Keywords: bile acids, caloric intake, cholesterol, fat, obesity
Abstract
Obesity is associated with alterations in cholesterol and bile acid (BA) metabolism. However, the interaction among dietary intake, cholesterol absorption, and BA metabolism in patients with obesity remains unclear. We conducted a 4-wk nutritional intervention nonrandomized clinical trial with three different sequential diets for a week in the following order: regular diet (RD); high calorie, high-fat diet (HCHF), washout period on RD; and low-calorie, low-fat diet (LCLF). We provided participants with meal replacements during HCHF and LCLF diets. A total of 16 participants completed the study [n = 8 normal weight (NW); n = 8 with obesity (OB)]. Overall, there was a significant increase in intestinal cholesterol uptake when changing from RD to HCHF and a reduction in intestinal cholesterol uptake from HCHF to LCLF. When analyzing by BMI groups, these findings were similar in patients with NW (RD to HCHF: P < 0.007; HCHF to LCLF: P = 0.02); however, in patients with obesity, the change in intestinal cholesterol uptake was only observed when changing from RD to HCHF (P = 0.006). There was no correlation between cholesterol absorption and fecal bile acids or other markers of BA metabolism in all patients or the subgroups. Dietary caloric content had a significant effect on cholesterol absorption, however, this effect is blunted in patients with obesity. These data are consistent with the impaired effect of a low-fat diet on cholesterol absorption in obesity.
NEW & NOTEWORTHY We show how switching from a regular diet to an HCHF increases cholesterol absorption in patients with normal weight and obesity. The decrease in cholesterol absorption from an HCHF to an LCLF, on the other hand, was only seen in normal-weight controls, underlining the importance of body weight in this regulation. In addition, changes in caloric and fat content had an immediate and direct effect on hepatic bile acid production.
INTRODUCTION
Obesity is a chronic multifactorial disease associated with numerous comorbidities and increased mortality. Obesity has been characterized by low dietary cholesterol absorption efficiency and excessive cholesterol synthesis, contributing to the increased risk of several comorbidities such as dyslipidemia and cardiovascular disease (1, 2). Although the exact mechanism underlying this inverse relationship between body weight and cholesterol synthesis is unknown, it is thought that cholesterol synthesis is increased in the liver in conjunction with the increased hepatic fatty acid influx, high very-low-density lipoprotein (VLDL) formation, and increased low-density lipoprotein (LDL) apoprotein B turnover (3, 4). The lower cholesterol absorption efficiency, on the other hand, was significantly associated with biliary concentrations of cholesterol, bile acids (BAs), and phospholipids, emphasizing the relevance of enterohepatic bile acid circulation (5).
Cholesterol metabolism in humans is a complex and tightly regulated process. The total serum cholesterol level is determined mainly by endogenous cholesterol synthesis (6) and intestinal absorption (7). As almost 50% of the intestinal cholesterol is absorbed in healthy humans (8), dietary habits are factors altering serum cholesterol concentration (9). Modifying the dietary composition into a low fat/cholesterol diet has neutralized serum cholesterol levels (9). These preliminary data uncover the complexity of cholesterol regulation and its ability to be modified by various exogenous and endogenous elements. It is incompletely understood how obesity and macronutrient dietary intake independently affect total serum cholesterol and intestinal absorption.
Bile acids (BAs) are cholesterol-derived metabolites having a well-established involvement in lipid digestion and absorption. In the last decade, BAs have also been recognized for their ability to act as structural molecules, allosteric modulators, and signaling molecules affecting key aspects of metabolic homeostasis (10–13). BAs activate numerous signaling pathways after excretion and transit through the gastrointestinal system. In the enterocyte, BAs activate the farnesoid X receptor (FXR) to synthesize fibroblast growth factor-19 (FGF-19) and the G protein-coupled bile acid receptor 1 (GPBAR1) receptor [also known as takeda G receptor 5 (TGR5)]. Signaling via both receptors, FXR and TGR5, has been connected to the release of gastrointestinal hormones such as glucagon-like peptide-1 (GLP-1), which are essential for maintaining metabolic homeostasis (13–15). Recently, we reported that serum FGF-19 was decreased in obesity and diabetes compared with healthy weight controls (16). In addition, rectal administration of taurocholic acid (TCA), a primary BA that is deconjugated and then dehydroxylated by colonic bacteria to secondary BAs, activates TGR5 signaling, resulting in increased GLP-1 and PYY secretion and decreased appetite in healthy patients in a dose-dependent manner (17). Ileo-colonic delivery of conjugated bile acids enhanced GLP-1 secretion and improved glycemic patients with obesity and type 2 diabetes (16). Given the role of bile acids on appetite regulation, notably via the production of gastrointestinal hormones, modifying bile acid signaling seems logically anticipated to affect energy balance. However, it is unknown if the bile acid-mediated cholesterol absorption in patients with obesity is also altered. Thus, we aimed to evaluate the bile acid pathway cholesterol absorption in patients with obesity compared with healthy weight controls under different diet interventions. We hypothesize that the high dietary fat and caloric content will be positively correlated with cholesterol absorption mediated by changes in BA metabolism and that this correlation will be impaired in obesity.
Hence, we first aimed to assess how dietary changes affect cholesterol absorption in participants with normal weight compared with those with obesity. We also sought to evaluate how these dietary changes affect BA synthesis and fecal excretion in these two groups. Finally, we studied the changes in cholesterol absorption and BA synthesis.
