Abstract
Common lymphatic endothelial and vascular endothelial receptor-1 (Clever-1) is a multifunctional type-1 transmembrane protein that plays an important role in immunosuppression against tumors. Clever-1 is highly expressed in a subset of human tumor-associated macrophages and associated with poor survival. In mice, Clever-1 supports tumor growth and metastasis formation, and its deficiency or blockage induces T-cell–dependent killing of cancer cells. Therefore, targeting Clever-1 could lead to T-cell activation and restoration of immune response also in patients with cancer. This is studied in an on-going clinical trial [Macrophage Antibody To INhibit immune Suppression (MATINS); NCT03733990] in patients with advanced solid tumors where bexmarilimab, a humanized IgG4 antibody against human Clever-1, shows promising safety and efficacy.
Here, we report the humanization and nonclinical characterization of physicochemical properties, biological potency, and safety profile of bexmarilimab.
Bexmarilimab showed high affinity to Clever-1 on KG-1 cells and bound to Clever-1 on the surface of classical and intermediate monocytes derived from healthy human blood. Bexmarilimab inhibited the internalization of its natural ligand acetylated low-density lipoprotein into KG-1 cells and increased TNFα secretion from macrophages but did not impair phagocytic clearance. Bexmarilimab did not induce significant cytokine release in human whole-blood cultures, did not contain nonsafe immunogenic glycans, or show any significant binding to human Fcγ receptors or complement pathway component C1q. In vivo, bexmarilimab showed dose-dependent duration of monocyte Clever-1 receptor occupancy in cynomolgus monkeys but did not induce a cytokine storm up to a dose of 100 mg/kg.
In conclusion, these data support the clinical development of bexmarilimab for the restoration of immune response in cancers.
Introduction
Common lymphatic endothelial and vascular endothelial receptor-1 (Clever-1), encoded by the STAB1 gene also known as Stabilin-1, FEEL-1, or KIAA0246, is a 280-KDa multifunctional type-1 transmembrane protein. It has a large extracellular part containing clusters of EGF-like domains, seven fasciclin domains, and one X-link domain. Clever-1 is expressed on circulating monocytes, M2 tissue macrophages, and sinusoidal endothelial cells in human spleen, liver, adrenal cortex, lymph nodes, and bone marrow as well as on lymphatic endothelium (1–4). In pregnant women, placental macrophages express Clever-1 (4) and this has been hypothesized to facilitate immunosuppression allowing the maternal immune system to tolerate fetal antigens (3).
Clever-1 binds to and mediates the endocytic and phagocytic clearance of unwanted-self components such as acetylated low-density lipoprotein (acLDL; refs. 5, 6). Clever-1 also plays an important role in suppressing the immune response against tumors by mediating a fast clearance and degradation of these unwanted-self components including potential cancer antigens (7). Clever-1 expression on both macrophages and different subtypes of endothelial cells is induced during chronic inflammation. High expression of Clever-1 on the surface of monocytes or macrophages suppresses the activation of Th1-type T-lymphocytes. The blocking of Clever-1 on monocytes or macrophages converts immunosuppressive M2 macrophages into immunostimulatory M1 macrophages that support IFNγ production and decrease IL4 production in cocultures of T-cells and macrophages (3). Clever-1–deficient macrophages produce more TNFα and support T-cell–dependent and independent antibody production (8). Most importantly, Clever-1 is highly expressed in a subset of human tumor-associated macrophages (TAM), and the expression is associated with poor survival (9, 10). In mice, Clever-1 supports tumor growth and metastasis formation, and its deficiency or blockage induces CD8-positive (+) T-cell–dependent killing of cancer cells in melanoma, lymphoma, colon cancer, breast cancer, and lung cancer models (11–13).
We hypothesized that targeting Clever-1 in human cancers could lead to a similar T-cell activation that has been demonstrated in mice models and ex vivo human cells (3, 12, 13). Thus, we generated bexmarilimab (FP-1305), a humanized antibody against Clever-1 and characterized its physicochemical properties, biological potency, and safety profile. As hypothesized, bexmarilimab promotes phenotypic conversion of circulating monocytes, restores responses to proinflammatory stimuli, impairs lysosomal acidification, endorses antigen cross-presentation, and promotes clonal expansion of CD8+ effector T-cells with promising safety and efficacy in patients with late-stage cancer (7). Here, we report on the nonclinical characterization of bexmarilimab.
Methods
Compounds and cells
The humanized anti–Clever-1 antibody bexmarilimab was generated and produced by Abzena. The anti–Clever-1 parental antibody 3–372 (InVivo Biotech), the humanized anti–Clever-1 antibody FP-1304 without the L248E mutation (Antitope Ltd), rituximab (Roche), alemtuzumab (Genzyme), cetuximab (Merck Healthcare KGaA), and the isotype control antibodies human IgG4 (Biolegend) and rat IgG2a (Biolegend) were used as references and controls as indicated in the respective method descriptions.
KG-1 human acute myelogenous leukemia and human Jurkat cells were purchased from ATCC and NS0 murine myeloma cells from the European Collection of Authenticated Cell Cultures (ECACC). The cell lines were routinely tested for Mycoplasma with PCR Mycoplasma Test Kit I/C (Promocell). Cell line authentication was not performed as the cells were used from a master cell bank (<5 passages) created from the original vial.
Generation of humanized anti–Clever-1 antibody bexmarilimab and affinity to Clever-1
The parental anti–Clever-1 antibody 3–372 was originally generated in mice immunized with an isolated human lymphatic vessel suspension (2). The humanization was conducted utilizing Composite Human AntibodyTM technology (Abzena), and selected sequence segments of the antibody were designed to avoid T-cell epitopes (Supplementary Methods). The binding affinities of 3–372 and the chimeric antibodies were determined using a competitive ELISA and surface plasmon resonance (SPR) analysis (Biacore) at SYRINX Bioanalytics. The details of the protocols are described in the Supplementary Methods.
The S241P (Kabat numbering) mutation was included in the hinge of IgG4 for stability purposes and for the prevention of half-antibody formation (14). To ablate immune effector functions without affecting binding to the FcRn receptor that protects antibodies from lysosomal degradation, the chosen composite antibody VH3/VK5 (also called FP-1304) was further optimized by introducing the L248E mutation (according to Kabat numbering) to fully remove binding to Fcγ receptors (FcγR) and the first component of the classical complement pathway, C1q (15). This mutational modification resulted in the final, fully humanized bexmarilimab (FP-1305) antibody. The details of amino acid sequence determination by reduced peptide mapping using LC-MS/MS are described in the Supplementary Methods.
Epitope characterization
To identify the epitope of bexmarilimab on Clever-1, the human Clever-1 protein (STAB1; Q9NY15) was converted into a library of up to 10,000 overlapping synthetic peptide constructs using a combinatorial matrix design (Pepscan Presto BV). The matrix of linear peptides was shaped into spatially defined constructs using Chemically Linked Peptides on Scaffolds (CLIPS) technology at Pepscan Presto BV. The antibody binding to each of the synthesized peptides was determined by a Pepscan-based ELISA, and after the binding epitopes of bexmarilimab on human Clever-1 were identified, the human Clever-1 sequence was compared in silico to cynomolgus monkey, mouse, and rat Clever-1 sequences to examine the binding of bexmarilimab to Clever-1 in these species.
The details of the protocols are described in the Supplementary Methods.
Immunofluorescence
Optimal cutting temperature (OCT) compound-embedded sections were fixed with cold acetone for 5 minutes. The sections were blocked with 5% goat serum (Jackson ImmunoResearch), stained with primary antibodies for CD68 or Clever-1 (3–372) overnight at +4°C, and labeled with goat anti-mouse IgG (AF546, 1:300, Invitrogen). Then, the sections were blocked with 5% mouse serum (Jackson ImmunoResearch, catalog no. 015–000–120), stained with biotinylated anti-human CD31 antibody for 1 hour and labeled with streptavidin (AF488 conjugate, 1:300, Invitrogen). Finally, the sections were stained with AF647-conjugated anti–Clever-1 antibodies, nuclei were labeled for 10 minutes with 2 μmol/L Hoechst 33342 (Thermo Fisher Scientific) and the stained sections mounted with ProLong Gold antifade reagent (Thermo Fisher Scientific). Used primary antibodies and their isotype controls were: CD68 (0.6 μg/mL, clone PG-M1, Dako) and mouse IgG (Rockland); 3–372 (10 μg/mL, InVivo Biotech) and mouse IgG1 (InVivo Biotech); CD31-biotin (5 μg/mL, clone WM-59, Invitrogen). In-house Alexa Fluor 647-conjugated (Invitrogen, catalog no. A20173) antibodies were: 9–11 (10 μg/mL, InVivo biotech), rat IgG2a (1:100, clone R35–95, BD Pharmingen); bexmarilimab (FP-1305; 20 μg/mL, Abzena, FP-1305 humanized IgG4), and human IgG4 [Abzena, Irrelevant IgG4 (S241P, L248E)]. Images were acquired with Marianas spinning disk (Intelligent Imaging Innovations) connected to CSU-W1 scanning unit (Yokogawa) and Orca Flash 4 sCMOS camera (Hamatsu) using a 40x objective (water, NA 1.1, LD C-Apochromat, Carl Zeiss) and SlideBook 6.1 software (Intelligent Imaging Innovations). Images were analyzed with NIH ImageJ software (version 1.52p).
