Abstract
Extracellular vesicles (EVs) provide a mechanism for intercellular communication that transports complex signals in membrane delimited structures between cells, tissues and organisms. Cells secrete EVs of various subtypes defined by the pathway leading to release and by the pathological condition of the cell. Cilia are evolutionarily conserved organelles that can act as sensory structures surveilling the extracellular environment. Here we discuss the secretory functions of cilia and their biological implications. Studies in multiple species – from the nematode Caenorhabditis elegans and the chlorophyte alga Chlamydomonas reinhardtii to mammals – have revealed that cilia shed bioactive EVs (ciliary EVs or ectosomes) by outward budding of the ciliary membrane. The content of ciliary EVs is distinct from that of other vesicles released by cells. Peptides regulate numerous aspects of metazoan physiology and development through evolutionarily conserved mechanisms. Intriguingly, cilia-derived vesicles have recently been found to mediate peptidergic signaling. C. reinhardtii releases the peptide α-amidating enzyme (PAM), bioactive amidated products and components of the peptidergic signaling machinery in ciliary EVs in a developmentally regulated manner. Considering the origin of cilia in early eukaryotes, it is likely that release of peptidergic signals in ciliary EVs represents an alternative and ancient mode of regulated secretion that cells can utilize in the absence of dedicated secretory granules.
Keywords: Amidation, Chlamydomonas, Cilia, Ectosome, Peptidergic Signaling
1. Introduction
Intercellular communication plays essential and central roles throughout biology. These can range from information transfer between individual, even unicellular, organisms to internal signal transduction within complex animals that possess a closed circulatory system. One mode of signal transmission is through the release of extracellular vesicles (EVs); this general process appears to be an evolutionarily conserved mechanism with pre-metazoan origins. For example, EVs play a crucial part in regulating the development and function of multicellular organisms ranging from mammals to echinoderms, placozoans, nematodes, as well as unicellular organisms such as the chlorophyte green alga Chlamydomonas reinhardtii [1–5]. Secretion of EVs facilitates long-distance signal transduction between different tissues and even inter-organismal signaling. EVs have also been found to mediate information exchange between high-level taxonomic divisions such as occurs for example during host-pathogen and parasite-vector interactions [6, 7]. Depending on their source, these membrane-delimited structures have been found to contain a broad array of components including numerous and varied proteins and lipids, various classes of RNAs and in some cases even DNA [8–12].
In this review, we focus on the EVs released from specialized microtubule-scaffolded cellular extensions known as cilia. These EVs are formed by budding from the ciliary membrane and are known as ciliary ectosomes or cilia-derived vesicles (Fig. 1a). The release of EVs from cilia has been described in several systems including mammals, nematodes and C. reinhardtii [1, 4, 13, 14]. Ciliary EVs share some characteristics with microvesicles, derived by budding from the plasma membrane (Fig. 1b), and with exosomes that form as intraluminal vesicles (ILVs) within multi-vesicular bodies (MVBs) (Fig. 1c) and are released following fusion of MVBs with the plasma membrane. Although many studies have examined the properties of exosomes and microvesicles, especially those of mammalian origin, our understanding of the composition and signaling functions of ciliary EVs is much more limited. Studies in the nematode Caenorhabditis elegans and in C. reinhardtii, organisms where the unambiguous origin of cilia-derived EVs can be readily demonstrated, has started to shed light on their bioactive cargoes and to point towards their possible roles in mammals [1, 15, 16]. For example, components of the peptidergic signaling machinery have been found in ciliary ectosomes isolated from C. reinhardtii [16].
Figure 1 -. Extracellular vesicles of different origin.

The generation of three different types of extracellular vesicle based on their origin is illustrated. a) Ciliary extracellular vesicles. The ciliary membrane is very different from the plasma membrane in terms of both its lipid and protein content. Membrane proteins are transported to cilia via Golgi-derived vesicles or through lateral diffusion from the plasma membrane. Outward budding of the ciliary membrane forms a microdomain that contains membrane and soluble proteins that are present in the ciliary matrix. Fission of the ciliary membrane generates ciliary ectosomes, also known as cilia-derived vesicles, which carry specific protein cargoes that transmit signals and enzymatic activities over long distances. Importantly, ectosome content is controlled by regulated entry of proteins into cilia and by their subsequent sorting to sites of ectosome release. b) Ectosomes/microvesicles. These EVs form by outward budding of the plasma membrane. Receptors, membrane and soluble proteins, lipids and nucleic acids accumulate at the cytosolic face of the outwardly budding plasma membrane; subsequent fission leads to the formation of ectosomes. Ectosomes are larger (100 nm-1 µm in diameter) then exosomes, but surface receptors and membrane proteins (red and blue) share the same topology in both structures. c) Exosomes. Surface receptors and membrane proteins (red and green) internalize through the endosomal pathway. Inward budding of the endosomal membrane leads to the production of various intraluminal vesicles (ILVs), and thus the formation of multivesicular bodies (MVBs); MVBs can be also be formed from Golgi-derived vesicles. Subsequently, fusion of MVBs with the plasma membrane releases these ILVs into the extracellular milieu where they are termed exosomes. Exosomes are small (50–100 nm diameter) membrane limited structures that carry soluble proteins, membrane proteins and surface receptors, lipids and nucleic acid cargoes that are largely different from those of the endocytic system. Illustrations were created with BioRender.com.
In mammalian neurons and neuroendocrine cells, neuropeptides and peptide hormones are stored in secretory granules and released through the regulated secretory pathway [17]. Here, we describe recent evidence for a different route leading to the regulated transmission of intercellular signaling factors into the environment. This pathway relies first on sorting of signaling precursors into the cilium, which is a highly specialized and regulated process, and then on their targeting to vesicles that bud from the ciliary membrane in response to specific cellular and/or environmental cues. Regulated and altered secretion of EVs has been associated with various ciliopathies (complex syndromes affecting multiple organs that originate from ciliary dysfunction), organismal development and maintenance of physiological homeostasis under both normal and pathological conditions [18–20]. Furthermore, for cells and organisms that lack dedicated secretory granules, the ciliary release pathway provides an alternative mechanism for regulated secretion that likely is of ancient origin and predictably was present in early eukaryotes.
2. Cilia and Ciliary-derived Vesicles
Cilia are evolutionarily conserved membrane bound cellular projections that can serve as motile, sensory and secretory organelles involved in the regulation of a wide variety of cellular activities (Fig. 1a and 2) [21, 22]. Studies suggest that these structures were present in the last eukaryotic common ancestor and are found throughout extant eukaryotes with several notable exceptions, such as the angiosperms and red algae [23, 24]. In many systems, these organelles have become highly modified and specialized to perform dedicated sensory and signaling functions, for example, photoreceptor cells contain a highly modified primary cilium where the ciliary membrane is extended to form hundreds of disc-like structures that contain proteins involved in the phototransduction cascade [25].
Figure 2 -. Ectosome formation on cilia.

a) Three optical sections of a single C. reinhardtii cell showing the two ~12 µm cilia (one is indicated by an arrow); differential interference contrast microscopy. b) Thin-section electron micrograph through a C. reinhardtii cilium. The transition zone gate and a nascent ectosome budding from the ciliary membrane are indicated. c) Section through a pellet of isolated ectosomes derived from the cilia of mating C. reinhardtii gametes. These structures may be purified in sufficient quantity for detailed biochemical and functional analysis. d) Electron micrograph of the neuroepithelium from the third ventricle of a mouse. A single ectosome budding from the surface of a primary cilium and the basal body that templates cilia formation are indicated. A second orthogonally oriented primary cilium in an adjacent cell is visible in cross section. Bars represent 5 µm (a), 250 nm (b), and 500 nm (c and d).
2.1. General Organization of Cilia
The central core of the cilium is a microtubule-based structure known as the axoneme, which derives from, and is anchored to, the basal body at the ciliary base. In general, motile cilia, except those at the embryonic node, have a 9+2 arrangement of axonemal microtubules with a central pair of singlet microtubules surrounding the nine outer doublets. These are decorated with a complex array of protein complexes, such as dynein arms and radial spokes, needed to generate and propagate ciliary waveforms [26]. Motile cilia enable the movement of individual cells such as unicellular organisms and sperm, and when arrayed in large numbers on an epithelial surface, for example those lining the trachea, oviduct and ependymal lining of the brain, generate the flow needed for bulk fluid transport and the generation of extracellular gradients [27]. In contrast, primary or non-motile cilia generally have a 9+0 microtubule arrangement lacking the central pair complex; the nine outer doublets also exhibit a more variable inter-doublet spacing [28].
The cilium also contains non-axonemal or matrix components and is delimited from the extracellular environment by the ciliary membrane. Although the ciliary membrane is contiguous with the plasma membrane, its lipid and protein compositions are very distinct leading to functional specialization of different cilia types; for example, in mammals the cilia of olfactory neurons in the nose and photosensitive cells in the eye contain specific adaptions and dedicated membrane receptors that enable them to detect and report on different environmental stimuli with exquisite sensitivity [29, 30].
