Abstract
Purpose
To launch a pharmaceutical product in the US market, approval from the FDA is required. Pharmaceutical companies undergo FDA pre-approval inspection (PAI for small molecule products) or pre-license approval (PLI for biological products) at their manufacturing sites (including contract development and manufacturing organization, testing laboratories, and packaging labelling facilities) prior to approval. After the products are approved by the FDA, surveillance inspections are performed by the FDA which are risk based as which company and which site will be inspected. The present study examines the causes of warning letters issued by the Center for Drug Evaluation and Research (CDER), FDA to the pharmaceutical companies after post-approval inspections.
Methods
Warning letters issued from the time period 2010 to 2020 were obtained from the FDA website, and information about date of issuance, company, and type of violations was extracted for the study.
Results
Poor compliance to CGMP and misbranding were the most common reasons for the warning letters. Detailed analysis of CGMP warning letters elucidated three major types of violations, namely deficiencies in process validation, documentation practices (data integrity), and quality control corresponding to 26%, 21%, and 15% warning letters, respectively.
Conclusion
Review of the analysed letters demonstrates that the FDA’s major concern is over CGMP compliance. To avoid these warning letters, pharmaceutical manufacturers need to improve their quality compliance and focus on creating effective quality management systems that govern the entire manufacturing process, quality control, employee training, and documentation practice. Companies should develop an internal compliance check list and also be ready for corrective measures as and when required.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12247-022-09678-2.
Keywords: Warning letters, FDA, Process validation, CGMP, Documentation, Quality control
Introduction
The United States Food and Drug Administration (US FDA), a federal government agency, safeguards public health in USA by ensuring safety of pharmaceutical drugs, medical devices, animal drugs, cosmetics, food additives and radiation emitting products [1]. To warrant safety and quality of products launched in the US market, FDA investigates the companies for their adherence to regulatory compliance. FDA conducts four different types of inspections including pre-approval, surveillance CGMP, compliance follow-up post-approval, and for-cause inspections.
The FDA issues warning letter to a manufacturer upon observing violations during an inspection by agency. Warning letter is defined as “A correspondence that notifies regulated industry about violations that FDA has documented during its inspections or investigations" [2]. Before issuing a warning letter, the FDA requests the manufacturer to respond to the deficiencies that are typically listed in the form 483. If the FDA observes that these responses are unsatisfactory and violations are of regulatory importance and may impact safety and quality of the product, an official notification of the deficiency in the form of a warning letter is issued to the manufacturer. Upon receiving warning letter, company is expected to take appropriate measures to rectify the issues listed in the warning letter to have a close-out letter from the FDA office. In warning letters, FDA also delivers recommendations and guidance to the manufacturer for corrective measures that need to be taken for observed violations [3].
Warning letters issued by FDA can be an important source of data to study and analyse violations that occur in pharmaceutical manufacturing. To date, a number of studies have been published on assessment of these warning letters. Researchers have examined violations specifically with respect to misleading promotional claims of the products [4, 5]. Major violations identified under promotional claims were unjustified patient reported outcome, insufficient evidence of content validity, and excessive broadening of claims. A recent study reviewed the warning letters and notices of violation from 2012–2019 for economic, clinical, and humanistic claims made in pharmaceutical promotional materials [6]. Most frequently cited violations were omission and misleading risk information and also overstatement of efficacy.
The Food and Drug Administration’s Bioresearch Monitoring (BIMO) program inspects clinical investigators, institutional review boards, sponsors, monitors, and contract research organizations involved in clinical trials, and non-clinical laboratories subject to good laboratory practice regulations. An analysis of warning letters issued by BIMO reported major deficiencies in documentation as well as in following written procedures [7]. A recent study analysed the warning letters issued to over-the-counter drugs and identified significant violations in product, process, and laboratory controls [8].
In the present study, the objective was to evaluate warning letters issued by FDA and identify and understand the nature of violations occur in pharmaceutical companies. For this, we have performed analysis of warning letters issued by Center for Drug Evaluation and Research (CDER) to pharmaceutical in the last 10 years (2010–2020). The analysis elucidates the major categories of these objections as well as the FDA expectations during inspection of pharmaceutical manufacturers.