Receptor occupancy of bexmarilimab on classical and intermediate monocytes
The receptor occupancy of bexmarilimab was measured on classical and intermediate Clever-1–expressing human monocytes at Labcorp. Whole blood was collected from three healthy donors and occupancy was measured using unconjugated bexmarilimab, PE-labeled bexmarilimab, and a PE-labeled anti–Clever-1 mAb 9–11 (4) that recognizes a different epitope on Clever-1 compared with bexmarilimab. Human IgG4 and rat IgG2a antibodies were used as isotype controls for bexmarilimab and 9–11, respectively. The protocol is described in the Supplementary Methods.
Activity of bexmarilimab in inhibiting the binding of Clever-1 ligands
Malondialdehyde modified low density lipoprotein (MDA-LDL) is known to internalize into activated human monocytes via the Clever-1 receptor and the parental anti–Clever-1 antibody 3–372 inhibits this internalization (16). This phenomenon was utilized to assess the biological potency of bexmarilimab by setting up a flow cytometry–based competition assay that measured the ability of the antibody to inhibit the internalization of acLDL into Clever-1–expressing KG-1 cells at Abzena. The activity of two batches of bexmarilimab produced at different facilities was compared to the activity of the parental 3–372 antibody, the isotype control, and the mAb 9–11 (4) as described in the Supplementary Methods.
Heparinized blood was collected from healthy volunteers and treated with the indicated concentrations of bexmarilimab or 133 μg/mL Kiovig (human normal immunoglobulin, Baxter), together with or without AF488-conjugated acLDL (1 μg/mL, Invitrogen) in round bottom polystyrene tubes (Corning) for 24 hours at +37°C and 5% CO2. After the treatment, red blood cells were lysed with Pharm Lyse (BD Pharmingen) by two subsequent incubations at room temperature (10 + 5 minutes) and remaining cells (from 500 μL blood/well) were plated on U-bottom 96-well plate. Following blockade of Fc receptors with 0.2 mg/mL Kiovig for 15 minutes at +4°C, the cells were stained with anti-human CD14 Pacific Blue (1:200, clone M5E2, BD Pharmingen, catalog no. 558121) together with 9–11 A647 (10 μg/mL, InVivo Biotech, AB FUMM 9–11), rat IgG2a AF647 (1:100, described above), or bexmarilimab AF647 (20 μg/mL, described above) for 30 minutes at +4°C. Samples were fixed with 1% formaldehyde in PBS and run on LSRFortessa (BD Pharmingen). For quenching surface-bound AF488-cojugated acLDL, trypan blue solution was added to 0.63 mg/mL on samples shortly before sample acquisition. Samples were analyzed with FlowJo software (TreeStar, v. 10.7.2).
Effect of bexmarilimab on the phagocytosis of Staphylococcus aureus by monocytes
The potential interfering effects of bexmarilimab on the phagocytosis of Staphylococcus aureus by human CD14+ monocytes were assessed by flow cytometry. Peripheral blood mononuclear cells (PBMC) from three healthy donors were plated (1 × 106 cells/well) on ultra-low attachment 96-well plate (Corning) in Iscove's modified Dulbecco's medium (IMDM, Gibco) containing 1% AB human serum (Valley Biomedical, Inc). The cells were incubated with anti–Clever-1 antibodies bexmarilimab, 9–11, and their isotype controls IgG4 or rat IgG2a, respectively, at 10 μg/mL for 20 minutes. The incubation was performed at +37°C for measurement of the phagocytized particles and at +4 °C for the extracellular binding. Subsequently, AF488-labeled S. aureus particles (#S23371, Invitrogen) were fed to the cells in a 10:1 ratio for 1 hour. The uptake of the particles was analyzed with LSR Fortessa Flow Cytometer (BD Pharmingen).
Effect of bexmarilimab on TNFα secretion from primary human macrophages
To evaluate the effect of bexmarilimab on TNFα secretion by primary human macrophages, monocytes were enriched from buffy-coat PBMCs from 7 healthy donors (Finnish Red Cross) with CD14 Microbeads (Miltenyi Biotech) and induced to differentiate into M2 macrophages by a 7-day incubation with 50 ng/mL of human M-CSF (Biolegend) followed by a 48-hour incubation with 100 nmol/L dexamethasone (Merck) and 20 ng/mL of human IL4 (200–04–50UG; Peprotech). The cells were treated with bexmarilimab (0.1–50 μg/mL) or irrelevant isotype control (IgG4; Abzena) for 24 hours as described in the Supplementary Methods. After stimulation of TNFα production with a Toll-like receptor 4 (TLR4) agonist [lipopolysaccharide (LPS) EB-ultrapure, E. coli O111:B4; InvivoGen], TNFα levels were measured with human TNFα ELISA kit (Invitrogen) according to manufacturer's instructions and calculated as a mean from triplicate wells per each condition for each donor.
Glycosylation profile of bexmarilimab
Reduced peptide mapping was used to determine the glycosylation sites of bexmarilimab. The N-glycan profile of bexmarilimab was examined quantitatively to investigate the presence of potential immunogenic glycan structures on the antibody at Abzena. Bexmarilimab glycans (2 mg/mL) were prepared with GlycoWorks RapiFluor-MS N-Glycan Kit (Waters) according to manufacturer's instructions. The glycans were separated using Acquity UPLC Glycan BEH Amide column (Waters) followed by fluorescence detection and glycans were assigned based on retention time. RapiFluor-MS Dextran Calibration ladder and an irrelevant positive control mAb were used as standards. The analysis was performed in triplicate.
Binding of bexmarilimab to Fc receptors and C1q
Bexmarilimab contains the L248E mutation in the Fc region that is designed to eliminate Fc-mediated effector functions such as binding to FcγRs and complement pathway component C1q, but it does not affect binding to the FcRn receptor. To confirm that these mutations contribute to the desired properties of bexmarilimab, its binding to human Fc receptors and C1q were examined by SPR analysis and ELISA at Abzena, respectively, as described in the Supplementary Methods. Rituximab (IgG1), known to have a binding site on C1q (17), was used as a positive control.
Effect of bexmarilimab on cytokine release in cultures of human whole blood
The capability of bexmarilimab to induce secretion of cytokines IL6, IL8, IL10, IFNγ, and TNFα was assessed in cultures of human whole blood using the Cytokine Screen assay at Abzena. The whole blood was obtained from 21 healthy human donors. Whole blood cultures were treated with bexmarilimab (0.1–100 μg/mL), alemtuzumab (10 μg/mL), or cetuximab (100 μg/mL) for 24 hours. Cetuximab and alemtuzumab were selected as control antibodies because they are associated with low and high rates of cytokine-related reactions, respectively (18). Pokeweed mitogen (PWM, 100 μg/mL, Sigma-Aldrich) and PBS were used as positive and negative controls, respectively. All conditions were set up in duplicate. Following incubation, whole blood was centrifuged (1,800 g, 15 minutes, +4°C) to extract plasma. Plasma samples were analyzed for cytokine levels using high sensitivity magnetic Milliplex beads (Merck Millipore) and incubated with premixed capture beads for 1 hour, followed by an incubation with detection reagents according to the manufacturer's instructions. Data were acquired using a FLEXMap 3D (Bio-Rad).