Ciliary proteins are synthesized in the cytosol, and transported into the organelle through a transition zone (TZ) selective diffusion barrier, which serves as a gate to allow the entry and exit of defined ciliary cargoes. The TZ is characterized by the presence of Y-shaped linkers that connect the axoneme to the ciliary membrane, transition fibers anchored at the basal body, and several multi-molecular complexes that regulate protein trafficking into the ciliary membrane [31, 32] (Fig. 1a). The bi-directional transport of proteins along the length of the cilium is mediated by a complex intraflagellar transport (IFT) system which comprises several multi-subunit protein complexes - IFTA, IFTB and the BBSome (Bardet–Biedl syndrome protein complex) - driven by dedicated kinesin and dynein motors [33, 34].
Ciliary membranes in mammals are enriched in phosphatidylinositol-4-phosphate [PI(4)P], while phosphatidylinositol-4,5-bisphosphate (PI(4,5)P2 levels are much higher in the plasma membrane [35]. In retinal pigment epithelial cells, PI(4,5)P2 and PI(3,4,5)P3 are concentrated at the transition zone [36] and in photoreceptor cells, PI(4,5)P2 localizes to the inner segment [37, 38]. The presence of sphingolipids and gangliosides has been reported in primary cilia of Madin–Darby Canine Kidney (MDCK) epithelial cells [39] and cilia of Trypanosoma brucei [40]. The differences in lipid composition at the ciliary base compared to the plasma membrane is thought to drive changes in membrane curvature and act as a barrier to free diffusion between the ciliary and plasma membrane compartments [41].
One major function of cilia is to sense the extracellular environment and signal dependent changes in various ligands to the cell body. Key control systems mediated by these organelles include the Hedgehog pathway, canonical and non-canonical Wnt signaling, mTOR signaling for cortical development, HIPPO (MST1/2) signaling in ciliogenesis, platelet-derived growth factor receptor-α (PDGFRα) signaling in wound healing, Notch/Delta and receptor-mediated signaling and GPCR signaling in response to numerous hormones such as somatostatin and kisspeptin [22]. Furthermore, although the Hedgehog pathway has been attributed to both primary and motile cilia, in motile cilia it reduces intracellular cAMP levels through a non-canonical pathway [42]. Cilium loss or dysfunction can cause defects in signal transduction mediated through these pathways and result in various ciliopathies [22].
2.2. Ciliary Release of Bioactive Vesicles
Cells release EVs via several distinct pathways (Fig. 1). EVs are heterogeneous in nature; however, despite differences in the origin of ectosomes and exosomes, the topology of surface proteins on their membranes is similar. As multi-cellular organisms can release EVs via multiple routes from numerous tissues, in some systems defining the precise source of an EV becomes quite challenging. EVs can carry a wide variety of cargoes such as proteins, RNA, miRNA and a recent study even revealed the presence of DNA [9–12].
In addition to their roles as sensory and motile organelles, cilia also act as secretory structures, releasing EVs into the surrounding environment [1, 13, 43, 44]. Ciliary EVs are formed directly from the ciliary membrane and are topologically related to ectosomes that derive by budding from the plasma membrane (Fig. 1a and b). These plasma membrane-derived EVs are known as microvesicles or ectosomes. In general, ectosomes have diameters ranging from ~100 nm – 1 µm [16] (Fig. 1a, b and Fig. 2b–d). However, the “ciliary entry” step distinguishes ciliary ectosome formation from that of plasma membrane-derived ectosomes as they form from different membranes and their content arises from different cytosolic/matrix compartments. Importantly, not all proteins synthesized by cells are transported into cilia. Indeed, protein entry into these organelles is a highly selective process controlled by directed trafficking of components to the ciliary base as well as passage through the transition zone that acts as a gate whose permeability is affected by protein size and specific ciliary targeting signals [45]. Similarly, the protein cargo of ciliary ectosomes is tightly regulated as many ciliary proteins are excluded from these vesicles while others are specifically concentrated in them [13, 16, 43]. Thus, two distinct sorting systems – gated ciliary entry and regulated trafficking from the cilium proper into nascent ectosomes – provides a cellular pathway for the controlled environmental release of defined signaling factors.
Mammalian cells release small EVs known as exosomes, which are in the 40–100 nm diameter range. Exosomes are generated inside late endosomes by invagination of the endosomal membrane forming ILVs within larger MVB structures. When the limiting MVB membrane fuses with the plasma membrane, ILVs are released as exosomes directly into the environment (Fig. 1c). Control of exosome content may be conceptually related to that of ciliary ectosomes in that many opportunities for protein sorting occur on the way from endocytic vesicles to MVB formation and ultimately ILV secretion.
3. Analysis of Cilia-derived Vesicles
Although there have been numerous proteomic analyses of exosomes from mammalian cells, there is limited information available on the cargoes of ciliary EVs. A major reason for this is, at the organismal level, it can be difficult to determine the cell type of origin of any particular EV, and then differentiate and subsequently purify ciliary-derived EVs from cell body-derived microvesicles or ectosomes. Major insights into the cargoes of ciliary EVs have come from proteomic and other studies in organisms where the ciliary source of EVs can be readily demonstrated, including the chlorophyte alga C. reinhardtii and the nematode C. elegans [1, 4, 16, 43].
3.1. Studying Ciliary Vesicles in Green Algae
C. reinhardtii is an extremely useful and experimentally tractable model organism for the study of ciliary assembly and function [46]. This biciliate unicellular green alga (Fig. 2a) is usually haploid and divides mitotically within a mother cell wall from which progeny are released following cell wall breakdown. Upon nutrient deprivation, C. reinhardtii cells undergo a genetically encoded developmental process to become mating competent gametes. Gametes of opposite mating type interact initially via adhesion receptor molecules on their cilia, which instigates a complex signaling cascade that ultimately leads to cell fusion and zygote formation [47, 48]. When nutrient conditions improve, zygotes undergo meiosis and release haploid progeny.
The first evidence of EV release from cilia came from studies in C. moewusii (a member of the Chlamydomonadales clade distantly related to C. reinhardtii) which identified vesicles, termed “gamone”, derived from cilia that contained adhesion molecules important for gamete interactions [49]. In C. reinhardtii, a cilia-localized protease needed for digestion of the mother cell wall surrounding the mitotic progeny was later shown to be released in ectosomes derived from cilia of daughter cells, thereby allowing the enzyme access to its biological target [1, 50]. Importantly, C. reinhardtii is encased in a multi-layered cell wall and so only the exposed membrane surrounding the cilium is available for EV release into the environment. This provides a major experimental advantage as any EV isolated from a C. reinhardtii culture is of known origin (Fig. 2c). Furthermore, C. reinhardtii releases bioactive ectosomes not only from the ciliary membranes of vegetative cells [1, 43], but also from those of mating gametes where release is concentrated at or near the ciliary tip [13, 16]. For example, the membrane-associated plus agglutinin-derived protein fragment SAG1-C65 is released in EVs as a result of cilium-generated signaling during mating of C. reinhardtii gametes [13].
3.1.1. Ciliary Ectosome Content is Developmentally Regulated
In C. reinhardtii, bioactive ciliary ectosomes are released from both vegetative and gametic cells (Fig. 3). Ciliary ectosomes isolated from vegetative cell cilia are enriched in numerous components compared to the cilium from which they derive, as a consequence of the second step in the ciliary EV sorting pathway. These include high-molecular-weight ubiquitinated proteins, several proteases such as the subtilisin-like enzyme VLE1 and matrix metalloproteinases along with protein kinases, small Rab-like GTPases and glycoproteins including the major flagellar membrane glycoprotein FMG-1, a homologue of the polycystin-2 Ca2+ channel defects in which in mammals lead to polycystic kidney disease, and components of the endosomal sorting complex required for transport (ESCRT) system [43]. In contrast, α- and β-tubulin, several components of IFT complexes (including IFT88, IFT139 and IFT144) and some flagellar-associated proteins were much more prevalent in ciliary membrane fractions [43]. These observations demonstrate that ciliary ectosome content is not a random admixture of ciliary components but rather the result of a specific sorting process that occurs on the cilium as nascent ectosomes are forming.
Figure 3 -. Regulated release of PAM and amidated products in ciliary ectosomes.

The secretion of PAM and amidated peptide products in ectosomes released from the cilia of mating gametes but not those of vegetative cells is illustrated. The left panel show the presence of flagellar membrane glycoprotein 1 (FMG1) and PAM in vegetative cell cilia and the release of FMG1, but not PAM, in ciliary ectosomes. The PAM luminal domain is exposed to the extracellular environment while its cytosolic domain is tethered to the axoneme through unknown interactions (green box). The right panel shows the release of PAM, proGATI amidated product and FMG1 in ectosomes from cilia of mating gametes. During mating, PAM and proGATI precursor are trafficked via Golgi-derived vesicles to the ciliary membrane (black arrow). However, whether the C-terminal amidation of proGATI takes place in Golgi-derived vesicles or on the ciliary membrane or even the ectosomal surface is currently unknown. The proGATI-amide product mediates chemotactic responses in both minus and plus gametes, however the signaling pathway has not yet been determined. Illustrations were created with BioRender.com.
