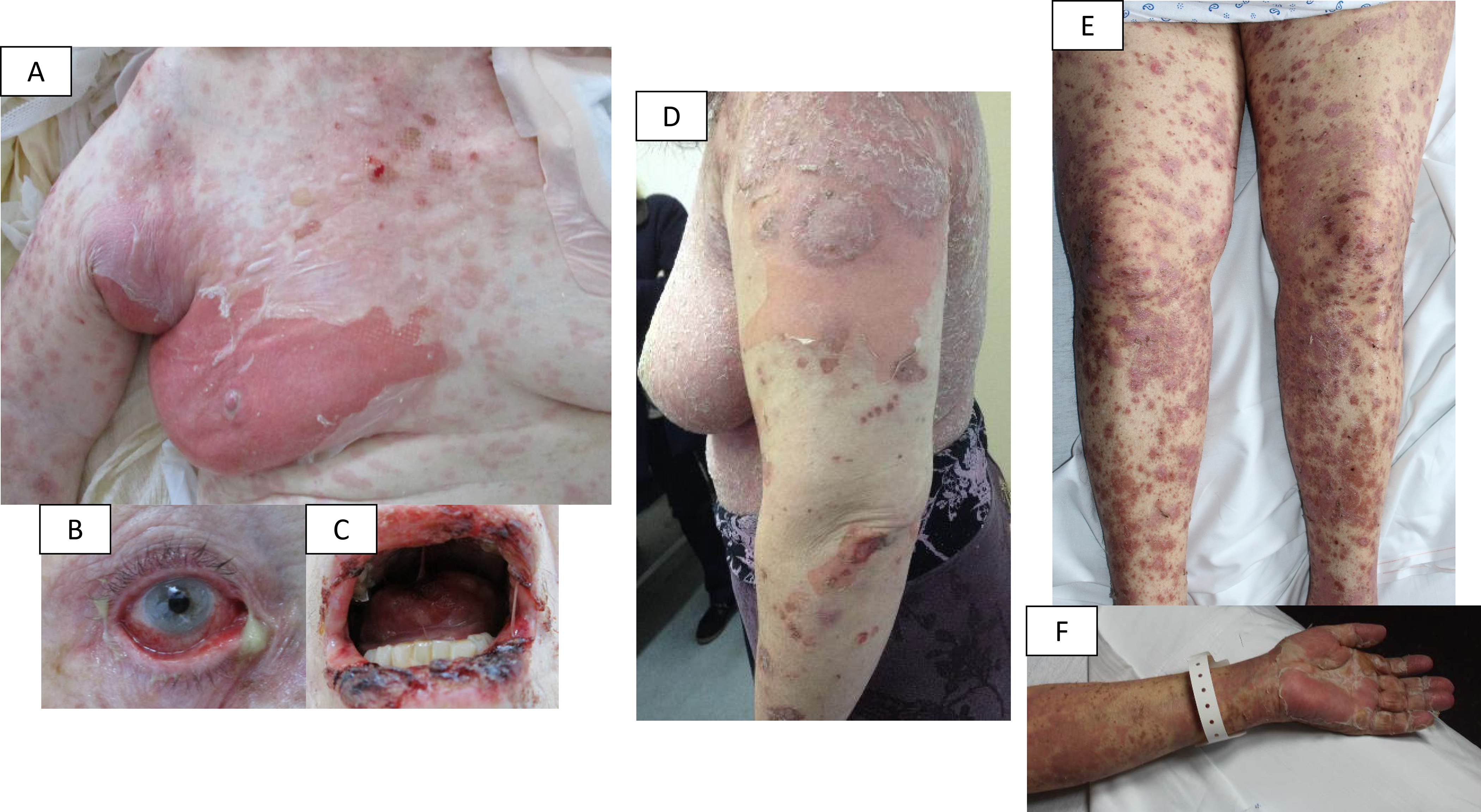
A-B-C: patient with a “true” epidermal necrolysis; D: patient with a lichenoid dermatosis (for these two cases, experts validated the initial diagnosis suggested by the participating center); E-F: patient with an initial diagnosis of epidermal necrolysis, reclassified as lichenoid dermatosis by experts.