Receptor occupancy and safety of bexmarilimab in cynomolgus monkey
The murine anti-human bexmarilimab does not recognize mouse or rat Clever-1, so rodent studies with bexmarilimab are not relevant for safety assessment. Hence, the receptor occupancy and preliminary safety of bexmarilimab was assessed in a general toxicology study using cynomolgus monkeys (male and female, 2-year-old, 1.85–2.98 kg, Silabe) during a single dose Good Laboratory Practice study conducted at Accelera. Briefly, a single dose of formulation buffer [histidine buffer (pH 6) with 0.3% L-Methionine, 10% trehalose and 0.02% polysorbate20] or bexmarilimab in formulation buffer was administered intravenously (radial vein; ∼ 4 mL/minute) at 3, 30, or 100 mg/kg in a volume of 4 mL/kg to cynomolgus monkeys (n = 6/group: 3 females and 3 males). The animals were observed for 3 weeks after treatment. Clinical signs (behavior and activity, appearance, major physiologic functions) and food intake were checked daily. Body weight was recorded before dosing and weekly afterwards. Ophthalmoscopic and electrocardiogram (ECG) examinations, urinalysis, hematologic analysis, and serum chemistry analyses including measurements of alanine aminotransferase (ALT), aspartate aminotransferase (AST), triglycerides, and bilirubin were performed at various time points during the study. Blood samples for serum preparation were allowed to clot for 30 minutes at room temperature. The clot was spun down by centrifugation (3 minutes, 10,000 g, +4°C). For each sample, serum was divided into two aliquots and stored in a freezer at -80°C pending analysis (within 3 months). Blood samples for systemic exposure analyses were collected predosing and at 0.5 hours, 3 hours, 8 hours, 24 hours (day 2), day 3, day 4, day 5, day 7, day 11, day 15, and day 20 of the study. The concentration was measured in serum by validated ELISA using recombinant anti-idiotypic capture antibody (Bio-Rad) and commercial horseradish peroxidase (HRP)-conjugated mouse anti-human IgG4 detection antibody (Thermo Fisher Scientific). Formation of anti-drug antibodies (ADA) was determined in serum using validated bridging ELISA assays as described in the Supplementary Methods using a custom made anti-idiotypic monoclonal antigen-binding (Fab) fragment antibody (AbD30054; Bio-Rad). At the end of the experimental phase, all animals were sacrificed on day 21 or 22 and a full necropsy was performed.
The receptor occupancy was determined in blood samples collected at 0 hours, 0.5 hours, day 2, day 3, day 7, day 11, and day 20 by immunofluorescence staining as described in the Supplementary Methods.
Ethical statement
Human tissue samples were obtained under the license ETMK 132/2016 and blood was collected from healthy donors under the license ETMK 43/1801/2015. Signed informed consent was obtained from all donors. Both studies were approved by local institutional review boards and conducted in accordance with the Declaration of Helsinki as revised 2002. All activities that fall under the Human Tissue Act 2004 were performed under Human Tissue Authority license. All the animal procedures and ethical revision were performed at Accelera S.r.l. and approved by the Service for Biotechnology and Animal Welfare at Istituto Superiore di Sanità according to the current Italian legislation (Legislative Decree March 4th, 2014 n. 26) enforcing the 2010/63/EU Directive on the protection of animals used for biomedical research.
Statistical analyses
The statistical analysis of the cytokine secretion data was performed using a paired Student t test or one-way ANOVA. The TNFα secretion experiment results were analyzed with one-way repeated measures ANOVA followed by Dunnett multiple comparison test.
Data availability statement
The data generated in this study are available within the article and its supplementary data files.
Results
Bexmarilimab shows high affinity to human and cynomolgus monkey Clever-1 in vitro
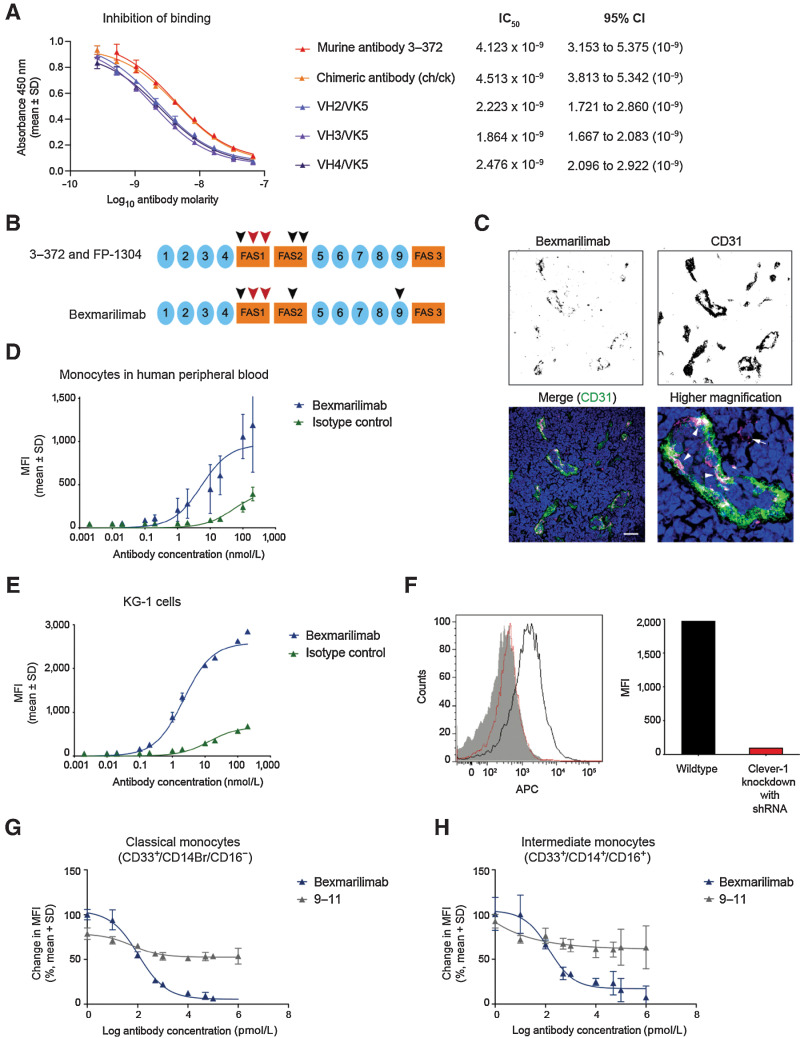
The chimeric antibody consisting of a human framework and mouse variable regions (IC50 4.51 × 10–9 M) showed similar affinity to Clever-1 as the parental murine anti-human antibody 3–372 (IC50 4.12 × 10–9 M) in the competitive ELISA assay (Fig. 1A). The three humanized antibodies VH2/VK5, VH3/VK5, and VH4/VK5 also bound to Clever-1 with increased affinity, with relative IC50 values of 2.22, 1.86, and 2.48 (x 10–9 M), respectively (Fig. 1A). The amino acid analysis of the selected bexmarilimab construct showed that the human genomic V and J genes closest in sequence to the variable regions of the humanized antibody were IGHV2–5*09 (91.8% similarity) - IGHJ4*01 (10.7.14; 1–122) and IGKV3–20*01 (80.2% similarity) - IGKJ2*01 (7.3.9; 1–108) for the heavy and light chains, respectively. The closest human genomic C gene for the heavy chain was IGHG4*01 (123–449) with the following structure: CH1 (123–220), hinge S10 > P (230; 221–232), CH2 L1.2 > E (237; 233–342), CH3 (343–447), CHS (448–449; see Supplementary Data for the sequence). Analysis of bexmarilimab binding to Clever-1 (0.75 × 10–9 M) in SPR showed similar affinity with 3–372 (0.55 × 10–9 M) and VH3/VK5 (0.65 × 10–9M) confirming good antigen recognition of the humanized antibody (Table 1A).
Figure 1.
Characterization of bexmarilimab binding in vitro. A, Binding of biotinylated parental anti–Clever-1 antibody 3–372 to human Clever-1 in the presence of nonbiotinylated 3–372, chimeric anti–Clever-1 antibody and three composite antibodies (VH2/VK5, VH3/VK5, VH4/VK5) as determined by a competitive ELISA. IC50 values were calculated by three-parameter nonlinear curve fitting with 95% confidence intervals (CI). B, Bexmarilimab-recognized region of Clever-1. The orange boxes and blue closed circles indicate fasciclin domains and EGF-like domains, respectively. The arrowheads indicate relative positions of identified binding motifs, the core epitopes are shown in red. C, Bexmarilimab immunoreactivity (magenta) in human lymph node with CD31 (green) and Hoechst (blue) co-staining. Scale bar 40 μm. Arrowheads point to Clever-1 expression on lymphatic endothelial cells and the arrow points to a single CD31-negative cell. Binding of bexmarilimab to human CD14+ cells (D) and human KG-1 acute myelogenous leukemia cells (E) as determined by flow cytometry. F, Binding and quantitation of bexmarilimab and the isotype control to KG-1 cells transfected with a Clever-1–targeting shRNA as determined by flow cytometry. The wild-type histogram is shown in black, the Clever-1 knockdown histogram in red, and the isotype control binding in solid gray. APC, allophycocyanin. Receptor occupancy of bexmarilimab in classical (G) and intermediate monocytes (H), shown as change in fluorescence intensity of CD14+ cells that bind to labeled bexmarilimab or mAb 9–11. Representative data are shown for 1 of 3 donors. MFI, mean fluorescence intensity.
