PURPOSE:
We sought to characterize patient-oncologist communication and decision making about continuing or limiting systemic therapy in encounters after an initial consultation, with a particular focus on whether and how oncologists foster shared decision making (SDM).
METHODS:
We performed content analysis of outpatient oncology encounters at two US National Cancer Institute–designated cancer centers audio recorded between November 2010 and September 2014. A multidisciplinary team used a hybrid approach of inductive and deductive coding and theme development. We used a combination of random and purposive sampling. We restricted quantitative frequency counts to the coded random sample but included all sampled encounters in qualitative thematic analysis.
RESULTS:
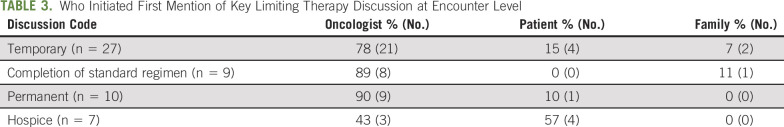
Among 31 randomly sampled dyads with three encounters each, systemic therapy decision making was discussed in 90% (84 of 93) encounters. Thirty-four (37%) broached limiting therapy, which 27 (79%) framed as temporary, nine (26%) as completion of a standard regimen, and five (15%) as permanent discontinuation. Thematic analysis of these 93 encounters, plus five encounters purposively sampled for permanent discontinuation, found that (1) patients and oncologists framed continuing therapy as the default, (2) deficiencies in the SDM process (facilitating choice awareness, discussing options, and incorporating patient preferences) contributed to this default, and (3) oncologists use persuasion rather than deliberation when broaching discontinuation.
CONCLUSION:
In this study of outpatient encounters between patients with advanced cancer and their oncologists, when discussing systemic therapy, there exists a default to continue systemic therapy, and deficiencies in SDM contribute to this default.
INTRODUCTION
Many patients with incurable cancer receive treatments toward the end of life (EOL) that are more aggressive than their preferences would support.1-3 Decisions regarding systemic therapy (ie, chemotherapy and immunotherapy) are inextricably linked to EOL quality both directly and indirectly through hospice enrollment since the Medicare hospice benefit requires forgoing cancer-directed treatments.4
Since the receipt of systemic therapy within the last two weeks of life is as an indicator of poor-quality EOL care,5,6 previous work has focused on (1) predictors and outcomes on the receipt of systemic therapy and (2) evaluating communication and decision making between patients and oncologists. Factors such as patient age,7 previous treatments received,8 race and ethnicity,9 prognostic awareness,10 pre-encounter preferences,11 and early integration of palliative care12 are associated with limiting systemic therapy. Medical record review suggests that definitive (explicit) decisions to discontinue systemic therapy occur only approximately 20% of the time; other processes (eg, deferred decisions or breaks and disruption for radiation or hospitalization) constitute the majority of instances of systemic therapy limitation.13 Although these studies helped identify both the scope and potential mediators of the problem, they often lacked granularity to identify specific communication behaviors contributing to the outcomes observed.
Best practice guidelines for oncology patient-clinician communication consider the principles of shared decision making (SDM) to be particularly important for systemic therapy decision making.14 SDM is a collaborative process with three key steps: creating choice awareness, discussing options, and then integrating patient preferences into the decision.15 Studies that include a focus on SDM when evaluating patient-oncologist communication on the initiation of chemotherapy for incurable cancer revealed deficiencies in all three steps.16-20 However, few studies describe communication and decision making in outpatient oncology visits for advanced cancer after the initial decision to pursue systemic therapy. This study aimed to characterize patient-oncologist communication and decision making about continuing or limiting systemic therapy in encounters after an initial consultation, with a particular focus on whether and how oncologists foster SDM.
METHODS
Overview
We conducted a secondary analysis of outpatient oncology encounters from two US National Cancer Institute–designated cancer centers that were audio recorded between November 2010 and September 2014 as part of a randomized controlled trial comparing two web-based communication tools. The trial protocol is described at ClinicalTrials.gov (NCT00994578),21 and both communication tools are described in detail elsewhere.22,23 These tools focused on recognizing and responding to emotion, not medical decision making. Eligible providers included medical, gynecological, and radiation oncologists. Eligible patients were those with stage IV malignancy, whom the oncologist would not be surprised if they were admitted to an intensive care unit or died within 1 year.24 Three consecutive encounters between each patient-oncologist dyad were audio recorded: one before study intervention and two postintervention. The trial was approved by local institutional review boards.
Sample
We summarize the sample in Figure 1. From available encounters with oncology providers (n = 615), we restricted analysis to patient-oncologist dyads with three consecutive encounters involving the study oncologist to increase the likelihood of finding dyads engaged in systemic therapy decision making. From these encounters (n = 141 dyads, n = 423; full sample), we randomly sampled all three encounters from 7 to 8 dyads from each of the four trial arms (n = 31 dyads, 93 clinical encounters; random sample). We included all three encounters to account for the longitudinal nature of cancer care and decision making.
FIG 1.
