Abstract
The objective of this study was to develop and evaluate newly improved, rapid, and reliable strategies based on real-time PCR to detect the most frequent beta-lactamase genes recorded in clinical Enterobacterales strains, particularly in Tunisia (blaSHV12, blaTEM, blaCTX-M-15, blaCTX-M-9, blaCMY-2, blaOXA-48, blaNDM-1, and blaIMP) directly from the urine. Following the design of primers for a specific gene pool and their validation, a series of real-time PCR reactions were performed to detect these genes in 78 urine samples showing high antibiotic resistance after culture and susceptibility testing. Assays were applied to DNA extracted from cultured bacteria and collected urine. qPCR results were compared for phenotypic sensitivity. qPCR results were similar regardless of whether cultures or urine were collected, with 100% sensitivity and specificity. Out of 78 multiresistant uropathogenic, strains of Enterobacterales (44 E. coli and 34 K. pneumoniae strains) show the presence of the genes of the bla group. In all, 44% E. coli and 36 of K. pneumoniae clinical strains harbored the bla group genes with 36.4%, 52.3%, 70.5%, 68.2%, 18.2%, and 4.5% of E. coli having blaSHV-12, blaTEM, blaCTX-M 15, blaCTX-M-9, blaCMY-2, and blaOXA-48 group genes, respectively, whereas 52.9%, 67.6%, 76.5%, 35.5%, 61.8, 14.7, and 1.28% of K. pneumoniae had blaSHV-12, blaTEM, blaCTX-M 15, blaCTX-M-9, blaCMY-2, blaOXA-48, and blaNDM-1 group genes, respectively. The time required to have a result was 3 hours by real-time PCR and 2 to 3 days by the conventional method. Resistance genes of Gram-negative bacteria in urine, as well as cultured bacteria, were rapidly detected using qPCR techniques. These techniques will be used as rapid and cost-effective methods in the laboratory. Therefore, this test could be a good candidate to create real-time PCR kits for the detection of resistance genes directly from urine in clinical or epidemiological settings.
1. Introduction
Urinary tract infections (UTIs) are among the most common infectious diseases in humans and represent a real public health problem in terms of both their frequency and their difficulty of treatment. Enterobacterales is primarily responsible for UTIs. Escherichia coli is the main pathogen responsible for cystitis and pyelonephritis as well as other species of Enterobacterales, such as Proteus mirabilis and especially Klebsiella pneumoniae [1, 2].
Βeta-lactams are the antibiotics preferentially used against these Enterobacterales. The emergence and spread of these bacteria producing extended-spectrum β-lactamases (ESBLs) or carbapenemases, have become a concern. In fact, the increase in the number of strains expressing β-lactamases (E. coli, K. pneumoniae) might be explained by the massive use of broad-spectrum cephalosporins (third-generation cephalosporins: C3G and fourth-generation cephalosporins: C4G). Moreover, this selection pressure adhered to the broadening of the TEM and SHV spectrum (by mutation of the blaTEM-1 and blaSHV-1 genes) and to the emergence of the CTX-M family, capable of hydrolyzing the penicillins, broad-spectrum cephalosporins, and aztreonam [3].
The dissemination of CTX-Ms enzymes has resulted in a generalization of their distribution throughout the world [4]. They are now the most extensively ESBLs in the world where the most common mutants are CTX-M-15 and CTX-M-14 belonging, respectively, to the CTX-M-1 and CTX-M-9 groups [5]. In Africa and Europe, recent studies have found a substantial increase in ESBL-producing Gram-negative bacteria causing community urinary tract infections, particularly harboring the blaCTX-M-15 allele [6, 7].
Furthermore, over the last two decades, extended-spectrum β-lactamase (ESBL) and plasmid-mediated AmpC- (pAmpC-) producing Enterobacterales exhibiting resistance to the 3GCs has been increasingly isolated in humans [8]. Among the AmpC β-lactamase genes, particularly blaCMY-2 and blaDHA-1 are the most common in E. coli and K. pneumoniae strains, respectively, of human and companion animal [9, 10].
On the other hand, additional enzymes called carbapenemases have been detected in E. coli and K. pneumoniae strains. The OXA-1 type enzymes are sometimes hosted alongside the CTX-M group exhibiting ESBL activity, and class D β-lactamases hydrolyzing carbapenems (for example OXA-23, OXA-24/40, OXA-48, OXA-51, and OXA-58) are commonly found in Pseudomonas aeruginosa and Acinetobacter baumannii [11, 12].
As elsewhere in the world, the spread of multidrug-resistant (MDR) E. coli, often ST131, complicates treatment and attributes to massive use of previously reserved antibiotics, such as carbapenems and colistin [13]. One potential way to address these issues is to switch from empirical therapy to early-targeted therapy, detecting antibiotic resistance genes directly from clinical samples, with no culture that can take 2 to 3 days to become available. This identification can be achieved by metagenomic sequencing [14] although there is skepticism about the implementation, depending on costs and workflows [15]. The polymerase chain reaction (PCR) system was more immediately deployable, was less expensive, and has been frequently studied for proliferate mecA or carbapenemase genes, making it easier to treat infections [16, 17].
For this reason, our work is aimed at developing a qPCR system for the detection of blaTEM, blaSHV-12, blaCTX-M-15, blaCTX-M-9, blaCMY-2, blaOXA-48, blaIMP-1, and blaNDM-1 group genes directly from urines and applying it in clinical E. coli and K. pneumoniae strains. An alternative to its methods through PCR can reduce the wait time to just a few hours.
2. Materials and Methods
2.1. Microbial Study
During the study period from December 2018 to December 2020, 2000 urine samples were collected from patients aged 20 to 65 years. Five hundred of them were culturally positive. Urine samples were collected from patients in health facilities as well as community patients in the towns of Sfax, south of Tunisia. All the strains collected were identified using the API 20E system (bioMérieux SA, Marcy l'Etoile, France).
