Abstract
Background:
Exposure to polycyclic aromatic hydrocarbons (PAH) occurs widely in occupational settings. We investigated the association between occupational exposure to PAH and lung cancer risk and joint effects with smoking within the SYNERGY project.
Methods:
We pooled 14 case–control studies with information on lifetime occupational and smoking histories conducted between 1985 and 2010 in Europe and Canada. Exposure to benzo[a]pyrene (BaP) was used as a proxy of PAH and estimated from a quantitative general population job-exposure matrix. Multivariable unconditional logistic regression models, adjusted for smoking and exposure to other occupational lung carcinogens, estimated ORs, and 95% confidence intervals (CI).
Results:
We included 16,901 lung cancer cases and 20,965 frequency-matched controls. Adjusted OR for PAH exposure (ever) was 1.08 (CI, 1.02–1.15) in men and 1.20 (CI, 1.04–1.38) in women. When stratified by smoking status and histologic subtype, the OR for cumulative exposure ≥0.24 BaP μg/m3-years in men was higher in never smokers overall [1.31 (CI, 0.98–1.75)], for small cell [2.53 (CI, 1.28–4.99)] and squamous cell cancers [1.33 (CI, 0.80–2.21)]. Joint effects between PAH and smoking were observed. Restricting analysis to the most recent studies showed no increased risk.
Conclusions:
Elevated lung cancer risk associated with PAH exposure was observed in both sexes, particularly for small cell and squamous cell cancers, after accounting for cigarette smoking and exposure to other occupational lung carcinogens.
Impact:
The lack of association between PAH and lung cancer in more recent studies merits further research under today's exposure conditions and worker protection measures.
Introduction
Polycyclic aromatic hydrocarbons (PAH) refer to a class of process-generated substances characterized by the presence of at least two benzene rings in their molecular structure. Benzo[a]pyrene (BaP), composed of five benzene rings, is often used as an indicator of carcinogenic PAHs (1). BaPs and other PAHs are widespread environmental pollutants formed during incomplete combustion or pyrolysis of organic materials. Individual exposure to PAH comes mainly from smoking, ambient air pollution, and PAH-containing foods.
Occupational exposure to PAH occurs primarily through inhalation and via skin contact. High levels of occupational exposure to PAH can occur during the conversion of coal to coke and coal tar, and during the processing and use of coal-tar–derived products. Industries where occupational exposure to BaP has been measured include coal liquefaction, coal gasification, coke production, wood impregnation, roofing and paving involving coal-tar pitch, aluminum production (including anode manufacture), carbon-electrode manufacture, chimney sweeping, and power plants (2). Several of these industries reviewed by the International Agency for Research on Cancer (IARC) have been classified as carcinogenic to humans (3). BaP was also classified as carcinogenic to humans (Group 1) by IARC based on strong and extensive experimental evidence for the carcinogenicity of BaP in several animal species, supported by consistent and coherent mechanistic evidence of genotoxicity from experimental and human studies that included exposed workers.
Here we report on the association between occupational BaP exposure as a proxy for exposure to PAH and lung cancer risk for various exposure metrics (ever/never, duration of exposure, level of cumulative exposure) by sex, smoking status, and histologic subtype. We also investigated the joint effects of exposure to PAH and cigarette smoking on multiplicative and additive scales.
Materials and Methods
SYNERGY study population
The SYNERGY project has been described in detail previously (4, 5). In the current analysis, data were pooled from 14 population- or hospital-based case–control studies of lung cancer from Europe and Canada, conducted between 1985 and 2010, that collected data on lifetime tobacco smoking and occupational history (6–19).
Some noteworthy design features of the included studies were: (i) most studies frequency-matched cases and controls on age and sex, and conducted face to face interviews (84%); (ii) the IARC study in Central and Eastern Europe, and the United Kingdom (INCO) was considered as one study in Supplementary Table S1, but was treated as separate studies by country in the current analysis; (iii) the LUCAS and LUCA studies were restricted to men and the PARIS study included only regular smokers; (iv) MORGEN is a case–control study nested in the prospective EPIC cohort in the Netherlands in which the subjects completed a questionnaire at recruitment, and where the mean time interval between enrolment and diagnosis or end of follow-up was 5.3 years (SD, 2.7); (v) all studies, except MORGEN, provided data on lifetime smoking habits and complete occupational history; (vi) generally, the occupational data were recoded from national classifications into the International Standard Classification of Occupations (ISCO-68); (vii) lung cancer subtypes were classified according to World Health Organization (WHO) guidelines after histologic or cytologic confirmation.
Ethical approval was obtained in accordance with the legislation in each country at the time of the study and by IARC/WHO Ethics Committee 2007 (IEC 07-05).
Occupational exposure assessment
An exposure database (ExpoSYN) was established to develop a job-exposure matrix (JEM) to enable data-driven quantitative exposure assessment within the SYNERGY project (20). The development of the quantitative JEM (SYN-JEM) for five lung carcinogens has been described previously (21).
For PAH exposure, about 4,500 BaP personal exposure measurements (1975–2009) from several European countries and Canada were available. A priori exposure rating was derived from a general population JEM (DOM-JEM), assigning no, low, or high exposure levels to all job titles listed in ISCO-68. Jobs that were a priori assessed as being unexposed but had measurements were reviewed and decided should not be rated differently, except the crane operators, who were assigned “low exposure” when in “Basic metal industries” (ISIC 3700). Forty-nine percent of the BaP measurements were below the limit of detection (LOD; ref. 21). Assuming these measurements followed the same log-normal probability distribution as the observed data the nondetected value was substituted with a random draw between 0 and the measurement-specific LOD (22). On the basis of the measurements, we estimated an overall linear time trend. The number of measurements was too small to estimate exposure to BaP at individual ISCO-68 job code level. Instead, each job was assigned an exposure level (geometric mean) based on the calibrated a priori DOM-JEM categories of “low” and “high” exposure. For 1980, estimated BaP concentration were 0.019 μg/m3 for low and 0.032 μg/m3 for high exposed jobs, while in 2000 these concentrations were respectively 0.015 μg/m3 and 0.025 μg/m3 based on an estimated downward time trend of −1.2% per year (95% CI, −3.1 to −0.7) between 1975 and 2010 (21).
The lifetime cumulative occupational exposure was then calculated among ever exposed as the sum of the exposure for each job and year held by a subject. Total duration of exposure was calculated with a similar method.