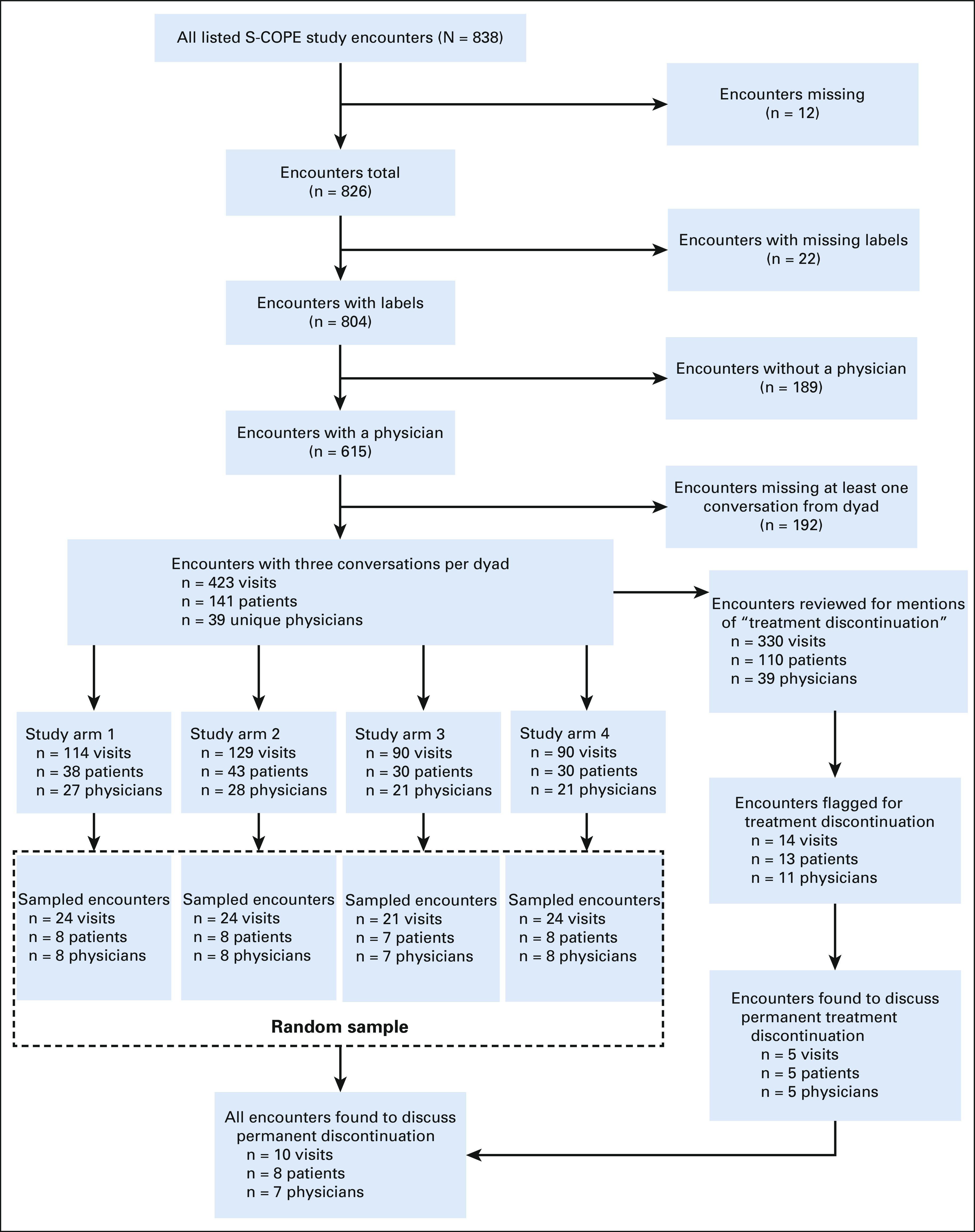
Flow diagram. Sampled encounters, encounter randomly selected from each study arm. Encounters found to discuss permanent treatment discontinuation are the additionally identified encounters for enrichment (aka purposive in the Methods).
In a separate strategy to enrich for encounters discussing permanent discontinuation of systemic therapy, we reviewed the remaining audio from the full sample (110 dyads, n = 330) for mentions of EOL topics, including treatment discontinuation. Details of the identification procedures are described elsewhere.25 The final purposive sample included all encounters discussing permanent discontinuation of systemic therapy. Audio files of included encounters were professionally transcribed for analysis.
