Abstract
Upper gastrointestinal (UGI) bleeding after percutaneous coronary intervention (PCI) for acute myocardial infarction (AMI) in ordinary patients is a common complication and poses a dilemma for clinical doctors to treat. In patients with renal impairment, that is more difficult and has rarely been reported. This case report involves an 82-year-old man who received regular hemodialysis and underwent PCI for acute inferior wall ST-segment elevation myocardial infarction. On the third day after PCI, the patient developed acute UGI bleeding, and gastroscopy confirmed that he had developed compound gastroduodenal ulcers (active stage) with hyperemia of the surrounding mucosa. After fasting, blood transfusion, acid inhibition, gastric protection and symptomatic support treatment, the patient’s UGI bleeding remained uncontrolled. Finally, upper gastrointestinal bleeding was stopped by empiric transcatheter arterial embolization (TAE). The patient’s condition was controlled through active treatment, and he was eventually discharged from the hospital. Bleeding complications after coronary stenting often present a dilemma, particularly in patients with renal impairment. Therefore, patients such as this should be thoroughly evaluated before any treatment. In the case of no obvious hemorrhagic spots found on endoscopic examination and failure of conservative medical treatment, empiric transcatheter arterial embolization TAE is a well-tolerated and effective treatment for UGI bleeding.
Keywords: acute myocardial infarction, acute upper gastrointestinal bleeding, empiric transcatheter arterial embolization, renal impairment
Introduction
In patients with renal impairment, the risk factors for acute myocardial infarction (AMI) include traditional risk factors as well as renal impairment specific-risk factors. As a result, patients with renal impairment are more likely to have AMI and higher in-hospital mortality rates [1]. Acute upper gastrointestinal (UGI) bleeding is a common complication during the perioperative period of interventional treatment for AMI. Studies have reported that hemodialysis is a risk factor for UGI bleeding [2]. Therefore, patients with renal impairment and AMI during perioperative intervention are more likely to experience acute UGI bleeding and higher mortality. More data from future studies are required to confirm this hypothesis. This case report involved a patient with acute renal impairment who experienced acute UGI bleeding after percutaneous coronary intervention (PCI) for AMI. It illustrates potential interactions between cardiovascular and metabolic diseases, that is AMI complicated by gastrointestinal hemorrhage superimposed on acute renal impairment. Here, we describe the patient’s rescue process and prognosis. Empiric transcatheter arterial embolization (TAE) may be the most reasonable treatment for these patients.
