Abstract
Introduction
Body composition as dynamic indices constantly changes in pregnancy. The use of body composition indices in the early stages of pregnancy has recently been considered. Therefore, the current meta-analysis study was conducted to investigate the relationship between body composition in the early stages of pregnancy and gestational diabetes.
Method
Valid databases searched for papers published from 2010 to December 2021 were based on PRISMA guideline. Newcastle Ottawa was used to assess the quality of the studies. For all analyses, STATA 14.0 was used. Mean difference (MD) of anthropometric indices was calculated between the GDM and Non-GDM groups. Pooled MD was estimated by “Metan” command, and heterogeneity was defined using Cochran’s Q test of heterogeneity, and I 2 index was used to quantify heterogeneity.
Results
Finally, 29 studies with a sample size of 56438 met the criteria for entering the meta-analysis. Pooled MD of neck circumference, hip circumference, waist hip ratio, and visceral adipose tissue depth were, respectively, 1.00 cm (95% CI: 0.79 to 1.20) [N = 5; I^2: 0%; p: 0.709], 7.79 cm (95% CI: 2.27 to 13.31) [N = 5; I2: 84.3%; P<0.001], 0.03 (95% CI: 0.02 to 0.04) [N = 9; I2: 89.2%; P<0.001], and 7.74 cm (95% CI: 0.11 to 1.36) [N = 4; I^2: 95.8%; P<0.001].
Conclusion
Increased neck circumference, waist circumference, hip circumference, arm circumference, waist to hip ratio, visceral fat depth, subcutaneous fat depth, and short stature increased the possibility of developing gestational diabetes. These indices can accurately, cost-effectively, and affordably assess the occurrence of gestational diabetes, thus preventing many consequences with early detection of gestational diabetes.
Introduction
Gestational diabetes mellitus (GDM) is defined as carbohydrate intolerance with varying degrees that is first diagnosed in pregnancy [1]. GDM usually begins in the second half of pregnancy when the mother is unable to secrete enough insulin to compensate for the nutritional increase in pregnancy and the possible increase in fat and anti-insulin hormones that occur during pregnancy (such as human placental hormone, cortisol, and prolactin) [2]. GDM has many maternal and fetal consequences that can be both short-term and long-term [3].
Several risk factors increase GDM, including aging, GDM history, body mass index (BMI) greater than 30 kg/m2, family history of diabetes, history of a macrosomic infant weighing 4.5 kg, and race [4]. Other maternal complications include shoulder dystocia, preeclampsia, cesarean section, type-2 diabetes, metabolic syndrome, and cardiovascular disease [5–7]. Neonatal complications also include macrosomia, neonatal trauma, hypoglycemia, and other metabolic disorders of the neonatal period [8, 9].
Many maternal and neonatal complications can be improved by careful monitoring of blood glucose during pregnancy, medical treatments (insulin and metformin), diet, physical activity, and lifestyle changes [10, 11].
In 2010, the International Association of Diabetes and Pregnancy Study Groups (IADPSG) developed new diagnostic criteria for GDM, based for the first time on adverse pregnancy outcomes [12]. In 2013, the World Health Organization (WHO) defined the IADPSG criteria adjusted during the 75 g OGTT threshold to 1.75 times the odds ratio for adverse pregnancy outcomes by reducing fasting glucose concentrations by 5.1 ≥, 1-h ≥ 10, and/or 2-h ≥ 8.5 mmol per liter [13].
The global prevalence of gestational diabetes is estimated 1 to 28%; this difference is due to differences in the criteria for measuring GDM, age, race, ethnicity, lifestyle, and history of the populations in which the prevalence was measured [14–16].
Normal pregnancy is characterized by a physiological reduction of 50–60% in insulin sensitivity [17]. Studies have reported that the likelihood of GDM increases with maternal weight gain, especially in early pregnancy. Numerous studies have been conducted worldwide to identify effective risk predictors to support early prevention or treatment [18, 19].
Measurement of body composition seems to be a practical method for potential screening of GDM [20]. Body composition is a risk factor for conditions such as diabetes, preeclampsia, and gestational hypertension [21, 22]. Obesity is a powerful predictor of GDM, and abdominal obesity is a powerful factor in the development of GDM and future diabetes [23, 24]. However, obesity is a complex process in which the distribution of body fat is involved, and body fat leads to adverse metabolic and cardiovascular consequences [25]. Studies show that increasing body composition, especially body fat, is closely related to glucose metabolism in humans [26]. But data on body composition and anthropometric indices are low. Studies show that weight gain in the first 2–3 months is composed of more fat mass, and patients with higher BMI gain more fat mass [15, 16] which can affect subsequent maternal insulin resistance [27].
However, there are other anthropometric indices that have been considered recently. In addition to showing more accurate information about body composition, they can also predict pregnancy outcomes, including GDM in pregnant women. For example, measurement of visceral abdominal adipose tissue (VAT) [28], neck circumference (NC), hip circumference (HC) and waist circumference (WC) [29], percentage of skeletal muscle mass and percentage of fat mass [30], and central obesity [31] can be used as an approach to predict occurrence GDM. Previous meta-analysis studies have shown a direct relationship with indices of general body obesity including WC, waist to hip ratio (WHR), and VAT with GDM [32].
In this study, according to the time period searched (1985–2020), a small number of studies were analyzed; in addition, a small number of anthropometric indices indicating the body composition were examined. Therefore, the present study was performed by reviewing the updated studies and all anthropometric indices expressed in the studies and using an accurate model in the early stages of pregnancy in a systematic review and meta-analysis to investigate the relationship between anthropometric indices expressing body composition and GDM.
Materials and methods
This study was approved by Alborz University of Medical Sciences (ethnical code: IR.ABZUMS.REC.1400.241). Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were observed in the report of the study. PRISMA contains 27 items related to the content of a systematic and meta-analysis, and includes abstracts, methods, results, discussions, and financial resource [33, 34]. Participant consent for this study is not applicable. This study was registered on PROSPERO website by "CRD42022302813" ID.
Search strategy
PubMed, Web of Science, Scopus, Google Scholar, and ProQuest were searched from 2010 to December 2021. MESH keywords and search strategy were as below:
’Gestational diabetes’ [tiab], OR ’GD’ [tiab], OR ’Gestational Diabetes Mellitus’ [tiab], OR ’GDM’ [tiab], OR ’pregnancy induced diabetes’[tiab]
’Anthropometric indicators’ [tiab], ’Anthropometric indices’ [tiab], OR ’body size’[tiab], OR ’body composition’ [tiab] OR, ’Waist/Hip Ratio’ [tiab], OR ’WHR’ [tiab], OR ’ visceral fat mass’ [tiab], OR ’VFM’ [tiab], OR ’ Neck circumference’ [tiab], OR ’hip circumference’ [tiab], OR ’ waist circumference’ [tiab], OR ’ subcutaneous adipose tissue’ [tiab], OR ’ skeletal muscle mass percentage’ [tiab], ’total adipose tissue thickness’ [tiab], OR ’subcutaneous adipose tissue’[tiab], OR ’Subcutaneous fat thickness’ [tiab], OR ’visceral adipose tissue depth’ [tiab], OR ’skinfold thickness’ [tiab], OR ’mid upper arm circumference’ [tiab], OR ’subcutaneous fat thickness’ [tiab], OR ’fat mass percentage’ [tiab], OR ’fat mass index’ [tiab], OR ’muscle mass percentage’ [tiab], OR ’Skinfold Thickness’ [tiab]
’Pregnancy’ [tiab], OR ’Pregnancies’ [tiab], OR ’Gestation’[tiab], OR ’early pregnancy’ [tiab]
#1 AND #2
#1 AND #2 AND #3
Eligibility criteria
Inclusion and exclusion criteria
We set our inclusion and exclusion criteria based on PICO criteria (population, intervention, comparison, outcome, and study design) (Table 1).
Table 1. PICO criteria.
| Selection criteria | Inclusion criteria | Exclusion criteria |
|---|---|---|
| Population | Healthy pregnant women with single fetus and at reproductive age group, GDM based on the diagnostic criteria, Gestational age considered for each study based on ultrasound, Studies were published until December 2021, Full-text available and with no language restrictions | Multiple pregnancies, women taking steroids, pre-pregnancy diabetes, maternal medical disorders such as liver, kidney, thyroid, fetal abnormalities, ovarian cysts, and maternal age less than 18 years |
| Exposure | Body composition (WHR, visceral adipose mass, NC, HCWC, subcutaneous adipose tissue (SAT), skeletal muscle mass percentage(SMMP), total adipose tissue thickness(TAT), VAT, skinfold thickness, mid upper arm circumference(MUAC), fat mass percentage(FMP), fat mass index(FMI), muscle mass percentage(MMP), skinfold thickness | Other body composition |
| Comparison | Healthy control group | GDM was combined with other maternal pregnancy complications (HDP, eclampsia, and pre-eclampsia); ethnicity, food habits, and separation were difficult. |
| Outcome | GDM according to different screening protocols | - |
| Study design | Cohort, case control, and cross sectional | Case study, case series, case report, lack of access to full text articles, review articles, letter to editor |
Study selection
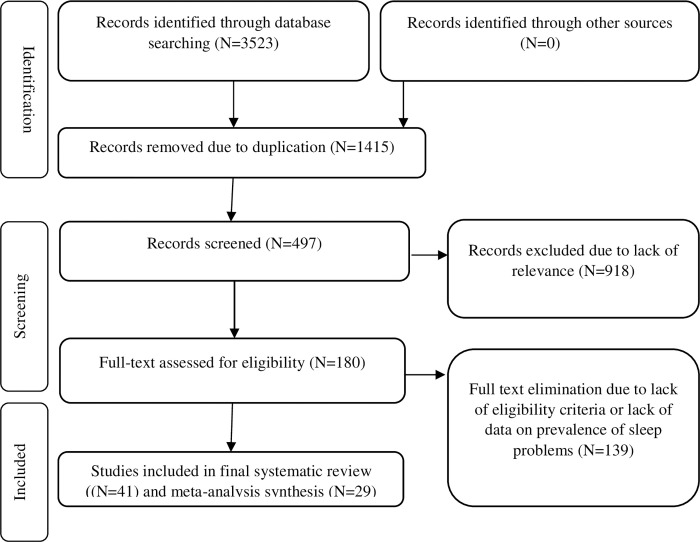
The initial search yielded 3523 results. The eligibility of these articles was independently evaluated by two authors, and disagreements were resolved by consensus. In the first stage, 2108 irrelevant or duplicate articles were excluded. After reviewing the titles and abstracts of the remaining articles, 918 more papers were excluded. In the evaluation of the full texts, 139 ineligible articles were excluded out of the remaining 180 articles. Finally, a total of 41 eligible articles were reviewed and 29 articles meets criteria to meta-analysis (Fig 1).