Treatment of the skin lesions mainly included corticosteroids (topical, n=16, 50% and/or systemic, n=26, 81%), for a variable duration (median 30 days [3–120]). Three patients (9%) received supportive care only. Topical corticosteroids were more frequently used in LD/UD than in EN (n=15, 71%, vs n=1, 9%, p=0.002). Five patients (24%, EN n=2, LD/UD n=3) required mechanical ventilation and 11 (34%, EN n=4, LD/UD n=7) had infectious complications during the acute phase. Eight patients (25%, 4 in each group, p=0.40) died within the first 6 weeks (4 patients of the skin drug reaction, one of immune-related pneumonitis, 2 of the underlying cancer).
Among the 24 patients alive at 6 weeks, the suspect ICI was not resumed in 22 cases (92%). One patient with LD due to ipilimumab + nivolumab resumed nivolumab alone but LD relapsed immediately. Another patient with LD induced by nivolumab relapsed after resuming the same drug. Among the 22 patients in which the offending ICI was not resumed, three (EN n=1, LD n=2) were switched to another ICI with a good tolerance: one was switched from ipilimumab to pembrolizumab and 2 were switched from one anti-PD1 to another (nivolumab to pembrolizumab, or the contrary).
Allergological tests were performed in three cases (lymphocyte transformation test n=2, +/− patch tests n=1, not specified n=1) and were negative in all cases.
Skin biopsies were reviewed for 21 patients (final diagnosis EN n=6, LD/UD n=15). Histopathological findings did not reveal differences between groups concerning epidermal changes, density, location, and type of dermal inflammatory infiltrates. In most biopsies (6/6 EN and 9/15 LD/UD, p=0.12), we observed an interface dermatitis with confluent apoptotic bodies (epidermal necrolysis-like pattern, acute syndrome of pan-epidermolysis, ASAP) [13]. An interface dermatitis without confluent apoptosis of keratinocytes, sometimes with a lichen planus-like pattern was observed in 0/6 EN cases and 5/15 LD/UD cases (p=0.26). To note, a spongiotic pattern was never individualized and only one biopsy showed pustules. Finally, eosinophils/neutrophils were either absent (n=12/18) or scarce (n=4/18).
Discussion
We report herein the largest series to our knowledge of ICI-related potentially life-threatening blistering eruptions to date, excluding auto-immune bullous diseases. In contrast with other more common eruptions of mild intensity [1,14], severe blistering dermatoses are rare (32 cases collected within four large international groups after a retrospective analysis of 7 years). In our study, ICI-related severe blistering eruptions were not related to a specific type of cancer, and were associated with any kind of ICI, used alone or in combination. The time from the first ICI-infusion to the onset of the cutaneous eruption was variable, ranging from 3 to 420 days (median 52 days), which is similar to previous reports [6,7]. Thus, facing such a cutaneous eruption with ICI, the search for causative drugs should not be restricted to the classical time to onset of drug reactions, especially in case of EN presentation (for which the usual time to onset is 4–28 days) [11].
According to the clinical presentation as judged on high-quality patient photographs, experts reclassified almost half of initial diagnoses of EN (10/21) as LD/UD. Indeed, lesions in patients with LD/UD were mainly maculopapular or lichenoid, desquamative, with only superficial detachment. Moreover, lesions were predominantly located to palms and soles. Mucosal surfaces were also involved, with lichenoid lesions of the mouth and/or lips in some cases. By contrast, ocular involvement was discrete. Finally, general status was only slightly altered.
ICI are known to be associated with an increased risk of SJS/TEN (OR= 4.33, 95%CI:1.90–9.87) [15]. However, our results confirm that ICI-related SJS/TEN is very likely overdiagnosed, and some of these EN cases correspond in fact to severe blistering lichenoid reactions [8]. However, such severe lichenoid eruptions and “true” EN could belong to the same spectrum of immune-related adverse events. Indeed, recently, a new terminology was suggested for this group of severe bullous cutaneous eruptions triggered by ICI, progressive immunotherapy-related mucocutaneous eruption (PIRME), which underlines the heterogeneous framework of these mucocutaneous reactions induced by ICIs [7].