Analysis
Our content analysis followed the model described by Elo and Kyngas,26 where, after code development, we counted systemic therapy discussion at the encounter level and finally characterized the discussion. We used a hybrid approach of inductive and deductive coding and theme development to describe systemic therapy decision making, as outlined by Fereday and Muir-Cochrane.27 The three talk model of SDM formed the basis of deductive coding.15 A multidisciplinary research team with expertise in medical oncology, palliative medicine, public health, medical anthropology, and decision science was engaged in analysis. After careful review of 10 randomly selected encounters, four investigators (G.T.W., K.E.K., M.A.L., and A.E.B.) developed a preliminary codebook. The team reviewed a subset of 10 dyads from the random sample (30 encounters) using the preliminary codebook, and new codes were inductively added to capture emergent themes. Existing codes were also refined. Five investigators contributed to the development of the final codebook (G.T.W., K.E.K. G.F.M., O.C.B.-B., and A.E.B.; see the Data Supplement [online only] for codebook). One investigator (K.E.K.) independently reviewed the entire random sample (n = 93 encounters) while a second investigator (G.T.W.) reviewed 45% of the sample (n = 42 encounters). Both investigators reviewed all encounters discussing permanent discontinuation. Encounter-level counts of discussions regarding therapy continuation and limitation, and who initiated the limitation discussion, were affirmed by one investigator who is a practicing medical oncologist (G.T.W.). The study team iteratively discussed their findings to develop themes and refine interpretations.27 Any disagreement among team members was resolved through discussion; we did not calculate inter-rater reliability. We performed coding using Atlas.ti version 8.4.4 (ATLAS.ti Scientific Software Development GmbH) qualitative data analysis software, and we adhered to the Consolidated Criteria for Reporting Qualitative Research Checklist.28
RESULTS
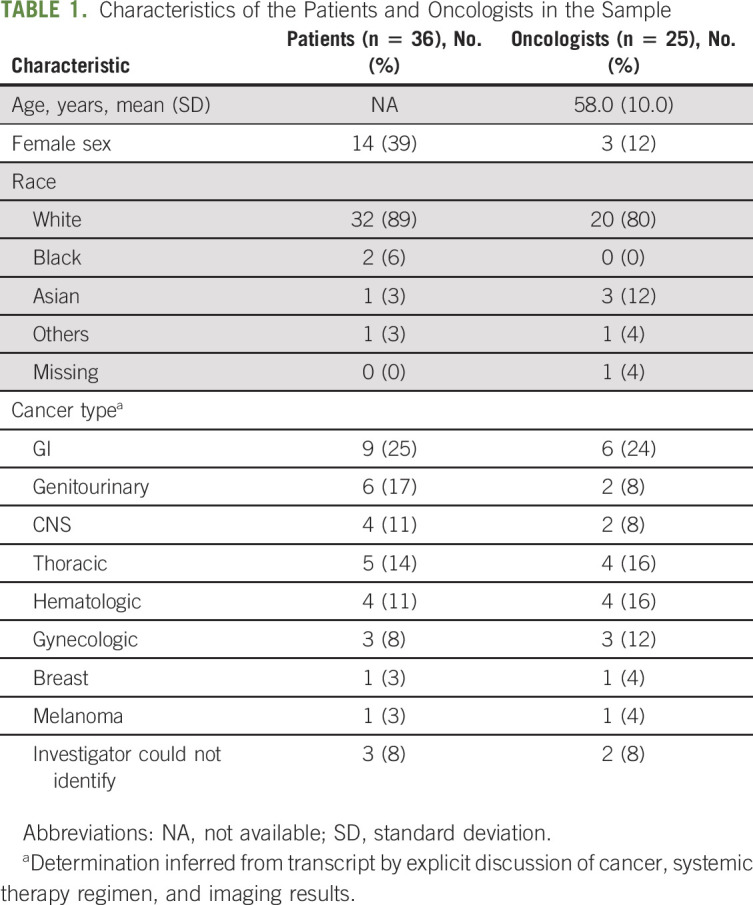
Table 1 describes the demographics and clinical characteristics of patients and oncologists. Of the 36 patients included in this analysis, 39% (12) were women and 89% (32) were White. Of the 25 oncologists, 12% (3) were women and 80% (20) were White, with a mean age of 58.0 years (standard deviation 10.0). The median duration of conversations was 33 minutes (interquartile range 20-44 min, 4-113 min range). All encounters included a physician, most (n = 59; 60%) included an additional clinician (eg, nurse practitioner, fellow, or medical student), and most (n = 63; 68%) also included a patient support person (eg, family member).
TABLE 1.
Characteristics of the Patients and Oncologists in the Sample
Frequency of Systemic Therapy Talk
In the random sample, systemic therapy decision making was discussed in 90% (n = 84 of 93) of encounters (Table 2). Conversations about continuing systemic therapy (n = 76 of 93) were more frequent than limiting therapy (n = 34 of 93), with both continuing and limiting being discussed in 28% (n = 26 of 93) encounters. Among encounters in which systemic therapy decision making was discussed (n = 84), discussing limiting exclusively occurred in only 10% (n = 8 of 84), whereas discussing continuation occurred exclusively in 60% (n = 50 of 84). When a new line of systemic therapy was discussed (n = 26 of 93 encounters), 50% (n = 13 of 26) of encounters exclusively discussed continuing therapy (Table 2).
TABLE 2.