METHODS
Study Design and Population
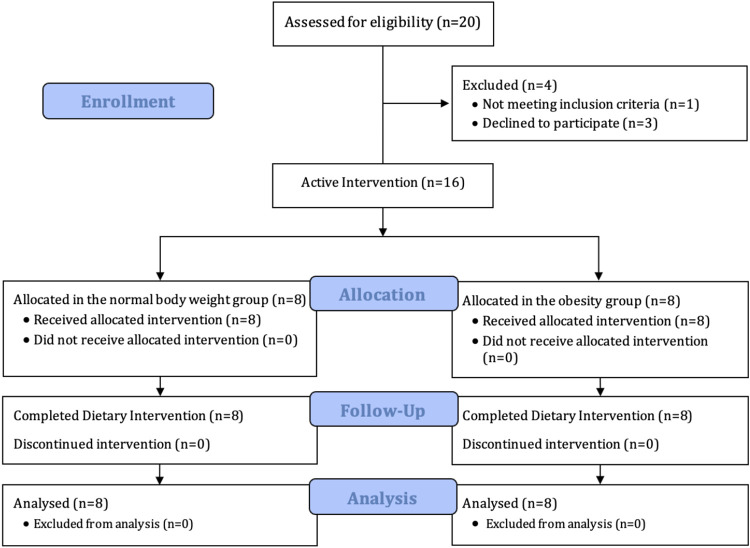
This is a 4-wk nutritional interventional clinical trial in which we evaluated the effect of three different sequential diets on metabolically healthy (i.e., lack diabetes mellitus, hypertension, dyslipidemia, or atherosclerotic cardiovascular diseases) (18) adults participants between 18 and 65 yr with normal weight (NW) or with obesity (OB). We invited 114 individuals from the community to this study. We excluded patients with the following: 1) history of abdominal surgery (including cholecystectomy and other than an appendectomy, caesarian section, or tubal ligation); 2) active use of medications that may alter gastrointestinal motility, appetite, or absorption (e.g., orlistat, phentermine); 3) positive history of chronic gastrointestinal diseases; 4) taking medication, (except multivitamins, birth control pill, estrogen replacement therapy, and thyroxin replacement therapy), within 7 days of the study; and 5) significant untreated psychiatric dysfunction. The first 20 to reply were screened, and 16 subjects who met inclusion criteria were enrolled [8 NW, i.e., body-mass index (BMI) 18.5–24.9 kg/m2, and 8 with OB, i.e., BMI >30 kg/m2] (Fig. 1). Pregnancy was ruled out in all females of childbearing potential. The study was reviewed and approved by the Mayo Clinic Institutional Review Board (IRB No.: 16-007060) and registered in clinical trials.gov (NCT03341052). All subjects provided written consent.
Figure 1.
The study flow chart revealing the distribution of our participants.
Study Intervention
During the first (baseline) and third (washout) weeks of the study, participants were on their regular diet (RD). The RD consisted of the participants’ regular eating habits, and a 24-h food diary was tracked. For the second [high caloric/high-fat diet (HCHF)] and fourth [low caloric/low-fat diet (LCLF)] interventional weeks, participants were provided with meals by the Mayo Clinic Clinical Research and Trials Unit (CRTU) metabolic kitchen daily (breakfast, lunch, dinner, and snacks). The HCHF meals consisted of 2,700 kcal/day and were composed of 100 g total fat (33%), 350 g carbohydrates (52%), 100 g protein (15%), and 300 mg cholesterol. The LCLF meals consisted of 1,200 kcal/day and were composed of 26 g fat (20%), 150 g carbohydrates (50%), 100 g protein (30%), and 78 mg cholesterol. All uneaten food was returned to the metabolic kitchen and weighed by the registered dietitian to determine the exact daily caloric intake (DCI) and macronutrient consumption. Throughout the study, participants completed a 24-h food diary daily. A nutritionist provided instructions on answering the food diary correctly and adding the consumed items objectively to reduce inaccuracy. A registered dietitian calculated the total daily caloric and macronutrient intake by the end of each week.
Cholesterol Absorption Test
The following protocol was used to measure cholesterol absorption. Subjects reported to the CRTU between 6:30 and 7:30 AM, after overnight fasting of 8 h, on day 5 of each dietary phase (RD, HCHF, and LCLF). A plastic cannula was inserted into a peripheral arm or hand vein upon arrival for blood sampling before the meal. After sample collection, participants ate a cholesterol-enriched meal (testing meal) at breakfast mixed (consisting of eggs, ham, milk, and toast) with a 100-mg of cholesterol stable isotope (cholesterol-d7) (CDN Isotopes, Quebec, Canada). Breakfast during HCHF consisted of 653 kcal (82 g carbohydrates, 28.4 g protein, and 23.93 g fat) versus LCLF, which consisted of 270 kcal (35 g carbohydrates, 20 g protein, and 6.7 g fat). Stable isotope-labeled cholesterol (cholesterol-d7) feeding method (19, 20) was utilized as an approximate tracer for the intestinal cholesterol absorption process. In humans, cholesterol absorbed by the small intestine after a meal comes from two sources: dietary cholesterol (range 0.1–1 g/day) and biliary cholesterol (1–4 g/day) (21). Cholesterol absorption may thus be reliably determined by measuring deuterium-enriched cholesterol. Subjects were discharged from the CRTU after completing their testing day. On day 7 (48 h later), subjects returned to the CRTU in the morning (between 6:30 and 7:30 AM), after overnight fasting, and a blood sample was obtained to measure free and total cholesterol-d7 and regular cholesterol. Cholesterol tracer is given orally, peaks in plasma within 2 days, and then slowly decline. Determination of the change (delta Δ) of the cholesterol tracers in plasma after 2 days of equilibration in the rapidly miscible pool of body cholesterol allows calculation of the fractional absorption of cholesterol from the test meal (22). The differences (delta Δ) in the baseline and 48-h serum levels of cholesterol-d7 were used to estimate intestinal cholesterol absorption.
BA Synthesis Assessment
To measure BA synthesis, 7α-hydroxy-4-cholesten-3-one (C4) was utilized as a biomarker, which is a by-product of the rate-limiting step of hepatic BA synthesis (23). Participants collected stool from every bowel movement throughout 48 h from days 5 to 7 after their meal test with cholesterol-d7 to estimate the fecal excretion of the total bile acid (TBA). Fecal BA amount, concentration, and fat were analyzed from these samples.
Sample Analyses
We used and adapted Galman et al. (24) method, using gas chromatography mass spectrometry (GCMS), to measure serum levels of cholesterol and cholesterol-d7 (mg/dL) in both free and total moieties. For BA synthesis, serum levels of C4 were measured (ng/mL) (25). The stool sample is weighed and frozen at −25°C until analysis. Fecal fat (FF) was measured by nuclear magnetic resonance spectrometry. Free BA and conjugated fecal BA concentration (BAC) in stool were quantified with HPLC-tandem mass spectrometry (26). Values were recorded as absolute values (µmol/48 h).