2.1.1. Phenotypic Characterization
Urine analysis and strain identification were performed by conventional methods. Antimicrobial susceptibility study was determined using the standard disk diffusion method on Mueller Agar-Hinton (Oxoid) according to Clinical Laboratory Guidelines and Institute Standards [18]. Tested antibiotics (Bio-Rad) were as follows: amoxicillin-clavulanate, (10 μg) (AMX); amoxicillin, (20 μg/10 μg) (AMC); cefotaxime, (30 μg) (CTX); ceftazidime, (30 μg) (CAZ); cefoxitin (30 μg) (FOX); amikacin (30 μg) (AN); ciprofloxacin (10 μg) (CIP); nalidixic acid (10 μg) (NA); gentamicin (10 μg) (GEN); netilmicin (30 μg) (NET); tobramycin (10 μg) (NN); fosfomycine (10 μg) (FFL); trimethoprim + sulfamide (10 μg) (SXT); imipenem (10 μg) (IPM); and colistin (10 μg) (CL). The diameters of the zones of inhibition were interpreted according to the recommendations of the CLSI [18]. All strains isolated were screened for extended-spectrum β-lactamase (ESBL) production by the double-disk synergy test [19].
2.2. Molecular Methods
2.2.1. DNA Extraction
The DNA template was prepared according to a previously reported method with some modification [20, 21]. A loop of bacteria colonies harvested from a McConkey agar plate was suspended in 250 μl of sterile distilled water and heated at 100°C for 10 minutes. After centrifugation at 15000 rpm for 5 min, the supernatant containing the harvested DNA was collected and stored at -20°C until its use in the PCR experiments.
The preparation of the DNA template from urine samples was conducted as follows: the urine (4–10 ml) was centrifuged at 12,000 rpm for 10 min, with the resulting bacterial pellet resuspended in 100 μl of PVG and treated with an ADNucleis extraction and purification kit (ADNucleis, veterinary diagnostic platform, Lyon, France), for the lysis of the bacterial cells and to eliminate their DNA. Bacterial lysis buffer, lyophilized enzyme powder (PE), and enzyme mix suspension buffer (TME) were added, and after incubation for 10 min at 58°C, the DNA was purified using the BM Nucleic Acid Isolation Kit Based on Magnetic Beads [22].
2.2.2. Primer Design
Based on the literature, primer design was performed using the BLAST (English Basic Local Alignment Search Tool) program to detect β-lactamase genes encoding extended-spectrum β-lactamases (blaSHV-12, blaTEM, and blaCTX-M-15) [23], Plasmid-mediated AmpC-lactamases (blaCMY-2) [24], and classes B (blaIMP-1, blaNDM-1) and D (blaOXA-48) carbapenemases [25]. These primers were verified by Primer 3 (Table 1).
Table 1.
List of primers used for qPCR amplification of ESBLs and carbapenemase genes.
| Genes | Primer sequence (5′→3′) FW: Forward RV: Reverse |
T°m | Product size (bp) | GenBank |
|---|---|---|---|---|
| blaSHV-12 | FW: AGCCGCTTGAGCAAATTAAA | 59.99 | 77 | LC229232.1 |
| RV: GCTGGCCAGATCCATTTCTA | 60.18 | |||
|
| ||||
| blaTEM | FW: GATAAATCTGGAGCCGGTGA | 60.04 | 78 | MG860488.1 |
| RV: GATACGGGAGGGCTTACCAT | 60.17 | |||
|
| ||||
| blaCTX-M-15 | FW: CACCAATGATATTGCGGTGA | 60.34 | 77 | MG288677.1 |
| RV: GTTGCGGCTGGGTAAAATAG | 59.61 | |||
|
| ||||
| blaCTX-M-9 | FW: TACTTCACCCAGCCTCAACC | 60.11 | 78 | CP028990.1 |
| RV: ACCGTCGGTGACGATTTTAG | 59.99 | |||
|
| ||||
| blaCMY-2 | FW: CCAGAACTGACAGGCAAACA | 59.87 | 65 | LC229227 |
| RV: CCTGCCGTATAGGTGGCTAA | 60.11 | |||
|
| ||||
| blaOXA-48 | FW: GTAGTCAGCGCATCGTGAAA | 60.02 | 73 | MN654469.1 |
| RV: CCCGTTTTAGCCCGAATAAT | 60.12 | |||
|
| ||||
| blaIMP-1 | FW: GCCAAAGTCCGCCAAATTAT | 60.17 | 92 | MK088089.1 |
| RV: TCAAGAGTGATGCGTCTCCA | 65.56 | |||
|
| ||||
| blaNDM-1 | FW: ATGGAGACTGGCGACCAAC | 61.10 | 87 | LC413788.2 |
| RV: GGCATGTCGAGATAGGAAGG | 59.65 | |||
2.2.3. Real-Time PCR Amplification Program
The qPCR assay was performed on a CFX96™ real-time PCR thermocycler (BioRad, France).
Each reaction was carried out in a 20 μl reaction mixture containing 1 μl of template AND extracted directly from urine or from strains (50 ng/μl), 100 nM of each primer, and 10 μl of the SYBR green.
The optimal program of the qPCR includes an initial denaturation at 95°C for 3 min, followed by 40 cycles of: 95°C for 10 s; a hybridization temperature of 56°C for the genes blaTEM, blaSHV-12, blaCTX-M-15, blaCTX-M-9, and blaCMY-2 and 60°C for the genes of group blaOXA-48, blaIMP-1, and blaNDM-1 for 10 s and 72°C for 30 s. A melting step was performed at the end of the amplification; it was performed using the following cycling parameters: 60°C for 30 s and 5°C temperature changes to the end temperature of 95°C. The amount of amplified product was monitored by detecting the fluorescence energy emitted by SYBR green. Each PCR run included a negative control (no template control).
2.3. Determination of Specificity and Sensitivity of the qPCR Using Cultured Bacteria or Bacteria Harvested from Urine
Specificity and sensitivity were determined using the following formula: specificity = (D/C + D) × 100 and sensitivity = (A/A + B) × 100, where A is true positive, B is false negative, C is false positive, and D is true negative (Table 2). The values of the correlation coefficients R2 were calculated by the standard curve method. These values (R2) were 0.98 for all genes.
Table 2.
