Abstract
Background:
A human study entitled “Learning Early About Peanut Allergy (LEAP)” showed that early introduction of peanut products decreases the prevalence of peanut allergy among children. However, the immunological mechanisms mediating the protective effects of consuming peanut products are not well understood.
Objective:
The objective was to develop a mouse model that simulates the LEAP study and investigate the underlying mechanisms for the study observations.
Methods:
Adult naïve BALB/c mice were fed a commercial peanut butter product (Skippy™) or buffer control and concomitantly exposed to peanut flour through the airway or skin to mimic environmental exposure. The animals were analyzed for anaphylactic reaction and by molecular and immunologic approaches.
Results:
Following exposure to peanut flour through the airway or skin, naïve mice developed peanut allergy, as demonstrated by acute and systemic anaphylaxis in response to challenge with peanut extract. Ingestion of Skippy™, however, nearly abolished the increase in peanut-specific immunoglobulin (Ig)E and IgG and protected animals from developing anaphylaxis. Skippy™-fed mice showed reduced numbers of T follicular helper (Tfh) cells and germinal center B cells in their draining lymph nodes, and single-cell RNA sequencing revealed a CD4+ T cell population expressing cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) in these animals. Critically, blocking CTLA-4 with antibody increased levels of peanut-specific antibodies and reversed the protective effects of Skippy™.
Conclusion:
Ingestion of a peanut product protects mice from peanut allergy induced by environmental exposure to peanuts, and the CTLA-4 pathway, which regulates Tfh responses, likely plays a pivotal role in this protection.
Keywords: Follicular T cells, IgE, allergy, allergens, peanuts, CTLA-4
Graphical Abstract
INTRODUCTION
Peanut allergy is one of the most common food allergies,1 the prevalence of which has increased over the past two decades among children in societies with Westernized lifestyles.2 In those with peanut allergy, accidental exposure to peanut-containing foods can trigger allergic responses involving multiple organ symptoms and systemic anaphylactic reactions.3 Due to both the prevalence and severity of peanut allergy, a major effort has been undertaken by both the biomedical community and the United States government to develop safe and effective strategies for preventing and treating peanut allergy. For this to be successful, however, it is critical to elucidate the mechanism(s) through which children develop peanut allergy and determine how the pathway can be disrupted to prevent disease.
Generally, development of peanut allergy is affected by several factors, including the microbiome,4 genetics,5, 6 and the environment.7 In particular, the routes of peanut exposure early in life are likely one of the key environmental factors that determine clinical outcomes of peanut allergy.4, 8, 9 Indeed, a clinical study showed that an individual is likely to become sensitized to peanuts if they are exposed to peanut allergens in the environment, perhaps through the skin or airway.7 Intriguingly, in a double-blind, placebo-controlled study, entitled “Learning Early About Peanut Allergy” (LEAP), children who consumed peanut products did not show increased allergy prevalence but rather were protected from developing peanut allergy at 5 years of age.10 Notably, however, peanut allergens were detected in house dust from not only the families who consumed peanuts but also from those who avoided them, albeit at lower levels,10 suggesting that all if not a majority of children in the study were exposed to peanut dust via inhalation or through skin.8, 9 Based on these observations, an expert panel of the National Institute of Allergy and Infectious Diseases (NIAID) recommended early introduction of peanut foods to infants to prevent development of peanut allergy.11
Critically, however, we have only limited understanding of the immunological mechanisms that explain this apparent paradox in which children who avoid peanuts are sensitized, and those who consume peanuts are protected. Therefore, the goal of this project was to begin to fill this knowledge gap by creating a mouse model that recapitulates key elements of the LEAP study and using this model to investigate the mechanisms underlying the study’s observations. It was previously shown that wild-type (WT) laboratory mice provide an ideal model for studying early life events in humans, as adult laboratory mice housed in specific pathogen-free (SPF) conditions are immunologically comparable to human infants.12 Here, we simulated conditions from the LEAP study by feeding naïve WT adult mice a popular commercial peanut butter product (Skippy™, Hormel Foods, Austin, MN) by oral gavage or ad lib; to mimic environmental exposure, mice were then exposed to peanut flour particles without any adjuvants by painting on the skin or airway inhalation.13, 14 We found that, unlike control animals, Skippy™-fed mice do not become sensitized and are protected from developing peanut allergy induced by skin or airway exposure. Further, our data suggest a pivotal role for the immune checkpoint molecule cytotoxic T-lymphocyte-associated protein (CTLA-4), which is expressed on CD4+ T cells in draining lymph nodes (dLNs), in regulating T follicular helper (Tfh) cell and germinal center (GC) B cell responses and suppressing production of peanut-specific immunoglobulin (Ig)E and IgG antibodies. Thus, using an experimental mouse model of peanut allergy development, we provide evidence that the CTLA-4 pathway may be involved in protection from peanut allergy conferred by oral ingestion of peanut products.
MATERIALS AND METHODS
Mice
BALB/c, C.Cg-Foxp3tm2Tch/J (Foxp3eGFP), C.129-Il4tm1Lky/J (Il4eGFP or 4get) and C57BL/6 mice were purchased from Jackson Laboratory (Bar Harbor, ME, USA) and maintained under SPF conditions at the Mayo Clinic (Rochester, MN, USA and Scottsdale, AZ, USA). Mice used in this study were females, 7 to 12 weeks of age, unless specified in the text or figure legends. All protocols and procedures for the handling of mice were reviewed and approved by the Mayo Institutional Animal Care and Use Committee, Mayo Clinic.
Allergens
Peanut flour was purchased from the Golden Peanut Company (Alpharetta, GA, USA). Endotoxin was undetectable in flour (<0.5 EU/mg flour), as noted previously.13 Crude peanut extract for intraperitoneal (i.p.) challenge was purchased from Greer Laboratories (Lenoir, NC, USA).13 Skippy™ peanut butter (22% peanut protein) and Skippy™ Peanut Butter (P.B.) Bites (18% peanut protein) was purchased from local grocery stores.
Ingestion of the peanut product
Prior to intranasal (i.n.) or epicutaneous (e.c.) exposure to peanut flour (see below), naïve mice were orally administered either 200 mg Skippy™ peanut butter slurry diluted in calcium bicarbonate buffer (pH 8.0) or calcium bicarbonate buffer alone by oral gavage, once daily, 5 days per week, for 1 week (Fig. 1A). Subsequently, during peanut flour exposure, mice were fed either 330 mg peanut butter diluted in calcium bicarbonate buffer or buffer alone by gavage, 3 times per week; thus, the dose of peanut butter product was 1 g/week (i.e. 220 mg peanut protein/week). This dosage per mouse was based on infant consumption recommendations made by the NIAID11 and adapted for the surface area of mice15 using the average for a 20-g mouse.
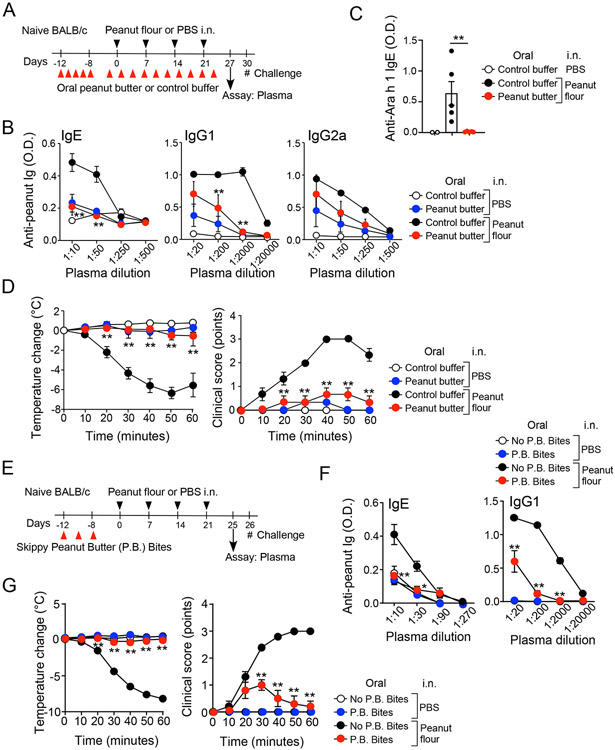
Figure 1.
Oral administration of peanut butter prevents peanut allergy induced by intranasal (i.n.) exposure to peanut flour. (A) Schematic overview of the experimental protocol. (B) Plasma levels of peanut-specific IgE, IgG1, and IgG2a are shown. Serial dilution of plasma was used to determine antibody titers. (C) Plasma levels of Ara h 1-specific IgE are shown. Each dot represents one mouse. (D) After intraperitoneal challenge with peanut extract, changes in rectal temperature and clinical scores were monitored. Data are presented as the mean ± SEM (n=4–6 in each group) and are representative of six experiments. **P<0.01 compared to mice fed control buffer and subjected to i.n. peanut flour exposure or between the groups indicated by horizonal lines. (E) Schematic overview of the experimental protocol for Panels F and G. (F) Plasma levels of peanut-specific IgE and IgG1 are shown. (G) Changes in rectal temperature and clinical scores were monitored. Data are presented as the mean ± SEM (n=6 in each group). *P<0.05 and **P<0.01 compared to mice that did not consume P.B. Bites and subjected to i.n. peanut flour exposure. P.B. Bites, Skippy™ Peanut Butter (P.B.) Bites.
Alternatively, mice were fed Skippy™ peanut butter ad lib. To do this, regular mouse chow was replaced with 15-20 pieces of Skippy™ P.B. Bites for 24 hours and replaced back to the regular chow. During this 24-hour period, mice had free access to P.B. Bites. In average, a mouse consumed approximately 1.1 g P.B. Bites/24 hours (i.e. 200 mg peanut protein/24 hours). The experimental protocols using P.B. Bites were largely identical to those using peanut butter slurry oral gavage except mice were fed P.B. Bites three times per week before i.n. peanut flour exposure and twice per week during the exposure. No significant differences in body weight were observed between mice that consumed P.B. Bites and those kept in mouse chow for the entire experimental period.
