Abstract
SpoIIE is a bifunctional protein which controls ςF activation and formation of the asymmetric septum in sporulating Bacillus subtilis. The spoIIE gene of B. subtilis has now been overexpressed in Escherichia coli, and SpoIIE has been purified by anion-exchange chromatography and affinity chromatography. Kinetic studies showed that the rate of dephosphorylation of SpoIIAA-P by purified SpoIIE in vitro was 100 times greater, on a molar basis, than the rate of phosphorylation of SpoIIAA by SpoIIAB. The intracellular concentrations of SpoIIE and SpoIIAB were measured by quantitative immunoblotting between 0 and 4 h after the beginning of sporulation. The facts that these concentrations were very similar at hour 2 and that SpoIIE could be readily detected before asymmetric septation suggest that SpoIIE activity may be strongly regulated.
The successful completion of sporulation in the gram-positive bacterium B. subtilis depends on the activation of the first sporulation-specific transcription factor, ςF. The activation of ςF in the prespore, soon after asymmetric septation, not only induces the prespore-specific program of gene expression (13) but also mediates an intracellular signal across the septum which activates the first mother cell-specific transcription factor, ςE (27).
ςF, together with the two regulatory proteins SpoIIAA and SpoIIAB, is synthesized before asymmetric septation (for a review, see references 14 and 27). In the predivisional cell, the anti-ς factor SpoIIAB holds ςF in an inactive complex (2, 10, 21) and also maintains the anti-anti-ς factor SpoIIAA in an inactive form by phosphorylating it on a specific serine residue (2, 9, 18, 21, 23). After asymmetric septation, activation of ςF in the prespore is triggered by the action of the specific phosphatase SpoIIE, which catalyzes the dephosphorylation of SpoIIAA-P (11). When dephosphorylated, SpoIIAA releases ςF activity by binding to and sequestering the anti-ς factor SpoIIAB (4, 11, 12, 15, 19, 25).
SpoIIE is a membrane-bound protein containing, in its N-terminal region, 10 membrane-spanning segments inserted into the asymmetric septum that separates the prespore from the mother cell (3, 6) and, in its C-terminal region, a large cytoplasmic domain which includes the phosphatase activity (11). The phosphatase domain of SpoIIE has some sequence similarity to the PP2C family of eukaryotic Ser/Thr protein phosphatases (1), especially in the metal binding residues, suggesting a conserved metal binding site and a common mechanism of phosphate recognition by the metal ions (8). The fact that the serine phosphatase activity is fused to a membrane domain may help to ensure a specific orientation of the protein, which provides activation of ςF only in the prespore (3, 11). Indeed, recent immunofluorescence studies provide evidence that SpoIIE may be localized exclusively to the prespore face of the septum (16, 29). In addition, SpoIIE is required for the proper formation of the asymmetric septum itself (7, 15). It has been suggested that SpoIIE acts to couple asymmetric septation to ςF activity (11, 15).
Given the apparent importance of SpoIIE in the establishment of ςF activity and thus of differential gene expression in sporulation, we have been studying the mechanism by which SpoIIE activity is itself regulated. In the present paper, we describe the purification of the full-length protein SpoIIE to apparent homogeneity and some characteristics of the reaction in which SpoIIE catalyzes the dephosphorylation of SpoIIAA-P. We have also determined the intracellular concentration of the enzyme in samples taken at various times during sporulation.
MATERIALS AND METHODS
Cloning.