Methods
All data for warning letters were collected from the public database available on the FDA webpage [9]. The list of warning letters issued from January 2010 to December 2020 were imported in a Microsoft Excel document using the ‘Search and Export Warning Letters to Excel’ function. The gathered data included the issuing date, issuing office, manufacturer’s name as well as the subject of the warning letter. Screening of these warning letters was performed to fetch relevant letters. The primary screening of the letters was performed using the keywords: medical devices, biological product, finished pharmaceuticals, active pharmaceutical ingredient, CDER and CDRH. Center for Drug Evaluation and Research (CDER) and Center for Devices and Radiological Health (CDRH) issues warning letters to pharmaceutical and medical companies, respectively. Warning letters related to foods, tobacco products, animal products, and cosmetics were excluded after the primary screening. Next, secondary screening was performed using the following keywords: GMP, CGMP, good manufacturing practices, misbranding, and promotional claim violations. At last, warning letters issued to pharmaceutical manufacturers pertaining to CGMP violations were downloaded. The text of each letter was reviewed to determine specific nature of violations and trends of these violations over the 10-year study period.
Results
Analysis of Warning Letters Issued between 2010 and 2020
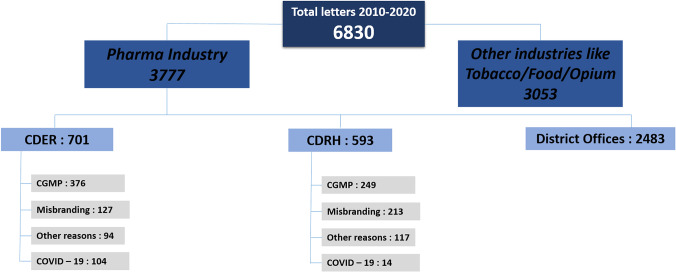
Figure 1 presents categorization of all the warning letters extracted from the FDA website for the period 2010 to 2020. A total of 6830 warning letters were issued in this time period, of which 3777 were issued to pharmaceutical and medical device manufacturers. Of these 3777 letters, letters issued by CDER and CDRH were considered further and letters issued by district offices were excluded from this study. Next, the warning letters issued by CDER and CDRH were categorized based on the type of violation. As can be observed from Fig. 1, non-compliance to CGMP practices and misbranding of product were the top two reasons for the issuance of the warning letters. Misbranding violations include labelling or packaging form are considered misleading or false. Other reasons included unusual tamper evident packaging, prescription drug marketing act violation, pharmacy compounding issues, and investigational drug use violations.
Fig. 1.
Categorization of all warning letters issued by FDA in the time period 2010 to 2020
Analysis of CGMP and Misbranding Warning Letters
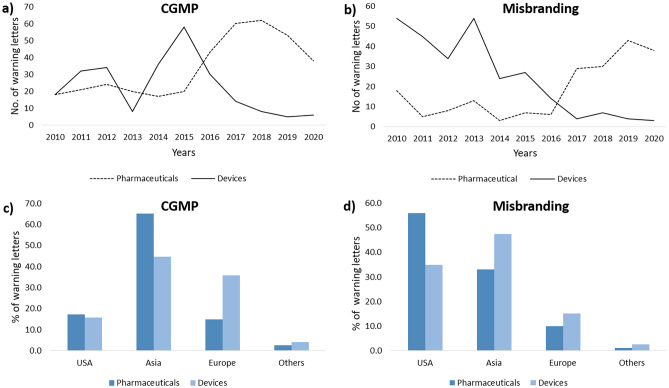
Figure 2a and b illustrates the number of warning letters issued to pharmaceutical and medical devices manufacturers from 2010 to 2020 for CGMP and misbranding violations, respectively. In the case of medical devices, it is observed that the CGMP violations increased up to year 2015, after which there is a steep decrease over the next five years up to 2020 (Fig. 2a). A similar trend is observed in misbranding violations with medical devices as well (Fig. 2b). The significant decrease in CGMP and misbranding violations in warning letters to the medical device manufacturers can be attributed to a combination of scientific developments and increased awareness of the expectations of the regulatory authorities.
Fig. 2.
