Abstract
COVID-19 spread in two pandemic waves in Italy between 2020 and 2021. The aim of this study is to compare the first with the second COVID-19 wave, analyzing modifiable and non-modifiable factors and how these factors affected mortality in patients hospitalized in Internal Medicine wards. Consecutive patients with SARS-CoV-2 infection and dyspnea requiring O2 supplementation were included. The severity of lung involvement was categorized according to the patients’ oxygen need. Six hundred and ten SARS-CoV-2 hospitalized patients satisfied the inclusion criteria. The overall estimated 4-week mortality was similar in the two pandemic waves. Several variables were associated with mortality after univariate analysis, but they lacked the significance after multivariable adjustment. Steroids did not exert any protective effect when analyzed in time-dependent models in the whole sample; however, steroids seemed to exert a protective effect in more severe patients. When analyzing the progression to different states of O2 supplementation during hospital stay, mortality was almost exclusively associated with the use of high-flow O2 or CPAP. The analysis of the transition from one state to the other by Cox–Markov models confirmed that age and the severity of lung involvement at admission, along with fever, were relevant factor for mortality or progression.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11739-022-03052-3.
Keywords: COVID-19, SARS-CoV-2, Internal Medicine, Survival, O2 supplementation, LMWH
Introduction
In December 2019, a new coronavirus was recognized as the cause of an international outbreak of respiratory diseases, which began in Wuhan, the capital of central China’s Hubei province, and rapidly spread around the world. The virus was named SARS coronavirus 2 (SARS-CoV-2, severe acute respiratory syndrome coronavirus 2) and the related disease was termed by the World Health Organization (WHO), COVID-19 (coronavirus disease 2019).
SARS-CoV-2 has been reported to cause an extensive spectrum of clinical features, ranging from mild flu-like symptoms to severe interstitial pneumonia (15–20% of cases) eventually leading to acute respiratory distress syndrome that may require intensive care support (5–6% of cases) [1]. Common symptoms include fever, cough, fatigue and dyspnea, but even olfactory and gustatory dysfunction, myalgia, sore throat, diarrhea, ocular symptoms, headache and skin rash have been reported.
At the end of May 2021, approximately 165,770,000 confirmed cases have been reported worldwide, accounting for approximately 1,134,000 deaths; in Italy, more than 4,159,000 cases and about 124,000 deaths were reported [2]. Several comorbidities have been associated with an increased risk of death secondary to COVID-19: age, hypertension, diabetes, chronic obstructive pulmonary diseases or cardiovascular diseases [3–5]. SARS-CoV-2 infection is plagued by high hospitalization rates [6], especially in frail subjects, putting the health-care systems at stake. During the COVID-19 pandemic wave(s), several treatments have been eagerly tested and repurposed and their overall efficacy or inefficacy was reported on the basis of retrospective case series or, sometimes, of controlled trials. In most cases, the efficacy of these drugs on 28-day mortality was not unequivocal, that is the case of remdesivir [7–13], hydroxychloroquine [14], and lopinavir–ritonavir [15]. On the contrary, the efficacy of dexamethasone was suggested in randomized trials [16–18] and other studies have tried to elucidate if other steroids could be useful in treating SARS-CoV-2 infection [19, 20] or the optimal strategy to treat hospitalized patients with dexamethasone [21]. Some more recent studies showed the benefit of other drugs in severely ill hospitalized patients, and namely tocilizumab [22] or low molecular weight heparin (LMWH), especially in those with increased D-Dimer levels [23].
COVID-19 spread in two pandemic waves, the first hit Italy from March to June 2020 and the second from October 2020 to February 2021. It is thought that the first wave hit harder because of the limited knowledge of the disease and the lack of established therapeutic protocols as well as of specialized equipment. Nonetheless, during the second wave, a much higher number of infections were observed, thus challenging the capabilities of health-care systems. Some estimates indicate that the mortality between the two waves was similar in intensive care unit (ICU) patients [24] but detailed data are scant. The aim of this study is to compare the first with the second COVID-19 wave, analyzing modifiable and non-modifiable factors such as gender, age, comorbidities prescribed therapies and how these factors affected mortality in hospitalized patients.
Methods
Patients
Consecutive patients referring to Internal Medicine wards of our institution with a confirmed SARS-CoV-2 infection by molecular nasopharyngeal swab were considered for inclusion. All the patients referred to the emergency ward in the period spanning from the beginning of March 2020 to the end of April 2020 (1st pandemic wave) or in November 2020 (2nd pandemic wave) and were hospitalized due to the persistence of dyspnea requiring O2 supplementation because of a severe lung involvement, defined according to the local guidelines published by the AGenzia NAzionale per i Servizi sanitari regionali (AGENAS) [25] as a respiratory rate ≥ 30 breaths/min OR oxygen saturation ≤ 92% at rest OR PaO2/fraction of inspired oxygen (FIO2) < 300 mmHg AND/OR lung infiltrates ≥ 50% on chest X-rays. Anamnestic data regarding the presence of the following comorbidities (according to literature) at baseline were collected: diabetes, cardiovascular diseases (coronary artery disease, heart failure and atrial fibrillation), neurological diseases (Alzheimer’s disease, Parkinson’s disease and dementia), renal diseases, hypertension, malignancy and chronic obstructive pulmonary disease [4, 26]. Baseline laboratory variables, including C-reactive protein (CRP), ferritin, lymphocyte absolute count [26, 27] and D-Dimer were retrieved, where available.
The daily pulse oximetric saturation (SpO2)/FIO2 (S/F) was recorded along with the daily presence of fever and exposure to hydroxychloroquine, remdesivir, steroids (methylprednisolone mg/day or equivalents), antibiotics and low molecular weight heparin (LMWH) given at prophylactic or anticoagulant dosing. The severity of SARS-CoV-2-related lung involvement was categorized according to the patients’ oxygen need: (1) no need, room air; (2) low-flow, supplemental oxygen up to 36% FIO2; (3) high-flow, supplemental oxygen ≥ 40% FIO2; (4) continuous positive airway pressure (CPAP); (5) mechanical ventilation or death [28].