The wild-type spoIIE gene was amplified by PCR (GeneAmp XL PCR kit; Perkin-Elmer) following the protocol given by the supplier. The oligonucleotides 5′-CAGGTGGGAGATGAGACATATGGAAAAAGC-3′, generating an NdeI restriction site (underlined) covering the start codon (ATG) of the spoIIE gene, and 5′-GCGGATCCCATATATTCCCATCTTCGCCAGAAG-3′, generating a BamHI restriction site (underlined) at the 3′ end of the spoIIE gene, were used to amplify the complete spoIIE gene from chromosomal DNA of B. subtilis SG38. The sequence of the 2,568-bp product was confirmed by means of the DNA sequencing kit, with Amplitaq DNA polymerase (Perkin-Elmer) and an ABI1373A automated sequencer (Applied Biosystems). The gene was cloned into NdeI/BamHI-digested pET11a (Novagen). The ATG start codon of the spoIIE gene was located 8 nucleotides downstream of the highly efficient Shine-Dalgarno sequence of the phage T7 major capsid protein encoded by the vector plasmid. The Escherichia coli strain C41(DE3) was transformed with the recombinant plasmid pRB1011.
Overexpression of spoIIE and membrane isolation.
To stimulate overproduction of SpoIIE, the E. coli clone containing pRB1011 was inoculated into 10 ml of 2YT medium containing ampicillin (100 μg/ml) and grown at 30°C for 1 to 2 h. This culture was used to inoculate a 3-liter volume of the same medium, which was induced with 0.7 mM IPTG (isopropyl-β-d-thiogalactopyranoside) when the A600 reached 0.6. The cells were harvested by centrifugation at 4°C after overnight growth for 15 h at room temperature. The cells from a 3-liter volume were resuspended in 50 ml of 100 mM Tris-HCl (pH 7.5) containing 5 mM EDTA, 5 mM EGTA, 1 mM dithiothreitol (DTT), 1 mM phenylmethylsulfonyl fluoride (PMSF), 10% glycerol, and 0.1% Triton X-100 and then disrupted in a precooled French press. The homogenate was centrifuged for 10 min at 3,000 × g to remove the cell debris, and the membrane fraction was recovered by centrifugation for 4 h at 40,000 × g. To remove peripheral membrane proteins, the membrane pellet was resuspended in the same buffer supplemented with 3 M KCl and was recentrifuged for 4 h. The washed membranes were suspended in a solution containing 50 mM Tris-HCl (pH 7.5), 1 mM DTT, 1 mM PMSF, 10% glycerol, and 8% sucrose and stored at −70°C.
Purification.
Proteins were extracted by treatment of the membrane fraction with a solution containing 5% Triton X-100, 50 mM Tris-HCl (pH 8.0), 50 mM NaCl, 1 mM DTT, 1 mM PMSF, and 10% glycerol for 3 h at 4°C. After centrifugation for 4 h at 40,000 × g, the supernatant was loaded onto a 30-ml DEAE-Sepharose column equilibrated in 50 mM Tris-HCl (pH 8.0) containing 0.5 mM EDTA, 0.5 mM DTT, 0.5 mM PMSF, 10% glycerol, and 1% Triton X-100 (buffer A). Proteins were eluted with a linear gradient (0 to 0.7 M) of NaCl in the same buffer. The fractions containing SpoIIE, which eluted between 0.2 and 0.3 M NaCl, were pooled and applied to a small column (1 by 2 cm) of Affi-Gel blue (Pharmacia) equilibrated in a solution containing 50 mM Tris-HCl (pH 7.5), 200 mM NaCl, 0.5 mM EDTA, 0.5 mM DTT, 0.5 mM PMSF, 10% glycerol, and 1% Triton X-100. The column was washed with 30 ml of the same buffer, and SpoIIE was eluted with 30 ml of a linear NaCl gradient (0.2 to 2 M). Fractions of 1.5 ml were collected. The enzymically active fractions, eluting between 0.8 and 1 M NaCl, were combined, dialyzed against a solution containing 50 mM Tris-HCl (pH 6.8), 50 mM NaCl, 0.5 mM EDTA, 0.5 mM DTT, 0.5 mM PMSF, 10% glycerol, and 1% Triton X-100, and applied to cation-exchange SP Hi-trap (Pharmacia) which had been equilibrated in the same buffer. The column was developed at a flow rate of 1 ml/min with a 30-ml linear gradient (0 to 0.8 M NaCl). The fractions containing purified SpoIIE eluted at about 0.35 M NaCl. They were dialyzed against buffer A (pH 7.5), concentrated with the use of Centriplus concentrators (Amicon), and stored in 50% glycerol at −70°C.