Table 1.
Affinity of bexmarilimab for human and cynomolgus monkey Clever-1 as determined by SPR analysis.
| A. Antibody | K D x 10–9 (M) | K a x 105 (1/Ms) | K d x 10–4 (1/Ms) |
|---|---|---|---|
| 3–372 | 0.55 | 2.3 | 1.2 |
| VH3/VK5 | 0.65 | 3.7 | 2.4 |
| Bexmarilimab | 0.75 | 3.1 | 2.4 |
| B. Antigen | K D x 10–9 (M) | K a x 105 (1/Ms) | K d x 10–4 (1/Ms) |
| Human Clever-1 | 0.75 | 3.1 | 2.8 |
| Cynomolgus Clever-1 | 0.92 | 3.6 | 3.3 |
Abbreviations: KD, affinity constant; Ka, association constant; Kd, dissociation constant.
In the epitope characterization, the final, humanized bexmarilimab was observed to bind to a discontinuous epitope on human Clever-1, which was different from the Clever-1 epitope recognized by mAb 9–11. The epitope mapping showed that bexmarilimab recognized the FAS1 and FAS2 regions and the EGF-like domain 9 of Clever-1 (Fig. 1B). The in silico analysis suggested that the binding sequence of the core epitope was similar in primates (human and cynomolgus monkey) but different between primates and rodents. Accordingly, bexmarilimab showed similar affinity for purified human Clever-1 and cynomolgus Clever-1 (Table 1B).
In an analysis of human lymph node tissues, bexmarilimab showed specific immunoreactivity in the luminal side of lymphatic vessels and high-endothelial venules as identified by CD31 co-staining (Fig. 1C). The staining pattern was similar to the parental antibody 3–372, indicating that the humanized antibody had retained intact Clever-1–recognizing complementarity-determining regions (CDR; Supplementary Fig. S1A). Bexmarilimab and 3–372 showed a patchy-like staining pattern on lymphatic vessels compared with more uniform immunoreactivity seen with the 9–11 antibody (Supplementary Fig. S1A). The difference is most likely due to possible epitope masking by alternative conformations, ligand binding, or other means that prevent binding of bexmarilimab to all Clever-1 molecules. Because very few macrophages were Clever-1+ in the lymph node we studied bexmarilimab immunoreactivity in breast cancer tissue with CD68 co-staining. Indeed, bexmarilimab signal mostly colocalized with CD68 staining (Supplementary Fig. S1B). In addition, bexmarilimab and 3–372 showed similar binding to cynomolgus monkey lymph node tissue in an immunofluorescence assay (Supplementary Fig. S1C), indicating that bexmarilimab was cross-reactive with cynomolgus monkey Clever-1. Absence of the staining in rodent lymph nodes confirmed that the antibody did not recognize rodent Clever-1 (Supplementary Fig. S2).
Expression of Clever-1 on monocytes has been shown previously (3). Therefore, we investigated the binding of bexmarilimab to monocytes in human peripheral blood: the observed mean EC50 value was 5.28 nmol/L with variation between donors (Fig. 1D). In contrast to the human monocytes, bexmarilimab showed strong binding (EC50 2.20 nmol/L) to the Clever-1–expressing KG-1 human acute myelogenous leukemia cells used as a positive control (Fig. 1E). Clever-1 was further confirmed as the binding target on the surface of KG-1 cells, as knockdown of Clever-1 in the cells by specific short hairpin (shRNA) abolished this binding (Fig. 1F). The receptor occupancy analysis of bexmarilimab in Clever-1–expressing classical (CD33+/CD14Br/CD16-) and intermediate (CD33+/CD14+/CD16+) monocytes in human blood from healthy donors showed that bexmarilimab bound to its target on the cell surface. This was evidenced by the finding that unlabeled bexmarilimab inhibited the binding of labeled bexmarilimab but not that of labeled anti–Clever-1 mAb 9–11, which has a different epitope on Clever-1 than bexmarilimab (Fig. 1G–H).
Low dose of bexmarilimab inhibits acLDL uptake and increases TNFα secretion after LPS stimulus
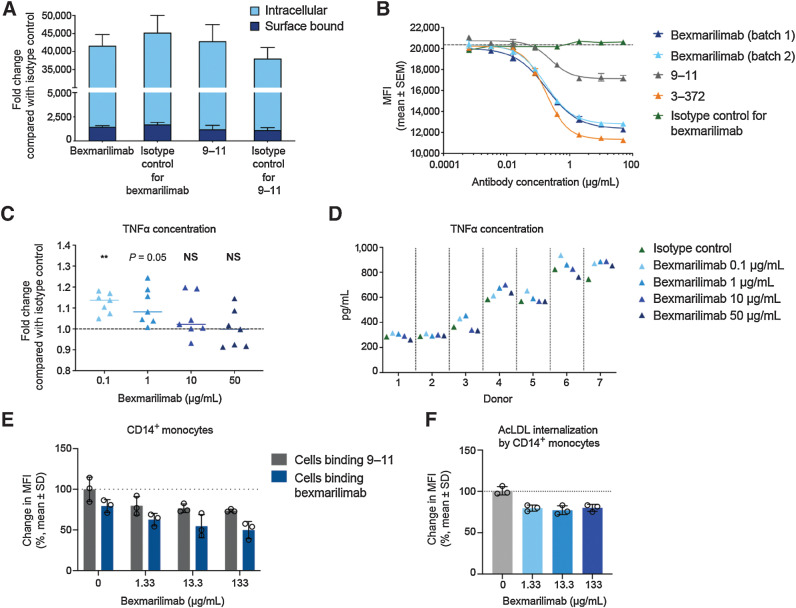
As Clever-1 acts in phagocytic clearance, the potential phagocytosis-interfering effects of bexmarilimab were first assessed using fluorescently labeled S. aureus particles and flow cytometry. No interfering effect was observed in monocytes from healthy human donors, as neither bexmarilimab nor 9–11 decreased the phagocytized amounts of S. aureus when compared with their isotype controls (Fig. 2A). The parental anti–Clever-1 antibody 3–372 is, however, known to inhibit the internalization of MDA-LDL into human monocytes (16). Here, the biological potency of bexmarilimab was measured in a flow cytometry-based competition assay where bexmarilimab was shown to block the Clever-1–mediated internalization of acLDL into KG-1 cells. Two different batches of humanized bexmarilimab showed similar blocking activity which was comparable with the activity of 3–372 (Fig. 2B). The antibody mAb 9–11, which detects a different epitope in Clever-1, and the isotype control showed weaker or no activity, respectively.
Figure 2.
Bexmarilimab binds to Clever-1 on monocytes and inhibits internalization of acLDL while inducing TNFα secretion. A, Internalized and surface-bound S. aureus particles in monocytes from healthy human donors after treatment with bexmarilimab, mAb 9–11, or their respective isotype controls. B, Internalization of acLDL into KG-1 cells via Clever-1 receptor in the presence of two batches of bexmarilimab, mAb 9–11, parental mouse antibody 3–372, and an isotype control as determined by a competition assay and flow cytometry. The dotted line indicates the level of internalization without a competitor compound present. C, Mean fold changes in TNFα secretion by primary human macrophages upon treatment with 0.1–50 μg/mL bexmarilimab compared with an isotype control. The macrophages were induced in vitro from CD14+ monocytes obtained from seven healthy donors. **, P < 0.01; NS, not significant compared with isotype control. The statistical analysis was performed using one-way repeated measures ANOVA followed by Dunnett test. D, Absolute levels of TNFα secretion by primary human macrophages (C) upon treatment with 0.1 to 50 μg/mL bexmarilimab or an isotype control. Each donor is shown separately. E, Whole blood from 3 healthy donors was incubated with bexmarilimab or Ig-control for 24 hours at concentrations of 1.33, 13.3, or 133 μg/mL. These correspond to the initial estimated concentrations after injection of the antibodies into a blood volume of 4.5 L at doses of 0.1, 1, or 10 mg/kg, respectively, in a 60-kg person. Receptor occupancy of bexmarilimab on human CD14+ cells was measured by flow cytometry. The binding of fluorescently labeled bexmarilimab on CD14+ cells after administration of nonlabeled bexmarilimab is shown in blue and the binding of fluorescently labeled 9–11 antibody is shown in grey. Data normalized to Ig-treated cells. F, Flow cytometry analysis of acLDL internalization in CD14+ cells after bexmarilimab treatment (E). The blood was spiked with 1 μg/mL of AF488-acLDL during bexmarilimab incubation. Data normalized to Ig-treated cells.