In our series, a majority of patients was treated with systemic corticosteroids. Treatment regimens were not homogeneous, neither with regard to the dose nor duration of steroid therapy (median 30 days [3–120]). In « true » EN with extensive detachment, the benefit-to-risk balance must be considered because of the risk of infection. However, when facing such eruptions, especially with a lichenoid presentation, corticosteroids should be proposed as the first-line therapy without delay, with a short-term reassessment [1,9,16].
The suspect ICI was resumed in two patients with LD, with relapse of the same skin reaction in both cases. Three other cases were switched to another ICI without relapse. However, our experience limited to 3 cases and the absence of previously published similar cases does not allow any strong conclusion regarding the safety of reintroduction of another ICI in this context.
In our series, the mortality rate during the acute phase and at last news was high (25% and 62% respectively), depending both on the severity of the skin reaction and the underlying cancer. It was higher in « true » EN (36% and 82%) than in LD/UD (19% and 52%), without reaching significance probably due to the limited number of patients in each group. In the most severe cases, the decision to admit such patients into intensive care units may be impacted by the underlying advanced cancer and its perceived prognosis.
Reviewed histologically cases revealed no major differences between « true » EN and LD/UD, showing that both manifestations belong to the ASAP spectrum, similar to classical drug-induced EN, bullous fixed drug eruption, lupus-like-TEN or Mycoplasma pneumoniae-induced severe erythema multiforme [13,17,18].
A transcriptomic analysis by Goldinger et al. in a small series of skin biopsies of anti-PD1 related cutaneous eruptions, including non-blistering reactions, showed a similar gene expression profile to “classic” drug-induced SJS-TEN, emphasizing the role of PD-1 in the regulation of cytotoxic T-cells in the skin [14]. Nevertheless, whether blistering diseases occurring after ICI administration are driven by an immuno-allergic reaction towards the compound or the dysregulation and activation of auto-immune T-cells remains an open question.
Although our results remain limited by the small number of patients, the multicenter and international design of the study and the independent blinded review of clinical features performed by a panel of experts allow to consolidate the proposed conclusions. Furthermore, we cannot exclude drug interactions in the 10 patients with associated chemotherapy or targeted therapy.
Severe blistering cutaneous drug reactions induced by ICI are often overdiagnosed as EN due to the presence of some extent of epidermal detachment associated with mucosal involvement. Consensus for management is still needed. The safety of switching from one ICI to another cannot be strongly assessed in our study.
Acknowledgement:
G. Chaby, S. Bethembos, JF. Cadranel, A. Pascale, P. Vuagnat, A. Belatti, M. Echeverria, J. Cura, N. Steven, F. Shah for the care provided to the patients.
Footnotes
Conflict of interest: None declared
References:
- [1].Apalla Z, Nikolaou V, Fattore D, Fabbrocini G, Freites-Martinez A, Sollena P, et al. European recommendations for management of immune checkpoint inhibitors-derived dermatologic adverse events. The EADV task force ‘Dermatology for cancer patients’ position statement. J Eur Acad Dermatol Venereol 2021. Dec 15. doi: 10.1111/jdv.17855. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- [2].Geisler AN, Phillips GS, Barrios DM, Wu J, Leung DYM, Moy AP, et al. Immune checkpoint inhibitor-related dermatologic adverse events. J Am Acad Dermatol 2020;83:1255–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Nayar N, Briscoe K, Fernandez Penas P. Toxic Epidermal Necrolysis-like Reaction With Severe Satellite Cell Necrosis Associated With Nivolumab in a Patient With Ipilimumab Refractory Metastatic Melanoma. J Immunother 2016;39:149–52. [DOI] [PubMed] [Google Scholar]