Removal of Triton X-100 by Extracti-gel D.
The purified enzyme was applied to a 1-ml Extracti-gel D column (Pierce) equilibrated at room temperature in a solution containing 50 mM Tris-HCl (pH 7.5), 50 mM NaCl, 10% glycerol, 0.5 mM EDTA, 0.5 mM DTT, and 0.5 mM PMSF. Elution was performed with the same buffer. SpoIIE, which eluted in the void volume, was assayed for activity in the presence of different detergents.
Purification of the C-terminal fragment of SpoIIE.
The histidine-tagged, carboxy-terminal fragment of SpoIIE was overexpressed as previously described (29) and purified from inclusion bodies by metal chelation chromatography after being solubilized with 6 M guanidine according to the Qiagen protocol. To recover the activity of the C-terminal fragment, the protein was renatured on the column (by means of a linear gradient of 6 to 0 M guanidine) over a period of 10 h, before elution with 200 mM imidazole.
Determination of protein concentration.
Protein concentration was determined after electrophoresis of purified proteins and SYPRO orange staining. The gel was scanned with a FluorImager (Molecular Dynamics), and the ImageQuant software package was used to calculate the quantities of SpoIIE and C-terminal fragment in the unknown samples, with known protein molecular weight standards used for calibration.
Western blot analysis.
To raise antibodies against SpoIIE, the C-terminal fragment of SpoIIE was purified from inclusion bodies by a Prep Cell (model 491; Bio-Rad) as previously described (29). The antibodies were purified by protein A Sepharose (Pharmacia), and the purified antiserum was used at a dilution of 1/2,000 for Western blot analysis.
Assay for dephosphorylation of SpoIIAA-P.
SpoIIAA (50 μM final concentration) was first phosphorylated in a 1-ml reaction volume containing 50 mM Tris-HCl (pH 7.5), 50 mM KCl, 1 mM DTT, 750 μM MgCl2, 100 μM ATP (including 100 μCi of [γ-32P]ATP), and 5 μM SpoIIAB. After complete phosphorylation (2 h at 37°C and overnight at 4°C), SpoIIAA-P was separated from SpoIIAB on a 1-ml DEAE-Sepharose column equilibrated in a solution containing 50 mM Tris-HCl (pH 8.0) and 1 mM DTT. The fractions containing SpoIIAA-P (eluted with 200 mM NaCl) were dialyzed extensively against a solution containing 50 mM Tris-HCl (pH 7.5), 50 mM NaCl, and 1 mM DTT to remove the [γ-32P]ATP.
Dephosphorylation was carried out at 30°C in a 300-μl volume containing 50 mM Tris-HCl (pH 7.5), 50 mM NaCl, 1 mM DTT, 10 mM MnCl2, and 5 or 15 μM [32P]SpoIIAA-P. The reaction was started by the addition of either SpoIIE or the C-terminal fragment of SpoIIE (2 to 60 nM). Samples (20 μl) were taken at different times and assayed as previously described (24).
Quantification of SpoIIE and SpoIIAB during sporulation.
B. subtilis cell extracts were prepared by incubating 1-ml cell pellets in 250 μl of 50 mM Tris-HCl (pH 7.5) containing 5 mg of lysozyme per ml, 1 mM DTT, 1 mM PMSF, and 10% glycerol for 10 min at 37°C and sonicating them briefly. These extracts were then subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotted with purified antibodies raised either against the C-terminal fragment of SpoIIE or against SpoIIAB. Each blot included known volumes of standard solutions of purified protein. The quantities of SpoIIE and SpoIIAB in the samples were determined by the method of Lord et al. (17).
RESULTS AND DISCUSSION
Overproduction and purification of the full-length protein SpoIIE.