Statistical analysis
Unconditional logistic regression models were fit to calculate ORs and 95% CIs of lung cancer associated with various metrics of occupational exposure to PAH: ever versus never, duration of exposure in years, and lifetime cumulative exposure. Cumulative exposure was categorized in four categories based on the PAH exposure distribution among all control subjects with the upper level of category 1 = 0.10 μg/m3-years, category 2 = 0.24 μg/m3-years, and category 3 = 0.52 μg/m3-years, while total duration was categorized into 1–9, 10–19, 20–29, and 30+ years. Subjects never occupationally exposed to PAH were the reference category in all analyses. Linear trends in ORs across categories of PAH exposure, starting from never occupationally exposed were examined by treating categories as equally spaced ordinal values in the logistic regression models.
Analyses were performed both overall and separately by lung cancer subtype as well as by smoking status (never, former, current smoker). Analyses were also stratified by sex because biological and social correlates of sex could plausibly have led to effect modification (Table 2).
Table 2.
Overall lung cancer risk associated with various metrics of occupational PAH exposure.
| Men | Women | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PAH exposure metric | Cases | % | Controls | % | OR (95% CI) | Cases | % | Controls | % | OR (95% CI) | ||
| Occupational exposure | ||||||||||||
| Never | 9,584 | 70.4 | 12,374 | 75.2 | 1.00 (ref) | 2,678 | 81.2 | 3,878 | 85.9 | 1.00 (ref) | ||
| Ever | 4,021 | 29.6 | 4,077 | 24.8 | 1.08 (1.02–1.15) | 618 | 18.8 | 636 | 14.1 | 1.20 (1.04–1.38) | ||
| Duration (years) among exposed | ||||||||||||
| 1–9 | 1,671 | 12.3 | 1,759 | 10.7 | 1.03 (0.94–1.11) | 323 | 9.8 | 359 | 8.0 | 1.18 (0.99–1.43) | ||
| 10–19 | 846 | 6.2 | 832 | 5.1 | 1.13 (1.01–1.27) | 157 | 4.8 | 157 | 3.5 | 1.20 (0.92–1.56) | ||
| 20–29 | 613 | 4.5 | 602 | 3.7 | 1.10 (0.97–1.26) | 81 | 2.5 | 69 | 1.5 | 1.25 (0.86–1.81) | ||
| 30+ | 891 | 6.5 | 884 | 5.4 | 1.12 (1.00–1.25) | 57 | 1.7 | 51 | 1.1 | 1.23 (0.79–1.92) | ||
| Test for trend, P value a | 0.004 | 0.021 | ||||||||||
| Test for trend, P value (exposed only) a | 0.38 | 0.99 | ||||||||||
| Cumulative exposure [(BaP) μg/m3-years] among exposed | ||||||||||||
| <0.10 | 859 | 6.3 | 950 | 5.8 | 0.98 (0.88–1.09) | 199 | 6.0 | 214 | 4.7 | 1.23 (0.98–1.55) | ||
| <0.24 | 967 | 7.1 | 984 | 6.0 | 1.06 (0.96–1.18) | 177 | 5.4 | 207 | 4.6 | 1.10 (0.87–1.40) | ||
| <0.52 | 1,021 | 7.5 | 1,024 | 6.2 | 1.15 (1.04–1.27) | 172 | 5.2 | 155 | 3.4 | 1.25 (0.96–1.61) | ||
| 0.52–1.83 | 1,174 | 8.6 | 1,119 | 6.8 | 1.11 (1.01–1.23) | 70 | 2.1 | 60 | 1.3 | 1.31 (0.88–1.96) | ||
| Test for trend, P value a | 0.002 | 0.016 | ||||||||||
| Test for trend, P value (exposed only) a | 0.15 | 0.83 | ||||||||||
Note: OR adjusted by study, age-group, List-A job, cigarette pack-years, time since quitting smoking.
Abbreviations: BaP, benzo[a]pyrene; CI, confidence intervals; OR, odds ratio; PAH, polycyclic aromatic hydrocarbons; Ref, reference category.
a P value obtained using the ordinal variable for respective exposure index.
All models were adjusted for study, age group (<45, 45–49, 50–54, 55–59, 60–64, 65–69, 70–74, and > 74 years), and ever-employment in a “List-A job” (yes/no), as in previous SYN-JEM papers (4, 5). “List A” is a list of occupations associated with lung cancer that includes, among others, jobs in metal production and processing, construction, mining, the chemical industry, asbestos production, and jobs potentially exposed to PAH (23, 24). For analyses that were not stratified by smoking status, models were also adjusted for cigarette pack-years [log(cigarette pack-years+1)] and time-since-quitting smoking cigarettes (current smokers; stopping smoking 2–7, 8–15, 16–25, ≥ 26 years before interview/diagnosis; and never-smokers). Current smokers were defined as having smoked at least one cigarette per day for 1 or more years and included those who had stopped smoking within the last 2 years before diagnosis or interview. The cut-off points used for former smokers in the “time-since-quitting smoking” variable were based on the quartile distribution among the control subjects. The cigarette pack-year was calculated as follows: Σ duration (years) × average cigarette smoking intensity per day/20 (cigarettes per pack).
We assessed the robustness of the overall results via sensitivity analyses as follows: studies with end of data collection before and after 1995 (∼ mid-point between 1985 and 2010); studies with population- and hospital-based controls (exploring the impact of study design); workers started working in 1960 or later (for which period exposure measurements were available); including and excluding adjustment for List A (exploring potential overadjustment), stratifying by geographic area (exploring potential disparities in regulatory and industrial practices); and excluding specific industries/jobs with exposure to PAH (to explore if specific industries/jobs largely influenced the results), one at a time.
Interactions on a multiplicative scale were assessed using an interaction term between exposure to PAH (never vs. ever exposed) and smoking status (never vs. ever smoker) in logistic regression models. Interactions on an additive scale, a more appropriate measure in public health, were assessed by fitting linear OR models and calculating the relative excess risk due to interaction (RERI) to test the departure from additivity of the effects of both risk factors (PAH and smoking status). RERI estimates along with CIs based on the delta method are reported. Never smokers and never occupationally exposed to PAH were considered as the reference category. The RERI was calculated as follows”: OR11 (doubly exposed) − OR10 (only PAH) − OR01(only smoking) +1. A RERI > 0 indicates a positive additive interaction where the effect of both exposures together exceeds the sum of the two exposures considered separately. All analyses were performed using R statistical software (version 3.6.1). P values are two sided and a significance level was set to 0.05.
Results
Table 1 displays selected characteristics of the study population (16,901 lung cancer cases and 20,965 control subjects) by lung cancer status and ever exposure to PAH. Among controls, the proportion of females, never smokers and never employed in a List-A job were higher among those never exposed to PAH at work compared with those exposed to PAH.
Table 1.