- [4].Hwang A, Iskandar A, Dasanu CA. Stevens-Johnson syndrome manifesting late in the course of pembrolizumab therapy. J Oncol Pharm Pract 2019;25:1520–2. [DOI] [PubMed] [Google Scholar]
- [5].Cai ZR, Lecours J, Adam J-P, Marcil I, Blais N, Dallaire M, et al. Toxic epidermal necrolysis associated with pembrolizumab. J Oncol Pharm Pract 2020;26:1259–65. [DOI] [PubMed] [Google Scholar]
- [6].Maloney NJ, Ravi V, Cheng K, Bach DQ, Worswick S. Stevens-Johnson syndrome and toxic epidermal necrolysis-like reactions to checkpoint inhibitors: a systematic review. Int J Dermatol 2020;59:e183–8. [DOI] [PubMed] [Google Scholar]
- [7].Molina GE, Yu Z, Foreman RK, Reynolds KL, Chen ST. Generalized bullous mucocutaneous eruption mimicking Stevens-Johnson syndrome in the setting of immune checkpoint inhibition: A multicenter case series. J Am Acad Dermatol 2020;83:1475–7. [DOI] [PubMed] [Google Scholar]
- [8].Reschke R, Mockenhaupt M, Simon J-C, Ziemer M. Severe bullous skin eruptions on checkpoint inhibitor therapy - in most cases severe bullous lichenoid drug eruptions. J Dtsch Dermatol Ges 2019;17:942–8. [DOI] [PubMed] [Google Scholar]
- [9].Logan IT, Zaman S, Hussein L, Perrett CM. Combination Therapy of Ipilimumab and Nivolumab-associated Toxic Epidermal Necrolysis (TEN) in a Patient With Metastatic Melanoma: A Case Report and Literature Review. J Immunother 2020;43:89–92. [DOI] [PubMed] [Google Scholar]
- [10].Choi EC-E, Heng YK, Lim YL. Immune checkpoint inhibitor-related Stevens-Johnson syndrome/toxic epidermal necrolysis-like reactions. J Am Acad Dermatol 2021;85:e109. [DOI] [PubMed] [Google Scholar]
- [11].Duong TA, Valeyrie-Allanore L, Wolkenstein P, Chosidow O. Severe cutaneous adverse reactions to drugs. Lancet 2017;390:1996–2011. [DOI] [PubMed] [Google Scholar]
- [12].Mockenhaupt M, Viboud C, Dunant A, Naldi L, Halevy S, Bouwes Bavinck JN, et al. Stevens-Johnson syndrome and toxic epidermal necrolysis: assessment of medication risks with emphasis on recently marketed drugs. The EuroSCAR-study. J Invest Dermatol 2008;128:35–44. [DOI] [PubMed] [Google Scholar]
- [13].Ting W, Stone MS, Racila D, Scofield RH, Sontheimer RD. Toxic epidermal necrolysis-like acute cutaneous lupus erythematosus and the spectrum of the acute syndrome of apoptotic pan-epidermolysis (ASAP): a case report, concept review and proposal for new classification of lupus erythematosus vesiculobullous skin lesions. Lupus 2004;13:941–50. [DOI] [PubMed] [Google Scholar]
- [14].Goldinger SM, Stieger P, Meier B, Micaletto S, Contassot E, French LE, et al. Cytotoxic Cutaneous Adverse Drug Reactions during Anti-PD-1 Therapy. Clin Cancer Res 2016;22:4023–9. [DOI] [PubMed] [Google Scholar]
- [15].Zhu J, Chen G, He Z, Zheng Y, Gao S, Li J, et al. Stevens-Johnson syndrome/toxic epidermal necrolysis in patients treated with immune checkpoint inhibitors: A safety analysis of clinical trials and FDA pharmacovigilance database. EClinicalMedicine 2021;37:100951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Choi J, Anderson R, Blidner A, Cooksley T, Dougan M, Glezerman I, et al. Multinational Association of Supportive Care in Cancer (MASCC) 2020 clinical practice recommendations for the management of severe dermatological toxicities from checkpoint inhibitors. Support Care Cancer 2020;28:6119–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Amode R, Ingen-Housz-Oro S, Ortonne N, Bounfour T, Pereyre S, Schlemmer F, et al. Clinical and histologic features of Mycoplasma pneumoniae-related erythema multiforme: A single-center series of 33 cases compared with 100 cases induced by other causes. J Am Acad Dermatol 2018;79:110–7. [DOI] [PubMed] [Google Scholar]
- [18].Perron E, Viarnaud A, Marciano L, Karkouche R, Wechsler J, De Prost N, et al. Clinical and histological features of fixed drug eruption: a single-centre series of 73 cases with comparison between bullous and non-bullous forms. Eur J Dermatol 2021;31:372–80. [DOI] [PubMed] [Google Scholar]