Allergic sensitization to peanut by i.n. and e.c. exposure
For i.n. exposure, naïve mice were lightly anesthetized with isoflurane and exposed intranasally to either 100 μg peanut flour in 50 μl sterile phosphate-buffered saline (PBS) or PBS alone, as previously described.13 Mice were exposed once a week for 4 weeks. For e.c. exposure, the fur from an approximately 5-cm2 area of abdominal skin was removed by clipping, followed by application of depilatory cream once a week. Tape stripping was then applied to the exposed abdominal skin seven times using 3M (Maplewood, MN, USA) Durapore® Surgical Tape, once a week. The mice were lightly anesthetized using isoflurane, and the abdominal area was exposed to either 200 μg peanut flour in 20 μl PBS or PBS alone, once a week for 4 weeks.
Plasma antibody analysis and induction of anaphylaxis
Protocols for plasma antibody analysis and anaphylaxis induction have been previously described.13 Briefly, 4 to 7 days after the final i.n. or e.c. peanut flour exposure, mice were lightly anesthetized with isoflurane, and peripheral blood for measurement of peanut-specific IgE and IgG isotypes in plasma was collected by retro-orbital bleeding. The plasma levels of peanut-specific IgE and IgG isotypes were measured by ELISA as previously described.13 The plasma levels of Ara h 1-specific IgE were measured by coating the ELISA plates with purified natural Ara h 1 (Indoor Biotechnologies, Charlottesville, VA) and by detecting bound specific IgE with biotinylated rat anti-mouse IgE (BD Biosciences, Franklin Lakes, NJ) and poly-HRP Streptavidin (Thermo Fisher Scientific, Waltham, MA) as previously described.16
One day later, mice were challenged by i.p. injection of 2.5 mg crude peanut extract in 500 mL sterile PBS. Rectal temperature was monitored by an electronic thermometer (Oakton Instruments, Vernon Hills, IL, USA), equipped with a RET-3 rectal probe (Physitemp Instruments, Clifton, NJ, USA), immediately before challenge (0 min) and every 10 min afterward for 1 h. Clinical symptoms were scored based on published criteria13 as follows: 0, no symptoms; 1, scratching of ear and mouth; 2, puffiness around eyes and mouth, pilar erection, labored breathing; 3, prolonged period of motionlessness; 4, severely reduced motility, tremors, severe respiratory distress; 5, death; ear scratching, labored breathing and prolonged motionlessness were commonly observed in mice experiencing systemic anaphylaxis.
In some experiments, CTLA-4 was blocked using a monoclonal antibody.17-19 Mice were administered either anti-CTLA-4 antibody (9H10) or polyclonal Syrian Hamster isotype control antibody (both from Bio X Cell, West Lebanon, NH, USA) via i.p. injection (200 μg) and i.n. administration (70 μg) one day before and one day after each i.n. peanut flour exposure.
Fluorescence-activated cell sorting (FACS) analysis
Mediastinal lymph nodes (mLNs) or mesenteric LNs were collected on day 4 or day 11 after the initiation of i.n. peanut flour exposure, as described in the Figure Legends. T cell and B cell populations from mLNs and mesenteric LNs were analyzed by flow cytometry, as described,20 with some modifications. Briefly, single-cell suspensions of mLN cells were first stained with a viability stain (Tonbo, San Diego, CA, USA), followed by pre-incubation with Fc-receptor blockers for 30 min at 4°C. Cells were then stained with a combination of FITC-conjugated anti-CD45.2 (clone 104, BioLegend, San Diego, CA, USA), PE-conjugated anti-PD-1 (J43), PerCP-conjugated anti-CD4 (L3T4), and biotin-conjugated anti-CXCR5 (clone 2G8), followed by streptavidin-APC. To analyze GC B cells, the cells were stained with FITC-conjugated peanut agglutinin (PNA; Vector Laboratories, Burlingame, CA, USA), PerCP-conjugated anti-B220 (RA3-6B2), and biotin-conjugated anti-CD95 (FAS; Jo2), followed by streptavidin-APC. After washing with PBS, the cells were resuspended in PBS containing 1% bovine serum albumin and 0.1% NaN3, fixed with 1% paraformaldehyde, and analyzed using a BD FACSAria Flow Cytometer (BD Biosciences, Franklin Lakes, NJ). The lymphocytic cell population was gated on live cells and by using a forward and side scatter plot. Antibodies were obtained from BD Biosciences, unless otherwise described.
In vitro mLN cell culture and cytokine production
Naïve BALB/c mice were fed peanut butter or control buffer and exposed i.n. to peanut flour on days 0 and 7. On day 11, mLN cells were collected and cultured (4x105 cells per well) in a 96-well round bottom plate in RPMI medium with 10% fetal bovine serum with or without 100 μg/ml crude peanut extract (final volume 200 μl/well) at 37°C for 4 days. Cell supernatants were analyzed for concentrations of IL-4 and IL-21 using commercial ELISA kits (R&D Systems) according to the manufacturer’s instructions.
Single-cell RNA sequencing (scRNAseq) and data analysis
CD3+CD4+ T cells were sorted from single-cell suspensions of mLNs stained with a viability dye (Tonbo) and antibodies to CD45.2, CD3, and CD4 (positive stain) and CD19 and CD11b (negative stain) (BioLegend), using a BD FACSAria Flow Cytometer (BD Biosciences). Freshly isolated CD4+ T cells were then shipped to SingulOmics (Bronx, NY), where they were subjected to scRNAseq using the 10X Genomics (Pleasanton, CA, USA) Chromium platform. Data were analyzed by Mayo Clinic biostatisticians; the 10X Genomics Cell Ranger Single Cell Software Suite (v2.0.2) was used to perform FASTQ reads alignment to the mm10 mouse genome, filtering, barcode counting, and unique molecular identifier (UMI) counting. For subsequent analyses, we followed the standard integrated analysis workflow in the Seurat package (v2.3.4).21, 22 Genes expressed in fewer than three cells and cells expressing fewer than 200 genes and >35% mitochondrial genes were excluded from downstream analysis in each sample. Each dataset was preprocessed using log-normalization and scaled for each gene across all cells. All datasets were integrated, scaled, and clustered on the low-dimensional space. Enriched gene markers in a cluster, conserved across two experimental conditions, were identified, and differentially expressed genes within each cluster between the two conditions were detected.
Gene expression analysis
Expression of CTLA-4 in CD3+CD4+Foxp3+ regulatory T (Treg) cells was analyzed by quantitative reverse transcription polymerase chain reaction (qRT-PCR). Briefly, Foxp3eGFP mice were fed peanut butter or control buffer by oral gavage. mLNs were collected on day 11 after the initiation of i.n. peanut flour, and CD4+ T cells were enriched from mLNs using EasySep mouse CD4+ T cell isolation kit (Stemcell Technologies). The cells were stained with CD3-BV421, CD4-PerCP, Ghost Red 780, and sorted by a BD FACSAria Flow Cytometer. cDNAs were synthesized using an iScript™ cDNA synthesis kit (Bio-Rad, Hercules, CA). The real-time PCR contained cDNA, TaqMan® Universal PCR Master Mix (Applied Biosystems, Foster City, CA), and TaqMan® gene expression assay for Ctla4 (Applied Biosystems) as per the manufacturer’s instructions. The transcription levels were normalized to 18S rRNA in each sample and expressed relative to CD3+CD4+Foxp3eGFP+ Treg cells from mice fed control buffer.
Statistical analysis
Data are presented as mean ± standard error of the mean (SEM) for the number of mice or experiments, as indicated. Statistical significance for differences between the various treatment groups was assessed by the unpaired two-tailed Student’s t-test (for two-group comparisons) or analysis of variance (ANOVA) with a post hoc Tukey’s test (for multiple group comparisons). P-values less than 0.05 were considered statistically significant.
RESULTS
Ingestion of a peanut product protects from peanut allergy development in response to airborne peanut exposure
To investigate the immunologic mechanisms underlying observations from the LEAP study,10 we fed naïve WT BALB/c mice Skippy™ peanut butter, a popular commercial product, by oral gavage. Throughout the study, we used adult WT mice to mimic the immune responses of healthy human infants.12 NIAID guidelines recommend a total amount of 6–7 g of peanut protein per week over three or more feedings in infants.11 By translating the human dose to the mouse dose,15 naïve mice were fed 220 mg of peanut protein (i.e., 1.0 g of Skippy™ peanut butter slurry) or calcium carbonate buffer (i.e., control buffer) per week, divided into three or five doses, for a total of 6 weeks (Fig. 1A).
In a previous study, high levels of peanut proteins in household dust were associated with peanut sensitization and likely an increased risk of peanut allergy.23 The association could be explained by exposure through airway, skin, or both.7, 8, 9 Here, to mimic environmental airway exposure, peanut butter-fed and control mice were concomitantly exposed intranasally to peanut flour in PBS or PBS-only control, once a week for 4 weeks. We found that mice fed control buffer and subjected to i.n. peanut flour exposure produced peanut-specific IgE, as well as IgG1 and IgG2a isotypes (Fig. 1B). Notably, however, mice fed peanut butter and exposed i.n. to peanut flour showed 10% and <1% levels of peanut-specific IgE and IgG1, respectively, relative to those fed control buffer (P<0.01). No significant difference in the levels of IgG2a isotype was observed. Similarly, no differences were observed in the levels of peanut-specific IgE and IgG1 between peanut butter-fed mice and control buffer-fed mice without i.n. exposure to peanut flour (i.e., PBS), suggesting that mice do not produce IgE by feeding peanut butter. In addition, mice fed control buffer and exposed i.n. to peanut flour produced IgE to a major peanut allergen Ara h 1, and the levels were significantly lower in mice fed peanut butter (P<0.01, Fig. 1C).