Charting of warning letters issued to pharmaceutical and medical device companies due to CGMP and misbranding from year 2010–2020 (a, b) and for different regions (c, d)
Amongst pharmaceutical manufacturers, however, a contrasting trend is observed. CGMP violation-related warning letters were hovering around an average of 20 letters per year from 2010 to 2015. However, a significant increase in number of issued warning letters is observed from 2015 to 2019. This increase in number of warning letters from 2015 can be attributed to increase in number of inspections especially foreign inspections. With the globalization of FDA’s drug inspection program, FDA conducted more foreign inspections than domestic drug inspections since 2015 [10, 11]. The relatively small number of warning letters issued in 2020 can be due to impact of Covid on FDA investigation. Factually, the decline in number of warning letters was not drastic, but moderate reduction was observed from 2018–2019. In March 2020, FDA decided to postpone its inspections with the exception of mission- critical inspection work [12] and made interim measures such as remote inspection, assessment, leveraging information provided by its trusted partners, testing of products at borders to ensure safety of products imported in USA. Despite this, decision on 49 drug applications has been reported to delay due to pending on-site inspection or facility assessment [13]. In financial year 2020, FDA planned to conduct 79 domestic follow-up activities related to compliance follow-up to meet their performance target and was able to complete 90% of these, delaying eight in 2020; however, only 61% of surveillance inspection were completed in 2020 [13]. For misbranding also, a sudden increase in number of warning letters is observed from 2017 to 2020 for pharmaceutical products. Misbranding includes fake promotion of the product, broadening of claims beyond approval, and marketing of unapproved product by the FDA.
Next, we sorted the warning letters issued by CDER and CDRH with CGMP and misbranding as the major issue based on geography. Figure 2c and d presents the outcome of this analysis. Majority of the pharmaceutical/medical manufacturers that accounted for CGMP violations belonged to Asian countries (Fig. 2c). In pharmaceutical sector, over 65% of total warning letters were issued to Asian companies and most of the recipients are in India and China. Amongst misbranding violations, highest percentage of violations (56%) in pharmaceutical were from manufacturers based in USA. In medical sector, highest percentage of violations (47%) were for manufacturers based in Asia (Fig. 2d). With the increase in FDA’s foreign inspections, foreign countries have contributed to the increase of warning letters issued [14].
Analysis of CGMP Warning Letters
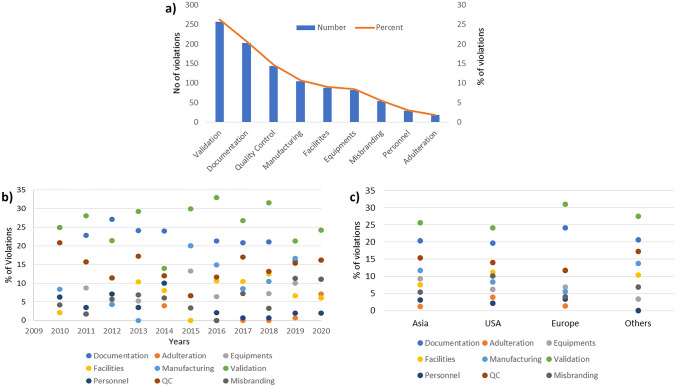
All the warning letters issued by CDER having CGMP as major violation were read carefully, and the nature of violations mentioned in letters was sub-categorized. These categories included documentation, adulteration, equipment, facilities, manufacturing, validation, personnel, quality control, misbranding (applications having both CGMP and misbranding issues were analysed with misbranding as secondary issue and CGMP as primary violation). During analysis, it was observed that almost all warning letters contain more than one violation. A letter with multiple violations was counted in all the applicable categories for analysis. Figure 3a demonstrates the total count and percent for each category. Major issues identified were validation, documentation, and quality control, corresponding to 26%, 21%, and 15% warning letters, respectively. Similar reasons have been identified in a previous study [15].
Fig. 3.
a Sub-categorization of violations observed in CGMP warning letters issued to pharmaceutical manufacturers and its trend analysis based on b year and c regions
Furthermore, we examined the trend for each of these subcategories in year-wise manner from 2010 to 2020 as well as with respect to geography (Fig. 3b and c). The total number for each violation from 2010 to 2020 and geography wise is summarized in the Supplementary Tables 1 and 2. It is observed that validation consistently ranks as one of the most common violations that is cited in the warning letters for all the years as well as across geographies. Concerns pertaining to process control, analytical test methods, material identity and equipment qualification were all included under validation category. Poor documentation and quality control related issues hold the next two spots both in data over the years and across geographies.
