Abstract
Purpose:
Phase I results of this phase I/II study showed that pamiparib 60 mg twice a day had antitumor activity and an acceptable safety profile in Chinese patients with advanced cancer, including epithelial ovarian cancer.
Patients and Methods:
This open-label phase II study was conducted in China and enrolled adult (≥18 years) patients with platinum-sensitive ovarian cancer (PSOC; disease progression occurring ≥6 months after last platinum treatment) or platinum-resistant ovarian cancer (PROC; disease progression occurring <6 months after last platinum treatment). Eligible patients had known or suspected deleterious germline BRCA mutation (gBRCAmut) and had previously received ≥2 lines of therapy. Pamiparib 60 mg orally twice a day was administered until disease progression, toxicity, or patient withdrawal. The primary endpoint was objective response rate (ORR) assessed by independent review committee (IRC) per RECIST version 1.1.
Results:
In the total patient population (N = 113; PSOC, n = 90; PROC, n = 23), median age was 54 years (range, 34–79) and 25.6% of patients received ≥4 prior systemic chemotherapy lines. Median study follow-up was 12.2 months (range, 0.2–21.5). Eighty-two patients with PSOC and 19 patients with PROC were evaluable for efficacy. In patients with PSOC, 8 achieved a complete response (CR) and 45 achieved a partial response (PR); ORR was 64.6% [95% confidence interval (CI), 53.3–74.9]. In patients with PROC, 6 achieved a PR; ORR was 31.6% (95% CI, 12.6–56.6). Frequently reported grade ≥3 adverse events were hematologic toxicities, including anemia and decreased neutrophil count.
Conclusions:
Pamiparib 60 mg twice a day showed antitumor activity with durable responses in patients with PSOC or PROC with gBRCAmut, and had a manageable safety profile.
Translational Relevance.
First-in-human (NCT02361723) results for the selective oral PARP1/2 inhibitor pamiparib established a recommended phase II dose of 60 mg twice a day. Phase I dose escalation of this phase I/II study (NCT03333915) showed that adverse events with pamiparib 60 mg twice a day were manageable/tolerable, and durable responses were observed in patients with ovarian cancer. Phase II dose expansion results, presented here, show that pamiparib 60 mg twice a day has antitumor activity with durable responses in platinum-sensitive or platinum-resistant ovarian cancer (PSOC or PROC) with germline BRCA1/2 mutation. Although PARP inhibitors are widely used as maintenance therapy for patients with PSOC, further investigation of rechallenge with pamiparib in Chinese patients with PROC who previously received PARP inhibitor therapy warrants further investigation. Frequently reported hematologic toxicities were manageable and resolved with medical intervention and laboratory monitoring during treatment. Plasma drug exposure of pamiparib 60 mg twice a day in phase II was consistent with that observed in phase I.
Introduction
Poly(ADP-ribose) polymerase (PARP) 1/2 proteins are involved in the repair of single- and double-strand DNA breaks (1, 2). Normal cells repair DNA breaks using base excision repair and homologous recombination (HR) pathways; HR-deficient (HRD) cancer cells are unable to repair double-strand DNA breaks (1). Tumor suppressor proteins BRCA1/2 are involved in the repair of double-strand DNA breaks (1), and loss of BRCA1/2 function leads to inhibition of HR-mediated repair of double-strand DNA breaks, making cells susceptible to DNA lesions caused by inhibition of PARP proteins (e.g., synthetic lethality; ref. 3).
Small-molecule PARP inhibitors are a class of therapeutic agents used to treat various malignancies, including tumors harboring BRCA1/2 mutations (4, 5). PARP inhibition impairs DNA repair and traps PARP proteins on damaged DNA, resulting in cytotoxicity that is exacerbated in HRD cells (5). Although PARP inhibitors have different potencies for PARP trapping of DNA, the cytotoxic effects of PARP inhibitors occur through modulation of the PARylation activity of PARP and PARP–DNA complex trapping (2, 5). Results from several randomized or single-arm clinical trials have shown that patients with platinum-sensitive ovarian cancer (PSOC), including those with tumors harboring BRCA1/2 mutations, can derive substantial clinical benefit from PARP inhibitor therapy (5, 6).
Pamiparib is a potent, selective, oral PARP1/2 inhibitor. In preclinical models, pamiparib demonstrated PARP–DNA complex trapping, brain penetration, antitumor activity, and inhibition of PARylation (7). Results of the first-in-human (FIH) study (NCT02361723) showed that pamiparib was generally well tolerated and had antitumor activity, notably in patients with epithelial ovarian cancer (8). The FIH phase I dose-escalation study defined the initial safety profile of pamiparib and established a recommended phase II dose (RP2D) as 60 mg orally twice a day and a maximum tolerated dose as 80 mg orally twice a day (8). The RP2D of pamiparib 60 mg twice a day was based on the overall adverse event (AE) profile and the lower incidence of nausea compared with the 80 mg twice a day dose (54.5% at 60 mg vs. 80.0% at 80 mg) as well as the clinical response observed throughout dose levels investigated in the FIH study (8). The phase I dose-escalation stage of this phase I/II study enrolled Chinese patients with advanced nonmucinous high-grade ovarian cancer or triple-negative breast cancer whose disease progressed despite standard therapy, or for which there is no standard therapy (NCT03333915; ref. 9). The phase I stage demonstrated that pamiparib's RP2D of 60 mg twice a day was well tolerated and durable responses were observed in patients with nonmucinous high-grade ovarian cancer (9). Here, we present primary results of the phase II expansion stage in patients with germline BRCA1/2 mutation–positive (gBRCA1/2mut) PSOC or platinum-resistant ovarian cancer (PROC).
Patients and Methods
Study design and patient population
This phase I/II open-label, multicenter study in China assessed the antitumor activity and safety of pamiparib in adult (≥18 years) female patients with advanced solid tumors whose disease progressed despite standard therapy or for which there is no standard therapy (Supplementary Fig. S1). Phase I evaluated the safety/tolerability, pharmacokinetic (PK) profile, and preliminary antitumor activity of pamiparib and established the RP2D in Chinese patients (9). Phase II assessed antitumor activity and further evaluated safety/tolerability of pamiparib. Phase II enrolled patients with PSOC (disease progression that occurred ≥6 months after last platinum treatment) or PROC (disease progression that occurred <6 months after last platinum treatment) and either known deleterious gBRCAmut or suspected deleterious gBRCAmut identified by central testing. The study was conducted in compliance with the protocol approved by the Institutional Review Board (IRB) or Independent Ethics Committee (IEC) and in accordance with Good Clinical Practice and all applicable regulatory requirements, including the Declaration of Helsinki. All patients provided written informed consent.
