Abstract
Exposure to early-life adversity (ELA) is associated with elevated risk for depression and anxiety disorders in adolescence. Identifying mechanisms through which ELA contributes to the emergence of depression and anxiety is necessary to design preventive interventions. One potential mechanism linking exposure to ELA with psychopathology is accelerated pubertal development. Exposure to trauma—specifically interpersonal violence—is associated with earlier pubertal timing, which in turn predicts adolescent-onset depression and anxiety disorders. We review the recent literature on adversity and accelerated pubertal development, exploring specific associations between trauma and accelerated pubertal development as a mechanism linking adversity with depression and anxiety disorders in adolescence. Finally, we suggest future directions for research exploring mechanisms linking ELA with accelerated pubertal development as well as pubertal timing and psychopathology in adolescence.
1. Introduction
Exposure to early-life adversity (ELA) is associated with elevated risk for numerous forms of psychopathology across the lifespan, including depression and anxiety disorders. Approximately one-third of adolescent depression onsets and one-sixth of anxiety disorder onsets are explained by exposure to ELA [1]. Identifying mechanisms through which ELA contributes to the emergence of depression and anxiety is imperative to designing preventive interventions.
One potential mechanism linking exposure to ELA with psychopathology is accelerated biological aging. Specifically, exposure to ELA may alter the pace of development, resulting in faster aging of physiological systems. One salient metric of biological aging early in development is pubertal timing, or the timing of the onset of pubertal development. Early pubertal timing is consistently associated with elevated risk for mood and anxiety disorders [2]. In this paper, we review evidence for the role of individual differences in pubertal timing as a mechanism underlying vulnerability to depression and anxiety following ELA. Specifically, we highlight recent research suggesting that exposure to trauma—specifically interpersonal violence—is associated with earlier pubertal timing, which in turn predicts adolescent-onset depression and anxiety disorders. We discuss potential mechanisms that may underlie these associations and potential future directions for this research.
2. Early Life Adversity and Accelerated Pubertal Development
The idea that early environmental experiences impact the pace of biological aging is central in life history theory [3]. These models suggest that the timing of life history events—such as age of sexual maturity, number of offspring, length of parental investment, etc.—is determined by trade-offs in the prioritization of time and energy invested in growth relative to reproduction and survival [4]. Early environmental experiences are thought to modulate the pace of development in order to maximize the chances of survival and reproduction later in life. Environments characterized by harshness or threat (e.g., trauma or exposure to violence) may accelerate the onset of puberty in order to maximize the opportunity for reproduction prior to mortality. However, in environments characterized by material and social deprivation, the onset of puberty may be delayed in order to ensure adequate resources are available to support reproduction [3].
A commonly used metric of biological aging in youths is the timing and pace of pubertal development, including age of menarche in females and pubertal stage controlling for chronological age in males and females. Exposure to ELA is associated with earlier pubertal timing in numerous studies [5]–[9], although others have reported no such associations [10], [11] or even delayed pubertal timing following ELA [7], [12], [13]. We have argued that discrepancies across studies may be due to the treatment of ELA as a homogenous construct [14].
2.1. Specificity to Trauma/Threat Exposure
One potential explanation for variability in the association of ELA with early pubertal timing is that distinct types of ELA influence the pace of development differently. Dimensional models of ELA argue that the wide range of experiences classified as ELA can be organized into underlying dimensions of environmental experience that have unique influences on development [3], [15]. These models identify core dimensions of environmental experience that occur in numerous forms of adversity [15]. Dimensions proposed in existing models including threat/harshness (which encompasses experiences involving trauma/threat of harm to the physical integrity of the child, such as abuse and exposure to violence), deprivation (which involves an absence of expected inputs from the environment during development, such as cognitive and social stimulation and responsive caregiving), and unpredictability (which involves temporal variation in caregiving). Dimensional models argue that these aspects of the early environment influence emotional, cognitive, and neural development through some shared pathways as well as others that are distinct and vary as a function of the type of adversity experienced [15]. It is important to note that a central issue in much work exploring the impact of specific dimensions of early life adversity on pubertal timing is a failure to assess and adjust for co-occurring forms of ELA. Such an approach is critical when evaluating potential specificity in associations with pubertal timing, because experiences of ELA are highly co-occurring [1][16].