Specificity and sensitivity of the qPCR system for bla group gene detection.
| Resistance gene target | Clinical urines (n = 78) | Strains (n = 78) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| True (+) = A | False (-) = B | False (+) = C | True (-) = D | Sensitivity; specificity, % | True (+) = A | False (-) = B | False (+) = C | True (-) = D | Sensitivity; specificity, % | |
| blaSHV-12 | 34 | 0 | 0 | 44 | 100; 100 | 34 | 0 | 0 | 44 | 100; 100 |
| blaTEM | 46 | 0 | 0 | 32 | 100; 100 | 46 | 0 | 0 | 32 | 100; 100 |
| blaCTX-M15 | 56 | 0 | 0 | 22 | 100; 100 | 56 | 0 | 0 | 22 | 100; 100 |
| blaCTX-M9 | 42 | 0 | 0 | 36 | 100; 100 | 42 | 0 | 0 | 36 | 100; 100 |
| blaCMY-2 | 29 | 0 | 0 | 49 | 100; 100 | 29 | 0 | 0 | 49 | 100; 100 |
| blaOXA-48 | 7 | 0 | 0 | 71 | 100; 100 | 7 | 0 | 0 | 71 | 100; 100 |
| blaIMP-1 | 0 | 0 | 0 | 78 | 100; 100 | 0 | 0 | 0 | 78 | 100; 100 |
| blaNDM-1 | 1 | 0 | 0 | 77 | 100; 100 | 1 | 0 | 0 | 77 | 100; 100 |
2.4. Statistical Analysis
Statistical tests including the χ2 test, multivariate logistic regression analysis to interpret the associations between the genes of the bla group and the different levels of antibiotic resistance, OR, and 95% CI were calculated as well as the analysis of Spearman's rank correlation. A p value of 0.05 was counted as statistically significant in this study. All data was done using IBM SPSS version 21.0.
3. Results
Of the 500 positive urine cultures, only the most predominant isolates were isolated, and among them, 90 isolates were considered multidrug-resistant strains, as these isolates were found to be resistant to at least two classes of antibiotics. Forty-four strains were identified as E. coli, which was predominant, followed by 34 strains of K. pneumoniae, 3 strains of Enterobacter cloacae, 2 strains of Pseudomonas aeruginosa, 2 strains of Enterococcus faecalis, 1 strain of Morganella morganii, 1 strain of Citrobacter koseri, 1 strain of Aeromonas hydrophila, 1 strain of Acinetobacter, and 1 strain of Staphylococcus aureus.
The qPCR system was used for the detection of antimicrobial resistance genes (Tables 2 and 3) in 78 uropathogenic Enterobacterales strains (44 strains of E. coli and 34 strains of K. pneumoniae included 40 strains of E. coli and 20 strains of K. pneumoniae ESBL producers), regardless of whether the DNA was extracted from bacteria in culture, or directly from urine.
Table 3.
The most frequent gene combinations in E. coli and K. pneumoniae strains.
| Genotype | E. coli | K. pneumoniae |
|---|---|---|
| Number of strains (n = 44) | Number of strains (n = 36) | |
| blaTEM/CTX-M-15/CTX-M-9 | 10 | 6 |
| blaTEM/SHV-12/CMY-2 | 10 | 7 |
| blaSHV-12/CTX-M-15/CTX M-9/TEM | 5 | 5 |
| blaCMY 2/SHV-12/CTX-M-15/CTX-M-9/TEM | 2 | 1 |
qPCR reactions were initially performed at different annealing temperatures designated for each primer pair (Table 1).
3.1. Evaluation of Specificity and Sensitivity of the qPCR Using Cultured Bacteria or Bacteria Harvested from Urine
The newly developed qPCR system was effective in detecting genes of the bla group. The use of this system has shown that all the Enterobacterales isolates tested have at least one gene of the bla group. Of these, 31 contained more than three genes from the bla group. qPCR amplification of DNA extracted directly from urine was also performed. A concordance of 100% was found between the results of resistance genes detected directly from urine to those using purely isolated colonies (Table 2).
For the targeted bla group genes, the specificity and sensitivity of qPCR were both determined to be 100%.
3.2. Distribution of the Types blaSHV, blaTEM, blaCTX-M-15, blaCTX-M-9, blaCMY-2, blaOXA-48, blaNDM-1, and blaIMP-1 Group Genes in Clinical Enterobacterales Strains
qPCR data show that among the E. coli clinical strains, group genes blaCTX-M-15 (70.5%, 31 strains), blaCTX-M-9 (68.2%, 30 strains), blaTEM (52.3%, 23 strains), and blaSHV-12 (34%, 16 strains) were the most prevalent followed by group genes blaCMY-2 (18.2%, 8 strains) and blaOXA-48 (4.54%, 2 strains). None of these stains show the presence of the genes of the group blaNDM-1 and blaIMP-1.
In K. pneumoniae, blaCTX-M-15 (76.5%, 26 strains) was the most detected followed by blaSHV-12 (52.9%, 18 strains), blaTEM (67.6%, 23 strains), blaCMY-2 (61.8%, 21 strains), blaCTX-M-9 (35.3%, 12 strains), blaOXA-48 (14.7%, 5 strains), and blaNDM1 (2.9%, 1 strains) group genes (Figure 1).
Figure 1.
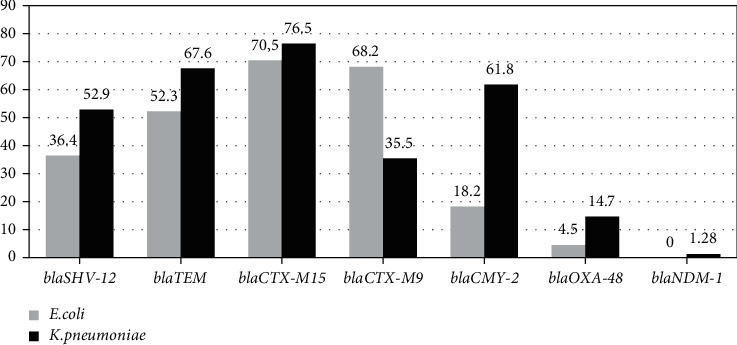
Distribution of the 7 resistance genes in the 44 E. coli and 34 K. pneumoniae clinical strains.
In addition, we found that 98.7% (n = 77) of the clinical E. coli and K. pneumoniae strains tested had at least one gene from the bla group with up to 34 different bla genotypes.