It is observed that almost every year from 2010 to 2020, 25–30% warning letters mentioned validation related violations as one of the key objections that include either process, method or material validation. In warning letters, major identified issues with validation included inadequate process validation for manufacturing consistent product, insufficient laboratory control mechanism and details for assuring maintenance of validated process, poor implementation of corrective actions for out of specification results, lacking validation of analytical test methods for active ingredient characterization, deficient materials validation received from different suppliers, inadequate equipment cleaning and maintenance validation. Geography-wise analysis also indicates that validation is a major issue in all regions with 25–31%. This suggests that globally manufacturers continue to struggle with respect to their understanding of the practices as well as regulatory expectations on the subject [16]. An example of a citation related to validation is “Your firm failed to establish adequate written procedures for production and process control designed to assure that the drug products you manufacture have the identity, strength, quality, and purity they purport or are represented to possess” [17, 18] and “Your firm also failed to validate and establish the reliability of component supplier analyses on which you rely in lieu of certain tests through appropriate validation of supplier’s test results at appropriate intervals” [19].
Documentation (data integrity) was the next most common issue highlighted in the warning letters. On an average, approximately 20–25% of warning letters cited subpar documentation as one of the major deficiencies. The last 2 years (2019 and 2020) have seen a slight decrease in this %. An analysis across the various geographies indicates around 20–24% of warning letters cited subpar documentation as one of the major deficiencies, highest amongst European manufacturers. Major documentation issues include failure of maintaining records of each batch produced, backdated records, and misplaced records and obtaining a government document subject to legalization. Some of the examples of related to poor documentation include: “Your firm failed to establish written procedures for production and process control designed to assure that the drug products you manufacture have the identity, strength, quality, and purity they purport or are represented to possess [20]” and “Your firm failed to establish and follow written procedures regarding storage and warehousing of drug products” [21].
Quality control was the next most cited issue in the warning letters. It is observed that about 12–15% of all the warning letters cited this as the major issue, with 2015 being an exception. Identified violations include failure of quality unit in providing assurance of testing of materials, deficiencies in proper control of batch records and lack of corrective and preventive actions (CAPA) in case of deviations with process performance and product quality. Examples of these violations include: “Your firm failed to establish a quality control unit with the responsibility and authority to approve or reject all components, drug product containers, closures, in-process materials, packaging materials, labelling, and drug products” [22], “Failure of your quality unit to exercise its responsibility to ensure the API manufactured at your facility are in compliance with CGMP [23] and Failure of your quality unit to ensure that critical deviations are investigated and resolved” [24].
Other Reasons
Other violations that were cited in the CGMP warning letters were related to manufacturing, equipment, facility, and personnel. Equipment and facilities-related issues accounted for an average of 8%, while that of manufacturing was at 10%. Adulteration was consistently at bottom of the list. Manufacturing issues typically included discrepancies observed during batch to batch production and inadequate microbiologic investigation. These microbial contamination issues were reported for both non-sterile and sterile drugs indicating poor microbiology practices at pharmaceutical industry. Facility issues included poor aseptic operations and poor monitoring of environmental conditions. Equipment issues highlighted were poor cleaning and maintenance practices, lack of cleaning validation, and sterilization of equipment.
COVID-19-related Warning Letters
During the COVID-19 pandemic of 2020, a large number of warning letters were related to Covid products which were not included in our study. Of the 265 warning letters issued by CDER and CDRH in 2020, 122 letters (46%) were related to Covid products. In most cases, reason for the warning letter was unapproved and misbranding of Covid-related products. Recipient companies mostly belonged to the USA, and a few were in Asia and Europe.
Discussion
Of all the global regulatory agencies, the USFDA is considered to be the pre-eminent regulatory body. The USA being the largest market for pharmaceutical products captivates manufacturing companies worldwide to have an approval from USFDA to launch product in US market. The present study presents a detailed analysis of the warning letters issued by CDER and CDRH units of the FDA to pharmaceutical and medical manufacturers in the last 10 years (2010 to 2020). All warning letters issued to pharmaceutical manufacturers were extracted followed by categorization of the violations and eventually data analysis. Validation, documentation, and quality control have been identified as the major CGMP violations as per the warning letters. Also, in our previous study with biosimilar regulatory applications, process validation and GMP compliance were among the top five reasons for denying the approval of product by regulatory authorities [25].