Study Endpoints
The primary end point was to compare cholesterol-d7 absorption and BA metabolism in patients with NW and OB in response to different fat and caloric content of diets (RD vs. HCHF vs. LCLF). The secondary endpoints were BA synthesis and fecal excretion of BA, fecal BAC, and fat excretion in response to different diets and among the normal weight or obese groups or the combined group.
Statistical Analyses
Categorical variables are expressed as frequency and percentage and are compared using Fisher’s exact. Continuous variables are summarized as means ± SD. Differences between groups were compared with the rank-sum test, whereas differences over different groups’ diets were compared using Wilcoxon signed-rank test. We used Pearson correlation to assess the relationship between the changes (delta Δ) in cholesterol absorption and 48-h fecal bile acids in NW and OB groups during HCHF and LCLF interventions. A P value <0.05 was considered statistically significant. We used JMP Pro version 14 software (SAS Institute, Cary, NC) to analyze the data.
RESULTS
Baseline Participants Characteristics
After a total of 20 participants were screened, 16 participants were found to be eligible, received the active interventions, and completed the study as outlined in the study flow chart (Fig. 1). Our participants were mostly females with a mean age of 35.6 ± 10.4 yr (Table 1). There were no significant differences in fasting and postprandial levels of total cholesterol, HDL, LDL, and triglycerides at baseline between groups.
Table 1.
The demographics and clinical characteristics of study participants at baseline with their body weight and caloric intake over different dietary interventions
| Variables | All | Normal Weight | Obesity | Difference (95% CI) | P Value |
|---|---|---|---|---|---|
| n | 16 | 8 | 8 | ||
| Demographics | |||||
| Age, yr | 35.6 ± 10.4 | 33 ± 8.9 | 38.3 ± 11.6 | 5.3 (−5.9 to 16.5) | 0.33 |
| Sex (F:M) | 10:6 | 4:4 | 6:2 | 0.30 | |
| Baseline weight, kg | 84.2 ± 16.8 | 71.2 ± 10.2 | 97.3 ± 10.6 | 26.1 (14.9–37.3) | <0.001 |
| Height, cm | 169.6 ± 8.9 | 171.5 ± 10 | 167.8 ± 8.1 | −3.7 (−13.5 to 6.1) | 0.43 |
| BMI, kg/m2 | 29.3 ± 5.9 | 24.1 ± 0.8 | 34.6 ± 3.4 | 10.5 (7.7–13.4) | <0.001 |
| Body weight, kg | |||||
| RD | 83.9 ± 17.2 | 70.3 ± 9.9 | 97.6 ± 10.4 | 27.3 (16.4–38.2) | <0.001 |
| HCHF | 83.6 ± 16.7 | 70.3 ± 9.8 | 96.8 ± 10.2 | 26.5 (15.8–37.2) | <0.001 |
| LCLF | 82.4 ± 17 | 68.8 ± 9.1 | 96.1 ± 10.7 | 27.2 (16.6–37.9) | <0.001 |
| Caloric intake, kcal | |||||
| RD | 1,952 + 456.1 | 1,963.5 ± 487.6 | 1,933.7 ± 455.2 | −29.8 (−632 to 573) | 0.91 |
| HCHF | 2,666.6 + 55.7 | 2,673.5 ± 48.5 | 2,659.7 ± 64.7 | −13.8 (−75.6 to 48) | 0.64 |
| LCLF | 1,103.4 + 68.6 | 1,123.6 ± 71.1 | 1,083.1 ± 63.9 | −40.5 (−113 to 32.1) | 0.25 |
Data are presented as means ± SD. BMI, body mass index; CI, confidence interval; HCHF, high-caloric/high-fat diet; LCLF, low-caloric/low-fat diet; RD, regular diet. Boldfaced text represents statistically significant values.
Caloric Intake
Overall, there was no difference in the daily caloric intake between NW and OB groups throughout the study period, regardless of the dietary intervention (Table 1). Participants’ body weight remained stable during RD to HCHF, however, they had a minor but statistically significant weight loss when shifting from HCHF to LCLF −1.1 [95% confidence interval (CI), −0.5 to −1.8 kg; P = 0.003].
Serum Cholesterol and Cholesterol Absorption
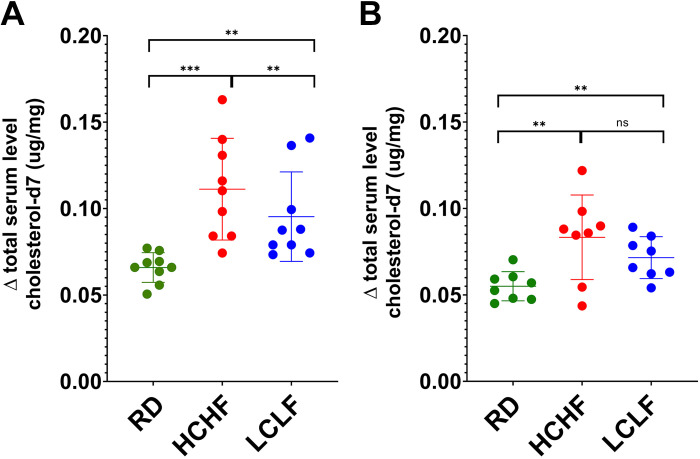
The cumulative and subgroups’ mean serum levels of the Δ free and Δ total cholesterol and cholesterol-d7 of each dietary intervention are listed in Table 2. In the whole cohort, there were no changes in the total regular cholesterol levels following various dietary interventions. The mean serum levels of Δ total cholesterol-d7 were significantly higher in NW when compared with OB after HCHF and LCLF. There were no differences in free or total cholesterol after any intervention (Table 2). The overall alterations in the estimated cholesterol absorption levels were significant after changing the dietary intervention among all participants, regardless of their body weight group (Table 2). There was a significant increase in intestinal cholesterol uptake when shifting from RD to HCHF 0.04 (95% CI, 0.03–0.05; P < 0.001) and a reduction from HCHF to LCLF −0.02 (95% CI, −0.03 to −0.003; P = 0.02). When subdividing patients according to their groups, NW participants maintained this trend (RD to HCHF: P < 0.007; HCHF to LCLF: P = 0.02) (Fig. 2A). The OB group showed a significant increase in intestinal cholesterol uptake when changing from RD to HCHF (P = 0.006), however, the significance was lost from HCHF to LCLF (P = 0.32) (Fig. 2B).