Frequency of Select Patient-Oncologist Discussion Codes at Encounter Level
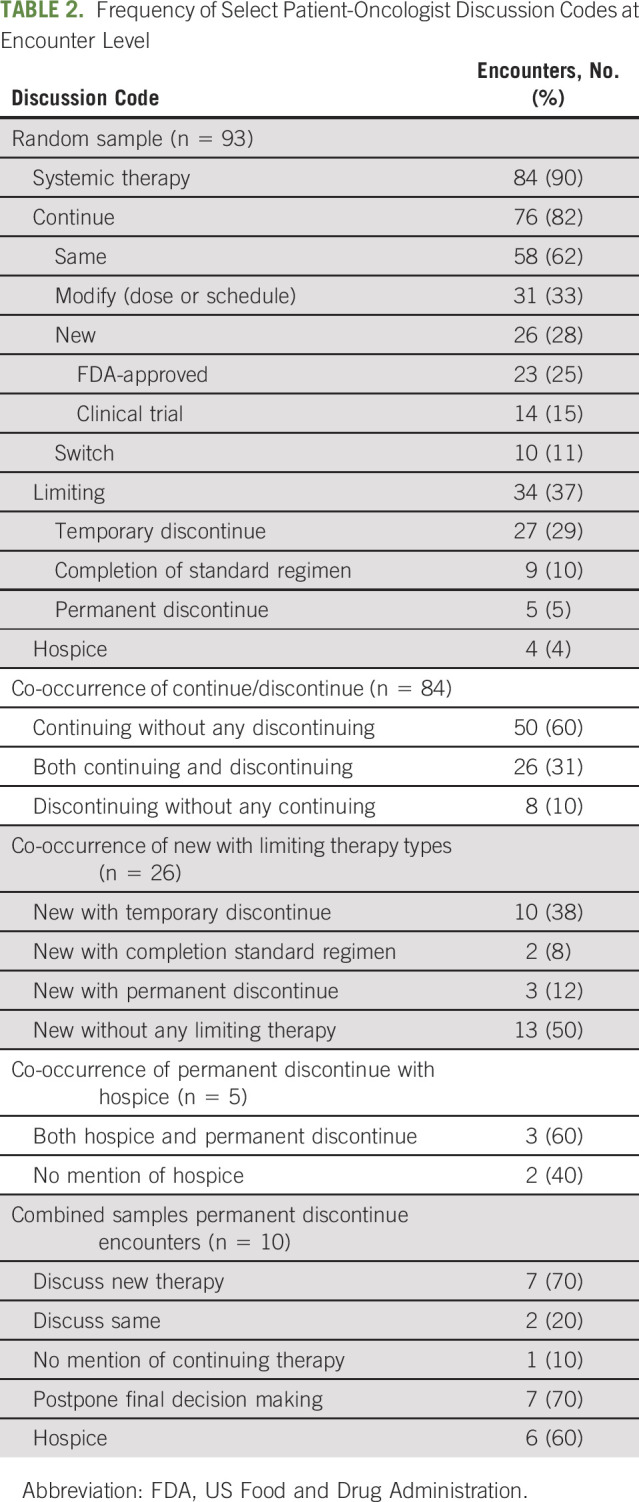
When discussed, limiting therapy was framed as temporary in 79% (n = 27 of 34), completion of a standard regimen in 26% (n = 9 of 34), or permanent discontinuation in 15% (n = 5 of 34) of encounters. Discussions of permanent discontinuation often co-occurred with mentions of hospice 60% (n = 3 of 5) in the random sample and 60% (n = 6 of 10) in the random-plus-purposive sample (Table 2). Oncologists initiated discussions of limiting therapy more frequently than patients or their family members (Table 3).
TABLE 3.
Who Initiated First Mention of Key Limiting Therapy Discussion at Encounter Level
Thematic Analysis
We found that (1) patients and oncologists framed continuing therapy as the default, (2) deficiencies in the SDM process (creating choice awareness, discussing options, and then integrating patient preferences into the decision) contributed to this default, and (3) oncologists used persuasion rather than deliberation in all three types of limiting systemic therapy discussions (temporary discontinuation, completion of standard regimen, and permanent discontinuation).
Continuing Therapy as the Default
Generally, continuing systemic therapy was framed as the default. Criteria for decisions on systemic therapy could be general—“nothing's convincingly worse, and you are doing well with it”—or specific—“we'll have to see how your neuropathy does in time because that is the one thing that frequently leads to dose changes.”
Deficiencies in SDM
Fostering choice awareness was uncommon. Oncologists infrequently offered anticipatory guidance regarding therapy discontinuation (ie, discussion of a future time when the patient may choose to permanently discontinue therapy).
Oncologist: We'll keep an eye on you. We'll keep watching you—any issues, any symptoms—obviously, this is not like a contract. You know it depends on our tolerance—our quality of life.
Patient: Sure.
Oncologist: So as long as we're tolerating, we'll continue. If we need to take a break, we'll take a break. If we need to change plans, we'll change plans.
Generally, decisions about systemic treatment were not framed as involving choices:
Patient: What is next? Is this the permanent protocol?
Oncologist: Generally, for our newly diagnosed Avastin protocol, we've been recommending a second year of what we call Avastin maintenance. It's not on the study, but it's just common sense that if we're significantly improving the number of people who are progression free at a year, continue the Avastin.
Discussing systemic therapy treatment options occurred as a monologue. Oncologists seldom invited patients to participate in deliberation about next steps; instead, they offered think aloud discussions of therapy alternatives, often invoking complex information from published and ongoing clinical trials. They typically concluded these narratives with a statement regarding their treatment recommendation, without referencing patient treatment preferences:
Oncologist: So this [clinical trial drug] could help with the rest of you. Other types of chemo could help with the rest of you. Other clinical trials that you may qualify for in the future, but not yet. Um, radiopharmaceuticals, so kinda liquid radiation called Samarium or Quadramet can be given IV. And then that kind of goes into your bones and radiates lots of different spots. I usually do that—do that if there's lots of spots that are painful.
Oncologists elicited and incorporated patient perceptions about symptoms from cancer and therapy into conversations about systemic therapy; however, discussions of broader patient values and preferences (eg, goals, abilities that are critical to their function, what brings them joy, etc) were much less frequent. Outside of permanent discontinuation of therapy, discussions of how patients perceived trade-offs between the potential for more time and quality-of-life costs to being on therapy were absent. In the context of permanent therapy discontinuation, trade-offs were used as a rhetorical device to convince patients that discontinuation would allow a focus on quality-of-life goals. Additional strategies oncologists used to address actual or anticipated patient resistance to therapy discontinuation included offering hope for future therapies, emphasizing either uncertainty or low benefit from continuing therapy, and reassuring that the patient received standard of care.