The time from the first symptoms attributable to SARS-CoV-2 infection to the referral to the emergency ward (time of hospitalization) was recorded. The total length of stay in the hospital was calculated from the time of hospitalization to death or to discharge if the patient had a stable SpO2 ≥ 94% at room air.
The study was approved by the local ethic committee (Comitato Etico Area B, approval number, 342_2020).
Statistical analysis
Baseline clinical variables of the 1st and the 2nd pandemic waves were compared by means of the t-Student’s or the Chi-square test, in case of continuous or categorical data, respectively.
Time-to-event analysis was conducted to determine the variables associated with mortality. The total length of hospital stay until death was used as primary outcome and cases requiring mechanical ventilation or dismissed to rehabilitation wards with oxygen supplementation were right-censored. Cox-regression analysis with time-dependent covariates was used to model survival and variables with an uncorrected p value < 0.1 were entered in a multivariable model. Cox–Markov Models [29] were used to model transitions from one lung severity state to the other. Survival analysis was restricted to 4 weeks.
For explorative purposes, the effect of steroids was assessed in multivariable Cox-regression models where the use 6 mg dexamethasone or equivalent [16] was evaluated along with age and the S/F ratio at admission.
For all the analyses, the R statistical software and related libraries were used [30].
Results
Baseline demographic characteristics are summarized in Table 1. Overall, 610 SARS-CoV-2 hospitalized patients satisfied the inclusion criteria, 397 from the 1st and 213 from the 2nd pandemic waves. Patients from the 2nd pandemic wave were older and with a higher prevalence of comorbidities. The severity of lung involvement at referral to the emergency ward was similar with a S/F ratio equal to 275.2 ± 109.5 and 272.3 ± 111.3 in the 1st and 2nd pandemic waves, respectively.
Table 1.
Baseline demographic characteristics
| Variable | Overall (n = 610) | 1st wave (n = 397) | 2nd wave (n = 213) | p |
|---|---|---|---|---|
| Age, years | 66.8 ± 15.4 | 64.4 ± 15.2 | 71.2 ± 14.7 | 1.75 × 10 − 7 |
| Age by groups, n (%) | ||||
| 18–29 | 6 (1%) | 6 (1.5%) | 0 (0%) | |
| 30–39 | 16 (2.6%) | 14 (3.5%) | 2 (0.9%) | |
| 40–49 | 60 (9.8%) | 44 (11.1%) | 16 (7.5%) | |
| 50–64 | 199 (32.6%) | 143 (36%) | 56 (26.3%) | |
| 65–74 | 199 (32.6%) | 127 (32%) | 72 (33.8%) | |
| 75–84 | 85 (13.9%) | 42 (10.6%) | 43 (20.2%) | |
| ≥ 85 | 45 (7.4%) | 21 (5.3%) | 24 (11.3%) | |
| Males, n (%) | 410 (67.2%) | 264 (66.5%) | 146 (68.5%) | 0.608 |
| SaO2 to FIO2 ratio | 274.2 ± 110.1 | 275.2 ± 109.5 | 272.3 ± 111.3 | 0.761 |
| SaO2 to FIO2 ratio by groups, n (%) | ||||
| < 200 | 229 (37.5%) | 155 (39%) | 74 (34.7%) | |
| 200–299 | 128 (21%) | 78 (19.6%) | 50 (23.5%) | |
| 300–399 | 137 (22.5%) | 87 (21.9%) | 50 (23.5%) | |
| ≥ 400 | 116 (19%) | 77 (19.4%) | 39 (18.3%) | |
| Time to hospitalization, days | 6.5 ± 4.7 | 6.8 ± 5.1 | 5.8 ± 3.8 | 0.018 |
| Hypertension, n (%) | 262 (43%) | 169 (42.6%) | 93 (43.7%) | 0.795 |
| Diabetes, n (%) | 108 (17.7%) | 70 (17.6%) | 38 (17.8%) | 0.949 |
| Cardiac involvement, n (%) | 116 (19%) | 64 (16.1%) | 52 (24.4%) | 0.013 |
| Malignancy, n (%) | 77 (12.6%) | 43 (10.8%) | 34 (16%) | 0.069 |
| COPD, n (%) | 71 (11.6%) | 49 (12.3%) | 22 (10.3%) | 0.46 |
| Neurological involvement, n (%) | 50 (8.2%) | 25 (6.3%) | 25 (11.7%) | 0.02 |
| Renal involvement, n (%) | 34 (5.6%) | 20 (5%) | 14 (6.6%) | 0.431 |
| N comorbidities | 0.292 | |||
| 0 | 215 (35.2%) | 143 (36%) | 72 (33.8%) | |
| 1 | 194 (31.8%) | 131 (33%) | 63 (29.6%) | |
| 2 | 114 (18.7%) | 77 (19.4%) | 37 (17.4%) | |
| 3 + | 82 (13.4%) | 46 (11.6%) | 36 (16.9%) | |
| CRP, mg/dL | 9.3 ± 7.1 | 8.9 ± 7 | 10 ± 7.1 | 0.06 |
| Lymphocytes, cells/mm3 | 1013.3 ± 571.5 | 1035 ± 590.2 | 972.3 ± 533.2 | 0.2 |
| Ferritin, pg/mL | 831.5 (460.5–1391) | 916 (465–1528) | 731 (457.5–1140.5) | 5.12 × 10 − 5 |
| D-Dimer, ng/mL | 936 (560.5–1580.5) | 875 (534–1412) | 1009 (613–1918) | 0.027 |
| 4-week crude mortality | 128 (21%) | 74 (18.6%) | 54 (25.4%) | 0.052 |
All continuous values expressed as mean ± standard deviation or by median (interquartile range)
Significant p values (<0.05) are highlighted in bold
The overall estimated 4-week mortality was similar in the two pandemic waves (Fig. 1) albeit a trend toward a worse long-term survival in the 2nd pandemic wave could be inferred from the graphical analysis. At 4 weeks from admittance, the estimated survival in the whole cohort was 0.621 (CI95 = 0.552–0.699).