Case presentation
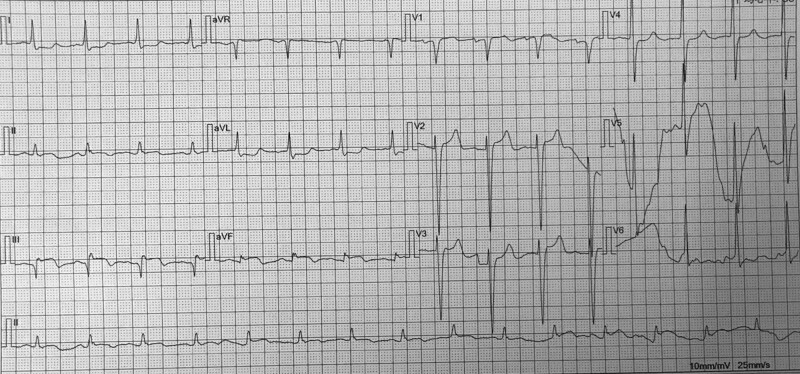
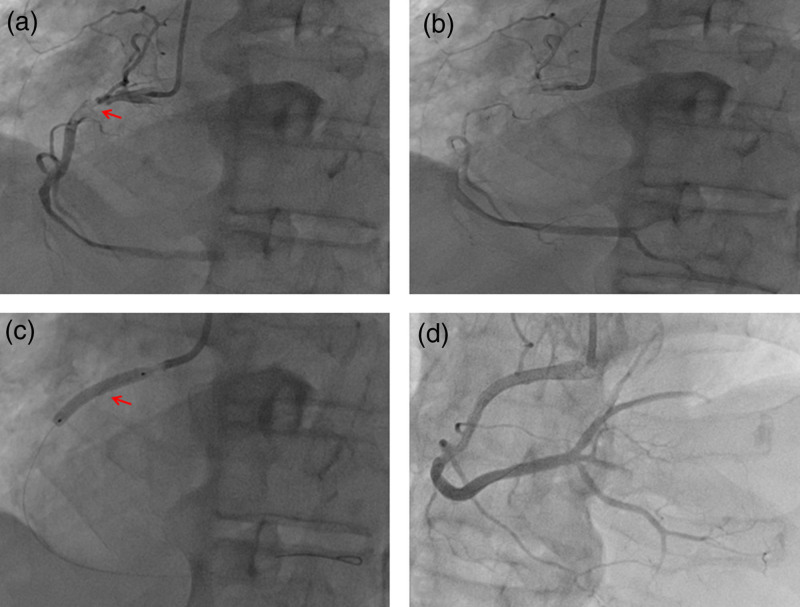
An 82-year-old male patient undergoing hemodialysis for more than 7 months was admitted to Chenzhou Third People’s Hospital (Chenzhou, Hunan, China) for repeated low back pain for more than 2 months on 11 November 2019. His medical history included renal impairment, renal anemia, osteoporosis, lumbar disease (intermittent use of nonsteroidal and glucocorticoids anti-inflammatory drugs for pain management), hypertension, chronic bronchitis and multiple lacunar cerebral infarctions. He had no history of diabetes. In 2011, renal artery stenting was performed for the stenosis of the right renal artery. He had a 30-pack-year smoking history and no family cardiovascular diseases. On the night of admission, the patient suffered a sudden syncope, which lasted for approximately 30 s, and gradually became conscious. Laboratory test results showed hemoglobin (70 g/L), creatinine (368 µmol/L), urea (9.09 mmol/L), serum albumin (27.43 g/L), myohemoglobin (1126.6 ng/mL), troponin I (1.6 ug/L) and N-terminal precursor brain natriuretic peptide (25000 pg/mL) levels. ECG results showed ST elevation in the inferior wall leads (Fig. 1). Emergency coronary angiography (CAG) showed subtotal occlusion and thrombus of the proximal right coronary artery (RCA) (forward flow thrombolysis in myocardial infarction [TIMI] grade I) (Fig. 2a,b). After the emergency CAG, PCI was immediately performed. The RCA was recanalized after thrombus aspiration, and a 3.5 mm × 24 mm drug-coated stent (JW Medical Systems, China) was implanted in the proximal RCA (Fig. 2c,d). During the operation, 6500 U of ordinary heparin and 150 ml of contrast agent were used. Dual antiplatelet therapy was initiated immediately (aspirin 300 mg loading and 100 mg/day maintenance; clopidogrel 300 mg loading and 75 mg/day maintenance) and he was administered a proton pump inhibitor.
Fig. 1.
Patient electrocardiogram (ECG) characteristics showed ST elevation in the inferior wall leads.
Fig. 2.
Angiographic findings: (a,b) Coronary angiography (CAG) revealed subtotal occlusion and thrombus of the proximal right coronary artery (RCA) (forward flow TIMI grade I). (c) A 3.5 mm × 24 mm drug-coated stent (JW Medical Systems, China) was implanted in the proximal RCA. (d) The RCA was recanalized. (a) Red arrow means the stenosis and thrombus. (c) Red arrow means the stent. TIMI, thrombolysis in myocardial infarction.