Persuasion Over Deliberation
Oncologists used persuasion rather than SDM deliberation in all three patterns for how limiting systemic therapy was discussed: (1) temporary discontinuation, (2) completion of standard regimen, and (3) permanent discontinuation.
Temporary discontinuation.
Discontinuation with the possibility of resumption was described with word choices such as vacation, break, or holiday, signaling both the temporary nature and positive valence of these stops. Whether the temporary stop was introduced by the oncologist or the patient, the basic structure was to (1) suggest some amount of time to wait, (2) outline a criterion to inform decision (eg, tumor marker, imaging, and cancer-related symptoms), and (3) establish consensus to re-evaluate at that point in the future. To help ease the anxiety some patients expressed about discontinuing therapy, some providers referenced future clinical therapy opportunities. For one patient who was hesitant to stop therapy, the oncologist framed the discontinuation of therapy as a pathway for opening new therapy possibilities: “I think that if we took a couple months off, we'd get a better feel for how the cancer's behaving … We have other options like clinical trials, phase one trials, other trials. That may be an option, but you can't really go on any of those with a CT scan that's not showing anything.” Through this lens, discontinuing therapy does not appear as a resignation to stop treating the patient's current cancer but may allow the patient to access therapy options that would otherwise remain inaccessible. Reasons patients initiated temporary discontinuation discussions included schedule conflicts (eg, “we're going on vacation”) or concerns about treatment side effects (eg, “afraid [neuropathy] was gonna be permanent”).
Completion of a standard therapy regimen.
When framing limiting therapy around completion of a standard regimen, oncologists discussed a desire to avoid therapy harms: “But now that because of the weight issues, you know, and the poor appetite, and the blood level, I think it's okay to stop here.” The framing of completing a standard regimen also offered further reassurance: “I usually give additional chemotherapy for two to three cycles. So I shoot for two … we've gotten what the majority of patients get.”
Permanent discontinuation.
When discussing permanent therapy discontinuation, emphasizing harm and/or limited benefit was universal. One clinical rationale was to explain systemic therapy was no longer safe by referencing either patient tolerability or therapy toxicity. The second was to acknowledge that although more systemic therapy was possible, the oncologist believed it was of low benefit and more likely to be harmful. In some encounters, the oncologist explicitly provided this assessment: “if we're giving him chemotherapy—and this happens a lot—and it's not working, what we're doing is getting the side effects without any benefit.” In other encounters, oncologists pursued strategies to have the patient verbalize how he or she might evaluate these trade-offs in light of inherent uncertainties. In one instance, an oncologist used a normalization of extremes by describing hypothetical patients: Someone who would “do anything … [to] eke out a few more months…[and] pursue further therapy at all cost” compared with another who would “say the best way of treating me is to leave me off therapy.” Both strategies involved naming that continuing therapy was of limited or no benefit.
DISCUSSION
In this analysis of communication between outpatients with advanced cancer and oncologists around decisions to continue or limit systemic therapy, we had two main findings. The first was continuing systemic therapy as the default was pervasive and that deficiencies in SDM process—inadequate fostering of choice awareness and integration of patient preferences—contributed to this default. This finding is inconsistent with best practice guidelines for discussing systemic therapy in advanced cancer.14,29 Previous studies restricted to a smaller number of cancer diagnoses also confirm this finding.30,31 Second, we found that discussion of permanently discontinuing systemic therapy was infrequent and oncologist often emphasize both avoidance of harm and marginal benefit in these conversations. Uniquely, our study was able to provide a relative measure of its infrequency versus other systemic therapy decision-making talk (random sample), as well as review a sufficient number of encounters to better characterize these infrequently studied encounters (purposive sample).