Table 2.
The cumulative and subgroups’ mean serum levels of the Δ free and Δ total cholesterol and cholesterol-d7 (mg/dL) of each dietary intervention
| Interventional Weeks | Type of Serum Cholesterol | All | Normal Weight | Obesity | Difference (95% CI) | P Value |
|---|---|---|---|---|---|---|
| Regular diet | ||||||
| Δ 48 h—Baseline (week 1, day 7) | Free cholesterol-d7 | 0.07 ± 0.01 | 0.08 ± 0.01 | 0.07 ± 0.01 | −0.01 (0.0006 to −0.02) | 0.062 |
| Total cholesterol-d7 | 0.06 ± 0.01 | 0.07 ± 0.01 | 0.06 ± 0.01 | −0.01 (−0.001 to −0.02) | 0.03 | |
| Free cholesterol | −2.1 ± 12.2 | −3.4 ± 12.5 | −0.71 ± 12.5 | 2.7 (16.1 to −10.7) | 0.67 | |
| Total cholesterol | −10 ± 32.7 | −17.1 ± 35.9 | −2.9 ± 29.7 | 14.2 (49.6 to −21.3) | 0.40 | |
| High caloric/fat diet | ||||||
| Δ 48 h—Baseline (week 2, day 7) | Free cholesterol-d7 | 0.12 ± 0.04 | 0.13 ± 0.03 | 0.1 ± 0.03 | −0.033 (0.0005 to −0.07) | 0.05 |
| Total cholesterol-d7 | 0.1 ± 0.031 | 0.11 ± 0.03 | 0.08 ± 0.03 | −0.03 (−0.002 to −0.06) | 0.037 | |
| Free cholesterol | −6.8 ± 10.9 | −5 ± 13.8 | −8.7 ± 7.5 | −3.7 (8.6 to −15.9) | 0.52 | |
| Total cholesterol | −15.7 ± 32.2 | −5.2 ± 38.4 | −26.2 ± 22.4 | −20.9 (13.5 to −55.5) | 0.21 | |
| Low caloric/fat diet | ||||||
| Δ 48 h—Baseline (week 4, day 7) | Free cholesterol-d7 | 0.1 ± 0.03 | 0.11 ± 0.009 | 0.085 ± 0.0052 | −0.028 (−0.004 to −0.051) | 0.03 |
| Total cholesterol-d7 | 0.09 ± 0.02 | 0.1 ± 0.01 | 0.07 ± 0.004 | −0.026 (−0.002 to −0.049) | 0.03 | |
| Free cholesterol | −5.1 ± 17.4 | −8.2 ± 21.9 | −1.9 ± 12 | 6.2 (25.7 to −13.3) | 0.50 | |
| Total cholesterol | −15.5 ± 51.8 | −16.7 ± 59.8 | −14.3 ± 46.5 | 2.5 (60.3 to −55.3) | 0.93 |
Δ cholesterol-d7 is considered as a rough estimation of intestinal cholesterol absorption. P value <0.05 is considered significant. CI, confidence interval. Boldfaced text represents statistically significant values.
Figure 2.
Variation in the estimated levels of absorbed cholesterol over three different dietary interventions. A: patients with normal weight. B: patients with obesity. Data are presented as means and SD. Significant values: **P < 0.01, and ***P < 0.001. ns, nonsignificant. HCHF, high calorie, high-fat diet; LCLF, low-calorie, low-fat diet; RD, regular diet.
Bile Acid Metabolism
The mean C4 level was significantly higher after HCHF than LCLF (23.3 ± 21.9 ng/mL vs. 8.3 ± 5.9 ng/mL; P = 0.01) (Fig. 3). BAC and FF excretion were measured at the end of each dietary intervention for all subjects (Table 3). There were no differences in the TBA, BAC, and fat excretion over various dietary interventions for each weight group.
Figure 3.
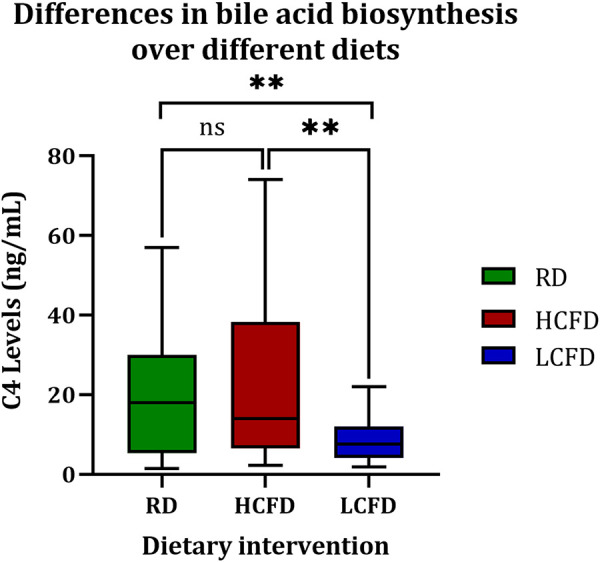
The level of bile acids synthesis according to the dietary intervention. **P < 0.01; ns, nonsignificant. C4, 7α-hydroxy-4-cholesten-3-one; HCHF, high calorie, high-fat diet; LCLF, low-calorie, low-fat diet; RD, regular diet.
Table 3.
