Abstract
Background:
Radiation therapy (RT) is used to treat many adolescent and young adult (AYA) and childhood cancer patients and is a risk factor for secondary breast cancer (BC). While premenopausal BC is inherently more aggressive, no studies to date have evaluated the characteristics and breast cancer specific survival (BCSS) of premenopausal secondary BC after RT in AYA and childhood cancer survivors.
Methods:
Female patients ages 12–50 diagnosed with primary BC during 1988–2014 (n=107,751) were obtained from the California Cancer Registry and compared with similar aged secondary BC patients who were treated with RT for their primary tumor (n=1,147) from ages 12–39. We examined BCSS using multivariable Cox proportional hazards regression.
Results:
The secondary BC cohort was more likely to be Hispanic or Black, 35–45 years of age, have earlier stage tumors, be higher grade, have no lymph node (LN) involvement, and hormone-receptor negative. All women showed worse BCSS for large tumor size, LN involvement, and hormone-receptor negative status. BCSS was worse for women with secondary BC both overall (HR: 1.98; 95% CI 1.77–2.23) and in all subgroups considered. Associations were most pronounced in Hispanics, Asian/Pacific Islanders, and younger women as well as those with earlier stage, lymph node negative, and hormone-receptor positive disease.
Conclusions:
BCSS is significantly decreased among all survivors of childhood and AYA cancer treated with RT that develop a secondary BC, including women with good prognostic features.
Impact:
Therefore, we may need to consider alternative and even more aggressive treatment in what were considered low risk populations previously.
Keywords: children, adolescent, young adult, secondary breast cancer, survival
Introduction:
In the adolescent and young adult (AYA) female population, breast cancer is the most common cause of cancer-related death.1 This is compounded when the breast cancer is a secondary malignancy.2 Multiple studies have shown that the AYA population has the highest absolute excess risk for secondary malignancies of any age group, including most commonly breast cancer.3, 4 Sadler and Goldfarb showed that within this demographic primary and secondary malignancies have different characteristics, including that secondary breast cancers present at earlier stages.5 However, even with improved stage, being diagnosed with a secondary breast cancer was an independent risk factor for worse overall survival in multivariable analysis.
Radiation therapy is integral to the multidisciplinary therapy used to treat common AYA and childhood cancers, such as Hodgkin lymphoma, sarcomas, and breast cancer. Even though radiation is central to the treatment of these malignancies, it is well-established that it is also a strong risk factor for a secondary breast malignancy, especially when used to treat childhood or AYA cancer patients.3, 6 Additionally it has been shown that for women treated for a primary breast cancer prior to 40 years of age, this risk of secondary malignancy applies to not only the ipsilateral breast, but also to the contralateral breast at a much higher rate than their older counterparts.7 These secondary breast malignancies after radiation are thought to have unique clinical characteristics, as well as distinct gene expression profiles.3, 8, 9 However, little is known about how much of these distinct features are due to being diagnosed while still being premenopausal versus the radiation therapy itself.
Therefore, we sought to better understand how radiation used to treat primary malignancies affects the clinical characteristics of secondary breast malignancies developed in the premenopausal setting. Using data from the large population-based California Cancer Registry (CCR), we examine demographic and clinical characteristics and breast cancer-specific survival (BCSS) in premenopausal patients with secondary breast malignancies compared to similar aged counterparts with primary breast cancer. Findings from this study will help us to better understand this unique group of secondary cancers allowing us in future to better tailor treatments for this population.
Methods:
Patients
Our data was extracted from the CCR which is estimated to represent more than 99% of all invasive cancers diagnosed in California. We included in our analysis female residents of California diagnosed with an invasive first and only invasive breast cancer or breast cancer as a second primary invasive cancer (hereafter referred to as secondary breast cancer) between 12–50 years of age during the period January 1, 1988, through December 31, 2014 (International Classification of Disease for Oncology, 3rd Edition, (ICD-O-3) site codes C50.0–50.9 (excluding codes for sarcoma, melanoma, neuroendocrine tumors, sweat gland tumors, and lymphoma). This age range was chosen to capture premenopausal breast cancer based on approximations of age at menarche and menopause.10 Women with secondary breast cancers were limited to those who had a known first primary cancer treated with radiation between the ages of 12–39 years to capture radiation exposure during breast development. Secondary breast cancers diagnosed within 2 months of the first primary cancer were excluded to remove what were likely multiple primary tumors. In addition, for women with two breast cancers, the secondary breast cancer had to have a different histology from the first primary, occur in the contralateral breast, or occur >5 years from the first primary.2, 11
From the CCR database, we obtained information routinely recorded in the medical record at diagnosis for each primary and secondary breast cancer patient on age at diagnosis, race/ethnicity (non-Hispanic White, Hispanic, non-Hispanic Black, and Asian or Pacific Islander, other/unknown), American Joint Committee on Cancer (AJCC) stage, tumor grade, histology, tumor size, lymph node involvement, estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER-2) tumor-expression status and sequence of primary cancer. The CCR has collected information on ER and PR since 1990 and on HER-2 since 1999.8, 12 Because of HER-2 data completeness, we limited our analyses of HER-2 data to women diagnosed after 2003. ER/PR was defined as positive if either ER or PR was reported as positive and triple-negative was defined as ER, PR, and HER-2 negative.
We also obtained registry information for each primary and secondary breast cancer patient on initial course of treatment (both initial and subsequent if applicable), census-block group of residence at diagnosis (used to determine socioeconomic status (SES)) 12–14, and vital status (routinely determined by the CCR through hospital follow-up and database linkages, including the Social Security Administration) as of December 31, 2014, and, for the deceased, the underlying cause of death.