Fig. 1.
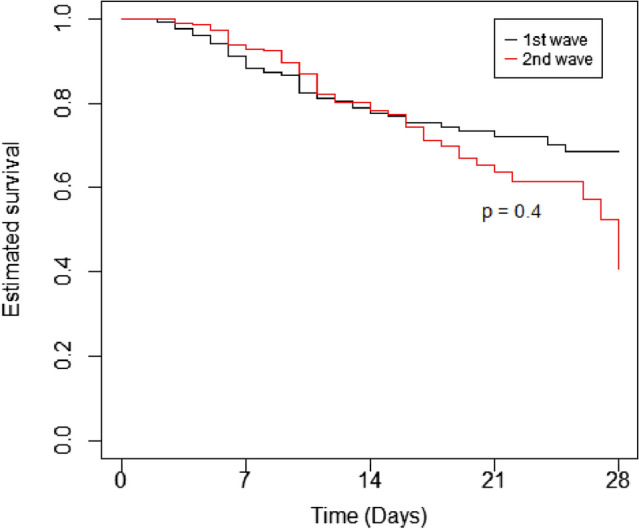
Estimated survival. Estimated survival (Kaplan–Meier curves) in the 1st (black line) and 2nd (red line) pandemic waves
HCQ was mostly used during the 1st pandemic wave while remdesivir during the 2nd. Exposure to steroids was much higher in the 2nd pandemic wave than in the 1st (steroids yes: 2452/2863 days [86.5%] vs 1268/5083 days [24.9%], p < 0.0001); similarly, a higher use of LMWH was observed in the 2nd pandemic wave than in the 1st (LMWH yes: 2545/2863 days [82.2%] vs 3285/5083 days [64.6%], p < 0.0001).
Several variables were associated with mortality after univariate analysis (Table 2). In determining the independent contribution of explanatory variables in multivariable analysis (Table 3), we observed that the severity of SARS-CoV-2 infection at presentation as assessed by the S/F ratio (the higher the better) and age (the lower the better) were the strongest predictors of mortality; distribution densities for age and S/F ratio are reported in Supplemental Fig. 1. The persistence of fever was associated with a nearly twofold relative risk of fatal outcome compared to defervescence. The use of LMWH approximately halved the risk of death, while other therapies did not have any influence on survival. HCQ use was mostly reserved to younger patients (HCQ yes: 53 ± 4.2 vs HCQ no: 71.6 ± 14.6 years, p = 1.03 × 10 − 12) while remdesivir (according to Italian prescription guidelines) to patients with a higher S/F ratio at admittance (remdesivir yes: 350.5 ± 82.6 vs remdesivir no: 270.7 ± 111.2 S/F ratio, p = 1.41 × 10 − 6), thus explaining their protective effect after univariate analysis and their consequent lack of significance after multivariable adjustment. The use of remdesivir in patients with milder disease was also testified by lower CRP (remdesivir yes: 7.3 ± 5 vs remdesivir no: 9.4 ± 7.2 mg/dL, p = 0.022) and D-Dimer values (remdesivir yes: 1136.6 ± 1213.6 vs remdesivir no: 2737.2 ± 7388.6, p = 7.2 × 10 − 5) in treated patients. It is worth noting that according to local prescribing guidelines, remdesivir is indeed reserved to patients with mild disease, with disease duration ≤ 10 days and undergoing low-flow oxygen therapy. Restricting the analysis to such cases (n = 183), we just observed 10 deaths (5.4%), none in the remdesivir group (p = not significant). Similarly, antibiotics had an apparent detrimental effect because used in patients with worse baseline respiratory conditions (antibiotics yes: 265 ± 110.5 vs antibiotics no: 314.6 ± 105.7 S/F ratio, p = 4.77 × 10 − 6) and increased CRP values (antibiotics yes: 10.4 ± 7.2 vs antibiotics no: 5.2 ± 5.1 mg/dL, p = 4.49 × 10 − 18). CRP, along with D-Dimer, was indeed the main laboratory parameter associated with an increased mortality in SARS-CoV-2 patients (Table 4).
Table 2.