Otherwise, 25 (56.8%) of E. coli and 23 (61.5%) of K. pneumoniae had more than 2 genes. The most frequent combinations of 3 or more genes from the bla group of isolates tested have been summarized in Table 3.
3.3. Antibiotic Resistance Rates of the Clinical Enterobacterales Strains
The antimicrobial susceptibility testing performed for 78 Enterobacterales isolates (44 E. coli and 34 K. pneumoniae) showed that 60 (90.9%) of isolates were ESBL producers (40 E. coli and 20 K. pneumoniae).
Multidrug-resistant E. coli and K. pneumoniae strains show strong resistance to penicillin-family antibiotics such as amoxicillin, amoxicillin-clavulanate, ticarcillin, and 3rd-generation cephalosporin antibiotics such as cefotaxime and ceftazidime.
According to the test of the sensitivity of multiresistant E. coli, a high level of resistance was also recorded to ofloxacin, ciprofloxacin and nalidixic acid with a percentage of 95.5%, 93.2% (% CI [81.34% -98.57%]), and 88.6% (% CI [75.44% -96.20%]), respectively. Strains of K. pneumoniae also showed a high level of resistance to ofloxacin 85.3% (% CI [68.94% -95.04%]), ciprofloxacin 82.4% (% CI [65.46% -93.23%]), and nalidixic acid 85.3% (% CI [68.94% -95.04%]). A relative low resistance was recorded for imipenem with a percentage of 2.3% for E. coli and 29.4% (% CI [15.09% -47.47%]) for K. pneumoniae.
Otherwise, resistance to cefoxitin can also be considered to be low in strains of E. coli and K. pneumoniae with a percentage of 9.1% (% CI [2.53%-21.66%]) and 41.2 (% CI [24.64% -59.30%]), respectively. Clinical strains of E. coli were also resistant to other non-β-lactam antibiotics such as netilmicin 59.1% (% CI [43.24% -73.66%]), gentamicin 45.5% (% CI [30.39% -61.15%]), tobramycin 63.6% (% CI [47.77% -77.59%]), amikacin 25% (% CI [13.19% -40.33%]), bactrim 68.2% (% CI [52.42% -81.39%]), and fosfomycin 9.1% (% CI [2.53%-21.66%]). In addition, the 34 strains of K. pneumoniae showed resistance to netilmicin, gentamicin, tobramycin, amikacin, bactrim, and fosfomycin with a percentage of 52.9% (% CI [35.12% -70.22%]), 61.8% (% CI [43.56% -77.83%]), 61.8% (% CI [43.56% -77.83%]), 17.6% (% CI [6.76% -34.53%]), 67.6% (% CI [49.47% -82.61%]), and 50% (% CI [32.42% -67.57%]), respectively. None of these strains shows resistance to colistin (Table 4).
Table 4.
Antibiotic susceptibility profile of multidrug E. coli and K. pneumoniae.
| Antibiotics |
E. coli (n = 44) (95% CI) |
K. pneumoniae (n = 34) (95% CI) |
||
|---|---|---|---|---|
| Resistant | Sensitive | Resistant | Sensitive | |
| AMX | 100% (44) | 0 | 100%(34) | 0 |
| AMC | 97.7% (43) [87.99%-99.92%] | 2.3% (1) | 100%(34) | 0 |
| TIC | 100% (44) | 0 | 100%(34) | 0 |
| CF | 100% (44) | 0 | 100%(34) | 0 |
| FOX | 9.1% (4) [2.53%-21.66%] | 90.9% (40) [78.33%-97.46%] | 41.2% (14) [24.64%-59.30%] | 58.8% (20) [40.69%-75.35%] |
| CFM | 97.7% (43) [87.99%-99.92%] | 2.3% (1) | 97.1% (33)[84.67%-99.92%] | 2.9% (1) |
| CAZ | 97.7% (43) [87.99%-99.92%] | 2.3% (1) | 94.1% (32) [80.32%-99.27%] | 5.9% (2) [0.72%-1.96%] |
| CTX | 97.7% (43) [87.99%-99.92%] | 2.3% (1) | 97.1% (33) [84.67%-99.92%] | 2.9% (1) |
| IMP | 2.3% (1) | 97.7%(43) [87.99%-99.92%] | 29.4% (10) [15.09%-47.47%] | 70.6% (24) [52.52%-84.90%] |
| AN | 25% (11) [13.19%-40.33%] | 75% (33) [59.66%-86.80%] | 17.6% (6) [6.76%-34.53%] | 82.4% (28) [65.46%-93.23%] |
| GM | 45.5% (20) [30.39%-61.15%] | 54.5% (24)[38.84%-69.60%] | 61.8% (21) [43.56%-77.83%] | 38.2% (13) [22.16%-56.43%] |
| NET | 59.1% (26) [43.24%-73.66%] | 40.9% (18)[26.33%-56.75%] | 52.9% (18) [35.12%-70.22%] | 47.1% (16) [29.77%-64.87%] |
| NN | 63.6% (28) [47.77%-77.59%] | 36.4% (16)[22.40%-52.2%] | 61.8% (21) [43.56%-77.83%] | 38.2% (13) [22.16%-56.43%] |
| NA | 88.6% (39) [75.44%-96.20%] | 11.4% (5)[11.36%-37.94%] | 85.3% (29) [68.94%-95.04%] | 14.7% (5) [4.95%-31.05%] |
| OFX | 95.5% (42) [84.52%-99.44%] | 4.5% (2)[0.5%-1.54%] | 85.3% (29) [68.94%-95.04%] | 14.7% (5) [4.95%-31.05%] |
| CIP | 93.2% (41) [81.34%-98.57%] | 6.8% (3)[1.42%-18.65%] | 82.4% (28) [65.46%-93.23%] | 17.6% (6) [6.76%-34.53%] |
| FFL | 9.1% (4) [2.53%-21.66%] | 90.9% (40)[78.33%-97.46%] | 50% (17) [32.42%-67.57%] | 50% (17) [32.42%-67.57%] |
| SXT | 68.2% (30) [52.42%-81.39%] | 31.8% (14)[18.60%-47.57%] | 67.6% (23)[49.47%-82.61%] | 32.4% (11) [17.38%-50.52%] |
| CL | 0 | 100% (44) | 0 | 100%(34) |
AMX: amoxicillin; AMC: amoxicillin + clavulanic acid; TIC; ticarcillin; CF: cephalexin; CFM: cefixime; FOX: cefoxitin; CTX: cefotaxime; CAZ: ceftazidime; IMP: imipenem; NA: nalidixic acid; OFX: ofloxacin; CIP: ciprofloxacin; AN: amikacin, GM: gentamycin; NET: netilmicin; NN: tobramycin; FFL: fosfomycine; SXT: trimethoprim + sulfamide; CL: colistin.