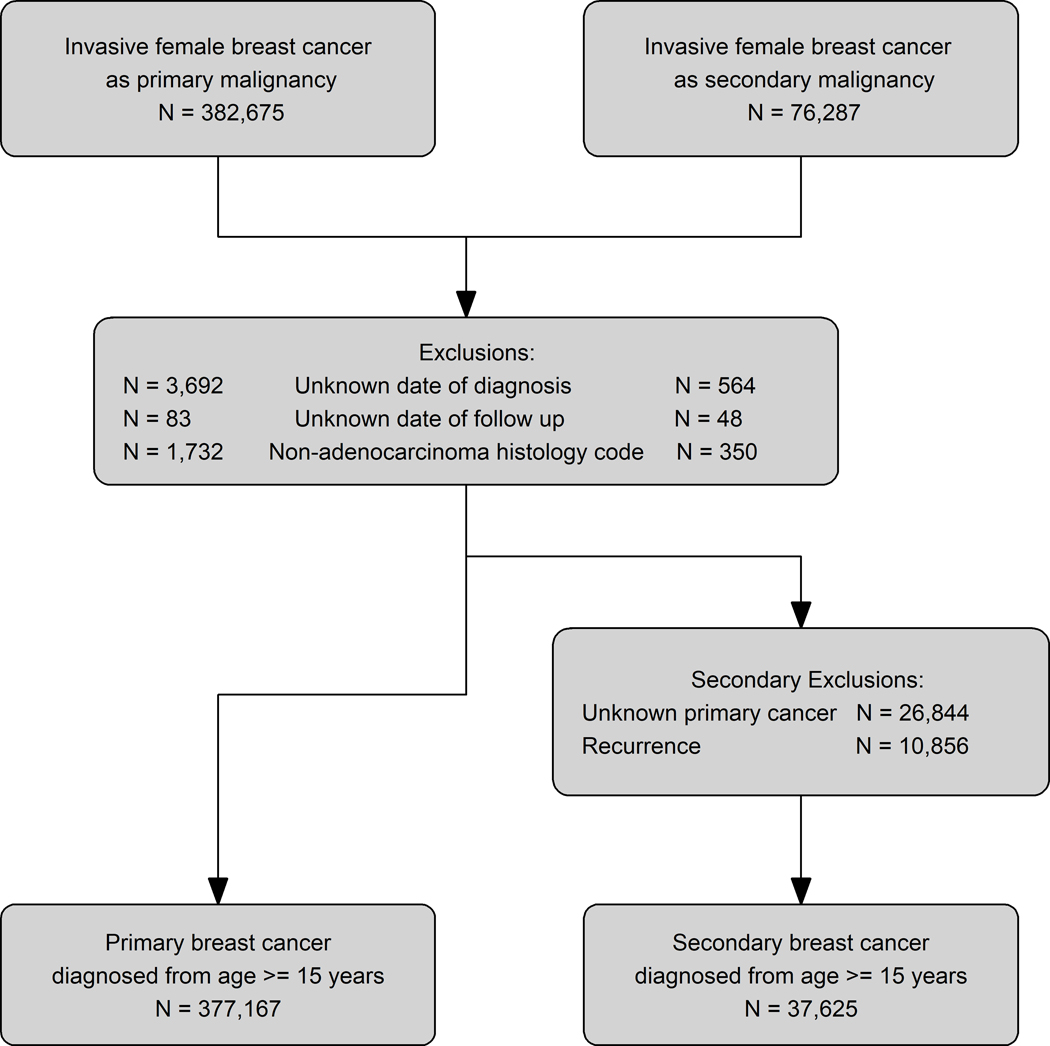
Of the 476,130 California females diagnosed with a first and only, or secondary, invasive breast cancer, we excluded patients with unknown date of diagnosis/follow-up and the noted above histology codes. The resulting study population of 12–50 year old females included 107,751 women with a first and only breast cancer and 1,147 women with a secondary breast cancer after being treated between ages 12 and 39 years with radiation for their primary malignancy (Figure 1). This study was approved by University of California, Davis Institutional Review Board and by the California Committee for the Protection of Human Subjects.
Figure 1: Selection of Primary and Secondary Breast Cancer Cohorts from the California Cancer Registry (1988–2014).
Exclusions are for the primary and secondary breast cancer diagnoses. Secondary exclusions are for women with secondary breast cancers.
Statistical Analysis
Multivariable logistic regression was used to compare demographic (race/ethnicity, age) and clinical (grade, histology, tumor size, lymph node involvement, ER, PR and HER-2 status) factors between women with secondary versus primary breast cancer. Results are presented as odds ratio (OR) and corresponding 95% confidence intervals (CI).
Multivariable Cox proportional hazards regression models were used to evaluate associations of demographic and clinical factors with breast cancer-specific survival (BCSS) for women with primary and secondary breast cancer separately. In addition, multivariable Cox proportional hazards regression models assessed the associations of secondary versus primary breast cancer on BCSS for all women and in subgroups defined by race/ethnicity, age, AJCC stage, lymph node involvement, and ER, PR and HER-2 status. Effect modification was assessed between secondary breast cancer and each subgroup by including interaction terms in the multivariable models. For deceased patients, survival time was measured from diagnosis date of the primary or secondary breast cancer to the date of death from breast cancer. Patients who died from other causes were censored at the time of death. Patients alive at the study end date (12/31/2014) were censored at this date or at last known contact.
In all survival models, the proportional hazards assumption was assessed numerically based on cumulative sums of Martingale residuals and visually based on inspection of the survival curves [log (−log) of the survival distribution function by log (months)]; variables that violated this assumption were included as stratifying variables to allow for differing baseline hazards associated with these variables (chemotherapy, radiation, surgery, regional lymph nodes examined). Sensitivity analyses of BCSS examined: 1) death from other causes as a competing risk; and 2) the impact of excluding patients who were unlikely to receive chest or total body radiation. Results are presented as hazard ratios (HR) and 95% confidence intervals (CI). Statistical analyses were performed using SAS statistical software (version 9.4), and a 2-sided P value of less than 0.05 was considered statistically significant.