To examine the clinical outcomes of peanut feeding and peanut flour exposure, we challenged mice by i.p. injection of peanut extract. Mice fed peanut butter and exposed to i.n. PBS showed no or minimal signs of anaphylactic response, even when challenged with i.p. peanut extract, suggesting that consuming peanut products alone does not sensitize mice for peanut allergy. Conversely, in response to i.p. injection of peanut extract, mice fed control buffer and subjected to i.n. peanut flour exposure showed an up to 6°C decrease in body temperature and demonstrate clinical symptoms consistent with systemic anaphylaxis within 10 min, including ear scratching, labored breathing, and a prolonged period of motionlessness (Fig. 1D). Critically, mice similarly exposed to i.n. peanut flour but previously fed peanut butter showed minimal decrease in body temperature and demonstrate significantly reduced clinical symptoms compared to those fed control buffer in response to i.p. peanut extract (P<0.01). These findings suggest that feeding of a peanut product does not sensitize naïve mice but rather protects from allergic sensitization induced by inhaled exposure to peanuts.
Repeated oral gavage could be stressful to mice. Furthermore, oral mucosa may also play an immunoregulatory role.24 Therefore, to mimic the condition of human “LEAP” study further, we fed peanut butter ad lib through the mouth. To do this, mice were allowed to have free access to a solid peanut butter product Skippy™ Peanut Butter (P.B.) Bites for 24 hours on defined days before and during i.n. exposure to peanut flour (Supplemental Figure E1A). The mice that were not allowed to consume P.B. Bites produced IgE and IgG1 antibodies to peanut when they were exposed i.n. to peanut flour (Supplemental Fig. E1B); they demonstrated systemic anaphylaxis when challenged i.p. with peanut extract (Supplemental Fig. E1C). In contrast, the mice that consumed P.B. Bites periodically produced significantly less titers of IgE, IgG1 and IgG2a antibodies (P<0.01) and showed no decrease in body temperature and minimal anaphylactic symptoms when challenged i.p. with peanut extract. Similar protective effects of consuming P.B. Bites were also observed in male mice (Supplemental Fig. E2), suggesting that both males and females are protected.
To examine whether the protective effects of peanut consumption represent temporally desensitization or immunological tolerance, we provided P.B. Bites to mice during airway exposure to peanut flour, allowed them to rest without any manipulations by switching back to regular mouse chow for 4 weeks and then challenged by i.p. peanut extract (Supplemental Fig. E3A). The mice fed only mouse chow and exposed to peanut flour produced peanut-specific IgE, IgG1 and IgG2a antibodies (Supplemental Fig. E3B) and showed 6.5 °C decrease in body temperature and apparent clinical symptoms of systemic anaphylaxis when challenged (Supplemental Fig. E3C). In contrast, the mice that consumed P.B. Bites produced 80% and >99% less IgE and IgG1/IgG2a antibodies, respectively. They also showed significantly less decrees in body temperature and milder clinical symptoms (P<0.01), suggesting that consumption of peanut butter products induce immunologic tolerance, rather than desensitizing the animals to peanut for a limited period.
To examine the roles for immunological tolerance further, we provided mice with P.B. Bites only for one week before they were subsequently exposed i.n. to peanut flour (Fig. 1E). The mice that did not consume P.B. Bites produced peanut-specific IgE and IgG1 antibodies (Fig. 1F) and showed approximately 8 °C decrease in body temperature and demonstrated clinical symptoms of systemic anaphylaxis when challenged with peanut extract (Fig. 1G). In contrast, the mice that consumed P.B. Bites produced significantly less IgE and IgG1 antibodies (P<0.01 and P<0.05) and were protected from developing systemic anaphylaxis. These findings suggest that initial oral consumption of peanut products plays a pivotal role in preventing the development of peanut allergy by subsequent environmental exposure.
Feeding a peanut product protects from developing peanut allergy in response to cutaneous exposure to peanuts
The skin has been considered a barrier surface through which sensitization to peanuts may occur, particularly in those with a history of atopic dermatitis.23, 25 Therefore, to examine the reproducibility of results from the airway model in naïve mice exposed to peanut flour through the skin, BALB/c mice were fed Skippy™ peanut butter or control buffer, as described above. Mice were then concomitantly exposed to peanut flour via e.c. administration over the abdominal skin, once a week for 4 weeks (Fig. 2A). Similar to the airway model, mice fed control buffer and exposed to e.c. peanut flour developed peanut-specific IgE and IgG1 (Fig. 2B). In contrast, peanut butter-fed mice produced 90% less IgE and IgG1 antibodies to peanut in response to e.c. peanut flour exposure as compared to control buffer-fed mice (P<0.01). The levels of peanut-specific IgG2a tended to be higher in peanut butter-fed mice as compared to control buffer-fed mice (P=0.06). Similarly, mice fed control buffer produced IgE to Ara h 1, and the levels were significantly lower in mice fed peanut butter (P<0.05, Fig. 2C)
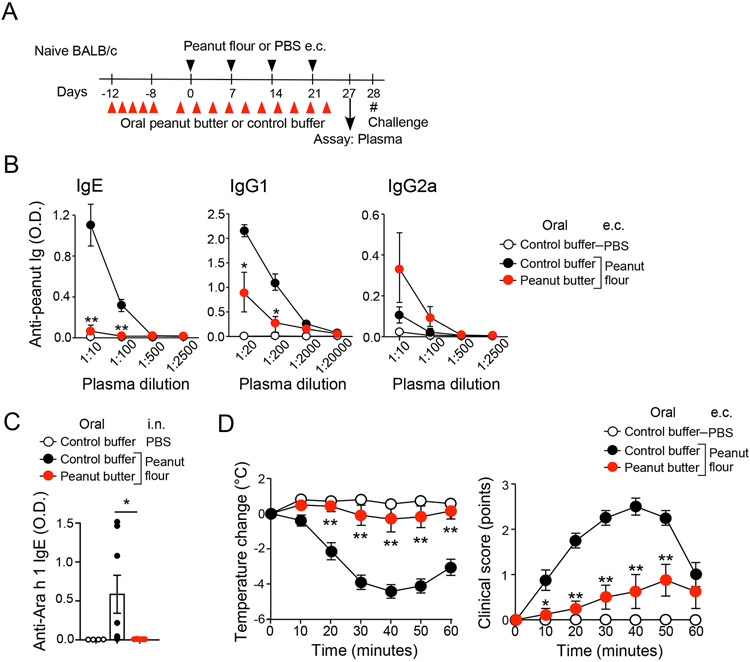
Figure 2.
Oral administration of peanut butter prevents peanut allergy induced by epicutaneous (e.c.) exposure to peanut flour. (A) Schematic overview of the experimental protocol. (B) Plasma levels of peanut-specific IgE, IgG1, and IgG2a are shown. (C) Plasma levels of Ara h 1-specific IgE are shown. Each dot represents one mouse. (D) After intraperitoneal challenge with peanut extract, changes in rectal temperature and clinical scores were monitored. Data are presented as the mean ± SEM (n=4–8 in each group) and are representative of two experiments. *P<0.05, **P<0.01 compared to mice fed control buffer and subjected to i.n. peanut flour exposure or between the groups indicated by a horizonal line.
We then challenged mice with i.p. injection of peanut extract and found that mice fed control buffer and exposed to e.c. peanut flour developed signs of acute and systemic anaphylaxis, including an up to 4.5°C decrease in body temperature and manifestation of anaphylactic clinical symptoms (Fig. 2D). In contrast, peanut butter-fed mice exposed to e.c. peanut flour showed minimal decrease in body temperature in response to i.p. peanut extract (P<0.01). Clinical symptoms of acute anaphylaxis were also significantly milder in these animals relative to mice fed control buffer (P<0.01). Altogether, these findings suggest that feeding peanut butter protects mice from developing peanut allergy, irrespective of whether sensitization occurs through the airway or skin.
Development of Tfh cells in dLNs is suppressed by peanut butter feeding
To investigate the immunologic mechanisms that mediate the protective effects of feeding peanut butter against peanut allergy, we chose to use the airway model, because dLNs, which are critical for in-depth analyses of humoral immune responses26, are readily available within the mediastinal compartment. As interleukin (IL)-4 plays a pivotal role in production of IgE and IgG1,27 we fed IL-4eGFP reporter (Il4eGFP) mice28 peanut butter or control buffer, and exposed them to peanut flour or PBS via i.n. administration for 7 days (Fig. 3A). On day 11, mice fed control buffer and exposed to i.n. peanut flour showed an approximately 7-fold increase in total cell number in mLNs relative to those exposed to i.n. PBS (Fig. 3B). Mice fed peanut butter and exposed to i.n. peanut flour showed significantly less mLN cell numbers as compared to those fed control buffer (P<0.01). Feeding peanut butter alone without i.n. exposure to peanut flour had no effects on mLN cell numbers.
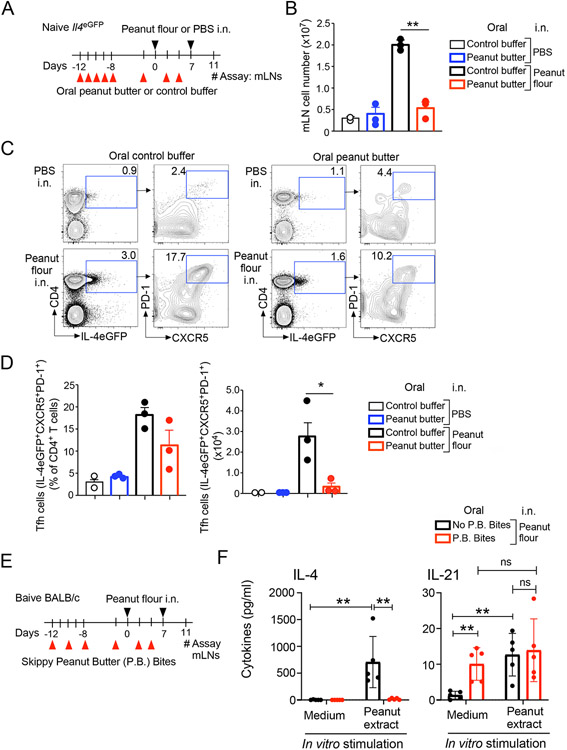
Figure 3.