Selected study population characteristics by lung cancer status and occupational exposure to PAH.
| Ever exposed to PAH | Never exposed to PAH | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Category | Cases | % | Controls | % | Cases | % | Controls | % |
| Gender | Female | 618 | 13.3 | 636 | 13.5 | 2,678 | 21.8 | 3,878 | 23.9 |
| Male | 4,021 | 86.7 | 4,077 | 86.5 | 9,584 | 78.2 | 12,374 | 76.1 | |
| Age group | <45 years | 184 | 4.0 | 320 | 6.8 | 531 | 4.3 | 1,051 | 6.5 |
| 45–49 years | 280 | 6.0 | 287 | 6.1 | 792 | 6.5 | 1,028 | 6.3 | |
| 50–54 years | 545 | 11.7 | 497 | 10.5 | 1,269 | 10.3 | 1,638 | 10.1 | |
| 55–59 years | 720 | 15.5 | 714 | 15.1 | 1,958 | 16.0 | 2,436 | 15.0 | |
| 60–64 years | 896 | 19.3 | 850 | 18.0 | 2,303 | 18.8 | 2,904 | 17.98 | |
| 65–69 years | 983 | 21.2 | 1,033 | 21.9 | 2,487 | 20.3 | 3,348 | 20.6 | |
| 70–74 years | 783 | 16.9 | 789 | 16.7 | 2,182 | 17.8 | 3,058 | 18.8 | |
| 75+ years | 248 | 5.3 | 223 | 4.7 | 740 | 6.0 | 789 | 4.9 | |
| Smoking | Median (±SD) | 38.00 | 26.67 | 24.20 | 22.99 | 37.75 | 26.82 | 22.01 | 23.02 |
| pack-years | |||||||||
| Smoking status and | Never smoker | 219 | 4.7 | 1,242 | 26.4 | 1,150 | 9.4 | 5,911 | 36.4 |
| years-since- | 26+ | 184 | 4.0 | 606 | 12.9 | 520 | 4.2 | 1948 | 12.0 |
| quitting smoking | 16–25 | 306 | 6.6 | 588 | 12.5 | 865 | 7.1 | 1,758 | 10.8 |
| among former | 8–15 | 441 | 9.5 | 485 | 10.3 | 1,090 | 8.9 | 1,413 | 8.7 |
| Smokers | <7 | 557 | 12.0 | 355 | 7.5 | 1,469 | 12.0 | 1,067 | 6.6 |
| Current smoker | 2,932 | 63.2 | 1,437 | 30.5 | 7,168 | 58.5 | 4,155 | 25.6 | |
| “List-A” job | Never employment | 3,841 | 82.8 | 4,122 | 87.5 | 11,273 | 91.9 | 15,479 | 95.2 |
| Ever employment | 798 | 17.2 | 591 | 12.5 | 989 | 8.1 | 773 | 4.8 | |
| Lung cancer | SqC | 1,980 | 42.7 | 4,523 | 36.9 | ||||
| Subtype | SCLC | 767 | 16.5 | 1,963 | 16.0 | ||||
| AC | 1,124 | 24.2 | 3,628 | 29.6 | |||||
| LCC | 223 | 4.8 | 587 | 4.8 | |||||
| Other/unspecified | 519 | 11.2 | 1,493 | 12.2 | |||||
| Not available | 26 | 0.6 | 68 | 0.6 | |||||
Abbreviations: AC, adenocarcinoma; LCC, large-cell carcinoma; PAH, polycyclic aromatic hydrocarbons; SqC, squamous cell cancers; SCLC, small cell lung cancer.
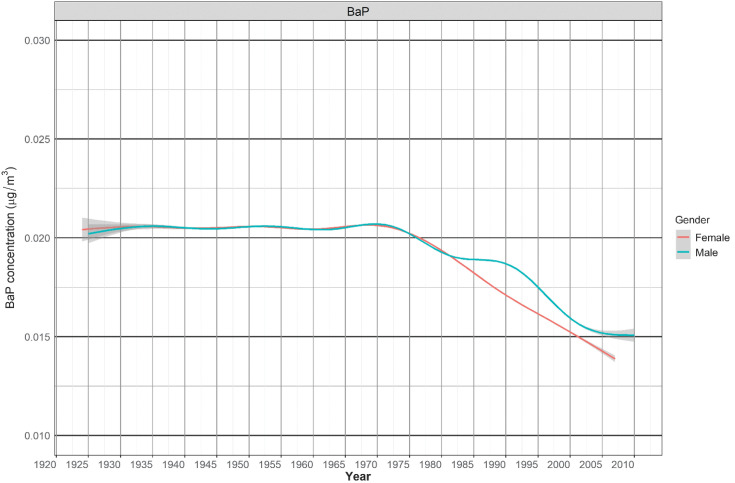
Figure 1 shows the average PAH (μg/m3) among exposed male and female workers each year from 1922 to 2010. The annual percent change of PAH exposure during this period was −0.5%.
Figure 1.
BaP (μg/m3) concentration levels (smoothing by cubic regression spline function) among female (red line) and male (green line) exposed workers between 1922 and 2010.
The prevalence of occupational PAH exposure among control subjects was 25% in men and 14% in women. ORs for ever PAH exposure versus never was 1.08 (CI, 1.02–1.15) in men and 1.20 (CI, 1.04–1.38) in women. Table 2 shows moderately elevated ORs for lung cancer associated with occupational PAH exposures in both men and women, as measured by additional exposure metrics (duration of exposure, cumulative exposure). Statistically significant exposure–response relationships were observed for both total duration and cumulative exposures (Ptrend < 0.05), although there was no exposure–response trend among the exposed only. These main results without adjustment for List-A jobs are displayed in Supplementary Table S2, and show similar risk estimates as compared with those with the adjustment for List-A jobs for both men and women.
Table 3 displays results of additional sensitivity analyses without adjustment for “ever employment in List-A jobs.” The ORs for ever PAH exposure versus never changed only slightly to 1.11 (CI, 1.05–1.18) in men and 1.21 (CI, 1.05–1.39) in women. The type of controls seemed to affect the risk estimates: ORs were null for studies using hospital-based controls while studies using population-based controls showed an increased risk of overall lung cancer in both men and women. However, for the studies with population-based controls, the results differed in studies ending data collection before and after 1995. The OR for PAH exposure in the older population-based studies was 1.36 (CI, 1.23–1.50) in men and 1.96 (CI, 1.44–2.67) in women, while in the population-based studies ending data collection after 1995, the OR was 1.02 (CI, 0.92–1.13) in men and 1.12 (CI, 0.93–1.35) in women. ORs differed significantly by time of data collection (<1995 vs. ≥1995) in both men and women (Pinteraction terms between PAH exposure and time: 0.004 and 0.001, respectively). Stratifying the results by geographic area revealed that results were mainly driven by Western European studies accounting for 41.6% of SYNERGY's study population. There was no or very small effect of PAH exposure on overall lung cancer risk for persons who started working 1960 or later, or when restricting the analysis to blue-collar workers. The overall ORs did not change much when leaving out specific industries and jobs with PAH exposure, one at a time.