The composition and rate of release of ciliary ectosomes is also developmentally regulated [16]. For example, ciliary adhesion between mating gametes triggers a 25-fold increase in the rate of ectosome release from the ciliary tip compared to cilia of vegetative cells [13, 16]. Furthermore, the peptide-amidating enzyme (PAM) and components of the peptidergic signaling machinery (see section 4) were found in ciliary ectosomes released by mating gametes but not in those derived from vegetative cell cilia (Fig. 3) [16, 43]. The specificity and selectivity with which other ciliary membrane proteins, such as the flagellar membrane glycoprotein (FMG1), are released into ciliary ectosomes is also regulated [16]. Intriguingly, PAM and FMG1 were found to be differentially localized such that some ectosomes were PAM-positive, some FMG1-positive, while a third subset contained both proteins [16]. Additionally, cell wall-related proline- and hydroxyproline-rich proteins, various metabolic enzymes, ER/Golgi-related proteins, several proteases and some flagellar-associated proteins were identified specifically in ectosomes from mating gametes [16]. These observations suggest that cells can secrete distinct subtypes of ciliary ectosomes that vary depending on specific cellular requirements, and further supports the concept that an active EV sorting machinery selects some cargoes but not others for packaging into EVs at distinct life cycle stages. Better understanding of the intra-ciliary trafficking of these proteins and its regulation may shed light on the mechanisms that control entry of ciliary proteins into ectosomes.
3.2. Ciliary Vesicle Release from Nematodes
Another model system that has been used to study the release of ciliary ectosomes is C. elegans. This nematode is a nonparasitic organism that lives in temperate soil environments, and has immotile ciliated sensory neurons located at the head and tail that monitor the internal and external environments; C. elegans hermaphrodites have 60 ciliated sensory neurons while males have an additional 52 neurons [51]. The released ciliary EVs were found to transmit signals between individuals and alter male mating behavior [52, 53]. In male C. elegans, the orthologue of polycystin-2, termed PKD-2, was localized by GFP-tagging in ciliary EVs shed by cephalic neuron (CEM) cilia located at the head and from cilia of bilateral ray B type (RnB) neurons located at the tail [52, 53]. Importantly, mutant nematodes with defects in ciliary assembly lack PKD-2-positive EVs [4]. The passage of ciliary EVs containing PKD-2 into the external milieu is complex. Initially, these vesicles bud from the sensory cilia into a lumen formed by glial cells surrounding the ciliated neuronal cells that is topologically outside the animal. Fully formed EVs then pass from this lumen into the environment in a process that requires enzymes involved in tubulin polyglutamylation – whether this reflects altered ciliary ultrastructure or other regulatory processes in the glutamylation-deficient nematodes remains to be determined [54].
Specific modifications of α- and β-tubulin also play a role in ciliary EV biogenesis. For example, the α-tubulin isotype TBA6 regulates the cargoes and velocities of kinesin-2-driven IFT without affecting kinesin-3 (KLP-6)-mediated motility along the same microtubular structures. TBA6 is required for EV release and affects cargo sorting and size of the released EVs [55]. EV biogenesis is also negatively regulated by the Rab28 small GTPase and by the multi-component BBSome whose loss results in altered EV production in C. elegans [56]. Recent studies suggest that mechanical or chemical stimulation causes ciliary EV release from sensory neuronal cilia of male C. elegans, which mediate directional information transfer to the vulva of another, hermaphrodite, animal [57]. These sensory neurons release EVs from both the ciliary base and tip, by budding of the ciliary membrane, not by fusion of MVBs. Release of EVs at the ciliary tip requires cargo enrichment, IFT and both kinesin-2 and kinesin-3 motors [15]. Furthermore, formation of ciliary EVs in nematodes is regulated by the small GTPase Rab28 and the BBSome complex; these vesicles may provide a mechanism for signaling between the cilia and surrounding glial cells in the sensory organ [56].
3.3. Vesicle release from Mammalian Cilia
In mammals, cilia are present on nearly all cell types, except lymphoid and myeloid lineages, and are intimately involved in the regulation of development, homeostasis and physiology. Mammalian cilia release vesicles from their surface (Fig. 2d). Several proteins found in cilia such as polycystin-2, the exocyst, and regulators of the exocyst (e.g. CDC42) have been identified in the proteomes of kidney epithelial cell EVs and urinary EVs [58, 59]. In mammals, both polycystin-1 and −2 are present in normal kidney and retinal epithelial cells as well as EVs released into urine [59]. In vitro analysis showed that PKD-containing exosome-like vesicles interact with the primary cilia of kidney epithelial cells, suggesting an interaction between cilia and EVs [60]. The Exo5 component of the exocyst complex localizes to primary cilia and ciliary EVs, and alteration in Exo5 expression affects ciliary biogenesis and EV production in MDCK cells [44, 58].
There is limited information about the role of ciliary vesicles in mammals in either physiological or pathological conditions. However, several studies implicate them in the regulation of cilia length [61, 62], cell cycle resumption [61, 62], cell proliferation in tumor growth [63], and receptor-mediated signaling to maintain cells homeostasis (discussed in section 3.3.1). Furthermore, ciliary EVs have been linked to sperm maturation in the turtle Pelodiscus sinensis [64], and they also play a role in cardiovascular function (see section 3.3.2). Abnormalities in ciliary signaling are associated with various pathological conditions in mammals and abnormal or mutant protein release in ciliary ectosomes has been reported in some of these disease conditions [60, 65, 66]. Defining the function of ciliary EVs in these situations will further enhance understanding of these complex processes and holds significant potential for identifying therapeutic targets.
3.3.1. Ciliary Homeostasis through Shedding of Ciliary Ectosomes
Several receptors are shed in ciliary ectosomes to maintain ciliary homeostasis. For example, the shedding (the term is used here to indicate removal of an activated receptor to terminate ligand-induced signaling) of G protein-coupled receptors (GPCRs), such as GPR161 (an orphan receptor over-expressed in triple negative breast cancer cells) and a somatostatin receptor (SSTR3), into ciliary ectosomes has been observed in mammalian cilia [14]. When the machinery (BBSome and β-arrestin) needed for retrieval to the cell body is compromised in murine inner medullary collecting duct (IMCD3) kidney cells, these GPCRs are not returned upon activation but rather concentrate at the tip of the cilia before being packaged into nascent ectosomes and subsequently released into the environment (Fig. 4). In this case, ciliary loss of activated GPCRs in ectosomes has been suggested as a mechanism to shut down or limit hormone-stimulated signaling to the cell body [14]. Similarly, the GPCR, neuropeptide Y receptor type 2 (NPY2R) that lacks the sequence determinants needed for retrieval of the activated GPCR to the cell body, is released in a vesicle from the ciliary tip in a process termed ectocytosis thereby downregulating signal transduction [14].
Figure 4 -. Shedding of GPCRs in ciliary ectosomes.
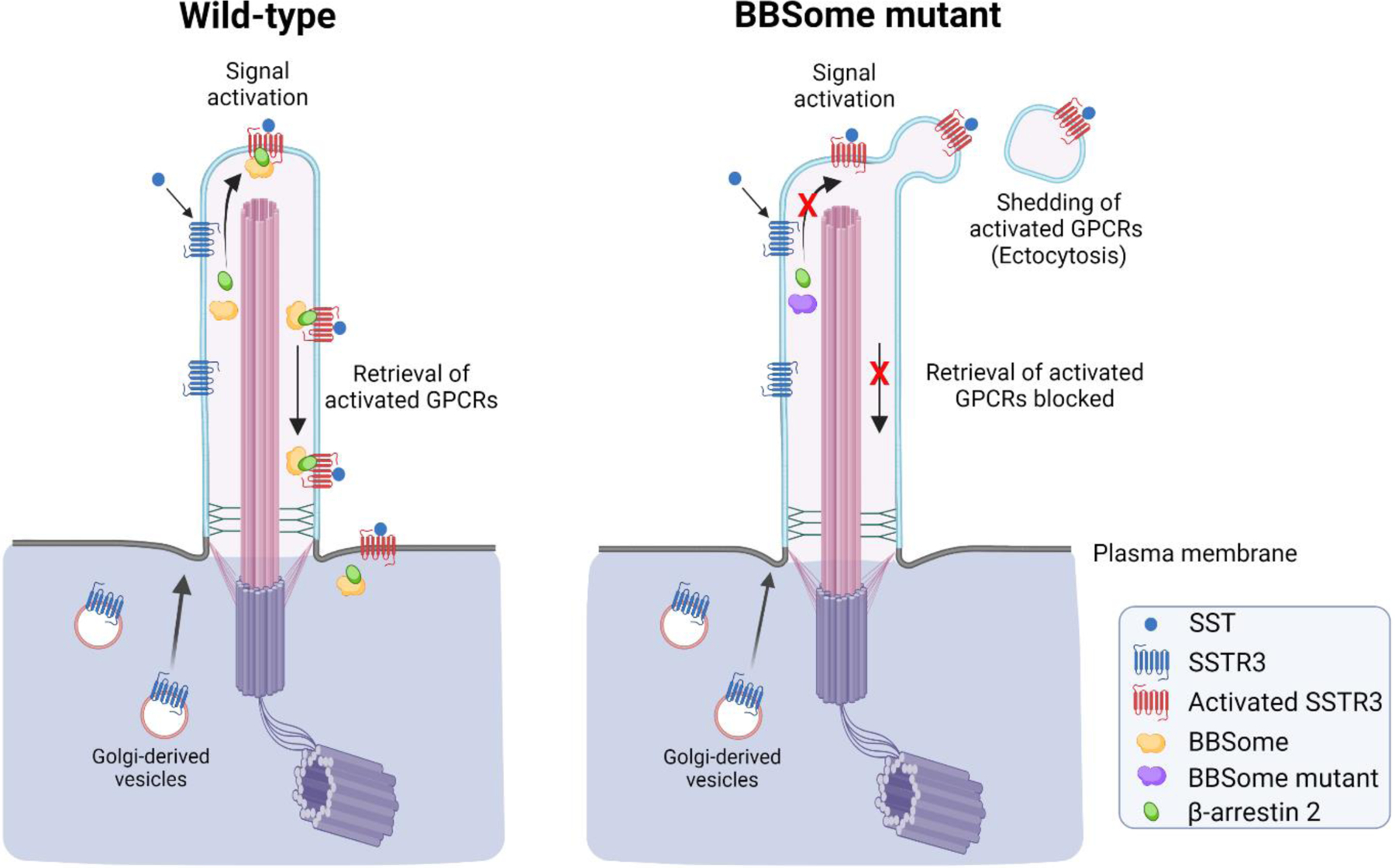
Illustration of somatostatin receptor (SSTR3) signaling in primary cilia of wild-type and BBSome mutant cells. SSTR3 is a G-protein coupled receptor (GPCR) localized to primary cilia of hippocampal neurons and contains a motif that interacts with components of the BBSome complex [130] (reviewed in [131]). In wild-type cells, upon receptor activation following binding of somatostatin ligand (SST), SSTR3 is retrieved back into the cell body with the assistance of β-arrestin 2 and the BBSome protein complex (left panel). However, when the retrieval machinery is absent or compromised, as occurs in a BBSome mutant that lacks the BBS2 and BBS4 subunits, activated SSTR3 is shed from the ciliary tip by ectocytosis (right panel). A similar release process occurs in cells lacking β-arrestin 2, Arl6 (an Arf-like GTPase) or IFT27, which act as BBSome regulators [14]. For some GPCRs, such as NPY2R, that lack a retrieval motif, cells employ ectocytosis as a primary mechanism to remove activated receptors from cilia, thus reducing receptor signaling and thereby maintaining cellular homeostasis. Illustrations were created with BioRender.com.