Results:
A total of 1,147 females ages 12–50 were diagnosed with an invasive breast cancer as a secondary malignancy after being treated with radiation for their primary malignancy. When comparing this group with women of the same age who were diagnosed with primary breast cancer (n=107,751), some differences were seen between the cohorts (Table 1). The secondary malignancy cohort was more likely of non-Hispanic Black (10.3% vs 7.4%) or Hispanic (26.4% vs. 22.2%) race/ethnicity, between 35–45 years of age, have earlier stage tumors (stage I, 45.5% vs 33.4%), be higher grade (grade III, 49.3% vs 39.5%), have no lymph node involvement (65.7% vs 53.4%), and be ER/PR negative (ER, 36.2% vs 22.2%; PR, 41.8% vs 26.8%). For treatment, the secondary malignancy cohort was less likely to be treated with chemotherapy (55.4% vs 62.2%) or radiation (26.3% vs 45.5%), but more likely get a mastectomy (63.0% vs 50.2%).
Table 1:
Characteristics and Treatment of Patients with Primary and Secondary Breast Cancer from the California Cancer Registry (1988–2014)
| Only primary Breast Cancer | Secondary Breast Cancer | |
|---|---|---|
| N= 107,751 | N= 1,147 | |
| Characteristics | N (%) | N (%) |
|
| ||
| Race/ethnicity | ||
| Non-Hispanic White | 59,430 (55.2) | 588 (51.3) |
| Non-Hispanic Black | 7,990 (7.4) | 118 (10.3) |
| Hispanic | 23,950 (22.2) | 303 (26.4) |
| Asian/Pacific Islander | 15,009 (13.9) | 137 (11.9) |
| Other/Unknown | 1,372 (1.3) | 1 (0.1) |
|
| ||
| Age at Diagnosis | ||
| 12–24 | 320 (0.3) | 5 (0.4) |
| 25–29 | 2,041 (1.9) | 19 (1.7) |
| 30–34 | 6,915 (6.4) | 78 (6.8) |
| 35–39 | 15,468 (14.4) | 268 (23.4) |
| 40–44 | 30,333 (28.2) | 430 (37.5) |
| 45–50 | 52,674 (48.9) | 347 (30.3) |
|
| ||
| Year of diagnosis | ||
| 1988–1994 | 21,639 (20.1) | 62 (5.4) |
| 1995–2001 | 26,914 (25.0) | 251 (21.9) |
| 2002–2008 | 31,180 (28.9) | 412 (35.9) |
| 2009–2014 | 28,018 (26.0) | 422 (36.8) |
|
| ||
| Neighborhood socioeconomic status | ||
| Low SES | 53,483 (49.6) | 551 (48.0) |
| High SES | 54,268 (50.4) | 596 (52.0) |
|
| ||
| AJCC Stage | ||
| Stage I | 35,991 (33.4) | 522 (45.5) |
| Stage II | 45,942 (42.6) | 355 (31.0) |
| Stage III | 12,886 (12.0) | 117 (10.2) |
| Stage IV | 4,555 (4.2) | 85 (7.4) |
| Unknown | 8,377 (7.8) | 68 (5.9) |
|
| ||
| Chemotherapy | ||
| Yes | 66,975 (62.2) | 635 (55.4) |
| No/Unknown | 40,776 (37.8) | 512 (44.6) |
| Radiation | ||
| Yes | 49,032 (45.5) | 302 (26.3) |
| No/Unknown | 58,719 (54.5) | 845 (73.7) |
| Surgery | ||
| Lumpectomy | 46,960 (43.6) | 321 (28.0) |
| Mastectomy | 54,092 (50.2) | 723 (63.0) |
| None | 5,960 (5.5) | 99 (8.6) |
| Unknown | 739 (0.7) | 4 (0.3) |
|
| ||
| Tumor grade | ||
| Grade I | 13,390 (12.4) | 112 (9.8) |
| Grade II | 36,023 (33.4) | 328 (28.6) |
| Grade III | 42,538 (39.5) | 566 (49.3) |
| Undifferentiated | 2,349 (2.2) | 33 (2.9) |
| Unknown | 13,451 (12.5) | 108 (9.4) |
|
| ||
| Histology | ||
| Ductal | 83,516 (77.5) | 897 (78.2) |
| Lobular | 14,644 (13.6) | 142 (12.4) |
| Other | 9,591 (8.9) | 108 (9.4) |
|
| ||
| Tumor size | ||
| T1a: ≤0.5cm | 5,925 (5.5) | 112 (9.8) |
| T1b: >0.5–1cm | 11,763 (10.9) | 179 (15.6) |
| T1c: >1–2cm | 33,970 (31.5) | 359 (31.3) |
| T2: >2–5cm | 38,180 (35.4) | 322 (28.1) |
| T3: >5.00 cm | 9,421 (8.7) | 70 (6.1) |
| Diffuse | 1,725 (1.6) | 18 (1.6) |
| Other | 6,767 (6.3) | 87 (7.6) |
|
| ||
| Regional lymph node involvement | ||
| Positive | 46,089 (42.8) | 327 (28.5) |
| Negative | 57,562 (53.4) | 754 (65.7) |
| Unknown | 4,100 (3.8) | 66 (5.8) |
|
| ||
| Regional nodes examined | ||
| Sentinel Lymph Node Biopsy (SLNB) | 31,986 (29.7) | 413 (36.0) |
| Axillary Lymph Node Dissection (ALND) | 65,484 (60.8) | 418 (36.4) |
| No nodes examined/unknown | 10,281 (9.5) | 316 (27.6) |
|
| ||
| Estrogen Receptor(ER)status | ||
| Positive | 63,749 (59.2) | 583 (50.8) |
| Negative | 23,926 (22.2) | 415 (36.2) |
| Unknown | 20,076 (18.6) | 149 (13.0) |
| Progesterone Receptor(PR)status | ||
| Positive | 57,160 (53.0) | 510 (44.5) |
| Negative | 28,884 (26.8) | 479 (41.8) |
| Unknown | 21,707 (20.1) | 158 (13.8) |
| HER-2* | ||
| Positive | 12,056 (21.9) | 151 (19.1) |
| Negative | 37,222 (67.6) | 541 (68.6) |
| Unknown | 5,800 (10.5) | 97 (12.3) |
|
| ||
| Vital status | ||
| Alive | 83,346 (77.4) | 799 (69.7) |
| Death from breast cancer | 20,558 (19.1) | 298 (26.0) |
| Death from other causes | 3.847 (3.6) | 50 (4.4) |
HER-2 data is limited to 2003+ diagnoses, N (only primary) = 55,078, N (secondary) = 789
SES=Socioeconomic status; AJCC=American Joint Committee on Cancer; HER-2=human epidermal growth factor receptor-2.