Clinical variables associated with mortality by time-dependent Cox regression by univariate analysis
| Overall (n = 610) | 1st wave (n = 397) | 2nd wave (n = 213) | ||||
|---|---|---|---|---|---|---|
| Variable | HR (CI95) | p | HR (CI95) | p | HR (CI95) | p |
| SaO2/FiO2 ratioa | 0.992 (0.99–0.994) | 3.04 × 10 − 12 | 0.993 (0.99–0.997) | 2.16 × 10 − 4 | 0.993 (0.99–0.996) | 1.92 × 10 − 5 |
| Age | 1.076 (1.059–1.093) | 2.22 × 10 − 15 | 1.088 (1.065–1.112) | 2.56 × 10 − 14 | 1.063 (1.035–1.092) | 6.17 × 10 − 6 |
| Gender (male) | 0.75 (0.52–1.077) | 0.1192 | 0.588 (0.367–0.942) | 0.027 | 0.758 (0.432–1.331) | 0.336 |
| Hypertension | 1.623 (1.444–2.30) | 0.007 | 1.986 (1.245–3.168) | 0.004 | 1.148 (0.671–1.963) | 0.615 |
| Diabetes | 1.437 (0.959–2.153) | 0.079 | 1.779 (1.07–2.954) | 0.026 | 0.937 (0.471–1.865) | 0.852 |
| Cardiovascular disease | 2.947 (2.061–4.212) | 3.04 × 10 − 9 | 3.895 (2.049–6.297) | 2.88 × 10 − 8 | 2.096 (1.208–3.638) | 0.008 |
| Malignancy | 1.639 (1.071–2.508) | 0.023 | 1.675 (0.948–2.959) | 0.07 | 1.474 (0.775–2.803) | 0.236 |
| COPD | 2.04 (1.333–3.125) | 0.001 | 2.137 (1.228–3.719) | 0.007 | 1.951 (0.993–3.832) | 0.053 |
| Neurological disease | 2.128 (1.32–3.432) | 0.002 | 3.277 (1.761–6.098) | 1.79 × 10 − 4 | 1.272 (0.6–2.695) | 0.53 |
| Renal disease | 2.482 (1.47–4.193) | 6.68 × 10 − 4 | 2.779 (1.38–5.595) | 0.004 | 3.03 (1.355–6.775) | 0.007 |
| Time to hospitalization | 0.952 (0.909–0.998) | 0.04 | 0.96 (0.903–1.02) | 0.184 | 0.938 (0.862–1.021) | 0.141 |
| Fever | 1.613 (2.176–2.136) | 8.6 × 10 − 4 | 1.756 (1.048–2.942) | 0.033 | 1.419 (0.842–2.39) | 0.188 |
| HCQb | 0.571 (0.40–0.815) | 0.002 | 0.357 (0.211–0.602) | 1.14 × 10–4 | NA | NA |
| Remdesivirb | 0.146 (0.02–1.015) | 0.052 | NA | NA | 0.134 (0.019–0.974) | 0.047 |
| Use of antibioticsb | 2.19 (1.251–3.837) | 0.006 | 1.473 (0.75–2.895) | 0.262 | 2.068 (0.742–5.764) | 0.165 |
| LMWH | ||||||
| No (reference) | – | – | – | – | – | – |
| Low dose | 0.488 (0.321–0.741) | 7.57 × 10 − 4 | 0.637 (0.376–1.079) | 0.094 | 0.171 (0.085–0.346) | 4.55 × 10 − 7 |
| High dose | 0.47 (0.292–0.757) | 0.002 | 0.361 (0.183–0.712) | 0.003 | 0.303 (0.152–0.603) | 6.78 × 10 − 4 |
| Steroid | 0.995 (0.989–1.002) | 0.168 | 0.99 (0.981–0.999) | 0.031 | 0.992 (0.98–1.004) | 0.206 |
Significant p values (<0.05) are highlighted in bold
aAt admission
bAt least 1 day of treatment during hospitalization
Table 3.
Clinical variables associated with mortality by time-dependent Cox regression, multivariate analysis
| Overall (n = 610) | 1st wave (n = 397) | 2nd wave (n = 213) | ||||
|---|---|---|---|---|---|---|
| Variable | HR (CI95) | p | HR (CI95) | p | (CI95) | p |
| SaO2/FiO2 ratioa | 0.993 (0.991–0.995) | 5.61 × 10 − 9 | 0.996 (0.992–0.999) | 0.008 | 0.996 (0.993–0.999) | 0.01 |
| Age | 1.06 (1.04–1.08) | 1.24 × 10 − 9 | 1.082 (1.052–1.112) | 3.13 × 10 − 8 | 1.046 (1.016–1.077) | 0.002 |
| Gender (male) | NA | NA | 0.912 (0.525–1.584) | 0.745 | NA | |
| Hypertension | 1.217 (0.852–1.739) | 0.280 | 1.281 (0.768–2.136) | 0.342 | NA | |
| Diabetes | 1.058 (0.694–1.612) | 0.794 | 1.243 (0.703–2.196) | 0.454 | NA | |
| Cardiovascular diseases | 1.408 (0.939–2.11) | 0.097 | 2.428 (1.392–4.232) | 0.002 | 1.055 (0.548–2.034) | 0.872 |
| Malignancy | 1.115 (0.715–1.736) | 0.632 | 1.63 (0.889–2.989) | 0.114 | NA | |
| COPD | 1.162 (0.746–1.81) | 0.507 | 1.496 (0.832–2.687) | 0.178 | 0.748 (0.36–1.553) | 0.436 |
| Neurological diseases | 1.333 (0.794–2.237) | 0.276 | 1.542 (0.694–3.429) | 0.287 | NA | |
| Renal diseases | 1.61 (0.922–2.813) | 0.094 | 1.641 (0.77–3.495) | 0.199 | 2.703 (1.047–6.978) | 0.039 |
| Time to hospitalization | 0.979 (0.938–1.022) | 0.338 | NA | NA | NA | |
| Fever | 1.965 (1.398–2.762) | 9.96 × 10 − 5 | 1.666 (0.957–2.902) | 0.071 | NA | |
| HCQb | 1.06 (0.702–1.603) | 0.78 | 0.884 (0.463–1.688) | 0.708 | NA | |
| Remdesivirb | 0.464 (0.062–3.452) | 0.454 | NA | NA | 0.293 (0.037–2.348) | 0.248 |
| Use of antibioticsb | 1.347 (0.749–2.421) | 0.319 | NA | NA | NA | |
| LMWH | ||||||
| No (reference) | – | – | – | – | – | – |
| Low dose | 0.465 (0.304–0.713) | 4.36 × 10 − 4 | 0.675 (0.388–1.174) | 0.164 | 0.253 (0.123–0.521) | 1.92 × 10 − 4 |
| High dose | 0.427 (0.265–0.688) | 4.72 × 10 − 4 | 0.303 (0.147–0.626) | 0.001 | 0.401 (0.196–0.82) | 0.012 |
| Steroid | NA | NA | 1 (0.989–1.01) | 0.931 | NA | NA |
Significant p values (<0.05) are highlighted in bold
aAt admission
bAt least 1 day of treatment during hospitalization
Table 4.