We have argued that threatening early environments (i.e., environments characterized by trauma/violence/threat of harm) may be particularly likely to lead to accelerated pubertal development, as they signal that the environment is dangerous and that morbidity and mortality risk is high [14]. In contrast, environments characterized by deprivation may lead to delayed pubertal development, as they signal that environmental resources may not be adequate to support reproduction. Indeed, across two independent samples children exposed to trauma exhibited earlier pubertal timing, whereas children who experienced deprivation did not, controlling for co-occurring ELA [13], [17]. In a meta-analysis spanning 43 studies and over 100,000 participants, ELA experiences characterized by threat (i.e., trauma/violence exposure/threat of harm) were associated with earlier pubertal timing, but no association with pubertal timing was observed for poverty or experiences characterized by deprivation (i.e., neglect or institutional rearing) [14]. These findings support our hypotheses regarding threatening early environments and accelerated development and highlight the importance of considering the nature of the early environmental experiences and controlling for co-occurring dimensions of adversity, when examining the influence of adversity on the pace of development.
These findings highlight one potential pathway through which trauma contributes to risk for adolescent depression and anxiety. Early pubertal timing is associated with elevations in depression and anxiety during adolescence [2], [18]–[20]. Our work suggests that early pubertal timing may be one mechanism that accounts for the powerful association between trauma and adolescent psychopathology, demonstrating that earlier pubertal timing helps to explain the link between trauma and later depression and anxiety [13], [17].
3. Mechanisms Linking ELA and Accelerated Pubertal Development
The mechanisms through which threatening early environments influence pubertal timing remain unknown. One plausible mechanism involves alterations in physiological stress response systems, such as the hypothalamic-pituitary-adrenal (HPA) axis, that in turn influence biological systems responsible for sexual development, including the hypothalamic-pituitary-gonadal (HPG) axis. Most work examining associations between HPA and HPG axis function in early development has been done in animal models. These studies suggest that higher levels of corticosterone suppress the release of sex steroids and can even halt ovulation (i.e. Kamel & Kubajak, 1987). The few studies examining these associations in humans are consistent with animal models. For instance, Shi et al. (2011) found that lower daily cortisol output in childhood (pre-puberty) was associated with earlier pubertal timing in females but not males. Similarly, lower daily cortisol output predicted earlier pubertal development in females only (Negriff, Saxbe, et al. 2015; Saxbe et al., 2015).
There is mixed evidence for how ELA influences HPA-axis responsivity. The strongest support exists for the association between early-life trauma and blunted cortisol response both diurnally and in response to stressors [24]. Given associations between trauma and blunted cortisol reactivity and diurnal patterns [25], [26], it is plausible that trauma-related alterations of the HPA-axis may influence regulation of the HPG-axis in ways that accelerate pubertal development (Belsky, Ruttle, Boyce, Armstrong, & Essex, 2015; Negriff, Saxbe, et al., 2015; Saxbe et al., 2015). More specifically, blunted HPA-axis function following early-life trauma could lead to an earlier influx of adrenal and gonadal hormones responsible for pubertal onset. Future research should directly examine whether altered HPA-axis function is a mechanism linking ELA with HPG-axis function and pubertal development.
4. Potential Mechanisms Linking Accelerated Pubertal Development to Psychopathology
Extensive evidence suggests that earlier pubertal timing is associated with elevated risk for psychopathology in adolescence, including depression and anxiety disorders [2], [28]. Numerous mechanisms are likely involved in this association (for reviews see Ge & Natsuaki, 2009; Graber, 2013). Early conceptual models focused on differences in the pace of maturation among physical, emotional, and cognitive systems as underlying the association between early pubertal timing and psychopathology [31]. For example, earlier pubertal timing may lead to changes in physical appearance and body size and shape that are not in line with societal ideals, leading to psychological distress and risk for psychopathology [32]. More recently, these ideas have been applied to theories of brain development—suggesting that earlier puberty leads to changes in neural networks involved in emotion processing before the maturation of prefrontal control networks that increases risk for adolescent psychopathology [33], [34].