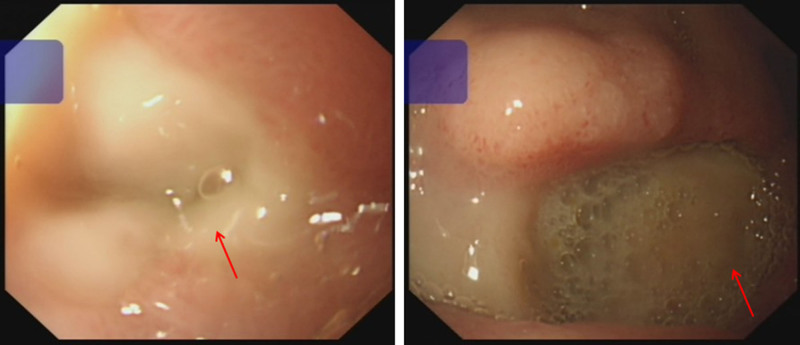
The patient underwent hemodialysis on the first day after PCI, during which heparin was used. On the third day after PCI, The patient suddenly developed black stool. The immediate hemoglobin level was 51 g/L, which was as low as 46 g/L. Pulse was 87 b.p.m. Blood pressure was 107/57 mmHg. Considering the acute UGI bleeding and stable hemodynamics, he underwent electronic gastroscopy immediately, which revealed compound gastroduodenal ulcers (active stage) with hyperemia of the surrounding mucosa (Fig. 3), and dual antiplatelet therapy was discontinued. After fasting, blood transfusion, fluid rehydration, gastric protection and symptomatic support treatment, the patient’s condition gradually stabilized and he had no black stool. His hemoglobin level was 77 g/L.
Fig. 3.
Electronic gastroscopy revealed compound gastroduodenal ulcers (active stage) with hyperemia of the surrounding mucosa.
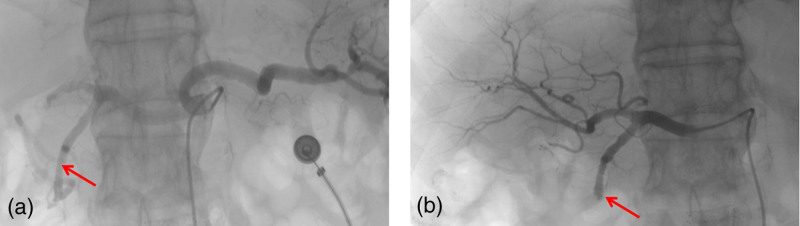
Clopidogrel antiplatelet aggregation therapy was added on the third day after the occurrence of UGI bleeding. Two days later, the patient again had a black stool, and recurrence of bleeding was considered. We immediately organized a multidisciplinary consultation. Finally, empiric embolization of the gastroduodenal artery was performed with polyvinyl alcohol foam embolization particles and embolization spring coils (Cook Incorporated, USA) (Fig. 4). The patient had no black stool after empiric TAE, and his condition was stable. No complications were associated with ischemia. On the 16th day of hospitalization, the patient was discharged. At the last visit, his hemoglobin level was 83 g/L.
Fig. 4.
Angiographic findings: (a) This is an image of a celiac artery. (b) Embolization of the gastroduodenal artery was performed with polyvinyl alcohol foam embolization particles and embolization spring coils. Red arrow means the gastroduodenal artery.
Discussion
We describe the process of diagnosis and salvage treatment for a case of acute UGI bleeding after PCI for AMI in a patient with acute renal impairment. When acute UGI bleeding occurs after PCI for AMI, the in-hospital and long-term mortality rates can substantially increase to 25.9 and 34.1%, respectively [3]. Then hospitalization rates and mortality rates are much higher for patients with acute renal impairment.
In this case, the risk factors for AMI included traditional cardiovascular risk factors common to the general population, such as age, sex, smoking, and hypertension. Patients with renal impairment also have unique risk factors for renal impairment. Among these renal impairment-specific risk factors, it is speculated that dialysis patterns may contribute to varying degrees of susceptibility to the development of AMI due to differences in hemodynamic stability during dialysis procedures, myocardial injury, endothelial dysfunction, left ventricular hypertrophy remodeling and solute clearance [4]. Epidemiological data show that the incidence of AMI is significantly higher in the dialysis population than in the nondialysis population [5,6]. Research from Taiwan showed that, compared to peritoneal dialysis, hemodialysis was significantly associated with a higher risk of developing AMI, especially 4 years after dialysis was initiated [4]. The differential risk of AMI for different dialysis modalities might be explained by repetitive myocardial injury and worsen the mismatch of oxygen demand and supply in the myocardium during the hemodialysis process.