Oral administration of peanut butter suppresses T follicular helper (Tfh) cell development in mediastinal lymph nodes (mLNs). (A) Schematic overview of the experimental protocol. (B) Total number of mLN cells. (C) Representative scattergrams from FACS analysis of mLN cells are shown. (D) The proportions and total numbers of Tfh cells. Data are presented as the mean ± SEM and are representative of two experiments. *P<0.05, **P<0.01 between the groups indicated by horizonal lines. (E) Schematic overview of the experimental protocol for Panel F. (F) Cytokine production by mLN cells in vitro. Data are presented as the mean ± SEM. **P<0.01 between the groups indicated by horizonal lines. P.B. Bites, Skippy™ Peanut Butter (P.B.) Bites; n.s., not significant.
FACS analyses of mLN cells revealed increased expression of IL-4eGFP in CD4+ T cells from mice fed control buffer and exposed to peanut flour via i.n. administration (Fig. 3C). Approximately 17% of these IL-4eGFP+CD4+ cells highly expressed both PD-1 and CXCR5, suggesting they are IL-4-competent mature Tfh cells.26 In contrast, mice fed peanut butter and then exposed to peanut flour showed a smaller proportion of IL-4eGFP+ T cells within the CD4+ T cell population relative to those fed control buffer prior to peanut flour exposure. A smaller proportion of PD-1+CXCR5+ cells within this IL-4eGFP+ population was also present in peanut butter-fed mice relative to control buffer-fed mice while the difference was not statistically significant (Fig. 3D). These animals contained approximately 10-fold fewer total number of IL-4eGFP+PD-1+CXCR5+ mature Tfh cells relative to buffer-fed mice (Fig. 3D) (P<0.05).
To examine the cellular functions, equal numbers of mLNs cells (i.e., 4x105 cells/well) from mice that consumed P.B. Bites and those kept in regular mouse chow were stimulated in vitro with peanut extract for 4 days (Fig. 3E). No IL-4 was detected from mLN cells when they were cultured with medium alone (Fig. 3F). mLN cells from the mice that did not consume P.B. Bites and were exposed to peanut flour produced a large quantity of IL-4 when stimulated with peanut extract in vitro (P<0.01); the levels were significantly lower in mLN cells from the mice that consumed P.B. Bites (P<0.01). In contrast, IL-21 was detected in mLN cells from the mice that consumed P.B. Bites regardless of whether they were stimulated with peanut extract in vitro. Thus, our findings suggest that feeding peanut butter products inhibits proliferation, differentiation and/or functions of IL-4-competent Tfh cells within dLNs in mice exposed intranasally to peanut flour.
The protective effects of oral peanut butter are associated with expression of T regulatory (Treg) cell-associated molecules in dLNs
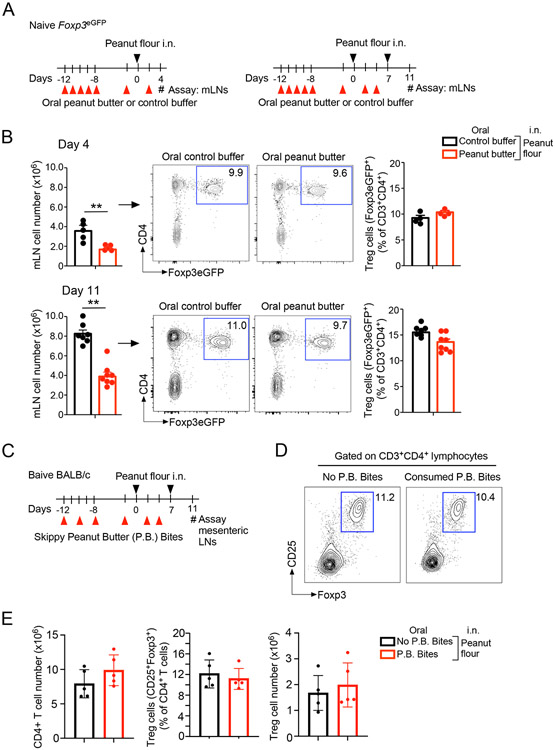
In several previous studies, the immunologic mechanisms of oral tolerance were investigated by immunizing mice systemically via i.p. injection of the model antigen ovalbumin (OVA) together with alum adjuvant.29-31 As Treg cells were shown to be involved in oral tolerance in these mouse models29-31, we hypothesized that protection from developing peanut allergy in peanut butter-fed mice results from an increased number of Treg cells within dLNs. To test this hypothesis, we fed Foxp3eGFP reporter (i.e., Foxp3eGFP) mice with peanut butter or control buffer, as described above, and exposed them to peanut flour via i.n. administration at two different timepoints (Fig. 4A); mLNs were then analyzed 4 days after the last peanut flour exposure. Consistent with results from the above experiments (Fig. 3B), in mice exposed to i.n. peanut flour, significantly lower total numbers of mLN cells were detected in peanut butter-fed mice compared to those fed control buffer, irrespective of whether they were analyzed after one-time or two-time i.n. exposure (P<0.01, Fig. 4B). FACS analyses of CD4+ T cells revealed that approximately 10% were positive for Foxp3eGFP, suggesting that Foxp3-positive Treg cells develop in dLNs in response to i.n. peanut flour exposure (Fig. 4B). However, no differences in the proportion of Foxp3eGFP+Treg cells among total CD4+ T cells were observed in mice fed peanut butter and those fed control buffer (Fig. 4B).
Figure 4.
Oral administration of peanut butter does not affect the proportion of Foxp3+ T regulatory (Treg) cells. (A) Schematic overview of the experimental protocol. (B) Representative scattergrams from FACS analysis of mediastinal LN (mLN) cells and the proportions of Treg cells are shown. Data are presented as the mean ± SEM in each group and are representative of three experiments. **P<0.01 between the groups indicated by horizonal lines. (C) Schematic overview of the experimental protocol for Panels D and E. (D) Representative scattergrams from FACS analysis of mesenteric LN cells are shown. (E) The proportions and total numbers of Treg cells are shown. Data are presented as the mean ± SEM in each group. P.B. Bites, Skippy™ Peanut Butter (P.B.) Bites.
We also examined CD4+ T cells and Foxp3+ Treg cells in day 11 mesenteric LNs, which drain the gastrointestinal tract (Fig. 4C). No differences were observed in the total number of CD4+ T cells between the mice consumed P.B. Bites and those kept in regular mouse chow (Fig. 4D and 4E). Furthermore, no differences were observed in the proportion or total number of Foxp3+ Treg cells between these two groups.
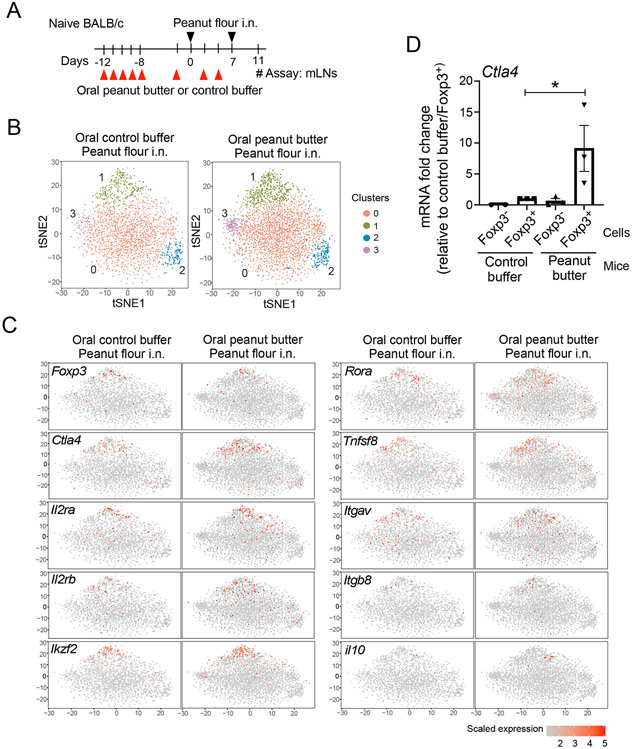
These findings led us to speculate that protection against developing peanut allergy in peanut-butter fed mice may not be due to an increase in Treg cells per se but rather might be associated with changes in their quality or function. Therefore, we analyzed gene expression in CD4+ T cells from dLNs by scRNAseq. To this end, WT BALB/c mice were fed peanut butter or control buffer, as described above, and exposed to peanut flour twice via i.n. administration. On day 11, CD4+ T cells in mLNs were sorted and analyzed by scRNAseq (Fig. 5A). Unsupervised analysis of the sequencing data revealed four distinct clusters of CD4+ T cells (Fig. 5B). Upon inspection, two clusters—Cluster 1 and a smaller Cluster 3—were found to be more pronounced in peanut butter-fed mice compared buffer control-fed animals. Transcripts in Cluster 1 include those expressed in conventional Treg cells, such as Foxp3, CTLA-4, Il2ra, and Il2rb (Fig. 5C).32 The transcription factors Ikzf2 and Rora, which are associated with Treg function, were also found to be enriched in Cluster 1.33-35 Interestingly, transcripts of two integrins associated with Treg cells in the gut, Itgav and Itgb8,36, 37 were also detected in Cluster 1. However, transcripts for cytokines that mediate the inhibitory functions of Treg cells were undetectable (e.g., transforming growth factor-β [Tgfb1]; data not shown) or represented minimally (e.g., il10) (Fig. 5C) in the sequencing data from either group of mice.
Figure 5.