Laboratory variables (at admission) associated with mortality by Cox regression
| Variable | Overall (n = 610) | 1st wave (n = 397) | 2nd wave (n = 213) | |||
|---|---|---|---|---|---|---|
| HR (CI95) | p | HR (CI95) | p | HR (CI95) | p | |
| Univariate | ||||||
| CRP, mg/dL | 1.064 (1.041–1.088) | 3.49 × 10 − 8 | 1.046 (1.016–1.077) | 0.003 | 1.059 (1.022–1.09) | 0.002 |
| Lymphocytes, cells/mm3 | 0.9999 (0.9996–1.0002) | 0.585 | 0.9997 (1.00026–1.00028) | 0.345 | 1.0001 (0.9997–1.0006) | 0.475 |
| Ferritin | 0.9999 (0.9997–1.00001) | 0.426 | 0.9998 (0.9996–1.00003) | 0.099 | 0.9999 (0.9996–1.0003) | 0.744 |
| D-Dimer | 1.00003 (1.00002–1.00005) | 1.11 × 10 − 5 | 1 (1.00002–1.00009) | 0.003 | 1.00004 (1.00002–1.00005) | 3.73 × 10 − 5 |
| Multivariate | ||||||
| CRP, mg/dL | 1.057(1.030–1.084) | 2.2 × 10 − 5 | 1.046 (1.0125–1.081) | 0.007 | 1.043 (1.0006–1.089) | 0.047 |
| D-Dimer | 1.00002 (1.00001–1.00004) | 9.84 × 10 − 4 | 1.00006 (1.00002–1.0001) | 0002 | 1.00003 (1.00001–1.00005) | 0.006 |
Upper panel: univariate analysis; lower panel: multivariate analysis
Significant p values (<0.05) are highlighted in bold
Steroids did not exert any protective effect when analyzed in time-dependent models. In the exploratory analysis with time-independent models that accounted for age and S/F ratio at steroid initiation, a trend toward a protective role of dexamethasone ≥ 6 mg was observed (HR = 0.735, CI95 = 0.515–1.051, p = 0.092); this effect was found to be significant when the analysis was restricted to patients with an S/F ratio ≤ 315, that correspond to a P/F ratio equal to 300 according to [31] (HR = 0.634, CI95 = 0.43–0.935, p = 0.0214).
When analyzing the progression to different states (Fig. 2) it can be observed that all the patients required O2 supplementation during the hospital stay and that the vast proportion of subjects required high-flow O2 or CPAP; differences between the two waves are detailed in Supplemental Fig. 2. Notably, when considering the number of days spent in each state, mortality was almost exclusively associated with the use high-flow O2 or CPAP (Supplemental Tables 1–3). The analysis of transition from one state to the other by Cox–Markov models confirmed that age and the severity of lung involvement at admission, along with the persistence of fever, were relevant factor for mortality or progression (Supplemental Table 4).
Fig. 2.
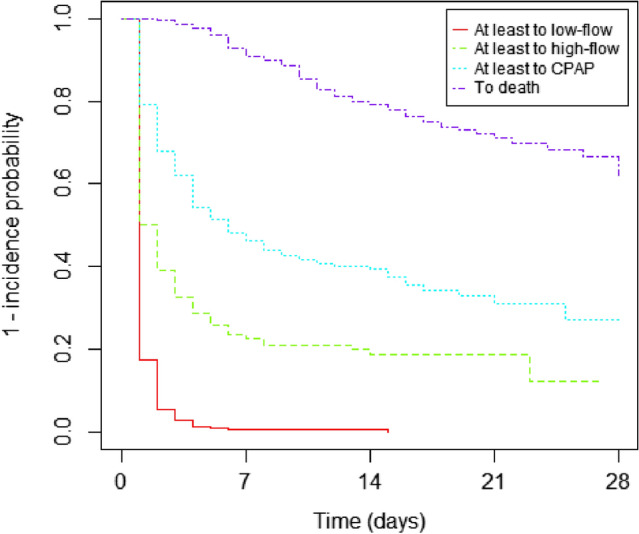
Progression to different states
Discussion
In our retrospective observational study, we evaluated 610 consecutive patients admitted to Internal Medicine wards of our Institution during two pandemic waves of SARS-CoV-2 infection (beginning of March 2020 to the end of April 2020 and November 2020). All the patients were first evaluated in the emergency room and then hospitalized due to the presence of respiratory insufficiency according to previously published criteria [32] while patients requiring mechanical ventilation were excluded from the analysis or right-censored. Our results are hardly comparable with previously published reports due to different inclusion criteria or characteristics of patients. Subjects with a negligible lung involvement, not requiring hospitalization or O2 supplementation due to respiratory insufficiency are often included in other studies, or conversely subjects requiring invasive support may constitute a relevant proportion of analyzed cases. These characteristics may explain the wide mortality range (14–65%) described in the literature as summarized in [33], and indeed, we found that the progression to severe form of disease state requiring high-flow O2 supplementation or CPAP use is relevant in determining the mortality risk (Supplemental Table 4). The mortality we observed in patients requiring noninvasive ventilation (n = 313) is similar, yet slightly higher, to in-hospital fatality rates previously reported [34] (at 3 weeks = 0.414; data not shown). The fact that CPAP was the ceiling of treatment in the majority of our patients may justify the high mortality we observed in patients who underwent noninvasive ventilation, according to previously published evidences [35].
To evaluate hypoxemia as well to determine the presence of acute respiratory distress syndrome (ARDS), we used the S/F ratio, an easy-to-use and noninvasive tool that also allowed us to daily monitor patients’ respiratory condition. Its use in COVID-19 patients has already been advocated by others [36, 37], and indeed, we were able to confirm, as shown in literature, that a decrease in the S/F ratio, as a surrogate of the P/F ratio, increases the mortality risk [37, 38]. Similarly, the increased risk associated with age is in accordance with the literature, including the first data collected in China [26, 39], worldwide [40, 41] and also in the Italian population[42–44].