Although, a few studies have shown that PCI for native vessel stenosis has poorer outcomes in hemodialysis patients than in nonhemodialysis patients. The probability of repeat revascularization after stent implantation to treat new lesions was reported to be as high as up to 19% in hemodialysis patients [7]. However, we had no choice but to implant a stent. The reasons for this are as follows. First, the patient’s emergency coronary angiography revealed acute subtotal occlusion of the proximal right coronary artery, and significant residual stenosis was observed after thrombus aspiration. Second, to date, there are no guidelines at home or abroad that define the strategy for coronary revascularization treatment of AMI in hemodialysis patients. Clinical studies from Japan have reported that drug-coated balloons for in-stent restenosis in hemodialysis patients have worse outcomes [8]. Future clinical studies are needed to determine whether drug-coated balloons can benefit from primary revascularization therapy in hemodialysis patients. Third, according to a consensus document from the Academic Research Consortium for High Bleeding Risk [9,10], this patient was defined as having a high bleeding risk. Because it can reduce the duration of dual antiplatelet aggregation therapy from 6–12 months to 1 month, after years of clinical practice and evidence-based research, the Biofreedom stent has proven to be the first choice for PCI treatment in patients with high bleeding risk, and has become the gold standard for PCI treatment in patients with high bleeding risk [11,12]. Unfortunately, the Biofreedom stent was only recently approved for marketing in China by China’s National Medical Products Administration.
Dual antiplatelet therapy (which combines aspirin with a platelet P2Y12 ADP-receptor inhibitor) is the cornerstone of the therapeutic strategy for the management of patients with acute myocardial infarction undergoing PCI. A 300 mg loading dose of aspirin and a 300 mg loading dose of clopidogrel were administered to the patient immediately after the diagnosis of AMI. Considering the risk of bleeding, guidelines do not recommend ticagrelor as an antithrombotic treatment strategy for end-stage renal disease. However, ticagrelor has a more rapid onset of action and induces more potent and reproducible platelet inhibition than clopidogrel, including in patients with chronic kidney disease [13]. Pharmacodynamic and pharmacokinetic studies have demonstrated that ticagrelor can be safely used in patients with kidney failure, including in those undergoing dialysis [14]. For hemodialysis patients, whether ticagrelor is more beneficial, perhaps the TROUPER trial will provide an answer [15].
Unfortunately, on the third day after PCI, the patient developed acute UGI bleeding and gastroscopy findings confirmed that he had developed compound gastroduodenal ulcers (active stage) with hyperemia of the surrounding mucosa. During a retrospective review of his UGI bleeding complications, we identified that he was at risk in relation to the following risk factors: (1) old age; (2) antiplatelet drugs such as clopidogrel and aspirin further increased the risk of UGI bleeding; (3) the patient had long back pain and had been taking nonsteroidal anti-inflammatory drugs; (4) history of chronic kidney disease; (5) stress; (6) the patient used glucocorticoids intermittently because of low back pain; (7) moderate anemia due to chronic kidney disease; (8) the patient who was treated with heparin underwent hemodialysis therapy after PCI and (9) the patient may have been infected with Helicobacter pylori. Acute UGI bleeding can cause a management dilemma for clinicians who need to balance the discontinuation of antithrombotic drugs to prevent bleeding and possible acute thrombosis of the stent. Considering the patient’s active bleeding, antiplatelet therapy with aspirin and clopidogrel was immediately discontinued. Of course, we administered proton pump inhibitor therapy immediately after the patient developed AMI, which has been proven to reduce the risk of gastrointestinal