These studies also emphasize the intraciliary sorting of specific components to discrete regions of primary cilia dedicated to ectosome budding. Ectocytosis sheds single vesicles from the ciliary tip and thus appears to be a spatially restricted form of ectosome release, which in other cilia often occurs along the length of the ciliary shaft, utilized to terminate signaling from activated ciliary GPCRs.
The shedding of ciliary ectosomes is also critical for maintenance of photoreceptor cells, as continuous replacement of older outer segment discs is essential for viability. As older discs are shed via ciliary ectosomes, new discs are added at the outer segment base by plasma membrane evagination. Studies in the retinal degeneration slow (rds) mouse, which lacks peripherin-2, revealed that photoreceptor cilia are unable to restore the outer segment. Rather, they are surrounded by large amounts of rhodopsin-containing ciliary ectosomes. This study suggests that photoreceptor outer segments are maintained by the retention of ciliary ectosomes (reviewed in [67]).
Ciliary EVs also impact on key developmental pathways. For example, in mammals, primary cilia detect the extracellular sonic hedgehog (SHH) ligand and transduce that signal to regulate a variety of developmental pathways [68]. Morphine stimulated astrocyte-derived EVs have increased levels of SHH that activate primary cilia signaling and contribute to development of morphine tolerance in mice [69].
EVs are also released by other cellular extensions such as the microvilli present on the apical membrane of epithelial cells [70]. The pentaspanin transmembrane protein prominin-1 binds to cholesterol present in the microvillar membrane and plays a role in the biogenesis of EVs released from microvilli [71]. Intriguingly, the secretion of SHH in small EVs derived from actin-based microvilli is required for wing imaginal disc development in Drosophila. This suggests that EV-mediated signaling through ligands sorted to dedicated cellular extensions may be of broad significance in many areas of biology.
3.3.2. Distinguishing Extracellular Vesicles from Different Sources
Multiple routes lead to formation and release of EVs, and thus defining EV origin in the complex extracellular milieu becomes important for assessing biological pathways. Depending upon their origin, developmental state and/or pathological condition, cells can secrete EVs of various subtypes containing different bioactive components [16, 19, 72, 73]. EVs may be enriched in cytoskeletal, cytosolic, secreted, and/or plasma membrane proteins (e.g. adhesion proteins, receptors, tetraspanins, and transporters), as well as nucleic acids including DNA fragments, mRNAs, and miRNAs [16, 43, 74, 75]. The topology of proteins in ciliary EV membranes is similar to that in exosomes and ectosomes, and indeed these different EVs classes may share some overlapping cargoes.
Ectosomal cargoes are enriched in extracellular matrix-degrading proteases, i.e. matrix metalloproteinase such as MMP2 and MMP9, tetraspanins, integrins, surface receptors, adhesion, metabolic and even nuclear proteins, as well as those involved in cell growth and maintenance [75–77]. A recent study in HeLa cells revealed that EVs containing the tetraspanins CD9 and CD81 but lacking CD63 are ectosomes, while those bearing CD63, other tetraspanins and late endosomal molecules such as LAMP1/2 correspond to MVB-derived exosomes [78]. Other surface proteins such as the basigin receptor and the amino acid transporter SLC3A2 are also specific markers of ectosomes [78].
The IFT machinery first identified in C. reinhardtii [79] mediates transport of components into, and in some cases out of, cilia and plays an essential role in ciliogenesis [80] while not significantly affecting cell growth or division [81, 82]. Defects in IFT components such as the core subunit IFT88 lead to the failure of ciliary assembly [83]. Proteomic analysis of EVs from endothelial cells of ciliated wildtype and non-ciliated IFT88 knockout mice revealed that ciliary and cytosolic EVs are distinct [19]. This study identified NADPH-cytochrome P450 reductase and the cell adhesion molecule CD166 in ciliary and cytosolic EVs, respectively, and suggested they may be useful as potential biomarkers.
Multiple signaling proteins including the receptor kinase BMPR2, the transferrin receptor TFRC, a cell adhesion protein F11R, the receptor tyrosine phosphatase PTPRS, and the progesterone receptor PGRMC2 were identified and localized to ciliary EVs obtained by shear fluid flow from isolated primary cilia [84]. Knockdown studies of these proteins in zebrafish and mice highlighted their physiological role in cardiovascular function, including cardiac arrhythmogenic defects and edema, as well as hydrocephalus, randomized heart looping, and cystic kidneys; intriguingly, these latter phenotypes are often associated with ciliary dysfunction [84, 85].
In contrast, CD63, CD9, CD81, ALIX, TSG101, HSP70 and HSP90, and flotillin-1 and −2 have been widely used in multiple studies as exosomal biomarkers, although their heterogeneous expression in different human cell lines potentially limits their utility [86]. A recent proteomic analysis of exosomes derived from cell lines of human and other species identified and proposed syntenin-1 as a universal exosome biomarker [86]; to date, this protein has not been described in either primary or motile mammalian cilia [87–90].
4. Cilia as Organelles for Peptidergic Transmission and Reception
The use of secreted peptides to mediate intercellular communication is widespread in eukaryotes; for example, in both mammals and invertebrates, peptides play fundamental roles in regulating numerous aspects of physiology and behavior [91]. To become bioactive and exhibit high affinity interactions with their cognate receptors, many peptides (such as oxytocin, vasopressin, and neuropeptide Y) require a specific post-translational modification that converts a C-terminal glycine residue into an α-amide; this also makes the secreted peptides less susceptible to proteolytic degradation by carboxypeptidases [92]. The two-step α-amidation reaction is catalyzed only by the enzyme peptidylglycine α-amidating monooxygenase (PAM) (EC1.14.17.3), which is a type-1 membrane protein that functions in the lumen of the secretory pathway [93]. Phylogenetic analysis identified a PAM-like gene in C. reinhardtii [94], and subsequent biochemical studies found both PAM protein and its enzyme activity in cilia and uncovered that this enzyme plays an essential role in ciliogenesis [95, 96]. The ciliary localization of PAM and its role in ciliogenesis was also observed in metazoans [96, 97]. Recently, bioactive PAM protein, α-amidated products and other proteins/enzymes involved in peptidergic signaling were described in vesicles released from cilia of mating C. reinhardtii gametes [16]. As both cilia and peptide-based communication were present in the last eukaryotic common ancestor [94, 95], these observations suggest that peptidergic signaling through ciliary EVs may be an ancient process that likely occurs in many eukaryotes including multicellular metazoans.