Despite substantial interest in how puberty shapes neurodevelopment, evidence for the impact of pubertal timing specifically on brain development is sparse. Findings from the Imaging brain development in Childhood to Adolescence Transition Study (iCATS; Simmons et al., 2014) reveal wide ranging effects of early adrenarche on brain function and structure. For instance, early adrenarche was associated with decreased frontal white matter [36], changes in neural responses to emotional faces in the salience network and ventromedial prefrontal cortex [37], and altered fronto-amygdala connectivity [38], with divergent patterns for males and females. These patterns of neural function, along with increased volume of the pituitary gland, mediated the association between early adrenarche and increases in anxiety symptoms [38], [39]. Altogether, these findings suggest that alterations in pubertal timing influence brain structure and function, particularly fronto-limbic circuitry. Work in this area remains due to the difficulty of disentangling the influence of alterations in pubertal timing from normative changes associated with pubertal development and chronological age. More research is needed to determine how other facets of pubertal timing, such as changes in body morphology or sex steroids associated with gonadarche, influence neural function and structure in ways that contributes to risk for adolescent psychopathology.
5. Limitations and Future Directions
Despite substantial evidence linking ELA and early pubertal timing with risk for psychopathology in adolescence, much work is needed to identify mechanisms underlying these associations. For example, symptoms of psychopathology may contribute to accelerated biological aging following ELA [40]–[42]. Understanding whether interventions, designed to treat psychopathology in children and adolescents exposed to adversity, lead to changes in pubertal timing will help to clarify the direction of these associations.
Understanding of the impact of pubertal timing on neurodevelopment remains limited, due in part to gaps in knowledge of normative patterns of brain development. For instance, reliable patterns of neurodevelopmental maturation in networks frequently examined in relation to puberty, such as amygdala-PFC connectivity, have not been established (although see Bloom et al., 2021). Establishing these normative patterns is essential in order to understand how pubertal timing influences these trajectories. Alternatively, more global metrics such as “BrainAGE” may do a better job of distinguishing departure from typical developmental trajectories (Figure 1). These methods will allow investigation into whether pubertal development accelerates the pace of brain development globally or only in