Functionally, Clever-1 inhibition either genetically or by 3–372 has been shown to increase the secretion of CCL3 (16), IL12 (13) and TNFα (3) by monocytes and macrophages. Thus, we studied the potency of bexmarilimab in increasing TNFα secretion by M2-polarized primary human macrophages after LPS stimulus. Bexmarilimab was most effective at lower concentrations (0.1–1 μg/mL) demonstrating a bell-shaped dose curve in TNFα release (Fig. 2C). There was substantial variation in the absolute TNFα levels induced by LPS between the donors, which may explain some of the response differences also seen with bexmarilimab (Fig. 2D). To understand the dose effect better, we treated human blood with bexmarilimab for 24 hours and measured the uptake of spiked acLDL in CD14+ monocytes. A concentration of 1.33 μg/mL bexmarilimab was able to inhibit acLDL internalization by 20.2% ±3.6 with a receptor occupancy (RO) of 21.2% ±2.1 (relative to 0 μg/mL; Fig. 2E–F). The inhibition did not increase despite higher bexmarilimab concentration (13.3 μg/mL, 22.8% ±5.3; RO: 31.5% ±14.2 and 133 μg/mL; 20.0% ±4.2; RO: 37.8% ±7.7) suggesting that maximal inhibition of Clever-1–mediated acLDL uptake can be achieved with relatively low dose and low receptor occupancy on human monocytes.
Bexmarilimab does not contain nonsafe immunogenic glycans
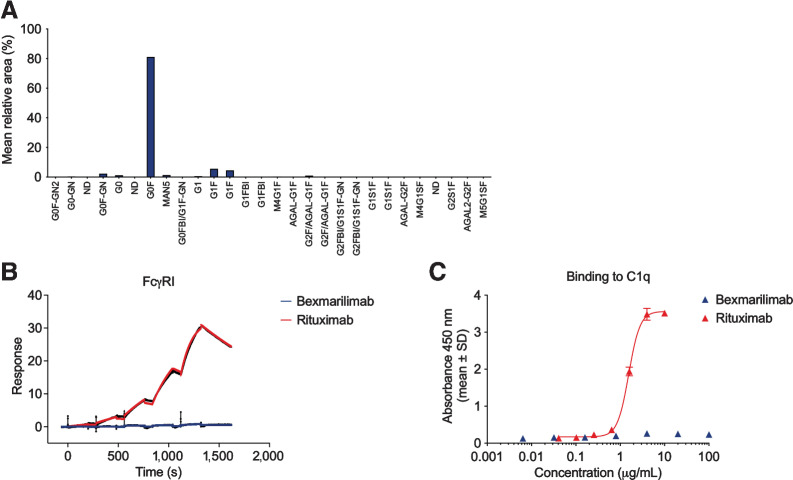
The quantitative analysis of the N-glycan profile of bexmarilimab showed that the major antibody glycoform was glycosylated with a core fucosylated G0 biantennary glycan and reduced peptide mapping indicated that the only glycosylation site was at the canonical (N299) site of the heavy chain (Fig. 3A). Sialylated oligosaccharides represented less than 0.6% of the total oligosaccharide composition of bexmarilimab. Thus, it is unlikely that bexmarilimab would contain Neu5Gc at a level that would be of any safety concern. Only ≤1% of the glycans in bexmarilimab contained galactose-α-1,3-galactose. In addition, the amino acids that may have been glycosylated in the Fab of the antibody were removed from bexmarilimab and the reduced peptide mapping analysis confirmed that the Fab of bexmarilimab was not glycosylated. Thus, the risk of the glycosylation site inducing hypersensitivity reactions due to galactose-α-1,3-galactose was not present in bexmarilimab.
Figure 3.
Bexmarilimab does not contain immunogenic glycans or bind to human FcγRI or complement 1q. A, N-glycoform distribution on bexmarilimab as determined by quantitative N-glycan profiling. The analysis was performed in triplicate and glycans were assigned according to retention time. B, Binding of bexmarilimab and the positive control IgG rituximab to FcγRI as determined by SPR analysis. Colored lines correspond raw data, black lines correspond fitted data. C, Binding of bexmarilimab and rituximab to component C1q of the complement pathway as determined by ELISA; a representative graph of three experiments. s, seconds.
Bexmarilimab shows no significant binding to human Fc receptors or complement pathway component
To examine the potential induction of Fc- or complement-mediated functions by bexmarilimab, its binding to human FcγRs and the complement pathway component C1q was determined by SPR. Bexmarilimab demonstrated reduced binding to the high- and low- affinity receptors FcγRI, FcγRIIa, FcγRIIIa, and FcγRIIIb when compared with the positive control huIgG1 antibody rituximab (Fig. 3B; Supplementary Fig. S3A–S3F). Low-affinity bexmarilimab binding to the inhibitory FcγRIIb was detected (KD = 1.14 × 10–5 M). However, the binding was outside the concentration range tested and the affinity was 2.2-fold lower than that of rituximab (KD = 5.13 × 10–6 M). Overall, these results suggest that bexmarilimab is unlikely to induce Fc-mediated modulation of immune cell functions but the possible inhibition of B-cell function due to the interaction with FcγRIIb cannot be ruled out especially when high doses of bexmarilimab is administered.
Interaction with FcRn is known to affect human IgG recycling into the bloodstream and is essential for preventing rapid clearance of the antibody (19). Here, bexmarilimab showed pH-dependent binding to FcRn but at weaker levels compared with that of rituximab control (Supplementary Fig. S3G–S3H). Bexmarilimab was not able to bind to human C1q at the concentration range tested in the two replicate experiments (Fig. 3C). In contrast, the positive control antibody rituximab showed binding to C1q at EC50 values of 1.4 and 1.6 μg/mL. This indicates that bexmarilimab is unlikely to trigger complement activation when bound to its antigen.
Bexmarilimab does not induce significant cytokine release in human whole blood in vitro
The ability of bexmarilimab to induce secretion of cytokines IL6, IL8, IL10, IFNγ, and TNF α was assessed in whole blood from healthy donors and compared with the known cytokine release rates of two mAbs in clinical use, cetuximab and alemtuzumab. Bexmarilimab-treated cultures showed similarly low release levels of IL6, IL10, IFNγ, and TNFα as cultures treated with cetuximab (Fig. 4A–D). Cetuximab is known to be associated with low cytokine release (20–22). However, the IL8 level was significantly reduced in cultures treated with 0.1 to 10 μg/mL bexmarilimab when compared with cetuximab (Fig. 4E). As expected, the positive control PWM induced a significant increase in the levels of all cytokines measured as compared with PBS cultures (Fig. 4F), indicating that the cells were viable and functional. In addition, cultures treated with alemtuzumab showed similar IL10 release and significant increase in the levels of IL6, IL8, IFNγ, and TNFα compared with cetuximab, which is in line with the rate and severity of the infusion reaction to alemtuzumab seen in the clinic (22, 23). These ex vivo data suggest that bexmarilimab does not spontaneously provoke overt immune system activation.
Figure 4.
Bexmarilimab does not induce cytokine release in whole blood. Release of cytokines (A) IL6 (A), IL10 (B), IFNγ (C), TNFα (D), and IL8 (E) upon bexmarilimab treatment of human whole blood as determined by Cytokine ScreenTM assay. Alemtuzumab and cetuximab were used as reference compounds. Each data point represents one donor (n = 21) and the graphs show the median, minimum, and maximum values. F, Cytokines induced by positive control [pokeweed mitogen (PWM)] compared with PBS. Statistical analysis was performed using one-way ANOVA followed by paired Student t test. ***, P < 0.001; ****, P < 0.0001 as compared with cetuximab.