Validation is an important and essential parameters to be compliant with CGMPs. In order to improve and assure the quality of pharmaceutical products, FDA has its guidance on “Good Manufacturing Practice Guidance for Active Pharmaceutical Ingredients” [26]. Separately, FDA issued its guidance on process validation early in 1987 entitled “Guidelines on General Principles of Process Validation” [27]. Later in 2011, it revised this guidance [28]. Process validation has been defined as “the collection and evaluation of data, from the process design stage through commercial production, which establishes scientific evidence that a process is capable of consistently delivering quality product” [28]. Due to complexity and inherent variability associated with pharmaceutical process, it is important to understand process in depth and also factors affecting the performance of process [29, 30]. Process validation in the pharmaceutical industry ensures protection of process from variance that can interfere with the quality of product. Three important steps in process validation include process design, process qualification, and continued process verification. With advancement in technology, quality by design (QbD) and risk management approaches have gained momentum in pharmaceutical industry [31–33]. Major challenges in implementing the QbD approach include–establishing appropriate level of details required to design QbD, sharing detailed information with regulatory agencies, and last but not the least, the associated costs and workload to implement QbD approach for product development and manufacturing unit operations. However, implementation of QbD approach reduces product variability and enhances understanding of product and process design and post-approval change management. Furthermore, these approaches enable faster approval of products with reduced numbers of batch recalls and rejects. Regulatory bodies also advocate implementation of quality by design and risk management in manufacturing process [34]. FDA clearly mandates that manufacturing practices and product quality should be reviewed periodically and the process should be adjusted if needed. If the manufacturing process is not consistently maintained to deliver pre-determined quality of the finished product, the manufacturer is liable for action from the regulator. Not only the manufacturing process needs to be validated but also manufacturing facilities and equipment need to be validated and calibrated as per specifications [16]. Analytical methods used to characterize drug substance or drug products should also be validated [35]. For analytical platforms also, a risk-based approach is recommended to ensure the actions and control strategy required for a method during its entire life-cycle [36]. Successful process validation provides high degree of assurance of maintaining consistent quality in each unit of the finished product. Validation of process or system helps to determine risks and deviations in batch to batch production.
Another major reason identified in CGMP warning letter was inadequate documentation. Documentation or data record is an integral component for CGMP compliance and allows regulators to assess all the necessary steps involved in drug manufacturing. In CGMP, it is considered as if it is not written, then it did not happen. Due to increasing number of violations in data integrity, FDA already has guidance for industry on data integrity and compliance [37]. The WHO has also issued a guidance on good data and management practice [38]. Data integrity issues can be unintentional because of lack of understanding, but at times can also be intentional due to economically motivated or cultural behaviour of personnel. To tackle these data integrity issues, pharmaceutical industries should instil culture of integrity, conduct work ethics training, and have transparent and effective quality training agreements. Companies need to understand that maintaining proper documentation or data integrity is a must task for pharmaceutical companies to get an approval from regulatory authorities. In many of the warning letters, FDA has recommended that the recipient companies hire a consultant with expertise in data management and GMP compliance. Companies should conduct internal audits in a timely manner to identify gaps and consult experts in case of lack of in-house knowledge.