particular brain networks (e.g., those involved in emotional processing or that have higher concentrations of sex steroid receptors). With the help of large longitudinal samples such as the Adolescent, Brain, Cognition and Development Study (Jernigan, Brown, & Dowling, 2018) and the Human Connectome Project-Development [45], we will be better able to distinguish how pubertal timing influences neurodevelopmental trajectories in ways that contribute to risk for adolescent psychopathology.
Figure 1.
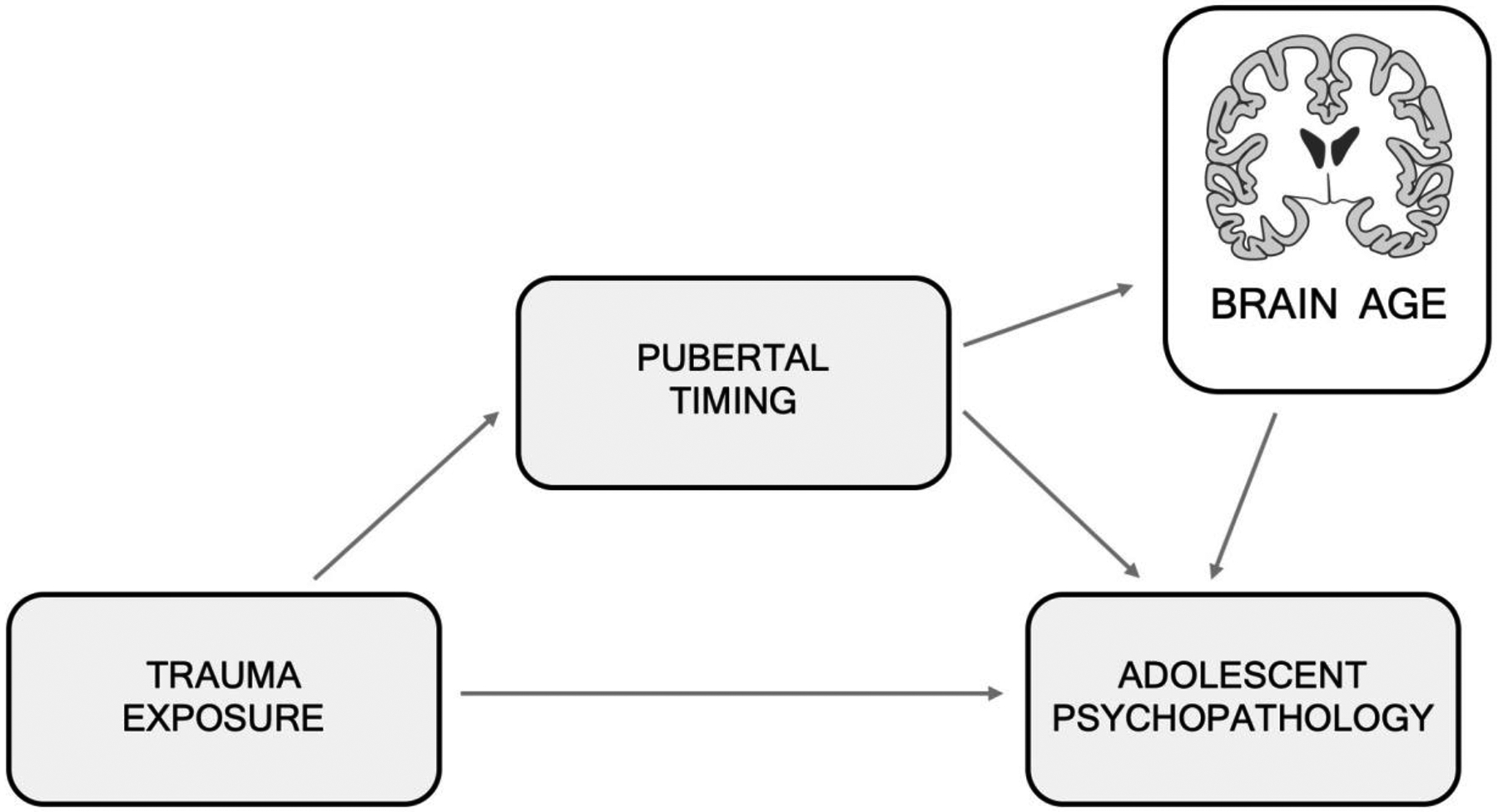
A visual representation of our theoretical model linking childhood trauma exposure and adolescent psychopathology through accelerated pubertal development.
Additionally, it is important to consider the possibility of sex differences in the associations among ELA, pubertal timing, and different domains of psychopathology. A full review of the literature on sex differences in the impact of pubertal timing is beyond the score of this review; however, it is important to note that a recent meta-analysis did not find support for sex differences in the association between pubertal timing and psychopathology generally [2].However, that analysis did not examine whether the association of pubertal timing with different types of psychopathology, such as internalizing and externalizing problems, varied by sex. Given significant sexual dimorphisms in the development of biological systems beginning in early development and continuing throughout sexual maturation and adulthood [46] as well as sex differences in rates of depression and anxiety that emerge in adolescence [47], it is likely that there may be sex differences in the association between pubertal timing and depression and anxiety in adolescence.
As with any body of research, it is important to consider the methodological limitations inherent in the subject under study. For instance, there are well documented recall biases associated with retrospective reporting of childhood experiences in adulthood [48]–[50]. Indeed, a recent meta-analysis comparing retrospective and prospective methods for measuring ELA exposure demonstrates very little overlap in the groups identified by each of these methods, suggesting that prospective and retrospective assessments identify fundamentally different groups of people [50]. Thus, it is important to account for the way that ELA experiences are measured when interpreting study results. In contrast, self-report of age of menarche, even retrospectively, shows relatively high reliability [51], [52] and does not suffer from retrospective recall to the same degree [53], [54]. However, different metrics of pubertal timing have differing degrees of reliability. For best practices in measuring pubertal development see [55]–[57].