In phase II, eligible patients received 2 or more lines of prior therapy. Patients were required to have measurable disease per Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 (10), an Eastern Cooperative Oncology Group (ECOG) performance status of 0–1, and adequate organ function. Patients were not permitted to have received therapies targeting PARP, chemotherapy, biologic therapy, immunotherapy, investigational agent, anticancer Chinese medicine or anticancer herbal remedies, or to have undergone radiotherapy for any cause within 14 days of the first dose of study drug. Patients with untreated and/or active brain metastases were excluded; patients with treated brain metastases must not have received corticosteroids for ≥14 days and have no signs or symptoms of the progression of brain metastases.
Pamiparib 60 mg was administered orally twice a day starting on day 1 of cycle 1 (21-day cycle) and continuously in all subsequent cycles until disease progression, toxicity, or patient withdrawal. Any deviation from the prescribed dose frequency (e.g., one dose missed, including a dose missed due to noncompliance) was classified as a dose interruption. Any dose that was reduced from starting dose level (60 mg) as determined by investigators was classified as a dose reduction. Pamiparib was administered without food restrictions.
Endpoints and assessments
The phase II primary objective was to evaluate antitumor response; the primary endpoint was objective response rate (ORR) by IRC. Secondary endpoints were progression-free survival (PFS), duration of response (DoR), disease control rate [DCR; complete response (CR) + partial response (PR) + stable disease (SD)], best overall response (BOR), and clinical benefit rate (CBR; CR + PR + SD ≥24 weeks) by IRC and investigator review; overall survival (OS) by investigator review; CA-125 response rate; PK endpoints including area under the concentration–time curve (AUC), maximal plasma concentration (Cmax), and time to maximal plasma concentration Tmax; and safety/tolerability.
Assessments of tumor response and CA-125 response were performed every 6 weeks after the first dose of pamiparib for the first 18 weeks, then every 9 weeks through the first year, and every 12 weeks from the second year onward. Tumor response was assessed by IRC based on RECIST version 1.1 and CA-125 responses were assessed by Gynecologic Cancer Intergroup (GCIG) criteria (11). Serial blood samples for pamiparib PK assessment were collected in 15 patients at predose, 0.5, 1, 2, 4, 6, 9, and 12 hours postdose on cycle 1, day 1 and on cycle 2, day 1; sparse blood samples were collected in 19 patients at predose and 2 hours postdose on cycle 1, day 1 and on cycle 2, day 1. Blood samples were analyzed using a validated liquid chromatography–tandem mass spectrometry (LC/MS-MS) method.
Safety/tolerability assessments were based on monitoring of AEs, as well as on vital signs, electrocardiograms, physical examinations, and clinical laboratory results. The incidence of AEs, including serious AEs, overall and by severity, was assessed according to National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03. All dose modifications were based on the worst preceding toxicity, and all dose interruptions or treatment discontinuation were based on treatment-related AEs; each patient was allowed up to 2 dose reductions. A protocol amendment (protocol version 5.0) initiated a more proactive dose-modification algorithm for instances of hematologic AEs (Supplementary Table S1).
Analysis populations
Antitumor activity (RECIST v1.1) was assessed in all evaluable patients; patients were considered efficacy evaluable if they had measurable disease at baseline and had ≥1 postbaseline tumor assessment, unless treatment had been discontinued due to clinical progression or death prior to tumor assessment. Patients were evaluated according to CA-125 only if they had a baseline sample that was at least twice the upper limit of the reference range within 2 weeks before starting treatment. The PK population included all patients for whom valid pamiparib PK parameters could be estimated. Safety/tolerability was evaluated in all patients who received ≥1 dose of pamiparib.
Statistical analysis
Approximately 100 evaluable patients (n = ∼80 PSOC; n = ∼20 PROC) were planned for enrollment in the phase II stage of the study. An ORR of 52% was assumed with pamiparib treatment of previously treated patients with PSOC with BRCAmut. A total of 80 evaluable patients with PSOC were expected to give 98% power to demonstrate a statistical difference versus a historical response rate of 30% (chemotherapy) using a binomial exact test at an alpha of 0.025 (1-sided). The two-sided exact 95% CI is 40.5%–63.3% when the observed ORR is 52%. The planned number of patients with PROC was not based on statistical design. The PROC cohort was exploratory and is summarized descriptively.
Continuous variables were either reported as median and ranges or dichotomized as categorical variables. Categorical variables were summarized by their frequencies and percentages. Time-to-event variables were estimated using the Kaplan–Meier method. Standard PK parameters (AUC, Cmax, Tmax) were estimated using standard noncompartmental methods with Phoenix WinNonlin (version 8.1 or higher).
Results
Patient disposition
Phase II enrolled 113 female patients (PSOC, n = 90; PROC, n = 23). The efficacy-evaluable population included 101 patients (PSOC, n = 82; PROC, n = 19) with measurable disease at baseline and ≥1 postbaseline tumor assessment at data cutoff. As of February 2, 2020, 74 patients had discontinued treatment due to progressive disease (n = 47), AEs (n = 14), patient withdrawal (n = 10), and investigator's decision (n = 3). Median study follow-up was 12.2 months (range, 0.2–21.5). In the total population, median age was 54 years (range, 34–79) and 89.4% of patients were younger than 65 years (Table 1). Overall, 25.6% of patients received ≥4 prior systemic chemotherapy lines (21.1% in patients with PSOC; 43.4% in patients with PROC) and 86.7% and 13.3% of patients had gBRCA1mut and gBRCA2mut, respectively. Most patients (95.6%) had serous epithelial tumors.
Table 1.