In the multivariable logistic regression model, non-Hispanic Black women were more likely (OR: 1.35; 95% CI: 1.11–1.66) and Asian/Pacific Islander women were less likely (OR: 0.76; CI 0.63–0.92) to have a secondary breast cancer than non-Hispanic White women (Table 2). In addition, secondary breast cancers were more likely to be diagnosed at an earlier tumor size (T3 vs T1a, OR: 0.38; 95% CI 0.28–0.52) and without lymph node involvement (lymph node positive vs negative, OR: 0.58; 95% CI 0.51–0.67), but more likely to be ER/PR negative (OR: 1.76; 95% CI 1.52–2.03) and have a higher grade (grade III vs I, OR: 1.76; 95% CI 1.41–2.21). Differences by age and histology were not observed among young women with primary versus secondary breast cancer.
Table 2.
Adjusted* Logistic Regression Model of Factors Associated with having Secondary Breast Cancer compared with Primary Breast Cancer
| Characteristics | Odds ratios |
|---|---|
|
| |
| Race/ethnicity | |
| Non-Hispanic White | Reference |
| Non-Hispanic Black | 1.35 (1.11, 1.66) |
| Hispanic | 1.05 (0.91, 1.21) |
| Asian/Pacific Islander | 0.76 (0.63, 0.92) |
|
| |
| Age (years) | |
| 12–24 | Reference |
| 25–29 | 0.62 (0.23, 1.67) |
| 30–34 | 0.79 (0.32, 1.98) |
| 35–39 | 1.23 (0.50, 3.01) |
| 40–44 | 0.96 (0.39, 2.34) |
| 45–50 | 0.42 (0.17, 1.04) |
|
| |
| Year of diagnosis | |
| 1988–1994 | Reference |
| 1995–2001 | 3.47 (2.60, 4.62) |
| 2002–2008 | 5.34 (4.02, 7.09) |
| 2009–2014 | 6.56 (4.91, 8.75) |
|
| |
| Tumor grade | |
| Grade I | Reference |
| Grade II | 1.29 (1.03, 1.60) |
| Grade III | 1.76 (1.41, 2.21) |
| Undifferentiated | 1.82 (1.22, 2.73) |
|
| |
| Histology | |
| Ductal | Reference |
| Lobular | 1.16 (0.97, 1.40) |
|
| |
| Tumor size | |
| T1a: ≤0.5cm | Reference |
| T1b: >0.5–1cm | 0.97 (0.76, 1.24) |
| T1c: >1–2cm | 0.61 (0.49, 0.76) |
| T2: >2–5cm | 0.43 (0.34, 0.55) |
| T3: >5.00 cm | 0.38 (0.28, 0.52) |
| Diffuse | 0.58 (0.34, 0.99) |
|
| |
| Regional lymph node involvement | |
| Positive | 0.58 (0.51, 0.67) |
| Negative | Reference |
|
| |
| ER and PR status | |
| Positive | Reference |
| Negative | 1.76 (1.52, 2.03) |
The estimates of odds ratios for unknown groups are not present.
Models adjusted for all variables in the table.
CI=confidence interval; ER=estrogen receptor; PR=progesterone receptor.
Table 3 shows two separate multivariable cox models for women with primary and secondary breast cancer. For primary breast cancer, non-Hispanic Black women (HR: 1.41; 95% CI 1.34–1.47) had worse breast cancer specific survival (BCSS), while non-Hispanic Asian/Pacific Islander (HR: 0.89; 95% CI 0.85–0.94) and Hispanic (HR: 0.96; 95% CI 0.92–0.99) women had improved BCSS compared with non-Hispanic White women. However, among women with secondary breast cancer, Hispanic women experienced worse BCSS (HR: 1.38; 95% CI 1.02–1.87) and Asian/Pacific Islander women had similar BCSS compared with non-Hispanic White women. The associations for non-Hispanic Black women were similar to primary breast cancers; however, the HR for non-Hispanic Black women with secondary BC is not statistically significant possibly due to the much smaller size of the secondary BC population. In both the models, larger tumor size and lymph node involvement demonstrated worse BCSS. In examining the effect of tumor markers on BCSS, ER/PR negative (when HER-2 status was not clarified) and triple negative tumors were found to have worse prognosis, whereas HER-2 negativity worsened BCSS only for primary breast cancers (HR: 1.31; 95% CI 1.22–1.40). Additionally, tumor grade made no difference on BCSS for secondary breast cancers.
Table 3.