Bexmarilimab shows dose-dependent duration of receptor occupancy in vivo
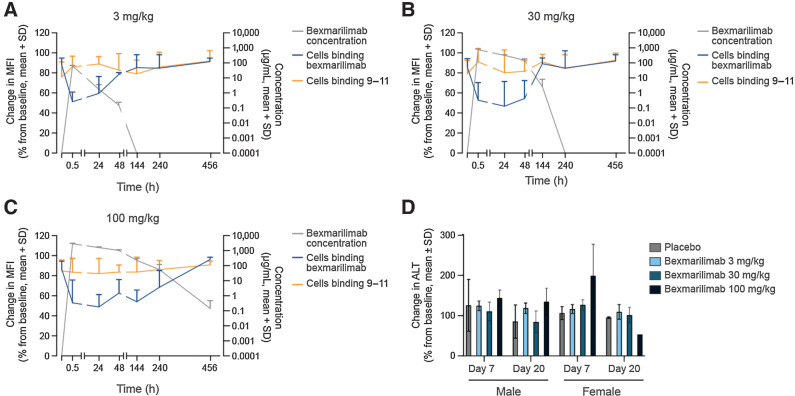
The receptor occupancy of bexmarilimab was studied in vivo in cynomolgus monkeys by measuring the binding of fluorescently labeled bexmarilimab to CD14+ cells by flow cytometry. At predosing, 75% to 98% of CD14+ monocytes bound to labeled bexmarilimab. Starting from 0.5 hours after treatment, CD14+ monocytes demonstrated reduced binding to the labeled bexmarilimab in all treated animals compared with the predosing samples, suggesting binding of bexmarilimab to its receptor Clever-1 on the monocytes (Fig. 5A–C). The decrease of binding initially ranged from 16% to 90% and no relation to bexmarilimab dose was observed. The recovery of binding, however, took place in a dose-dependent manner mirroring the clearance of the antibody: on day 3 (48 hours) for animals dosed at 3 mg/kg and on day 7 (144 hours) for animals dosed at 30 mg/kg. For animals dosed with 100 mg/kg, the duration of receptor blockage lasted the longest with a complete recovery of receptor binding observed on day 20. The mAb 9–11 clone was used for detection of Clever-1 expression on the surface of CD14+ monocytes. Like with human cells ex vivo, the expression of Clever-1 on the cell surface remained stable over the course of the study, suggesting that the receptor was present on the cell surface and not internalized.
Figure 5.
Bexmarilimab shows dose-dependent duration of receptor occupancy in vivo and only moderate increase in ALT levels in cynomolgus monkeys. Receptor occupancy of bexmarilimab in cynomolgus monkey monocytes after administration of a single i.v. dose of bexmarilimab at 3 mg/kg (A), 30 mg/kg (B), or 100 mg/kg (C; n = 6 animals/dosing group). Change in MFI of CD14+ cells binding to fluorescently labeled bexmarilimab after administration of nonlabeled bexmarilimab is shown in blue and binding to fluorescently labeled 9–11 antibody is shown in orange. Concentration of the bexmarilimab in circulation (grey) was measured at the same time points as the receptor occupancy. D, Effect of bexmarilimab on ALT levels in cynomolgus monkeys on days 7 and 20 compared with predose levels. h, hours.
Pharmacokinetics and safety of bexmarilimab in cynomolgus monkey
The safety of bexmarilimab was assessed in cynomolgus monkeys treated with a single 0, 3, 30, or 100 mg/kg dose of bexmarilimab. No mortality was observed during the study. No toxicologically relevant changes were observed in respiration, central nervous system, body weight, food intake, ECG, ophthalmoscopic examinations, or in gross or histologic pathology examinations. Antidrug antibody formation was not detected, and signs of immune responses were not observed in any animal at any dose tested. A moderate and dose-dependent but transient increase in serum ALT levels was observed in females on day 7 (Fig. 5D). However, AST and bilirubin levels were not affected, and the level of serum triglycerides did not change during the observation period (Supplementary Fig. S4A–S4C). The pharmacokinetic assessments of bexmarilimab in cynomolgus monkeys indicated that the compound was characterized by quite rapid serum clearance and limited volume of distribution (Supplementary Table S1). The terminal half-life of bexmarilimab was in the range of 10.7 to 25.1 hours in males and 16.9 to 24.3 hours in females. The AUC∞ value increased more than dose-proportionally, rising approximately 200-fold over the 33-fold increase in dose.
Discussion
In recent years, the role of the tumor microenvironment as a key element in tumor response has garnered significant interest. One of the main contributors to an immunosuppressive, and consequently a tumor-promoting, microenvironment are the TAMs. In particular, M2 TAMs have been demonstrated to promote tumor progression by stimulating tumor cell motility, angiogenesis, growth, and immune evasion (24, 25). As a result, the depletion of M2 TAMs has been shown to correlate with reduced tumor growth in murine models (26, 27) but these results have not been replicated in human studies (28). The conversion of immunosuppressive M2 TAMs into immunostimulatory M1 macrophages thus presents a valuable opportunity to modulate the immunologic landscape of the tumor microenvironment. Clever-1 as a known immunosuppressive driver offers one potential therapeutic target, and its inhibition has been demonstrated to convert M2 macrophages into M1 macrophages and to improve the secretion of proinflammatory cytokines in cocultures of T-cells and macrophages (3). Here, we characterize the physicochemical properties, binding, and safety profile of the anti–Clever-1 humanized antibody bexmarilimab.
Bexmarilimab demonstrated high binding affinity to Clever-1 on both human and cynomolgus peripheral lymph nodes but it did not recognize rodent Clever-1. This suggests that cynomolgus monkey is a relevant species to study bexmarilimab. The binding of bexmarilimab to Clever-1 was also demonstrated to inhibit the internalization of acLDL, a natural ligand of Clever-1, into KG-1 cells and human monocytes. This outcome is vital to the prospective efficacy of Clever-1 blockade. Indeed, a significant downregulation of the tolerogenic liver X receptor (RXR) and PPAR pathways related to modified LDL scavenging has been detected in monocytes of patients with cancer after bexmarilimab treatment (7). Clever-1 mediates the internalization of proimmunogenic and harmful substances, and if these substances remain in the extracellular space following Clever-1 inhibition, the result is a proimmunogenic effect. Clever-1 inhibition by bexmarilimab can therefore be expected to induce immunostimulatory signaling. However, although acLDL internalization was inhibited by bexmarilimab, it was shown not to impair bacterial uptake in monocytes, and thus not likely to impair the capacity of the host to control bacterial replication (29). Bexmarilimab has also been demonstrated not to impair phagocytosis of apoptotic cells, and instead can actively promote the cross-presentation of scavenged antigens (7).
The binding of bexmarilimab to Clever-1 also resulted in an increase in TNFα production in primary human macrophages, showing that the blockade of Clever-1 indeed leads to the establishment of an immunostimulatory microenvironment. The effect was evident only when the macrophages were stimulated with LPS suggesting that bexmarilimab activity is supported by secondary signals such as pathogen or danger associated molecular patterns; components that are abundant in the tumor microenvironment. This could also explain the good safety and tolerability reported for bexmarilimab in patients with cancer (30) and the cynomolgus monkeys studied here. One peculiar phenomenon with bexmarilimab was the bell-shaped dose curve for TNFα, which counteracts the general concept that more drug is better. In fact, a study investigating recombinant IFNγ to activate the antitumor properties of monocytes in patients with cancer shows that, while appropriate amounts of IFNγ activate tumor cytotoxic monocytes, high doses suppress this effect (31). Indeed, the therapeutic efficacy of biologicals with immunostimulatory activity can be hampered by negative feedback loops suppressing their desired outcome. For example, LPS stimulus has been shown to transiently activate inflammatory responses in macrophages but preexposure to LPS rather induces tolerance and thereby resistance to subsequent TLR4 stimulation (32). Thus, it is possible that preexposure of cells in donors prior isolation may account for the variability in end-point assays performed with primary human monocytes due to epigenetic differences and chromatin remodeling.
Bexmarilimab was demonstrated to not bind significantly to FcγRs nor to C1q. The binding of antibodies to FcγRs is known to induce antibody-dependent cellular cytotoxicity (33), but the introduced L237E mutation was able to completely remove the activating FcγR binding (15), and thus, the binding of bexmarilimab is not expected to lead to target cell lysis. The low affinity to inhibitory FcγIIb could potentially have on immunologic impact. However, the amount of endogenous IgG molecules in circulation (10 g/L) far exceeds that of bexmarilimab even with 10 mg/kg dose (Cmax is less than 0.3 g/L). Thus, the possibility that bexmarilimab replaces endogenous IgG in FcγIIb binding and exerts immunologic functions through this interaction is extremely low. The low binding of bexmarilimab to C1q, the initiator of the classical complement pathway (34), suggests that bexmarilimab is also unlikely to trigger complement-dependent cytotoxicity.