Patient demographics and baseline characteristics.
| Patient demographics/characteristics | PSOC | PROC | Total |
|---|---|---|---|
| (n = 90) | (n = 23) | (N = 113) | |
| Median age, years (range) | 54 (39–79) | 54 (34–66) | 54 (34–79) |
| <65 years, n (%) | 80 (88.9) | 21 (91.3) | 101 (89.4) |
| ECOG score, n (%) | |||
| 0 | 42 (46.7) | 10 (43.5) | 52 (46.0) |
| 1 | 48 (53.3) | 13 (56.5) | 61 (54.0) |
| Number of prior lines of systemic chemotherapy (grouped), n (%) | |||
| 2 | 52 (57.8) | 3 (13.0) | 55 (48.7) |
| 3 | 19 (21.1) | 10 (43.5) | 29 (25.7) |
| 4 | 8 (8.9) | 6 (26.1) | 14 (12.4) |
| 5 | 4 (4.4) | 1 (4.3) | 5 (4.4) |
| ≥6 | 7 (7.8) | 3 (13.0) | 10 (8.8) |
| gBRCA status, n (%) | |||
| BRCA1 mutation | 79 (87.8) | 19 (82.6) | 98 (86.7) |
| BRCA2 mutation | 11 (12.2) | 4 (17.4) | 15 (13.3) |
| Years from initial diagnosis, median (range) | 3.9 (1.4–13.6) | 3.6 (1.1–7.1) | 3.9 (1.1–13.6) |
| Primary tumor location at initial diagnosis, n (%) | |||
| Ovary | 81 (90.0) | 20 (87.0) | 101 (89.4) |
| Fallopian tube | 7 (7.8) | 3 (13.0) | 10 (8.8) |
| Peritoneum | 1 (1.1) | 0 | 1 (0.9) |
| Undesignated | 1 (1.1) | 0 | 1 (0.9) |
| Histology, n (%) | |||
| Serous epithelial tumors | 85 (94.4) | 23 (100.0) | 108 (95.6) |
| Endometrioid epithelial tumors | 4 (4.4) | 0 | 4 (3.5) |
| Mixed epithelial tumors | 1 (1.1) | 0 | 1 (0.9) |
| Target lesion diameter per IRC at study entry (mm), n (%) | |||
| <50 | 41 (45.6) | 8 (34.8) | 49 (43.4) |
| ≥50 | 41 (45.6) | 11 (47.8) | 52 (46.0) |
| Missing | 8 (8.9) | 4 (17.4) | 12 (10.6) |
| CA-125 value at study entry (kU/L), n (%) | |||
| <70 | 16 (17.8) | 2 (8.7) | 18 (15.9) |
| ≥70 | 74 (82.2) | 21 (91.3) | 95 (84.1) |
Abbreviations: BRCA, breast cancer susceptibility gene; ECOG, Eastern Cooperative Oncology Group.
Clinical response
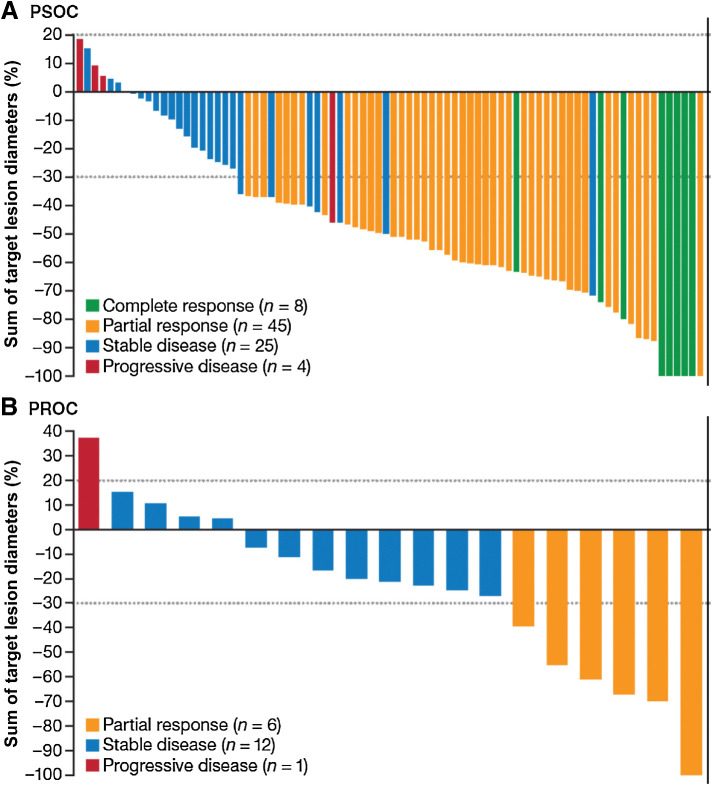
In both groups, most patients had a reduction in target lesions from baseline (Fig. 1A and B). In the efficacy-evaluable population (PSOC, n = 82; PROC, n = 19), by IRC assessment (RECIST v1.1), 8 patients with PSOC achieved a CR and 45 achieved a PR, resulting in an ORR of 64.6% (95% CI, 53.3–74.9; Table 2). Twenty-five patients with PSOC achieved SD, yielding a DCR of 95.1% and a CBR of 74.4%. By IRC assessment, 6 patients with PROC achieved a confirmed PR, resulting in an ORR of 31.6% (95% CI, 12.6–56.6). Twelve patients with PROC achieved SD, which yielded a DCR of 94.7% and a CBR of 52.6%. Responses for patients with PSOC and PROC occurred at approximately the first tumor assessment; median time to response was 1.68 months (range, 1.3–6.3) among PSOC responders (n = 53) and 1.38 months (range, 1.2–1.4) among PROC responders (n = 6). Clinical response (RECIST v1.1) was similar between IRC and investigator assessment (Table 2).
Figure 1.
Clinical response per IRC assessment (RECIST version 1.1) in patients with PSOC (A) and PROC (B) in the efficacy-evaluable population (n = 101). Patients were considered efficacy-evaluable if they had measurable disease at baseline and ≥1 postbaseline tumor assessment, unless treatment had been discontinued due to clinical progression or death prior to tumor assessment.
Table 2.
Tumor response by patient cohort in the efficacy-evaluable population (N = 101) by IRC and investigator assessment based on RECIST version 1.1.
| Tumor response | PSOC (n = 82) | PROC (n = 19) |
|---|---|---|
| IRC assessment | ||
| BOR, n (%) | ||
| CR (confirmed) | 8 (9.8) | 0 |
| PR (confirmed) | 45 (54.9) | 6 (31.6) |
| SD | 25 (30.5) | 12 (63.2) |
| PD | 4 (4.9) | 1 (5.3) |
| ORR, % (95% CI) | 64.6 (53.3–74.9) | 31.6 (12.6–56.6) |
| DCR, % (95% CI) | 95.1 (88.0–98.7) | 94.7 (74.0–99.9) |
| CBR, % (95% CI) | 74.4 (63.6–83.4) | 52.6 (28.9–75.6) |
| Investigator assessment | ||
| BOR, n (%) | ||
| CR (confirmed) | 5 (6.1) | 0 |
| PR (confirmed) | 46 (56.1) | 5 (26.3) |
| SD | 28 (34.1) | 10 (52.6) |
| PD | 3 (3.7) | 3 (15.8) |
| ORR, % (95% CI) | 62.2 (50.8–72.7) | 26.3 (9.1–51.2) |
| DCR, % (95% CI) | 96.3 (89.7–99.2) | 78.9 (54.4–93.9) |
| CBR, % (95% CI) | 72.0 (60.9–81.3) | 52.6 (28.9–75.6) |
Note: ORR was based on IRC per RECIST version 1.1. Patients were considered response-evaluable if they had measurable disease at baseline and had ≥1 postbaseline tumor assessment, unless treatment had been discontinued due to clinical progression or death prior to tumor assessment.