Finally, it is important to determine whether associations between ELA and pubertal timing extend to additional metrics of biological aging, or whether this association is specific to puberty. We recently found consistency in the association among threat-related adversity and early pubertal timing, cellular aging, and structural metrics of cortical development [14]. Recent evidence suggests that ELA is associated with changes in brain aging [42], [58] and the timing of the emergence of permanent molars in early childhood [59]. More work is needed to integrate across measures of biological aging, in order to fully understand the mechanisms underlying associations among ELA, biological aging and risk for adolescent psychopathology.
6. Conclusions
Accelerated pubertal development is a potential mechanism linking exposure to threat-related adversity with the onset of depression and anxiety disorders in adolescents. Mechanisms underlying these associations are still under investigation, including associations between stress systems and reproductive hormones, and the impact of alterations in pubertal timing on brain development in childhood and adolescents. These findings and future research directions present novel potential to inform early interventions for children and adolescents who have experienced ELA.
Acknowledgements
The authors would like to Nessa Bryce for creating the illustration in Fig. 1. This work was supported by the National Institute of Mental Health at the National Institute of Health (F32-MH114317 to N.L.C and R01-MH106482, R56-MH119194, and R37-MH119194 to K.A.M).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declare no conflict of interest.
References
- [1].McLaughlin KA, Green JG, Gruber MJ, Sampson NA, Zaslavsky AM, and Kessler RC, “Childhood Adversities and First Onset of Psychiatric Disorders in a National Sample of US Adolescents,” Arch. Gen. Psychiatry, vol. 69, no. 11, p. 1151, Nov. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ullsperger JM and Nikolas MA, “A meta-analytic review of the association between pubertal timing and psychopathology in adolescence: Are there sex differences in risk?,” Psychol. Bull, vol. 143, no. 9, pp. 903–938, Sep. 2017. [DOI] [PubMed] [Google Scholar]
- [3].Ellis BJ, Figueredo AJ, Brumbach BH, and Schlomer GL, Fundamental dimensions of environmental risk: The impact of harsh versus unpredictable environments on the evolution and development of life history strategies, vol. 20, no. 2. 2009. [DOI] [PubMed] [Google Scholar]
- [4].Hill K and Kaplan H, “Life History Traits in Humans: Theory and Empirical Studies,” Annu. Rev. Anthropol, vol. 28, no. 1, pp. 397–430, Oct. 1999. [DOI] [PubMed] [Google Scholar]
- [5].Deardorff J, Abrams B, Ekwaru JP, and Rehkopf DH, “Socioeconomic status and age at menarche: an examination of multiple indicators in an ethnically diverse cohort,” Ann. Epidemiol, vol. 24, no. 10, pp. 727–733, Oct. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Graber J, Brooks-Gunn J, and Warren MP, “The Antecedents of Menarcheal Age: Heredity, Family Environment, and Stressful Life Events,” Child Dev, vol. 66, no. 2, p. 346, Apr. 1995. [DOI] [PubMed] [Google Scholar]
- [7].Negriff S, Blankson AN, and Trickett PK, “Pubertal Timing and Tempo: Associations With Childhood Maltreatment,” J. Res. Adolesc, vol. 25, no. 2, pp. 201–213, Jun. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Noll JG et al. , “Childhood Sexual Abuse and Early Timing of Puberty,” J. Adolesc. Heal, vol. 60, no. 1, pp. 65–71, Jan. 2017. [DOI] [PubMed] [Google Scholar]
