Abstract
Background:
Social and physical environmental factors affect real-world walking activity in individuals with stroke. However, environmental factors are often non-modifiable, presenting a challenge for clinicians working with individuals with stroke whose real-world walking is limited due to environmental barriers.
Objective:
The purpose of this work was to test a model hypothesizing the relationships among environmental factors (specifically, living situation and area deprivation), modifiable factors, and real-world walking activity to understand opportunities for intervention. We hypothesized that balance self-efficacy would mediate the relationship between the environment and real-world walking and that physical capacity would moderate this mediation.
Methods:
This was a cross-sectional study of 282 individuals with chronic (≥6 months) stroke. We tested the indirect effect to determine if mediation was present. Multiple group structural equation modeling was used to test if physical capacity moderated this mediation. A χ2 difference test was used to compare the moderation model against the null (no moderation) model.
Results:
Balance self-efficacy mediated the relationship between area deprivation and real-world walking (indirect effect: β = −0.04, p=0.04). Both the moderation and null models fit the data equally well statistically (χ2 (5)=6.9, p=0.23). We therefore accepted the simpler (null) model and concluded that the mediation was not moderated.
Conclusions:
Targeting balance self-efficacy may be an effective approach to improving real-world walking in persons with stroke who experience barriers within the physical environment. A stroke survivor’s physical capacity may not impact this approach. Future work should consider utilizing more specific measures of the social and physical environment to better understand their influences on real-world walking activity in individuals with stroke. However, the results of this work provide excellent targets for future longitudinal studies targeting real-world walking activity in stroke.
Keywords: Rehabilitation, Physical Activity, Theoretical Model
INTRODUCTION:
Stroke is a leading cause of disability in the United States and leads to numerous deficits, including impairments in physical capacity and balance self-efficacy.1-4 Consequently, many individuals with stroke are inactive and spend the majority of time in sedentary behaviors.5-7 This is problematic because reduced activity and increased sedentary time are risk factors for cardiovascular events, mortality, and other negative health consequences, such as loss of muscle strength and increased risk for deep venous thromboembolism.8-11 Thus, it is critically important that rehabilitation professionals understand what factors influence activity levels in individuals with stroke and how these factors are related to understand opportunities for intervention.
Previous studies suggest that environmental factors affect walking activity outside the clinical or laboratory setting (i.e., real-world walking activity) after stroke.12-15 This includes both social environmental factors, defined as factors related to social connectedness and social support,16 and physical environmental factors, defined as factors within the physical (i.e., built) environment.16 For example, qualitative work suggests that the supportiveness and availability of a caregiver and/or family member is an important factor that contributes to a stroke survivor’s activity levels.15,17 Similarly, physical environmental factors, such as the quality of sidewalks and roadways, availability of park benches, and crowdedness of an area, also influence real-world walking activity in persons post stroke.12,15,17,18
While previous work has shed light on the role of social and physical environmental factors,12-15,17,19 previous theoretical models in stroke accounting for environmental factors have either not been empirically tested12,20 or focused predominantly on disability,21 not on activity, presenting a gap in the literature as to how the environment may influence real-world walking activity in stroke. In addition, a recent meta-analysis examining factors associated with physical activity post-stroke did not examine the role of environmental factors,22 demonstrating that additional studies are needed to improve our understanding of how the environment affects real-world walking activity in individuals with stroke.
Despite our growing understanding of the role of environmental barriers on real-world activity levels, physical and social environmental factors are often difficult, if not impossible, to modify in clinical practice, presenting a challenge for clinicians working with individual’s post stroke whose activity is limited due to environmental barriers. We know, however, that there may be complex interactions between these environmental factors and other factors that are modifiable. For example, measures of physical capacity, such as the 6-Minute Walk Test,22-24 and balance self-efficacy, such as the Activities Specific Balance Confidence Scale,22,25,26 are strongly related to real-world walking activity in individuals with stroke. Moreover, Danks et al. found that in the context of improving steps per day, balance self-efficacy may be particularly important for stroke survivors with lower physical capacity, suggesting a complex relationship between physical capacity and balance self-efficacy as it relates to walking activity.25 Finally, our recent work in people with chronic stroke suggests that environmental factors, balance self-efficacy and physical capacity may be related in terms of their associations with real-world walking activity.27 However the nature of those relationships has not been examined. Therefore, the purpose of this work was to test a model hypothesizing the relationships among the social and physical environment, balance self-efficacy, physical capacity, and real-world walking activity to determine if balance self-efficacy and physical capacity could be targeted to overcome environmental barriers to improve real-world walking in individuals with stroke. Based on previous studies,22,25,26 we had three hypotheses: 1) Our overall hypothesized model will fit the data; (2) Balance self-efficacy (Activities Specific Balance Confidence Scale) will mediate the relationship between the environment (social and physical) and real-world walking activity (measured using average steps/day)25,26; (3) Physical capacity (6-Minute Walk Test) would moderate this mediation.25
METHODS:
Study Design and Participants:
This study was a cross-sectional analysis of baseline data from a larger clinical trial aimed at understanding which interventions are most effective at improving real-world walking activity post stroke (NCT02835313).28 The clinical trial included four sites: University of Delaware, University of Pennsylvania, Christiana Care Health System and Indiana University28; thus, participants in the dataset used for this analysis represented a variety of geographical regions within the United States. Recruitment methods included advertisements in newspapers and websites, contacting local stroke support groups, physical therapy clinics and physician offices, and leveraging existing clinical databases. The following eligibility criteria were applied to be included in the database used for this analysis: Inclusion Criteria: (1) Ages 21-85, (2) ≥6 months post stroke, (3) Able to walk at a self-selected gait speed of ≥0.3 m/s without assistance from another person (assistive devices allowed), (4) Resting heart rate between 40-100 beats/minute, (5) Resting blood pressure between 90/60 to 170/90 mmHg; Exclusion Criteria: (1) Evidence of cerebellar stroke, (2) Other potentially disabling neurologic conditions in addition to stroke, (3) Lower limb Botulinum toxin injection <4 months earlier, (4) Current participation in physical therapy, (5) Inability to walk outside the home prior to stroke, (6) Coronary artery bypass graft, stent placement or myocardial infarction within the past 3 months, (7) Musculoskeletal pain limiting activity, (8) Unable to provide informed consent as indicated by an inability to answer at least 1 orientation question correctly (item 1b on the NIH Stroke Scale) and inability to follow at least one, two-step command (item 1c on the NIH Stroke Scale). Participants were eligible for the study if they self-reported a stroke during the initial evaluation and this was verified via imaging (MRI or CT scan). In addition, we only included participants that did not have missing data for the 6-Minute Walk Test and steps/day for this analysis. All participants signed informed consent approved by the Human Subjects Review Board at the University of Delaware or their respective institution prior to study participation (protocol number 878153-50).