bleeding [16]. Considering the patient’s progressive decline in hemoglobin levels to 46 g/L, we administered two transfusions. In fact, blood transfusions during the perioperative period of PCI treatment are controversial. The REALITY trial provided a reasonable answer. The trial confirms that a ‘restrictive’ transfusion strategy (triggered by hemoglobin ≤80 g/L) is clinically noninferior to a ‘liberal’ transfusion strategy (triggered by hemoglobin ≤100 g/L), but is less costly [17]. Because the patient temporarily had no active gastrointestinal bleeding, clopidogrel antiplatelet aggregation therapy was added on the third day after the occurrence of UGI bleeding. Unfortunately, the patient again presented with black stools, and active bleeding was considered 2 days later. Considering that no obvious hemorrhagic spots were observed on endoscopy, we immediately organized a multidisciplinary consultation and decided to perform TAE. Because the angiogram did not show any bleeding vessels, empiric embolization of the gastroduodenal artery was performed with polyvinyl alcohol foam embolization particles and embolization spring coils. After empiric TAE, the patient’s condition gradually stabilized and he was discharged successfully. A meta-analysis also reported that empiric embolization is well-tolerated and appears to be as effective as targeted embolization in preventing rebleeding and mortality in patients with angiographically negative acute UGI bleeding [18]. Despite a recent reported 1 month of dual antiplatelet therapy was noninferior to the continuation of therapy for at least 2 additional months with regard to the occurrence of net adverse clinical events and major adverse cardiac or cerebral events and the abbreviated therapy also resulted in a lower incidence of major or clinically relevant nonmajor bleeding in patients at high bleeding risk [19]. Considering the high risk of bleeding, long-term therapy with clopidogrel alone prevented platelet aggregation in this case. At the latest return visit, the patient’s hemoglobin level was 83 g/L, and all indicators were relatively stable.
In conclusion, the treatment of UGI bleeding includes conservative treatment, endoscopic hemostasis, TAE and surgical treatment. Patients at a high risk of bleeding should be thoroughly evaluated before any treatment. Empiric TAE may be well-tolerated and effective for patients with renal impairment during perioperative coronary intervention when both conservative and endoscopic therapies have failed.
Acknowledgements
This research was supported by Scientific research Project of Hunan Provincial Health Commission (No. 202103011188) and Chenzhou Municipal Science and Technology Bureau (ZDYF2020225).
Compliance with ethical standards
Written informed consent for publication was obtained from the patient.
All patient information was obtained from the Department of Cardiology, Chenzhou Third People’s Hospital. All data used and analyzed in the current study are included in this article.
Investigation: J.L. Supervision: Y.G. Writing – original draft; review & editing: R.W.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Chou MT, Wang JJ, Sun YM, Sheu MJ, Chu CC, Weng SF, et al. Epidemiology and mortality among dialysis patients with acute coronary syndrome: Taiwan National Cohort Study. Int J Cardiol 2013; 167:2719–2723. [DOI] [PubMed] [Google Scholar]
- 2.Niikura R, Aoki T, Kojima T, Kawahara T, Yamada A, Nakamura H, et al. Natural history of upper and lower gastrointestinal bleeding in hemodialysis patients: a dual-center long-term cohort study. J Gastroenterol Hepatol 2021; 36:112–117. [DOI] [PubMed] [Google Scholar]
- 3.Chen YL, Chang CL, Chen HC, Sun CK, Yeh KH, Tsai TH, et al. Major adverse upper gastrointestinal events in patients with ST-segment elevation myocardial infarction undergoing primary coronary intervention and dual antiplatelet therapy. Am J Cardiol 2011; 108:1704–1709. [DOI] [PubMed] [Google Scholar]