The wild-type spoIIE gene from B. subtilis was amplified by PCR and cloned into the pET11a vector as described in Materials and Methods. E. coli C41(DE3) was selected for SpoIIE overproduction. This strain has been used successfully for overproduction of membrane proteins. It reproducibly yields a higher level of expression and less toxicity than BL21(DE3) (22).
To determine the conditions for maximal overexpression of spoIIE, a Western blot was performed on cell extracts prepared after cells were grown in various conditions and induced by IPTG. Maximal expression of spoIIE occurred when the cells were grown at 30°C and harvested approximately 15 h after induction (data not shown). About 75% of the total SpoIIE was membrane associated. The membrane-bound phosphatase could not be released by treatment with 3 M KCl, confirming that the enzyme is an integral membrane protein. We therefore tested various detergents {3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate [CHAPS], octoglucoside, and Triton X-100} for their ability to solubilize the enzyme. Triton X-100 (5%) was found to be the most effective. To keep the protein in solution, only a 1% final concentration of Triton X-100 needed to be added to the buffers during the purification.
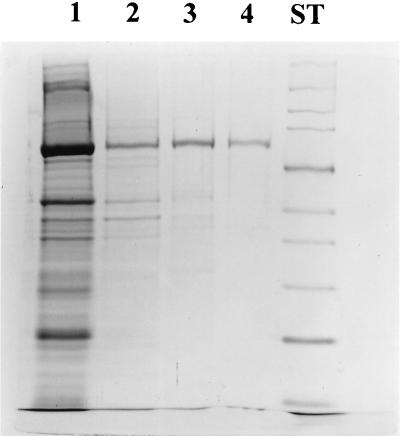
The solubilized membrane extract from a 3-liter culture was purified through a number of steps as described in Materials and Methods. Figure 1 shows the purity of the preparation at each stage. The final preparation was more than 95% pure as estimated from an overloaded gel stained by silver staining.
FIG. 1.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of the results of SpoIIE purification stained with Coomassie blue. The membrane extract was treated with 5% Triton X-100, and the solubilized proteins (lane 1) were applied to a DEAE-Sepharose column. The fractions containing SpoIIE were pooled (lane 2) and applied to an Affi-Gel blue column. After dialysis, the active fractions (lane 3) were loaded on a cation-exchange column. The fractions containing purified SpoIIE (lane 4) were concentrated and stored in 50% glycerol. The molecular mass standards (lane ST) are, from the top, myosin (212 kDa), MBP-β-galactosidase (158 kDa), β-galactosidase (116 kDa), phosphorylase b (97 kDa), bovine serum albumin (66 kDa), glutamate dehydrogenase (55 kDa), MBP2 (42 kDa), lactate dehydrogenase M (36 kDa), triosephosphate isomerase (26 kDa), and trypsin inhibitor (20 kDa).
Apparent molecular weight of the purified SpoIIE.
To measure the apparent molecular weight of SpoIIE, we analyzed the final enzyme preparation by gel filtration chromatography on Superose 12. The elution position of the native enzyme corresponded to an apparent Mr of 250,000 (results not shown) even when chromatography was performed in the presence of 1 M NaCl. Assuming that the micellar weight of Triton X-100 is 66,700 at 4°C (28), these results imply that the Mr of SpoIIE is about 180,000. However, the Mr of SpoIIE calculated from the gene sequence is 91,500. Since SpoIIE has a large membrane-spanning domain, the enzyme may be aggregated even in the presence of Triton X-100. Alternatively, this finding may indicate that SpoIIE is dimeric, as was recently reported for PP2C of Plasmodium falciparum (20).
Kinetic properties of the dephosphorylation of SpoIIAA-P.