Theoretical Model:
Figure 1 displays our theoretical model hypothesizing the relationships among the social (living situation) and physical (Area Deprivation Index) environment, balance self-efficacy (Activities Specific Balance Confidence Scale) and real-world walking activity (average steps/day). Based on previous work, the physical and social environments would have a direct effect on average steps/day (pathways a and d, respectively).12-15,17-19,29 Past work suggesting that physical and social environmental factors influence balance self-efficacy (pathways b and e, respectively) 13-15,17,18 coupled with evidence demonstrating that Activities Specific Balance Confidence Scale is a strong predictor of walking activity (pathway c)22,25,26 served as the theoretical basis for our hypothesis that Activities Specific Balance Confidence Scale would mediate the relationship between the social and physical environment and real-world walking activity. This hypothesis is also supported by past work demonstrating that the Activities Specific Balance Confidence Scale may serve as a mediator in understanding walking activity post stroke26 and also by past work demonstrating that affective factors mediate the relationship between the social and physical environment and physical activity in the general population30,31 and individuals with obesity.32 Thus, there are two mediated relationships (i.e., indirect effects)33 in this model, one of which is displayed as the arrows from Area Deprivation Index to average steps/day through Activities Specific Balance Confidence Scale, and the other as arrows from living situation to average steps/day also through Activities Specific Balance Confidence Scale. Finally, we also considered past work that suggests that balance self-efficacy may be particularly important for individuals with stroke with lower physical capacity.25 This led us to hypothesize that our mediation model would be conditional on physical capacity status. We therefore tested if the mediation model differed for individuals with higher and lower physical capacity.
Figure 1. Theoretical Model.
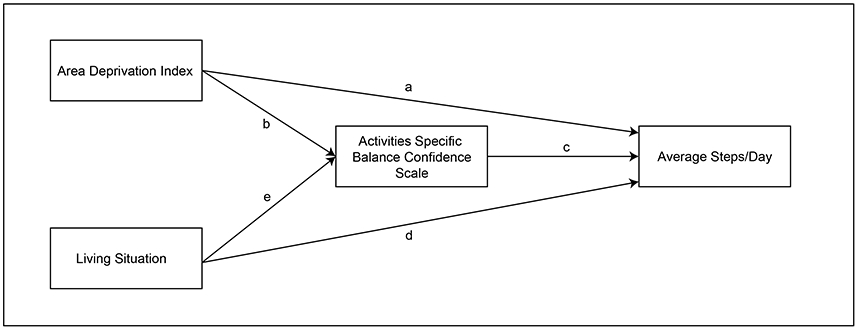
The model depicts that the physical environment (Area Deprivation Index) has a direct effect on average steps/day (pathway a) as well as an indirect effect through Activities Specific Balance Confidence Scale (pathways b and c). Similarly, the social environment (living situation) has a direct effect on average steps/day (pathway d) and an indirect effect through Activities Specific Balance Confidence Scale (pathways e and c). We hypothesized that the Activities Specific Balance Confidence Scale would mediate the relationship between the environmental variables and average steps/day in individuals with stroke and that physical capacity would moderate this mediated relationship.
Measures:
Participants completed a baseline evaluation that included measures of their physical capacity (6-Minute Walk Test), balance self-efficacy (Activities Specific Balance Confidence Scale), and real-world walking activity (average steps/day).28 Participants’ living situation was used to represent the social environment, and the Area Deprivation Index was used to represent the physical environment.
During the 6-Minute Walk Test, participants were instructed to walk as far as possible around a rectangular path for 6 minutes. The 6-Minute Walk Test is a valid and reliable test of walking endurance in individuals with stroke.34,35 In this study, distance traveled on the 6-Minute Walk Test was used to examine the role of physical capacity as a moderator in our mediation model. Individuals whose 6-Minute Walk Test was ≤312 m (the median of our sample) were categorized as having “lower” physical capacity, and participants whose 6-Minute Walk Test was >312 meters were categorized as having “higher” physical capacity.