CBR = CR + PR + SD ≥24 weeks; DCR = CR + PR + SD; ORR = CR + PR.
The CA-125 response rate was 79.7% (95% CI, 68.8–88.2) in patients with PSOC and 38.1% (95% CI, 18.1–61.6) in patients with PROC (Supplementary Table S2). Median time to response was 1.4 months (range, 1.3–4.2) in patients with PSOC and 1.4 months (range, 1.3–2.8) in patients with PROC.
Durable clinical responses were observed in patients with PSOC and PROC. Median IRC-assessed DoRs were 14.5 months (95% CI, 11.10–not reached) and 11.1 months (95% CI, 4.21–not reached), respectively (Table 3; Supplementary Fig. S2A and S2B); in both groups, the upper limit of the 95% CI for median DoR was not reached. Median DoR among responders in the PSOC population was 16.6 months (95% CI, 9.03–not estimable) for patients who achieved a CR (n = 8) and 13.2 months (95% CI, 9.00–20.73) for patients who achieved a PR (n = 48). For patients with PSOC, the median follow-up was 12.25 months with a censoring rate of 75.5%; among patients with PROC, the median follow-up was 11.63 months with a censoring rate of 50.0%. Event-free rates at 9 and 12 months were 76.9% and 66.3%, respectively, in patients with PSOC, and 66.7% and 33.3%, respectively, in patients with PROC.
Table 3.
Duration of response by IRC assessment in the efficacy-evaluable population.
| Duration of Response | PSOC (n = 82) | PROC (n = 19) |
|---|---|---|
| Responders, n | 53 | 6 |
| Duration of response per RECIST version 1.1, n (%) | ||
| Events | 13 (24.5) | 3 (50.0) |
| Disease progression | 12 (22.6) | 3 (50.0) |
| Death | 1 (1.9) | 0 |
| Censored | 40 (75.5) | 3 (50.0) |
| New anticancer therapy | 11 (20.8) | 2 (33.3) |
| No disease progression or death | 29 (54.7) | 1 (16.7) |
| Duration of response, months | ||
| Median (95% CI)a | 14.5 (11.10–NE) | 11.1 (4.21–NE) |
| Q1–Q3a | 9.0–NR | 4.9–NR |
| Event-free rateb (%, 95% CI) of responders at: | ||
| 3 months | 97.8 (85.55–99.69) | 100.0 (NE–NE) |
| 6 months | 86.1 (71.61–93.53) | 66.7 (19.46–90.44) |
| 9 months | 76.9 (59.87–87.41) | 66.7 (19.46–90.44) |
| 12 months | 66.3 (45.08–80.94) | 33.3 (1.37–75.48) |
Note: Percentages were based on the number of responders.
Abbreviations: NE, not estimable; NR, not reached.
aMedians and other quartiles for DoR were estimated by Kaplan–Meier method with 95% CIs estimated using the method of Brookmeyer and Crowley.
bEvent-free rates were estimated by Kaplan–Meier method with 95% CIs estimated using the Greenwood's formula.
Analysis of patients with PSOC showed that high level of response, as assessed by the primary endpoint of ORR per IRC, was generally consistent across all subgroups analyzed (Supplementary Fig. S3). Because of limited sample size, no subgroup analyses were performed in patients with PROC.
PFS and OS
In patients with PSOC (n = 90) and PROC (n = 23), median PFS per IRC assessment was 15.2 months (95% CI, 10.35–not estimable) and 6.2 months (95% CI, 4.11–not estimable), respectively (Supplementary Table S3; Supplementary Fig. S2C and S2D). Event-free rates for PFS at 6 and 12 months were 78.6% and 57.4%, respectively, in patients with PSOC, and 51.2% and 38.4%, respectively, in patients with PROC. While median OS was not mature at data cutoff for patients with PSOC, median OS was estimated as 13.6 months (95% CI, 7.13–not estimable) in patients with PROC (Supplementary Table S3). Event-free rates for OS at 6 and 12 months were 93.2% and 83.5%, respectively, in patients with PSOC, and 73.4% and 50.5%, respectively, in patients with PROC.
Pharmacokinetic profile of pamiparib
Mean pamiparib plasma concentration–time profiles after a single dose (cycle 1, day 1) and at steady-state (cycle 2, day 1) are presented in Supplementary Fig. S4. A summary of pamiparib PK parameters is presented in Supplementary Table S4. Median Tmax was approximately 2 hours; steady-state geometric mean (% coefficient of variation) AUC from time of drug administration to 12 hours or to the last measurable concentration and Cmax were 48,802.4 (24.3) ng/mL/h and 5,251.5 (22.7) ng/mL, respectively. The accumulation ratios based on AUC and Cmax of pamiparib 60 mg twice a day were 3.0 and 2.4, respectively (Supplementary Table S5).
Safety/tolerability profile
Across PSOC (n = 90) and PROC (n = 23) groups, all patients (100.0%) experienced at least one AE and 71.1% and 87.0% of patients, respectively, experienced an AE of grade ≥3 (Supplementary Table S6). Among patients with PSOC and PROC, the most frequently reported AEs of any grade were hematologic toxicities (anemia, decreased neutrophil count, and decreased white blood cell count) and gastrointestinal disorders (nausea and vomiting; Table 4). The most frequently reported grade ≥3 AEs were hematologic toxicities, including anemia and decreased neutrophil count. There were no reports of significant complications, such as grade ≥3 or serious hemorrhage or infection potentially related to hematologic toxicity, myelodysplastic syndrome or acute myeloid leukemia, or life-threatening or fatal outcomes related to hematologic toxicity. There was one case of grade 3 febrile neutropenia unlikely related to study drug, which was reported in a patient in the PSOC population. Most hematologic AEs were manageable with medical intervention (e.g., dose modification and/or supportive care). Treatment-emergent serious AEs were reported in 37.8% and 65.2% of patients with PSOC and PROC, respectively (Supplementary Table S6). Across PSOC and PROC groups, all patients (100.0%) experienced at least one treatment-related AE and 67.8% and 82.6% of patients, respectively, experienced a treatment-related AE of grade ≥3 (Supplementary Table S6).