3.4. Relationship between Genotypic and Phenotypic Results of Resistance of Strains to Antibiotics
qPCR was carried out for the 78 uropathogenic strains of Enterobacteriaceae (44 E. coli and 34 K. pneumoniae) to analyze the ESBL genes as well as the determinants of resistance to drugs conferring resistance to β-lactam. The detailed associations of drug resistance with bla group detected in K. pneumoniae and E. coli isolates have been summarized in supplement Tables 5–7).
Table 5.
Correlation between bla genes and antibiotic resistance in the 78 clinical strains (by the χ2 test).
| Antibiotics | blaTEM | blaSHV-12 | blaCTX-M9 | blaCTX-M15 | blaCMY-2 | blaOXA-48 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| + | - | P value | + | - | P value | + | - | P value | + | - | P value | + | - | P value | + | - | P value | |
| Resistance to ≥4 β-lactam antibiotics | 32.60% (15/46) | 6.25% (2/32) |
0.006 | 23.52% (8/34) |
20.45% (9/44) |
0.744 | 33.33% (14/42) |
8.33% (3/36) |
0.008 | 17.85% (10/56) |
31.81% (7/22) |
0.179 | - - |
- - |
— | 66.66% (4/6) |
18.05% (13/72) |
0.006 |
Table 6.
Relationship between bla genes and antibiotic resistance in the 78 clinical strains (by multiple logistic regression analysis).
| Antibiotics | Resistance rate % (positive/total) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| blaTEM | blaSHV-12 | blaCTX-M9 | blaCTX-M15 | blaCMY-2 | blaOXA-48 | |||||||||||||
| χ 2 | P value | OR (95% CI) |
χ 2 | P value | OR (95% CI) |
χ 2 | P value | OR (95% CI) |
χ 2 | P value | OR (95% CI) |
χ 2 | P value | OR (95% CI) |
χ 2 | P value | OR (95% CI) |
|
| Resistance to ≥4 β-lactam antibiotics | 5.921 | 0.015 | 9.372 (1.545-56.834) |
— | — | — | 3.764 | 0.05 | 4.448 (0.985-20.087) |
— | — | — | — | — | — | 5.049 | 0.025 | 13.100 (1.389-123.552) |
| FOX | — | — | — | — | — | — | — | — | — | — | — | — | 9.835 | 0.002 | 11.136 (2.469-50.226) |
— | — | — |
Table 7.
Correlation between bla genes and antibiotic resistance in the 78 clinical strains (by Spearman's rank correlation analysis).
| Antibiotics | Resistance rate % (positive/total) | |||||
|---|---|---|---|---|---|---|
| blaTEM | blaSHV-12 | blaCTX-M9 | blaCTX-M15 | blaCMY-2 | blaOXA-48 | |
| P value | P value | P value | P value | P value | P value | |
| Resistance to ≥4 beta-lactam antibiotics | 0.002 | 0.404 | 0.024 | 0.264 | — | 0.002 |
| FOX | — | — | — | — | 0.022 | 0.008 |
Resistance to four or more β-lactam antibiotics was associated with the presence of four genes from the bla group. Indeed, the analysis of the present data using the chi-square test showed a highly significant correlation between the resistance to four or more -lactams and blaTEM (P value = 0.006), blaCTX-M9 (P value = 0.008), and blaOXA-48 (P value = 0.006) (Table 5). Many strains harboring the blaTEM genes have also cohosted the genes of the blaCTX-M-9 groups. This positive association between the gene(s) of the blaTEM and/or blaCTX-M-9 group and resistance to four or more antibiotics was confirmed by multiple logistic regression analysis (Table 6) and by Spearman's rank correlation analysis (Table 7). In addition, a positive correlation was also recorded between the blaCMY-2 gene and the agent FOX. This association was proved by the chi-square test (P < 0.001; Table 5), multiple logistic regression (P = 0.002; Table 6), and Spearman's rank correlation analysis (P < 0.001; Table 7).
4. Discussion
qPCR system is a faster and more efficient technology for detection sensitivity to antibiotics compared to classical phenotypic and conventional methods [26]. It has been widely used for the research of resistance genes in clinical samples, but principally to support infection controlling rather than guiding therapy [21]. We explored its potential to detect important genes for antibioresistance to Enterobacterales in the clinic urine without culture.
Unlike most tests currently available (conventional methods), our qPCR system offers the advantage of detecting the target group of 7 bla genes (blaSHV, blaTEM, blaCTX-M-1, blaCTX-M-9, blaCMY-2, blaOXA-48, and blaNDM) after 2 hours with similar sensitivity and specificity which was obtained for both urine and cultured bacteria. For this reason, the detection of antibioresistance genes directly from biological samples could be a useful tool in Tunisia and other countries where the blaCTX-M gene clusters were predominant. The CTX-M, TEM, and SHV enzymes were among the most common variants in Tunisia [27], in Palestine, in Egypt [28], and in European region [29] with varying prevalence rates. These results are similar to our qPCR data. Among the clinical strains of E. coli, blaCTX-M-15 (70.5%) and blaCTX-M-9 (68.2%) group genes were the most prevalent followed by blaTEM (52.3%), blaSHV (36.4%), blaCMY-2 (18.2%), and blaOXA-48 (4.5%) group genes. In K. pneumonia strains, blaCTX-M-15 (85.3%) was the most detected followed by blaSHV-12 group (52.9%), blaTEM (67.6%), blaCMY-2 (61.2%), blaCTX-M-9 (35.3%), blaOXA-48 (14.7%), and blaNDM-1 (2.9%) group genes.