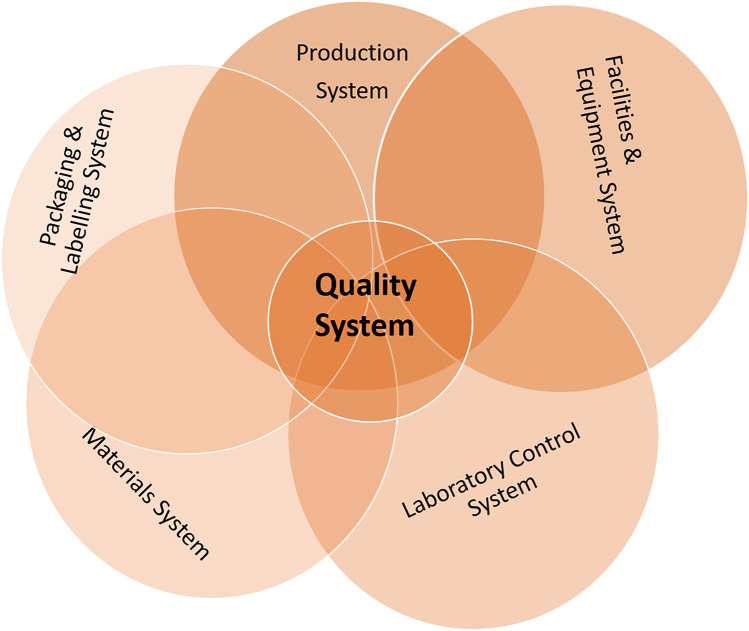
The quality control unit of a pharmaceutical manufacturer ensures testing and maintenance of product quality of each batch produced. FDA as well as most other regulatory agencies inspect the QC unit and its work processes as ultimately, they are responsible for testing and issuing the certificate to each batch that the manufacturer produces and sends to market. During the FDA audit, quality of each batch of the drug product along with methods and technology equipped to ensure quality are checked thoroughly. All the procedures employed should be scientifically sound and appropriate to ensure that the raw materials and API conform to established standards of quality. As per the FDA guideline, six system inspection model is described as shown in Fig. 4 with quality system at core and five manufacturing systems, namely production, facilities and equipment, laboratory control system, materials system, and packaging and labelling systems linked to it [39]. In the warning letters, the FDA has recommended the company to follow guidelines establishing and following CGMP compliant quality systems including Q8(R2) Pharmaceutical Development [40], Q9 Quality Risk Management [41], and Q10 Pharmaceutical Quality System [42]. These guidelines provide guidance on defining product critical quality attributes, design space, the manufacturing process and the control strategy in a structured manner which can be used to identify the studies to be performed during product development and initial commercial production. FDA highlights the importance of four elements in Q10 Pharmaceutical Quality System guidelines during different stages of pharmaceutical product life cycle for improving product quality. These elements include process performance and product quality monitoring system, corrective action and preventive action (CAPA) system, change management system and management review of process performance and product quality [42]. ICH Q12 guideline, recently released in 2020, provides a framework to facilitate the management of post-approval CMC changes [43]. This will help in the management of post-approval changes, supporting continual improvement in a more efficient manner and have better transparency with regulatory authorities.
Fig. 4.
The six-step inspection model in FDA
FDA guideline also emphasizes on top-down management approach in building appropriate quality culture in company. Effective training program should be organized to percolate the required culture of quality amongst the employees. A robust quality unit in place ensures not only good manufacturing practices within the organization but also contributes towards the quality and safety of product. It is expected that with careful assessment of the past year’s warning letters, manufacturers can efficiently work on their shortcomings and manage future FDA inspection in an efficient manner. The findings of the present study offer guidance to pharmaceutical manufacturers to avoid common mistakes in order to meet FDA expectations [Box1].
Conclusions
The present study offers detailed analysis of the warning letters issued by CDER and CDRH of the FDA to pharmaceutical and medical devices manufacturers in the last 10 years (2010 to 2020). The study observed that a larger number of warning letters were issued to pharmaceutical manufacturers as compared to medical device manufacturers between 2015 and 2020. The major reasons for this were poor CGMP compliance and misbranding. Content analysis of reviewed letters issued to pharmaceutical manufacturers shows that FDA closely monitor operations of quality control unit, validation of manufacturing process, and data record and integrity. Overall, the present study underscores the importance of CGMP compliance in pharmaceutical manufacturing not only to have an approval of product but also to ensure safety and quality of the manufactured drug product. To have a smooth approval process, pharmaceutical companies should focus on structured quality management system and also keep themselves updated with guidance documents to be aware with expectation and thinking of regulatory agency.
Supplementary Information
Below is the link to the electronic supplementary material.
Funding
This work was funded by the Department of Biotechnology, Ministry of Science and Technology (BT/COE/34/SP15097/2015).
Declarations
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
The present study highlights the importance of CGMP compliance in pharmaceutical manufacturing for FDA approval. Validation, documentation, and quality control were identified as the major CGMP violations as per the warning letters.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.US Food and Drug Administration. What we do. 2018. https://www.fda.gov/about-fda/what-we-do. Accessed 14 Jun 2021.
- 2.US Food and Drug Administration. About Warning and Close-Out Letters. 2019. https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/warning-letters/about-warning-and-close-out-letters. Accessed 10 Jun 2021.
- 3.US Food and Drug Administration. Regulatory Procedures Manual. Chapter 4 Advisory actions. 2022. https://www.fda.gov/media/71878/download. Accessed 10 Jun 2021.