The Activities Specific Balance Confidence Scale is a 16-item questionnaire that measures an individual’s balance self-efficacy. Participants rate how confident they are performing various tasks on a scale from 0-100 in which lower scores reflect lower balance confidence. The scores for each item are then averaged to provide an overall score that reflects the individual’s balance self-efficacy. The Activities Specific Balance Confidence Scale is a valid and reliable measure in persons with stroke.36,37
To measure real-world walking activity, participants were provided with a FitBit One or Zip at the baseline evaluation to wear on their non-paretic ankle. The FitBit has demonstrated acceptable accuracy in detecting steps in individuals with stroke.38-42 Participants were instructed to go about their normal routine while wearing the monitor and to remove it for sleep and water-based activities. A minimum of three days of step data was collected to reliably estimate real-world walking activity43; however, participants were encouraged to wear the device for seven days if possible.43 Average steps/day was calculated by summing the total number of steps taken over all valid recording days and dividing this sum by the number of valid recording days.
At the baseline evaluation, participants were queried about their living situation, which was used to represent the social environment, defined as factors related to social connectedness and social support. Under this definition, an individual’s living situation may reflect one aspect of the social environment. In this analysis, living situation was coded as, 0= living alone, with or without outside assistance, or 1= living with a family member or significant other.
Participants provided the investigators with their address during the baseline evaluation which was used to obtain their respective Area Deprivation Index score. The Area Deprivation Index is a composite index of neighborhood disadvantage that includes various indicators of housing quality and crowding, poverty, education, and employment.44-46 Because the Area Deprivation Index includes aspects of the built environment, we therefore considered it a measure of physical environment. In addition, prior work suggests that more deprived neighborhoods tend to exhibit less favorable physical environmental characteristics, such as less cleanliness, lower aesthetic quality and fewer safe and accessible green spaces for engaging in physical activity.47,48 Thus, while the Area Deprivation Index was designed to measure area deprivation, we considered it a measure of the physical environment in the current work based on prior evidence demonstrating that areas of greater deprivation may exhibit unique physical environmental characteristics. However, the use of this measure to quantify the physical environment is a limitation of the current work which is discussed in the Limitations section. The Area Deprivation Index provides a national percentile ranking from 1-100 where lower values represent lower levels of disadvantage within the nation and higher values represent the higher levels of disadvantage.44-46 Our previous work found a significant relationship between the Area Deprivation Index and real-world walking activity where greater area deprivation was associated with lower real-world walking activity.19,27
Statistical Analysis:
To test that balance self-efficacy (Activities Specific Balance Confidence Scale) mediates the relationship between the social (living situation) and physical (Area Deprivation Index) environment and real-world walking activity (average steps/day), we tested the two indirect effects for significance.33,49,50 To test if the mediation differed between individuals with higher and lower physical capacity, a multiple group structural equation model was used.51,52 High and low physical capacity membership was determined using the median split of distance traversed on the 6-Minute Walk Test (≤312m). The median split was used for two reasons; first, there is no gold standard or consensus as to what constitutes “sufficient” physical capacity in the post-stroke rehabilitation literature; and second, using the median split ensured a balanced sample size for groups. Any potentially relevant covariates that were significantly different (p<0.05) between the higher and lower physical capacity groups were included in the multiple group structural equation model. Continuous variables were compared using an independent t-test or Mann-Whitney U test for normal or non-normal data, respectively. Categorical variables were compared between groups using a chi-square test.
Using multiple group structural equation modeling to test for moderation, we tested two models, a null model reflecting that no moderation is present and an experimental model reflecting that moderation is present. In the null model, we constrained all pathway estimates to be the same between the higher and lower physical capacity groups; in the experimental model these paths were free to vary between groups.51,52 A χ2 difference test was used to compare the null and experimental models to determine if freeing the pathways significantly improved model fit.52,53 Model fit was examined using fit statistics, including root mean square error of approximation (RMSEA), comparative fit index (CFI), Tucker-Lewis index (TLI), and standardized root mean square residual (SRMR).53 The software package MPlus was used to conduct the statistical analysis.54 Maximum likelihood with robust standard errors (MLM) was used to estimate the models of interest.54 Chi square difference testing was conducted using the Satorra-Bentler method.55,56
RESULTS:
Two hundred and eighty-two participants completed the baseline phase of the clinical trial and met eligibility criteria at the time this analysis was conducted. The demographic and clinical characteristics of our full sample and each physical capacity subgroup are displayed in Table 1. Assistive device use (p<0.001) and the Charlson-Comorbidity Index (age-adjusted, p=0.01) were significantly different between the physical capacity groups and therefore included in the multiple group structural equation model.
Table 1.
Demographic and Clinical Characteristics of Study Sample*
| All (n = 282) |
Lower Physical Capacity (n = 141) |
Higher Physical Capacity (n = 141) |
P value** |
|
|---|---|---|---|---|
| Age (years) | 63.43 (12.63) | 63.58 (13.08) | 63.27 (12.19) | 0.63 |
| Time Since Initial Stroke (months) | 46.99 (61.5) | 51.79 (70.48) | 42.20 (50.78) | 0.44 |
| Gender | Male: n = 146 (51.8%) Female: n = 136 (48.2%) |
Male: n = 74 (52.5%) Female: n = 67 (47.5%) |
Male: n = 72 (51.1%) Female: n = 69 (48.9%) |
0.81 |
| Hemiparesis | Left: n = 148 (52.5%) Right: n = 128 (45.4%) Bilateral: n = 6 (2.1%) |
Left: n = 79 (56.0%) Right: n = 60 (42.6%) Bilateral: n = 2 (1.4%) |
Left: n = 69 (48.9%) Right: n = 68 (48.2%) Bilateral: n = 4 (2.8%) |
0.40 |
| Body Mass Index | 30.31 (6.36) | 29.87 (6.86) | 30.75 (5.80) | 0.09 |
| Assistive Device Use (yes/no) | No: n = 149 (52.8%) Yes: n = 133 (47.2%) |
No: n = 32 (22.7%) Yes: n = 109 (77.3%) |
No: n = 117 (83.0%) Yes: n = 24 (17.0%) |
<0.001 |
| Charlson Comorbidity Index (age-adjusted) | 6.09 (2.46) | 6.46 (2.54) | 5.72 (2.33) | 0.01 |
| 6-Minute Walk Test (m) | 302.13 (115.54) | 205.87 (67.67) | 398.39 (59.60) | <0.001 |
| Activities Specific Balance Confidence Scale | 75.55 (18.27) | 69.0 (18.21) | 82.06 (15.90) | <0.001 |
| Living Situation | Alone: n = 59 (20.9%) Living with family member/significant other: n = 223 (79.1%) |
Alone: n = 33 (23.4%) Living with family member/significant other: n = 108 (76.6%) |
Alone: n = 26 (18.4%) Living with family member/significa nt other: n = 115 (81.6%) |
0.31 |
| Area Deprivation Index | 36.29 (23.64) | 40.48 (24.70) | 32.12 (21.84) | 0.001 |
| Average steps/day | 4563.73 (2696.78) | 3283.88 (2026.96) | 5843.59 (2681.26) | <0.001 |
Data displayed as mean (standard deviation) for continuous variables and count (percentage) for categorical variables
P value reflects comparisons between the lower physical capacity and higher physical capacity groups. Continuous variables were compared using an independent t-test for normally distributed data, or a Mann Whitney U Test for non-normal data. Categorical variables were compared using a chi-squared test.