The mRNA transcript for T-lymphocyte-associated protein 4 (Ctla4) and transcripts of other Treg cell-related genes are enriched in CD4+ T cells from mLNs of peanut-butter fed mice. (A) Schematic overview of the experimental protocol. (B) Unsupervised t-distributed stochastic neighbor embedding (t-SNE) plots of CD4+ T cells are shown. (C) t-SNE plots of selected genes are shown. (D) Foxp3eGFP mice were treated as described in (A), and mRNA transcript for Ctla4 in sorted Foxp3− and Foxp3+ cells was analyzed by qRT-PCR. Data are presented as the mean ± SEM in each group. *P<0.05 between the groups indicated by a horizonal line.
To verify the observations, we sorted CD4+Foxp3+ Treg cells from day 11 mLNs of Foxp3eGFP mice and analyzed CTLA-4 expression by qRT-PCR. Foxp3+ Treg cells from mice fed peanut butter and exposed to peanut flour expressed approximately 9-fold higher levels of CTLA-4 mRNA as compared to Foxp3+ Treg cells from mice fed control buffer and exposed to peanut flour (Fig. 5D).
Blocking CTLA-4 with monoclonal antibody promotes development of Tfh cells and GC B cells in response to i.n. peanut flour exposure
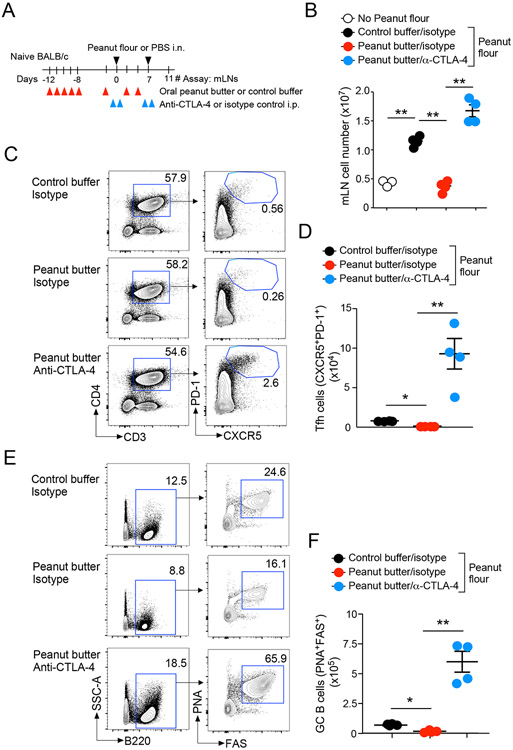
Mice lacking CTLA-4 develop spontaneous lymphoproliferative disorders.38 We therefore speculated that CTLA-4 might play a regulatory role in development of Tfh cells and GC B cells in mLNs of mice exposed to peanut flour. To address this question, we examined the effects of treatment with anti-CTLA-4 blocking antibody in our model. Peanut butter-fed and buffer control-fed mice were administered anti-CTLA-4 antibody17-19 or isotype control antibody during the 7-day period of i.n. exposure to peanut flour or control (Fig. 6A). Consistent with the results in Figure 3A, mice fed control buffer and administered control antibody showed a significant increase in the number of total mLN cells in response to i.n. peanut flour exposure relative to mice exposed to i.n. PBS (P<0.01, Fig. 6B). Peanut butter feeding significantly decreased the number of mLN cells in control antibody-treated mice exposed to i.n. peanut flour (P<0.01); however, these inhibitory effects of peanut butter were reversed by administration of anti-CTLA-4 (P<0.01).
Figure 6.
Anti-CTLA-4 treatment promotes development of Tfh and germinal center (GC) B cells in mLNs. (A) Schematic overview of the experimental protocol. (B) Total number of cells in mLNs. (C) Representative scattergrams from FACS analysis of mLN cells are shown. (D) Total numbers of Tfh cells in mLNs. (E) Representative scattergrams from FACS analysis of mLN cells are shown. (F) Total numbers of GC B cells in mLNs. Data are presented as the mean ± SEM (n=4) in each group and are representative of two experiments. *P<0.05 and **P<0.01 between the groups indicated by horizonal lines.
The effects of anti-CTLA-4 treatment became more pronounced when Tfh and GC B cell populations were analyzed by FACS. In mice treated with isotype control antibody, the proportion of CD3+CD4+PD-1+CXCR5+ mature Tfh cells showed a partial decrease in peanut butter-fed mice compared to those fed control buffer in response to i.n. peanut flour exposure (Fig. 6C), whereas anti-CTLA-4 significantly increased the proportion of mature Tfh cells in peanut butter-fed mice. Notably, the total number of Tfh cells in anti-CTLA-4-treated peanut butter-fed mice was even greater than in control buffer-fed mice treated with isotype control antibody (Fig. 6D). In addition, anti-CTLA-4-treated mice showed an approximately 4-fold increase in the proportion of B220+PNA+FAS+ GC B cells relative to peanut butter-fed mice treated with control antibody in response to i.n. peanut flour (Fig. 6E). Further, the total numbers of GC B cells in peanut butter-fed mice treated with anti-CTAL4 were approximately 20-fold higher than in peanut butter-fed mice treated with control antibody and 5-fold higher than in mice fed control buffer after i.n. peanut flour exposure (Fig. 6F). Collectively, these findings suggest that anti-CTLA-4 treatment promotes the development of Tfh cells and GC B cells in dLNs of mice exposed intranasally to peanut flour and attenuates the tolerogenic effects of feeding peanut butter.
Anti-CTLA-4 ameliorates the protective effect of feeding peanut butter on development of peanut allergy
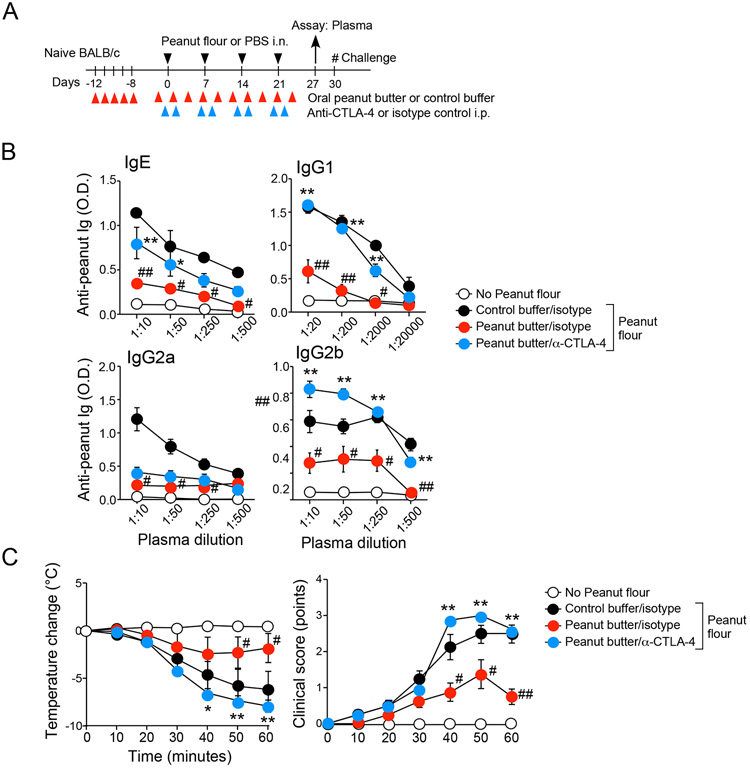
To further test this hypothesis, we extended anti-CTLA-4 treatment throughout the 4-week period when mice were fed peanut butter or control buffer and exposed intranasally to peanut flour (Fig. 7A), administering anti-CTLA-4 or control antibody the day before and after each i.n. peanut flour exposure. Similar to the results shown in Figure 1, we found that, in mice treated with isotype control antibody and exposed to i.n. peanut flour, peanut butter feeding significantly decreased serum levels of peanut-specific IgE and all IgG isotypes relative to buffer control feeding (P<0.01, Fig. 7B). However, anti-CTLA-4 reversed the inhibitory effects of peanut butter on serum levels of IgE and IgG1 antibodies. Levels of IgG2a antibody are unaffected by anti-CTLA-4, whereas anti-CTLA-4 increased production of IgG2b in peanut butter-fed mice above the levels observed in buffer control-fed mice.
Figure 7.
Anti-CTLA-4 treatment reverses the tolerogenic effects of oral peanut butter. (A) Schematic overview of the experimental protocol. (B) Titers of peanut-specific IgE, IgG1, IgG2a, and IgG2b antibodies in plasma are shown. (C) After intraperitoneal challenge with peanut extract, changes in rectal temperatures and clinical scores were monitored. Data are presented as the mean ± SEM (n=4 in each group) and are representative of two experiments. #P<0.05 and ##P<0.01 compared to mice fed control buffer, treated with isotype control, and subjected to i.n. peanut flour exposure. *P<0.05 and **P<0.01 compared to mice fed peanut butter, treated with isotype control, and subjected to i.n. peanut flour exposure.
We then examined the clinical consequences of anti-CLTA4 treatment by challenging these mice with i.p. injection of peanut extract (Fig. 7C). As observed in Figure 1, peanut butter feeding significantly inhibited the decrease in body temperate and manifestation of clinical anaphylaxis observed in buffer-fed mice that have been subjected to i.n. peanut flour exposure (P<0.05 and p<0.01). However, the protective effects of peanut butter were reversed by anti-CTLA-4 (p<0.05 and P<0.01). Thus, we show that blocking CTLA-4 promotes peanut allergy, even in mice protected by oral administration of peanut butter. Therefore, our findings suggest that this protein plays a pivotal regulatory role in development of peanut allergy in response to environmental exposure to peanuts.