Factors Associated with Breast Cancer-Specific Survival* among Patients with Invasive Breast Cancer
| Characteristics | Only primary | Secondary |
|---|---|---|
| HR (95% CI) | HR (95% CI) | |
|
| ||
| Race/ethnicity | ||
| Non-Hispanic White | Reference | Reference |
| Non-Hispanic Black | 1.41 (1.34, 1.47) | 1.33 (0.88, 1.99) |
| Hispanic | 0.96 (0.92, 0.99) | 1.38 (1.02, 1.87) |
| Asian/Pacific Islander | 0.89 (0.85, 0.94) | 1.22 (0.78, 1.89) |
|
| ||
| Age (years) | ||
| 12–24 | Reference | Reference |
| 25–29 | 1.19 (0.95, 1.48) | 0.87 (0.15, 5.09) |
| 30–34 | 1.08 (0.88, 1.33) | 0.63 (0.13, 3.10) |
| 35–39 | 1.05 (0.85, 1.28) | 0.90 (0.19, 4.33) |
| 40–44 | 0.92 (0.75, 1.13) | 0.49 (0.10, 2.36) |
| 45–50 | 0.92 (0.75, 1.13) | 0.27 (0.06, 1.36) |
|
| ||
| Year of diagnosis | ||
| 1988–1994 | Reference | Reference |
| 1995–2001 | 0.75 (0.72, 0.77) | 0.66 (0.43, 1.02) |
| 2002–2008 | 0.55 (0.53, 0.58) | 0.57 (0.36, 0.90) |
| 2009–2014 | 0.46 (0.43, 0.49) | 0.53 (0.31, 0.90) |
|
| ||
| Neighborhood socioeconomic status | ||
| Low SES | 1.22 (1.19, 1.26) | 1.42 (1.09, 1.86) |
| High SES | Reference | Reference |
|
| ||
| Tumor grade | ||
| Grade I | Reference | Reference |
| Grade II | 2.04 (1.87, 2.23) | 1.77 (0.89, 3.49) |
| Grade III | 2.86 (2.61, 3.12) | 1.96 (0.99, 3.85) |
| Undifferentiated | 2.77 (2.47, 3.11) | 2.05 (0.79, 5.34) |
|
| ||
| Histology | ||
| Ductal | Reference | Reference |
| Lobular | 1.02 (0.97, 1.06) | 2.09 (1.45, 3.01) |
|
| ||
| Tumor size | ||
| T1a: ≤ 0.5cm | Reference | Reference |
| T1b: > 0.5–1cm | 1.26 (1.08, 1.47) | 1.33 (0.62, 2.87) |
| T1c: > 1–2cm | 1.93 (1.68, 2.22) | 2.07 (1.04, 4.10) |
| T2: > 2–5cm | 2.89 (2.52, 3.32) | 2.92 (1.46, 5.82) |
| T3: > 5.00 cm | 4.16 (3.61, 4.79) | 4.30 (1.99, 9.33) |
| Diffuse | 6.42 (5.51, 7.48) | 10.16 (3.74, 27.59) |
|
| ||
| Regional lymph node involvement | ||
| Positive | 2.52 (2.43, 2.61) | 2.10 (1.50, 2.95) |
| Negative | Reference | Reference |
|
| ||
| ER and PR status | ||
| Positive | Reference | Reference |
| Negative | 1.39 (1.34, 1.44) | 1.51 (1.11, 2.07) |
|
| ||
| HER-2** | ||
| Positive | Reference | Reference |
| Negative | 1.31 (1.22, 1.40) | 1.15 (0.68, 1.93) |
|
| ||
| Triple negative** | ||
| Yes | Reference | Reference |
| No | 0.51 (0.48, 0.55) | 0.42 (0.27, 0.67) |
Adjusted for all the variables in the table and stratified by chemotherapy, radiation, surgery and regional nodes examined; The estimates of hazard ratios (HRs) for unknown groups are not present.
HER-2 and triple-negative are limited to 2003+ diagnoses
CI= confidence interval; SES= socioeconomic status; ER= Estrogen Receptor; PR= Progesterone Receptor; HER-2= human epidermal growth factor receptor-2.
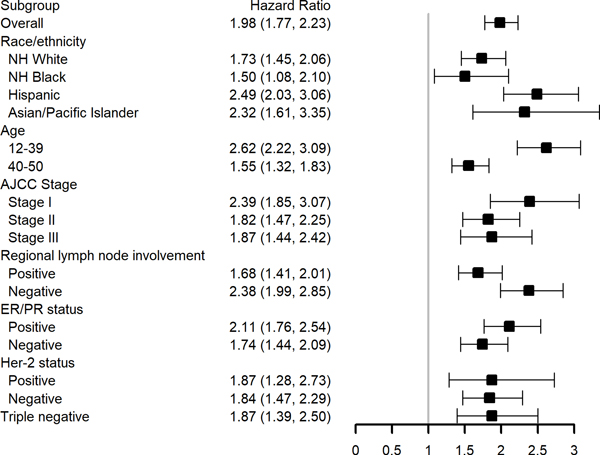
In multivariable survival models evaluating the impact of secondary versus primary breast cancer in demographic and clinical subgroups, we found that the hazard of breast cancer death was higher for women with secondary breast cancers both overall (HR: 1.98; 95% CI 1.77–2.23) and in all subgroups considered (Figure 2). We observed significant interaction by race/ethnicity (p=0.02), age group (p<0.001), and stage at diagnosis (p<0.001), with associations most pronounced in Hispanics, Asian/Pacific Islanders, and younger women (age 12–39), as well as those with earlier AJCC stage. Although not statistically significant, additionally associations appeared to be more pronounced in women with lymph node negative disease (p=0.06).
Figure 2: Adjusted* Breast Cancer-Specific Survival associated with having Secondary Breast Cancer compared with Primary Breast Cancer by Demographic and Clinical Patient Subgroups.
NH=non-Hispanic; ER=estrogen receptor; PR=progesterone receptor; HER-2= human epidermal growth factor receptor-2
HER-2 and triple-negative are limited to 2003+ diagnoses
* Each subgroup is evaluated in a separate model. Models adjusted for race/ethnicity, age at diagnosis, year of diagnosis, neighborhood socioeconomic status, T stage, lymph node involvement, grade, estrogen receptor and progesterone receptor status, and histology and stratified by chemotherapy, radiation, surgery and regional nodes examined.
In sensitivity analyses considering non-breast cancer causes of death as a competing risk in our BCSS analyses, the findings were similar to those presented in Table 3 and Figure 2, suggesting that competing risks were not impacting our findings. In addition, sensitivity analyses excluding 3.8% of patients who were unlikely to receive chest or total body radiation for their primary cancer (female genital system, eye and orbit, brain, oral, and colorectal; Table 4) did not change the findings presented in Table 3 and Figure 2.
Table 4.