Both the constrained and unconstrained models demonstrated acceptable model fit, supporting hypothesis 1 (Constrained: RMSEA 0.04 (90% CI 0-0.1), CFI 0.91, TLI 0.83, SRMR 0.06, χ2 (15, N=282)=18.44, p=0.24; Unconstrained: RMSEA 0.04 (90% CI 0-0.1), CFI 0.96, TLI 0.88, SRMR 0.04, χ2 (10, N=282)=11.64, p=0.31 ).57-59 Comparing these two models resulted in a non-significant difference test, χ2 (5)=6.9, p=0.23, suggesting that both models fit the data equally well statistically. We therefore concluded that the mediation was not moderated, refuting hypothesis 3, and the simpler (null) model is reported in Figure 2. For completeness, the results of the unconstrained (moderation) model are discussed in Appendix 1 and shown in Supplemental Figure 1.
Figure 2. Results of Null (No Moderation) Model.
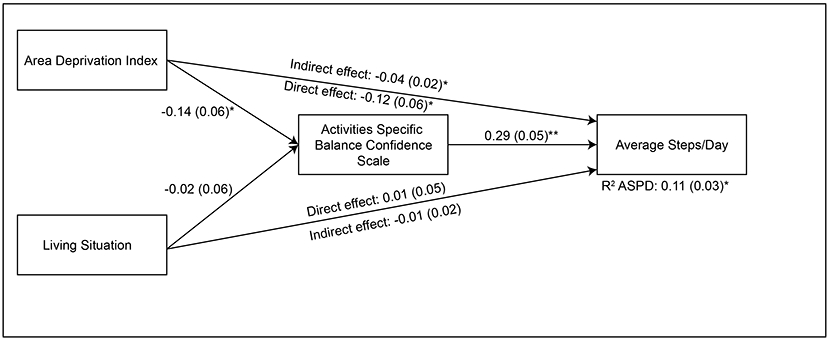
The direct effects of Area Deprivation Index and living situation on average steps/day are displayed as solid black lines. The indirect effects of Area Deprivation Index and living situation on average steps/day through Activities Specific Balance Confidence Scale are displayed next to the line representing the direct effect. Standardized coefficients are displayed with standard errors in parentheses. We found that the Activities Specific Balance Confidence Scale mediated the relationship between the Area Deprivation Index and average steps/day in our sample of individuals with chronic stroke. *p<0.05, **p<0.001.
Supporting hypothesis 2, there was a significant indirect effect of Area Deprivation Index on average steps/day through Activities Specific Balance Confidence Scale (β = −0.04, p=0.04), suggesting that Activities Specific Balance Confidence Scale does mediate the relationship between Area Deprivation Index and average steps/day in our sample of individuals with chronic stroke. The direct effects of Area Deprivation Index on average steps/day and Activities Specific Balance Confidence Scale were significant (β = −0.12, p=0.04; β = −0.14, p=0.02, respectively). Lower area deprivation was associated with greater steps per day and higher balance self-efficacy. We also found a significant positive relationship between Activities Specific Balance Confidence Scale and average steps/day (β = 0.29, p<0.001), suggesting higher balance self-efficacy was associated with greater steps per day. There was not a significant indirect effect of living situation on average steps/day through Activities Specific Balance Confidence Scale, (β = −0.01, p=0.69). The direct effects of living situation on Activities Specific Balance Confidence Scale (β = −0.02, p=0.69) and average steps/day (β = 0.01, p=0.86) were not significant.
Overall, these findings provided partial support for our theoretical model. First, we did find that Activities Specific Balance Confidence Scale mediated the relationship between Area Deprivation Index and average steps/day. However, there was no moderation of the mediated effect and living situation’s effect on average steps/day was not mediated by Activities Specific Balance Confidence Scale.