The qPCR test achieves a sensitivity of 100% and 100% specificity of the β-lactamase genes in clinical urine and cultured strains. Our results are in agreement with those of Schmidt et al. [14] who developed a multiplex tandem PCR (MT-PCR) for the detection of 16 genes of the ESBLs family in clinical urine and isolates cultured with a sensitivity of 100% and a specificity of 95-100% [30].
Uniformly, these results using urine directly are comparable to that proven by others using analogous methodology on cultivated isolates. Chavada and Maley [31] evaluated the MT-PCR to look for 12 β-lactamases genes in cultured Gram-negative isolates, achieving 95% sensitivity and 96.7% specificity [31].
Singh et al. [32] have developed a real-time multiplex PCR test to detect 10 β-lactamases, such as ESBLs, AmpCs, and carbapenemases genes. The diversity of genes sought was higher than in our study, although the blaCTX-M 9 group was neglected [32].
Moreover, Willemsen et al. [33] used qPCR to detect ESBL-encoding genes (blaCTX-M-like, blaTEM, and blaSHV) accessing 98.9% sensitivity and 100% specificity compared to a reference chip [33].
In this study, we also investigated the relationship between phenotypic and genotypic results of ESBL-producing clinical isolates of E. coli and K. pneumoniae. We detected a relatively high percentage of genes previously shown to be associated with resistance to antibiotics belonging to the β-lactams [34].
The strains which carry the SHV and TEM genes show only relatively low resistance to third-generation cephalosporins such as cefixime, cefotaxime, ceftazidime, and monobactam; when coexisting with the genes of the blaCTX-M group, the rate resistance of these bacteria to these antibiotics will be significantly increased [35]. Apart from the many variants of CTX-M that have been reported in recent years, CTX-M-15 belongs to a specific group of these genes, which is defined by increased hydrolysis activity of ceftazidime [36].
Strains with the blaCTX-M-9 gene group only are characterized by a relatively low level of resistance to CAZ, but in combination with blaCTX-M-15, this resistance was markedly increased. The resistance of strains to CTX and CAZ may be explained by the presence of blaCTX-M15 [3].
The blaTEM and blaCTX-M-9 group genes were positively related with resistance to more than four β-lactams, according to statistical analysis of our data.
The CMY-2 gene encoded by the plasmid was detected by qPCR in 51.7% of cefoxitin resistant isolates and this result was statistically significant (P < 0.05). These results were consistent with two studies conducted in Egypt by Rensing et al. [8] and Fam et al. [37] in which CMY-2 was detected in 86.9% and 76.5%, respectively.
Three of K. pneumoniae and one E. coli strains carrying blaOXA-48 are resistant to imipenem. β-Lactamase OXA-48 hydrolyze significantly carbapenems such as imipenem and penicillins, but not extended-spectrum cephalosporins [38].
These findings support the idea that the qPCR technique can reveal the link between certain bla group genes and resistance to multiple β-lactam drugs. Multiple resistance genes coexisting in a single strain increase the risk of them spreading to new strains, and the diversity of their resistance complicates molecular detection and treatment.
However, despite the sensitivity of 100% was achieved for resistance tested in clinical urine and culture isolates, the qPCR system could not detect new or currently rare determinants if their number increases over time, which limits the number of targets that can be screened. In addition, this system could not distinguish the genes which code for ESBLs and non-ESBLs from blaTEM and blaSHV, as long as they are 10 times rarer than blaCTX-M among E. coli causing urinary tract infections [39].
Thus, our system does not interfere with the collapse antherotherapy, indeed during our work we have used qPCR to detect, only, the variants of ESBL frequently found in our region.
Finally, qPCR cannot predict resistance to cephalosporins and carbapenems in Enterobacterales isolates which is caused only by β-lactamases, but by other resistance mechanisms such as changes in membrane permeability and efflux pump [40]. These limitations could be linked with those of the conventional method, which only gives results for at least 2 days. If resistance prevails, this shows that most patients are undertreated either by a random treatment, by an agent with limitations but little resistance, or by an antibiotic which would usually be reserved (such as ertapenem) becomes the standard of empirical care.
5. Conclusion
In conclusion, the real-time PCR system accurately detected β-lactamase-producing Enterobacterales directly from biological samples or using purely isolated colonies.
Our results showed a concordance between the results found by the classical method and those by the molecular method. Here, we showed the high prevalence of ESBLs in Tunisia. In summary, an efficient detection system could be put in place to have a diagnosis, a rapid, specific, and reliable antibiotic therapy, and the management of infection control programs. All the tests validated during our work will be soon be marketed as a rapid and cost-effective methods in the laboratory.
Acknowledgments
This research has been funded by the Scientific Research Deanship at University of Ha'il, Saudi Arabia, through the project number RG-20 230. Special thanks are due to all the members of the private medical laboratory Salem Riguen and Clinique El alya in particular the pharmacist biologist Salem Rigan and Ghazie Boujelben and the two technicians Mouna Cheikhrouhou and Houda Ellouze for their help in the collection of urine samples.
Data Availability
All data and additional information regarding this study are available to third parties under reasonable request.
Ethical Approval
Approval and consent were not required.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Authors' Contributions
Mariem Yengui was responsible for the conception and design of the work and writing of the manuscript. Rahma Trabelsi and Nourelhouda Mathlouthi were responsible for the interpretation of data for the work and writing and reviewing of the manuscript. Radhouane Gdoura, Mejdi, and Lamia Khannous were responsible for the supervision of the project, important intellectual contributions, and final approval of the version to be published. All authors read and approved the final manuscript.