Table 3.
Sensitivity analyses for overall lung cancer risk associated with ever occupational exposure to PAH, by sex and subgroup.
| Men | Women | |
|---|---|---|
| Occupational PAH exposure | OR (95%CI) | OR (95%CI) |
| Never exposed | 1.00 (Ref.) | 1.00 (Ref.) |
| All studies | 1.11 (1.05–1.18) | 1.21 (1.05–1.39) |
| Population controls | 1.17 (1.09–1.25) | 1.30 (1.11–1.53) |
| Population controls data collection ended before 1995 | 1.36 (1.23–1.50) | 1.96 (1.44–2.67) |
| Population controls data collection ended after 1995 | 1.02 (0.92–1.13) | 1.12 (0.93–1.35) |
| Hospital controls | 1.00 (0.87–1.14) | 0.77 (0.52–1.16) |
| Started working 1960 or later | 0.95 (0.84–1.07) | 1.08 (0.86–1.37) |
| Data collection ended before 1995 | 1.23 (1.13–1.34) | 1.79 (1.37–2.34) |
| Data collection ended after 1995 | 1.02 (0.94–1.11) | 1.04 (0.88–1.22) |
| Blue-collar workers only | 1.02 (0.96 –1.09) | 1.11 (0.93 –1.34) |
| Omitting specific industries/jobs | ||
| Construction | 1.14 (1.07–1.23) | 1.20 (1.04V1.38) |
| Mining | 1.10 (1.04–1.17) | 1.21 (1.05–1.39) |
| Metal workers | 1.10 (1.03–1.17) | 1.18 (1.03–1.36) |
| Transport | 1.11 (1.04–1.18) | 1.20 (1.04–1.38) |
| Farmer | 1.14 (1.07–1.22) | 1.17 (1.01–1.35) |
| Vehicle mechanic | 1.10 (1.03–1.17 | 1.21 (1.05–1.40) |
| By geographic areaa | ||
| Canada | 1.14 (0.91–1.42) | 1.17 (0.85–1.61) |
| Western Europe | 1.20 (1.09–1.31) | 1.50 (1.20–1.87) |
| Eastern Europe | 1.03 (0.88–1.20) | 0.85 (0.58–1.25) |
| Northern Europe | 1.10 (0.92–1.31) | 0.99b (0.62–1.58) |
| Southern Europe | 1.01 (0.89–1.14) | 1.05 (0.74–1.50) |
Note: OR adjusted for study, age-group, cigarette pack years, time since quitting smoking.
Abbreviations: CI, confidence intervals; OR, odds ratio; PAH, polycyclic aromatic hydrocarbons; Ref, reference category.
aWestern Europe: France, Germany, and the Netherlands; Eastern Europe: Czechia, Hungary, Poland, Romania, Russia, and Slovakia; Northern Europe: Sweden and the UK; and Southern Europe: Italy and Spain.
bAmong women, OR was adjusted for age group, cigarette pack-years, time since quitting smoking only (due to sample size).
We also assessed the lung cancer risk associated with cumulative PAH exposure by histologic lung cancer subtype and smoking status (Table 4). When restricted to never smokers, ORs were significantly elevated for small cell lung cancer (SCLC) in the highest cumulative exposure category (> 0.24 BaP μg/m3-years) for men (OR = 2.53; CI, 1.28–4.99) and women (OR = 3.02; CI, 1.21–7.59), although there were few cases exposed and the CIs overlapped between categories. Among current smokers, the association between PAH and lung cancer risk was stronger for squamous cell carcinomas (SqC) and SCLC when compared with adenocarcinoma (AC) for both men and women.
Table 4.
Risk of lung cancer associated with occupational cumulative PAH exposure by smoking status, gender, and histologic subtype.
| Cumulative PAH exposure | Never smokersa | Former smokersb | Current smokersc | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Histology | (BaP μg/m3-years) | Case | Control | OR (95% CI) | Case | Control | OR (95% CI) | Case | Control | OR (95% CI) |
| Men | ||||||||||
| All | Unexposed | 379 | 3,547 | 1 | 3,419 | 5,424 | 1 | 5,786 | 3,403 | 1 |
| <0.24 | 46 | 401 | 1.06 (0.76–1.48) | 631 | 919 | 1.02 (0.91–1.16) | 1,149 | 614 | 1.00 (0.89–1.12) | |
| >0.24 | 65 | 489 | 1.31 (0.98–1.75) | 737 | 985 | 1.08 (0.96–1.21) | 1,393 | 669 | 1.15 (1.04–1.28) | |
| Test for trend, P value d | 0.08 | 0.21 | 0.02 | |||||||
| Without reference group | 0.31 | 0.52 | 0.04 | |||||||
| AC | Unexposed | 140 | 3,547 | 1 | 937 | 5,424 | 1 | 1,352 | 3,403 | 1 |
| <0.24 | 18 | 401 | 1.03 (0.62–1.72) | 153 | 919 | 0.91 (0.75–1.10) | 258 | 614 | 1.03 (0.86–1.22) | |
| >0.24 | 21 | 489 | 1.07 (0.66–1.73) | 182 | 985 | 1.00 (0.84–1.20) | 264 | 669 | 0.99 (0.84–1.17) | |
| Test for trend, P value d | 0.78 | 0.78 | 1.00 | |||||||
| Without reference group | 0.92 | 0.42 | 0.76 | |||||||
| SqC | Unexposed | 112 | 3,547 | 1 | 1,452 | 5,424 | 1 | 2,440 | 3,403 | 1 |
| <0.24 | 10 | 401 | 0.83 (0.42–1.63) | 302 | 919 | 1.19 (1.02–1.39) | 498 | 614 | 1.01 (0.88–1.16) | |
| >0.24 | 19 | 489 | 1.33 (0.80–2.21) | 342 | 985 | 1.14 (0.99–1.33) | 653 | 669 | 1.24 (1.09–1.41) | |
| Test for trend, P value d | 0.40 | 0.03 | 0.0026 | |||||||
| Without reference group | 0.24 | 0.70 | 0.02 | |||||||
| SCLC | Unexposed | 37 | 3,547 | 1 | 453 | 5,424 | 1 | 1,069 | 3,403 | 1 |
| <0.24 | 7 | 401 | 1.59 (0.68–3.72) | 73 | 919 | 0.88 (0.67–1.16) | 200 | 614 | 0.99 (0.82–1.20) | |
| >0.24 | 12 | 489 | 2.53 (1.28–4.99) | 101 | 985 | 1.17 (0.92–1.49) | 248 | 669 | 1.21 (1.02–1.44) | |
| Test for trend, P value d | 0.01 | 0.36 | 0.06 | |||||||
| Without reference group | 0.35 | 0.09 | 0.09 | |||||||