DISCUSSION:
The purpose of this work was to test a model hypothesizing the relationships among the social and physical environment, balance self-efficacy, physical capacity, and real-world walking activity in individuals with stroke. We found a significant effect of the physical environment (measured using the Area Deprivation Index) on real-world walking activity and that balance self-efficacy, measured by the Activities Specific Balance Confidence Scale, mediated the relationship between the Area Deprivation Index and real-world walking activity in our sample of individuals with chronic stroke. Conversely, we did not find a significant effect of the social environment (measured using an individual’s living situation) on real-world walking activity nor a significant indirect effect through Activities Specific Balance Confidence Scale. Contrary to our hypothesis, we found that physical capacity, defined using a cut-off score of 312 meters on the 6-Minute Walk Test, was not a moderator in our mediation model, suggesting that our mediation model did not differ for stroke survivors with higher (>312 meters) versus lower (≤312 meters) physical capacity. Overall, the results of this work provide insights for potential opportunities for intervention to overcome environmental barriers to improving real-world walking activity in individuals with stroke without cerebellar involvement whose comfortable gait speeds are ≥0.3 m/s.
Our finding that the Area Deprivation Index had a significant direct effect on real-world walking activity in our full sample corroborates past qualitative12,13,15,17,18,60 and quantitative19,27 work suggesting that physical aspects of the environment play an important role on walking activity and participation in persons with stroke. This work also adds a new contribution to the post stroke rehabilitation literature by demonstrating that balance self-efficacy mediates the relationship between area deprivation and real-world walking activity in persons with stroke without cerebellar involvement whose comfortable gait speeds are ≥0.3 m/s. This suggests that the effect of area deprivation on real-world walking activity is partly due to the fact that area deprivation influences balance self-efficacy which in turn influences real-world walking activity. This idea is also supported by past work suggesting that aspects of the physical environment, such as the quality of sidewalks and crowdedness of an area, affect balance self-efficacy in persons with stroke.13,15,18 The finding that psychosocial factors mediate the relationship between the environment and physical activity has also been found in previous work in the general population30,31 and individuals with obesity32, lending credence to the idea that the environment affects activity behavior through psychosocial influences. Importantly, the finding in the present work suggests that targeting balance self-efficacy may be an effective approach to improving real-world walking activity in persons with stroke (without cerebellar involvement whose comfortable gait speeds are ≥0.3 m/s) who experience barriers within the physical environment. For example, an individual with stroke living in a highly populated area of high deprivation may not feel confident walking outdoors on sidewalks that are uneven or not well maintained or having to navigate around others, particularly if bumped into. In this case, the results of this work suggest that improving an individual’s balance confidence while walking over uneven terrains and during perturbations might be an effective approach to overcoming this barrier to improve their real-world walking activity. These results also highlight the critical importance of balance self-efficacy and corroborate past work demonstrating that balance self-efficacy is an important predictor of activity and participation,4,15,26,61,62 community ambulation (measured via self-report questionnaire),63 sedentary behavior,13 and real-world walking activity (measured with a performance-based measure) in individuals with stroke.25 In addition, the fact that physical capacity (defined using a cut-off score of 312 meters on the 6-Minute Walk Test) was not a moderator in our mediation model suggests that targeting balance self-efficacy to overcome physical environmental barriers to increasing real-world walking activity will likely not be impacted by a stroke survivor’s physical capacity.
Unlike the physical environment, we did not find support for our hypothesis that the relationship between who a stroke survivor lives with (which was our measure of the social environment) and real-world walking activity was mediated by balance self-efficacy in our cohort of participants with stroke. There may be several reasons for this. First, we conceptualized the social environment as factors related to social connectedness and social support which may not have been adequately captured by solely an individual’s living situation. Past work suggests that other aspects of the social environment, such as the supportiveness13 and comfort levels of a caregiver15, social support for exercise,17 and social roles within the household,15 influence activity levels in individuals with stroke that were not measured in this study. In addition, recent work suggests that the individual providing social support for physical activity (e.g., a partner, family member, friend, colleague, or other) may be important for individuals with stroke29 which may not have been captured by solely measuring an individual’s living situation. Thus, an individual’s living situation likely only reflects one aspect of the social environment and future studies should consider measuring multiple aspects of the social environment to better understand its influence on real-world walking activity in stroke.
Using a cut-off score of 312 meters on the 6-Minute Walk Test, we did not find evidence that physical capacity was a moderator in our mediation model, suggesting that our mediation model was not different for individuals with higher (>312 meters) and lower (≤312 meters) physical capacity in the cohort of participants with stroke meeting our eligibility criteria. Past work suggests that measures of physical capacity, and in particular the 6-Minute Walk Test, are likely the strongest predictors of real-world walking activity in persons with stroke.22 This finding, coupled with the finding that balance self-efficacy may be particularly important for stroke survivors with lower physical capacity,25 led us to hypothesize that our mediation model would be conditional on physical capacity. While we did find a stronger relationship between Activities Specific Balance Confidence Scale and average steps/day in individuals with lower physical capacity compared to those with higher physical capacity (Supplemental Figure 1), corroborating the work by Danks and colleagues,25 the results of the chi square difference test refuted our moderation hypothesis, suggesting that the mediation model was not conditional on physical capacity using a cut-off of 312 meters. There are several possible reasons for this finding, one of which relates to our decision to use the median split for scores on the 6-Minute Walk Test to define physical capacity as a moderator. While there is no consensus as to what constitutes “sufficient” physical capacity in the stroke rehabilitation literature, it is possible that our decision to use the median split was not sufficient for detecting if differences in the mediation model exist. In addition, any potential limitations of our theoretical model, such as the variables used to reflect the social and physical environment, persist through the moderation step. Thus, future work may consider examining alternative variables to represent these environmental domains to better understand their potential direct and indirect effects on real-world walking activity.