Biomarkers of the hepatic bile acid synthesis, fecal bile acids amount and concentration, and fat excretion over different dietary interventions
| Variables | All | Normal Weight | Obesity | Difference (95% CI) | P Value |
|---|---|---|---|---|---|
| Hepatic bile acid biosynthesis [7α‐hydroxy‐4‐cholesten‐3‐one (C4)], ng/mL | |||||
| RD | 20.1 ± 17.2† | 15.3 ± 18.9 | 24.9 ± 14.9† | 9.6 (−8.7 to 28) | 0.28 |
| HCHF | 23.3 ± 21.9‡ | 19.1 ± 22 | 27.4 ± 22.4 | 8.3 (−15.5 to 32.1) | 0.47 |
| LCLF | 8.3 ± 5.9†‡ | 5.6 ± 3.3 | 10.6 ± 6.7‡ | 5 (−1.1 to 11.1) | 0.09 |
| Total bile acids, µmol/48 h | |||||
| RD | 517.8 ± 478.3 | 480.5 ± 489.2* | 555.1 ± 497.9 | 74.6 (−454.6 to 603.9) | 0.77 |
| HCHF | 444.3 ± 386.4 | 344.8 ± 445* | 543.9 ± 313.6 | 199.1 (−214.3 to 612.5) | 0.32 |
| LCLF | 493.3 ± 515.2 | 297 ± 237.3 | 717.6 ± 666.1 | 420.6 (−121.5 to 962.7) | 0.12 |
| Total fecal fat, g/day | |||||
| RD | 3.5 ± 1.4 | 4 ± 1.5* | 3 ± 1.2 | −1 (−2.3 to 0.47) | 0.16 |
| HCHF | 3.2 ± 1.8 | 2.4 ± 1.2* | 4.4 ± 1.6‡ | 2.1 (0.42 to 3.7) | 0.06 |
| LCLF | 2.4 ± 1.2 | 2.6 ± 1.4 | 2.1 ± 1.1‡ | −0.48 (−1.9 to 0.93) | 0.47 |
| Bile acid concentration, µmol/g/48 h | |||||
| RD | 2.5 ± 1.8 | 2.2 ± 1.7 | 2.8 ± 1.9 | 0.6 (−1.3 to 2.5) | 0.51 |
| HCHF | 2.2 ± 1.6 | 2.1 ± 2.2 | 2.2 ± 0.8 | 0.17 (−1.7 to 2.1) | 0.40 |
| LCLF | 2.4 ± 1.8 | 1.9 ± 0.8 | 2.9 ± 1.3 | 0.96 (−0.36 to 2.3) | 0.14 |
Data are presented as means ± SD. BMI, body mass index; CI, confidence interval; HCHF, high-caloric/high-fat diet; LCLF, low-caloric/low-fat diet; RD, regular diet.
RD vs. HCHF P < 0.05; †RD vs. LCLF P < 0.05; ‡LCLF vs. HCHF P < 0.05.
Interaction of Cholesterol Absorption and BA Metabolism
Table 4 details the correlation between cholesterol absorption and fecal bile acids and BA metabolism in all patients and groups. Based on the study results, the changes in cholesterol-d7 absorption (Δ Cholesterol-7d) between RD and HCHF were not significantly correlated, and they are independent when considering all patients in this study. However, the NW patients with higher changes in cholesterol-d7 absorption tend to show higher changes in C4 levels when going from RD to HCHF (R2 = 0.50; 0.04). The change in Cholesterol-7d absorption when ingesting RD compared with LCLF were not significantly correlated, but there was a nonsignificant numerical association between change in Cholesterol-7d and change in 48-h fecal bile acids. However, this trend was not observed when analyzed within the two patient groups.
Table 4.
Correlation of cholesterol absorption and fecal bile acids in normal weight and obesity
| Variables | All | Normal Weight | Obesity |
|---|---|---|---|
| Changes from baseline to high calories, high-fat diet | |||
| Δ Cholesterol-d7 vs. Δ 48 h fecal BA | R2 = 0.13, P = 0.17 | R2 = 0.17, P = 0.29 | R2 = 0.07, P = 0.41 |
| Δ Cholesterol-d7 vs. Δ C4 | R2 = 0.08, P = 0.28 | R2 = 0.50, P = 0.04 | R2 = 0.01, P = 0.79 |
| Δ Cholesterol-d7 vs. Δ fecal fat | R2 = 0.004, P = 0.79 | R2 = 0.17, P = 0.29 | R2 = 0.02, P = 0.72 |
| Changes from baseline to low calories, low-fat diet | |||
| Δ Cholesterol-d7 vs. Δ 48 h fecal BA | R2 = 0.29, P = 0.054 | R2 = 0.32, P = 0.14 | R2 = 0.17, P = 0.28 |
| Δ Cholesterol-d7 vs. Δ C4 | R2 = 0.004, P = 0.97 | R2 = 0.003, P = 0.89 | R2 = 0.01, P = 0.69 |
| Δ Cholesterol-d7 vs. Δ fecal fat | R2 = 0.004, P = 0.79 | R2 = 0.01, P = 0.80 | R2 = 0.16, P = 0.32 |
Data are presented as means ± SD. BA, bile acid; C4, 7α-hydroxy-4-cholesten-3-one. Boldfaced text represents statistically significant values.
Changes in Cholesterol-7d and fecal fat and C4 were not significantly correlated when changing from an RD to LCLF in all patients, and the two groups were analyzed separately.
DISCUSSION
Our nutritional interventional study demonstrates that intestinal cholesterol absorption is shifted after a short-term dietary modification in fat and caloric content. The increase in cholesterol absorption when shifting from a regular diet to a HCHF is observed in patients with normal weight and obesity. The reduction in cholesterol absorption from a HCHF to a LCLF, on the other hand, was only observed in normal-weight controls, emphasizing the role of body weight in this regulation. Furthermore, the modification of caloric and fat content had a direct and immediate impact on hepatic bile acid synthesis. Finally, changes in cholesterol absorption seen from a RD to a HCHF negatively correlated with the hepatic bile acid synthesis pathway in patients with normal weight.
We evaluated the relationship between macronutrient composition and cholesterol absorption and found that short-term dietary cholesterol modification can only impact intestinal absorption in patients with normal weight. This might be explained by improved absorption due to increased secretion and pancreatic sterol ester hydrolase activity, as found in patients with high-cholesterol diets (27, 28). The HCHF meals used in our study contained high cholesterol levels (300 mg cholesterol), in contrast to the LCLF (78 mg cholesterol), adding to the evidence that dietary cholesterol intake and intestinal absorption are linked. This was also observed by the study of Sehayek et al. (29), who examined the change in dietary cholesterol absorption in patients with normal weight in response to low-fat, low-cholesterol, high-fat, low-cholesterol, and high-fat, high-cholesterol diets and noticed that increased cholesterol intake increased dietary cholesterol mass absorption but not percentage dietary cholesterol absorption.