In addition to the binding properties of bexmarilimab, the results showed that bexmarilimab incubation does not provoke cytokine production by healthy human peripheral blood cells in comparison with alemtuzumab, a known inducer of cytokine storms, i.e., harmful systemic inflammatory events triggered by elevated levels of circulating cytokines and immune-cell hyperactivation (35). Taken together with the fact that bexmarilimab does not contain nonsafe immunogenic glycans, our results suggest that there is little reason to expect significant toxicity issues to arise in connection to bexmarilimab. This is well in line with the observations that bexmarilimab is well-tolerated and does not provoke any adverse reactions despite clearly binding to the target on circulating monocytes in cynomolgus monkeys. The results are consistent with the observations in Clever-1 deficient mice, which present enhanced antibody production (8), but are otherwise normal in phenotype. However, mice deficient of both Clever-1 and homologous Stabilin-2 protein exhibit premature mortality (36). Stabilin-2, like Clever-1, is also expressed on the sinusoidal endothelial cells of liver, lymph nodes, and spleen in humans (The Human Protein Atlas; ref. 37), mice (36, 38), and rats (39) and these proteins demonstrate redundant functions (6, 36, 38, 40, 41). This may well explain the absence of pathologic phenotype when either of the genes is deleted, while double-deficient mice show a lethal phenotype with impaired clearance of unwanted and most likely toxic self-molecules. Because Stabilin-2 is not expressed in monocytes/macrophages, targeting Clever-1 should mostly impact the function of monocytes/macrophages, and the binding to the endothelial cells does not provoke significant undesired adverse reactions. However, since placental macrophages express Clever-1 (4), the possibility of side effects by bexmarilimab on pregnant women should not go unnoted.
The pharmacokinetics in cynomolgus monkey suggest that bexmarilimab is cleared relatively fast from the circulation and thereafter Clever-1 is no longer occupied on circulating monocytes. Clever-1 is a scavenger receptor, and it is expressed not only on monocytes, which are quite abundant in the circulation but also on the hepatic sinusoids. Thus, the receptor mediated clearance can be quite fast. This accompanied with the modest affinity to the FcRn may significantly affect the clearance of the antibody from the circulation. The exposure of bexmarilimab over time is not dose proportional. These data suggest that either high doses or frequent administration with small doses would be required for sustained Clever-1 receptor occupancy if the pharmacokinetics in humans are similar to those observed in cynomolgus monkey.
Taken together, it can be concluded that bexmarilimab could induce an immunostimulatory tumor microenvironment that leads to antitumor efficacy without suspected major safety concerns. Bexmarilimab binds to Clever-1 and inhibits the internalization of its ligands without impairing bacterial uptake but does not bind to FcγRs, complement components, or nontarget tissues, and does not induce excessive cytokine production in normal or healthy tissues. This suggests that there is a solid rationale for the continuing development of bexmarilimab for the treatment of difficult-to-treat cancers by providing permanent immune stimulation through targeting myeloid cell function. Indeed, results from a currently on-going clinical trial [Macrophage Antibody To INhibit immune Suppression (MATINS); NCT03733990] have shown promising safety and efficacy for bexmarilimab in patients with advanced solid tumors (42).
Supplementary Material
Acknowledgments
We thank Mari Parsama for excellent technical assistance. We also acknowledge the contract research organizations for excellent collaboration and technical expertise. Aurexel Life Sciences Ltd. (www.aurexel.com) is acknowledged for editorial support funded by Faron Pharmaceuticals. M Hollmén was supported by a research fellowship (no. 316340) from the Academy of Finland.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
Note: Supplementary data for this article are available at Molecular Cancer Therapeutics Online (http://mct.aacrjournals.org/).
Authors’ Disclosures
M. Hollmén reports other support from Faron Pharmaceuticals; and personal fees from Faron Pharmaceuticals during the conduct of the study; in addition, M. Hollmén has a patent for Diagnosis of immune activation using Clever-1, TNF-alpha and HLA-DR binding agents issued. M. Maksimow reports patents for US2021332128 and US2019064180 issued to Faron Pharmaceuticals. M.K. Karvonen reports other support from Faron Pharmaceuticals outside the submitted work. M. Vainio reports personal fees from Faron Pharmaceuticals during the conduct of the study; personal fees from Faron Pharmaceuticals outside the submitted work; in addition, M. Vainio has a patent for WO2017182705 issued to Faron Pharmaceuticals; and a patent for WO2021255336 pending to Faron Pharmaceuticals. S. Jalkanen reports grants from Finnish Academy during the conduct of the study; in addition, S. Jalkanen has a patent for US 7354577 issued; and S. Jalkanen owns stocks of Faron Pharmaceuticals. M. Jalkanen reports grants from Business Finland during the conduct of the study; personal fees from Faron Pharmaceuticals; and other support from Faron Pharmaceuticals outside the submitted work; in addition, M. Jalkanen has a patent for US 2021/0332128 issued; and Bexmarilimab is a proprietary humanized antibody under clinical development by Faron Pharmaceuticals. J. Mandelin reports personal fees from Faron Pharmaceuticals; and other support from Faron Pharmaceuticals during the conduct of the study; in addition, J. Mandelin has a patent for Method for determining potency of therapeutic anti-Clever-1 antibody pending, a patent for Anti-clever-1 agents for controlling the expression of cell surface markers on leucocytes, and using these to guide anti-clever-1 based cancer treatment pending, and a patent for Stable anti-clever-1 antibody formulation issued. No disclosures were reported by the other authors.
Authors’ Contributions
M. Hollmén: Conceptualization, resources, formal analysis, validation, investigation, visualization, methodology, writing–review and editing. M. Maksimow: Conceptualization, resources, investigation, methodology, project administration. J.H. Rannikko: Formal analysis, investigation, methodology, writing–review and editing. M.K. Karvonen: Data curation, investigation, project administration, writing–review and editing. M. Vainio: Resources, data curation, formal analysis, investigation, project administration, writing–review and editing. S. Jalkanen: Conceptualization, resources, supervision, investigation, writing–review and editing. M. Jalkanen: Conceptualization, resources, supervision, funding acquisition, investigation, project administration, writing–review and editing. J. Mandelin: Resources, data curation, formal analysis, validation, investigation, methodology, project administration, writing–review and editing.
References
- 1. Goerdt S, Walsh LJ, Murphy GF, Pober JS. Identification of a novel high molecular weight protein preferentially expressed by sinusoidal endothelial cells in normal human tissues. J Cell Biol 1991;113:1425–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Irjala H, Elima K, Johansson EL, Merinen M, Kontula K, Alanen K, et al. The same endothelial receptor controls lymphocyte traffic both in vascular and lymphatic vessels. Eur J Immunol 2003;33:815–24. [DOI] [PubMed] [Google Scholar]
- 3. Palani S, Elima K, Ekholm E, Jalkanen S, Salmi M. Monocyte stabilin-1 suppresses the activation of Th1 lymphocytes. J Immunol 2016;196:115–23. [DOI] [PubMed] [Google Scholar]
- 4. Palani S, Maksimow M, Miiluniemi M, Auvinen K, Jalkanen S, Salmi M. Stabilin-1/CLEVER-1, a type 2 macrophage marker, is an adhesion and scavenging molecule on human placental macrophages. Eur J Immunol 2011;41:2052–63. [DOI] [PubMed] [Google Scholar]
- 5. Kzhyshkowska J. Multifunctional receptor stabilin-1 in homeostasis and disease. ScientificWorldJournal 2010;10:2039–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shetty S, Weston CJ, Oo YH, Westerlund N, Stamataki Z, Youster J, et al. Common lymphatic endothelial and vascular endothelial receptor-1 mediates the transmigration of regulatory T cells across human hepatic sinusoidal endothelium. J Immunol 2011;186:4147–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Virtakoivu R, Rannikko JH, Viitala M, Vaura F, Takeda A, Lönnberg T, et al. Systemic blockade of Clever-1 elicits lymphocyte activation alongside checkpoint molecule downregulation in patients with solid tumors: Results from a phase I/II clinical trial. Clin Cancer Res 2021;27:4205–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dunkel J, Viitala M, Karikoski M, Rantakari P, Virtakoivu R, Elima K, et al. Enhanced antibody production in clever-1/stabilin-1-deficient mice. Front Immunol 2018;9:2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yin SP, Gao Y, Xie XS, Xu DD, Riabov V, Du WD. Accumulation of stabilin-1 positive macrophages in the early stage of gastric cancer is associated with short cumulative survival. Oncol Lett 2020;19:2404–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Algars A, Irjala H, Vaittinen S, Huhtinen H, Sundström J, Salmi M, et al. Type and location of tumor-infiltrating macrophages and lymphatic vessels predict survival of colorectal cancer patients. Int J Cancer 2012;131:864–73. [DOI] [PubMed] [Google Scholar]