Numerous GPCRs that act as receptors for neuropeptides and neurotransmitters have been localized to neuronal primary cilia, including the neuropeptide Y (NPY) receptors NPY2R and NPY5R, somatostatin receptor 3 (SSTR3), melanin-concentrating hormone receptor 1 (MCHR1), melanocortin 4 receptor (MC4R) that binds α-melanocyte stimulating hormone, serotonin receptor 6 (HTR6), dopamine receptors D1, D2, and D5, and GPR161 which negatively regulates hedgehog signaling [14, 98–101]. The presence of these receptors enables cilia to receive peptidergic signals and thus mediate long distance communication. Abnormalities in ciliary GPCRs can result in various ciliopathies. For example, altered signaling by NPY2R and NPY5R receptors has been linked to Bardet-Biedl syndrome and other monogenic obesity syndromes [102]; defective ciliary localization of MC4R affects food intake and causes obesity in human-inherited obesity syndromes and ciliopathies [103]; and, mice lacking primary cilia on their β-cells exhibit impaired glucose homeostasis and develop diabetes [104]. Pharmacological manipulation of SSTR3 signaling bidirectionally modulates excitatory synapses in neocortical pyramidal neurons and regulates a subset of behavioral and cognitive disorders [105]. Thus, cilia play key and diverse roles in the reception of peptidergic signals and thereby regulate many aspects of mammalian physiology.
4.1. Amidation of Peptide Signals
In a typical neuroendocrine cell, α-amidated peptides are synthesized from larger inactive precursors in the lumen of the secretory pathway, stored in dense core secretory granules and secreted upon receipt of an appropriate stimulus. Following removal of the signal peptide from prepropeptide precursors, the resulting propeptides are cleaved as they transverse the secretory pathway at paired basic amino acid sites by the endoproteolytic action of subtilisin-like prohormone convertases (PCs). Subsequently, carboxypeptidase-like exoproteases remove the C-terminal basic residues. If this activity exposes a C-terminal–Gly, the peptide becomes a substrate for PAM which converts the –Gly into an –NH2 (amide) [17, 93]. Once secreted, α-amidated peptides bind with high affinity in many cases to GPCRs to initiate downstream signaling.
PAM is a bifunctional enzyme and has two catalytic cores, peptidylglycine α-hydroxylating monooxygenase (PHM) and peptidyl α-hydroxyglycine α-amidating lyase (PAL), followed by a transmembrane domain and a C-terminal cytosolic domain which is involved in routing PAM protein through the secretory pathway. PHM and PAL act sequentially on the substrate. PHM requires copper for its activity and catalyzes the hydroxylation of the C-terminal -Gly Cα atom to yield α-hydroxyglycine in a reaction that consumes molecular oxygen and needs a single electron donor, usually ascorbate. The resulting hydroxyglycine residue is then cleaved at the Cα-N bond by the Zn-dependent PAL enzymatic core to yield the final α-amidated peptide and the release of glyoxylate as a byproduct [93].
The α-amidation of peptides is a highly conserved post-translational modification that predates evolution of the nervous system, and α-amidated peptides have been reported throughout the metazoa, including placozoans, where they control shape, movement and behavior [106], nematodes, echinoderms and in the venoms of cone snails, scorpions, ants and vespids [107–112]. As phylogenetic studies indicate that PAM-like proteins are present in many other eukaryotic groups including sponges, choanoflagellates, filastereans, haptophytes and dinoflagellates, we can infer that these organisms also likely generate α-amidated peptide products [94, 113].
4.2. Ciliary Localization of PAM and its Conserved Role in Ciliogenesis
The C. reinhardtii genome encodes a bioactive, bifunctional PAM that shares many features with mammalian PAM including an absolute requirement for copper [95]. Although most PAM protein is present in the Golgi, it also localizes along the length of the cilia. Importantly, the ciliary localization of PAM has been conserved in metazoans including planaria, mice and zebrafish [95–97]. In mammals, PAM is present in the Golgi and secretory pathway, but was also found in the primary cilia of mouse embryonic fibroblasts and retinal pigment epithelial cells, as well as the motile cilia of tracheal and ependymal cells and both along the tail and in the acrosome of spermatozoa [95].
The PAM catalytic domains are located within the secretory pathway lumen. Consequently, when Golgi-derived vesicles insert into the membrane at the ciliary base, the enzymatic domains are exposed to the external environment. Furthermore, it was found that PAM is tethered to the ciliary axoneme through unknown interactions [95]. This PAM-axoneme interaction, which can be disrupted in vitro by treating isolated axonemes with 0.6 M NaCl, is regulated as axoneme-bound PAM is released in ciliary ectosomes during mating but is absent from ectosomes derived from vegetative cells [16].
In C. reinhardtii cells, knockdown of PAM expression resulted in the failure of ciliary assembly [96]. Similarly, reduction of PAM (and the separate soluble PHM enzyme found in many invertebrates) in planaria led to the almost complete loss of the ventral motile cilia that are used for whole animal locomotion [114]. In mice, PAM-null mutations are embryonic lethal; the animals die at embryonic day E14.5 and exhibit massive edema, a poorly formed vasculature and ventricular hypertrophy [115]. In addition, they have short or stunted cilia protruding from cells on the developing neuroepithelium [96]. Similarly, pam-null zebrafish embryos develop edema and several defects related to primary ciliary dyskinesia, including hydrocephalus and formation of cyst-like structures on the pronephros [97]. As pam mRNA is highly maternally loaded into early zebrafish zygotes [116], the mutants do not exhibit some classic motile ciliopathy phenotypes such as situs inversus because the left-right body axis is determined very early in development when the embryos can still make PAM due to the maternal contribution.
Ciliogenesis is a highly complex and multifaceted process, and PAM is clearly essential for its normal progression. However, the mechanism by which PAM contributes to this highly complex pathway and to ciliary maintenance remains unknown. Although experiments in C. reinhardtii and planaria suggest that amidating activity per se is important [96], an amidated product needed for ciliogenesis has yet to be identified, and thus a key role for PAM in protein-protein and/or other interactions that directly affect ciliogenesis and/or ciliary homeostasis cannot be ruled out.
4.3. Peptidergic Machinery and Bioactive Amidated Products in Ciliary Ectosomes
Despite differences in the size and sequence of bioactive peptides, they all share a common biosynthetic pathway involving signal sequence removal, cleavage at dibasic or multi-basic sites and in many cases C-terminal amidation [17]. In mammals, following synthesis, mature peptides are stored in large dense core secretory granules and released into the environment in a regulated manner upon receipt of an appropriate stimulus. Although dense core secretory granules have not been identified in C. reinhardtii, the developmental stage-specific secretion of proteins under nutrient-limiting conditions has been observed [16, 117]. Intriguingly, the presence of PAM in HEK293 and Chinese hamster ovary (CHO) cells that do not produce secretory granules has been reported. Indeed, these cells amidate -Gly extended peptide substrates during their passage through the secretory pathway [118, 119].
PAM is present in both primary and motile cilia of various mammalian cell types suggesting that peptidergic signaling is significant in these cellular compartments [95]. Defects in cilia and cilia-mediated signaling pathways lead to various human genetic disorders including ciliopathies. However, the presence of PAM and its amidated product(s) in ciliary ectosomes released by primary cilia of neuronal and kidney cells has yet to be demonstrated. PAM has been localized in the MVBs of murine corticotrope tumor cells (AtT-20) and human embryonic kidney cells (HEK293), where it is present in intraluminal vesicles [118, 120]. Mammalian PAM is also found in exosomes purified from blood, saliva and urine [121–123], and in those isolated from a glioblastoma cell line [124].
In C. reinhardtii, bioactive ciliary ectosomes secreted from mating gametes contain all the components needed for peptidergic signaling including PAM, bioactive amidated products, proteases involved in cleavage of propeptide precursors such as subtilisin-like endoproteases, and a carboxypeptidase B-like exoprotease (Fig. 3) [16]. In addition, multiple receptors were found in these vesicles including the blue light receptor phototropin, and several scavenger receptors. Scavenger receptors have also been localized on the flagellum of sea urchin sperm and bind to egg-derived amidated sperm-activating peptide-1 used to attract sperm in a species/genus specific manner [125]. In addition, C. reinhardtii ciliary ectosomes contain a seven transmembrane “GPCR-like” protein (Cre12.g523950) [16] that exhibits considerable similarity to human GPR107, which has been described as a GPCR for the short peptide neuronostatin; this peptide is produced from the N-terminal region of prosomatostatin, and stimulates glucagon release and attenuates glucose-stimulated insulin secretion [126]. Related GPCR-like proteins are also present in angiosperms such as Arabidopsis (AT5G42090). The sequence similarity between this putative C. reinhardtii receptor and mammalian GPR107 is mainly confined to the C-terminal seven transmembrane region, while the presumptive ligand binding domains are quite distinct. Importantly though, the C. reinhardtii genome does not encode classic trimeric Gαβγ protein subunits; a protein recently described as a Gα subunit [127] is actually an ADP ribosylation factor-like protein. Furthermore, plants in general lack canonical GPCRs that act as receptor-guanine nucleotide exchange factors as their Gα subunits have a rate of GDP→GTP exchange several hundred times that of mammalian Gαs and thus are essentially always in the GTP-bound form [128].
A synthetic peptide containing the amidated C-terminus from one identified amidated peptide precursor (Cre03.g204500 termed preproGATI) found in C. reinhardtii gametic ciliary ectosomes was found to act as a chemotactic modulator, attracting minus gametes while repelling plus gametes [16]. This observation directly demonstrates that cilia provide a source of amidated signaling peptides/products. Dissecting how the proGATI precursor is processed and trafficked into ciliary ectosomes will be essential to define the mechanisms involved in peptidergic signaling through ciliary ectosomes. These observations also highlight another key question – how does an amidated PAM product derived from a precursor that lacks transmembrane domains associate with the ectosomal membrane?