Obesity has been deemed to result in derangements in serum cholesterol levels, increasing endogenous cholesterol production by the liver proportional to body weight. Here, as previously reported, we observed a decreased absorption of total cholesterol-d7 (5). This might be explained by increased biliary lipids preventing tagged dietary cholesterol from entering the micellar phase, hence lowering labeled cholesterol absorption in obesity (5). In addition, decreased cholesterol absorption has been linked to insulin resistance, and the effects of insulin resistance on cholesterol absorption may be mediated by BMI (30). The decrease in dietary cholesterol absorption is accompanied by multiple molecular mechanisms that maintain cholesterol homeostasis. Particularly, the oxyterol feedback mechanism results in the activation of the sterol regulatory-element binding protein 2 (SREBP-2), which induces all enzymes of the cholesterol biosynthetic pathway (31).
We did not see a change in cholesterol absorption from an HCHF to an LCLF in patients with obesity, which is consistent with earlier studies that indicate that the quantity of dietary cholesterol had a more pronounced influence on cholesterol synthesis but no correlation with fractional cholesterol absorption. The observed lack of changes in cholesterol absorption from a HCHF to a LCLF can be explained by an already decreased cholesterol absorption and a dilution of the cholesterol-d7 resulting in reduced absorption efficiency of labeled cholesterol. Previously, it has been reported that patients with obesity and hypertriglyceridemia tended to increase cholesterol absorption efficiency after a low-calorie diet (32). However, the changes in cholesterol absorption observed in this cohort might be explained by weight loss rather than fat content modification.
Cholesterol and BA metabolism are strongly connected, as BA synthesis results from hepatic cholesterol catabolism. The intimately interconnected whole body cholesterol homeostasis involves the following major components: intestinal cholesterol absorption, hepatic de novo cholesterol synthesis, and cholesterol excretion from the body (33). In our study during HCHF, C4 levels were significantly elevated compared with LCLF, indicating that an increase in dietary fat/cholesterol will reduce intracellular cholesterol in the liver, thus stimulate de novo synthesis of cholesterol to maintain cholesterol and bile acid homeostasis. The increase in de novo synthesis of cholesterol will result in an increase in fecal bile acid secretion. Previously, low-caloric diets that resulted in weight loss were linked to decreased cholesterol synthesis in those who were overweight or had obesity (30, 34). We demonstrated that this impact might be mediated not just by weight loss but also by intestinal fat content. This increase in intestinal fat content with HCHF will ultimately enhance the secretion of higher levels of BAs from the liver to the duodenum compared with LCLF. Therefore, the higher release of BAs requires a higher hepatic production. Previously, Haeusler et al. (35) found that obesity was positively associated with increased hepatic BA biosynthesis. Increasing BA synthesis increases fecal bile acid excretion; nevertheless, we found no change in patients with obesity.
The analysis of changes in cholesterol-d7 and changes in BA metabolism and excretion showed that 50% of the variability in cholesterol absorption (serum cholesterol-d7) was explained by BA synthesis (serum C4 levels) in patients with normal weight. Here, we showed that in patients with normal weight, the change from a RD to HCHF, increased cholesterol absorption is correlated to increased bile acid synthesis. However, we did not observe this correlation in patients with obesity, suggesting different effects of short-term dietary cholesterol increase in the diet in patients with obesity. Finally, our study found a trend in the variability of cholesterol absorption and bile acid excretion when changing from RD to a LCLF diet. Previous research attempted to demonstrate the influence of dietary supplements on fecal bile acid excretion to lower cholesterol concentrations. We detected an intriguing link with a show-term calorie diet in our study; however, this association was not observed when we studied the individual groups. Because the sample size of BMI groups is restricted in this study, future research should establish a clear mechanistic cause-and-effect correlation between cholesterol absorption and BA production.
There was no association between the estimated cholesterol absorption and BA synthesis, regardless of the dietary intervention. This observation suggests that the production of BAs is positively linked with the biliary secretion of BA and cholesterol rather than intestinal cholesterol absorption (36).
Strengths and Limitations
Our study has multiple strengths. First, the fact that in our study meals are provided by the study team, we were able to minimize any confounding variables that might alter our results. We intentionally chose participants without any metabolic syndrome features other than obesity to rule out the effect of insulin resistance, dyslipidemia, and hypertension on cholesterol absorption and hepatic production of BAs and their fecal excretion. Second, we used precise and accurate methods to collect data regarding the exposure (e.g., dietary intake) and various end point results (e.g., cholesterol absorption) to establish an association between both.
Our study also has limitations. A major one was the sample size of our cohort. There were multiple results by which the statistical significance was not revealed, likely due to our study’s lack of adequate power. The serum levels of cholesterol biosynthesis were not measured as part of our protocol, this reduced the accuracy of our findings as the absorbed cholesterol might be affected by the endogenous production of cholesterol. Our protocol attempted to study the effects of diets on cholesterol absorption independent of weight loss in obesity compared with health; however, there was a statistically significant change in body weight between the HCHF and the LCLF, which could have influenced some of the findings. In addition to the shift in cholesterol percentage, there was a change in caloric composition, which may have contributed to the observed alterations.
Conclusions
In summary, a short-term decrease in dietary-fat content is associated with a decrease in intestinal absorption of dietary cholesterol; however, this response is blunted in patients with obesity. These dietary modifications also resulted in changes in bile acid synthesis, which were not associated with cholesterol absorption. Our findings highlight the effect of body weight and dietary intake on cholesterol absorption and the bile acid pathway.
GRANTS
M. Camilleri is supported by National Institutes of Health Grant RO1-DK67071. A. Acosta is supported by National Institutes of Health Grant K23-DK114460.
DISCLOSURES
Dr. Acosta is a stockholder in Gila Therapeutics, Phenomix Sciences; he is a consultant for Rhythm Pharmaceuticals, Amgen, Bausch Health, and General Mills. Dr. Camilleri is a stockholder in Phenomix Sciences and serves as a consultant to Takeda, Arena, Enterin.