There are several potential reasons why patients and oncologists frame continuing systemic therapy as the default. Our study included SDM as a focus, and our results align well with others, and specifically, we found that (1) patients were infrequently invited to deliberate about therapy decisions,17,32 (2) inadequate rates (0%-50%) of discussing no therapy as an option,18,20 and (3) patient values were not frequently assessed when making systemic therapy decisions.20,30 We infer that these behaviors contribute to continuing systemic therapy as the default, yet they are not the only explanations. Brom et al30 identified four patterns of daily oncology practice as potential contributors to this default: (1) Presenting the full therapy sets the standard, (2) focus on standard evaluation moments hampers evaluation of care goals, (3) opening question guides toward focus on symptoms, and (4) treatment is perceived as the only option. There are also potential benefits that support maintaining the possibility of future therapy (eg, arrival of a better therapy, maintaining hope, time for clinical improvement, and decision postponement). Our observation that oncologists attempted to either amplify positive emotions (eg, hope) or mitigate negative ones (eg, worry) when discussing limiting therapy lends support to the role that actual or perceived patient emotion has in these discussions. Given the theorized benefits of using defaults in therapy decision making,33 we speculate that both patients and oncologists might use this by default to simplify a set of complex and emotionally challenging decisions. Regardless of why it occurs, the unintended consequences of this default to continue systemic therapy are more hospital admissions in the later stages of cancer and delays in hospice enrollment, which are both linked to lower EOL quality.34,35
Our second finding that discussing permanent discontinuation was infrequent and oncologists tend to emphasize both harm avoidance and marginal benefit also finds support in the literature. Since our study was the first to quantify permanent discontinuation talk in this context, we needed to look at studies using documentation or specialty palliative care to assess our finding. Pirl et al13 found only a minority of patients with advanced cancer (16 of 81, 20%) had any oncology encounter where a definitive decision to permanently discontinue chemotherapy was documented. Looking at the intervention arm of a specialty palliative care trial for patients with advanced cancer, only 6% (31 of 497) of these encounters included discussion on either permanent discontinuation of systemic therapy or hospice enrollment.36 Given that palliative care clinicians discuss EOL issues more frequently than oncologists,37 the true estimate of discussing permanent discontinuation in oncology encounters is likely lower than 6%. By using a purposive sample to increase our number of permanent discontinuation encounters under review, we feel confident that oncologists tend to discuss both harm avoidance and marginal benefit in these conversations. This is significant because in their analysis of medical documentation, Pirl et al concluded, “in weighing the potential benefits and risks of additional chemotherapy, a decision may be guided more by the potential for harm.”13 Although our findings support harm avoidance as an important rationale for permanently discontinuing treatment, we could not conclude that it was more important than perceived marginal benefit since both were mentioned. More work is needed to identify the salience of harm avoidance v perceived marginal benefit surrounding decision making for systemic therapy.
There were limitations in the study design. As a secondary analysis, we did not have the ability target which visits were recorded or interview participants to further probe potential influences on decision making. We could not independently verify the cancer and EOL care patients received outside of what was discussed in encounters beyond the available data. We cannot comment on communication differences between chemotherapy and immunotherapy discussion since the latter became a major therapeutic option after our study period. We speculate that emergence of more therapeutic options would only exacerbate the tendency to continue systemic therapy as the default as published literature in this area suggests that oncologists find immunotherapy presents additional challenges to discussing prognosis and treatment uncertainties with their patients.38 Three sequential encounters are likely insufficient to fully evaluate the longitudinal nature of the patient-oncologist relationship or changes in communication over time. Since participants knew they were being recorded, the potential that they may behave differently exists (Hawthorne effect). However, since encounters were recorded using personal recording devices and not a separate human observer, we infer this influence was negible.39 We could not analyze nonverbal communication. Our study participants were selected from two large National Cancer Institute–designated cancer centers, although upward of 80% of patients who die with advanced cancer in the United States receive their cancer care at community hospitals.40 Furthermore, since some protected health information was removed from the data because of participant confidentiality, our approach of inferring cancer type from transcripts opens up the possibility of misattribution for cancer diagnosis and treating clinician subspecialty.
The default decision to continue systemic therapy and infrequent discussion of permanent discussion likely contribute to the receipt of systemic therapy and hospitalizations closer to death and delayed hospice enrollment. Communication guidelines are useful practices to implement,14 and a growing literature suggests outpatient interventions targeting patients and clinicians can improve communication in this context.41-44 Future work should focus on continuing to improve the effectiveness of patient-clinician communication and understanding the processes outside the oncology visit that contribute to patients receiving goal-aligned care throughout their continuum of cancer care.
ACKNOWLEDGMENT
We thank Raina H. Jain, Lindsay A. Holdcroft, Judy Li, Isabelle Hentschel, and John Speicher for their assistance listening to audio-recorded encounters and identifying instances of permanent treatment discontinuation. Megan Murphy, MS, assisted in analyzing patient and provider demographic information. We also thank Shama S. Alam, PhD, MSc, for leading early project work in codebook development.
Matthew A. Liu
Stock and Other Ownership Interests: Apollo Medical Holdings (Inst)
Yael Schenker
Honoraria: UpToDate
No other potential conflicts of interest were reported.
See accompanying article on page 543
SUPPORT
Supported by NCI R01—2R01CA100387-06A1. The transcription and analysis of audio recordings was funded by the Susan J. and Richard M. Levy Academic Cluster in Health Care Delivery at Dartmouth College.
AUTHOR CONTRIBUTIONS
Conception and design: Garrett T. Wasp, Kristin E. Knutzen, Matthew A. Liu, Kathryn I. Pollak, James A. Tulsky, Amber E. Barnato
Financial support: Amber E. Barnato
Administrative support: Amber E. Barnato
Provision of study materials or patients: James A. Tulsky, Amber E. Barnato
Collection and assembly of data: Garrett T. Wasp, Matthew A. Liu, James A. Tulsky, Amber E. Barnato
Data analysis and interpretation: Garrett T. Wasp, Kristin E. Knutzen, Genevra F. Murray, Olivia C. Brody-Bizar, Kathryn I. Pollak, James A. Tulsky, Yael Schenker, Amber E. Barnato
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Systemic Therapy Decision Making in Advanced Cancer: A Qualitative Analysis of Patient-Oncologist Encounters
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/op/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Matthew A. Liu
Stock and Other Ownership Interests: Apollo Medical Holdings (Inst)
Yael Schenker
Honoraria: UpToDate
No other potential conflicts of interest were reported.