| Women | ||||||||||
| All | Unexposed | 771 | 2,364 | 1 | 525 | 762 | 1 | 1,382 | 752 | 1 |
| <0.24 | 73 | 231 | 0.96 (0.73–1.28) | 71 | 92 | 1.27 (0.88–1.84) | 232 | 98 | 1.31 (0.99–1.74) | |
| >0.24 | 35 | 121 | 0.83 (0.56–1.23) | 49 | 38 | 1.94 (1.19–3.17) | 158 | 56 | 1.35 (0.95–1.90) | |
| Test for trend, P value d | 0.37 | 0.005 | 0.02 | |||||||
| Without reference group | 0.52 | 0.15 | 0.90 | |||||||
| AC | Unexposed | 461 | 2364 | 1 | 239 | 762 | 1 | 499 | 752 | 1 |
| <0.24 | 44 | 231 | 1.00 (0.71–1.42) | 25 | 92 | 0.95 (0.57–1.57) | 77 | 98 | 1.09 (0.76–1.56) | |
| >0.24 | 16 | 121 | 0.61 (0.35–1.06) | 16 | 38 | 1.37 (0.71–2.65) | 50 | 56 | 1.19 (0.77–1.84) | |
| Test for trend, P value d | 0.15 | 0.51 | 0.39 | |||||||
| Without reference group | 0.13 | 0.36 | 0.76 | |||||||
| SqC | Unexposed | 103 | 2364 | 1 | 116 | 762 | 1 | 300 | 752 | 1 |
| <0.24 | 8 | 231 | 0.76 (0.36–1.59) | 27 | 92 | 2.05 (1.17–3.59) | 60 | 98 | 1.72 (1.14–2.59) | |
| >0.24 | 5 | 121 | 0.75 (0.29–1.95) | 15 | 38 | 3.40 (1.55–7.45) | 41 | 56 | 1.98 (1.22–3.22) | |
| Test for trend, P value d | 0.38 | 0.0002 | 0.0005 | |||||||
| Without reference group | 0.98 | 0.27 | 0.64 | |||||||
| SCLC | Unexposed | 42 | 2,364 | 1 | 66 | 762 | 1 | 296 | 752 | 1 |
| <0.24 | 7 | 231 | 1.61 (0.69–3.72) | 6 | 92 | 0.78 (0.30–2.06) | 55 | 98 | 1.54 (1.02–2.34) | |
| >0.24 | 6 | 121 | 3.02 (1.21–7.59) | 8 | 38 | 3.84 (1.40–10.54) | 44 | 56 | 1.94 (1.18–3.18) | |
| Test for trend, P value d | 0.01 | 0.06 | 0.002 | |||||||
| Without reference group | 0.29 | 0.02 | 0.46 | |||||||
Abbreviations: AC, adenocarcinoma; BaP, benzo[a]pyrene; CI, confidence intervals; OR, odds ratio; PAH, polycyclic aromatic hydrocarbons; SCLC, small cell lung cancer; SqC, squamous cell cancers.
aAdjusted for study, age-group, List-A job.
bAdjusted for study, age-group, List-A job, pack-years and time since quitting.
cAdjusted for study, age-group, List-A job, and pack-years.
d P value obtained using the ordinal variable for respective exposure index.
Joint effects of ever/never occupational exposure to PAH and smoking status in relation to lung cancer risk overall and by subtype are shown in Table 5. In men, we observed no multiplicative interactions between PAH exposure and smoking, while modest positive additive interactions for lung cancer overall and the SqC subtype were observed. In women, we observed multiplicative interactions between PAH exposure and smoking for lung cancer overall, and the AC and SqC histologic subtypes. The additive interaction between PAH exposure and smoking was elevated for lung cancer overall, and for all major histologic subtypes with the highest RERI for SCLC (RERI = 11.5; CI, 3.91–19.1) followed by SqC (RERI 8.63; CI, 4.54–12.71). Supplementary Figure S1 graphically illustrates this synergism between smoking and occupational exposure to PAH.
Table 5.
Joint effects of occupational PAH exposure and smoking for lung cancer overall and by histologic subtype.
| All lung cancer types | Adenocarcinoma | Squamous cell carcinoma | Small cell lung cancer | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Exposure status | Controls | Cases | OR (95%CI) | Cases | OR (95%CI) | Cases | OR (95%CI) | Cases | OR (95%CI) |
| MEN | |||||||||
| Never smoker and nNever PAH exposed | 3,547 | 379 | 1.0 (Ref.) | 140 | 1.0 (Ref.) | 112 | 1.0 (Ref.) | 37 | 1.0 (Ref.) |
| Never smoker and PAH exposed | 890 | 111 | 1.10 (0.85–1.34) | 39 | 1.05 (0.66–1.43) | 29 | 0.94 (0.55–1.33V | 19 | 1.97 (0.87–3.07) |
| Ever smoker and never PAH exposed | 8,827 | 9,205 | 9.30 (8.27–10.33) | 2,289 | 6.48 (5.34–7.62) | 3892 | 12.79 (10.32–15.26) | 1,522 | 16.01 (10.74–21.29) |
| Ever smoker and PAH exposed | 3,187 | 3,910 | 10.59 (9.34–11.83) | 857 | 6.68 (5.43–7.94) | 1795 | 15.38 (12.33–18.44V | 622 | 18.2 (12.06–24.35) |
| P-value multiplicative interaction | 0.74 | 0.94 | 0.23 | 0.07 | |||||
| RERI | 1.19 (0.57–1.81) | 0.16 (−0.55 to 0.87) | 2.66 (1.51–3.81) | 1.23 (−0.84 to 3.29) | |||||
| WOMEN | |||||||||
| Never smoker and never PAH exposed | 2,364 | 771 | 1.0 (Ref.) | 461 | 1.0 (Ref.) | 103 | 1.0 (Ref.) | 42 | 1.0 (Ref.) |
| Never smoker and PAH exposed | 352 | 108 | 0.90 (0.69–1.12) | 60 | 0.86 (0.60–1.11V | 13 | 0.75 (0.30–1.20) | 13 | 2.06 (0.74–3.38) |
| Ever smoker and never PAH exposed | 1,514 | 1,907 | 4.41 (3.91–4.90) | 738 | 2.71 (2.33–3.10) | 416 | 8.05 (6.14–9.96V | 362 | 14.84 (9.87–19.80) |
| Ever smoker and PAH exposed | 284 | 510 | 6.81 (5.61–8.01) | 168 | 3.50 (2.71–4.30) | 143 | 16.43 (11.42–21.44V | 113 | 27.4 (16.64–38.16) |
| P-value multiplicative interaction | 0.0002 | 0.03 | 0.001 | 0.76 | |||||
| RERI | 2.5 (1.40–3.60) | 0.93 (0.16–1.71) | 8.63 (4.54–12.71) | 11.5 (3.91–19.1) | |||||
Note: OR adjusted by study, age-group, List-A job.