AUTHOR CONTRIBUTIONS
A.M.H., G.C., M.L.R.-S., D.G.-I., A.M., M.D.H., M.C., and A.A. conceived and designed research; G.C., M.L.R.-S., D.G.-I., A.C., A.M., S.F., M.D.H., D.D.B., X.-M.P., I.R.L., and A.A. performed experiments; A.M.H., L.C., A.C., A.M., M.D.H., X.-M.P., I.R.L., and A.A. analyzed data; A.M.H., L.C., G.C., M.L.R.-S., D.G.-I., M.C., and A.A. interpreted results of experiments; A.M.H., L.C., and A.A. and prepared figures; A.M.H., L.C., and A.A. and drafted manuscript; A.M.H., L.C., A.C., S.F., M.D.H., D.D.B., X.-M.P., I.R.L., M.C., and A.A. edited and revised manuscript; A.M.H., L.C., G.C., M.L.R.-S., D.G.-I., A.C., A.M., S.F., M.D.H., D.D.B., X.-M.P., I.R.L., M.C., and A.A. approved final version of manuscript.
REFERENCES
- 1. Miettinen TA, Kesäniemi Y. Cholesterol absorption: regulation of cholesterol synthesis and elimination and within-population variations of serum cholesterol levels. Am J Clin Nutr 49: 629–635, 1989. doi: 10.1093/ajcn/49.4.629. [DOI] [PubMed] [Google Scholar]
- 2. Paramsothy P, Knopp RH, Kahn SE, Retzlaff BM, Fish B, Ma L, Ostlund RE. Plasma sterol evidence for decreased absorption and increased synthesis of cholesterol in insulin resistance and obesity. Am J Clin Nutr 94: 1182–1188, 2011. doi: 10.3945/ajcn.110.006668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nestel P, Goldrick B. Obesity: changes in lipid metabolism and the role of insulin. Clin Endocrinol Metab 5: 313–335, 1976. doi: 10.1016/s0300-595x(76)80024-2. [DOI] [PubMed] [Google Scholar]
- 4. Angelin B, Backman L, Einarsson K, Eriksson L, Ewerth S. Hepatic cholesterol metabolism in obesity: activity of microsomal 3-hydroxy-3-methylglutaryl coenzyme A reductase. J Lipid Res 23: 770–773, 1982. [PubMed] [Google Scholar]
- 5. Miettinen TA, Gylling H. Cholesterol absorption efficiency and sterol metabolism in obesity. Atherosclerosis 153: 241–248, 2000. doi: 10.1016/S0021-9150(00)00404-4. [DOI] [PubMed] [Google Scholar]
- 6. Pinal-Fernandez I, Casal-Dominguez M, Mammen AL. Statins: pros and cons. Med Clin (Barc) 150: 398–402, 2018. doi: 10.1016/j.medcli.2017.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Patel J, Sheehan V, Gurk-Turner C. Ezetimibe (Zetia): a new type of lipid-lowering agent. Proc (Bayl Univ Med Cent) 16: 354–358, 2003. doi: 10.1080/08998280.2003.11927928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sudhop T, LüTjohann D, Kodal A, Igel M, Tribble DL, Shah S, Perevozskaya I, von Bergmann K. Inhibition of intestinal cholesterol absorption by ezetimibe in humans. Circulation 106: 1943–1948, 2002. doi: 10.1161/01.CIR.0000034044.95911.DC. [DOI] [PubMed] [Google Scholar]
- 9. Ehnholm C, Huttunen JK, Pietinen P, Leino U, Mutanen M, Kostiainen E, Pikkarainen J, Dougherty R, Iacono J, Puska P. Effect of diet on serum lipoproteins in a population with a high risk of coronary heart disease. N Engl J Med 307: 850–855, 1982. doi: 10.1056/NEJM198209303071403. [DOI] [PubMed] [Google Scholar]
- 10. Camilleri M, Gores GJ. Therapeutic targeting of bile acids. Am J Physiol Gastrointest Liver Physiol 309: G209–G215, 2015. doi: 10.1152/ajpgi.00121.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chiang JY. Bile acid metabolism and signaling. Compr Physiol 3: 1191–1212, 2013. doi: 10.1002/cphy.c120023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ahmad TR, Haeusler RA. Bile acids in glucose metabolism and insulin signalling—mechanisms and research needs. Nat Rev Endocrinol 15: 701–712, 2019. doi: 10.1038/s41574-019-0266-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Xie C, Huang W, Young RL, Jones KL, Horowitz M, Rayner CK, Wu T. Role of bile acids in the regulation of food intake, and their dysregulation in metabolic disease. Nutrients 13: 1104, 2021. doi: 10.3390/nu13041104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. de Boer JF, Bloks VW, Verkade E, Heiner-Fokkema MR, Kuipers F. New insights in the multiple roles of bile acids and their signaling pathways in metabolic control. Curr Opin Lipidol 29: 194–202, 2018. doi: 10.1097/MOL.0000000000000508. [DOI] [PubMed] [Google Scholar]
- 15. Mertens KL, Kalsbeek A, Soeters MR, Eggink HM. Bile acid signaling pathways from the enterohepatic circulation to the central nervous system. Front Neurosci 11: 617, 2017. doi: 10.3389/fnins.2017.00617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Calderon G, McRae A, Rievaj J, Davis J, Zandvakili I, Linker-Nord S, Burton D, Roberts G, Reimann F, Gedulin B, Vella A, LaRusso NF, Camilleri M, Gribble FM, Acosta A. Ileo-colonic delivery of conjugated bile acids improves glucose homeostasis via colonic GLP-1-producing enteroendocrine cells in human obesity and diabetes. EBioMedicine 55: 102759, 2020. doi: 10.1016/j.ebiom.2020.102759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wu T, Bound M, Standfield S, Gedulin B, Jones K, Horowitz M, Rayner C. Effects of rectal administration of taurocholic acid on glucagon-like peptide-1 and peptide YY secretion in healthy humans. Diabetes Obes Metab 15: 474–477, 2013. doi: 10.1111/dom.12043. [DOI] [PubMed] [Google Scholar]