- 4.Sun CY, Li CY, Sung JM, Cheng YY, Wu JL, Kuo YT, Chang YT. A comparison of the risk of acute myocardial infarction in patients receiving hemodialysis and peritoneal dialysis: a population-based, propensity score-matched cohort study. Atherosclerosis 2020; 307:130–138. [DOI] [PubMed] [Google Scholar]
- 5.Yeh RW, Sidney S, Chandra M, Sorel M, Selby JV, Go AS. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med 2010; 362:2155–2165. [DOI] [PubMed] [Google Scholar]
- 6.Roberts MA, Polkinghorne KR, McDonald SP, Ierino FL. Secular trends in cardiovascular mortality rates of patients receiving dialysis compared with the general population. Am J Kidney Dis 2011; 58:64–72. [DOI] [PubMed] [Google Scholar]
- 7.Shroff GR, Solid CA, Herzog CA. Long-term survival and repeat coronary revascularization in dialysis patients after surgical and percutaneous coronary revascularization with drug-eluting and bare metal stents in the United States. Circulation 2013; 127:1861–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kiriyama H, Kodera S, Minatsuki S, Kaneko H, Kikuchi H, Kiyosue A, et al. Short-term and long-term efficacy of drug-coated balloon for in-stent restenosis in hemodialysis patients with coronary artery disease. Int Heart J 2019; 60:1070–1076. [DOI] [PubMed] [Google Scholar]
- 9.Urban P, Mehran R, Colleran R, Angiolillo DJ, Byrne RA, Capodanno D, et al. Defining high bleeding risk in patients undergoing percutaneous coronary intervention. Circulation 2019; 140:240–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corpataux N, Spirito A, Gragnano F, Vaisnora L, Galea R, Svab S, et al. Validation of high bleeding risk criteria and definition as proposed by the academic research consortium for high bleeding risk. Eur Heart J 2020; 41:3743–3749. [DOI] [PubMed] [Google Scholar]
- 11.Urban P, Meredith IT, Abizaid A, Pocock SJ, Carrié D, Naber C, et al.; LEADERS FREE Investigators. Polymer-free drug-coated coronary stents in patients at high bleeding risk. N Engl J Med 2015; 373:2038–2047. [DOI] [PubMed] [Google Scholar]
- 12.Windecker S, Latib A, Kedhi E, Kirtane AJ, Kandzari DE, Mehran R, et al.; ONYX ONE Investigators. Polymer-based or polymer-free stents in patients at high bleeding risk. N Engl J Med 2020; 382:1208–1218. [DOI] [PubMed] [Google Scholar]
- 13.Bliden KP, Tantry US, Storey RF, Jeong YH, Gesheff M, Wei C, Gurbel PA. The effect of ticagrelor versus clopidogrel on high on-treatment platelet reactivity: combined analysis of the ONSET/OFFSET and RESPOND studies. Am Heart J 2011; 162:160–165. [DOI] [PubMed] [Google Scholar]
- 14.Butler K, Teng R. Pharmacokinetics, pharmacodynamics, and safety of ticagrelor in volunteers with severe renal impairment. J Clin Pharmacol 2012; 52:1388–1398. [DOI] [PubMed] [Google Scholar]
- 15.Laine M, Lemesle G, Burtey S, Cayla G, Range G, Quaino G, et al. TicagRelor Or Clopidogrel in severe or terminal chronic kidney patients Undergoing PERcutaneous coronary intervention for acute coronary syndrome: the TROUPER trial. Am Heart J 2020; 225:19–26. [DOI] [PubMed] [Google Scholar]
- 16.Sehested TSG, Carlson N, Hansen PW, Gerds TA, Charlot MG, Torp-Pedersen C, et al. Reduced risk of gastrointestinal bleeding associated with proton pump inhibitor therapy in patients treated with dual antiplatelet therapy after myocardial infarction. Eur Heart J 2019; 40:1963–1970. [DOI] [PubMed] [Google Scholar]
- 17.Ducrocq G, Calvo G, González-Juanatey JR, Durand-Zaleski I, Avendano-Sola C, Puymirat E, et al.; REALITY investigators. Restrictive vs liberal red blood cell transfusion strategies in patients with acute myocardial infarction and anemia: Rationale and design of the REALITY trial. Clin Cardiol 2021; 44:143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu Q, Funaki B, Navuluri R, Zangan S, Zhang A, Cao D, et al. Empiric transcatheter embolization for acute arterial upper gastrointestinal bleeding: a meta-analysis. AJR Am J Roentgenol 2021; 216:880–893. [DOI] [PubMed] [Google Scholar]
- 19.Valgimigli M, Frigoli E, Heg D, Tijssen J, Jüni P, Vranckx P, et al.; MASTER DAPT Investigators. Dual antiplatelet therapy after PCI in patients at high bleeding risk. N Engl J Med 2021; 385:1643–1655. [DOI] [PubMed] [Google Scholar]