When comparing survival estimates between the two pandemic waves, we did not find any substantial difference, albeit ties seem to diverge indicating a worse long-term prognosis in the second wave (Fig. 1), accounting for higher overall crude mortalities (Table 1). This could due to a more severe long-term prognosis in frail patients, that is elderly people, whose prevalence was higher in the second wave (Table 1 and Supplemental Fig. 1, left panel). Indeed, age emerged as the strongest predictor of mortality, along with the severity of lung involvement, in both waves and in the combined population. Nonetheless, the effect of age seems largely mitigated by other factors as, for instance LMWH therapy, whose exposure was higher in the 2nd than in 1st wave (82.2% vs 64.4%) and globally associated with a reduced mortality risk in multivariable models (Table 3). These findings are in line with previous reports showing that LMWH, given at prophylactic dose, is associated with prolonged survival [45–47]. Of interest, in our population, the use of LMWH at anticoagulant doses yielded no superior benefit compared to the prophylactic dosage. These results are at variance with another report [48]; the reasons for these differences are unknown and may be related to the type of analysis (Cox regression vs Cox regression with time-dependent covariates) and to the severity of analyzed patients as indeed the same authors observed that the effect of high-dose LMWH was restricted to high-intensity care wards, or in general to the adjustment we made by different covariates and confounding factors.
Besides LMWH, no other therapy seemed to influence survival in the time-dependent model. Remdesivir had an apparent protective effect, yet its use was restricted to patients with mild disease, as testified by a higher S/F ratio at admittance, lower CRP and/or D-Dimer values, that is the two laboratory variables associated with poor survival. Of interest and despite previous reports indicating a survival benefit associated with the use of steroids [17, 18], we could not observe a substantial effect on mortality by this class of drugs, even if it should be observed that our study is retrospective and not specifically designed to evaluate the effect of steroids in COVID-19 patients. It should be observed that post hoc analysis of the RECOVERY trial showed that the benefit of dexamethasone is related to the severity of illness, at least in those patients receiving respiratory support and that it is restricted to subjects experiencing symptoms > 7 days [21]. In our population, exposure to steroids was much higher in the 2nd wave than in the 1st wave (97.2% vs 24.7%) and unrestricted to the severity of the disease, age at presentation or disease duration; moreover, we did not discriminate between dexamethasone or methylprednisolone as the effect on COVID-19 is not theoretically related to a specific class [49]. We cannot tell whether these aspects could have influenced our results and we cannot completely rule out a potential benefit of steroids, albeit this benefit in unselected hospitalized COVID-19 patients seems dwarfed by other factors. Indeed, in the exploratory analysis we conducted, 6 mg dexamethasone or equivalent reduced the mortality risk only in severely ill patients and in relation to age and the severity of lung involvement. This finding is in accordance with published guidelines that suggest the possible use of dexamethasone 6 mg/day in inpatients with COVID-19 requiring oxygen supplementation (strength of recommendation: weak; certainty of evidence: low) [50].
Fever is one of the cardinal presenting symptoms of COVID-19, yet data about its significance as a predictor of mortality are scant. Previous reports have described the persistence of fever as a prognostic factor for progression of COVID-19 in patients with mild or moderate disease [51] that is related with overall mortality in hospitalized [52] as well as in ventilated patients [53]. Our results shed new light on these findings as fever seems the only predictive clinical symptom predictive of poor prognosis after correction for other relevant risk factors, such as age or the severity of hypoxia. Of interest, it has been observed that targeted temperature management did not have an effect on outcomes in critically ill COVID-19 patients [38], yet we cannot exclude that pursuing normothermia in non-ICU patients is beneficial just because in the time-dependent analysis defervescence is associated with a reduced mortality.
The present study has advantages and limitations. Among the strengths of our research, we can list the through and daily collection of data to better evaluate in a time-dependent manner the medical intervention conditioned on the patient’s evolving state; moreover, we confronted the first and second waves to understand possible difference in clinical management and risk factors between two different periods for the same disease before the availability of SARS-CoV-2 vaccines. A shortcoming of our study is the relatively small sample size of the second wave, this is mainly due because we collected who arrived during its peak weeks to represent the main population characteristics of that period. We cannot tell for sure whether the different sample size between the two waves could have influenced the results, even though this seems unlikely as the main predictors of mortality do not change between the two waves (Table 2). This is also a retrospective single-center study and hence the studied population could be biased by local referral rules of patient to the hospital as well as local practice guidelines. In our analysis, we focused on risk factors known by the literature during data collection, hence other variables of potential interest could have been overlooked, yet their role cannot be further explored because medical records and data were manually extracted and not systematically collected. In our study, we limited the observation to 4 weeks, being this a reasonable time in survival studies of hospitalized patients and because just only a fraction of patients was hospitalized for longer period (n = 34, 5.5%), not allowing reliable long-term estimates. Lastly, frail patients were enriched in the second wave for some unknown reason; we may only speculate that a better preparedness of the health system as well as a more appropriate domiciliary use of therapies could have prevented the hospitalization of younger patients in the second wave [54].
In conclusion, despite the evolving knowledge about COVID-19 pandemic, the overall outcome of SARS-CoV-2 infection seemed unchanged over time between the 1st and 2nd pandemic waves. An improved knowledge and better use of available therapies as well as a timely assistance of affected subjects could actually improve the overall outcomes at the net effect of frailty of patients. Novel therapies as those listed in the a portfolio of 10 potential COVID-19 therapeutics released by the European Commission and including antiviral monoclonal antibodies, oral antivirals and immunomodulators [55] may offer new possibilities and improve the overall outcome of hospitalized subjects; further efforts are needed to elucidate their effect on the bedside in unselected patients as those we describe in our paper and as the pandemic keep on striking the world with new variants that may have a clinical and epidemiological impact [56].
Supplementary Information
Below is the link to the electronic supplementary material.
Declarations
Conflict of interest
The authors declare they have no conflicts of interest. This work was not supported by any grant.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Ospedale Maggiore Policlinico - Comitato Etico Area B (approval number, 342_2020).
Human and animal rights statement
The authors state that this research was conducted in accordance with the Helsinki Declaration as revised in 2008.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Deborah Blanca, Email: deborah.blanca@unimi.it.
Lorenzo Beretta, Email: lorenzo.beretta@policlinico.mi.it.
References
- 1.World Health Organization, Mission China Joint (2020) Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19). WHO-China Jt Mission Coronavirus Dis 2019. 2019.