Table 4.
Treatment-emergent adverse events of any grade (≥10% in the total population) and of grade ≥3 by PSOC and PROC cohorts and by preprotocol amendment and postprotocol amendment subgroups.
| PSOC | PROC | Total | Preprotocol amendment | Postprotocol amendment | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (n = 90) | (n = 23) | (N = 113) | (n = 74) | (n = 39) | ||||||
| Adverse event | All grades | Grade ≥3 | All grades | Grade ≥3 | All grades | Grade ≥3 | All grades | Grade ≥3 | All grades | Grade ≥3 |
| Anemia | 80 (88.9) | 34 (37.8) | 21 (91.3) | 13 (56.5) | 101 (89.4) | 47 (41.6) | 65 (87.8) | 37 (50.0) | 36 (92.3) | 10 (25.6) |
| Nausea | 61 (67.8) | 1 (1.1) | 16 (69.6) | 0 | 77 (68.1) | 1 (0.9) | 48 (64.9) | 1 (1.4) | 29 (74.4) | 0 |
| Decreased neutrophil count | 56 (62.2) | 28 (31.1) | 13 (56.5) | 10 (43.5) | 69 (61.1) | 38 (33.6) | 46 (62.2) | 29 (39.2) | 23 (59.0) | 9 (23.1) |
| Decreased white blood cell count | 54 (60.0) | 17 (18.9) | 14 (60.9) | 5 (21.7) | 68 (60.2) | 22 (19.5) | 44 (59.5) | 16 (21.6) | 24 (61.5) | 6 (15.4) |
| Vomiting | 46 (51.1) | 4 (4.4) | 11 (47.8) | 1 (4.3) | 57 (50.4) | 5 (4.4) | 42 (56.8) | 5 (6.8) | 15 (38.5) | 0 |
| Decreased platelet count | 25 (27.8) | 4 (4.4) | 10 (43.5) | 1 (4.3) | 35 (31.0) | 5 (4.4) | 22 (29.7) | 2 (2.7) | 13 (33.3) | 3 (7.7) |
| Decreased appetite | 29 (32.2) | 0 | 5 (21.7) | 0 | 34 (30.1) | 0 | 21 (28.4) | 0 | 13 (33.3) | 0 |
| Asthenia | 26 (28.9) | 1 (1.1) | 6 (26.1) | 0 | 32 (28.3) | 1 (0.9) | 20 (27.0) | 1 (1.4) | 12 (30.8) | 0 |
| Diarrhea | 19 (21.1) | 3 (3.3) | 6 (26.1) | 0 | 25 (22.1) | 3 (2.7) | 19 (25.7) | 1 (1.4) | 6 (15.4) | 2 (5.1) |
| Increased AST | 20 (22.2) | 0 | 4 (17.4) | 1 (4.3) | 24 (21.2) | 1 (0.9) | 15 (20.3) | 1 (1.4) | 9 (23.1) | 0 |
| Decreased lymphocyte count | 19 (21.1) | 6 (6.7) | 5 (21.7) | 2 (8.7) | 24 (21.2) | 8 (7.1) | 19 (25.7) | 7 (9.5) | 5 (12.8) | 1 (2.6) |
| Increased ALT | 18 (20.0) | 1 (1.1) | 5 (21.7) | 0 | 23 (20.4) | 1 (0.9) | 15 (20.3) | 1 (1.4) | 8 (20.5) | 0 |
| Leukopenia | 20 (22.2) | 9 (10.0) | 3 (13.0) | 3 (13.0) | 23 (20.4) | 12 (10.6) | 17 (23.0) | 11 (14.9) | 6 (15.4) | 1 (2.6) |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Median treatment duration was 8.3 (range, 0.1–19.3) months in patients with PSOC and 4.1 (range, 0.1–19.9) months in patients with PROC, with median relative dose intensities of 91.2% (range, 41.9–100.0) and 90.9% (range, 54.8–100.0), respectively. Dose interruptions were reported in 93.3% of patients with PSOC and 91.3% of patients with PROC; subsequent dose reductions were reported in 76.7% and 47.8% of patients, respectively. Management of AEs was the primary reason for dose modifications (dose reductions: 68.9%, PSOC; 47.8%, PROC; and dose interruptions: 75.6%, PSOC; 69.6%, PROC). Anemia was the most frequently reported AE that led to dose modifications (72.2%, PSOC; 56.5%, PROC). In the total population, 65.8% (73/111) of patients required dose reductions to manage anemia. Other AEs that led to dose reductions were decreased neutrophil count (n = 12); decreased white blood cell count (n = 5); leukopenia (n = 4); decreased platelet count, nausea, thrombocytopenia, and vomiting (each n = 2); and asthenia, decreased lymphocyte count, fatigue, hypokalemia, intestinal obstruction, increased gamma-glutamyl transferase, malaise, neutropenia, and pancytopenia (each n = 1). Most of these AEs resolved with dose reductions (91.9%, n = 102/111). Six cases of anemia (grade 2, n = 5 and grade 3, n = 1) and one case each of decreased lymphocyte count (grade 3), malaise (grade 2), and thrombocytopenia (grade 3) were ongoing at the time of analysis.
To improve the safety of pamiparib in this study, a more proactive dose-management algorithm with closer hematologic monitoring was instituted via a protocol amendment to be more consistent with hematologic toxicity management employed with other PARP inhibitors. Subsequently, the percentage of patients who experienced grade ≥3 AEs of anemia, decreased white blood cell count, decreased lymphocyte count, and leukopenia was lower in the postprotocol amendment subgroup than in the preprotocol amendment subgroup (Table 4). In addition, the percentage of patients who experienced serious hematologic AEs was lower in the postprotocol amendment subgroup than in the preprotocol amendment subgroup (7.7% vs. 35.1%), as was the percentage of patients who received erythropoietin (58.1% vs. 33.3%) and red blood cell transfusion (37.8% vs. 12.8%). No patient in the postprotocol amendment subgroup experienced a hematologic AE that led to treatment discontinuation. There were two fatal AEs; both were unlikely to be related to study treatment.