REFERENCES
- 1. Wright AA, Keating NL, Ayanian JZ, et al. Family perspectives on aggressive cancer care near the end of life. JAMA. 2016;315:284. doi: 10.1001/jama.2015.18604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Weeks JC. Relationship between cancer patients’ predictions of prognosis and their treatment preferences. JAMA. 1998;279:1709. doi: 10.1001/jama.279.21.1709. [DOI] [PubMed] [Google Scholar]
- 3. Zafar SY, Malin JL, Grambow SC, et al. Chemotherapy use and patient treatment preferences in advanced colorectal cancer. Cancer. 2013;119:854–862. doi: 10.1002/cncr.27815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Casarett DJ, Fishman JM, Lu HL, et al. The terrible choice: Re-evaluating hospice eligibility criteria for cancer. J Clin Oncol. 2009;27:953–959. doi: 10.1200/JCO.2008.17.8079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Seow H, Snyder CF, Shugarman LR, et al. Developing quality indicators for cancer end-of-life care. Cancer. 2009;115:3820–3829. doi: 10.1002/cncr.24439. [DOI] [PubMed] [Google Scholar]
- 6.National Quality Forum . National Quality Forum Quality Positioning System. 2021. http://www.qualityforum.org/QPS/ [Google Scholar]
- 7. Elkin EB, Kim SHM, Casper ES, et al. Desire for information and involvement in treatment decisions: Elderly cancer patients’ preferences and their physicians’ perceptions. J Clin Oncol. 2007;25:5275–5280. doi: 10.1200/JCO.2007.11.1922. [DOI] [PubMed] [Google Scholar]
- 8. Grunfeld EA, Maher EJ, Browne S, et al. Advanced breast cancer patients’ perceptions of decision making for palliative chemotherapy. J Clin Oncol. 2006;24:1090–1098. doi: 10.1200/JCO.2005.01.9208. [DOI] [PubMed] [Google Scholar]
- 9. Blackhall LJ. Ethnicity and attitudes toward patient autonomy. JAMA. 1995;274:820. [PubMed] [Google Scholar]
- 10. Mack JW, Weeks JC, Wright AA, et al. End-of-life discussions, goal attainment, and distress at the end of life: Predictors and outcomes of receipt of care consistent with preferences. J Clin Oncol. 2010;28:1203–1208. doi: 10.1200/JCO.2009.25.4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Koedoot CG, de Haan RJ, Stiggelbout AM, et al. Palliative chemotherapy or best supportive care? A prospective study explaining patients’ treatment preference and choice. Br J Cancer. 2003;89:2219–2226. doi: 10.1038/sj.bjc.6601445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non–small-cell lung cancer. N Engl J Med. 2010;363:733–742. doi: 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]
- 13. Pirl WF, Greer JA, Irwin K, et al. Processes of discontinuing chemotherapy for metastatic non–small-cell lung cancer at the end of life. JCO Oncol Pract. 2015;11:e405–e412. doi: 10.1200/JOP.2014.002428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gilligan T, Coyle N, Frankel RM, et al. Patient-clinician communication: American Society of Clinical Oncology consensus guideline. J Clin Oncol. 2017;35:3618–3632. doi: 10.1200/JCO.2017.75.2311. [DOI] [PubMed] [Google Scholar]
- 15. Elwyn G, Durand MA, Song J, et al. A three-talk model for shared decision making: Multistage consultation process. BMJ. 2017;359:j4891. doi: 10.1136/bmj.j4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kunneman M, Engelhardt EG, ten Hove FL, et al. Deciding about (neo-)adjuvant rectal and breast cancer treatment: Missed opportunities for shared decision making. Acta Oncol. 2016;55:134–139. doi: 10.3109/0284186X.2015.1068447. [DOI] [PubMed] [Google Scholar]
- 17. Leppin AL, Humeniuk KM, Fernandez C, et al. Was a decision made? An assessment of patient-clinician discordance in medical oncology encounters. Healt Expect. 2015;18:3374–3381. doi: 10.1111/hex.12340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Koedoot C, Oort F, de Haan R, et al. The content and amount of information given by medical oncologists when telling patients with advanced cancer what their treatment options are palliative chemotherapy and watchful-waiting. Eur J Cancer. 2004;40:225–235. doi: 10.1016/j.ejca.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 19. Gattellari M. When the treatment goal is not cure: Are cancer patients equipped to make informed decisions? J Clin Oncol. 2002;20:503–513. doi: 10.1200/JCO.2002.20.2.503. [DOI] [PubMed] [Google Scholar]
- 20. Brom L, De Snoo-Trimp JC, Onwuteaka-Philipsen BD, et al. Challenges in shared decision making in advanced cancer care: A qualitative longitudinal observational and interview study. Health Expect. 2017;20:69–84. doi: 10.1111/hex.12434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Library of Medicine, ClinicalTrials.gov . Intervention Study of Communication in Oncologist-Patient Encounters (COPE) 2009. https://clinicaltrials.gov/ct2/show/study/NCT00994578 [Google Scholar]