Limitations:
In addition to the limitations discussed above, the most important limitation of this work is that it is cross-sectional, and we therefore cannot infer causality. This cross-sectional analysis provides excellent guidance for targets of future longitudinal work that is needed to fully understand the potential mechanisms by which the environment influences real-world walking activity in persons with stroke. A second important limitation is that our findings are only applicable to persons with stroke that meet our eligibility criteria, particularly those that do not have cerebellar involvement and whose comfortable gait speeds were ≥0.3 m/s. Thus, our results may not generalize to individuals with stroke that do not meet these criteria. In a similar vein, this gait speed criteria likely limited our representation of stroke survivors with “lower” physical capacity. Finally, the Area Deprivation Index was considered a measure of the physical environment based on prior work demonstrating that more deprived neighborhoods tend to have unique physical environmental characteristics47,48; however, the Area Deprivation Index also includes indicators of socioeconomic status.46 Because the Area Deprivation Index is a composite measure,46 it is not known which aspects of this measure were contributing to the relationships observed in the current work. Past work suggests that the physical and socioeconomic environments are intricately linked in terms of their effects on health outcomes64,65; thus, additional studies are needed to disentangle these relationships and understand their effects on outcomes in individuals with stroke.
Supplementary Material
FUNDING SOURCES:
The authors disclose receipt of the following financial support for the research, authorship and/or publication of this article: This work was supported in part by the Foundation for Physical Therapy Research [Florence P. Kendall Doctoral Scholarship, Promotion of Doctoral Studies I Scholarship, Promotion of Doctoral Studies II Scholarship]; and the National Institutes of Health [grant number R01HD086362].
APPENDIX 1.
For completeness, we display the results of the unconstrained (i.e., moderation) model in Supplemental Figure 1 which show the model results for individuals with lower and higher physical capacity. In stroke survivors with lower physical capacity (≤312 m on 6MWT), the direct effect of ADI on ASPD was significant (β = −0.18, p=0.01), suggesting that lower area deprivation is associated with higher ASPD. The effect of ABC on ASPD (β=0.23, p=0.001) was also significant implying that higher balance self-efficacy is associated with higher ASPD in persons with stroke with lower physical capacity. All other path coefficients were smaller in magnitude and not statistically significant. For individuals with greater physical capacity (>312 m on 6MWT), all path coefficients were relatively small in magnitude, and none were statistically significant.
Footnotes
DECLARATION OF CONFLICTING INTERESTS:
The authors declare that there is no conflict of interest.
REFERENCES:
- 1.Benjamin EJ, Virani SS, Callaway CW, et al. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation. 2018;137(12):e67–e492. [DOI] [PubMed] [Google Scholar]
- 2.Mayo NE, Wood-Dauphinee S, Cote R, Durcan L, Carlton J. Activity, participation, and quality of life 6 months poststroke. Archives of physical medicine and rehabilitation. 2002;83(8):1035–1042. [DOI] [PubMed] [Google Scholar]
- 3.Michael KM, Allen JK, Macko RF. Reduced ambulatory activity after stroke: the role of balance, gait, and cardiovascular fitness. Archives of physical medicine and rehabilitation. 2005;86(8):1552–1556. [DOI] [PubMed] [Google Scholar]
- 4.Salbach NM, Mayo NE, Robichaud-Ekstrand S, Hanley JA, Richards CL, Wood-Dauphinee S. Balance self-efficacy and its relevance to physical function and perceived health status after stroke. Archives of physical medicine and rehabilitation. 2006;87(3):364–370. [DOI] [PubMed] [Google Scholar]
- 5.Butler EN, Evenson KR. Prevalence of physical activity and sedentary behavior among stroke survivors in the United States. Topics in stroke rehabilitation. 2014;21(3):246–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.English C, Healy GN, Coates A, Lewis L, Olds T, Bernhardt J. Sitting and Activity Time in People With Stroke. Physical therapy. 2016;96(2):193–201. [DOI] [PubMed] [Google Scholar]
- 7.Paul L, Brewster S, Wyke S, et al. Physical activity profiles and sedentary behaviour in people following stroke: a cross-sectional study. Disability and rehabilitation. 2016;38(4):362–367. [DOI] [PubMed] [Google Scholar]
- 8.Billinger SA, Arena R, Bernhardt J, et al. Physical activity and exercise recommendations for stroke survivors: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(8):2532–2553. [DOI] [PubMed] [Google Scholar]
- 9.Kang S-M, Kim S-H, Han K-D, Paik N-J, Kim W-S. Physical activity after ischemic stroke and its association with adverse outcomes: A nationwide population-based cohort study. Topics in stroke rehabilitation. 2020:1–11. [DOI] [PubMed] [Google Scholar]
- 10.Lee CD, Folsom AR, Blair SN. Physical Activity and Stroke Risk. Stroke. 2003;34(10):2475–2481. [DOI] [PubMed] [Google Scholar]
- 11.O'Donnell MJ, Xavier D, Liu L, et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case-control study. Lancet. 2010;376(9735):112–123. [DOI] [PubMed] [Google Scholar]
- 12.Barclay R, Ripat J, Mayo N. Factors describing community ambulation after stroke: a mixed-methods study. Clin Rehabil. 2015;29(5):509–521. [DOI] [PubMed] [Google Scholar]