References
- 1.Livermore D. M., Canton R., Gniadkowski M., et al. Incidence, risk factors, and outcomes of Klebsiella pneumoniae bacteremia. The American Journal of Medicine . 2009;122(9):866–873. doi: 10.1016/j.amjmed.2009.03.034. [DOI] [PubMed] [Google Scholar]
- 2.Zhao L., Chen X., Zhu X., et al. Prevalence of virulence factors and antimicrobial resistance of uropathogenic Escherichia coli in Jiangsu province (China) Urology . 2009;74(3):702–707. doi: 10.1016/j.urology.2009.01.042. [DOI] [PubMed] [Google Scholar]
- 3.Paterson D. L., Bonomo R. A. Extended-spectrum β-lactamases: a clinical update. Clinical Microbiology Reviews . 2005;18(4):657–686. doi: 10.1128/CMR.18.4.657-686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cantón R., González-Alba J. M., Galán J. C. CTX-M enzymes: origin and diffusion. Frontiers in Microbiology . 2012;3:p. 110. doi: 10.3389/fmicb.2012.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zeynudin A., Pritsch M., Schubert S., et al. Prevalence and antibiotic susceptibility pattern of CTX-M type extended-spectrum β-lactamases among clinical isolates of gram-negative bacilli in Jimma, Ethiopia. BMC Infectious Diseases . 2018;18(1):p. 524. doi: 10.1186/s12879-018-3436-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barguigua A., El Otmani F., Talmi M., Zerouali K., Timinouni M. Prevalence and types of extended spectrum β-lactamases among urinary Escherichia coli isolates in Moroccan community. Microbial Pathogenesis . 2013;61:16–22. doi: 10.1016/j.micpath.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 7.Hammami S., Saidani M., Ferjeni S., Aissa I., Slim A., Boutiba-Ben Boubaker I. Characterization of extended spectrum β-lactamase-producing Escherichia coli in community-acquired urinary tract infections in Tunisia. Microbial Drug Resistance . 2013;19(3):231–236. doi: 10.1089/mdr.2012.0172. [DOI] [PubMed] [Google Scholar]
- 8.Rensing K. L., Abdallah H. M., Koek A., et al. Prevalence of plasmid-mediated AmpC in Enterobacteriaceae isolated from humans and from retail meat in Zagazig, Egypt. Antimicrobial Resistance & Infection Control . 2019;8(1):p. 45. doi: 10.1186/s13756-019-0494-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harada K., Shimizu T., Mukai Y., et al. Phenotypic and molecular characterization of antimicrobial resistance in Klebsiella spp. isolates from companion animals in Japan: clonal dissemination of multidrug-resistant extended-spectrum β-lactamase-producing Klebsiella pneumoniae. Frontiers in Microbiology . 2016;7 doi: 10.3389/fmicb.2016.01021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wohlwend N., Endimiani A., Francey T., Perreten V. Third-generation-cephalosporin-resistant Klebsiella pneumoniae isolates from humans and companion animals in Switzerland: spread of a DHA-producing sequence type 11 clone in a veterinary setting. Antimicrobial Agents and Chemotherapy . 2015;59(5):2949–2955. doi: 10.1128/AAC.04408-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perez F., Hujer A. M., Hujer K. M., Decker B. K., Rather P. N., Bonomo R. A. Global challenge of multidrugresistant Acinetobacter baumannii. Antimicrobial Agents and Chemotherapy . 2007;51:3471–3484. doi: 10.1128/AAC.01464-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun T., Nukaga M., Mayama K., Braswell E. H., Knox J. R. Comparison of β-lactamases of classes A and D: 1.5-A crystallographic structure of the class D OXA-1 oxacillinase. Protein Science . 2003;12(1):82–91. doi: 10.1110/ps.0224303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hawkey P. M., Warren R. E., Livermore D. M., et al. Treatment of infections caused by multidrug-resistant Gram-negative bacteria: report of the British Society for Antimicrobial Chemotherapy/Healthcare Infection Society/British Infection Association Joint Working Party. The Journal of Antimicrobial Chemotherapy . 2018;73(Supplement_3):iii2–iii78. doi: 10.1093/jac/dky027. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt K., Mwaigwisya S., Crossman L. C., et al. Identification of bacterial pathogens and antimicrobial resistance directly from clinical urines by nanopore-based metagenomic sequencing. The Journal of Antimicrobial Chemotherapy . 2017;72(1):104–114. doi: 10.1093/jac/dkw397. [DOI] [PubMed] [Google Scholar]
- 15.Ellington M. J., Ekelund O., Aarestrup F. M., et al. The role of whole genome sequencing in antimicrobial susceptibility testing of bacteria: report from the EUCAST subcommittee. Clinical Microbiology and Infection . 2017;23(1):2–22. doi: 10.1016/j.cmi.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 16.Moore N. M., Canton R., Carretto E., Peterson L. R., Sautter R. L., Traczewski M. M. Rapid identification of five classes of carbapenem resistance genes directly from rectal swabs by use of the Xpert Carba-R assay. Journal of Clinical Microbiology . 2017;55(7):2268–2275. doi: 10.1128/JCM.00137-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palavecino E. L. Rapid methods for detection of MRSA in clinical specimens. Methods in Molecular Biology . 2014;1085:71–83. doi: 10.1007/978-1-62703-664-1_3. [DOI] [PubMed] [Google Scholar]
- 18.CLSI. Performance Standards for Antimicrobial Susceptibility Testing. Clinical Lab Standards Institute . 2016;35(3):16–38. [Google Scholar]
- 19.Drieux L., Brossier F., Sougakoff W., Jarlier V. Phenotypic detection of extended-spectrum β-lactamase production in Enterobacteriaceae: review and bench guide. Clinical Microbiology and Infection . 2008;14(Supplement 1):90–103. doi: 10.1111/j.1469-0691.2007.01846.x. [DOI] [PubMed] [Google Scholar]
- 20.Chia J. H., Chu C., Su L. H., et al. Development of a multiplex PCR and SHV melting-curve mutation detection system for detection of some SHV and CTX-M β-lactamases of Escherichia coli, Klebsiella pneumoniae, and Enterobacter cloacae in Taiwan. Journal of Clinical Microbiology . 2005;43:4486–4491. doi: 10.1128/JCM.43.9.4486-4491.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y., Guo G., Wang H., et al. Comparison of multiplex real-time PCR and PCR-reverse blot hybridization assay for the direct and rapid detection of bacteria and antibiotic resistance determinants in positive culture bottles. Journal of Medical Microbiology . 2016;65(9):962–974. doi: 10.1099/jmm.0.000319. [DOI] [PubMed] [Google Scholar]