- 22. Tulsky JA, Arnold RM, Alexander SC, et al. Enhancing communication between oncologists and patients with a computer-based training program. Ann Intern Med. 2011;155:593. doi: 10.1059/0003-4819-155-9-201111010-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Porter LS, Pollak KI, Farrell D, et al. Development and implementation of an online program to improve how patients communicate emotional concerns to their oncology providers. Support Care Cancer. 2015;23:2907–2916. doi: 10.1007/s00520-015-2656-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Downar J, Goldman R, Pinto R, et al. The “surprise question” for predicting death in seriously ill patients: A systematic review and meta-analysis. Can Med Assoc J. 2017;189:E484–E493. doi: 10.1503/cmaj.160775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Knutzen KE, Sacks OA, Brody-Bizar OC, et al. Actual and missed opportunities for end of life care discussions with oncology patients: A qualitative study. JAMA Netw Open. 2021;4:e2113193. doi: 10.1001/jamanetworkopen.2021.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Elo S, Kyngäs H. The qualitative content analysis process. J Adv Nurs. 2008;62:107–115. doi: 10.1111/j.1365-2648.2007.04569.x. [DOI] [PubMed] [Google Scholar]
- 27. Fereday J, Muir-Cochrane E. Demonstrating rigor using thematic analysis: A hybrid approach of inductive and deductive coding and theme development. Int J Qual Methods. 2006;5:80–92. [Google Scholar]
- 28. Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): A 32-item checklist for interviews and focus groups. Int J Qual Heal Care. 2007;19:349–357. doi: 10.1093/intqhc/mzm042. [DOI] [PubMed] [Google Scholar]
- 29. Levy MH, Weinstein SM, Carducci MA. NCCN palliative care practice guidelines panel. NCCN: Palliative care. Cancer Control. 2001;8(6 suppl 2):66–71. [PubMed] [Google Scholar]
- 30. Brom L, Onwuteaka-Philipsen BD, Widdershoven GA, et al. Mechanisms that contribute to the tendency to continue chemotherapy in patients with advanced cancer. Qualitative observations in the clinical setting. Support Care Cancer. 2016;24:1317–1325. doi: 10.1007/s00520-015-2910-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chou WS, Hamel LM, Thai CL, et al. Discussing prognosis and treatment goals with patients with advanced cancer: A qualitative analysis of oncologists’ language. Health Expect. 2017;20:1073–1080. doi: 10.1111/hex.12549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Winkler EC, Reiter-Theil S, Lange-Riess D, et al. Patient involvement in decisions to limit treatment: The crucial role of agreement between physician and patient. J Clin Oncol. 2009;27:2225–2230. doi: 10.1200/JCO.2008.17.9515. [DOI] [PubMed] [Google Scholar]
- 33. Reyna VF, Nelson WL, Han PK, et al. Decision making and cancer. Am Psychol. 2015;70:105–118. doi: 10.1037/a0036834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang B, Nilsson ME, Prigerson HG. Factors important to patients’ quality of life at the end of life. Arch Intern Med. 2012;172:1133–1142. doi: 10.1001/archinternmed.2012.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Prigerson HG, Bao Y, Shah MA, et al. Chemotherapy use, performance status, and quality of life at the end of life. JAMA Oncol. 2015;1:778. doi: 10.1001/jamaoncol.2015.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Traeger L, Rapoport C, Wright E, et al. Nature of discussions about systemic therapy discontinuation or hospice among patients, families, and palliative care clinicians during care for incurable cancer: A qualitative study. J Palliat Med. 2020;23:542–547. doi: 10.1089/jpm.2019.0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Thomas TH, Jackson VA, Carlson H, et al. Communication differences between oncologists and palliative care clinicians: A qualitative analysis of early, integrated palliative care in patients with advanced cancer. J Palliat Med. 2019;22:41–49. doi: 10.1089/jpm.2018.0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Elias R, Odejide O. Immunotherapy in older adults: A checkpoint to palliation? Am Soc Clin Oncol Ed Book. 2019;39:e110–e120. doi: 10.1200/EDBK_238795. [DOI] [PubMed] [Google Scholar]
- 39. Henry SG, Jerant A, Iosif A, et al. Analysis of threats to research validity introduced by audio recording clinic visits: Selection bias, Hawthorne effect, both, or neither? Patient Educ Couns. 2015;98:849–856. doi: 10.1016/j.pec.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wasp GT, Alam SS, Brooks GA, et al. End-of-life quality metrics among medicare decedents at minority-serving cancer centers: A retrospective study. Cancer Med. 2020;9:1911–1921. doi: 10.1002/cam4.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bernacki R, Hutchings M, Vick J, et al. Development of the serious illness care program: A randomised controlled trial of a palliative care communication intervention. BMJ Open. 2015;5:e009032. doi: 10.1136/bmjopen-2015-009032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Curtis JR, Downey L, Back AL, et al. Effect of a patient and clinician communication-priming intervention on patient-reported goals-of-care discussions between patients with serious illness and clinicians: A randomized clinical trial. JAMA Intern Med. 2018;178:930–940. doi: 10.1001/jamainternmed.2018.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Temel JS, Greer JA, Gallagher ER, et al. Electronic prompt to improve outpatient code status documentation for patients with advanced lung cancer. J Clin Oncol. 2013;31:710–715. doi: 10.1200/JCO.2012.43.2203. [DOI] [PubMed] [Google Scholar]
- 44. Paladino J, Bernacki R, Neville BA, et al. Evaluating an intervention to improve communication between oncology clinicians and patients with life-limiting cancer: A cluster randomized clinical trial of the serious illness care program. JAMA Oncol. 2019;5:801–809. doi: 10.1001/jamaoncol.2019.0292. [DOI] [PubMed] [Google Scholar]