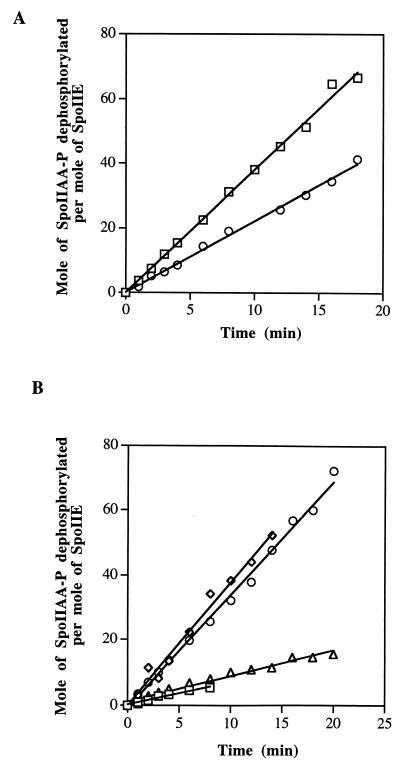
As previously described (11), SpoIIE catalyzes hydrolysis of SpoIIAA-P, a reaction dependent on either Mn2+ or Mg2+. The reaction was completely inhibited by inorganic phosphate or by EDTA (data not shown). To study the kinetics of this reaction, we incubated SpoIIE with excess purified [32P]SpoIIAA-P and measured the amount of Pi produced. We first studied the kinetics in the presence of 1% Triton X-100. Figure 2A shows that dephosphorylation of SpoIIAA-P appeared to be linear with time. The turnover number (moles of SpoIIAA-P dephosphorylated per mole of SpoIIE) was 6 × 10−2 to 7 × 10−2 s−1 and was similar for all concentrations of SpoIIE from 2 to 60 nM.
FIG. 2.
(A) Time course of dephosphorylation of SpoIIAA-P by SpoIIE (squares) and by the C-terminal fragment of SpoIIE (circles). (B) Time course of dephosphorylation of SpoIIAA-P by SpoIIE in the absence of detergents (squares) and in the presence of Triton X-100 (diamonds), CHAPS (circles), and octoglucoside (triangles). The assays were as described in Materials and Methods.
We then tested the effect of various detergents on the phosphatase activity of the full-length protein, after first removing Triton X-100 with an Extracti-gel D column (see Materials and Methods) (Fig. 2B). The rate of dephosphorylation in CHAPS was similar to that found with the initial preparation in Triton X-100, but there was little activity in octoglucoside. With no detergent in the reaction mixture, the protein was still active, but at low concentrations the rate of hydrolysis of SpoIIAA-P was not proportional to the concentration of the enzyme (results not shown). The presence of either CHAPS or Triton X-100 abolished this nonlinearity of response.
The specific activity of the C-terminal fragment of SpoIIE (purified from inclusion bodies and renatured) was about 70% of that of the full-length protein (Fig. 2A). One possible interpretation of the data is that the purified full-length protein is fully active and that we recovered 70% of the activity from the C-terminal fragment by renaturation from inclusion bodies. Alternatively, it may be that all of the activity of the C-terminal fragment has been recovered but that the N terminus of the full-length protein plays a role in modulating its activity in the cell.
Intracellular concentration of the SpoIIE compared to SpoIIAB.
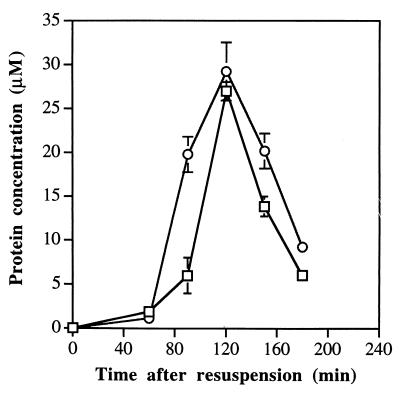
To see whether we could find evidence for the suggestion that the phosphatase activity of SpoIIE is regulated in the cell, we determined the concentration of SpoIIE in sporulating B. subtilis cells and compared it with that of SpoIIAB. Samples were taken at various times during the first 4 h of sporulation and assayed by quantitative immunoblotting with purified antibodies raised against either SpoIIAB or the C-terminal fragment of SpoIIE. At 0 h of sporulation (t0), both proteins were barely detectable. As sporulation proceeded, the intracellular concentration of both proteins increased quickly until about t2 (Fig. 3). Thereafter, the concentration of SpoIIE declined, and the protein was hardly detectable at t4, suggesting that it had been degraded (26). A degradation product with a molecular weight of 50,000 was detectable with anti-SpoIIE antibodies at t4. As Fig. 3 shows, the molar ratios of SpoAB/SpoIIE were close to unity both at t1 and at t2. However, in the sample taken at t1.5, the concentration of SpoIIAB exceeded that of SpoIIE by around threefold. To check whether this observation was reproducible, we took samples from another, independent sporulation experiment. In this second experiment, the molar ratios of SpoAB/SpoIIE were 1.1 at t1, 2.8 at t1.5, and 1.3 at t2. We conclude that, shortly after t1, the concentration of SpoIIAB rises much more sharply than that of SpoIIE.