- [9].Stenson AF, Michopoulos V, Stevens JS, Powers A, and Jovanovic T, “SexSpecific Associations Between Trauma Exposure, Pubertal Timing, and Anxiety in Black Children,” Front. Hum. Neurosci, vol. 15, no. June, pp. 1–12, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Negriff S and Trickett PK, “Peer substance use as a mediator between early pubertal timing and adolescent substance use: Longitudinal associations and moderating effect of maltreatment,” Drug Alcohol Depend, vol. 126, no. 1–2, pp. 95–101, Nov. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Negriff S, Saxbe DE, and Trickett PK, “Childhood maltreatment, pubertal development, HPA axis functioning, and psychosocial outcomes: An integrative biopsychosocial model,” Dev. Psychobiol, vol. 57, no. 8, pp. 984–993, Dec. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Johnson DE et al. , “Caregiving Disruptions Affect Growth and Pubertal Development in Early Adolescence in Institutionalized and Fostered Romanian Children: A Randomized Clinical Trial,” J. Pediatr, vol. 203, pp. 345–353.e3, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sumner JA, Colich NL, Uddin M, Armstrong D, and McLaughlin KA, “Early Experiences of Threat, but Not Deprivation, Are Associated With Accelerated Biological Aging in Children and Adolescents,” Biol. Psychiatry, vol. 85, no. 3, pp. 268–278, Feb. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Colich NL, Rosen ML, Williams ES, and McLaughlin KA, “Biological aging in childhood and adolescence following experiences of threat and deprivation: A systematic review and meta-analysis.,” Psychol. Bull, no. August, p. 642405, Aug. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].McLaughlin KA, Sheridan MA, Humphreys KL, Belsky J, and Ellis BJ, “The Value of Dimensional Models of Early Experience: Thinking Clearly About Concepts and Categories,” Perspect. Psychol. Sci, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kessler RC et al. , “Childhood adversities and adult psychopathology in the WHO world mental health surveys,” Br. J. Psychiatry, vol. 197, no. 5, pp. 378–385, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Colich NL, Platt JM, Keyes KM, Sumner JA, Allen NB, and McLaughlin KA, “Earlier age at menarche as a transdiagnostic mechanism linking childhood trauma with multiple forms of psychopathology in adolescent girls,” Psychol. Med, vol. 50, no. 7, pp. 1090–1098, May 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hamilton JL, Hamlat EJ, Stange JP, Abramson LY, and Alloy LB, “Pubertal timing and vulnerabilities to depression in early adolescence: differential pathways to depressive symptoms by sex.,” J. Adolesc, vol. 37, no. 2, pp. 165–74, Feb. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Mendle J, Leve LD, Van Ryzin M, and Natsuaki MN, “Linking Childhood Maltreatment With Girls’ Internalizing Symptoms: Early Puberty as a Tipping Point,” J. Res. Adolesc, vol. 24, no. 4, pp. 689–702, Dec. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Platt B, Waters AM, Schulte-Koerne G, Engelmann L, and Salemink E, “A review of cognitive biases in youth depression: attention, interpretation and memory,” Cogn. Emot, vol. 31, no. 3, pp. 462–483, Nov. 2017. [DOI] [PubMed] [Google Scholar]
- [21].Kamel F and Kubajak CL, “Modulation of gonadotropin secretion by corticosterone: Interaction with gonadal steroids and mechanism of action,” Endocrinology, vol. 121, no. 2, pp. 561–568, 1987. [DOI] [PubMed] [Google Scholar]
- [22].Shi L, Wudy SA, Buyken AE, Maser-Gluth C, Hartmann MF, and Remer T, “Prepubertal glucocorticoid status and pubertal timing,” J. Clin. Endocrinol. Metab, vol. 96, no. 6, pp. 891–898, 2011. [DOI] [PubMed] [Google Scholar]
- [23].Saxbe DE, Negriff S, Susman EJ, and Trickett PK, “Attenuated hypothalamic–pituitary–adrenal axis functioning predicts accelerated pubertal development in girls 1 year later,” Dev. Psychopathol, vol. 27, no. 3, pp. 819–828, Aug. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hosseini-Kamkar N, Lowe C, and Morton JB, “The differential calibration of the HPA axis as a function of trauma versus adversity: A systematic review and p-curve meta-analyses,” Neurosci. Biobehav. Rev, vol. 127, no. January, pp. 54–135, 2021. [DOI] [PubMed] [Google Scholar]