Abbreviations: CI, confidence intervals; OR, odds ratio; PAH, polycyclic aromatic hydrocarbons; RERI, relative excess risk due to interaction.
Discussion
This analysis of 14 pooled case–control studies showed that occupational PAH exposure was modestly associated with an increased risk of lung cancer in both men and women, after adjusting for potential confounders including smoking and employment in occupations with exposure to established lung carcinogens. A PAH-related increased risk in never smokers was present in SCLC cases, for both sexes. Joint effects of occupational PAH exposure and smoking were present on an additive scale for SqC lung cancer both in men and women, and in addition for SCLC and AC in women.
The sensitivity analyses aimed at exploring the robustness of our results revealed that more recent studies (≥1995), workers starting working 1960 or later, and analyses restricted to blue-collar workers resulted in no or minor association between occupational exposure to PAH and increased risk of lung cancer.
Our main results are in line with previous studies, although cumulative exposure levels and risk estimation per exposure unit are not directly comparable with industrial cohorts (25–28). In our study, the most frequent occupations with PAH exposure among women were waiters, cooks, and machine-tool operators, and among men blacksmiths, chimney sweeps, and boiler firemen. The maximum cumulative exposure of 1.83 μg/m3-years BaP was estimated for a male blacksmith. For reference, Armstrong and colleagues reported very high cumulative exposure levels in various industrial cohorts including aluminum production workers (max. 413 μg/m3-years BaP) and coke oven workers (max. 805 μg/m3-years BaP; ref. 29). A contributing reason to our different results is that there were too few exposure measurements to allow job specific estimates of exposure. Therefore, we could only calibrate a priori high and low exposed jobs that would result in lowered assigned exposures for individuals experiencing very high exposure concentrations (e.g., coke oven workers, n = 19).
Some relative risk estimates in women in relation to occupational exposure to PAH are higher among women than in men. However, risk estimates in women are less precise and confidence intervals often overlap widely. If there are truly higher risks in women, it is unlikely that these are due to higher exposures in women or lesser use of personal protection equipment. As illustrated in Fig. 1, in this analysis men were exposed to higher PAH levels than women. The estimated median cumulative exposure to BaP among men was 0.27 μg/m3-years, while 0.16 μg/m3-years in women. Regarding physiologic differences, there is currently only weak evidence that CYP1A1 expression involved in bioactivation and DNA adduct formation of PAH is higher in women, or that women are more susceptible than men to oxidative stress and chromosome damage induced by PAHs (30–32). Alternative explanations for the higher relative risk in women could be the lower background lung cancer rate in women compared with men, or that the reference group in the female population was cleaner in terms of exposure to PAH than the reference group in the male population.
When restricting the analyses to blue-collar workers there was no association between occupational PAH exposure and lung cancer risk in men and an attenuated risk in women. In previous SYNERGY analyses, the exposure–response relationship between diesel engine exhaust exposure and lung cancer in the SYNERGY male population was robust and present in various sensitivity analyses, including when we limited analyses to blue-collar workers (33). Likewise, in analyses of occupational exposure to asbestos and respirable crystalline silica restricted to blue-collar workers we observed an increased lung cancer risk, although somewhat attenuated compared with the main analyses (4, 5). Therefore, we think that the “no/attenuated association” between occupational PAH exposure and lung cancer in blue-collar workers in the current analyses may be related to the reduced exposure contrasts resulting from the lack of exposure measurements for PAH (BaP) which resulted in a cruder exposure assignment compared with the other exposures included in SYN-JEM (21), which may have been aggravated by restriction to blue-collar workers.
Stratified analysis by geographic area showed that studies from Western Europe stood out with higher ORs than other areas, although with largely overlapping CIs. This result may reflect the larger study population 42% in Western Europe compared with 14% in Eastern Europe, 12% in Northern Europe, 22% in Southern Europe, and 10% from Canada. Another less probable explanation could be varying regulatory and industrial practices leading to differences in exposure and use of personal protective equipment.
Work after 1960 resulted in lower exposure concentrations, especially in recent years, and therefore limited our statistical power to detect increased risks. In addition, the actual exposures may be lower than reported here due to increased use of personal protective equipment, although these data were not available for the SYN-JEM nor for the analyses.
In interpreting these results, it is important to consider several limitations in the SYNERGY project. The participation rates were relatively low for some of the studies, especially among the control subjects, which may have led to selection bias if blue-collar workers were underrepresented among the control subjects. Previous analyses in SYNERGY restricted to blue-collar workers have resulted in robust associations, although attenuated. Thus, selection bias in SYNERGY does not seem to be a major issue. We used BaP concentrations as a proxy for carcinogenic PAHs in the workplace. It is not known to what extent our findings are representative of co-occurring PAHs. However, BaP is commonly used as a proxy for PAH, as evidenced in several industrial cohort studies (27, 28). Although, occupational histories were self-reported in SYNERGY, they are less prone to recall bias compared with self-reported exposure histories (34). We cannot exclude the possibility of residual confounding by other occupational exposures, even after adjusting for “List-A jobs.” However, the magnitude of the pooled OR did not change markedly after excluding single industries or jobs one at a time from the analysis. Given that “List-A jobs” include jobs with potential PAH exposure, overadjustment could be a potential concern. However, there was little difference in the results when comparing ORs for PAH in Table 2 (adjusted for ever employment in “List-A jobs”) with the results in Supplementary Table S2 and Table 3 (without adjustment for “List-A” jobs).
A major strength of SYNERGY is the large size resulting in reasonable precision in estimating effects in subgroups by sex, smoking status, and histologic subtype. All but one study collected lifelong occupational histories, which is important considering the long latency from exposure to the development of lung cancer.
Our results show a modest elevation in lung cancer risk, particularly for SqC and SCLC, in relation to occupational exposure to PAH after accounting for cigarette smoking and exposure to other occupational lung carcinogens. Joint effects of cigarette smoking and PAH exposure was observed for SqC in both men and women, and in addition for SCLC and AC in women. The lack of association in more recent studies merits further research under today's exposure conditions and worker protection measures.