FIG. 3.
Changes in intracellular concentration of SpoIIE (squares) and SpoIIAB (circles) during the first 4 h of sporulation. Samples were collected and assayed as described in Materials and Methods. Each point shown is the mean of three determinations. The error bars give the range of the three estimates at each time point; no error bars are shown where the range of three determinations is too small to fit conveniently on the figure.
SpoIIE activity may be regulated in the cell.
We have previously proposed that after asymmetric septation, nonphosphorylated SpoIIAA, generated in the prespore by the activity of SpoIIE, sequesters SpoIIAB in the kinase reaction and keeps the latter from inhibiting ςF (19). For this mechanism to be effective, the activity of SpoIIE in hydrolyzing SpoIIAA-P to SpoIIAA would need to be no less than that of SpoIIAB in phosphorylating SpoIIAA to SpoIIAA-P, but one would not expect the activities of the two enzymes to differ enormously. In fact, however, under the conditions of the assays in vitro, the rate of dephosphorylation by SpoIIE was about 100 times higher than the rate of phosphorylation by SpoIIAB. Moreover, the time course of accumulation of nonphosphorylated SpoIIAA, previously published by Magnin et al. (19), closely follows that for the appearance of SpoIIE, which suggests that SpoIIE activity is not present in great excess. Again, the results given in Fig. 3 show that SpoIIE is detectable by immunoblotting 1 h after the initiation of sporulation, which is well before the formation of the asymmetric septum. It seems unlikely that this SpoIIE is fully active; if it were, nonphosphorylated SpoIIAA would be generated in the predivisional cell and ςF would be liberated prematurely.
These arguments suggest that SpoIIE activity is likely to be regulated in vivo and/or that the high activity that is observed in vitro is an artifact of our having the enzyme in a soluble, rather than a membrane-bound, form. It has been suggested that in the recently discovered Spalten protein of Dictyostelium, the membrane-associated N-terminal domain regulates the phosphatase activity that is located in the soluble C-terminal domain (5). If SpoIIE is confined to the prespore side of the asymmetric septum of the sporulating cell (3, 6, 29), its local concentration will be substantially higher than that shown in Fig. 3. The high concentration of SpoIIE is likely to result in the rapid hydrolysis of all the SpoIIAA-P in the prespore, which will in turn ensure the rapid activation of ςF in that compartment.
ACKNOWLEDGMENTS
We thank Julie Wickson for outstanding technical assistance and A. Feucht and J. Errington for their comments on the manuscript.
The Biotechnology and Biological Sciences Research Council supported this work.