- 2.WHO COVID-19 global table data May 5th 2021 at 10
- 3.Xu XW, Wu XX, Jiang XG, et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ. 2020;368:1–7. doi: 10.1136/bmj.m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA J Am Med Assoc. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ejaz H, Alsrhani A, Zafar A, et al. COVID-19 and comorbidities: deleterious impact on infected patients. J Infect Public Health. 2020;13:1833–1839. doi: 10.1016/j.jiph.2020.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petersen E, Koopmans M, Go U, et al. Comparing SARS-CoV-2 with SARS-CoV and influenza pandemics. Lancet Infect Dis. 2020;20:e238–e244. doi: 10.1016/S1473-3099(20)30484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh S, Khera D, Chugh A, et al. Efficacy and safety of remdesivir in COVID-19 caused by SARS-CoV-2: a systematic review and meta-analysis. BMJ Open. 2021;11:1–9. doi: 10.1136/bmjopen-2020-048416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gholamhoseini MT, Yazdi-Feyzabadi V, Goudarzi R, Mehrolhassani MH. Safety and efficacy of remdesivir for the treatment of COVID-19: a systematic review and meta-analysis. J Pharm Pharm Sci. 2021;24:237–245. doi: 10.18433/jpps31870. [DOI] [PubMed] [Google Scholar]
- 9.Rezagholizadeh A, Khiali S, Parvin Sarbakhsh TE-M. Remdesivir for treatment of COVID-19; an updated systematic review and meta-analysis. Eur J Pharmacol. 2021;896:173926. doi: 10.1016/j.ejphar.2021.173926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Piscoya A, Ng-Sueng LF, del Riego AP, et al. Efficacy and harms of remdesivir for the treatment of COVID-19: a systematic review and meta-analysis. PLoS ONE. 2020;15:1–19. doi: 10.1371/journal.pone.0243705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elsawah HK, Elsokary MA, Abdallah MS, ElShafie AH. Efficacy and safety of remdesivir in hospitalized Covid-19 patients: systematic review and meta-analysis including network meta-analysis. Rev Med Virol. 2021 doi: 10.1002/rmv.2187. [DOI] [PubMed] [Google Scholar]
- 12.Shrestha DB, Budhathoki P, Syed N-I-H, et al. Remdesivir: a potential game-changer or just a myth? A systematic review and meta-analysis. Life Sci. 2021;264:118663. doi: 10.1016/j.lfs.2020.118663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gebrie D, Getnet D, Manyazewal T. Efficacy of remdesivir in patients with COVID-19: a protocol for systematic review and meta-analysis of randomised controlled trials. BMJ Open. 2020;10:1–5. doi: 10.1136/bmjopen-2020-039159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The RECOVERY Collaborative Effect of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. 2020;383:2030–2040. doi: 10.1056/nejmoa2022926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cao B, Wang Y, Wen D, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382:1787–1799. doi: 10.1056/nejmoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.The RECOVERY Collaborative Group Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/nejmoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sterne JAC, Murthy S, Diaz JV, et al. Association between administration of systemic corticosteroids and mortality among critically Ill patients with COVID-19: a meta-analysis. JAMA J Am Med Assoc. 2020;324:1330–1341. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tomazini BM, Maia IS, Cavalcanti AB, et al. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: the CoDEX randomized clinical trial. JAMA J Am Med Assoc. 2020;324:1307–1316. doi: 10.1001/jama.2020.17021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ranjbar K, Moghadami M, Mirahmadizadeh A, et al. Correction to: methylprednisolone or dexamethasone, which one is superior corticosteroid in the treatment of hospitalized COVID-19 patients: a triple-blinded randomized controlled trial. BMC Infect Dis. 2021;21:1–8. doi: 10.1186/s12879-021-06130-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hasan SS, Kow CS, Mustafa ZU, Merchant HA. Does methylprednisolone reduce the mortality risk in hospitalized COVID-19 patients? A meta-analysis of randomized control trials. Expert Rev Respir Med. 2021 doi: 10.1080/17476348.2021.1925546. [DOI] [PubMed] [Google Scholar]
- 21.Calzetta L, Aiello M, Frizzelli A, et al. Dexamethasone in patients hospitalized with COVID-19: whether, when and to whom. J Clin Med. 2021;10:1607. doi: 10.3390/jcm10081607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghosn L, Chaimani A, Evrenoglou T, Davidson M, Graña C, Schmucker C, Bollig C, Henschke N, Sguassero Y, Nejstgaard CH, Menon S, Nguyen TV, Ferrand G, Kapp P, Riveros C, Ávila C, Devane D, Meerpohl JJ, Rada G, Hróbjartsson A, Grasselli G, Tovey D, Ravaud P BI (2021) Association Between Administration of Systemic Corticosteroids and Mortality Among Critically Ill Patients With COVID-19: A Meta-analysis. 10.1002/14651858.CD013881.www.cochranelibrary.com
- 23.Sadeghipour P, Talasaz AH, Rashidi F, et al. Effect of intermediate-dose vs standard-dose prophylactic anticoagulation on thrombotic events, extracorporeal membrane oxygenation treatment, or mortality among patients with COVID-19 admitted to the intensive care unit: the INSPIRATION randomized clinic. JAMA J Am Med Assoc. 2021;325:1620–1630. doi: 10.1001/jama.2021.4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Contou D, Fraissé M, Pajot O, et al. Comparison between first and second wave among critically ill COVID-19 patients admitted to a French ICU: no prognostic improvement during the second wave? Crit Care. 2021;25:1–4. doi: 10.1186/s13054-020-03449-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Criteri di appropriatezza per i setting assistenziali di gestione ospedaliera dei pazienti affetti da COVID-19 – Ver. 2.0. https://www.agenas.gov.it/images/agenas/covid-19/Appropriatezza_setting_ospedalieri__COVID_2.0_17_6_2021.pdf