Fig 1. PRISMA flowchart of selected studies.
Quality assessment
Newcastle Ottawa scale (NOS) was used to measure the quality of studies. This scale is used to measure the quality of cohort and case control studies. The validity and reliability of this tool have been proven in various studies [35, 36].
Data extraction
Two authors independently performed the study selection and validity assessment and resolved any disagreements by consulting a third researcher. Author, year, study design, geographic region, maternal age, diagnostic criteria of GDM, anthropometric indices, accompanying factors, results, and quality assessment scores were extracted from articles.
Statistical analysis
All analyses were conducted with STATA 14.0 (College Station, Texas). For each study, mean value and standard deviation (SD) of anthropometric indices were extracted; if IQR was reported, we changed it to SD with IQR/1.35. Then, the mean difference (MD) of anthropometric indices was calculated between GDM and non-GDM group for each study. Then, standard error (SE) of MD was calculated for each study using the following formula:
Where, , n1, , and n2 are variance values, and samples size in GDM and control groups, respectively. Then, pooled MD was calculated by “Metan” command [37]. Heterogeneity was determined using Cochran’s Q test of heterogeneity, and the I 2 index was used to quantify heterogeneity. In accordance with Higgins classification approach, I 2 values above 0.7 were considered as having high heterogeneity. To estimate the pooled MD for anthropometric indices, the fixed-effect model was used; when heterogeneity was greater than 0.7, the random effects model was used. The meta-regression analysis was used to examine the effect of publication year, age, sample size, and study design as factors affecting heterogeneity among studies. The “meta bias” command [38] was used to check for publication bias, and if there was any publication bias, the pooled MD was adjusted with the “meta trim” command using the trim-and-fill method [39]. In all analyses, significance level was considered 0.05.
Results
Twenty-nine studies with a sample size of 56,438 met the meta-analysis inclusion criteria (Table 1). Fig 1 shows the flowchart of the study selection process. Anthropometric indices values for the groups with and without GDM of included studies are given in Table 5.
Table 5. Details of studies included in the systematic review.
| ID | References | Study design | Sample size | Geographic region | Age(year) | Diagnostic criteria of GDM | Anthropometric indices | applying Time | Accompanying factors | Results | QS |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Jitngamsujarit et al.(2021) [43] | Cross-sectional | 212 | Thailand | 27.1 ± 6.7 | WHO | WC≥82: (OR 7.85, 95%CI 1.80–34.32 | <18 | • maternal age • history of diabetes in family • history of giving birth to a fetal anomaly • History of giving birth to an infant ≥ 4,000 gm |
Significant | 8 |
| 2 | Saif Elnasr et al.2021 [44] | Cohort | 83 | Egypt | 26.8 | ADA | VAT: 5.85 ± 0.47 cm SAT:1.80±0.57 cm |
11–14 | BMI | VAT depth ranged from 1.4 to 9.1 cm, with a mean of 3.9 ± 1.6 cm is associated with GDM. | 8 |
| 3 | Cremona et al.2021 [45] | Cohort | 187 | Ireland | 18–50 | IADPSG | • abdominal SAT:1.99 (1.64–2.31) mm • abdominal VAT:1.41 (1.11–1.65) mm • FMP: 45.6 (39.2–49.0) • MUAC:32.9 (30.1–36.4) cm • WC = 90.3 (85.9–96.2) cm • HC: 108.6 (99.9–111.6) cm total SFT:226.4 (184.1–244.7) mm |
10–16 | • BMI • Parity >3 • Family Hx diabetes Age >40 • Smoking • High risk ethnicity • Previous perinatal death • Glucosuria • Previous baby ≥4.0 kg • Previous macrosomia (≥4.5 kg) |
Significant for VAT, SAT, WC, HC and total SFT | 7 |
| 4 | Barforoush et al.2021 [46] | cohort | 372 | Iran | 28.1 ±4.4 | ADA | NC: 35.1 ±2.7 cm | 14–16 | Age Gravidity Family -history of type 2 diabetes Pre-pregnancy weight Height |
NC ≥34.3 cm can be deemed as a predictor of GDM | 8 |
| 5 | Aydin et al.2021 [41] | Cohort | 142 | Turkey | 31.24±5.11 | IADPSG | • Intraperitoneal fat thickness:51.59 ± 22.49 mm • SAT: 19.79 ± 12.52 mm • WC:95.25±15 cm HC:115.38±15.41 cm WHR: 0.82±0.06 cm Perirenal fat thickness: 11.77±8.79 mm, SFTmax: 19.79±12.52 mm |
11–14 | • Pre-pregnancy BMI • BMI • smoking • history of DM in the first degree relatives • GDM during previous pregnancy |
Significant for all except Perirenal fat thickness | 7 |
| 6 | Zhang et al.2020 [47] | Cohort | 22,223 | China | 28.09 ± 4.48 | IADPSG | FM: 17.95 ± 5.65 kg, 1.085 (1.079–1.091) FFM: 40.56 ± 4.92 kg, 1.080 (1.100–1.115) Fat mass percentage: 30.09 ± 5.69%, 1.057 (1.052–1.063) MM:21.87 ± 2.96 kg, 1.114 (1.106–1.121) VF level:8.48 ± 0.56, 2.604 (2.459–2.758) Lean trunk mass: 18.32 ± 2.47 kg, 1.226 (1.209–1.243) |
<17 | • BMI • Total body water • Proteins • Bone minerals • Basal metabolic rate |
Significant | 7 |
| 7 | Rocha et al.2020 [48] | Cohort | 133 | Brazil | 26±6.2 | IADSPG | VAT: 55.4 ±11.4 mm | ≤20 | BMI | Significant | 9 |
| 8 | Alves et al.2020 [28] | cohort | 518 | Brazil | 26.25±5.8 | IADPSG | VAT: 5.44 ±1.27mm | 14 | • age • Pre-pregnancy BMI |
significant | 8 |
| 9 | Hancerliogullari et al.2020 [29] | cohort | 525 | Turkey | 27 (18–44) | Carpenter and Coustan | NC:37.14 ± 3.34 cm WC: 91.78 ± 11.41 cm |
11–14 | • Age • Parity • BMI |
Significant | 8 |
| 10 | Liu et al.2020 [30] | cohort | 1318 | China | 32.6±5.1 | IADPSG | FMI: 7.14±2.26 SMMP: 40.0±8.3 FMP: 30.1±5.8 |
13 | • Age • pre-pregnancy BMI • Pre-pregnancy weight |
Significant | 8 |
| 11 | Thaware et al.2019 [49] | Cohort | 80 | UK | 18–40 | IADPSG /WHO | VAT: 4.36±1.31 cm SAT: 2.24±1.01 cm |
9–18 | • Early pregnancy BMI ≥30 kg/m2 • Family history of diabetes in first-degree relative |
Significant for VAT of ≥ 4.27 cm (p = 0.03) | 8 |
| 12 | Takmaz et al.2019 [50] | cohort | 261 | Turkey | 30.57±5.78 | IADPSG | WC: 103.91±14.13 cm 8.36(0.74–0.84) |
20–24 | • Age • Parity • Weight gain • PPBMI • BMI |
Significant | 7 |
| 13 | Budak et al.2019 [42] | Case control | 100 | Turkey | 33.5 (27–37) | Carpenter and Coustan | SFT: 21.1 (16.6–26.4)**mm | 24–28 | • Age • Parity • Weight gain |
Significant | 9 |
| 14 | Kawanabe et al.2019 [51] | Cohort | 96 | Japan | 34.4 ± 4.8 | IADPSG | ASM: 17.0 ± 2.1 kg FM: 18.8 ± 8.2 kg ASM/FM ratio: 1.02 ± 0.34 |
16–30 | • ISI • Age • HbA1c • pre-pregnancy BMI • Family history of diabetes |
Significant | 8 |
| 15 | Marshall et al.2019 [52] | cohort | 1,775,984 | California | 18–40 | ICD-9 | MH: 1.68 (1.58–1.66) m | nine months prior to birth | • Age • BMI |
Taller women were less likely to have GDM 0.81 (0.80, 0.82)*. | 8 |
| 16 | Ulubasoglu et al.2019 [53] | cohort | 148 | Turkey | 28.4±3.8 | ADA | WC = 87.7 ±13.6 cm | 11–14 | • Total triglycerides • BMI |
Significant | 8 |
| 17 | Wang et al.2019 [54] | Case-control | 2698 | China | 30.95± 4.01 | IADPSG | • FFMP: 68.45±4.81 • FMP: 31.55±4.81 FMI: 7.00±1.81 WHR: 0.86±0.04 MUAC: 27.64±2.30 cm FM/FFM ratio: 0.47 ±0.14 |