Impact of dose reduction on efficacy
Of the 82 efficacy-evaluable patients with PSOC, 52 (63.4%) required a pamiparib one-level dose reduction to 40 mg twice a day, and 12 (14.6%) patients required a two-level dose reduction to 20 mg twice a day. Eighteen (22.0%) patients did not require a reduction from the starting dose of 60 mg twice a day during the study. Median time to first dose reduction was 9.1 and 10.1 weeks for patients with PSOC and PROC, respectively. Confirmed ORR was 74.5% (95% CI, 60.4–85.7) in patients with PSOC who required a dose reduction to 40 mg twice a day and 69.2% (95% CI, 38.6–90.9) in patients whose dose was reduced to 20 mg twice a day. Given the relatively limited sample size in each subgroup and the overlapping CIs, the ORRs among patients who required dose reductions were generally consistent with the ORRs of patients who did not require a dose reduction [ORR for 60 mg twice a day, 50.0% (95% CI, 26.0–74.0)]. Clinical activity, as measured by DoR and PFS, was generally consistent in the PSOC group with and without a dose reduction (Supplementary Fig. S5A and S5B). Because of limited sample size, analyses of the impact of dose reduction on clinical activity was not performed in the PROC group.
Discussion
These phase II results demonstrated that pamiparib is highly active, with durable responses observed in BRCAmut patients with PSOC and PROC. Activity observed in Chinese patients with PROC is particularly notable, as this population has not been studied extensively in trials of PARP inhibitors. Although PARP inhibitors are widely used as maintenance therapy for patients with PSOC, further investigation of rechallenge with pamiparib in Chinese patients with PROC who previously received PARP inhibitor therapy warrants further investigation. The ORRs observed with pamiparib (31.6%) are comparable with ORRs observed with current treatment options for patients with PROC (12). In addition, results of this study showed durability of response (median DoR, 11.1 months) and PFS benefit (median PFS, 6.2 months) with pamiparib treatment of patients with PROC. It is important to note that results of this study represent the first readout for a PARP inhibitor used to treat Chinese patients with PROC. This is also the first indication for PARP inhibitor use in this patient population. Although the number of patients with PROC in this study was small, results of the study suggest that pamiparib may fill an unmet medical need for this patient population. This is of particular importance as patients who were sensitive to platinum agents later progress to becoming resistant to platinum agents, thus increasing the population of patients with PROC.
Results of this study showed that pamiparib's safety profile is similar to those of other PARP inhibitors in phase II studies of the ovarian cancer population (13–15). Gastrointestinal and hematologic toxicities were the most commonly reported AEs in this study. Anemia, the most commonly reported hematologic toxicity in the current study, might be an on-target effect related to PARP2 inhibition and erythrogenesis (16). Anemia was the most common reason for dose modification (69.9%) and was considered related to pamiparib treatment. Although the overall number of patients who required dose reduction (n = 73) or dose interruption (n = 84) to manage AEs was relatively high, few AEs led to treatment discontinuation (n = 13, 11.5%) and few patients discontinued treatment because of anemia (n = 5, 4.4%), indicating that most patients continued to receive pamiparib while being managed for hematologic AEs with dose modification or supportive care. No life-threatening or fatal outcomes related to hematologic toxicity were reported. Neither myelodysplastic syndrome nor acute myeloid leukemia were reported, although these events have been observed with other PARP inhibitors. As expected, most dose reductions occurred in the first 3 months of treatment. Despite the high rate of hematologic AEs observed during pamiparib treatment, the median duration of treatment was 8.3 months (range, 0.1–26.0) and relative dose intensity was 91.2% (range, 41.9–100.0) in the PSOC population, based on the prescribed dose level, indicating that patients remained on treatment with high compliance to study protocol.
The relatively high incidence of hematologic AEs was partially a result of the current study being conducted entirely in China, where PARP inhibitors for ovarian cancer were only approved in 2018 (17, 18) and medical management of PARP inhibitor–related hematologic toxicities had become more of a standard practice following the initial 2014 approval of PARP inhibitors in the United States (19). To better manage the hematologic AEs induced by pamiparib, a protocol amendment was put into place to more proactively assess and manage hematologic AEs in the current study. The new dose modification plan required a mandatory dose interruption/reduction at first occurrence of hemoglobin <9.0 g/dL. Of the total safety population (N = 113), 74 patients were assessed before the protocol amendment and 39 patients were assessed after the protocol amendment. After following this per-protocol proposed dose modification algorithm, the incidence of grade ≥3 hematologic AEs and serious hematologic AEs was reduced, as was the use of erythropoietin and red blood cell transfusions. No patient experienced a hematologic AE leading to treatment discontinuation after the protocol amendment was put into place. Although hematologic toxicities remained the most significant safety events observed during study treatment, no grade ≥3 or serious hemorrhage or infection potentially related to hematologic toxicity was reported (only one reported case of grade 3 febrile neutropenia unlikely related to study drug). Most hematologic toxicities were considered manageable and resolved with medical intervention and close laboratory monitoring during treatment, which is also consistent with management of these toxicities that occur with other PARP inhibitors. Overall, the management of hematologic AEs was improved with a more proactive dose modification plan and closer hematologic monitoring.
Pamiparib single-dose and steady-state plasma exposure was similar to plasma exposure observed in phase I of this study (9). Plasma exposure after administration of a single dose of pamiparib in the current study was also similar to plasma exposure observed in the FIH study (20), but steady-state plasma exposure was slightly higher.
Clinical studies of PARP inhibitors in patients with advanced ovarian cancer who had received at least two prior therapies reported ORRs ranging from 39% with niraparib to 80% with rucaparib in patients with PSOC and 25% with rucaparib to 30% with olaparib in patients with PROC (14, 21–25). In the current study, patients with PSOC who received pamiparib achieved an ORR of 64.6%, which falls within the range of ORRs reported with other PARP inhibitors. However, patients with PROC in the current study achieved an ORR of 31.6%, which falls above the higher end of the range of ORRs reported with other PARP inhibitors. In the current study, median PFS was 15.2 months in patients with PSOC and 6.2 months in patients with PROC, which is in line with median PFS values reported for the approved PARP inhibitors (PSOC, 9.4–16.5 months; PROC, 5.5–7.4 months; refs. 14, 21–25). In addition, patients with PROC who received pamiparib achieved a DoR of 11.1 months, which also falls above the reported DoR for other PARP inhibitors (14, 21, 23). This DoR is likely related to pamiparib not serving as a substrate for P-glycoprotein (7). Of note, outcomes of these studies cannot be directly compared because of differences in patient populations and study designs. Hematologic AEs, including anemia, neutropenia, and thrombocytopenia, were frequently reported in studies of patients with advanced ovarian cancer who received PARP inhibitor therapy (14, 21–25).