- 13.Hall J, Morton S, Fitzsimons CF, et al. Factors influencing sedentary behaviours after stroke: findings from qualitative observations and interviews with stroke survivors and their caregivers. BMC Public Health. 2020;20(1):967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nicholson S, Sniehotta FF, van Wijck F, et al. A systematic review of perceived barriers and motivators to physical activity after stroke. Int J Stroke. 2013;8(5):357–364. [DOI] [PubMed] [Google Scholar]
- 15.Outermans J, Pool J, van de Port I, Bakers J, Wittink H. What's keeping people after stroke from walking outdoors to become physically active? A qualitative study, using an integrated biomedical and behavioral theory of functioning and disability. BMC Neurol. 2016;16(1):137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Institute of Medicine Committee on the R, Assessment of the NsSRP, Budget to R, Ultimately Eliminate Health D. The National Academies Collection: Reports funded by National Institutes of Health. In: Thomson GE, Mitchell F, Williams MB, eds. Examining the Health Disparities Research Plan of the National Institutes of Health: Unfinished Business. Washington (DC): National Academies Press (US) Copyright © 2006, National Academy of Sciences.; 2006. [PubMed] [Google Scholar]
- 17.Damush TM, Plue L, Bakas T, Schmid A, Williams LS. Barriers and facilitators to exercise among stroke survivors. Rehabil Nurs. 2007;32(6):253–260, 262. [DOI] [PubMed] [Google Scholar]
- 18.Prakash V, Ganesan M. What matters to patients with stroke in India and why: a qualitative study. Disability and rehabilitation. 2021;43(18):2585–2592. [DOI] [PubMed] [Google Scholar]
- 19.Miller A, Pohlig RT, Reisman DS. Social and physical environmental factors in daily stepping activity in those with chronic stroke. Topics in stroke rehabilitation. 2020:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van der Ploeg HP, van der Beek AJ, van der Woude LH, van Mechelen W. Physical activity for people with a disability: a conceptual model. Sports Med. 2004;34(10):639–649. [DOI] [PubMed] [Google Scholar]
- 21.Brenner AB, Burke JF, Skolarus LE. Moving Toward an Understanding of Disability in Older U.S. Stroke Survivors. J Aging Health. 2018;30(1):75–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thilarajah S, Mentiplay BF, Bower KJ, et al. Factors Associated With Post-Stroke Physical Activity: A Systematic Review and Meta-Analysis. Archives of physical medicine and rehabilitation. 2018;99(9):1876–1889. [DOI] [PubMed] [Google Scholar]
- 23.Fulk GD, He Y, Boyne P, Dunning K. Predicting Home and Community Walking Activity Poststroke. Stroke. 2017;48(2):406–411. [DOI] [PubMed] [Google Scholar]
- 24.Fulk GD, Reynolds C, Mondal S, Deutsch JE. Predicting home and community walking activity in people with stroke. Archives of physical medicine and rehabilitation. 2010;91(10):1582–1586. [DOI] [PubMed] [Google Scholar]
- 25.Danks KA, Pohlig RT, Roos M, Wright TR, Reisman DS. Relationship Between Walking Capacity, Biopsychosocial Factors, Self-efficacy, and Walking Activity in Persons Poststroke. J Neurol Phys Ther. 2016;40(4):232–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.French MA, Moore MF, Pohlig R, Reisman D. Self-efficacy Mediates the Relationship between Balance/Walking Performance, Activity, and Participation after Stroke. Topics in stroke rehabilitation. 2016;23(2):77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller A, Pohlig RT, Wright T, Kim H, Reisman DS. Beyond Physical Capacity: Factors Associated with Real-world Walking Activity Post Stroke. Archives of physical medicine and rehabilitation. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wright H, Wright T, Pohlig RT, Kasner SE, Raser-Schramm J, Reisman D. Protocol for promoting recovery optimization of walking activity in stroke (PROWALKS): a randomized controlled trial. BMC Neurol. 2018;18(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pauly T, Ashe MC, Murphy R, et al. Active With Whom? Examining the Social Context of Physical Activity in Individuals After Stroke and Their Partners. Front Public Health. 2021;9:754046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ishii K, Shibata A, Oka K. Environmental, psychological, and social influences on physical activity among Japanese adults: structural equation modeling analysis. Int J Behav Nutr Phys Act. 2010;7:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McNeill LH, Wyrwich KW, Brownson RC, Clark EM, Kreuter MW. Individual, social environmental, and physical environmental influences on physical activity among black and white adults: a structural equation analysis. Ann Behav Med. 2006;31(1):36–44. [DOI] [PubMed] [Google Scholar]
- 32.Boyle HK, Dunsiger SI, Bohlen LC, et al. Affective response as a mediator of the association between the physical and social environment and physical activity behavior.J Behav Med. 2020;43(5):773–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51(6):1173–1182. [DOI] [PubMed] [Google Scholar]
- 34.Eng JJ, Dawson AS, Chu KS. Submaximal exercise in persons with stroke: test-retest reliability and concurrent validity with maximal oxygen consumption. Archives of physical medicine and rehabilitation. 2004;85(1):113–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Flansbjer UB, Holmback AM, Downham D, Patten C, Lexell J. Reliability of gait performance tests in men and women with hemiparesis after stroke. J Rehabil Med. 2005;37(2):75–82. [DOI] [PubMed] [Google Scholar]
- 36.Botner EM, Miller WC, Eng JJ. Measurement properties of the Activities-specific Balance Confidence Scale among individuals with stroke. Disability and rehabilitation. 2005;27(4):156–163. [DOI] [PubMed] [Google Scholar]
- 37.Salbach NM, Mayo NE, Hanley JA, Richards CL, Wood-Dauphinee S. Psychometric evaluation of the original and Canadian French version of the activities-specific balance confidence scale among people with stroke. Archives of physical medicine and rehabilitation. 2006;87(12):1597–1604. [DOI] [PubMed] [Google Scholar]