The release of ciliary ectosomes appears to represent an ancient mechanism for regulated secretion where, depending upon environmental conditions or life cycle stage, cells would sort peptide precursors or fully formed peptidergic signals to cilia and release them following appropriate stimulation. This pathway may be especially important in unicellular organisms where, given the complexity of potential signaling requirements, it might not be feasible to dedicate secretory granules to many different individual peptides. For example, C. reinhardtii, a cell only ~10 µm in diameter, encodes hundreds of putative prepropeptides with multiple paired basic cleavage sites and classical –Gly-Arg-Arg amidation sites [93]. Although in neurons, granules containing different peptides are assembled, shipped to dendrites or to axons, and their release differentially controlled [129], these large cells are highly differentiated and sorting/trafficking places the granules in very distinct and spatially restricted subcellular compartments. Currently, it is uncertain whether peptidergic communication through cilia derived EVs plays a critical role in neuronal function.
5. Conclusions
Recent studies have revealed that cilia from various species secrete vesicles into the extracellular environment. Although, these cilia-derived vesicles share many features with microvesicles and exosomes, their content is unique and developmentally regulated. The process of ectosome release from cilia represents a regulated mode of secretion that cells can utilize in the absence of dedicated secretory granules. However, how bioactive cargoes are selected for entry into the ciliary membrane, moved to sites of ectosome release and subsequently sorted for entry into nascent EVs as they bud from the ciliary surface remain key questions that need to be addressed. Due to technical issues, purifying populations of ciliary ectosomes from mammalian systems remains challenging. Studies in nematodes and green algae have provided a route for biochemical analysis of ciliary EV content and have allowed for direct measurements of bioactivity. Alterations in signaling by proteins present in these vesicles can affect ciliogenesis and result in various ciliopathies. In C. reinhardtii, ciliary ectosomes contain PAM and other components of the peptidergic signaling machinery, including an amidated peptide product that acts as a chemotactic modulator for gametes. PAM has also been identified in primary and motile cilia of vertebrates and altered expression has major impacts on ciliogenesis; whether PAM and its amidated products are released in ciliary ectosomes by mammals, and if they play a role in ciliopathy syndromes remains uncertain. However, the studies in C. reinhardtii and C. elegans clearly reveal that cells can release an array of signaling molecules in ciliary ectosomes that may initiate various physiological responses in other cells, tissues or individual animals. Considering the origin of cilia in early eukaryotes and the conserved nature of ciliary EV release, it seems clear that this process represents an ancient mode of regulated secretion that can be utilized to mediate intercellular communication as well as control of ciliary responses to extracellular signals.
Acknowledgements
We thank Dr. Dhivya Kumar for the micrographs shown in Figures 1b and d, and Drs. Betty Eipper and Richard Mains for insightful comments.
Funding
This work was supported by grants RO1-GM125606 and R35-GM140631 (to SMK) from the National Institutes of Health, USA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors declare no competing interests.
References
- [1].Wood CR, Huang K, Diener DR, Rosenbaum JL, The cilium secretes bioactive ectosomes, Curr. Biol 23 (2013), 906–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Wood CR, Rosenbaum JL, Ciliary ectosomes: transmissions from the cell’s antenna, Trends Cell Biol 25 (2015), 276–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Woith E, Fuhrmann G, Melzig MF, Extracellular vesicles-connecting kingdoms, Int. J. Mol. Sci 20 (2019), 5695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wang J, Silva M, Haas LA, Morsci NS, Nguyen KCQ, Hall DH, Barr MM, elegans C ciliated sensory neurons release extracellular vesicles that function in animal communication, Curr. Biol 24 (2014), 519–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wang J, Barr MM, Cell-cell communication via ciliary extracellular vesicles: clues from model systems, Essays Biochem 62 (2018), 205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Mantel PY, Marti M, The role of extracellular vesicles in Plasmodium and other protozoan parasites, Cell Microbiol 16 (2014), 344–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Silvester E, McWilliam KR, Matthews KR, The cytological events and molecular control of life cycle development of Trypanosoma brucei in the mammalian bloodstream, Pathogens 6 (2017), 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Skotland T, Sagini K, Sandvig K, Llorente A, An emerging focus on lipids in extracellular vesicles, Adv. Drug Delivery Rev 159 (2020), 308–321. [DOI] [PubMed] [Google Scholar]
- [9].Abels ER, Breakefield XO, Introduction to extracellular vesicles: biogenesis, RNA cargo selection, content, release, and uptake, Cell. Mol. Neurobiol 36 (2016), 301–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Malkin EZ, Bratman SV, Bioactive DNA from extracellular vesicles and particles, Cell Death Dis 11 (2020), 584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Veziroglu EM, Mias GI, Characterizing extracellular vesicles and their diverse RNA contents, Front. Genet 11 (2020), 700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Amintas S, Vendrely V, Dupin C, Buscail L, Laurent C, Bournet B, Merlio J-P, Bedel A, Moreau-Gaudry F, Boutin J, Dabernat S, Buscail E, Next-generation cancer biomarkers: extracellular vesicle DNA as a circulating surrogate of tumor DNA, Front, Cell Dev. Biol 8 (2021), 622048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Cao M, Ning J, Hernandez-Lara CI, Belzile O, Wang Q, Dutcher SK, Liu Y, Snell WJ, Unidirectional ciliary membrane protein trafficking by a cytoplasmic retrograde IFT motor and ciliary ectosome shedding, Elife 4 (2015), e05242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Nager AR, Goldstein JS, Herranz-Perez V, Portran D, Ye F, Garcia-Verdugo JM, Nachury MV, An actin network dispatches ciliary GPCRs into extracellular vesicles to modulate signaling, Cell 168 (2017), 252–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wang J, Nikonorova IA, Silva M, Walsh JD, Tilton PE, Gu A, Akella JS, Barr MM, Sensory cilia act as a specialized venue for regulated extracellular vesicle biogenesis and signaling, Curr. Biol 31 (2021), 3943–3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Luxmi R, Kumar D, Mains RE, King SM, Eipper BA, Cilia-based peptidergic signaling, PLoS. Biol 17 (2019), e3000566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kumar D, Mains RE, Eipper BA, 60 years of POMC: From POMC and α-MSH to PAM, molecular oxygen, copper, and vitamin C, J. Mol. Endocrinol 56 (2016), T63–T76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Cai Y, Fedeles SV, Dong K, Anyatonwu G, Onoe T, Mitobe M, Gao JD, Okuhara D, Tian X, Gallagher AR, Tang Z, Xie X, Lalioti MD, Lee AH, Ehrlich BE, Somlo S, Altered trafficking and stability of polycystins underlie polycystic kidney disease, J. Clin. Invest 124 (2014), 5129–5144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Mohieldin AM, Pala R, Beuttler R, Moresco JJ, Yates JR III, Nauli SM, Ciliary extracellular vesicles are distinct from the cytosolic extracellular vesicles, J. Extracellular Vesicles 10 (2021), e12086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Volz A-K, Frei A, Kretschmer V, de Jesus Domingues AM, Ketting RF, Ueffing M, Boldt K, Krämer-Albers E-M, May-Simera HL, Bardet-Biedl syndrome proteins modulate the release of bioactive extracellular vesicles, Nat. Comm 12 (2021), 5671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Marshall W, Basto R (eds), Cilia, Cold Spring Harbor, NY: Cold Spring Harb. Lab. Press; (2017). [Google Scholar]
- [22].Wheway G, Nazlamova L, Hancock JT, Signaling through the primary cilium, Front. Cell Dev. Biol 6 (2018), 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Mitchell DR, The evolution of eukaryotic cilia and flagella as motile and sensory organelles, Adv. Exp. Med. Biol 607 (2007), 130–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Moran J, McKean PG, Ginger ML, Eukaryotic flagella: variations in form, function, and composition during evolution, BioSci 64 (2014), 1103–1114. [Google Scholar]
- [25].Li L, Anand M, Rao KN, Khanna H, Cilia in photoreceptors, Methods Cell Biol 127 (2015), 75–92. [DOI] [PubMed] [Google Scholar]
- [26].King SM, Axonemal dynein arms, Cold Spring Harb. Perspect. Biol 8 (2016), a028100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hoyer-Fender S, Primary and motile cilia: their ultrastructure and ciliogenesis, in: Tucker KL, Caspary T (Eds.), Cilia and Nervous System Development and Function, Springer Netherlands, Dordrecht, 2013, pp. 1–53. [Google Scholar]