DISCUSSION
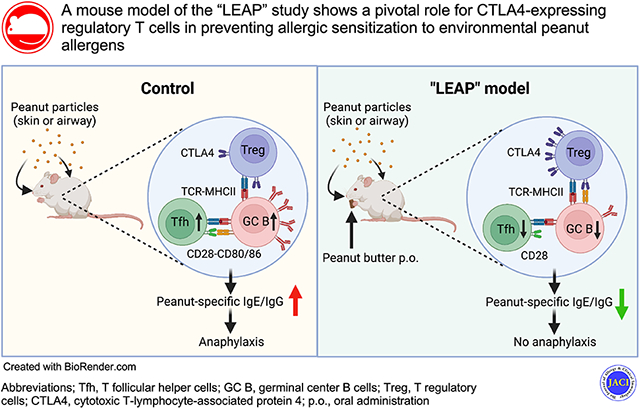
Traditionally, exposure through the gut has been considered to be a route of sensitization in the development of food allergy.39 However, the recent LEAP study revealed that children who consumed peanut products were protected from developing peanut allergy, whereas those who avoided peanut products were more prone to peanut allergy, perhaps through household exposure to peanut allergens.10 The goal of this project was to recreate the results of the LEAP study in mice and investigate the immunologic mechanisms by which consuming peanut products prevents peanut allergy development. Critically, mouse models allow collection and analysis of dLNs, which are difficult to obtain in humans, and antibody administration, which is difficult to justify in children, can also be performed. Consistent with the LEAP study, we found that oral feeding with commercial peanut products does not promote allergic sensitization to peanuts, but rather, mice developed signs of peanut allergy when exposed to peanuts through the airway or skin. Importantly, these mice were protected when they were fed peanut products prior to exposure. Furthermore, commercial roasted peanut butter products (i.e. from Hormel Foods) given orally prevented sensitization and anaphylaxis reactions from both raw (from Greer Laboratories) and peanut products (from Golden Peanut).Thus, our observations recapitulate key elements of the LEAP study and suggest that animals can be readily sensitized to peanuts through non-oral routes but are protected when they are orally administered peanut products.
One previous study found that high levels of peanut proteins in household dust were associated with peanut sensitization and likely an increased risk of peanut allergy.23 Further, peanut proteins, up to 1,000 μg per gram of dust, have been detected in infants’ bed sheets and play areas.40 In this study, we exposed mice to 100 μg peanut flour (approximately 14 μg peanut protein) without any adjuvants by either inhalation or e.c. painting, once a week for 4 weeks and found that both exposure models increase plasma levels of peanut-specific IgE and IgG1. In contrast, when these mice were orally fed 1.0 g Skippy™ peanut butter slurry (220 mg peanut protein) per week or consumed approximately 3.3 g Skippy™ P.B. Bites (600 mg peanut protein) per week, they did not produce peanut-specific IgE. On a weekly basis, we estimate that mice were given 4,000-fold or 10,000-fold more peanut protein orally as compared to through either airway or skin exposure. These findings are consistent with earlier observations from oral tolerance models in mice using systemic sensitization with OVA antigen plus adjuvant alum29-31 and suggest that the gut provides an environment that is highly protective against the development of an antigen-specific type 2 immune response. Conversely, non-oral routes, such as the airway and skin, likely provide a mechanism for allergic sensitization. Indeed, conditions such as asthma, allergic rhinitis, and atopic dermatitis are often predisposing factors for developing peanut allergy.41 In addition, CD4+ Th2 cells from peanut-allergic subjects express elevated levels of the skin- and lung-homing molecule CCR4, but not the gut-homing molecule α4β7,42 suggesting an association between both the skin and airway and type 2 immune responses.
Food allergy may result from breakdown of oral tolerance in the gut mucosa that occurs due to epithelial injury and/or production of immunogenic cytokines, such as IL-25, thymic stromal lymphopoietin, and IL-33, by gut epithelial cells.3, 4, 8 Indeed, oral administration of toxins, such as cholera toxin, to disrupt the mucosal barrier was found to be necessary for sensitizing WT mice to peanut antigens by oral administration.43, 44 Our findings suggest that the normal gut mucosa is not only resistant to allergic sensitization but also provides protection against allergic sanitization induced by exposure via other mucosal routes, such as the skin and airway. We also found that protection is observed in both males and females and in both BALB/c and CD57BL/6 mice. As judged by the duration of protection, the effects are likely mediated by immunological tolerance, rather than by temporally desensitization to peanuts (Fig. 1 and Supplemental Fig. E3). Indeed, the protective effects of oral peanut butter administration are associated with decreased Tfh and B cell responses (Fig. 3 and Fig. 6) in mLNs that drain lung lymphatics.45 Furthermore, mLN cells from the mice that consumed P.B. Bites produced significantly less IL-4 in response to peanut antigen in vitro (Fig. 3). Using Tfh-deficient Bcl6fl/flCd4-Cre mice, we previously found that Tfh cells in mLNs are indispensable for production of IgE and IgG s in response to inhaled peanut allergens.13 Thus, oral foods likely suppress antigen-specific Tfh and B cell responses in distant dLNs.
Protective roles for Treg cells mediated by their secreted products and cell surface molecules, such as TGF-β, IL-10, and CTLA-4, have been reported previously in mouse models of systemic sensitization with OVA.46-49 Although we did not detect an increased frequency of Foxp3+ Treg cells in dLNs for the lungs and gastrointestinal tract in our study, a CD4+ T cell cluster with distinct expression of Foxp3, Il2ra, Il2rb, CTLA-4, Ikzf2, and RORα was identified in mLNs from peanut butter-fed mice (Fig. 5). Expression of TGF-β and IL-10 was not apparent in this cell population.
Notably, previous studies have reported that CTLA-4 may be involved in controlling peanut allergy in mouse models of sensitization, although the results were rather conflicting. For example, in mice sensitized to peanuts by oral administration of allergen together with CT, anti-CTLA-4 antibody increased the levels of IgE antibody to peanuts;50 clinical anaphylactic response was not examined. Conversely, in a similar model of oral peanut exposure with CT, blocking with CTLA-4-Ig led to reduced levels of peanut-specific IgE and IgG1 antibodies and decreased splenocyte production of IL-4, IL-10, and interferon-γ,51 paradoxically suggesting that CTLA-4 is necessary for development of peanut allergy. We also note that in autoimmune disease models, CTLA-4-deficient mice show more severe collagen-induced arthritis but are protected from peptide-induced experimental autoimmune encephalitis.52 Thus, the role of CTLA-4 may vary depending on the use of adjuvants, route of sensitization, and disease model.
Critically, although a key role for CTLA-4 in preventing peanut allergy is apparent in our model (Fig. 7), a number of questions remain. In particular, the mechanism by which CTLA-4 protects animals from developing peanut allergy when they are exposed to peanut flour through the airway is not known. In a previous study, mice globally deficient in CTLA-4 were found to show dysregulated proliferation of Tfh and GC B cells,53 suggesting that CTLA-4 may generally play a role in regulating humoral immune responses. More recently, CTLA-4 expressed on Treg cells was shown to suppress B cell responses54 by restricting Tfh differentiation55 and depleting co-stimulatory molecules CD80/CD86 from dendritic cells (DCs) and/or B cells.54, 56-58 We also found significant increases in Tfh and GC B cells when CTLA-4 is blocked by antibody during airway exposure to peanut allergens (Fig. 6), suggesting a pivotal regulatory role for CTLA-4 in the Tfh and GC B cell pathway. Nonetheless, we should also note that a similar cluster of CD4+ T cells (e.g. Cluster 1) that express Ctla4, as well as other Treg cell-associated genes, such as Foxp3, Il2ra, and Il2rb, was detected in mLNs of mice exposed intranasally to peanut flour but fed control buffer, albeit less pronounced than in peanut butter-fed mice (Fig. 5). Therefore, presence of this CD4+ T cell population is not unique to oral administration of peanut butter but rather appears to be associated with airway exposure to allergens.
These findings led us to speculate that CTLA-4+ Treg cells may develop in lung dLNs as a homeostatic mechanism during inhaled allergen exposure, although they need to be licensed or activated to fully exert their suppressive functions. Indeed, mLN Foxp3+ Treg cells in mice fed peanut butter expressed higher levels of CTLA-4 mRNA as compared to those in mice fed control buffer (Fig. 5). This model is consistent with the observation that activated Treg cells highly expressing the adhesion molecule lymphocyte function-associated antigen 1 (LFA-1) strongly adhere to antigen-presenting cells and effectively suppress their expression of CD80 and CD86.59 Perhaps, antigen-bearing circulating DCs that originate from the gut mucosa during oral administration of peanut butter activate these Treg cells in lung-dLNs. Alternatively, highly active peanut-specific CD4+ Treg cells may be generated in the gut mucosa in response to oral administration of peanut butter, which then travel to lung-dLNs and suppress Tfh cell and B cell responses during airborne allergen exposure. On the other hand, we also found that adoptive transfer of CD4+ T cells or Foxp3+ Treg cells isolated from mesenteric LNs of mice fed peanut butter does not transfer protection (data not shown). Thus, further studies involving identification, isolation and adoptive transfer of these candidate cell types will be necessary to elucidate the precise mechanism by which CTLA-4+CD4+ T cells confer systemic tolerance to inhaled and likely e.c. allergens.
In summary, results of the present study suggest that oral administration of food products does not sensitize naïve and healthy adult mice, which show an immune system comparable to that of human infants.12 Rather, oral food products likely prevent development of IgE and IgG in response to exposure to the same antigens through the airway or skin. Furthermore, our data suggest that the co-inhibitory receptor CTLA-4 plays a pivotal role in regulating Tfh and GC B cell responses to inhaled antigens. Thus, it is reasonable to surmise that CTLA-4 expressed on CD4+ cells is critical for preventing development of peanut allergy in children who consume peanut products, as reported in the LEAP study. Indeed, CTLA4 polymorphisms are associated with increased serum levels of IgE and allergic sensitization, but not with airway hyperreactivity, in subjects with allergy and asthma.60 We expect that further studies investigating the role of the gut mucosa in regulating allergic immune responses and exploring the regulatory mechanisms of Treg cells and CTAL-4 will aid in the development of more effective and safer strategies for preventing peanut allergy and reversing the increasing prevalence of this disease.
Supplementary Material
Key Messages.
Environmental exposure to peanut fine particles through the airway or skin promotes allergic sensitization to peanuts in mice.