- [25].King LS et al. , “The impact of the severity of early life stress on diurnal cortisol: The role of puberty,” Psychoneuroendocrinology, vol. 77, pp. 68–74, Mar. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Jaffee SR, Mcfarquhar T, Stevens S, Ouellet-Morin I, Melhuish E, and Belsky J, “Interactive effects of early and recent exposure to stressful contexts on cortisol reactivity in middle childhood,” J. Child Psychol. Psychiatry Allied Discip, vol. 2, pp. 138–146, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Sandro Marini ECD, Kathryn A, Soare Thomas W., Suderman Matthew J., Simpkin Andrew J., Smith Andrew D.A.C., Wolf Erika J., Relton Caroline L., “Predicting cellular aging following exposure to adversity: Does accumulation, recency, or developmental timing of exposure matter?,” Dev. Psychol, vol. 51, no. 6, pp. 816–822, Jun. 2015.25915592 [Google Scholar]
- [28].Platt JM, Colich NL, McLaughlin KA, Gary D, and Keyes KM, “Transdiagnostic psychiatric disorder risk associated with early age of menarche: A latent modeling approach,” Compr. Psychiatry, vol. 79, pp. 70–79, Nov. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ge X and Natsuaki MN, “In Search of Explanations for Early Pubertal Timing Effects on Developmental Psychopathology,” Curr. Dir. Psychol. Sci, vol. 18, no. 6, pp. 327–331, Dec. 2009. [Google Scholar]
- [30].Graber J, “Pubertal timing and the development of psychopathology in adolescence and beyond,” Horm. Behav, vol. 64, no. 2, pp. 262–269, Jul. 2013. [DOI] [PubMed] [Google Scholar]
- [31].Brooks-Gunn J, Petersen AC, and Eichorn D, “The study of maturational timing effects in adolescence,” J. Youth Adolesc, vol. 14, no. 3, pp. 149–161, 1985. [DOI] [PubMed] [Google Scholar]
- [32].Magnusson D, Stattin H, and Allen VL, “Biological maturation and social development: A longitudinal study of some adjustment processes from midadolescence to adulthood,” J. Youth Adolesc, vol. 14, no. 4, pp. 267–283, 1985. [DOI] [PubMed] [Google Scholar]
- [33].Ladouceur CD, “Neural systems supporting cognitive-affective interactions in adolescence: the role of puberty and implications for affective disorders.,” Front. Integr. Neurosci, vol. 6, no. August, pp. 1–11, Jan. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Steinberg LD, Albert D, Cauffman E, Banich MT, Graham S, and Woolard J, “Age differences in sensation seeking and impulsivity as indexed by behavior and self-report: evidence for a dual systems model.,” Dev. Psychol, vol. 44, no. 6, pp. 1764–1778, 2008. [DOI] [PubMed] [Google Scholar]
- [35].Simmons JG et al. , “Study protocol: Imaging brain development in the Childhood to Adolescence Transition Study (iCATS).,” BMC Pediatr, vol. 14, no. 1, p. 115, Jan. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Klauser P et al. , “Reduced frontal white matter volume in children with early onset of adrenarche,” Psychoneuroendocrinology, vol. 52, no. 1, pp. 111–118, 2015. [DOI] [PubMed] [Google Scholar]
- [37].Whittle SL et al. , “Associations between early adrenarche, affective brain function and mental health in children,” Soc. Cogn. Affect. Neurosci, vol. 10, no. 9, pp. 1282–1290, Sep. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Barendse MEA et al. , “Adrenarcheal Timing Longitudinally Predicts Anxiety Symptoms Via Amygdala Connectivity During Emotion Processing,” J. Am. Acad. Child Adolesc. Psychiatry, no. June, 2019. [DOI] [PubMed] [Google Scholar]
- [39].Murray CR et al. , “Associations between dehydroepiandrosterone (DHEA) levels, pituitary volume, and social anxiety in children,” Psychoneuroendocrinology, vol. 64, pp. 31–39, 2016. [DOI] [PubMed] [Google Scholar]
- [40].Mensah FK, Bayer JK, Wake M, Carlin JB, Allen NB, and Patton GC, “Early puberty and childhood social and behavioral adjustment,” J. Adolesc. Heal, vol. 53, no. 1, pp. 118–124, 2013. [DOI] [PubMed] [Google Scholar]