Primary Cancer site among Patients with a Secondary Breast Cancer
| Primary cancer site | N (%) |
|---|---|
|
| |
| Oral cavity and pharynx | 9 (0.78) |
| Gastrointestinal tract | < 5 |
| Bones and soft tissue | 7 (0.61) |
| Breast | 927 (80.82) |
| Female genital system | 25 (2.18) |
| Eye and orbit | < 5 |
| Brain and nervous system | 14 (1.22) |
| Endocrine (including thyroid) | 77 (6.71) |
| Hematologic malignancies | 82 (7.15) |
| Other | < 5 |
Discussion:
The use of radiation as cancer treatment has been shown in multiple studies to increase breast cancer risk if given to female patients during their formative years.15, 16 However, this is the first study, to our knowledge, that compares the unique breast cancers found in this population to their age controlled counterparts without a prior cancer, looking at multiple aspects related to the subsequent breast cancer characteristics and outcomes. Consistent with prior work, we found that secondary breast cancers were more likely, to be diagnosed at an earlier stage, be ER/PR negative and higher grade than primary breast cancers.5 However, we additionally identified that non-Hispanic Blacks were more likely and Asian/Pacific Islanders were less likely than non-Hispanic Whites to have a secondary breast cancer. While BCSS was significantly decreased among all survivors of childhood and AYA cancer treated with radiation that develop a secondary breast cancer, the negative impact of the secondary cancers was particularly strong in subgroups of patients that have superior survival after primary breast cancer. These factors included Asian/Pacific Islanders and those with earlier AJCC stage, lymph node negative, and ER/PR positive disease. Our findings suggest that we may need to consider alternative and even more aggressive treatment in subgroups of women with secondary breast cancers that were considered low risk populations previously.
Many studies have shown differences in breast cancer rates and survival by race/ethnicity.1, 17–20 In particular, primary breast cancer in non-Hispanic Black women has been characterized by higher grade,21 later stage at diagnosis,21, 22 and worse survival after controlling for stage at diagnosis.17 Due to our study data representing over 99% of all cancers diagnosed in California, we include a very racially and ethnically diverse population. We found that non-Hispanic Black women are more likely to have a second breast cancer, and, while secondary breast cancer in this population is associated with worse survival than primary breast cancer, the negative impact of secondary breast cancer on survival in non-Hispanic Blacks is the smallest of all racial/ethnic groups. This is most likely due to non-Hispanic Black women having poorer survival after primary breast cancer compared with women of other race/ethnicities. Alternatively, Asian/Pacific Islanders and Hispanics have shown increased BCSS after primary breast cancers compared to non-Hispanic Whites.23–25 Although we observe these associations in the primary breast cancer population, Asian/Pacific Islanders no longer experience increased BCSS and Hispanics experience worse BCSS than non-Hispanic White women after secondary breast cancer. This results in a greater than two-fold increased hazard of breast cancer death among Asian/Pacific Islanders and Hispanics with secondary compared to primary breast cancer, which are the strongest associations of any racial/ethnic group. These data show that there is something innately different about secondary breast cancers that modifies the differences seen traditionally by race/ethnicity.
Certain tumor factors and characteristics of breast cancer are thought to provide improved survival, in so much that they serve as the foundation of the AJCC Staging Manual, Eighth Edition.26, 27 Small tumor size has been a long-standing correlate of better prognosis, as has been lymph node negativity.28, 29 Low histologic grade has also been shown to be a significantly beneficial prognostic factor when primary breast tumors have been examined.30, 31 In both the primary and secondary breast cancer cohorts, larger tumor size and lymph node involvement were associated with worse BCSS, but tumor grade was not associated with BCSS in women with secondary breast cancer.17 We hypothesize that grade, unlike in primary breast cancers, no longer adequately reflects intrinsic biological characteristics and clinical behavior of the secondary tumor.32, 33 In our subgroup analyses, the negative impact of secondary breast cancer was strongest among women with early AJCC stage and those without regional lymph node involvement. Multiple studies have also shown the lack of lymph node involvement and earlier stage at diagnosis in secondary breast cancer populations,11, 34 however, we are among the first to report an over two-fold increased hazard of breast cancer death among women with secondary compared to primary breast cancer in these subgroups.
Tumor markers in the modern era of breast cancer care are predominant factors that contribute to prognosis and treatment.12 Hormone receptor (ER/PR) positive tumors are thought to need less systemic treatment, be less overall aggressive at times leading to less local therapy, and ultimately have improved survival.35 Consistent with prior studies, ER/PR negative disease was associated with worse BCSS in both primary and secondary breast cancer cohorts; however, the negative impact of secondary breast cancer on BCSS was stronger for women with ER/PR positive disease, a significant findings given that secondary breast cancers in the childhood and AYA survivor population are more commonly ER and PR positive.11, 34 HER-2 positive breast cancer treatment changed with introduction of trastuzumab (Herceptin) in the late 1990s which led to a dramatic improvement in survival in primary breast cancer.36 In prior studies, secondary breast cancers after radiation therapy have showed decreased frequency of HER-2 positive tumors, which we also observed in our data.37 In our study, HER-2 negativity was only associated with worse BCSS in women with primary breast cancer and the impact of secondary breast cancer on BCSS was similar across HER-2 status, suggesting that regardless of phenotype of the tumor, breast cancers arising in patients treated prior with radiation therapy are more aggressive.
Overall the data shows that in secondary breast cancers after radiation, the negative impacts are most pronounced in subgroups of patients that have superior survival for primary breast cancer, which leads one to consider if treatment should be changed as a result. Do smaller and lower stage secondary breast cancers need more aggressive treatment? Our data show that women with secondary breast cancers are having more aggressive surgical therapy, but receiving less chemotherapy or radiation. The cause of this difference in treatment is not directly known, but consideration should be given to the treatments used for the primary cancer, such as chest radiation and prior anthracycline use, that may limit the ability to use them in the secondary setting. However, in recent randomized control trials, overall younger women have demonstrated more aggressive tumors and the need for more treatment.38 Additionally, studies suggest that young age is an independent predictor of poorer survival after primary breast cancer, even after adjustment for sociodemographic and tumor characteristics.39–41 The negative survival impact of age is more pronounced in secondary breast cancer. In our study, women who were diagnosed with a secondary breast cancer between 12–39 years of age were 2.6 times more likely to die from their breast cancer than women with a primary breast cancer at this age. However, we postulate that the known poorer survival, even with intensive therapy, seen in primary breast cancers in women under 40 is compounded by both the nature of secondary breast cancers in this population and the decreased treatment.