- 26.Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/nejmoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang I, Pranata R. Lymphopenia in severe coronavirus disease-2019 (COVID-19): systematic review and meta-analysis. J Intensive Care. 2020;8:1–10. doi: 10.1186/s40560-020-00453-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aliberti S, Amati F, Pappalettera M, et al. COVID-19 multidisciplinary high dependency unit: the Milan model. Respir Res. 2020;21:1–12. doi: 10.1186/s12931-020-01516-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meira-Machado LF, de Uña-Álvarez J, Cadarso-Suárez C, Andersen PK. Multi-state models for the analysis of time-to-event data. Stat Methods Med Res. 2009;18:195–222. doi: 10.1177/0962280208092301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.R Core Team. (2017) R: A language and environment for statistical computing. In: R Found Stat Comput. Vienna, Austria
- 31.Rice TW, Wheeler AP, Bernard GR, et al. Comparison of the SpO2/FIO2 ratio and the PaO2/FIO2 ratio in patients with acute lung injury or ARDS. Chest. 2007;132:410–417. doi: 10.1378/chest.07-0617. [DOI] [PubMed] [Google Scholar]
- 32.https://www.covid19treatmentguidelines.nih.gov/ (2021) Outpatient Management of Acute COVID-19. 1–8
- 33.Cosco TD, Best J, Davis D, et al. What is the relationship between validated frailty scores and mortality for adults with COVID-19 in acute hospital care? A systematic review. Age Ageing. 2021;50:608–616. doi: 10.1093/ageing/afab008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vaschetto R, Barone-Adesi F, Racca F, et al. Outcomes of COVID-19 patients treated with continuous positive airway pressure outside the intensive care unit. ERJ Open Res. 2021;7:00541–02020. doi: 10.1183/23120541.00541-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aliberti S, Radovanovic D, Billi F, et al. Helmet CPAP treatment in patients with COVID-19 pneumonia: a multicentre cohort study. Eur Respir J. 2020 doi: 10.1183/13993003.01935-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Catoire P, Tellier E, de Rivière C. Assessment of the SpO2/FiO2 ratio as a tool for hypoxemia screening in the emergency department. Am J Emerg Med. 2020;44:116–120. doi: 10.1016/j.ajem.2021.01.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu X, Jiang L, Chen T, et al. Continuously available ratio of SpO2/FiO2serves as a noninvasive prognostic marker for intensive care patients with COVID-19. Respir Res. 2020;21:1–4. doi: 10.1186/s12931-020-01455-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Corradini E, Ventura P, Ageno W, et al. Clinical factors associated with death in 3044 COVID-19 patients managed in internal medicine wards in Italy: results from the SIMI-COVID-19 study of the Italian society of internal medicine (SIMI) Intern Emerg Med. 2021;16:1005–1015. doi: 10.1007/s11739-021-02742-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. JAMA J Am Med Assoc. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA J Am Med Assoc. 2020;323:1775–1776. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 43.Unim B, Palmieri L, Lo Noce C, et al. Prevalence of COVID-19-related symptoms by age group. Aging Clin Exp Res. 2021 doi: 10.1007/s40520-021-01809-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ciceri F, Castagna A, Rovere-querini P, et al. Early predictors of clinical outcomes of COVID-19 outbreak in Milan Italy. Clin Immunol. 2020;217:108509. doi: 10.1016/j.clim.2020.108509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kakkar AK, Cimminiello C, Goldhaber SZ, et al. Low-molecular-weight heparin and mortality in acutely ill medical patients. N Engl J Med. 2011;365:2463–2472. doi: 10.1056/NEJMoa1111288. [DOI] [PubMed] [Google Scholar]
- 46.Grandone E, Tiscia G, Pesavento R, et al. Use of low-molecular weight heparin, transfusion and mortality in COVID-19 patients not requiring ventilation. J Thromb Thrombolysis. 2021 doi: 10.1007/s11239-021-02429-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rentsch CT, Beckman JA, Tomlinson L, et al. Early initiation of prophylactic anticoagulation for prevention of coronavirus disease 2019 mortality in patients admitted to hospital in the United States: cohort study. BMJ. 2021 doi: 10.1136/bmj.n311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martinelli I, Ciavarella A, Abbattista M, et al. Increasing dosages of low-molecular-weight heparin in hospitalized patients with Covid-19. Intern Emerg Med. 2021 doi: 10.1007/s11739-020-02585-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Prescott HC, Rice TW. Corticosteroids in COVID-19 ARDS: evidence and hope during the pandemic. JAMA J Am Med Assoc. 2020;324:1292–1295. doi: 10.1001/jama.2020.16747. [DOI] [PubMed] [Google Scholar]
- 50.Bassetti M, Giacobbe DR, Bruzzi P, et al. Clinical management of adult patients with COVID-19 outside intensive care units: guidelines from the italian society of anti-infective therapy (SITA) and the Italian society of pulmonology (SIP) Infect Dis Ther. 2021;10:1837–1885. doi: 10.1007/s40121-021-00487-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liao Y, Feng Y, Wang B, et al. Clinical characteristics and prognostic factors of COVID-19 patients progression to severe: a retrospective, observational study. Aging (Albany NY) 2020;12:18853–18865. doi: 10.18632/aging.103931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Drewry AM, Hotchkiss R, Kulstad E. Response to “body temperature correlates with mortality in COVID-19 patients”. Crit Care. 2020;24:4–6. doi: 10.1186/s13054-020-03186-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Choron RL, Butts CA, Bargoud C, et al. Fever in the ICU: a predictor of mortality in mechanically ventilated COVID-19 patients. J Intensiv Care Med. 2021;36:484–493. doi: 10.1177/0885066620979622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dorrucci M, Minelli G, Boros S, et al. excess mortality in Italy during the COVID-19 pandemic : assessing the differences between the first and the second wave, year 2020. Front Public Heal. 2021;9:1–9. doi: 10.3389/fpubh.2021.669209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.European Commission Press release October 2021. https://ec.europa.eu/commission/presscorner/detail/en/ip_21_5366
- 56.Planas D, Saunders N, Maes P, et al. Considerable escape of SARS-CoV-2 Omicron to antibody neutralization. Nature. 2021 doi: 10.1038/s41586-021-04389-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