13–20 | • Age • PPBMI |
Significant | 7 |
| 18 | Zhu et al.2019 [31] | Cohort | 1750 | California | 18–45 | Carpenter and Coustan | WHR = 0.91 ±0.06 WC = 102.4 ±18.5 cm |
10–13 | • Smoking • Family history of diabetes • Previous GDM • Preexisting hypertension • Physical inactivity in early pregnancy |
Significant | 7 |
| 19 | Nombo et al.2018 [55] | Cross sectional | 609 | Tanzania | 27.5 ± 5.0 | WHO | MUAC = 27.3± 3.8 cm | 20–38 | • Previous stillbirth • Family history of type 2 diabetes • Diet habits |
Significant | 9 |
| 20 | Anafcheh et al.2018 [56] | Case control | 195 | Iran | 32.35± 0.68 | WHO | H = 159.72±6.72 | <24–28 | • Blood group • GWG • Age • History of stillbirth • History of GDM • History of type 2 diabetes in first-degree relatives • Birth -History of a baby weighing≥ 4 kg • History of a birth with a congenital anomaly • History of PCO |
NS | 7 |
| 21 | Balani et al.2018 [57] | cohort | 302 | UK | 31 | WHO |
PBF VFM<210 WHR |
15 | Age BMI • History of PCOs • Family history of diabetes, • History of hypertension and Previous macrosomia |
Significant | 7 |
| 22 | Bourdages et al.2018 [58] | cohort | 1048 | Canada | 28.9 ± 4.1 | IADPSG | • SAT: 0.66 (0.59–0.73) • TAT:0.68 (0.61–0.76) • VAT: 0.65 (0.58–0.73)*** |
11–14 | • Age≥35 • BMI≥31.6 |
Significant | 8 |
| 23 | Kansu-Celik et al.2018 [40] | Cross sectional | 223 | Turkey | 27.46± 5.9 | Carpenter and Coustan | • SAT: 19 (11–28) mm • WC: 95 (72–111) cm • WHR: 0.89 ± 0.59 |
24–28 | • BMI |
Significant | 9 |
| 24 | KhushBakht et al. 2018 [59] | Cross sectional | 90 | Pakistan | 30.8 ± 3.2 | ADA | • NC: 36.1 ± 2.8 cm • H: 1.61 ± 0.03 m • WC: 104.2 ± 9.0 cm |
16 | • BMI • Fasting lipid profile • Serum albumin • Uric acid • Age Gravidity |
cut-off value of neck circumference for predicting GDM was 35.70 cm with a sensitivity of 51.4% and specificity of 81.2%. |
9 |
| 25 | Nassr et al.2018 [60] | cohort | 389 | USA | 29.7±4.67 | ACOG | Pre-peritoneal fat: 12 (9–16)**** mm SFT: 11 (8–14) mm BFI: 0.78 (0.42 - 1.26) |
18–24 | • Age>30 • Parity • History of GDM • History of bariatric surgery • Current gestational hypertension or preeclampsia |
Significant | 8 |
| 26 | D’Ambrosi et al.2017 [61] | Case control | 168 | Italy | 34.5±5.1 | IADPSG | SAT: 107±4.8 mm VAT: 10.1±3.0 mm |
24–28 | • Age • BMI • Family history of diabetes |
Significant | 8 |
| 27 | Han et al.2017 [62] | Cohort | 17803 | China | 28.5±2.8 | IADPSG | WC: 82.8±9.7 cm | 4–12 | • BP • BMI |
Significant | 7 |
| 28 | He et al. 2017 [63] | Case control | 255 | China | 29.1 ±3.7 | ADA | NC: 35.20 ±2.56 cm WC: 103.16±8.00 cm |
16 | • Age • Gravidity • HbA1c • Lipid profile • BMI |
Significant | 7 |
| 29 | Li et al.2017 [64] | cohort | 371 | china | 31.0±3.0 | IADPSG | NC: 34.3±1.5 cm | 11–13 | • Age • PPBMI • Lipid profile |
Significant | 7 |
| 30 | Yang et al.2017 [65] | cohort | 333 | Korea | 32±3.9 | National Diabetes Data Group | SFT:2.7±0.6 cm |
10–13 | • Age • PPBMI • GWG |
Significant | 7 |
| 31 | Alptekin et al.2016 [66] |
Cohort | 227 | Turkey | 28.8 ± 4.8 | Carpenter and Coustan | WC: 89.7 ± 11.9 cm HC: 105.8 ± 14.2 cm WHR: 0.84 ± 0.04 |
7–12 | • HOMA-IR • BMI WGDP |
Significant | 8 |
| 32 | Basraon et al.2016 [67] | Cohort | 2300 | USA | 23.3±4.9 | Guidelines of each clinical center | WHR: 0.88 ± 0.07 |
9–16 | • IR • BMI • Ethnicity |
Significant | 8 |
| 33 | White et al.2016 [68] | Cohort | 1303 | UK | 32.0 ±4.9 | IADPSG | • NC: 37.4 ±2.5 cm • WC: 110 (103–116) cm • MUAC:37 (35–40) cm • HC: 123 (116–130) cm • WHR: 0.89 ±0.07 |
15–18 | • Age • BP • Ethnicity • Parity • IR • Previous GDM • HgbA1C -Adiponectin • Sex hormone binding globulin • Triglycerides • PCOs • Smoking |
Significant | 8 |
| 34 | De Souza et al.2015 [69] | Cohort | 485 | Canada | 32.9 ±4.8 | IADPSG | • SAT: 1.9± 0.80 cm • VAT: 4.1±1.7 cm • TAT: 5.9±2.1 cm |
11–14 | • AgeSi • BMI |
Significant for TAT & VAT | |
| 35 | Kennedy et al.2015 [70] | Cohort | 1350 | Canada | 29.3 ± 5.1 | NR | • SAT1: 21.2 mm (6.9– • 73.9) SAT2: 20.3 mm (7.5–68.0) |
11–14 (SAT1) 18–22 (SAT2) |
• BMI | Significant | 7 |
| 36 | Sina et al.2015 [71] | Case control | 131 | Australia | 23.7 ±5.5 | ICD-9 and ICD -10 | ▪ WC:90.3 ±16.4 cm ▪ HC: 98.3 ±16.3 cm ▪ WHR: 0.92 ±0.05 |
- | • BMI | Significant for WC and HC | 9 |
| 37 | Balani et al.2014 [72] | Case control | 302 | UK | 32.1±5.5 | WHO | ▪ WHR: 1.02±0.07 ▪ TPBF: 49.8±3.5 ▪ VAT: 199.2±40.5 |
14–17 | • BMI | Significant for BMI, WHR, VFM | 7 |
| 38 | Bolognani et al.2014 [73] | Cross sectional | 240 | Brazil | 17–40 | WHO | WC: 93.548±8.873 cm | 20–24 | • PPBMI • BMI • GWG |
Significant | 8 |
| 39 | Gur et al. 2014 [74] | Cohort | 94 | Turkey | 43.4 | WHO | WC:65.3 cm minimum subcutaneous fat (Smin): 66.7 mm maximum pre-peritoneal visceral fat (Vmax):67.2 mm |
4–14 | • BMI • FBG • Metabolic • syndrome • Lipid profile • BP • HOMA-IR Smoking |
Significant | 8 |
| 40 | Mameghani et al.2013 [75] | Cohort | 1140 | Iran | 17–40 | WHO | WC: 81.84 ± 0.35 cm | <12 | • BMI | Significant | 8 |
| 41 | Suresh et al.2012 [76] | Cohort | 1200 | Australia | 17–45 | The Royal Australian and New Zealand College of Obstetricians and Gynaecologists. C-Obs guideline | -SAT: 18.2 mm (range 6.3–50.9 mm) | 18–22 | • BMI | Significant | 8 |
ICD9: International Classification of Diseases, 9th Revision-Clinical Modification, H: height, WGDP: weight gained during pregnancy, HOMA-IR: homeostasis model assessment insulin resistance, WHR: Waist/Hip Ratio, QUICKI: quantitative insulin sensitivity check index, VAD: Visceral Adipose Tissue Depth, BMI: Body Mass Index, VFM: visceral fat mass, PBF: percentage body fat, IR: insulin resistance, WC: waist circumference, SAT: subcutaneous tissues thickness, TAT: total adipose tissues thickness, VAT: visceral tissues thickness, ASFT: abdominal subcutaneous fat thickness, FBG: fasting blood glucose, NC: Neck circumference, ISI: insulin sensitivity index, ASM: appendicular skeletal muscle mass, FM: fat mass, HbA1c: glycosylated hemoglobin A1c,SFT: subcutaneous fat thickness, IADPSG: International Association of Diabetes and Pregnancy Study Groups, FMP: fat mass percentage, SMMP: skeletal muscle mass percentage, FMI: Fat mass index, BFI: Body Fat Index = (pre-peritoneal fat x subcutaneous fat/height), FFM: fat free mass, MM: muscular mass, PP: Pre pregnancy, PPBMI: Pre pregnancy BMI, ADA: American Diabetes Association, WHO: World health Organization, ACOG: American College of Obstetricians and Gynecologists, AC: arm circumference, NS: Not Significant
*: OR
**: median (IQR)
***: AUC (CI)
****: median (max-min)
Pooled MD of anthropometric indices
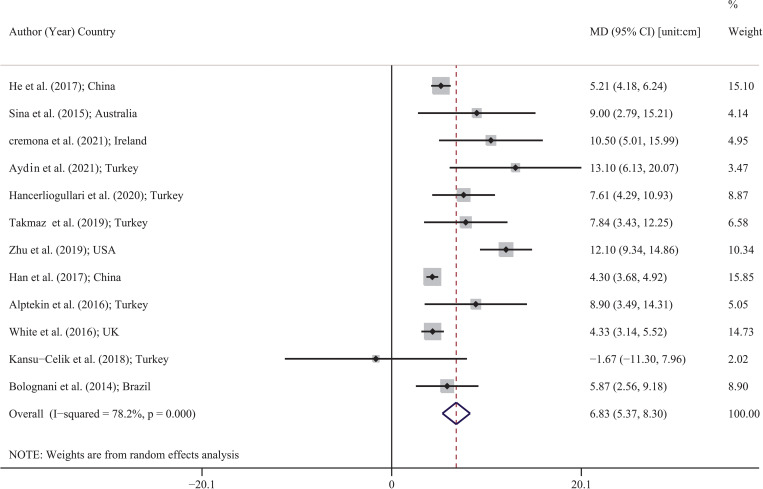
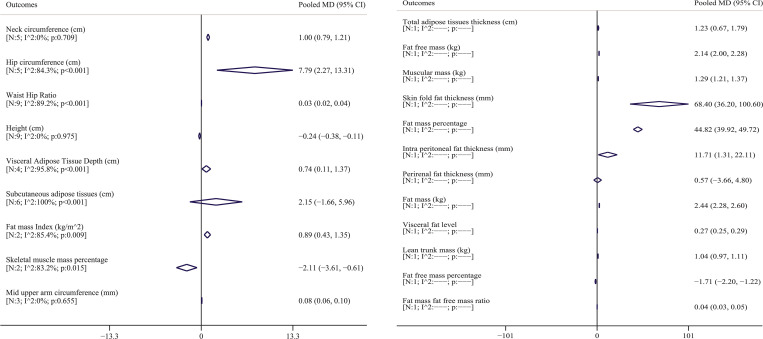