- 4.Stewart KA, Neumann PJ. FDA actions against misleading or unsubstantiated economic and quality-of-life promotional claims: An analysis of warning letters and notices of violation. Value Heal. 2002;5(5):390–397. doi: 10.1046/J.1524-4733.2002.55146.x. [DOI] [PubMed] [Google Scholar]
- 5.Symonds T, Hackford C, Abraham L. A review of FDA warning letters and notices of violation issued for patient-reported outcomes promotional claims between 2006 and 2012. Value Heal. 2014;17(4):433–437. doi: 10.1016/j.jval.2014.03.1718. [DOI] [PubMed] [Google Scholar]
- 6.Mohite N, Funtanilla V, Muzumdar J, Park T. Content Analysis of 2012–2019 FDA Warning Letters and Notices of Violations using the Economic, Clinical, and Humanistic Outcomes (ECHO) Model. Inov Pharm. 2021;12(1):4. doi: 10.24926/iip.v12i1.3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rogers CA, Ahearn JD, Bartlett MG. Data Integrity in the Pharmaceutical Industry: Analysis of Inspections and Warning Letters Issued by the Bioresearch Monitoring Program Between Fiscal Years 2007–2018. Ther Innov Regul Sci. 2020;54(5):1123–1133. doi: 10.1007/s43441-020-00129-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bai HK, Ahearn JD, Bartlett MG. Over-the-Counter Drugs: Regulatory Analysis of Warning Letters Between Fiscal Years 2015–2019. Ther Innov Regul Sci. 2021;55(2):426–436. doi: 10.1007/s43441-020-00231-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.US Food and Drug Administration. Warning Letters. https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/compliance-actions-and-activities/warning-letters. Accessed 14 Jun 2021.
- 10.US Food and Drug Administration. Securing the U.S. drug supply chain: Oversight of FDA’s foreign inspection program. 2019. https://www.fda.gov/news-events/congressional-testimony/securing-us-drug-supply-chain-oversight-fdas-foreign-inspection-program-12102019. Accessed 15 Jul 2022.
- 11.US Food and Drug Administration. Data Dashboard. Inspections. https://datadashboard.fda.gov/ora/cd/inspections.htm. Accessed 15 Jul 2022.
- 12.US Food and Drug Administration. Coronavirus Disease 2019 (COVID-19) Update: Foreign Inspections. 2020. https://www.fda.gov/news-events/press-announcements/coronavirus-disease-2019-covid-19-update-foreign-inspections. Accessed 14 Jun 2021.
- 13.US Food and Drug Administration. Resiliency Roadmap for FDA inspectional oversight. 2021. https://www.fda.gov/media/148197/download. Accessed 15 July 2022.
- 14.Valdova V, Sheckler RL. The dark side of pharma globalization. U.S. dependency on foreign pharmaceutical production imposes vulnerability to failure. https://www.linkedin.com/pulse/us-dependency-foreign-pharmaceutical-production-imposes-valdova/. Accessed 15 July 2022.
- 15.Jain SK, Jain RK. Review of fda warning letters to pharmaceuticals: Cause and effect analysis. Res J Pharm Technol. 2018;11(7):3219–3226. doi: 10.5958/0974-360X.2018.00592.9. [DOI] [Google Scholar]
- 16.Rathore AS, Sofer G. Process Validation in Manufacturing of Biopharmaceuticals. 3. Taylor and Francis; 2012. [Google Scholar]
- 17.US Food and Drug Administration. Warning Letter. Coral Pharmaceuticals LTD (MARCS-CMS 586576 – October 09, 2019). https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/warning-letters/coral-pharmaceuticals-ltd-586576-10092019. Accessed 14 Jun 2021.
- 18.US Food and Drug Administration. Warning Letter. Mayon's Pharmaceuticals Pvt Ltd (MARCS-CMS 607388 – September 04, 2020). https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/warning-letters/mayons-pharmaceuticals-pvt-ltd-607388-09042020. Accessed 14 Jun 2021.
- 19.US Food and Drug Administration.Warning Letter. Swabplus, L.P. (MARCS-CMS 584803 – October 31, 2019). https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/warning-letters/swabplus-lp-584803-10312019. Accessed 14 Jun 2021.
- 20.US Food and Drug Administration. Warning Letter. Dermameal Co., Ltd. (MARCS-CMS 582118 – September 12, 2019). https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/warning-letters/dermameal-co-ltd-582118-09122019. Accessed 14 Jun 2021.