REFERENCES
- 1.Adler E, Donella-Deana A, Arigoni F, Pinna L A, Stragier P. Structural relationship between a bacterial developmental protein and eucaryotic PP2C protein phosphatases. Mol Microbiol. 1997;23:57–62. doi: 10.1046/j.1365-2958.1997.1801552.x. [DOI] [PubMed] [Google Scholar]
- 2.Alper S, Duncan L, Losick R. An adenosine nucleotide switch controlling the activity of a cell type-specific transcription factor in B. subtilis. Cell. 1994;77:195–205. doi: 10.1016/0092-8674(94)90312-3. [DOI] [PubMed] [Google Scholar]
- 3.Arigoni F, Pogliano K, Webb C D, Stragier P, Losick R. Localization of protein implicated in establishment of cell type to sites of asymmetric division. Science. 1995;270:637–640. doi: 10.1126/science.270.5236.637. [DOI] [PubMed] [Google Scholar]
- 4.Arigoni F, Duncan L, Alper S, Losick R, Stragier P. SpoIIE governs the phosphorylation state of a protein regulating transcription factor ςF during sporulation in Bacillus subtilis. Proc Natl Acad Sci USA. 1996;93:3238–3242. doi: 10.1073/pnas.93.8.3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aubry L, Firtel R A. Spalten, a protein containing Gα-protein-like and PP2C domains, is essential for cell-type differentiation in Dictyostelium. Genes Dev. 1998;12:1525–1538. doi: 10.1101/gad.12.10.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barak I, Behari J, Olmedo G, Guzman P, Brown D P, Castro E, Walker D, Westpheling J, Youngman P. Structure and function of the Bacillus SpoIIE protein and its localization to sites of sporulation septum assembly. Mol Microbiol. 1996;19:1047–1060. doi: 10.1046/j.1365-2958.1996.433963.x. [DOI] [PubMed] [Google Scholar]
- 7.Barak I, Youngman P. SpoIIE mutants of Bacillus subtilis comprise two distinct phenotypic classes consistent with a dual functional role for the SpoIIE protein. J Bacteriol. 1996;178:4984–4989. doi: 10.1128/jb.178.16.4984-4989.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Das A K, Helps N R, Cohen P T W, Barford D. Crystal structure of the protein serine/threonine phosphatase 2C at 2.0 A resolution. EMBO J. 1996;15:6798–6809. [PMC free article] [PubMed] [Google Scholar]
- 9.Diederich B, Wilkinson J F, Magnin T, Najafi S M A, Errington J, Yudkin M D. Role of interactions between SpoIIAA and SpoIIAB in regulating cell-specific transcription factor ςF of Bacillus subtilis. Genes Dev. 1994;8:2653–2663. doi: 10.1101/gad.8.21.2653. [DOI] [PubMed] [Google Scholar]
- 10.Duncan L, Losick R. SpoIIAB is an anti-ς factor that binds to and inhibits transcription by regulatory protein ςF from Bacillus subtilis. Proc Natl Acad Sci USA. 1993;90:2325–2329. doi: 10.1073/pnas.90.6.2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duncan L, Alper S, Arigoni F, Losick R, Stragier P. Activation of cell-specific transcription by a serine phosphatase at the site of asymmetric division. Science. 1995;270:641–644. doi: 10.1126/science.270.5236.641. [DOI] [PubMed] [Google Scholar]
- 12.Duncan L, Alper S, Losick R. SpoIIAA governs the release of the cell-specific transcription factor ςF from its anti-sigma factor SpoIIAB. J Mol Biol. 1996;260:147–164. doi: 10.1006/jmbi.1996.0389. [DOI] [PubMed] [Google Scholar]
- 13.Errington J. Bacillus subtilis sporulation: regulation of gene expression and control of morphogenesis. Microbiol Rev. 1993;57:1–33. doi: 10.1128/mr.57.1.1-33.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Errington J. Determination of cell fate in Bacillus subtilis. Trends Genet. 1996;12:31–34. doi: 10.1016/0168-9525(96)81386-2. [DOI] [PubMed] [Google Scholar]
- 15.Feucht A, Magnin T, Yudkin M D, Errington J. Bifunctional protein required for asymmetric cell division and cell-specific transcription in Bacillus subtilis. Genes Dev. 1996;10:794–803. doi: 10.1101/gad.10.7.794. [DOI] [PubMed] [Google Scholar]