Table 2 shows the pooled MD of all anthropometric indices. As shown in Table 2, twelve studies were carried out for waist circumference, five studies for neck and hip circumference, nine studies for waist hip ratio and height, six studies for subcutaneous adipose tissues, four studies for visceral adipose tissue depth, three studies for mid upper arm circumference, two studies for fat mass index and skeletal muscle mass percentage, and one study for other indices. Fig 2 shows the pooled MD of waist circumference for included studies. The lowest and highest MDs were reported by Kansu-Celik et al. [40] in Turkey (MD: -1.67; 95% CI: -11.30 to 7.96) and Aydin et al. [41] in Turkey (MD: 13.10; 95% CI: 6.13 to 20.07). Based on random effects model, the pooled MD for waist circumference was 6.83 cm (95% CI: 5.37 to 8.30). In other words, the mean values of waist circumference in people with GDM were higher than that in non-GDM people. Forest plot of other anthropometric indices was provided in supplements 1 to 21, and pooled MD is shown in Table 2 and Fig 3. Pooled MD of neck circumference, hip circumference, waist hip ratio, and visceral adipose tissue depth was 1.00 cm (95% CI: 0.79 to 1.20) [N = 5; I^2: 0%; p: 0.709]; 7.79 cm (95% CI: 2.27 to 13.31) [N = 5; I^2: 84.3%; P<0.001]; 0.03 (95% CI: 0.02 to 0.04) [N = 9; I^2: 89.2%; p<0.001] and 7.74 cm (95% CI: 0.11 to 1.36) [N = 4; I^2: 95.8%; P<0.001], respectively, which indicates that the average of these indices was higher in the GDM group. An adverse pattern was observed for the height and skeletal muscle mass percentage, which pooled MD for the height, and skeletal muscle mass percentage was -0.24 cm (95% CI: -0.37 to -0.10) [N = 9; I^2: 0%; p:0.975]; and -2.11 (95% CI: -3.61 to -0.61) [N = 2; I^2: 83.2%; p:0.015], respectively, which indicates that the average of these indices was higher in the non-GDM group. In other words, in general, people with non-GDM had a mean height and skeletal muscle mass percentage higher than GDM people. Although pooled MD was higher for subcutaneous adipose tissues in the GDM group, this difference was not significant (2.15 [95% CI: -1.66 to 5.96]). The pooled MD of other indices are given in Table 2 and Fig 3.
Table 2. Pooled MD (95% confidence interval) and heterogeneity of anthropometric indices.
| Outcomes | Heterogeneity index | Number of studies | Pooled MD (95% CI) # |
|---|---|---|---|
| Waist circumference (cm) | I^2: 78.2%; p<0.001 | 12 | 6.83 (5.37 to 8.30) * |
| Neck circumference (cm) | I^2: 0%; p: 0.709 | 5 | 1.00 (0.79 to 1.20) * |
| Hip circumference (cm) | I^2: 84.3%; p<0.001 | 5 | 7.79 (2.27 to 13.31) * |
| Waist Hip Ratio | I^2: 89.2%; p<0.001 | 9 | 0.03 (0.02 to 0.04) * |
| Height (cm) | I^2: 0%; p: 0.975 | 9 | -0.24 (-0.37 to -0.10) * |
| Visceral Adipose Tissue Depth (cm) | I^2: 95.8%; p<0.001 | 4 | 0.74 (0.11 to 1.36) * |
| Fat mass percentage | I^2: ---; p: --- | 1 | 44.82 (39.92 to 49.72) * |
| Subcutaneous adipose tissues (cm) | I^2: 100%; p<0.001 | 6 | 2.15 (-1.66 to 5.96) |
| Total adipose tissues thickness (cm) | I^2: ---; p: --- | 1 | 1.23 (0.67 to 1.79) * |
| Fat mass Index (kg/m^2) | I^2: 85.4%; p: 0.009 | 2 | 0.89 (0.43 to 1.35) * |
| Skeletal muscle mass percentage | I^2: 83.2%; p: 0.015 | 2 | -2.11 (-3.61 to -0.61) * |
| Fat free mass (42) | I^2: ---; p: --- | 1 | 2.14 (2.00 to 2.28) * |
| Muscular mass [42] | I^2: ---; p: --- | 1 | 1.29 (1.21 to 1.37) * |
| Skin fold fat thickness (mm) | I^2: ---; p: --- | 1 | 68.40 (36.20 to 100.6) * |
| Mid upper arm circumference (mm) | I^2: 0%; p: 0.655 | 3 | 0.08 (0.06 to 0.10) * |
| Intra peritoneal fat thickness (mm) | I^2: ---; p: --- | 1 | 11.71 (1.31 to 22.11) * |
| Perirenal fat thickness (mm) | I^2: ---; p: --- | 1 | 0.57 (-3.66 to 4.80) |
| Fat mass [42] | I^2: ---; p: --- | 1 | 2.44 (2.28 to 2.60) * |
| Visceral fat level | I^2: ---; p: --- | 1 | 0.27 (0.25 to 0.29) * |
| Lean trunk mass [42] | I^2: ---; p: --- | 1 | 1.04 (0.97 to 1.11) * |
| Fat free mass percentage | I^2: ---; p: --- | 1 | -1.71 (-2.20 to -1.22) * |
| Fat mass fat free mass ratio | I^2: ---; p: --- | 1 | 0.04 (0.03 to 0.05) * |
CI: Confidence Interval
*: significant
# Positive pooled MD means the index was higher in GDM compared to non-GDM, and negative pooled MD means the index was lower in GDM compared to non-GDM.
Fig 2. Forest plot for MD of waist circumference (cm) between GMD and non-GDM group based on a random effects model.
Each study is distinguished by its author (year) and countries. Each line segment’s midpoint shows the MD estimate; the length of line segment indicates 95% confidence interval (CI) in each study, and the diamond mark illustrates the pooled estimate of MD.
Fig 3. Pooled MD and 95% confidence interval of anthropometric index.
The diamond mark illustrates the pooled MD, and the length of the diamond indicates 95% CI.
Heterogeneity and meta-regression results
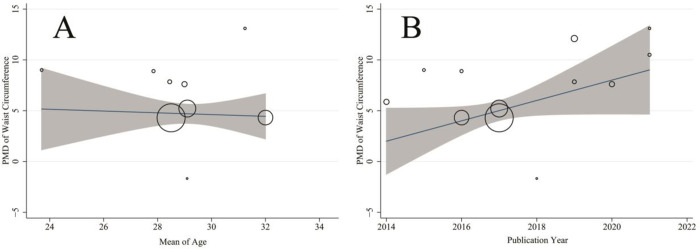
Table 2 shows significant heterogeneity between different studies for waist circumference, hip circumference, waist/hip ratio, visceral adipose tissue depth, subcutaneous adipose tissue (Cochran’s Q test P-value < 0.001 for all lipid profiles) so that the I2 index was above 70% for all mentioned indices. Table 3 shows the meta-regression results to investigate the effect of publication year, age, sample size, and study design on heterogeneity between studies. Accordingly, none of the variables had a significant role on heterogeneity between studies (P>0.05 for all). Fig 4 shows the result of meta-regression for association between pooled MD of waist circumference with age (A) and publication year (B).
Table 3. Results of the univariate meta-regression analysis on the heterogeneity of the determinant.
| variables | Publication Year (year) | Age | Sample size | Study Design* | ||||
|---|---|---|---|---|---|---|---|---|
| Coefficient 95% CI | p-value | Coefficient 95% CI | P-value | Coefficient 95% CI | P-value | Coefficient 95% CI | P-value | |
| Waist Circumference | 0.81 (-0.11 to 1.75) | 0.078 | -0.36 (-1.48 to 0.77) | 0.480 | 0.01 (-0.01 to 0.01) | 0.656 | -0.88 (-4.98 to 3.23) | 0.643 |
| Hip Circumference | 1.60 (-0.66 to 3.86) | 0.109 | -0.29 (-3.62 to 3.04) | 0.743 | 0.01 (-0.02 to 0.01) | 0.071 | 0.94 (-21.17 to 23.06) | 0.900 |
| Waist/Hip Ratio | -0.01 (-0.01 to 0.01) | 0.979 | -0.01 (-0.01 to 0.01) | 0.067 | 0.01 (-0.01 to 0.01) | 0.705 | 0.01 (-0.01 to 0.03) | 0.280 |
| Visceral Adipose Tissue Depth | 0.22 (-0.43 to 0.88) | 0.276 | -0.13 (-0.34 to 0.08) | 0.081 | 0.01 (-0.01 to 0.01) | 0.633 | 0.92 (-0.57 to 2.41) | 0.116 |
| Subcutaneous adipose tissue | -1.02 (-3.48 to 1.44) | 0.313 | 1.59 (-0.89 to 4.08) | 0.134 | -0.01 (-0.07 to 0.06) | 0.846 | -2.69 (-8.49 to 3.12) | 0.268 |
CI: Confidence Interval
*: Significant
Coding for study design: 1 = case control; 2 = cohort; 3 = cross-sectional
Fig 4.
Association between pooled mean difference (MD) of waist circumference with age (A) and publication year (B) by means of meta regression. The size of circles indicates the precision of each study. There is no significant association with respect to the pooled MD of waist circumference with age publication year.