Oral ingestion of peanut product inhibits generation of peanut-specific Tfh cells and protects mice from developing peanut allergy in response to environmental exposure.
The CTLA-4 pathway likely plays a key role in conferring protection from developing peanut allergy.
Capsule Summary.
Krempski et al. developed a novel mouse model recapitulating a human study showing that early ingestion of peanut products protects from peanut allergy and demonstrated the protective role for the CTLA-4 pathway.
ACKNOWLEDGEMENTS
This work was supported by grants from the National Institutes of Health (R37AI71106), the Mayo Graduate School of Biomedical Sciences, and the Mayo Foundation. All authors acknowledge no conflict of interest related to this manuscript.
Abbreviations
- APCs
antigen-presenting cells
- BAL
bronchoalveolar lavage
- CT
cholera toxin
- DC
dendritic cell
- dLNs
draining lymph nodes
- e.c.
epicutaneous
- eGFP
enhanced green fluorescent protein
- FACS
fluorescence-activated cell sorting
- GC
germinal center
- IL
interleukin
- i.n.
intranasal
- Ig
immunoglobulin
- IFN
interferon
- i.p.
intraperitoneal
- LFA-1
lymphocyte function-associated antigen 1
- mLN
mediastinal lymph node
- LNs
lymph nodes
- OVA
ovalbumin
- P.B
peanut butter
- qRT-PCR
quantitative reverse transcription polymerase chain reaction
- SPF
specific pathogen-free
- Tfh
T follicular helper
- TGF-β
transforming growth factor-β
- Treg
T regulatory
- UMI
unique molecular identifier
- WT
wild-type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All authors acknowledge no conflict of interest related to this manuscript.
REFERENCES
- 1.Dyer AA, Rivkina V, Perumal D, Smeltzer BM, Smith BM, Gupta RS. Epidemiology of childhood peanut allergy. Allergy Asthma Proc. 2015;36(1):58–64. Epub 2015/01/07. doi: 10.2500/aap.2015.36.3819.. [DOI] [PubMed] [Google Scholar]
- 2.Osborne NJ, Koplin JJ, Martin PE, Gurrin LC, Lowe AJ, Matheson MC, et al. Prevalence of challenge-proven IgE-mediated food allergy using population-based sampling and predetermined challenge criteria in infants. J Allergy Clin Immunol. 2011;127(3):668–76.e1-2. Epub 2011/03/08. doi: 10.1016/j.jaci.2011.01.039. [DOI] [PubMed] [Google Scholar]
- 3.Renz H, Allen KJ, Sicherer SH, Sampson HA, Lack G, Beyer K, et al. Food allergy. Nat Rev Dis Primers. 2018;4:17098. Epub 2018/01/05. doi: 10.1038/nrdp.2017.98. [DOI] [PubMed] [Google Scholar]
- 4.Chinthrajah RS, Hernandez JD, Boyd SD, Galli SJ, Nadeau KC. Molecular and cellular mechanisms of food allergy and food tolerance. J Allergy Clin Immunol. 2016;137(4):984–97. Epub 2016/04/10. doi: 10.1016/j.jaci.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hong X, Hao K, Ladd-Acosta C, Hansen KD, Tsai HJ, Liu X, et al. Genome-wide association study identifies peanut allergy-specific loci and evidence of epigenetic mediation in US children. Nat Commun. 2015;6:6304. Epub 2015/02/25. doi: 10.1038/ncomms7304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carter CA, Frischmeyer-Guerrerio PA. The Genetics of Food Allergy. Curr Allergy Asthma Rep. 2018;18(1):2. Epub 2018/01/28. doi: 10.1007/s11882-018-0756-z. [DOI] [PubMed] [Google Scholar]
- 7.Fox AT, Sasieni P, du Toit G, Syed H, Lack G. Household peanut consumption as a risk factor for the development of peanut allergy. J Allergy Clin Immunol. 2009;123(2):417–23. Epub 2009/02/11. doi: 10.1016/j.jaci.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 8.Du Toit G, Tsakok T, Lack S, Lack G. Prevention of food allergy. J Allergy Clin Immunol. 2016;137(4):998–1010. Epub 2016/04/10. doi: 10.1016/j.jaci.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 9.Kulis MD, Smeekens JM, Immormino RM, Moran TP. The airway as a route of sensitizaiton to peanut: An update to the dual allergen exposure hypothesis. J Allergy Clin Immunol. 2021;148(3):689–93. Epub 2021/06/07. doi: 10.1016/j.jaci.2021.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Du Toit G, Roberts G, Sayre PH, Bahnson HT, Radulovic S, Santos AF, et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372(9):803–13. Epub 2015/02/24. doi: 10.1056/NEJMoa1414850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Togias A, Cooper SF, Acebal ML, Assa'ad A, Baker JR Jr., Beck LA, et al. Addendum guidelines for the prevention of peanut allergy in the United States: Report of the National Institute of Allergy and Infectious Diseases-sponsored expert panel. J Allergy Clin Immunol. 2017;139(1):29–44. Epub 2017/01/10. doi: 10.1016/j.jaci.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beura LK, Hamilton SE, Bi K, Schenkel JM, Odumade OA, Casey KA, et al. Normalizing the environment recapitulates adult human immune traits in laboratory mice. Nature. 2016;532(7600):512–6. Epub 2016/04/21. doi: 10.1038/nature17655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dolence JJ, Kobayashi T, Iijima K, Krempski J, Drake LY, Dent AL, et al. Airway exposure initiates peanut allergy by involving the IL-1 pathway and T follicular helper cells in mice. J Allergy Clin Immunol. 2018;142(4):1144–58.e8. Epub 2017/12/17. doi: 10.1016/j.jaci.2017.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tordesillas L, Goswami R, Benedé S, Grishina G, Dunkin D, Järvinen KM, et al. Skin exposure promotes a Th2-dependent sensitization to peanut allergens. J Clin Invest. 2014;124(11):4965–75. Epub 2014/10/09. doi: 10.1172/jci75660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22(3):659–61. Epub 2007/10/19. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 16.Li XM, Serebrinsky D, Lee SY, Huang CK, Bardina L, Schofield BH, et al. A murine model of peanut anaphylaxis: T- and B-cell responses to a major peanut allergen mimic human repones. J Allergy Clin Immunol. 2000;106(1):150–8. [DOI] [PubMed] [Google Scholar]
- 17.Ariyan CE, Brady MS, Siegelbaum RH, Hu J, Bello DM, Rand J, et al. Robust Antitumor Responses Result from Local Chemotherapy and CTLA-4 Blockade. Cancer Immunol Res. 2018;6(2):189–200. Epub 2018/01/18. doi: 10.1158/2326-6066.Cir-17-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao J, Shi LZ, Zhao H, Chen J, Xiong L, He Q, et al. Loss of IFN-γ Pathway Genes in Tumor Cells as a Mechanism of Resistance to Anti-CTLA-4 Therapy. Cell. 2016;167(2):397–404.e9. Epub 2016/09/27. doi: 10.1016/j.cell.2016.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spranger S, Bao R, Gajewski TF. Melanoma-intrinsic β-catenin signalling prevents anti-tumour immunity. Nature. 2015;523(7559):231–5. Epub 2015/05/15. doi: 10.1038/nature14404. [DOI] [PubMed] [Google Scholar]
- 20.Kobayashi T, Iijima K, Dent AL, Kita H. Follicular helper T cells mediate IgE antibody response to airborne allergens. J Allergy Clin Immunol. 2017;139(1):300–13.e7. Epub 2016/06/22. doi: 10.1016/j.jaci.2016.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Butler A, Hoffman P, Smibert P, Papalexi E, Satija R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat Biotechnol. 2018;36(5):411–20. Epub 2018/04/03. doi: 10.1038/nbt.4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stuart T, Butler A, Hoffman P, Hafemeister C, Papalexi E, Mauck WM, 3rd, et al. Comprehensive Integration of Single-Cell Data. Cell. 2019;177(7):1888–902.e21. Epub 2019/06/11. doi: 10.1016/j.cell.2019.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brough HA, Liu AH, Sicherer S, Makinson K, Douiri A, Brown SJ, et al. Atopic dermatitis increases the effect of exposure to peanut antigen in dust on peanut sensitization and likely peanut allergy. J Allergy Clin Immunol. 2015;135(1):164–70. Epub 2014/12/03. doi: 10.1016/j.jaci.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park JY, Chung H, DiPalma DT, Tai X, Park JH. Immune quiescence in the oral mucosa is maintained by a uniquely large population of highly activated Foxp3+ regulatory T cells. Mucosal Immunol 11(4):1092–1102, 2018. doi: 10.1038/s41385-018-0027-2. Epub 2018 May 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tham EH, Rajakulendran M, Lee BW, Van Bever HPS. Epicutaneous sensitization to food allergens in atopic dermatitis: What do we know? Pediatr Allergy Immunol. 2020;31(1):7–18. Epub 2019/09/22. doi: 10.1111/pai.13127. [DOI] [PubMed] [Google Scholar]
- 26.Crotty S T Follicular Helper Cell Biology: A Decade of Discovery and Diseases. Immunity. 2019;50(5):1132–48. Epub 2019/05/23. doi: 10.1016/j.immuni.2019.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Finkelman FD, Holmes J, Katona IM, Urban JF Jr., Beckmann MP, Park LS, et al. Lymphokine control of in vivo immunoglobulin isotype selection. Annu Rev Immunol. 1990;8:303–33. Epub 1990/01/11. doi: 10.1146/annurev.iy.08.040190.001511. [DOI] [PubMed] [Google Scholar]