- 16.Lewis P J, Wu L J, Errington J. Establishment of prespore-specific gene expression in Bacillus subtilis: localization of SpoIIE phosphatase and initiation of compartment-specific proteolysis. J Bacteriol. 1998;180:3276–3284. doi: 10.1128/jb.180.13.3276-3284.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lord M, Barillà D, Yudkin M D. Replacement of vegetative ςA by sporulation-specific ςF as a component of the RNA polymerase holoenzyme in sporulating Bacillus subtilis. J Bacteriol. 1999;181:2346–2350. doi: 10.1128/jb.181.8.2346-2350.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Magnin T, Lord M, Errington J, Yudkin M D. Establishing differential gene expression in sporulating Bacillus subtilis: phosphorylation of SpoIIAA (anti-anti ςF) alters its conformation and prevents formation of a SpoIIAA/SpoIIAB/ADP complex. Mol Microbiol. 1996;19:901–907. doi: 10.1046/j.1365-2958.1996.434964.x. [DOI] [PubMed] [Google Scholar]
- 19.Magnin T, Lord M, Yudkin M D. Contribution of partner switching and SpoIIAA cycling to regulation of ςF activity in sporulating Bacillus subtilis. J Bacteriol. 1997;179:3922–3927. doi: 10.1128/jb.179.12.3922-3927.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mamoun C B, Sullivan D J, Jr, Banerjee R, Goldberg D E. Identification and characterization of an unusual double serine/threonine protein phosphatase 2C in the malaria parasite Plasmodium falciparum. J Biol Chem. 1998;273:11241–11247. doi: 10.1074/jbc.273.18.11241. [DOI] [PubMed] [Google Scholar]
- 21.Min K-T, Hilditch C M, Diederich B, Errington J, Yudkin M D. ςF, the first compartment-specific transcription factor of Bacillus subtilis, is regulated by an anti-ς factor that is also a protein kinase. Cell. 1993;74:735–742. doi: 10.1016/0092-8674(93)90520-z. [DOI] [PubMed] [Google Scholar]
- 22.Miroux B, Walker J E. Over-production of proteins in Escherichia coli: mutant hosts that allow synthesis of some membrane proteins and globular proteins at high levels. J Mol Biol. 1996;260:289–298. doi: 10.1006/jmbi.1996.0399. [DOI] [PubMed] [Google Scholar]
- 23.Najafi S M A, Willis A C, Yudkin M D. Site of phosphorylation of SpoIIAA, the anti-anti-sigma factor for sporulation-specific ςF of Bacillus subtilis. J Bacteriol. 1995;177:2912–2913. doi: 10.1128/jb.177.10.2912-2913.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Najafi S M A, Harris D A, Yudkin M D. The SpoIIAA protein of Bacillus subtilis has GTP-binding properties. J Bacteriol. 1996;178:6632–6634. doi: 10.1128/jb.178.22.6632-6634.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Najafi S M A, Harris D A, Yudkin M D. Properties of the phosphorylation reaction catalyzed by SpoIIAB that help to regulate sporulation of Bacillus subtilis. J Bacteriol. 1997;179:5628–5631. doi: 10.1128/jb.179.17.5628-5631.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pogliano K, Hofmeister A E M, Losick R. Disappearance of the ςE transcription factor from the forespore and the SpoIIE phosphatase from the mother cell contributes to establishment of cell-specific gene expression during sporulation in Bacillus subtilis. J Bacteriol. 1997;179:3331–3341. doi: 10.1128/jb.179.10.3331-3341.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stragier P, Losick R. Molecular genetics of sporulation in Bacillus subtilis. Annu Rev Genet. 1996;30:297–341. doi: 10.1146/annurev.genet.30.1.297. [DOI] [PubMed] [Google Scholar]
- 28.Weinhold P A, Rounsifer M E, Feldman D A. The purification and characterization of CTP:phosphorylcholine cytidyltransferase from rat liver. J Biol Chem. 1986;261:5104–5110. [PubMed] [Google Scholar]
- 29.Wu L J, Feucht A, Errington J. Prespore-specific gene expression in Bacillus subtilis is driven by sequestration of SpoIIE phosphatase to the prespore side of the asymmetric septum. Genes Dev. 1998;12:1371–1380. doi: 10.1101/gad.12.9.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]