Supplementary Material
Acknowledgments
We are grateful to Isabelle Stücker, who will be remembered for her professionalism and generosity regarding the SYNERGY project. The SYNERGY project was funded by the German Social Accident Insurance (DGUV) between 2007 and 2011.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
Note: Supplementary data for this article are available at Cancer Epidemiology, Biomarkers & Prevention Online (http://cebp.aacrjournals.org/).
Authors' Disclosures
The authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy or views of their affiliated institutes. S. Peters reports grants from DGUV Germany during the conduct of the study. P. Gustavsson reports grants from Swedish Council for Work Life Research during the conduct of the study. T. Behrens reports T. Behrens, B. Kendzia, and T. Brüning, as staff of the Institute for Prevention and Occupational Medicine (IPA), are employed by the study's main financing body, the German Social Accident Insurance (DGUV). IPA is an independent research institute of the Ruhr University Bochum. S. Karrasch reports grants from German Center for Lung Research (DZL), grant number 82DZL083B2 during the conduct of the study. D. Mirabelli reports grants from International Agency for Research on Cancer during the conduct of the study; grants from Ministry of Health and INAIL outside the submitted work; and served as expert witness for the public prosecution office in court cases of asbestos-related diseases. L. Richiardi reports grants from International Agency for Research on Cancer during the conduct of the study. K. Straif reports grants from DGUV, Germany during the conduct of the study. No disclosures were reported by the other authors.
Authors' Contributions
A. Olsson: Data curation, formal analysis, supervision, project administration, writing–review and editing. N. Guha: Writing–original draft. L. Bouaoun: Formal analysis. H. Kromhout: Conceptualization, supervision, methodology. S. Peters: Data curation, formal analysis, methodology, writing–review and editing. J. Siemiatycki: Data curation, investigation, methodology, writing–review and editing. V. Ho: Writing–review and editing. P. Gustavsson: Data curation, supervision, investigation, methodology, writing–review and editing. P. Boffetta: Data curation, investigation, methodology, writing–review and editing. R. Vermeulen: Conceptualization, supervision, methodology, writing–review and editing. T. Behrens: Investigation, methodology, writing–review and editing. T. Brüning: Conceptualization, funding acquisition, writing–review and editing. B. Kendzia: Data curation, writing–review and editing. P. Guénel: Data curation, investigation, methodology, writing–review and editing. D. Luce: Data curation, investigation, methodology, writing–review and editing. S. Karrasch: Data curation, investigation, methodology, writing–review and editing. H.-E. Wichmann: Data curation, investigation, methodology, writing–review and editing. D. Consonni: Data curation, investigation, methodology, writing–review and editing. M.T. Landi: Data curation, validation, methodology, writing–review and editing. N.E. Caporaso: Data curation, investigation, methodology, writing–review and editing. F. Merletti: Data curation, investigation, methodology, writing–review and editing. D. Mirabelli: Data curation, investigation, methodology, writing–review and editing. L. Richiardi: Data curation, investigation, methodology, writing–review and editing. K.-H. Jöckel: Data curation, investigation, methodology, writing–review and editing. W. Ahrens: Data curation, investigation, methodology, writing–review and editing. H. Pohlabeln: Data curation, investigation, methodology, writing–review and editing. A. Tardon: Data curation, investigation, methodology, writing–review and editing. D. Zaridze: Data curation, investigation, methodology, writing–review and editing. J.K. Field: Data curation, investigation, methodology, writing–review and editing. J. Lissowska: Data curation, investigation, methodology, writing–review and editing. B. Świątkowska: Data curation, investigation, methodology, writing–review and editing. J.R. McLaughlin: Data curation, investigation, methodology, writing–review and editing. P.A. Demers: Data curation, investigation, methodology, writing–review and editing. V. Bencko: Data curation, investigation, methodology, writing–review and editing. L. Foretova: Data curation, investigation, methodology, writing–review and editing. V. Janout: Data curation, investigation, methodology, writing–review and editing. T. Pándics: Data curation, investigation, methodology, writing–review and editing. E. Fabianova: Data curation, investigation, methodology, writing–review and editing. D. Mates: Data curation, investigation, methodology, writing–review and editing. F. Forastiere: Data curation, investigation, methodology, writing–review and editing. B. Bueno-de-Mesquita: Data curation, investigation, methodology, writing–review and editing. J. Schüz: Conceptualization, supervision, funding acquisition, writing–review and editing. K. Straif: Conceptualization, funding acquisition, methodology, writing–review and editing.
References
- 1. Boström CE, Gerde P, Hanberg A, Jernström B, Johansson C, Kyrklund T, et al. Cancer risk assessment, indicators, and guidelines for polycyclic aromatic hydrocarbons in the ambient air. Environ Health Perspect 2002;110:451–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Straif K, Baan R, Grosse Y, Secretan B, El Ghissassi F, Cogliano V. Carcinogenicity of polycyclic aromatic hydrocarbons. Lancet Oncol 2005;6:931–2. [DOI] [PubMed] [Google Scholar]
- 3. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans Volume 92. Some non-heterocyclic polycyclic aromatic hydrocarbons and some related occupational exposures. Lyon, France: IARC Press; Geneva: Distributed by World Health Organization; 2010. [PMC free article] [PubMed] [Google Scholar]
- 4. Olsson AC, Vermeulen R, Schuz J, Kromhout H, Pesch B, Peters S, et al. Exposure-response analyses of asbestos and lung cancer subtypes in a pooled analysis of case-control studies. Epidemiology 2017;28:288–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ge C, Peters S, Olsson A, Portengen L, Schüz J, Almansa J, et al. Respirable crystalline silica exposure, smoking, and lung cancer subtype risks. a pooled analysis of case-control studies. Am J Respir Crit Care Med 2020;202:412–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brenner DR, Boffetta P, Duell EJ, Bickeböller H, Rosenberger A, McCormack V, et al. Previous lung diseases and lung cancer risk: a pooled analysis from the International Lung Cancer Consortium. Am J Epidemiol 2012;176:573–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brüske-Hohlfeld I, Möhner M, Pohlabeln H, Ahrens W, Bolm-Audorff U, Kreienbrock L, et al. Occupational lung cancer risk for men in Germany: results from a pooled case-control study. Am J Epidemiol 2000;151:384–95. [DOI] [PubMed] [Google Scholar]