Table 4 shows the publication bias results based on the Egger’s test and the fill and trim method. There was a significant publication bias for waist circumference (coefficient: 1.95; P: 0.019) and hip circumference (coefficient: 3.06; P: 0.028). According to the fill and trim method, the value of adjusted pooled MD for waist circumference and hip circumference was 5.35 (95% CI: 3.81–6.88) and 7.80 (95% CI: 2.76–13.31), which was not significantly different from the pooled MD calculated for waist circumference (6.83 [95% CI: 5.37–8.30]) and hip circumference (7.79 [95% CI: 2.27–13.31]). In other words, the publication bias had no cosiderable effect on the result of meta analysis. No publication bias was observed for other anthropometric indices including neck circumference, waist/hip ratio, height, visceral adipose tissue depth, and subcutaneous adipose tissue. Details of the studies are listed in Table 5.
Table 4. Result of publication bias for anthropometric indices and fill and trim method result of adjusting publication bias.
| Variables | Publication bias | Trim and fill | ||
|---|---|---|---|---|
| Coefficient 95% CI | p-value | Coefficient 95% CI | p-value | |
| Waist Circumference | 1.95 (2.57 to 5.09) | 0.019* | 5.35 (3.81 to 6.88) | <0.001 |
| Neck Circumference | 0.26 (-2.59 to 3.12) | 0.788 | --- | |
| Hip Circumference | 3.06 (0.64 to 5.49) | 0.028* | 7.80 (2.76 to 13.31) | <0.001 |
| Waist/Hip Ratio | 2.83 (-0.48 to 6.15) | 0.083 | --- | |
| Height | 0.11 (-0.39 to 0.62) | 0.608 | --- | |
| Visceral Adipose Tissue Depth | 6.75 (-0.41 to 13.91) | 0.056 | --- | |
| Subcutaneous adipose tissue | -1.94 (-136.42 to 132.55) | 0.970 | --- | |
CI: Confidence Interval
*: Significant
Discussion
The current study set to investigate the relationship between body composition and GDM as a systematic review and meta-analysis. The results indicate that anthropometric indices such as WC, NC, HC, WHR, VAT, SAT, Height, and MUAC are associated with GDM; an increase in the indices of WC, NC, HC, WHR, VAT, SAT, and MUAC increase developing GDM, also short stature increases the susceptibility to GDM.
We investigated that VAT and SAT are associated with GDM. Alwash et al.(2021) found that all three obesity phenotypes were significantly associated with the risk of developing GDM. In addition, visceral obesity was a stronger risk factor for GDM than other obesity phenotypes [32]. Yao et al.(2020) also stated that the risk of GDM is associated with maternal central obesity in early pregnancy [77]. In the case of central and visceral body fats, Benevides et al.(2020) reported that the cut-off point for subcutaneous, visceral, and total abdominal fat to predict GDM varied between studies in the first and second trimesters of pregnancy. No study confirmed a model for predicting GDM using subcutaneous and visceral fat measurements [78].
De Souza et al.(2015) determined the relationship between SAT depth, TAT depth, and VAT depth in the first trimester of pregnancy and the occurrence of GDM in mid-pregnancy. It was observed that increasing the depth of VAT and TAT independently of BMI could predict the risk of dysglycemia in later stages of pregnancy [69]. Similarly, Balani et al. (2018) showed that visceral adipose mass in obese women can be a predictor of GDM [57]. Increased VAT depth, but not SAT depth, was associated with an increased risk of GDM after adjusting for confounding factors. VAT depth ≥ 4.27 cm is more sensitive compared to the National Institute of Health and Care Excellence criteria and similar feature for the diagnosis of GDM [79]. In addition, Alves et al.(2020) observed an increase in VAT depth in sonographic measurements in early pregnancy; GDM was associated with a higher risk [28]. One of the strengths of the present study is the assessment of most indices of body composition and their relationship with GDM and the large number of up-to-date studies that lead to the investigation of more samples.
The results of the present study also showed WC, HC and WHR are associated with GDM. Various studies have shown an association between WC and WHR-based central obesity around the hip with the occurrence of GDM [31]. However, the data are also contradictory; for example, Basraon et al.(2016) showed that WHR could not replace BMI as a risk factor in pregnancy for GDM [67]. But, Yao et al.(2020) in his subgroup analysis showed that higher levels of central maternal obesity in the first stage have a similar risk of GDM in the first and second trimesters of pregnancy [77]. However, Tornaghi et al.(1994) provided evidence of the superiority of maternal central obesity regarding mid-pregnancy (18–22 weeks) in identifying obesity-related complications in pregnancy. In other words, the factors expressing central obesity in the mother’s body can better predict the risk of GDM than BMI [80]. Central obesity is expressed as a risk factor for insulin resistance associated with deposition and abnormal fat function. WC as one of the indices of central obesity leads to an increased risk of GDM. Multivariate regression analysis with consideration of other risk factors showed that WC ≥ 80 cm could not predict the risk of GDM. However, Ebrahimi-Mameghani et al.(2013) concluded that WC≥88 cm is a significant predictor of GDM (OR: 3.77) [41, 75]. Han et al. (2018) also observed that the risk of GDM increases with WC≥78.5 cm increase [75]. WC at gestation weeks 20–24, pre-pregnancy BMI, and gestational BMI can predict the occurrence of GDM. WC 100 cm with 84% sensitivity and 70% specificity predicts GDM risk [50]. Although other studies have shown that at gestation weeks 20–24, WC: 85.5–88.5 cm was the optimal cut-off point for GDM prediction (Sens/Spec balance between 87.1/41.1% and 77.4/56.9%) [73].
Kansu-Celik et al.(2018) observed a significant relationship between 50g GCT and WC, and SAT thickness. He showed that SAT predicts thickness greater than 16.75 mm GDM with a sensitivity of 71.7% and a specificity of 87.6% [40]. In adults, WHR is independently associated with complications after relative weight adjustment, i.e. the use of relative weight and body shape at the same time provides a better estimate of the risk of disease than either alone [81]. In women with WHR<0.85, one or more risk factors increased the risk of GDM by 1.99 times, and in women with WHR≥0.85 but without fixed risk factors, the risk of GDM increased by 2.41 times, and in women with fixed risk factors, it increased by 6.22 times. Similar but weak results were observed for WC≥88 cm [31].
We have shown that increased NC also leads to GDM. Hancerliogullari et al.(2020) also stated that NC in women with GDM are significantly higher [29] and NC is assumed to be a better marker than WC for determining metabolic syndrome and its key features. It is also easy to measure and it is replicable [82, 83]. Barforoush et al.(2021) also stated that NC more than 34.3 cm in Iranian women could predict GDM [46].
In this study we reported that short stature increases the susceptibility to GDM. Height in adulthood is an indices of genetic, early and childhood factors and their interactions. Although the biological mechanism associated with adult height and GDM is unknown, several pathways have been suggested. For example, malnutrition of the fetus may lead to low birth weight, which is associated with shorter height in adulthood, and may also be associated with metabolic disorders in adulthood. Height has different variations in different populations [84, 85]. In an analysis of 135861 pregnant women, height was found to be inversely related to the occurrence of GDM. Of course, this relationship can also vary between different races [86].
Body composition in pregnancy has a dynamic process; for example, changes in weight gain and free body adipose mass during pregnancy are clearly observed [87].
Measuring maternal body composition during pregnancy is challenged by existing in-vivo measurement methods that cannot distinguish between maternal and fetal reserves [88] and look at the mother and fetus as a whole. In addition, some pregnancy-induced changes in body composition violate the assumptions that underlie many commonly available measurement methods and require special pregnancy modifications (which often vary at different gestational ages) [89].
The composition of the mother’s body changes during pregnancy to support optimal fetal growth. In the first few months of pregnancy, changes in the composition of the mother’s body indicate the readiness of the female body for fetal growth. Especially, the uterine and breast tissue that makes up the mother unit grows and the blood volume increases. In late pregnancy, more pronounced growth of the embryonic unit (including the fetus, amniotic fluid, and placenta) occurs along with the continued growth of maternal tissue and further increase in blood volume. At the time of delivery, the fetal unit accounts for approximately one-third of the total GWG [90].
Accordingly, central obesity is associated with more obesity-related complications [91]. In contrast, peripheral obesity has been suggested to eliminate or even protect against some of the risks associated with obesity [92]. CT, MRI, body densitometry, or WHR are better indices of central obesity than BMI but are impractical as screening tools in pregnancy. SAT measurement can be used as an alternative measure of central obesity [93] as it is associated with a wide range of cardiovascular and metabolic risk factors. SAT can be easily and accurately measured by ultrasound [94]. BMI can also be potentially useful as a direct and inexpensive method for assessing central fat distribution [95]. In adults, BMI can predict outcomes such as type-2 diabetes and hypertension[81]. Although a sufficient number of studies examining the relationship between BMI and GDM have been performed in the past [96, 97].
Conclusion
Body composition indices such as WC, HC, WHR, AC, VAT, SAT, and height can relate more effectively and accurately to GDM. These available anthropometric indices can be used as a tool to assess the occurrence of GDM in an accessible, cost-effective, and high-precision manner.
Limitation
One of the limitations of the study is the difference in the critical values of the criteria used to diagnose GDM, which may affect the decision on the absence or occurrence of GDM based on different indices. In addition, studies conducted in different populations and races, which is a determining factor in body composition and can affect both body composition and the occurrence of GDM, have not been considered in the present study. Also, the small number of studies performed on some anthropometric indices is another limitation of the study, which makes it difficult to draw conclusions about such indices.