- 21.US Food and Drug Administration. Warning Letter. Barox Co., Ltd. (MARCS-CMS 565314 – November 28, 2018). https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/warning-letters/barox-co-ltd-565314-11282018. Accessed 14 Jun 2021.
- 22.US Food and Drug Administration. Warning Letter. C. O. Truxton Inc. (MARCS-CMS 535005 – December19, 2017). https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/warning-letters/c-o-truxton-inc-535005-12192017. Accessed 14 Jun 2021.
- 23.US Food and Drug Administration. Warning Letter. B & B Pharmaceuticals, Inc. (MARCS-CMS 570613 – June 04, 2019). https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/warning-letters/b-b-pharmaceuticals-inc-570613-06042019. Accessed 14 Jun 2021.
- 24.US Food and Drug Administration. Warning Letter. IDT Australia Ltd. (MARCS-CMS 547605 – May 23, 2018). https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/warning-letters/idt-australia-ltd-547605-05232018. Accessed 14 Jun 2021.
- 25.Rathore AS, Chhabra H, Bhargava A. Approval of biosimilars: a review of unsuccessful regulatory filings. Expert Opin Biol Ther. 2021;21:19–28. doi: 10.1080/14712598.2020.1793954. [DOI] [PubMed] [Google Scholar]
- 26.US Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER). ICH Q7 Good Manufacturing Practice Guide for Active Pharmaceutical Ingredients (APIs). 2016.
- 27.Center for Drugs and Biologics, Center for Devices and Radiological Health Food and Drug Administration. Guidelines on General Principles of Process Validation. 1987.
- 28.US Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER) and Center for Veterinary Medicines (CVM). Process Validation: General Principles and Practices. 2011.
- 29.Rathore AS, Noferi JF, Arling ER, Sofer G, Watler P, O’Leary R. Process Validation: How Much and When. BioPharm. 2002;15.
- 30.Rathore AS, Bansal A, Hans J. Knowledge Management and Process Monitoring of Biopharmaceutical Processes in the Quality by Design Paradigm. In: Mandenius C-F, Hooker NT, editors. Measurement, Monitoring, Modelling and Control. Springer Verlag; 2013. pp. 217–247. [Google Scholar]
- 31.Mollah H, Bozzone S, Rathore AS. Process Lifecycle Validation: Applying Risk Management. BioPharm Int. 2013;26:28–33. [Google Scholar]
- 32.Rathore AS, Winkle H. Quality by Design for Biopharmaceuticals: Regulatory Perspective and Approach. Nat Biotechnol. 2009;27:26–34. doi: 10.1038/nbt0109-26. [DOI] [PubMed] [Google Scholar]
- 33.Yu LX. Pharmaceutical Quality by Design: Product and Process Development. Understanding and control. Pharm Res. 2008;25:781–791. doi: 10.1007/s11095-007-9511-1. [DOI] [PubMed] [Google Scholar]
- 34.US Food and Drug Administration. Pharmaceutical Quality for the 21st Century A Risk-Based Approach Progress Report. 2015. https://www.fda.gov/about-fda/center-drug-evaluation-and-research-cder/pharmaceutical-quality-21st-century-risk-based-approach-progress-report. Accessed 10 Jun 2021.
- 35.ICH Harmonized Tripartite Guideline. Validation of analytical procedures: Text and methodology Q2R1. 2005.
- 36.US Food and Drug Administration. QbD Considerations for Analytical Methods – FDA Perspective. 2013.
- 37.US Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER) and Center for Veterinary Medicines (CVM). Data Integrity and Compliance With Drug CGMP. 2016.
- 38.World Health Organization. Guidance on good data and record management practices. 2016.
- 39.US Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER), Center for Veterinary Medicines (CVM), ORA. Quality Systems Approach to Pharmaceutical CGMP Regulations. 2006.
- 40.US Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER). Q8(R2) Pharmaceutical Development. 2009.
- 41.US Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER), Center for Veterinary Medicines (CVM). Q9 Quality Risk Management. 2006.
- 42.US Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER), Center for Veterinary Medicines (CVM). Q10 Pharmaceutical Quality System. 2009.
- 43.International Council for Harmonization. ICH Q12 Technical and regulatory considerations for pharmaceutical product lifecycle management. 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.