- [41].Lindqvist D et al. , “Psychiatric disorders and leukocyte telomere length: Underlying mechanisms linking mental illness with cellular aging,” Neuroscience and Biobehavioral Reviews, vol. 55. Elsevier Ltd, pp. 333–364, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Keding TJ et al. , “Differential Patterns of Delayed Emotion Circuit Maturation in Abused Girls With and Without Internalizing Psychopathology,” Am. J. Psychiatry, no. 5, p. appi.ajp.2021.2, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Bloom PA et al. , “Age-related change in task-evoked amygdala-prefrontal circuitry: a multiverse approach with an accelerated longitudinal cohort aged 4–22 years,” 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Jernigan TL, Brown SA, and Dowling GJ, “The Adolescent Brain Cognitive Development Study,” J. Res. Adolesc, vol. 28, no. 1, pp. 154–156, Mar. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Somerville LH et al. , “The Lifespan Human Connectome Project in Development: A large-scale study of brain connectivity development in 5–21 year olds,” Neuroimage, vol. 183, no. July, pp. 456–468, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Cousminer DL, Widén E, and Palmert MR, “The genetics of pubertal timing in the general population,” Curr. Opin. Endocrinol. Diabetes Obes, vol. 23, no. 1, pp. 57–65, Feb. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Breslau J, Gilman SE, Stein BD, Ruder T, Gmelin T, and Miller E, “Sex differences in recent first-onset depression in an epidemiological sample of adolescents,” Transl. Psychiatry, vol. 7, no. 5, p. e1139, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Hardt J and Rutter M, “Validity of adult retrospective reports of adverse childhood experiences: review of the evidence,” J. Child Psychol. Psychiatry, vol. 45, no. 2, pp. 260–273, Feb. 2004. [DOI] [PubMed] [Google Scholar]
- [49].Widom CS, Raphael KG, and DuMont KA, “The case for prospective longitudinal studies in child maltreatment research: commentary on Dube, Williamson, Thompson, Felitti, and Anda (2004),” Child Abuse Negl., vol. 28, no. 7, pp. 715–722, Jul. 2004. [DOI] [PubMed] [Google Scholar]
- [50].Baldwin JR, Reuben A, Newbury JB, and Danese A, “Agreement between Prospective and Retrospective Measures of Childhood Maltreatment: A Systematic Review and Meta-analysis,” JAMA Psychiatry, vol. 76, no. 6, pp. 584–593, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Dorn LD, Sontag-Padilla LM, Pabst S, Tissot A, and Susman E, “Longitudinal reliability of self-reported age at menarche in adolescent girls: variability across time and setting.,” Dev. Psychol, vol. 49, no. 6, pp. 1187–93, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Lundblad MW and Jacobsen BK, “The reproducibility of self-reported age at menarche: The Tromsø Study,” BMC Womens. Health, vol. 17, no. 1, pp. 1–7, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Gilger JW, Geary DC, and Eisele LM, “Reliability and validity of retrospective self-reports of the age of pubertal onset using twin, sibling, and college student data.,” Adolescence, vol. 26, no. 101, pp. 41–53, 1991. [PubMed] [Google Scholar]
- [54].Cooper R et al. , “Validity of age at menarche self-reported in adulthood,” J. Epidemiol. Community Health, vol. 60, no. 11, pp. 993–997, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Shirtcliff EA, Dahl RE, and Pollak SD, “Pubertal development: correspondence between hormonal and physical development.,” Child Dev., vol. 80, no. 2, pp. 327–37, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Cheng TW et al. , “A Researcher’s Guide to the Measurement and Modeling of Puberty in the ABCD Study® at Baseline,” Front. Endocrinol. (Lausanne), vol. 12, no. May, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Dorn LD and Biro FM, “Puberty and Its Measurement: A Decade in Review,” J. Res. Adolesc, vol. 21, no. 1, pp. 180–195, Mar. 2011. [Google Scholar]
- [58].Gur RE et al. , “Burden of Environmental Adversity Associated With Psychopathology, Maturation, and Brain Behavior Parameters in Youths,” JAMA Psychiatry, vol. 19104, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].McDermott CL et al. , “Early life stress is associated with earlier emergence of permanent molars,” Proc. Natl. Acad. Sci. U. S. A, vol. 118, no. 24, pp. 3–5, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]