- 38.Duclos NC, Aguiar LT, Aissaoui R, Faria C, Nadeau S, Duclos C. Activity Monitor Placed at the Nonparetic Ankle Is Accurate in Measuring Step Counts During Community Walking in Poststroke Individuals: A Validation Study. PM R. 2019;11(9):963–971. [DOI] [PubMed] [Google Scholar]
- 39.Fulk GD, Combs SA, Danks KA, Nirider CD, Raja B, Reisman DS. Accuracy of 2 activity monitors in detecting steps in people with stroke and traumatic brain injury. Physical therapy. 2014;94(2):222–229. [DOI] [PubMed] [Google Scholar]
- 40.Hui J, Heyden R, Bao T, et al. Validity of the Fitbit One for Measuring Activity in Community-Dwelling Stroke Survivors. Physiother Can. 2018;70(1):81–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klassen TD, Semrau JA, Dukelow SP, Bayley MT, Hill MD, Eng JJ. Consumer-Based Physical Activity Monitor as a Practical Way to Measure Walking Intensity During Inpatient Stroke Rehabilitation. Stroke. 2017;48(9):2614–2617. [DOI] [PubMed] [Google Scholar]
- 42.Klassen TD, Simpson LA, Lim SB, et al. "Stepping Up" Activity Poststroke: Ankle-Positioned Accelerometer Can Accurately Record Steps During Slow Walking. Physical therapy. 2016;96(3):355–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tudor-Locke C, Burkett L, Reis JP, Ainsworth BE, Macera CA, Wilson DK. How many days of pedometer monitoring predict weekly physical activity in adults? Prev Med. 2005;40(3):293–298. [DOI] [PubMed] [Google Scholar]
- 44.Kind AJ, Jencks S, Brock J, et al. Neighborhood socioeconomic disadvantage and 30-day rehospitalization: a retrospective cohort study. Ann Intern Med. 2014;161(11):765–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kind AJH, Buckingham WR. Making Neighborhood-Disadvantage Metrics Accessible - The Neighborhood Atlas. The New England journal of medicine. 2018;378(26):2456–2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Singh GK. Area deprivation and widening inequalities in US mortality, 1969-1998. Am J Public Health. 2003;93(7):1137–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jones A, Hillsdon M, Coombes E. Greenspace access, use, and physical activity: understanding the effects of area deprivation. Prev Med. 2009;49(6):500–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mouratidis K. Neighborhood characteristics, neighborhood satisfaction, and well-being: The links with neighborhood deprivation. Land Use Policy. 2020;99:104886. [Google Scholar]
- 49.Jose PE. Doing statistical mediation and moderation. Guilford Press; 2013. [Google Scholar]
- 50.Preacher KJ, Rucker DD, Hayes AF. Addressing Moderated Mediation Hypotheses: Theory, Methods, and Prescriptions. Multivariate Behav Res. 2007;42(1):185–227. [DOI] [PubMed] [Google Scholar]
- 51.Vandenberg RJ, Lance CE. A Review and Synthesis of the Measurement Invariance Literature: Suggestions, Practices, and Recommendations for Organizational Research. Organizational Research Methods. 2000;3(1):4–70. [Google Scholar]
- 52.Hox JJ, Bechger TM. An introduction to structural equation modeling. 1998. [Google Scholar]
- 53.Widaman KF, Thompson JS. On specifying the null model for incremental fit indices in structural equation modeling. Psychological Methods. 2003;8(1):16–37. [DOI] [PubMed] [Google Scholar]
- 54.Mplus User's Guide [computer program]. Los Angeles, CA: Muthén & Muthén; 1998-2017. [Google Scholar]
- 55.Satorra A, Bentler PM. A scaled difference chi-square test statistic for moment structure analysis. Psychometrika. 2001;66(4):507–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bryant FB, Satorra A. Principles and Practice of Scaled Difference Chi-Square Testing. Structural Equation Modeling: A Multidisciplinary Journal. 2012;19(3):372–398. [Google Scholar]
- 57.Bentler PM. Comparative fit indexes in structural models. Psychol Bull. 1990;107(2):238–246. [DOI] [PubMed] [Google Scholar]
- 58.Hu Lt,Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling: A Multidisciplinary Journal. 1999;6(1):1–55. [Google Scholar]
- 59.Browne MW, Cudeck R. Single Sample Cross-Validation Indices for Covariance Structures. Multivariate Behavioral Research. 1989;24(4):445–455. [DOI] [PubMed] [Google Scholar]
- 60.Della Vecchia C, Viprey M, Haesebaert J, et al. Contextual determinants of participation after stroke: a systematic review of quantitative and qualitative studies. Disability and rehabilitation. 2021;43(13):1786–1798. [DOI] [PubMed] [Google Scholar]
- 61.Schmid AA, Van Puymbroeck M, Altenburger PA, et al. Balance and balance self-efficacy are associated with activity and participation after stroke: a cross-sectional study in people with chronic stroke. Archives of physical medicine and rehabilitation. 2012;93(6):1101–1107. [DOI] [PubMed] [Google Scholar]
- 62.Robinson CA, Shumway-Cook A, Ciol MA, Kartin D. Participation in community walking following stroke: subjective versus objective measures and the impact of personal factors. Physical therapy. 2011;91(12):1865–1876. [DOI] [PubMed] [Google Scholar]
- 63.Durcan S, Flavin E, Horgan F. Factors associated with community ambulation in chronic stroke. Disability and rehabilitation. 2016;38(3):245–249. [DOI] [PubMed] [Google Scholar]
- 64.Chaparro MP, Benzeval M, Richardson E, Mitchell R. Neighborhood deprivation and biomarkers of health in Britain: the mediating role of the physical environment. BMC Public Health. 2018;18(1):801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schüle SA, Bolte G. Interactive and Independent Associations between the Socioeconomic and Objective Built Environment on the Neighbourhood Level and Individual Health: A Systematic Review of Multilevel Studies. PloS one. 2015;10(4):e0123456. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