- 28.Mohrs K, Wakil AE, Killeen N, Locksley RM, Mohrs M. A two-step process for cytokine production revealed by IL-4 dual-reporter mice. Immunity. 2005;23(4):419–29. Epub 2005/10/18. doi: 10.1016/j.immuni.2005.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Russo M, Nahori MA, Lefort J, Gomes E, de Castro Keller A, Rodriguez D, et al. Suppression of asthma-like responses in different mouse strains by oral tolerance. Am J Respir Cell Mol Biol. 2001;24(5):518–26. Epub 2001/05/15. doi: 10.1165/ajrcmb.24.5.4320. [DOI] [PubMed] [Google Scholar]
- 30.van Halteren AG, van der Cammen MJ, Cooper D, Savelkoul HF, Kraal G, Holt PG. Regulation of antigen-specific IgE, IgG1, and mast cell responses to ingested allergen by mucosal tolerance induction. J Immunol. 1997;159(6):3009–15. Epub 1997/09/23. [PubMed] [Google Scholar]
- 31.Yamashita H, Takahashi K, Tanaka H, Nagai H, Inagaki N. Overcoming food allergy through acquired tolerance conferred by transfer of Tregs in a murine model. Allergy. 2012;67(2):201–9. Epub 2011/11/05. doi: 10.1111/j.1398-9995.2011.02742.x. [DOI] [PubMed] [Google Scholar]
- 32.Chinen T, Kannan AK, Levine AG, Fan X, Klein U, Zheng Y, et al. An essential role for the IL-2 receptor in T(reg) cell function. Nat Immunol. 2016;17(11):1322–33. Epub 2016/10/21. doi: 10.1038/ni.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim HJ, Barnitz RA, Kreslavsky T, Brown FD, Moffett H, Lemieux ME, et al. Stable inhibitory activity of regulatory T cells requires the transcription factor Helios. Science. 2015;350(6258):334–9. Epub 2015/10/17. doi: 10.1126/science.aad0616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kitagawa Y, Ohkura N, Kidani Y, Vandenbon A, Hirota K, Kawakami R, et al. Guidance of regulatory T cell development by Satb1-dependent super-enhancer establishment. Nat Immunol. 2017;18(2):173–83. Epub 2016/12/20. doi: 10.1038/ni.3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malhotra N, Leyva-Castillo JM, Jadhav U, Barreiro O, Kam C, O'Neill NK, et al. RORα-expressing T regulatory cells restrain allergic skin inflammation. Sci Immunol. 2018;3(21). Epub 2018/03/04. doi: 10.1126/sciimmunol.aao6923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Worthington JJ, Czajkowska BI, Melton AC, Travis MA. Intestinal dendritic cells specialize to activate transforming growth factor-β and induce Foxp3+ regulatory T cells via integrin αvβ8. Gastroenterology. 2011;141(5):1802–12. Epub 2011/07/05. doi: 10.1053/j.gastro.2011.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ye J, Qiu J, Bostick JW, Ueda A, Schjerven H, Li S, et al. The Aryl Hydrocarbon Receptor Preferentially Marks and Promotes Gut Regulatory T Cells. Cell Rep. 2017;21(8):2277–90. Epub 2017/11/23. doi: 10.1016/j.celrep.2017.10.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khattri R, Auger JA, Griffin MD, Sharpe AH, Bluestone JA. Lymphoproliferative disorder in CTLA-4 knockout mice is characterized by CD28-regulated activation of Th2 responses. J Immunol. 1999;162(10):5784–91. Epub 1999/05/07. [PubMed] [Google Scholar]
- 39.American Academy of Pediatrics. Committee on Nutrition. Hypoallergenic infant formulas. Pediatrics. 2000;106(2 Pt 1):346–9. Epub 2000/08/02. [PubMed] [Google Scholar]
- 40.Brough HA, Makinson K, Penagos M, Maleki SJ, Cheng H, Douiri A, et al. Distribution of peanut protein in the home environment. J Allergy Clin Immunol. 2013;132(3):623–9. Epub 2013/04/24. doi: 10.1016/j.jaci.2013.02.035. [DOI] [PubMed] [Google Scholar]
- 41.Yu W, Freeland DMH, Nadeau KC. Food allergy: immune mechanisms, diagnosis and immunotherapy. Nat Rev Immunol. 2016;16(12):751–65. Epub 2016/11/01. doi: 10.1038/nri.2016.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blom LH, Juel-Berg N, Larsen LF, Hansen KS, Poulsen LK. Circulating allergen-specific T(H)2 lymphocytes: CCR4(+) rather than CLA(+) is the predominant phenotype in peanut-allergic subjects. J Allergy Clin Immunol. 2018;141(4):1498–501.e5. Epub 2017/12/12. doi: 10.1016/j.jaci.2017.10.037. [DOI] [PubMed] [Google Scholar]
- 43.Orgel K, Smeekens JM, Ye P, Fotsch L, Guo R, Miller DR, et al. Genetic diversity between mouse strains allows identification of the CC027/GeniUnc strain as an orally reactive model of peanut allergy. J Allergy Clin Immunol. 2019;143(3):1027–37.e7. Epub 2018/10/22. doi: 10.1016/j.jaci.2018.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Orgel K, Kulis M. A Mouse Model of Peanut Allergy Induced by Sensitization Through the Gastrointestinal Tract. Methods Mol Biol. 2018;1799:39–47. Epub 2018/06/30. doi: 10.1007/978-1-4939-7896-0_4. [DOI] [PubMed] [Google Scholar]
- 45.Doki Y, Murakami K, Yamaura T, Sugiyama S, Misaki T, Saiki I. Mediastinal lymph node metastasis model by orthotopic intrapulmonary implantation of Lewis lung carcinoma cells in mice. Br J Cancer. 1999;79(7-8):1121–6. Epub 1999/03/31. doi: 10.1038/sj.bjc.6690178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fowler S, Powrie F. CTLA-4 expression on antigen-specific cells but not IL-10 secretion is required for oral tolerance. Eur J Immunol. 2002;32(10):2997–3006. Epub 2002/10/02. doi: . [DOI] [PubMed] [Google Scholar]
- 47.Mucida D, Kutchukhidze N, Erazo A, Russo M, Lafaille JJ, Curotto de Lafaille MA. Oral tolerance in the absence of naturally occurring Tregs. J Clin Invest. 2005;115(7):1923–33. Epub 2005/06/07. doi: 10.1172/jci24487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Samoilova EB, Horton JL, Zhang H, Khoury SJ, Weiner HL, Chen Y. CTLA-4 is required for the induction of high dose oral tolerance. Int Immunol. 1998;10(4):491–8. Epub 1998/06/10. doi: 10.1093/intimm/10.4.491. [DOI] [PubMed] [Google Scholar]
- 49.Chen Y, Ma Y, Chen Y. Roles of cytotoxic T-lymphocyte-associated antigen-4 in the inductive phase of oral tolerance. Immunology. 2002;105(2):171–80. Epub 2002/03/02. doi: 10.1046/j.1365-2567.2002.01348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Wijk F, Hoeks S, Nierkens S, Koppelman SJ, van Kooten P, Boon L, et al. CTLA-4 signaling regulates the intensity of hypersensitivity responses to food antigens, but is not decisive in the induction of sensitization. J Immunol. 2005;174(1):174–9. Epub 2004/12/22. doi: 10.4049/jimmunol.174.1.174. [DOI] [PubMed] [Google Scholar]
- 51.van Wijk F, Nierkens S, de Jong W, Wehrens EJ, Boon L, van Kooten P, et al. The CD28/CTLA-4-B7 signaling pathway is involved in both allergic sensitization and tolerance induction to orally administered peanut proteins. J Immunol. 2007;178(11):6894–900. Epub 2007/05/22. doi: 10.4049/jimmunol.178.11.6894. [DOI] [PubMed] [Google Scholar]
- 52.Klocke K, Sakaguchi S, Holmdahl R, Wing K. Induction of autoimmune disease by deletion of CTLA-4 in mice in adulthood. Proc Natl Acad Sci U S A. 2016;113(17):E2383–92. Epub 2016/04/14. doi: 10.1073/pnas.1603892113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang CJ, Heuts F, Ovcinnikovs V, Wardzinski L, Bowers C, Schmidt EM, et al. CTLA-4 controls follicular helper T-cell differentiation by regulating the strength of CD28 engagement. Proc Natl Acad Sci U S A. 2015;112(2):524–9. Epub 2014/12/31. doi: 10.1073/pnas.1414576112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wing JB, Ise W, Kurosaki T, Sakaguchi S. Regulatory T cells control antigen-specific expansion of Tfh cell number and humoral immune responses via the coreceptor CTLA-4. Immunity. 2014;41(6):1013–25. Epub 2014/12/20. doi: 10.1016/j.immuni.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 55.Sage PT, Paterson AM, Lovitch SB, Sharpe AH. The coinhibitory receptor CTLA-4 controls B cell responses by modulating T follicular helper, T follicular regulatory, and T regulatory cells. Immunity. 2014;41(6):1026–39. Epub 2014/12/20. doi: 10.1016/j.immuni.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Qureshi OS, Zheng Y, Nakamura K, Attridge K, Manzotti C, Schmidt EM, et al. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science. 2011;332(6029):600–3. Epub 2011/04/09. doi: 10.1126/science.1202947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tekguc M, Wing JB, Osaki M, Long J, Sakaguchi S. Treg-expressed CTLA-4 depletes CD80/CD86 by trogocytosis, releasing free PD-L1 on antigen-presenting cells. Proc Natl Acad Sci U S A. 2021;118(30). Epub 2021/07/25. doi: 10.1073/pnas.2023739118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322(5899):271–5. Epub 2008/10/11. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 59.Sakaguchi S, Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T. Regulatory T cells: how do they suppress immune responses? Int Immunol. 2009;21(10):1105–11. Epub 2009/09/10. doi: 10.1093/intimm/dxp095. [DOI] [PubMed] [Google Scholar]
- 60.Munthe-Kaas MC, Carlsen KH, Helms PJ, Gerritsen J, Whyte M, Feijen M, et al. CTLA-4 polymorphisms in allergy and asthma and the TH1/ TH2 paradigm. J Allergy Clin Immunol. 2004;114(2):280–7. Epub 2004/08/19. doi: 10.1016/j.jaci.2004.03.050. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.