- 22.Thatcher S. A. DNA/RNA preparation for molecular detection. Clinical Chemistry . 2015;61(1):89–99. doi: 10.1373/clinchem.2014.221374. [DOI] [PubMed] [Google Scholar]
- 23.Jena J., Sahoo R. K., Debata N. K., Subudhi E. Prevalence of TEM, SHV, and CTX-M genes of extended-spectrum β-lactamase-producing Escherichia coli strains isolated from urinary tract infections in adults. Biotech . 2017;7(4) doi: 10.1007/s13205-017-0879-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tavakoly T., Jamali S., Mojtahedi A., Mirzaei M. K., Shenagari M. The prevalence of CMY-2, OXA-48 and KPC-2 genes in clinical isolates of Klebsiella spp. Cellular and Molecular Biology . 2018;64(3):40–44. doi: 10.14715/cmb/2018.64.3.7. [DOI] [PubMed] [Google Scholar]
- 25.Smiljanic M., Kaase M., Ahmad-Nejad P., Ghebremedhin B. Comparison of in-house and commercial real time-PCR based carbapenemase gene detection methods in Enterobacteriaceae and non-fermenting gram-negative bacterial isolates. Annals of Clinical Microbiology and Antimicrobials . 2017;16(1):p. 48. doi: 10.1186/s12941-017-0223-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Espy M. J., Uhl J. R., Sloan L. M., et al. Real-time PCR in clinical microbiology: applications for routine laboratory testing. Clinical Microbiology Reviews . 2006;19(1):165–256. doi: 10.1128/CMR.19.1.165-256.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ben Tanfous F., Raddaoui A., Chebbi C., Achour W. Epidemiology and molecular characterisation of colistin-resistant Klebsiella pneumoniae isolates from immunocompromised patients in Tunisia. International Journal of Antimicrobial Agents . 2018;52(6):861–865. doi: 10.1016/j.ijantimicag.2018.08.022. [DOI] [PubMed] [Google Scholar]
- 28.Elsayed M. S. A. E., Roshdey T., Salah A., Tarabees R., Younis G., Eldeep D. Phenotypic and genotypic methods for identification of slime layer production, efflux pump activity, and antimicrobial resistance genes as potential causes of the antimicrobial resistance of some mastitis pathogens from farms in Menoufia, Egypt. Molecular Biology Reports . 2019;46(6):6533–6546. doi: 10.1007/s11033-019-05099-6. [DOI] [PubMed] [Google Scholar]
- 29.Cantón R., Novais A., Valverde A., et al. Prevalence and spread of extended-spectrum β-lactamase-producing Enterobacteriaceae in Europe. Clinical Microbiology and Infection . 2008;14(Supplement 1):144–153. doi: 10.1111/j.1469-0691.2007.01850.x. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt K., Stanley K. K., Hale R., et al. Evaluation of multiplex tandem PCR (MTPCR) assays for the detection of bacterial resistance genes among Enterobacteriaceae in clinical urines. The Journal of Antimicrobial Chemotherapy . 2019;74(2):349–356. doi: 10.1093/jac/dky419. [DOI] [PubMed] [Google Scholar]
- 31.Chavada R., Maley M. Evaluation of a commercial multiplex PCR for rapid detection of multi drug resistant gram negative infections. The Open Microbiology Journal . 2015;9(1):125–135. doi: 10.2174/1874285801509010125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh P., Pfeifer Y., Mustapha A. Multiplex real-time PCR assay for the detection of extended-spectrum β-lactamase and carbapenemase genes using melting curve analysis. Journal of Microbiological Methods . 2016;124:72–78. doi: 10.1016/j.mimet.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 33.Willemsen I., Hille L., Vrolijk A., Bergmans A., Kluytmans J. Evaluation of a commercial real-time PCR for the detection of extended spectrum β-lactamase genes. Journal of Medical Microbiology . 2014;63(4):540–543. doi: 10.1099/jmm.0.070110-0. [DOI] [PubMed] [Google Scholar]
- 34.Jemima S. A., Verghese S. Molecular characterization of nosocomial CTX-M type β-lactamase producing Enterobacteriaceae from a tertiary care hospital in south India. Indian Journal of Medical Microbiology . 2008;26(4):365–368. doi: 10.1016/S0255-0857(21)01816-8. [DOI] [PubMed] [Google Scholar]
- 35.Tzouvelekis L. S., Tzelepi E., Tassios P. T., Legakis N. J. CTX-M-type β-lactamases: an emerging group of extended-spectrum enzymes. International Journal of Antimicrobial Agents . 2000;14(2):137–142. doi: 10.1016/S0924-8579(99)00165-X. [DOI] [PubMed] [Google Scholar]
- 36.Baraniak A., Fiett J., Hryniewicz W., Nordmann P., Gniadkowski M. Ceftazidime-hydrolysing CTX-M-15 extended-spectrum beta-lactamase (ESBL) in Poland. The Journal of Antimicrobial Chemotherapy . 2002;50(3):393–396. doi: 10.1093/jac/dkf151. [DOI] [PubMed] [Google Scholar]
- 37.Fam N., Gamal D., El Said M., et al. Detection of plasmid-mediated AmpC beta-lactamases in clinically significant bacterial isolates in a research institute hospital in Egypt. Life Science Journal . 2013;10(2):2294–2304. [Google Scholar]
- 38.Poirel L., Héritier C., Tolün V., Nordmann P. Emergence of oxacillinase mediated resistance to imipenem in Klebsiella pneumoniae. Antimicrobial Agents and Chemotherapy . 2004;48(1):15–22. doi: 10.1128/AAC.48.1.15-22.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Calbo E., Romaní V., Xercavins M., et al. Risk factors for community-onset urinary tract infections due to Escherichia coli harbouring extended-spectrum β-lactamases. The Journal of Antimicrobial Chemotherapy . 2006;57(4):780–783. doi: 10.1093/jac/dkl035. [DOI] [PubMed] [Google Scholar]
- 40.Woodford N., Dallow J. W., Hill R. L., et al. Ertapenem resistance among Klebsiella and Enterobacter submitted in the UK to a reference laboratory. International Journal of Antimicrobial Agents . 2007;29(4):456–459. doi: 10.1016/j.ijantimicag.2006.11.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data and additional information regarding this study are available to third parties under reasonable request.