Our data set has limitations in that our knowledge of the treatment given for the primary tumor in our secondary breast cancer population is limited. Specifically, the radiation fields are not specified and chemotherapeutic agents are unknown. Similarly, we lack the specific, and at times full treatment details used for breast cancer treatment in both of our populations leading us to not know the specific influence treatment has on our survival outcomes, an important area of future research. In a similar way, there is no genetic information or comorbidities gathered on the patient population, which we acknowledge can have an influence on treatment decisions and risk for secondary breast cancers, especially for patients found to have a BRCA mutation. Additionally, due to the nature of the database there is no information about treatment failure, both for local regional as well as distant recurrence. Despite these limitations, the current study includes a large number of patients from population-based registries, increasing the generalizability of our results.
In conclusion, we found that BCSS is significantly decreased among all survivors of childhood and AYA cancer treated with radiation therapy that develop a secondary breast cancer, even in the setting of early-stage breast cancer and other characteristics, including low histologic grade and ER positivity, which are considered good prognostic factors. Treating secondary breast cancers is likely complicated by the unique genetic make-up of the tumors and prior treatment regimens received. These factors should be evaluated in more depth to determine if the differences seen in BCSS in the secondary breast cancer population should result in augmentation of the treatment algorithms in this specific population, such as possibly augmenting with novel treatments like PARP and CDK 4/6 inhibitors when able.
Acknowledgements:
The collection of cancer incidence data used in this study was supported by the California Department of Public Health pursuant to California Health and Safety Code Section 103885; Centers for Disease Control and Prevention’s National Program of Cancer Registries, under cooperative agreement 5NU58DP006344; the National Cancer Institute’s SEER Program under contract HHSN261201800032I awarded to the University of California, San Francisco, contract HHSN261201800015I awarded to the University of Southern California, and contract HHSN261201800009I awarded to the Public Health Institute. The ideas and opinions expressed herein are those of the author(s) and do not necessarily reflect the opinions of the State of California, Department of Public Health, National Cancer Institute, and Centers for Disease Control and Prevention or their contractors and subcontractors.
Funding:
Institutional support from the University of California, Davis and UC Davis Comprehensive Cancer Center Support Grant (P30CA093373-16).
Footnotes
Conflicts of Interest: The authors declare no potential conflicts of interest
References:
- 1.Noone AM HN, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER Cancer Statistics Review, 1975–2015. Available from URL: https://seer.cancer.gov/csr/1975_2015/. [Google Scholar]
- 2.Keegan THM, Bleyer A, Rosenberg AS, Li Q, Goldfarb M. Second Primary Malignant Neoplasms and Survival in Adolescent and Young Adult Cancer Survivors. JAMA Oncol. 2017;3: 1554–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee JS, DuBois SG, Coccia PF, Bleyer A, Olin RL, Goldsby RE. Increased risk of second malignant neoplasms in adolescents and young adults with cancer. Cancer. 2016;122: 116–123. [DOI] [PubMed] [Google Scholar]
- 4.Schaapveld M, Aleman BMP, van Eggermond AM, et al. Second Cancer Risk Up to 40 Years after Treatment for Hodgkin’s Lymphoma. New England Journal of Medicine. 2015;373: 2499–2511. [DOI] [PubMed] [Google Scholar]
- 5.Sadler C, Goldfarb M. Comparison of primary and secondary breast cancers in adolescents and young adults. Cancer. 2015;121: 1295–1302. [DOI] [PubMed] [Google Scholar]
- 6.Friedman DL, Whitton J, Leisenring W, et al. Subsequent Neoplasms in 5-Year Survivors of Childhood Cancer: The Childhood Cancer Survivor Study. JNCI: Journal of the National Cancer Institute. 2010;102: 1083–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stovall M, Smith SA, Langholz BM, et al. Dose to the contralateral breast from radiotherapy and risk of second primary breast cancer in the WECARE study. Int J Radiat Oncol Biol Phys. 2008;72: 1021–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer Registry. Cancer. 2007;109: 1721–1728. [DOI] [PubMed] [Google Scholar]
- 9.Kamran SC, Berrington de Gonzalez A, Ng A, Haas-Kogan D, Viswanathan AN. Therapeutic radiation and the potential risk of second malignancies. Cancer. 2016;122: 1809–1821. [DOI] [PubMed] [Google Scholar]
- 10.Forman MR, Mangini LD, Thelus-Jean R, Hayward MD. Life-course origins of the ages at menarche and menopause. Adolesc Health Med Ther. 2013;4: 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldfarb M, Rosenberg AS, Li Q, Keegan THM. Impact of latency time on survival for adolescents and young adults with a second primary malignancy. Cancer. 2018;124: 1260–1268. [DOI] [PubMed] [Google Scholar]
- 12.Keegan THM, Press DJ, Tao L, et al. Impact of breast cancer subtypes on 3-year survival among adolescent and young adult women. Breast Cancer Research. 2013;15: R95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keegan TH, DeRouen MC, Parsons HM, et al. Impact of Treatment and Insurance on Socioeconomic Disparities in Survival after Adolescent and Young Adult Hodgkin Lymphoma: A Population-Based Study. Cancer Epidemiol Biomarkers Prev. 2016;25: 264–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yost K, Perkins C, Cohen R, Morris C, Wright W. Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Causes & Control. 2001;12: 703–711. [DOI] [PubMed] [Google Scholar]