- 8. Consonni D, De Matteis S, Lubin JH, Wacholder S, Tucker M, Pesatori AC, et al. Lung cancer and occupation in a population-based case-control study. Am J Epidemiol 2010;171:323–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fortes C, Forastiere F, Farchi S, Mallone S, Trequattrinni T, Anatra F, et al. The protective effect of the mediterranean diet on lung cancer. Nutr Cancer 2003;46:30–7. [DOI] [PubMed] [Google Scholar]
- 10. Guida F, Papadopoulos A, Menvielle G, Matrat M, Févotte J, Cénée S, et al. Risk of lung cancer and occupational history: results of a French population-based case-control study, the ICARE study. J Occup Environ Med 2011;53:1068–77. [DOI] [PubMed] [Google Scholar]
- 11. Gustavsson P, Jakobsson R, Nyberg F, Pershagen G, Järup L, Schéele P. Occupational exposure and lung cancer risk: a population-based case-referent study in Sweden. Am J Epidemiol 2000;152:32–40. [DOI] [PubMed] [Google Scholar]
- 12. Jöckel KH, Ahrens W, Jahn I, Pohlabeln H, Bolm-Audorff U. Occupational risk factors for lung cancer: a case-control study in West Germany. Int J Epidemiol 1998;27:549–60. [DOI] [PubMed] [Google Scholar]
- 13. Kazma R, Babron MC, Gaborieau V, Génin E, Brennan P, Hung RJ, et al. Lung cancer and DNA repair genes: multilevel association analysis from the International Lung Cancer Consortium. Carcinogenesis 2012;33:1059–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. López-Cima MF, González-Arriaga P, García-Castro L, Pascual T, Marrón MG, Puente XS, et al. Polymorphisms in XPC, XPD, XRCC1, and XRCC3 DNA repair genes and lung cancer risk in a population of northern Spain. BMC Cancer 2007;7:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ramanakumar AV, Parent ME, Siemiatycki J. Risk of lung cancer from residential heating and cooking fuels in Montreal, Canada. Am J Epidemiol 2007;165:634–42. [DOI] [PubMed] [Google Scholar]
- 16. Riboli E, Kaaks R. The EPIC Project: rationaleand study design. European prospective investigation into cancer and nutrition. Int J Epidemiol 1997;26:S6–14. [DOI] [PubMed] [Google Scholar]
- 17. Richiardi L, Boffetta P, Simonato L, Forastiere F, Zambon P, Fortes C, et al. Occupational risk factors for lung cancer in men and women: a population-based case-control study in Italy. Cancer Causes Control 2004;15:285–94. [DOI] [PubMed] [Google Scholar]
- 18. Scélo G, Constantinescu V, Csiki I, Zaridze D, Szeszenia-Dabrowska N, Rudnai P, et al. Occupational exposure to vinyl chloride, acrylonitrile and styrene and lung cancer risk (Europe). Cancer Causes Control 2004;15:445–52. [DOI] [PubMed] [Google Scholar]
- 19. Stücker I, Hirvonen A, de Waziers I, Cabelguenne A, Mitrunen K, Cénée S, et al. Genetic polymorphisms of glutathione S-transferases as modulators of lung cancer susceptibility. Carcinogenesis 2002;23:1475–81. [DOI] [PubMed] [Google Scholar]
- 20. Peters S, Vermeulen R, Olsson A, Van Gelder R, Kendzia B, Vincent R, et al. Development of an exposure measurement database on five lung carcinogens (ExpoSYN) for quantitative retrospective occupational exposure assessment. Ann Occup Hyg 2012;56:70–9. [DOI] [PubMed] [Google Scholar]
- 21. Peters S, Vermeulen R, Portengen L, Olsson A, Kendzia B, Vincent R, et al. SYN-JEM: a quantitative job-exposure matrix for five lung carcinogens. Ann Occup Hyg 2016;60:795–811. [DOI] [PubMed] [Google Scholar]
- 22. Lubin JH, Colt JS, Camann D, Davis S, Cerhan JR, Severson RK, et al. Epidemiologic evaluation of measurement data in the presence of detection limits. Environ Health Perspect 2004;112:1691–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ahrens W, Merletti F. A standard tool for the analysis of occupational lung cancer in epidemiologic studies. Int J Occup Environ Health 1998;4:236–40. [DOI] [PubMed] [Google Scholar]
- 24. Mirabelli D, Chiusolo M, Calisti R, Massacesi S, Richiardi L, Nesti M, et al. [ Database of occupations and industrial activities that involve the risk of pulmonary tumors]. Epidemiol Prev 2001;25:215–21. [PubMed] [Google Scholar]
- 25. Boffetta P, Jourenkova N, Gustavsson P. Cancer risk from occupational and environmental exposure to polycyclic aromatic hydrocarbons. Cancer Causes Control 1997;8:444–72. [DOI] [PubMed] [Google Scholar]
- 26. Bosetti C, Boffetta P. Occupational exposures to polycyclic aromatic hydrocarbons, and respiratory and urinary tract cancers: a quantitative review to 2005. Ann Oncol 2007;18:431–46. [DOI] [PubMed] [Google Scholar]
- 27. Petit P, Maître A, Persoons R, Bicout DJ. Lung cancer risk assessment for workers exposed to polycyclic aromatic hydrocarbons in various industries. Environ Int 2019;124:109–20. [DOI] [PubMed] [Google Scholar]
- 28. Armstrong BG, Gibbs G. Exposure-response relationship between lung cancer and polycyclic aromatic hydrocarbons (PAHs). Occup Environ Med 2009;66:740–6. [DOI] [PubMed] [Google Scholar]
- 29. Armstrong B, Hutchinson E, Unwin J, Fletcher T. Lung cancer risk after exposure to polycyclic aromatic hydrocarbons: a review and meta-analysis. Environ Health Perspect 2004;112:970–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mollerup S, Berge G, Baera R, Skaug V, Hewer A, Phillips DH, et al. Sex differences in risk of lung cancer: expression of genes in the PAH bioactivation pathway in relation to smoking and bulky DNA adducts. Int J Cancer 2006;119:741–4. [DOI] [PubMed] [Google Scholar]
- 31. Uppstad H, Osnes GH, Cole KJ, Phillips DH, Haugen A, Mollerup S. Sex differences in susceptibility to PAHs is an intrinsic property of human lung adenocarcinoma cells. Lung Cancer 2011;71:264–70. [DOI] [PubMed] [Google Scholar]
- 32. Guo H, Huang K, Zhang X, Zhang W, Guan L, Kuang D, et al. Women are more susceptible than men to oxidative stress and chromosome damage caused by polycyclic aromatic hydrocarbons exposure. Environ Mol Mutagen 2014;55:472–81. [DOI] [PubMed] [Google Scholar]
- 33. Ge C, Peters S, Olsson A, Portengen L, Schüz J, Almansa J, et al. Diesel engine exhaust exposure, smoking, and lung cancer subtype risks. a pooled exposure-response analysis of 14 case-control studies. Am J Respir Crit Care Med 2020;202:402–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Teschke K, Olshan AF, Daniels JL, De Roos AJ, Parks CG, Schulz M, et al. Occupational exposure assessment in case-control studies: opportunities for improvement. Occup Environ Med 2002;59:575–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.