Supporting information
(DOC)
(DOC)
Abbreviations
- GDM
gestational diabetes mellitus
- GWG
gestational weight gain
- ICD9
International Classification of Diseases, 9th Revision-Clinical Modification
- H
height
- WGDP
weight gained during pregnancy
- HOMA-IR
homeostasis model assessment insulin resistance
- WHR
Waist/Hip Ratio
- QUICKI
quantitative insulin sensitivity check index
- VAD
Visceral Adipose Tissue Depth
- BMI
Body Mass Index
- VFM
visceral fat mass
- PBF
percentage body fat
- IR
insulin resistance
- WC
waist circumference
- SAT
subcutaneous tissues thickness
- TAT
total adipose tissues thickness
- VAT
visceral tissues thickness
- ASFT
abdominal subcutaneous fat thickness
- FBG
fasting blood glucose
- NC
Neck circumference
- ISI
insulin sensitivity index
- ASM
appendicular skeletal muscle mass
- FM
fat mass
- HbA1c
glycosylated hemoglobin A1c
- SFT
subcutaneous fat thickness
- IADPSG
International Association of Diabetes and Pregnancy Study Groups
- FMP
fat mass percentage
- SMMP
skeletal muscle mass percentage
- FMI
Fat mass index
- BFI
Body Fat Index = (pre-peritoneal fat x subcutaneous fat/height)
- FFM
fat free mass
- MM
muscular mass
- PP
Pre pregnancy
- PPBMI
Pre pregnancy BMI
- ADA
American Diabetes Association
- WHO
World health Organization
- ACOG
American College of Obstetricians and Gynecologists
- AC
arm circumference
- MUAC
mid upper arm circumference
- NS
Not Significant
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This study was funded by Alborz University of Medical Sciences and the funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.McIntyre HD, Catalano P, Zhang C, Desoye G, Mathiesen ER, Damm P. Gestational diabetes mellitus. Nature reviews Disease primers. 2019;5(1):1–19. [DOI] [PubMed] [Google Scholar]
- 2.Afkhami M, Rashidi M. Gestational diabetes mellitus. Hormozgan Medical Journal. 2007;11(1):1–12. [Google Scholar]
- 3.Wang H, Li N, Chivese T, Werfalli M, Sun H, Yuen L, et al. IDF diabetes atlas: estimation of global and regional gestational diabetes mellitus prevalence for 2021 by International Association of Diabetes in Pregnancy Study Group’s Criteria. Diabetes research and clinical practice. 2022;183:109050. doi: 10.1016/j.diabres.2021.109050 [DOI] [PubMed] [Google Scholar]
- 4.Ali AD, Mehrass AA-KO, Al-Adhroey AH, Al-Shammakh AA, Amran AA. Prevalence and risk factors of gestational diabetes mellitus in Yemen. International journal of women’s health. 2016;8:35. doi: 10.2147/IJWH.S97502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bellamy L, Casas J-P, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. The Lancet. 2009;373(9677):1773–9. doi: 10.1016/S0140-6736(09)60731-5 [DOI] [PubMed] [Google Scholar]
- 6.Fadl H, Magnuson A, Östlund I, Montgomery S, Hanson U, Schwarcz E. Gestational diabetes mellitus and later cardiovascular disease: a S wedish population based case–control study. BJOG: An International Journal of Obstetrics & Gynaecology. 2014;121(12):1530–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bener A, Saleh NM, Al-Hamaq A. Prevalence of gestational diabetes and associated maternal and neonatal complications in a fast-developing community: global comparisons. International journal of women’s health. 2011;3:367. doi: 10.2147/IJWH.S26094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Domanski G, Lange AE, Ittermann T, Allenberg H, Spoo RA, Zygmunt M, et al. Evaluation of neonatal and maternal morbidity in mothers with gestational diabetes: a population-based study. BMC pregnancy and childbirth. 2018;18(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prakash GT, Das AK, Habeebullah S, Bhat V, Shamanna SB. Maternal and neonatal outcome in mothers with gestational diabetes mellitus. Indian journal of endocrinology and metabolism. 2017;21(6):854. doi: 10.4103/ijem.IJEM_66_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. New England journal of medicine. 2005;352(24):2477–86. doi: 10.1056/NEJMoa042973 [DOI] [PubMed] [Google Scholar]
- 11.Au CP, Raynes-Greenow CH, Turner RM, Carberry AE, Jeffery HE. Body composition is normal in term infants born to mothers with well-controlled gestational diabetes mellitus. Diabetes care. 2013;36(3):562–4. doi: 10.2337/dc12-1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diabetes IAo Panel PSGC. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes care. 2010;33(3):676–82. doi: 10.2337/dc09-1848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nallaperumal S, Bhavadharini B, Mahalakshmi MM, Maheswari K, Jalaja R, Moses A, et al. Comparison of the world health organization and the International association of diabetes and pregnancy study groups criteria in diagnosing gestational diabetes mellitus in South Indians. Indian Journal of Endocrinology and Metabolism. 2013;17(5):906. doi: 10.4103/2230-8210.117241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Behboudi-Gandevani S, Amiri M, Bidhendi Yarandi R, Ramezani Tehrani F. The impact of diagnostic criteria for gestational diabetes on its prevalence: a systematic review and meta-analysis. Diabetology & metabolic syndrome. 2019;11(1):1–18. doi: 10.1186/s13098-019-0406-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hunt KJ, Schuller KL. The increasing prevalence of diabetes in pregnancy. Obstetrics and gynecology clinics of North America. 2007;34(2):173–99. doi: 10.1016/j.ogc.2007.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang C, Ning Y. Effect of dietary and lifestyle factors on the risk of gestational diabetes: review of epidemiologic evidence. The American journal of clinical nutrition. 2011;94(suppl_6):1975S-9S. doi: 10.3945/ajcn.110.001032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sorbye L, Skjaerven R, Klungsoyr K, Morken N-H. Gestational diabetes mellitus and interpregnancy weight change: A population-based cohort study. PLoS medicine. 2017;14(8):e1002367. doi: 10.1371/journal.pmed.1002367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hao M, Lin L. Fasting plasma glucose and body mass index during the first trimester of pregnancy as predictors of gestational diabetes mellitus in a Chinese population. Endocrine journal. 2017:EJ16-0359. doi: 10.1507/endocrj.EJ16-0359 [DOI] [PubMed] [Google Scholar]
- 19.Farina A, Eklund E, Bernabini D, Paladino M, Righetti F, Monti G, et al. A first-trimester biomarker panel for predicting the development of gestational diabetes. Reproductive sciences. 2017;24(6):954–9. doi: 10.1177/1933719116675057 [DOI] [PubMed] [Google Scholar]
- 20.Tatsukawa Y, Misumi M, Kim YM, Yamada M, Ohishi W, Fujiwara S, et al. Body composition and development of diabetes: a 15-year follow-up study in a Japanese population. European journal of clinical nutrition. 2018;72(3):374–80. doi: 10.1038/s41430-017-0077-7 [DOI] [PubMed] [Google Scholar]
- 21.Shao J-t Qi J-q. The relationship between body adiposity index and pregnancy-induced hypertension in third-trimester pregnant women. Blood pressure monitoring. 2017;22(5):279–81. doi: 10.1097/MBP.0000000000000273 [DOI] [PubMed] [Google Scholar]
- 22.Staelens AS, Vonck S, Molenberghs G, Malbrain ML, Gyselaers W. Maternal body fluid composition in uncomplicated pregnancies and preeclampsia: a bioelectrical impedance analysis. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2016;204:69–73. doi: 10.1016/j.ejogrb.2016.07.502 [DOI] [PubMed] [Google Scholar]
- 23.Neeland IJ, Turer AT, Ayers CR, Powell-Wiley TM, Vega GL, Farzaneh-Far R, et al. Dysfunctional adiposity and the risk of prediabetes and type 2 diabetes in obese adults. Jama. 2012;308(11):1150–9. doi: 10.1001/2012.jama.11132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Freemantle N, Holmes Ja, Hockey A, Kumar S. How strong is the association between abdominal obesity and the incidence of type 2 diabetes? International journal of clinical practice. 2008;62(9):1391–6. doi: 10.1111/j.1742-1241.2008.01805.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Després J-P. Body fat distribution and risk of cardiovascular disease: an update. Circulation. 2012;126(10):1301–13. doi: 10.1161/CIRCULATIONAHA.111.067264 [DOI] [PubMed] [Google Scholar]
- 26.PEIRIS AN, STRUVE MF, MUELLER RA, LEE MB, KISSEBAH AH. Glucose metabolism in obesity: influence of body fat distribution. The Journal of Clinical Endocrinology & Metabolism. 1988;67(4):760–7. doi: 10.1210/jcem-67-4-760 [DOI] [PubMed] [Google Scholar]
- 27.Vidanalage CK, Senarth U, Silva K, Lekamge U, Liyanage I. Effects of initial body mass index on development of gestational diabetes in a rural Sri Lankan population: A case-control study. Diabetes & Metabolic Syndrome: Clinical Research & Reviews. 2016;10(2):S110–S3. [DOI] [PubMed] [Google Scholar]
- 28.Alves JG, Souza ASR, Figueiroa JN, de Araújo CAL, Guimarães A, Ray JG. Visceral adipose tissue depth in early pregnancy and gestational diabetes mellitus-a cohort study. Scientific reports. 2020;10(1):1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hancerliogullari N, Kansu-Celik H, Asli Oskovi-Kaplan Z, Kisa B, Engin-Ustun Y, Ozgu-Erdinc AS. Optimal maternal neck and waist circumference cutoff values for prediction of gestational diabetes mellitus at the first trimester in Turkish population; a prospective cohort study. Gynecological Endocrinology. 2020:1–4. doi: 10.1080/09513590.2020.1750003 [DOI] [PubMed] [Google Scholar]