Pamiparib has potent PARP-trapping activity. Results of in vitro studies showed that pamiparib inhibits the enzyme activity of PARP1/2 with half-maximal inhibitory concentrations of 1.3 and 0.92 nmol/L, respectively (7). The PARP-trapping activity of pamiparib (half-maximal effective concentration, 13 nmol/L) is similar to that of olaparib (half-maximal effective concentration, 16 nmol/L), and 30-fold more potent than veliparib (7). Results of in vivo studies showed that the antitumor activity against a BRCA1 mutant mouse xenograft model was 16-fold more potent with pamiparib than with olaparib (7). In addition, pamiparib has shown strong penetration of the blood–brain barrier in nonclinical studies (7), which may lead to clinical benefit for patients with BRCAmut. Results of a large retrospective analysis of patients with ovarian cancer (N = 4515) showed that a higher percentage of patients with BRCAmut, compared with BRCA wild-type, developed brain metastases (3.0% vs. 0.6%; ref. 26). Of note, acquired resistance to PARP inhibitors has reportedly occurred in most patients with advanced cancer who are treated with these agents (4). The acquired resistance may, in part, result from a PARP inhibitor being a substrate of P-gp (P-glycoprotein) and BCRP (breast cancer resistance protein; refs. 27–29). Recently reported evidence has shown that pamiparib is not a substrate of P-glycoprotein or BCRP (7), thereby preventing potential drug resistance mechanisms commonly observed in other PARP inhibitors (30). An ongoing phase II study (NCT03933761, PRECISE, ANZGOG 1721/2018) being conducted in Australia is investigating pamiparib in patients with germline or somatic BRCA1/2-mutant advanced ovarian cancer who have progressed on P-gp substrate PARP inhibitor or chemotherapy. In addition, the bioavailability of pamiparib is high, with near complete absorption in humans (8, 9). These prominent differentiation factors of pamiparib may increase its utility in the treatment of patients with various solid tumors. As PARP inhibitors are now proving to be useful for much broader populations of patients with ovarian cancer and those with other solid tumors, additional studies of pamiparib, including in the maintenance setting for ovarian cancer (NCT03519230) and in various other solid tumors, are now under way.
Data sharing
On request, and subject to certain criteria, conditions, and exceptions, BeiGene, Ltd., will provide access to individual de-identified participant data from BeiGene-sponsored global interventional clinical studies conducted for medicines (1) for indications that have been approved or (2) in programs that have been terminated. BeiGene will also consider requests for the protocol, data dictionary, and statistical analysis plan. Data requests may be submitted to DataDisclosure@beigene.com.
Authors' Disclosures
X. Mu reports personal fees from BeiGene outside the submitted work. L. Li reports other support from BeiGene during the conduct of the study. S. Mu reports other support from BeiGene during the conduct of the study, as well as other support from BeiGene outside the submitted work. No disclosures were reported by the other authors.
Supplementary Material
Acknowledgments
The authors wish to acknowledge the investigative center study staff, the study patients, and their families. BeiGene, Ltd. provided financial support for this manuscript, including writing and editorial assistance by Cathy R. Winter, Amit Lugade, Regina Switzer, and Elizabeth Hermans (Peloton Advantage, LLC, an OPEN Health Company).
The study protocol was developed by BeiGene, Ltd. in collaboration with the study investigators. BeiGene, Ltd. was also involved in data collection, analysis, and interpretation of results. Statistical analyses were performed by statisticians at BeiGene, Ltd.
All authors were in agreement regarding the submission of this manuscript and vouch for the completeness and accuracy of the data. Professional medical writers, funded by BeiGene, Ltd., assisted with the development and submission of this manuscript under the authors' guidance. The corresponding author had full access to all of the study data and was responsible for the decision to submit the manuscript for publication.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Authors' Contributions
X. Wu: Conceptualization, supervision, investigation, methodology, writing–original draft, writing–review and editing. J. Zhu: Investigation, writing–review and editing. J. Wang: Investigation, writing–review and editing. Z. Lin: Investigation, writing–review and editing. R. Yin: Investigation, writing–review and editing. W. Sun: Investigation, writing–review and editing. Q. Zhou: Investigation, writing–review and editing. S. Zhang: Investigation, writing–review and editing. D. Wang: Investigation, writing–review and editing. H. Shi: Investigation, writing–review and editing. Y. Gao: Investigation, writing–review and editing. Y. Huang: Investigation, writing–original draft. G. Li: Investigation, writing–review and editing. X. Wang: Investigation, writing–review and editing. Y. Cheng: Investigation, writing–original draft. G. Lou: Investigation, writing–review and editing. Q. Gao: Investigation, writing–review and editing. L. Wang: Investigation, writing–review and editing. X. Du: Investigation, writing–review and editing. M. Pan: Investigation, writing–review and editing. X. Mu: Conceptualization, resources, data curation, formal analysis, methodology, project administration, writing–review and editing. L. Li: Conceptualization, resources, data curation, formal analysis, methodology, project administration, writing–review and editing. M. Li: Conceptualization, resources, data curation, formal analysis, supervision, methodology, project administration, writing–review and editing. S. Mu: Conceptualization, resources, data curation, formal analysis, supervision, methodology, project administration, writing–review and editing. B. Kong: Investigation, writing–review and editing.