- 18. Blüher M. Metabolically healthy obesity. Endocr Rev 41: 405–420, 2020. doi: 10.1210/endrev/bnaa004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Matthan NR, Lichtenstein AH. Approaches to measuring cholesterol absorption in humans. Atherosclerosis 174: 197–205, 2004. doi: 10.1016/S0021-9150(03)00248-X. [DOI] [PubMed] [Google Scholar]
- 20. Santosa S, Demonty I, Lichtenstein AH, Jones PJ. Cholesterol metabolism and body composition in women: the effects of moderate weight loss. Int J Obes 31: 933–941, 2007. doi: 10.1038/sj.ijo.0803549. [DOI] [PubMed] [Google Scholar]
- 21. Beaumier-Gallon G, Dubois C, Portugal H, Lairon D. Postprandial studies on dietary cholesterol in human subjects using stable isotopes and gas chromatography–mass spectrometry analysis. Atherosclerosis 141: S81–S85, 1998. doi: 10.1016/S0021-9150(98)00223-8. [DOI] [PubMed] [Google Scholar]
- 22. Bosner MS, Ostlund R, Osofisan O, Grosklos J, Fritschle C, Lange LG. Assessment of percent cholesterol absorption in humans with stable isotopes. J Lipid Res 34: 1047–1053, 1993. doi: 10.1016/S0022-2275(20)39690-5. [DOI] [PubMed] [Google Scholar]
- 23. Vijayvargiya P, Camilleri M, Taylor A, Busciglio I, Loftus EV Jr, Donato LJ. Combined fasting serum C4 and primary bile acids from a single stool sample to diagnose bile acid diarrhea. Gastroenterology 159: 1952–1954.e2, 2020. doi: 10.1053/j.gastro.2020.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gälman C, Angelin B, Rudling M. Bile acid synthesis in humans has a rapid diurnal variation that is asynchronous with cholesterol synthesis. Gastroenterology 129: 1445–1453, 2005. doi: 10.1053/j.gastro.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 25. Donato LJ, Lueke A, Kenyon SM, Meeusen JW, Camilleri M. Description of analytical method and clinical utility of measuring serum 7-alpha-hydroxy-4-cholesten-3-one (7aC4) by mass spectrometry. Clin Biochem 52: 106–111, 2018. doi: 10.1016/j.clinbiochem.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 26. Tagliacozzi D, Mozzi AF, Casetta B, Bertucci P, Bernardini S, Ilio CD, Urbani A, Federici G. Quantitative analysis of bile acids in human plasma by liquid chromatography-electrospray tandem mass spectrometry: a simple and rapid one-step method. Clin Chem Lab Med 41: 1633–1641, 2003. doi: 10.1515/CCLM.2003.247. [DOI] [PubMed] [Google Scholar]
- 27. Nissinen MJ, Gylling H, Miettinen TA. Effects of plant stanol esters supplied in a fat free milieu by pastilles on cholesterol metabolism in colectomized human subjects. Nutr Metab Cardiovasc Dis 16: 426–435, 2006. doi: 10.1016/j.numecd.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 28. Nissinen MJ, Gylling H, Miettinen TA. Responses of surrogate markers of cholesterol absorption and synthesis to changes in cholesterol metabolism during various amounts of fat and cholesterol feeding among healthy men. Br J Nutr 99: 370–378, 2008. doi: 10.1017/S0007114507811998. [DOI] [PubMed] [Google Scholar]
- 29. Sehayek E, Nath C, Heinemann T, McGee M, Seidman CE, Samuel P, Breslow JL. U-shape relationship between change in dietary cholesterol absorptionand plasma lipoprotein responsiveness and evidence for extreme interindividualvariation in dietary cholesterol absorption in humans. J Lipid Res 39: 2415–2422, 1998. doi: 10.1016/S0022-2275(20)33320-4. [DOI] [PubMed] [Google Scholar]
- 30. Simonen PP, Gylling H, Miettinen TA. Body weight modulates cholesterol metabolism in non-insulin dependent type 2 diabetics. Obes Res 10: 328–335, 2002. doi: 10.1038/oby.2002.46. [DOI] [PubMed] [Google Scholar]
- 31. Shimano H, Sato R. SREBP-regulated lipid metabolism: convergent physiology—divergent pathophysiology. Nat Rev Endocrinol 13: 710–730, 2017. doi: 10.1038/nrendo.2017.91. [DOI] [PubMed] [Google Scholar]
- 32. Kudchodkar BJ, Sodhi H, Mason D, Borhani N. Effects of acute caloric restriction on cholesterol metabolism in man. Am J Clin Nutr 30: 1135–1146, 1977. doi: 10.1093/ajcn/30.7.1135. [DOI] [PubMed] [Google Scholar]
- 33. Nemes K, Åberg F, Gylling H, Isoniemi H. Cholesterol metabolism in cholestatic liver disease and liver transplantation: from molecular mechanisms to clinical implications. World J Hepatol 8: 924–932, 2016. doi: 10.4254/wjh.v8.i22.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Di Buono M, Hannah JS, Katzel LI, Jones PJ. Weight loss due to energy restriction suppresses cholesterol biosynthesis in overweight, mildly hypercholesterolemic men. J Nutr 129: 1545–1548, 1999. doi: 10.1093/jn/129.8.1545. [DOI] [PubMed] [Google Scholar]
- 35. Haeusler RA, Camastra S, Nannipieri M, Astiarraga B, Castro-Perez J, Xie D, Wang L, Chakravarthy M, Ferrannini E. Increased bile acid synthesis and impaired bile acid transport in human obesity. J Clin Endocrinol Metab 101: 1935–1944, 2016. doi: 10.1210/jc.2015-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Li T, Matozel M, Boehme S, Kong B, Nilsson LM, Guo G, Ellis E, Chiang JY. Overexpression of cholesterol 7α-hydroxylase promotes hepatic bile acid synthesis and secretion and maintains cholesterol homeostasis. Hepatology 53: 996–1006, 2011. doi: 10.1002/hep.24107. [DOI] [PMC free article] [PubMed] [Google Scholar]