- 30.Liu Y, Liu J, Gao Y, Zheng D, Pan W, Nie M, et al. The body composition in early pregnancy is associated with the risk of development of gestational diabetes mellitus late during the second trimester. Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy. 2020;13:2367. doi: 10.2147/DMSO.S245155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu Y, Hedderson MM, Quesenberry CP, Feng J, Ferrara A. Central Obesity Increases the Risk of Gestational Diabetes Partially Through Increasing Insulin Resistance. Obesity (Silver Spring, Md). 2019;27(1):152–60. doi: 10.1002/oby.22339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alwash SM, McIntyre HD, Mamun A. The association of general obesity, central obesity and visceral body fat with the risk of gestational diabetes mellitus: Evidence from a systematic review and meta-analysis. Obesity Research & Clinical Practice. 2021;15(5):425–30. [DOI] [PubMed] [Google Scholar]
- 33.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. International Journal of Surgery. 2021;88:105906. doi: 10.1016/j.ijsu.2021.105906 [DOI] [PubMed] [Google Scholar]
- 34.Abdi F, Roozbeh N. The effects of Humulus lupulus L.) hops) on menopausal vasomotor symptoms: a systematic review and meta-analysis. The Iranian Journal of Obstetrics, Gynecology and Infertility. 2016;19(26):9–17. [Google Scholar]
- 35.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. European journal of epidemiology. 2010;25(9):603–5. doi: 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 36.Sedgh G, Bearak J, Singh S, Bankole A, Popinchalk A, Ganatra B, et al. Abortion incidence between 1990 and 2014: global, regional, and subregional levels and trends. The Lancet. 2016;388(10041):258–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rahnemaei FA, Pakzad R, Amirian A, Pakzad I, Abdi F. Effect of gestational diabetes mellitus on lipid profile: A systematic review and meta-analysis. Open Medicine. 2022;17(1):70–86. doi: 10.1515/med-2021-0408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Soltani S, Tabibzadeh A, Zakeri A, Zakeri AM, Latifi T, Shabani M, et al. COVID-19 associated central nervous system manifestations, mental and neurological symptoms: a systematic review and meta-analysis. Reviews in the Neurosciences. 2021. doi: 10.1515/revneuro-2020-0108 [DOI] [PubMed] [Google Scholar]
- 39.Hallajzadeh J, Khoramdad M, Izadi N, Karamzad N, Almasi-Hashiani A, Ayubi E, et al. The association between metabolic syndrome and its components with systemic lupus erythematosus: a comprehensive systematic review and meta-analysis of observational studies. Lupus. 2018;27(6):899–912. doi: 10.1177/0961203317751047 [DOI] [PubMed] [Google Scholar]
- 40.Kansu-Celik H, Karakaya BK, Tasci Y, Hancerliogullari N, Yaman S, Ozel S, et al. Relationship maternal subcutaneous adipose tissue thickness and development of gestational diabetes mellitus. Interventional medicine & applied science. 2018;10(1):13–8. doi: 10.1556/1646.10.2018.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aydın GA, Özsoy HG, Akdur PÖ, Özgen G. The predictive value of first‐trimester anthropometric and ultrasonographic adipose tissue measurements in gestational diabetes mellitus. Journal of Obstetrics and Gynaecology Research. 2021. doi: 10.1111/jog.14887 [DOI] [PubMed] [Google Scholar]
- 42.Budak MS, Kahramanoglu I, Vitale SG, Akgol S, Dilek ME, Kartal S, et al. Maternal abdominal subcutaneous fat thickness as a simple predictor for gestational diabetes mellitus. Journal of perinatal medicine. 2019;47(6):605–10. doi: 10.1515/jpm-2018-0431 [DOI] [PubMed] [Google Scholar]
- 43.Jitngamsujarit S, Pittyanont S, Thamrongwuttikul C. Waist Circumference at 18 weeks of gestation as a Predictor for Gestational Diabetes Mellitus in Women with Normal Pre-pregnancy Body Mass Index. Thai Journal of Obstetrics and Gynaecology. 2021:177–87. [Google Scholar]
- 44.Saif Elnasr I, Ammar H. Ultrasound markers for prediction of gestational diabetes mellitus in early pregnancy in Egyptian women: observational study. The Journal of Maternal-Fetal & Neonatal Medicine. 2021;34(19):3120–6. doi: 10.1080/14767058.2019.1678132 [DOI] [PubMed] [Google Scholar]
- 45.Cremona A, O’Gorman CS, Ismail KI, Hayes K, Donnelly AE, Hamilton J, et al. A risk-prediction model using parameters of maternal body composition to identify gestational diabetes mellitus in early pregnancy. Clinical Nutrition ESPEN. 2021;45:312–21. doi: 10.1016/j.clnesp.2021.08.002 [DOI] [PubMed] [Google Scholar]
- 46.Barforoush TS, Ghadimi R, Pahlevan Z, Ahmadi N, Delavar MA. The relationship between neck circumference and gestational diabetes mellitus in Iranian women. Clinical Diabetes and Endocrinology. 2021;7(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang R-Y, Wang L, Zhou W, Zhong Q-M, Tong C, Zhang T, et al. Measuring maternal body composition by biomedical impedance can predict risk for gestational diabetes mellitus: a retrospective study among 22,223 women. The Journal of Maternal-Fetal & Neonatal Medicine. 2020:1–8. doi: 10.1080/14767058.2020.1797666 [DOI] [PubMed] [Google Scholar]
- 48.Rocha AdS Bernardi JR, Matos S Kretzer DC, Schöffel AC Goldani MZ, et al. Maternal visceral adipose tissue during the first half of pregnancy predicts gestational diabetes at the time of delivery–a cohort study. PLoS One. 2020;15(4):e0232155. doi: 10.1371/journal.pone.0232155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thaware PK, Patterson CC, Young IS, Casey C, McCance DR. Clinical utility of ultrasonography-measured visceral adipose tissue depth as a tool in early pregnancy screening for gestational diabetes: a proof-of-concept study. Diabetic medicine: a journal of the British Diabetic Association. 2019;36(7):898–901. doi: 10.1111/dme.13906 [DOI] [PubMed] [Google Scholar]
- 50.Takmaz T, Yalvaç ES, Özcan P, Çoban U, Karasu AFG, Ünsal M. The predictive value of weight gain and waist circumference for gestational diabetes mellitus. Turkish journal of obstetrics and gynecology. 2019;16(3):199. doi: 10.4274/tjod.galenos.2019.03266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kawanabe S, Nagai Y, Nakamura Y, Nishine A, Nakagawa T, Tanaka Y. Association of the muscle/fat mass ratio with insulin resistance in gestational diabetes mellitus. Endocrine journal. 2019;66(1):75–80. doi: 10.1507/endocrj.EJ18-0252 [DOI] [PubMed] [Google Scholar]
- 52.Marshall NE, Biel FM, Boone-Heinonen J, Dukhovny D, Caughey AB, Snowden JM. The Association between Maternal Height, Body Mass Index, and Perinatal Outcomes. American journal of perinatology. 2019;36(6):632–40. doi: 10.1055/s-0038-1673395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ulubaşoğlu H, Bakay K, Özdemir AZ, Güven D, Batıoglu S. Can we use waist circumference in the first trimester to screen for gestational diabetes? Journal of Experimental and Clinical Medicine. 2019;36(1):9–12. [Google Scholar]
- 54.Wang Y, Luo BR. The association of body composition with the risk of gestational diabetes mellitus in Chinese pregnant women: A case-control study. Medicine. 2019;98(42):e17576. doi: 10.1097/MD.0000000000017576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nombo AP, Mwanri AW, Brouwer-Brolsma EM, Ramaiya KL, Feskens EJ. Gestational diabetes mellitus risk score: a practical tool to predict gestational diabetes mellitus risk in Tanzania. Diabetes research and clinical practice. 2018;145:130–7. doi: 10.1016/j.diabres.2018.05.001 [DOI] [PubMed] [Google Scholar]
- 56.Anafcheh M, Ahmadzadeh B, Albookordi M, Najafian M. Effect of blood group, height, and weight gain during pregnancy on gestational diabetes mellitus. The Iranian Journal of Obstetrics, Gynecology and Infertility. 2018;21(4):34–42. [Google Scholar]
- 57.Balani J, Hyer SL, Shehata H, Mohareb F. Visceral fat mass as a novel risk factor for predicting gestational diabetes in obese pregnant women. Obstetric medicine. 2018;11(3):121–5. doi: 10.1177/1753495X17754149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bourdages M, Demers M-É, Dubé S, Gasse C, Girard M, Boutin A, et al. First-Trimester Abdominal Adipose Tissue Thickness to Predict Gestational Diabetes. Journal of Obstetrics and Gynaecology Canada. 2018;40(7):883–7. doi: 10.1016/j.jogc.2017.09.026 [DOI] [PubMed] [Google Scholar]
- 59.KhushBakht D, Mazhar S, Bhalli A, Rashid A, Khan K, Jahanzaib U. Correlation Between Neck Circumference and Gestational Diabetes Mellitus and Associated Risk Factors During Pregnancy. Cureus. 2018;10(5):e2699. doi: 10.7759/cureus.2699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nassr AA, Shazly SA, Trinidad MC, El-Nashar SA, Marroquin AM, Brost BC. Body fat index: A novel alternative to body mass index for prediction of gestational diabetes and hypertensive disorders in pregnancy. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2018;228:243–8. doi: 10.1016/j.ejogrb.2018.07.001 [DOI] [PubMed] [Google Scholar]
- 61.D’Ambrosi F, Crovetto F, Colosi E, Fabietti I, Carbone F, Tassis B, et al. Maternal subcutaneous and visceral adipose ultrasound thickness in women with gestational diabetes mellitus at 24–28 weeks’ gestation. Fetal diagnosis and therapy. 2018;43(2):143–7. doi: 10.1159/000475988 [DOI] [PubMed] [Google Scholar]
- 62.Han Q, Shao P, Leng J, Zhang C, Li W, Liu G, et al. Interactions between general and central obesity in predicting gestational diabetes mellitus in Chinese pregnant women: A prospective population‐based study in Tianjin, China: 单纯性肥胖和中心性肥胖在预测中国孕妇妊娠糖尿病中的交互作用: 一个中国天津前瞻性人群队列研究. Journal of diabetes. 2018;10(1):59–67. doi: 10.1111/1753-0407.12558 [DOI] [PubMed] [Google Scholar]