- [28].Kiesel P, Alvarez Viar G, Tsoy N, Maraspini R, Gorilak P, Varga V, Honigmann A, Pigino G, The molecular structure of mammalian primary cilia revealed by cryo-electron tomography, Nat. Struct. Mol. Biol 27 (2020), 1115–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sobkowicz HM, Slapnick SM, August BK, The kinocilium of auditory hair cells and evidence for its morphogenetic role during the regeneration of stereocilia and cuticular plates, J. Neurocytol 24 (1995), 633–653. [DOI] [PubMed] [Google Scholar]
- [30].Axelrod JD, Basal bodies, kinocilia and planar cell polarity, Nat. Genet 40 (2008), 10–11. [DOI] [PubMed] [Google Scholar]
- [31].Weiss RL, Goodenough DA, Goodenough UW, Membrane particle arrays associated with the basal body and with contractile vacuole secretion in Chlamydomonas, J. Cell Biol 72 (1977), 133–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Dutcher SK, O’Toole ET, The basal bodies of Chlamydomonas reinhardtii, Cilia 5 (2016), 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ye F, Nager AR, Nachury MV, BBSome trains remove activated GPCRs from cilia by enabling passage through the transition zone, J. Cell Biol 217 (2018), 1847–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Pigino G, Intraflagellar transport, Curr. Biol 31 (2021), R530–R536. [DOI] [PubMed] [Google Scholar]
- [35].Jensen VL, Leroux MR, Gates for soluble and membrane proteins, and two trafficking systems (IFT and LIFT), establish a dynamic ciliary signaling compartment, Curr. Opin. Cell Biol 47 (2017), 83–91. [DOI] [PubMed] [Google Scholar]
- [36].Dyson JM, Conduit SE, Feeney SJ, Hakim S, DiTommaso T, Fulcher AJ, Sriratana A, Ramm G, Horan KA, Gurung R, Wicking C, Smyth I, Mitchell CA, INPP5E regulates phosphoinositide-dependent cilia transition zone function, J. Cell Biol 216 (2017), 247–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Finkelstein S, Gospe SM, Schuhmann K, Shevchenko A, Arshavsky VY, Lobanova ES, Phosphoinositide profile of the mouse retina, Cells 9 (2020), 1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Nasuhoglu C, Feng S, Mao J, Yamamoto M, Yin HL, Earnest S, Barylko B, Albanesi JP, Hilgemann DW, Nonradioactive analysis of phosphatidylinositides and other anionic phospholipids by anion-exchange high-performance liquid chromatography with suppressed conductivity detection, Anal. Biochem 301 (2002), 243–254. [DOI] [PubMed] [Google Scholar]
- [39].Janich P, Corbeil D, GM1 and GM3 gangliosides highlight distinct lipid microdomains within the apical domain of epithelial cells, FEBS Letts 581 (2007), 1783–1787. [DOI] [PubMed] [Google Scholar]
- [40].Serricchio M, Schmid AW, Steinmann ME, Sigel E, Rauch M, Julkowska D, Bonnefoy S, Fort C, Bastin P, Bütikofer P, Flagellar membranes are rich in raft-forming phospholipids, Biol. Open 4 (2015), 1143–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Garcia G 3rd, Raleigh DR, Reiter JF, How the ciliary membrane is organized inside-out to communicate outside-in, Curr. Biol 28 (2018), R421–R434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Mao S, Shah AS, Moninger TO, Ostedgaard LS, Lu L, Tang XX, Thornell IM, Reznikov LR, Ernst SE, Karp PH, Tan P, Keshavjee S, Abou Alaiwa MH, Welsh MJ, Motile cilia of human airway epithelia contain hedgehog signaling components that mediate noncanonical hedgehog signaling, Proc. Natl. Acad. Sci. USA 115 (2018), 1370–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Long H, Zhang F, Xu N, Liu G, Diener DR, Rosenbaum JL, Huang K, Comparative analysis of ciliary membranes and ectosomes, Curr. Biol 26 (2016), 3327–3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Zuo X, Guo W, Lipschutz JH, The exocyst protein Sec10 is necessary for primary ciliogenesis and cystogenesis in vitro, Mol. Biol. Cell 20 (2009), 2522–2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Long H, Huang K, Transport of ciliary membrane proteins, Front. Cell Dev. Biol 7 (2020), 381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Salomé PA, Merchant SS, A Series of fortunate events: Introducing Chlamydomonas as a reference organism, Plant Cell 31 (2019), 1682–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Goodenough U, Lin H, Lee JH, Sex determination in Chlamydomonas, Semin. Cell Dev. Biol 18 (2007), 350–361. [DOI] [PubMed] [Google Scholar]
- [48].Harris E, Stern DB., Witman GB, The Chlamydomonas Sourcebook, Academic Press, San Diego: (2nd edn.) (2009). [Google Scholar]
- [49].McLean RJ, Laurendi CJ, Brown RM, The Relationship of gamone to the mating reaction in Chlamydomonas moewusii, Proc. Natl. Acad. Sci. USA 71 (1974), 2610–2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Kubo T, Kaida S, Abe J, Saito T, Fukuzawa H, Matsuda Y, The Chlamydomonas hatching enzyme, sporangin, is expressed in specific phases of the cell cycle and is localized to the flagella of daughter cells within the sporangial cell wall, Plant Cell Physiol 50 (2009), 572–583. [DOI] [PubMed] [Google Scholar]
- [51].Perkins LA, Hedgecock EM, Thomson JN, Culotti JG, Mutant sensory cilia in the nematode Caenorhabditis elegans, Dev. Biol 117 (1986), 456–487. [DOI] [PubMed] [Google Scholar]
- [52].Barr MM, Sternberg PW, A polycystic kidney-disease gene homologue required for male mating behaviour in C. elegans, Nature 401 (1999), 386–389. [DOI] [PubMed] [Google Scholar]
- [53].Barr MM, DeModena J, Braun D, Nguyen CQ, Hall DH, Sternberg PW, The Caenorhabditis elegans autosomal dominant polycystic kidney disease gene homologs lov-1 and pkd-2 act in the same pathway, Curr. Biol 11 (2001), 1341–1346. [DOI] [PubMed] [Google Scholar]
- [54].Akella JS, Barr MM, The tubulin code specializes neuronal cilia for extracellular vesicle release, Develop. Neurobiol 81 (2021), 231–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Silva M, Morsci N, Nguyen KCQ, Rizvi A, Rongo C, Hall DH, Barr MM, Cell-specific alphatubulin isotype regulates ciliary microtubule ultrastructure, intraflagellar transport, and extracellular vesicle biology, Curr. Biol 27 (2017), 968–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Akella JS, Carter SP, Nguyen K, Tsiropoulou S, Moran AL, Silva M, Rizvi F, Kennedy BN, Hall DH, Barr MM, Blacque OE, Ciliary Rab28 and the BBSome negatively regulate extracellular vesicle shedding, eLife 9 (2020), e50580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Wang J, Nikonorova IA, Gu A, Sternberg PW, Barr MM, Release and targeting of polycystin-2-carrying ciliary extracellular vesicles, Curr. Biol 30 (2020), R755–R756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Zuo X, Kwon SH, Janech MG, Dang Y, Lauzon SD, Fogelgren B, Polgar N, Lipschutz JH, Primary cilia and the exocyst are linked to urinary extracellular vesicle production and content, J. Biol. Chem 294 (2019), 19099–19110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Pocsfalvi G, Raj DA, Fiume I, Vilasi A, Trepiccione F, Capasso G, Urinary extracellular vesicles as reservoirs of altered proteins during the pathogenesis of polycystic kidney disease, Proteomics Clin. Appl 9 (2015), 552–567. [DOI] [PubMed] [Google Scholar]
- [60].Hogan MC, Manganelli L, Woollard JR, Masyuk AI, Masyuk TV, Tammachote R, Huang BQ, Leontovich AA, Beito TG, Madden BJ, Charlesworth MC, Torres VE, LaRusso NF, Harris PC, Ward CJ, Characterization of PKD protein-positive exosome-like vesicles, J. Am. Soc. Nephrol 20 (2009), 278–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Wang G, Hu H-B, Chang Y, Huang Y, Song Z-Q, Zhou S-B, Chen L, Zhang Y-C, Wu M, Tu H-Q, Yuan J-F, Wang N, Pan X, Li A-L, Zhou T, Zhang X-M, He K, Li H-Y, Rab7 regulates primary cilia disassembly through cilia excision, J. Cell Biol 218 (2019), 4030–4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Phua SC, Chiba S, Suzuki M, Su E, Roberson EC, Pusapati GV, Schurmans S, Setou M, Rohatgi R, Reiter JF, Ikegami K, Inoue T, Dynamic remodeling of membrane composition drives cell cycle through primary cilia excision, Cell 168 (2017), 264–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Hoang-Minh LB, Dutra-Clarke M, Breunig JJ, Sarkisian MR, Glioma cell proliferation is enhanced in the presence of tumor-derived cilia vesicles, Cilia 7 (2018), 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Tarique I, Liu Y, Bai X, Haseeb A, Yang P, Huang Y, Qu W, Wu R, Vistro WA, Chen Q, Characterization of extracellular vesicles from cilia and epithelial cells of ductuli efferentes in a Turtle (Pelodiscus sinensis), Animals (Basel) 9 (2019), 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Bergmann C, Guay-Woodford LM, Harris PC, Horie S, Peters DJM, Torres VE, Polycystic kidney disease, Nat. Rev. Dis. Primers 4 (2018), 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Salinas RY, Pearring JN, Ding J-D, Spencer WJ, Hao Y, Arshavsky VY, Photoreceptor discs form through peripherin-dependent suppression of ciliary ectosome release, J. Cell Biol 216 (2017), 1489–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Spencer WJ, Lewis TR, Pearring JN, Arshavsky VY, Photoreceptor discs: built like ectosomes, Trends Cell Biol 30 (2020), 904–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Bangs F, Anderson KV, Primary cilia and mammalian hedgehog signaling, Cold Spring Harb. Perspect. Biol 9 (2017), a028175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Ma R, Kutchy NA, Hu G, Astrocyte-derived extracellular vesicle-mediated activation of primary ciliary signaling contributes to the development of morphine tolerance, Biological Psych 90 (2021), 575–585. [DOI] [PubMed] [Google Scholar]