- 11. Riabov V, Yin S, Song B, Avdic A, Schledzewski K, Ovsiy I, et al. Stabilin-1 is expressed in human breast cancer and supports tumor growth in mammary adenocarcinoma mouse model. Oncotarget 2016;7:31097–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Karikoski M, Marttila-Ichihara F, Elima K, Rantakari P, Hollmén M, Kelkka T, et al. Clever-1/stabilin-1 controls cancer growth and metastasis. Clin Cancer Res 2014;20:6452–64. [DOI] [PubMed] [Google Scholar]
- 13. Viitala M, Virtakoivu R, Tadayon S, Rannikko J, Jalkanen S, Hollmén M. Immunotherapeutic blockade of macrophage clever-1 reactivates the CD8+ T-cell response against immunosuppressive tumors. Clin Cancer Res 2019;25:3289–303. [DOI] [PubMed] [Google Scholar]
- 14. Angal S, King DJ, Bodmer MW, Turner A, Lawson AD, Roberts G, et al. A single amino acid substitution abolishes the heterogeneity of chimeric mouse/human (IgG4) antibody. Mol Immunol 1993;30:105–8. [DOI] [PubMed] [Google Scholar]
- 15. Reddy MP, Kinney CA, Chaikin MA, Payne A, Fishman-Lobell J, Tsui P, et al. Elimination of Fc receptor-dependent effector functions of a modified IgG4 monoclonal antibody to human CD4. J Immunol 2000;164:1925–33. [DOI] [PubMed] [Google Scholar]
- 16. Rantakari P, Patten DA, Valtonen J, Karikoski M, Gerke H, Dawes H, et al. Stabilin-1 expression defines a subset of macrophages that mediate tissue homeostasis and prevent fibrosis in chronic liver injury. Proc Natl Acad Sci U S A 2016;113:9298–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Idusogie EE, Presta LG, Gazzano-Santoro H, Totpal K, Wong PY, Ultsch M, et al. Mapping of the C1q binding site on rituxan, a chimeric antibody with a human IgG1 Fc. J Immunol 2000;164:4178–84. [DOI] [PubMed] [Google Scholar]
- 18. Bugelski PJ, Achuthanandam R, Capocasale RJ, Treacy G, Bouman-Thio E. Monoclonal antibody-induced cytokine-release syndrome. Expert Rev Clin Immunol 2009;5:499–521. [DOI] [PubMed] [Google Scholar]
- 19. Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol 2007;7:715–25. [DOI] [PubMed] [Google Scholar]
- 20. George TJ, Laplant KD, Walden EO, Davis AB, Riggs CE, Close JL, et al. Managing cetuximab hypersensitivity-infusion reactions: incidence, risk factors, prevention, and retreatment. J Support Oncol 2010;8:72–7. [PubMed] [Google Scholar]
- 21. Patel DD, Goldberg RM. Cetuximab-associated infusion reactions: pathology and management. Oncology (Williston Park) 2006;20:1373–82. [PubMed] [Google Scholar]
- 22. Stančič B, Qvarfordt B, Berglund MM, Brenden N, Sydow Bäckman M, Fransson M, et al. The blood endothelial cell chamber - An innovative system to study immune responses in drug development. Int Immunopharmacol 2021;90:107237. [DOI] [PubMed] [Google Scholar]
- 23. Thomas K, Eisele J, Rodriguez-Leal FA, Hainke U, Ziemssen T. Acute effects of alemtuzumab infusion in patients with active relapsing-remitting MS. Neurol Neuroimmunol Neuroinflamm 2016;3:e228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chanmee T, Ontong P, Konno K, Itano N. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers (Basel) 2014;6:1670–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity 2014;41:49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fritz JM, Tennis MA, Orlicky DJ, Lin H, Ju C, Redente EF, et al. Depletion of tumor-associated macrophages slows the growth of chemically induced mouse lung adenocarcinomas. Front Immunol 2014;5:587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wu X, Schulte BC, Zhou Y, Haribhai D, Mackinnon AC, Plaza JA, et al. Depletion of M2-like tumor-associated macrophages delays cutaneous T-cell lymphoma development in vivo. J Invest Dermatol 2014;134:2814–22. [DOI] [PubMed] [Google Scholar]
- 28. O'Brien SA, Orf J, Skrzypczynska KM, Tan H, Kim J, DeVoss J, et al. Activity of tumor-associated macrophage depletion by CSF1R blockade is highly dependent on the tumor model and timing of treatment. Cancer Immunol Immunother 2021;70:2401–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Karikoski M, Irjala H, Maksimow M, Miiluniemi M, Granfors K, Hernesniemi S, et al. Clever-1/Stabilin-1 regulates lymphocyte migration within lymphatics and leukocyte entrance to sites of inflammation. Eur J Immunol 2009;39:3477–87. [DOI] [PubMed] [Google Scholar]
- 30. Bono P, Virtakoivu R, Vaura F, Jaakkola P, Shetty S, Thibault A, et al. Immune activation in first-in-human anti-macrophage antibody (anti-Clever-1 mAb; FP-1305) phase I/II MATINS trial: Part 1 dose-escalation, safety and efficacy results. J Clin Oncol 38:15s, 2020. (suppl; abstr 3097). [Google Scholar]
- 31. Kleinerman ES, Kurzrock R, Wyatt D, Quesada JR, Gutterman JU, Fidler IJ. Activation or suppression of the tumoricidal properties of monocytes from cancer patients following treatment with human recombinant gamma-interferon. Cancer Res 1986;46:5401–5. [PubMed] [Google Scholar]
- 32. Seeley JJ, Ghosh S. Molecular mechanisms of innate memory and tolerance to LPS. J Leukoc Biol 2017;101:107–19. [DOI] [PubMed] [Google Scholar]
- 33. Stewart R, Hammond S, Oberst MW, Wilkinson R. The role of Fc gamma receptors in the activity of immunomodulatory antibodies for cancer. J Immunother Cancer 2014;2:29. [Google Scholar]
- 34. Meyer S, Leusen JH, Boross P. Regulation of complement and modulation of its activity in monoclonal antibody therapy of cancer. MAbs 2014;6:1133–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fajgenbaum DC, June CH. Cytokine storm. N Engl J Med 2020;383:2255–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schledzewski K, Géraud C, Arnold B, Wang S, Gröne HJ, Kempf T, et al. Deficiency of liver sinusoidal scavenger receptors stabilin-1 and -2 in mice causes glomerulofibrotic nephropathy via impaired hepatic clearance of noxious blood factors. J Clin Invest 2011;121:703–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Politz O, Gratchev A, McCourt PA, Schledzewski K, Guillot P, Johansson S, et al. Stabilin-1 and -2 constitute a novel family of fasciclin-like hyaluronan receptor homologues. Biochem J 2002;362:155–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Miller CM, Donner AJ, Blank EE, Egger AW, Kellar BM, Østergaard ME, et al. Stabilin-1 and Stabilin-2 are specific receptors for the cellular internalization of phosphorothioate-modified antisense oligonucleotides (ASOs) in the liver. Nucleic Acids Res 2016;44:2782–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhou B, Weigel JA, Fauss L, Weigel PH. Identification of the hyaluronan receptor for endocytosis (HARE). J Biol Chem 2000;275:37733–41. [DOI] [PubMed] [Google Scholar]
- 40. Tamura Y, Adachi H, Osuga J, Ohashi K, Yahagi N, Sekiya M, et al. FEEL-1 and FEEL-2 are endocytic receptors for advanced glycation end products. J Biol Chem 2003;278:12613–7. [DOI] [PubMed] [Google Scholar]
- 41. Jung MY, Park SY, Kim IS. Stabilin-2 is involved in lymphocyte adhesion to the hepatic sinusoidal endothelium via the interaction with alphaMbeta2 integrin. J Leukoc Biol 2007;82:1156–65. [DOI] [PubMed] [Google Scholar]
- 42. Bono P, Minchom A, Shetty S, Ma Y, Cruz R, de Jonge M, et al. LBA38 - Bexmarilimab, a novel macrophage re-programmer shows promising anti-tumour activity in phase I/II trial in several last line solid tumour types. Ann Oncol 2021:32:S1283–S346. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated in this study are available within the article and its supplementary data files.



![Figure 4. Bexmarilimab does not induce cytokine release in whole blood. Release of cytokines (A) IL6 (A), IL10 (B), IFNγ (C), TNFα (D), and IL8 (E) upon bexmarilimab treatment of human whole blood as determined by Cytokine ScreenTM assay. Alemtuzumab and cetuximab were used as reference compounds. Each data point represents one donor(N = 21) and the graphs show the median, minimum, and maximum values. F, Cytokines induced by positive control [pokeweed mitogen (PWM)] compared with PBS. Statistical analysis was performed using one-way ANOVA followed by paired Student t test. ***, P < 0.001; ****, P < 0.0001 as compared with cetuximab.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/8f45/9377746/cb2bac17ae4a/1207fig4.jpg)