References
- 1. Dziadkowiec KN, Gasiorowska E, Nowak-Markwitz E, Jankowska A. PARP inhibitors: review of mechanisms of action and BRCA1/2 mutation targeting. Prz Menopauzalny 2016;15:215–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Thomas C, Tulin AV. Poly-ADP-ribose polymerase: machinery for nuclear processes. Mol Aspects Med 2013;34:1124–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shen Y, Aoyagi-Scharber M, Wang B. Trapping poly(ADP-Ribose) polymerase. J Pharmacol Exp Ther 2015;353:446–57. [DOI] [PubMed] [Google Scholar]
- 4. Lord CJ, Ashworth A. PARP inhibitors: synthetic lethality in the clinic. Science 2017;355:1152–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mateo J, Lord CJ, Serra V, Tutt A, Balmaña J, Castroviejo-Bermejo M, et al. A decade of clinical development of PARP inhibitors in perspective. Ann Oncol 2019;30:1437–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Miller RE, Leary A, Scott CL, Serra V, Lord CJ, Bowtell D, et al. ESMO recommendations on predictive biomarker testing for homologous recombination deficiency and PARP inhibitor benefit in ovarian cancer. Ann Oncol 2020;31:1606–22. [DOI] [PubMed] [Google Scholar]
- 7. Xiong Y, Guo Y, Liu Y, Wang H, Gong W, Liu Y, et al. Pamiparib is a potent and selective PARP inhibitor with unique potential for the treatment of brain tumor. Neoplasia 2020;22:431–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lickliter J, Mileshkin L, Voskoboynik M, Millward M, Freimund A, Meniawy T, et al. Dose escalation/expansion study to investigate the safety, pharmacokinetics, food effect, and antitumor activity of BGB-290 in patients with advanced solid tumors. Ann Oncol 2017;28:v123. [Google Scholar]
- 9. Xu B, Yin Y, Dong M, Song Y, Li W, Huang X, et al. Pamiparib dose escalation in Chinese patients with non-mucinous high-grade ovarian cancer or advanced triple-negative breast cancer. Cancer Med 2021;10:109–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. [DOI] [PubMed] [Google Scholar]
- 11. Rustin GJS, Vergote I, Eisenhauer E, Pujade-Lauraine E, Quinn M, Thigpen T, et al. Definitions for response and progression in ovarian cancer clinical trials incorporating RECIST 1.1 and CA 125 agreed by the Gynecological Cancer Intergroup (GCIG). Int J Gynecol Cancer 2011;21:419–23. [DOI] [PubMed] [Google Scholar]
- 12. Pujade-Lauraine E, Banerjee S, Pignata S. Management of platinum-resistant, relapsed epithelial ovarian cancer and new drug perspectives. J Clin Oncol 2019;37:2437–48. [DOI] [PubMed] [Google Scholar]
- 13. Kaufman B, Shapira-Frommer R, Schmutzler RK, Audeh MW, Friedlander M, Balmaña J, et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol 2015;33:244–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moore KN, Secord AA, Geller MA, Miller DS, Cloven N, Fleming GF, et al. Niraparib monotherapy for late-line treatment of ovarian cancer (QUADRA): a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol 2019;20:636–48. [DOI] [PubMed] [Google Scholar]
- 15. Coleman RL, Sill MW, Bell-McGuinn K, Aghajanian C, Gray HJ, Tewari KS, et al. A phase II evaluation of the potent, highly selective PARP inhibitor veliparib in the treatment of persistent or recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer in patients who carry a germline BRCA1 or BRCA2 mutation - An NRG Oncology/Gynecologic Oncology Group study. Gynecol Oncol 2015;137:386–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bai P. Biology of Poly(ADP-Ribose) polymerases: the factotums of cell maintenance. Mol Cell 2015;58:947–58. [DOI] [PubMed] [Google Scholar]
- 17. AstraZeneca China and Merck China. First ovarian cancer targeting drug approved in China. Drug Discovery & Development [cited 2018]. Available from: https://www.drugdiscoverytrends.com/first-ovarian-cancer-targeting-drug-approved-in-china/. [Google Scholar]
- 18. European Pharmaceutical Manufacturer. Recent approval of Lynparza reflects quickening of Chinese drug approvals, says GlobalData. [cited 2018]. Available from: https://www.epmmagazine.com/news/approval-of-lynparza-reflects-quickening-of-chinese-approvals.
- 19. Franzese E, Centonze S, Diana A, Carlino F, Guerrera LP, Di Napoli M, et al. PARP inhibitors in ovarian cancer. Cancer Treat Rev 2019;73:1–9. [DOI] [PubMed] [Google Scholar]
- 20. Voskoboynik M, Mileshkin L, Gan H, Millward M, Au-Yeung G, Meniawy TM, et al. Safety, antitumor activity, and pharmacokinetics (PK) of pamiparib (BGB-290), a PARP1/2 inhibitor, in patients (pts) with advanced solid tumours: updated phase I dose-escalation/expansion results. Ann Oncol 2019;30:452PD. [Google Scholar]
- 21. Domchek SM, Aghajanian C, Shapira-Frommer R, Schmutzler RK, Audeh MW, Friedlander M, et al. Efficacy and safety of olaparib monotherapy in germline BRCA1/2 mutation carriers with advanced ovarian cancer and three or more lines of prior therapy. Gynecol Oncol 2016;140:199–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu JF, Barry WT, Birrer M, Lee J-M, Buckanovich RJ, Fleming GF, et al. Combination cediranib and olaparib versus olaparib alone for women with recurrent platinum-sensitive ovarian cancer: a randomised phase 2 study. Lancet Oncol 2014;15:1207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Oza AM, Tinker AV, Oaknin A, Shapira-Frommer R, McNeish IA, Swisher EM, et al. Antitumor activity and safety of the PARP inhibitor rucaparib in patients with high-grade ovarian carcinoma and a germline or somatic BRCA1 or BRCA2 mutation: Integrated analysis of data from Study 10 and ARIEL2. Gynecol Oncol 2017;147:267–75. [DOI] [PubMed] [Google Scholar]
- 24. Penson RT, Valencia RV, Cibula D, Colombo N, Leath CA, Bidziński M, et al. Olaparib versus nonplatinum chemotherapy in patients with platinum-sensitive relapsed ovarian cancer and a germline BRCA1/2 mutation (SOLO3): a randomized phase III trial. J Clin Oncol 2020;38:1164–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Swisher EM, Lin KK, Oza AM, Scott CL, Giordano H, Sun J, et al. Rucaparib in relapsed, platinum-sensitive high-grade ovarian carcinoma (ARIEL2 Part 1): an international, multicentre, open-label, phase 2 trial. Lancet Oncol 2017;18:75–87. [DOI] [PubMed] [Google Scholar]
- 26. Ratner E, Bala M, Louie-Gao M, Aydin E, Hazard S, Brastianos PK. Increased risk of brain metastases in ovarian cancer patients with BRCA mutations. Gynecol Oncol 2019;153:568–73. [DOI] [PubMed] [Google Scholar]
- 27. Lawlor D, Martin P, Busschots S, Thery J, O'leary JJ, Hennessy BT, et al. PARP inhibitors as P-glyoprotein substrates. J Pharm Sci 2014;103:1913–20. [DOI] [PubMed] [Google Scholar]
- 28. Januchowski R, Wojtowicz K, Sterzyſska K, Sosiſska P, Andrzejewska M, Zawierucha P, et al. Inhibition of ALDH1A1 activity decreases expression of drug transporters and reduces chemotherapy resistance in ovarian cancer cell lines. Int J Biochem Cell Biol 2016;78:248–59. [DOI] [PubMed] [Google Scholar]
- 29. Dufour R, Daumar P, Mounetou E, Aubel C, Kwiatkowski F, Abrial C, et al. BCRP and P-gp relay overexpression in triple negative basal-like breast cancer cell line: a prospective role in resistance to olaparib. Sci Rep 2015;5:12670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Klotz DM, Wimberger P. Overcoming PARP inhibitor resistance in ovarian cancer: what are the most promising strategies? Arch Gynecol Obstet 2020;302:1087–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.