- 63.He F, He H, Liu WQ, Lin JY, Chen BJ, Lin YC, et al. Neck circumference might predict gestational diabetes mellitus in Han Chinese women: A nested case-control study. Journal of Diabetes Investigation. 2017;8(2):168–73. doi: 10.1111/jdi.12574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li P, Lin S, Cui JH, Li L, Zhou SS, Fan JH. First-trimester neck circumference as a predictor of the development of gestational diabetes mellitus. Diabetes-Metabolism Research and Reviews. 2017;33. doi: 10.1016/j.amjms.2017.09.012 [DOI] [PubMed] [Google Scholar]
- 65.Yang SH, Kim C, An HS, An H, Lee JS. Prediction of Gestational Diabetes Mellitus in Pregnant Korean Women Based on Abdominal Subcutaneous Fat Thickness as Measured by Ultrasonography. Diabetes & metabolism journal. 2017;41(6):486–91. doi: 10.4093/dmj.2017.41.6.486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Alptekin H, Cizmecioglu A, Isik H, Cengiz T, Yildiz M, Iyisoy MS. Predicting gestational diabetes mellitus during the first trimester using anthropometric measurements and HOMA-IR. Journal of endocrinological investigation. 2016;39(5):577–83. doi: 10.1007/s40618-015-0427-z [DOI] [PubMed] [Google Scholar]
- 67.Basraon SK, Mele L, Myatt L, Roberts JM, Hauth JC, Leveno KJ, et al. Relationship of early pregnancy waist-to-hip ratio versus body mass index with gestational diabetes mellitus and insulin resistance. American journal of perinatology. 2016;2(01):114–22. doi: 10.1055/s-0035-1562928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.White SL, Lawlor DA, Briley AL, Godfrey KM, Nelson SM, Oteng-Ntim E, et al. Early antenatal prediction of gestational diabetes in obese women: Development of prediction tools for targeted intervention. PLoS ONE. 2016;11(12). doi: 10.1371/journal.pone.0167846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.De Souza LR, Berger H, Retnakaran R, Maguire JL, Nathens AB, Connelly PW, et al. First-trimester maternal abdominal adiposity predicts dysglycemia and gestational diabetes mellitus in midpregnancy. Diabetes Care. 2016;39(1):61–4. doi: 10.2337/dc15-2027 [DOI] [PubMed] [Google Scholar]
- 70.Kennedy NJ, Peek MJ, Quinton AE, Lanzarone V, Martin A, Benzie R, et al. Maternal abdominal subcutaneous fat thickness as a predictor for adverse pregnancy outcome: A longitudinal cohort study. BJOG: An International Journal of Obstetrics and Gynaecology. 2016;123(2):225–32. doi: 10.1111/1471-0528.13758 [DOI] [PubMed] [Google Scholar]
- 71.Sina M, Hoy WE, Callaway L, Wang Z. The associations of anthropometric measurements with subsequent gestational diabetes in Aboriginal women. Obesity research & clinical practice. 2015;9(5):499–506. doi: 10.1016/j.orcp.2015.02.005 [DOI] [PubMed] [Google Scholar]
- 72.Balani J, Hyer S, Johnson A, Shehata H. The importance of visceral fat mass in obese pregnant women and relation with pregnancy outcomes. Obstetric medicine. 2014;7(1):22–5. doi: 10.1177/1753495X13495192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bolognani CV, Reis L, de Souza SS, Dias A, Rudge MVC, Calderon IDP. Waist circumference in predicting gestational diabetes mellitus. Journal of Maternal-Fetal & Neonatal Medicine. 2014;27(9):943–8. [DOI] [PubMed] [Google Scholar]
- 74.Gur EB, Ince O, Turan GA, Karadeniz M, Tatar S, Celik E, et al. Ultrasonographic visceral fat thickness in the first trimester can predict metabolic syndrome and gestational diabetes mellitus. Endocrine. 2014;47(2):478–84. doi: 10.1007/s12020-013-0154-1 [DOI] [PubMed] [Google Scholar]
- 75.Ebrahimi-Mameghani M, Mehrabi E, Kamalifard M, Yavarikia P. Correlation between body mass index and central adiposity with pregnancy complications in pregnant women. Health Promotion Perspectives. 2013;3(1):73. doi: 10.5681/hpp.2013.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Suresh A, Liu A, Poulton A, Quinton A, Amer Z, Mongelli M, et al. Comparison of maternal abdominal subcutaneous fat thickness and body mass index as markers for pregnancy outcomes: A stratified cohort study. Australian and New Zealand Journal of Obstetrics and Gynaecology. 2012;52(5):420–6. doi: 10.1111/j.1479-828X.2012.01471.x [DOI] [PubMed] [Google Scholar]
- 77.Yao D, Chang Q, Wu Q-J, Gao S-Y, Zhao H, Liu Y-S, et al. Relationship between maternal central obesity and the risk of gestational diabetes mellitus: a systematic review and meta-analysis of cohort studies. Journal of diabetes research. 2020;2020. doi: 10.1155/2020/6303820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Benevides FT, Araujo Junior E, Maia CSC, Montenegro Junior RM, Carvalho FHC. Ultrasound evaluation of subcutaneous and visceral abdominal fat as a predictor of gestational diabetes mellitus: a systematic review. The Journal of Maternal-Fetal & Neonatal Medicine. 2020:1–11. doi: 10.1080/14767058.2020.1781808 [DOI] [PubMed] [Google Scholar]
- 79.Thaware P, Patterson C, Young I, Casey C, McCance D. Clinical utility of ultrasonography‐measured visceral adipose tissue depth as a tool in early pregnancy screening for gestational diabetes: a proof‐of‐concept study. Diabetic Medicine. 2019;36(7):898–901. doi: 10.1111/dme.13906 [DOI] [PubMed] [Google Scholar]
- 80.Tornaghi G, Raiteri R, Pozzato C, Rispoli A, Bramani M, Cipolat M, et al. Anthropometric or ultrasonic measurements in assessment of visceral fat? A comparative study. International journal of obesity and related metabolic disorders: journal of the International Association for the Study of Obesity. 1994;18(11):771–5. [PubMed] [Google Scholar]
- 81.Wells JCK, Fewtrell MS. Measuring body composition. Arch Dis Child. 2006;91(7):612–7. doi: 10.1136/adc.2005.085522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ben-Noun LL, Laor A. Relationship between changes in neck circumference and cardiovascular risk factors. Experimental & Clinical Cardiology. 2006;11(1):14. [PMC free article] [PubMed] [Google Scholar]
- 83.Zhou J-y, Ge H, Zhu M-f, Wang L-j, Chen L, Tan Y-z, et al. Neck circumference as an independent predictive contributor to cardio-metabolic syndrome. Cardiovascular diabetology. 2013;12(1):1–7. doi: 10.1186/1475-2840-12-76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yeung EH, Hu FB, Solomon C, Chen L, Louis G, Schisterman E, et al. Life-course weight characteristics and the risk of gestational diabetes. Diabetologia. 2010;53(4):668–78. doi: 10.1007/s00125-009-1634-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rudra CB, Sorensen TK, Leisenring WM, Dashow E, Williams MA. Weight characteristics and height in relation to risk of gestational diabetes mellitus. American journal of epidemiology. 2007;165(3):302–8. doi: 10.1093/aje/kwk007 [DOI] [PubMed] [Google Scholar]
- 86.Brite J, Shiroma E, Bowers K, Yeung E, Laughon S, Grewal J, et al. Height and the risk of gestational diabetes: variations by race/ethnicity. Diabetic medicine. 2014;31(3):332–40. doi: 10.1111/dme.12355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hutcheon JA, Platt RW, Abrams B, Himes KP, Simhan HN, Bodnar LM. A weight-gain-for-gestational-age z score chart for the assessment of maternal weight gain in pregnancy. The American of Clinical Nutrition. 2013;97(5):1062–7. doi: 10.3945/ajcn.112.051706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Forbes GB. Human body composition: growth, aging, nutrition, and activity: Springer Science & Business Media; 2012. [Google Scholar]
- 89.Widen E, Gallagher D. Body composition changes in pregnancy: measurement, predictors and outcomes. European journal of clinical nutrition. 2014;68(6):643–52. doi: 10.1038/ejcn.2014.40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Most J, Marlatt KL, Altazan AD, Redman LM. Advances in assessing body composition during pregnancy. European journal of clinical nutrition. 2018;72(5):645–56. doi: 10.1038/s41430-018-0152-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Björntorp P. " Portal" adipose tissue as a generator of risk factors for cardiovascular disease and diabetes. Arteriosclerosis: An Official Journal of the American Heart Association, Inc. 1990;10(4):493–6. [PubMed] [Google Scholar]
- 92.Hamdy O, Porramatikul S, Al-Ozairi E. Metabolic obesity: the paradox between visceral and subcutaneous fat. Current diabetes reviews. 2006;2(4):367–73. doi: 10.2174/1573399810602040367 [DOI] [PubMed] [Google Scholar]
- 93.Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu C-Y, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116(1):39–48. doi: 10.1161/CIRCULATIONAHA.106.675355 [DOI] [PubMed] [Google Scholar]
- 94.Ruhl CE, Everhart JE, Ding J, Goodpaster BH, Kanaya AM, Simonsick EM, et al. Serum leptin concentrations and body adipose measures in older black and white adults. The American journal of clinical nutrition. 2004;80(3):576–83. doi: 10.1093/ajcn/80.3.576 [DOI] [PubMed] [Google Scholar]
- 95.Cole TJ, Freeman JV, Preece MA. Body mass index reference curves for the UK, 1990. Arch Dis Child. 1995;73(1):25–9. doi: 10.1136/adc.73.1.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Najafi F, Hasani J, Izadi N, Hashemi‐Nazari SS, Namvar Z, Mohammadi S, et al. The effect of prepregnancy body mass index on the risk of gestational diabetes mellitus: A systematic review and dose‐response meta‐analysis. Obesity Reviews. 2019;20(3):472–86. doi: 10.1111/obr.12803 [DOI] [PubMed] [Google Scholar]
- 97.D’Souza R, Horyn I, Pavalagantharajah S, Zaffar N, Jacob C-E. Maternal body mass index and pregnancy outcomes: a systematic review and metaanalysis. American Journal of Obstetrics & Gynecology MFM. 2019;1(4):100041. doi: 10.1016/j.ajogmf.2019.100041 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOC)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.