- 15.Demoor-goldschmidt C, Supiot S, Mahé M-A, et al. Clinical and histological features of second breast cancers following radiotherapy for childhood and young adult malignancy. The British Journal of Radiology. 2018;91: 20170824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drooger JC, Hooning MJ, Seynaeve CM, et al. Diagnostic and therapeutic ionizing radiation and the risk of a first and second primary breast cancer, with special attention for BRCA1 and BRCA2 mutation carriers: A critical review of the literature. Cancer Treatment Reviews. 2015;41: 187–196. [DOI] [PubMed] [Google Scholar]
- 17.Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. Jama. 2006;295: 2492–2502. [DOI] [PubMed] [Google Scholar]
- 18.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59: 225–249. [DOI] [PubMed] [Google Scholar]
- 19.Boyer-Chammard A, Taylor TH, Anton-Culver H. Survival differences in breast cancer among racial/ethnic groups: a population-based study. Cancer Detect Prev. 1999;23: 463–473. [DOI] [PubMed] [Google Scholar]
- 20.Curtis E, Quale C, Haggstrom D, Smith-Bindman R. Racial and ethnic differences in breast cancer survival: how much is explained by screening, tumor severity, biology, treatment, comorbidities, and demographics? Cancer. 2008;112: 171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Furberg H, Millikan R, Dressler L, Newman B, Geradts J. Tumor characteristics in African American and white women. Breast Cancer Research and Treatment. 2001;68: 33–43. [DOI] [PubMed] [Google Scholar]
- 22.Eley JW, Hill HA, Chen VW, et al. Racial Differences in Survival From Breast Cancer: Results of the National Cancer Institute Black/White Cancer Survival Study. Jama. 1994;272: 947–954. [DOI] [PubMed] [Google Scholar]
- 23.McCracken M, Olsen M, Chen MS Jr., et al. Cancer Incidence, Mortality, and Associated Risk Factors Among Asian Americans of Chinese, Filipino, Vietnamese, Korean, and Japanese Ethnicities. CA Cancer J Clin. 2007;57: 190–205. [DOI] [PubMed] [Google Scholar]
- 24.Iqbal J, Ginsburg O, Rochon PA, Sun P, Narod SA. Differences in breast cancer stage at diagnosis and cancer-specific survival by race and ethnicity in the United States. Jama. 2015;313: 165–173. [DOI] [PubMed] [Google Scholar]
- 25.Miller KD, Goding Sauer A, Ortiz AP, et al. Cancer Statistics for Hispanics/Latinos, 2018. CA Cancer J Clin. 2018;68: 425–445. [DOI] [PubMed] [Google Scholar]
- 26.Amin MB, Edge SB, American Joint Committee on C. AJCC cancer staging manual. 2017. [Google Scholar]
- 27.Amin MB, Greene FL, Edge SB, et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67: 93–99. [DOI] [PubMed] [Google Scholar]
- 28.Fisher B, Bauer M, Wickerham DL, et al. Relation of number of positive axillary nodes to the prognosis of patients with primary breast cancer. An NSABP update. Cancer. 1983;52: 1551–1557. [DOI] [PubMed] [Google Scholar]
- 29.Elkin EB, Hudis C, Begg CB, Schrag D. The effect of changes in tumor size on breast carcinoma survival in the U.S.: 1975–1999. Cancer. 2005;104: 1149–1157. [DOI] [PubMed] [Google Scholar]
- 30.Héry M, Delozier T, Ramaioli A, et al. Natural history of node-negative breast cancer: are conventional prognostic factors predictors of time to relapse? The Breast. 2002;11: 442–448. [DOI] [PubMed] [Google Scholar]
- 31.Rosner D, Lane WW. Predicting recurrence in axillary-node negative breast cancer patients. Breast Cancer Research and Treatment. 1993;25: 127–139. [DOI] [PubMed] [Google Scholar]
- 32.Vogt A, Schmid S, Heinimann K, et al. Multiple primary tumours: challenges and approaches, a review. ESMO Open. 2017;2: e000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Broeks A, Braaf LM, Wessels LFA, et al. Radiation-Associated Breast Tumors Display a Distinct Gene Expression Profile. International Journal of Radiation Oncology*Biology*Physics. 2010;76: 540–547. [DOI] [PubMed] [Google Scholar]
- 34.Moskowitz CS, Chou JF, Neglia JP, et al. Mortality After Breast Cancer Among Survivors of Childhood Cancer: A Report From the Childhood Cancer Survivor Study. Journal of Clinical Oncology. 2019;37: 2120–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fragomeni SM, Sciallis A, Jeruss JS. Molecular Subtypes and Local-Regional Control of Breast Cancer. Surgical oncology clinics of North America. 2018;27: 95–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344: 783–792. [DOI] [PubMed] [Google Scholar]
- 37.Horst KC, Hancock SL, Ognibene G, et al. Histologic subtypes of breast cancer following radiotherapy for Hodgkin lymphoma. Annals of Oncology. 2014;25: 848–851. [DOI] [PubMed] [Google Scholar]
- 38.Sparano JA, Gray RJ, Makower DF, et al. Adjuvant Chemotherapy Guided by a 21-Gene Expression Assay in Breast Cancer. New England Journal of Medicine. 2018;379: 111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dubsky PC, Gnant MF, Taucher S, et al. Young age as an independent adverse prognostic factor in premenopausal patients with breast cancer. Clin Breast Cancer. 2002;3: 65–72. [DOI] [PubMed] [Google Scholar]
- 40.Gnerlich JL, Deshpande AD, Jeffe DB, Sweet A, White N, Margenthaler JA. Elevated breast cancer mortality in women younger than age 40 years compared with older women is attributed to poorer survival in early-stage disease. J Am Coll Surg. 2009;208: 341–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fredholm H, Eaker S, Frisell J, Holmberg L, Fredriksson I, Lindman H. Breast cancer in young women: poor survival despite intensive treatment. PLoS One. 2009;4: e7695. [DOI] [PMC free article] [PubMed] [Google Scholar]