- [70].Marzesco A-M, Wilsch-Bräuninger M, Dubreuil V, Janich P, Langenfeld K, Thiele C, Huttner WB, Corbeil D, Release of extracellular membrane vesicles from microvilli of epithelial cells is enhanced by depleting membrane cholesterol, FEBS Letts 583 (2009), 897–902. [DOI] [PubMed] [Google Scholar]
- [71].Florek M, Bauer N, Janich P, Wilsch-Braeuninger M, Fargeas CA, Marzesco A-M, Ehninger G, Thiele C, Huttner WB, Corbeil D, Prominin-2 is a cholesterol-binding protein associated with apical and basolateral plasmalemmal protrusions in polarized epithelial cells and released into urine, Cell Tissue Res 328 (2007), 31–47. [DOI] [PubMed] [Google Scholar]
- [72].Tauro BJ, Greening DW, Mathias RA, Mathivanan S, Ji H, Simpson RJ, Two distinct populations of exosomes are released from LIM1863 colon carcinoma cell-derived organoids, Mol. Cell Proteomics 12 (2013), 587–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Bobrie A, Théry C, Exosomes and communication between tumours and the immune system: are all exosomes equal?, Biochem. Soc. Trans 41 (2013), 263–267. [DOI] [PubMed] [Google Scholar]
- [74].Jeppesen DK, Fenix AM, Franklin JL, Higginbotham JN, Zhang Q, Zimmerman LJ, Liebler DC, Ping J, Liu Q, Evans R, Fissell WH, Patton JG, Rome LH, Burnette DT, Coffey RJ, Reassessment of exosome composition, Cell 177 (2019), 428–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Meldolesi J, Exosomes and ectosomes in intercellular communication, Curr. Biol 28 (2018), R435–R444. [DOI] [PubMed] [Google Scholar]
- [76].Choi DS, Kim DK, Kim YK, Gho YS, Proteomics of extracellular vesicles: exosomes and ectosomes, Mass Spectrom. Rev 34 (2015), 474–490. [DOI] [PubMed] [Google Scholar]
- [77].Kalra H, Drummen GPC, Mathivanan S, Focus on extracellular vesicles: introducing the next small big thing, Int. J. Mol. Sci 17 (2016), 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Mathieu M, Névo N, Jouve M, Valenzuela JI, Maurin M, Verweij FJ, Palmulli R, Lankar D, Dingli F, Loew D, Rubinstein E, Boncompain G, Perez F, Théry C, Specificities of exosome versus small ectosome secretion revealed by live intracellular tracking of CD63 and CD9, Nat. Comm 12 (2021), 4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Kozminski KG, Johnson KA, Forscher P, Rosenbaum JL, A motility in the eukaryotic flagellum unrelated to flagellar beating, Proc. Natl. Acad. Sci. USA 90 (1993), 5519–5523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Follit JA, Tuft RA, Fogarty KE, Pazour GJ, The intraflagellar transport protein IFT20 is associated with the Golgi complex and is required for cilia assembly, Mol. Biol. Cell 17 (2006), 3781–3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Colombo M, Raposo G, Théry C, Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles, Annu. Rev. Cell Dev. Biol 30 (2014), 255–289. [DOI] [PubMed] [Google Scholar]
- [82].Das RM, Storey KG, Apical abscission alters cell polarity and dismantles the primary cilium during neurogenesis, Science 343 (2014), 200–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Pazour GJ, Dickert BL, Vucica Y, Seeley ES, Rosenbaum JL, Witman GB, Cole DG, Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella, J. Cell Biol 151 (2000), 709–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Mohieldin AM, Pala R, Sherpa RT, Alanazi M, Alanazi A, Shamloo K, Ahsan A, AbouAlaiwi WA, Moresco JJ, Yates JR III, Nauli SM, Proteomic identification reveals the role of ciliary extracellular-like vesicle in cardiovascular function, Adv. Sci 7 (2020), 1903140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Reiter JF, Leroux MR, Genes and molecular pathways underpinning ciliopathies, Nat. Rev. Mol. Cell Biol 18 (2017), 533–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Kugeratski FG, Hodge K, Lilla S, McAndrews KM, Zhou X, Hwang RF, Zanivan S, Kalluri R, Quantitative proteomics identifies the core proteome of exosomes with syntenin-1 as the highest abundant protein and a putative universal biomarker, Nat. Cell Biol 23 (2021), 631–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Mick DU, Rodrigues RB, Leib RD, Adams CM, Chien AS, Gygi SP, Nachury MV, Proteomics of primary cilia by proximity labeling, Dev. Cell 35 (2015), 497–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Ishikawa H, Thompson J, Yates JR 3rd, Marshall WF, Proteomic analysis of mammalian primary cilia, Curr. Biol 22 (2012), 414–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Blackburn K, Bustamante-Marin X, Yin W, Goshe MB, Ostrowski LE, Quantitative proteomic analysis of human airway cilia identifies previously uncharacterized proteins of high abundance, J. Proteome Res 16 (2017), 1579–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].May EA, Kalocsay M, D’Auriac IG, Schuster PS, Gygi SP, Nachury MV, Mick DU, Time-resolved proteomics profiling of the ciliary hedgehog response, J. Cell Biol 220 (2021), e202007207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Bhat US, Shahi N, Surendran S, Babu K, Neuropeptides and Behaviors: How small peptides regulate nervous system function and behavioral outputs, Front. Mol. Neurosci 14 (2021), 786471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Kahns AH, Bundgaard H, Prodrugs of peptides. 13. Stabilization of peptide amides against alpha-chymotrypsin by the prodrug approach, Pharm. Res 8 (1991), 1533–1538. [DOI] [PubMed] [Google Scholar]
- [93].Luxmi R, Mains RE, King SM, Eipper BA, Peptidylglycine α-amidating monooxygenase (PAM), in: Jez J (Ed.), Encyclopedia of Biological Chemistry (Third Edition), Elsevier, Oxford, (2021), pp. 88–104. [Google Scholar]
- [94].Attenborough RMF, Hayward DC, Kitahara MV, Miller DJ, Ball EE, A “neural” enzyme in nonbilaterian animals and algae: preneural origins for peptidylglycine α-amidating monooxygenase, Mol. Biol. Evolution 29 (2012), 3095–3109. [DOI] [PubMed] [Google Scholar]
- [95].Kumar D, Blaby-Haas CE, Merchant SS, Mains RE, King SM, Eipper BA, Early eukaryotic origins for cilia-associated bioactive peptide-amidating activity, J. Cell Sci 129 (2016), 943–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Kumar D, Strenkert D, Patel-King RS, Leonard MT, Merchant SS, Mains RE, King SM, Eipper BA, A bioactive peptide amidating enzyme is required for ciliogenesis, eLife 6 (2017), e25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Kumar D, Thomason RT, Yankova M, Gitlin JD, Mains RE, Eipper BA, King SM, Microvillar and ciliary defects in zebrafish lacking an actin-binding bioactive peptide amidating enzyme, Sci. Rep 8 (2018), 4547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Berbari NF, Johnson AD, Lewis JS, Askwith CC, Mykytyn K, Identification of ciliary localization sequences within the third intracellular loop of G protein-coupled receptors, Mol. Biol. Cell 19 (2008), 1540–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Brailov I, Bancila M, Brisorgueil MJ, Miquel MC, Hamon M, Vergé D, Localization of 5-HT(6) receptors at the plasma membrane of neuronal cilia in the rat brain, Brain Res 872 (2000), 271–275. [DOI] [PubMed] [Google Scholar]
- [100].Domire JS, Green JA, Lee KG, Johnson AD, Askwith CC, Mykytyn K, Dopamine receptor 1 localizes to neuronal cilia in a dynamic process that requires the Bardet-Biedl syndrome proteins, Cell Mol. Life Sci 68 (2011), 2951–2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Mukhopadhyay S, Wen X, Ratti N, Loktev A, Rangell L, Scales SJ, Jackson PK, The ciliary G-protein-coupled receptor Gpr161 negatively regulates the sonic hedgehog pathway via cAMP signaling, Cell 152 (2013), 210–223. [DOI] [PubMed] [Google Scholar]
- [102].Loktev Alexander V., Jackson Peter K., Neuropeptide Y family receptors traffic via the Bardet-Biedl syndrome pathway to signal in neuronal primary cilia, Cell Rep 5 (2013), 1316–1329. [DOI] [PubMed] [Google Scholar]
- [103].Wang Y, Bernard A, Comblain F, Yue X, Paillart C, Zhang S, Reiter JF, Vaisse C, Melanocortin 4 receptor signals at the neuronal primary cilium to control food intake and body weight, J. Clin. Invest 131 (2021), e142064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Hughes JW, Cho JH, Conway HE, DiGruccio MR, Ng XW, Roseman HF, Abreu D, Urano F, Piston DW, Primary cilia control glucose homeostasis via islet paracrine interactions, Proc. Natl. Acad. Sci. USA 117 (2020), 8912–8923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Tereshko L, Gao Y, Cary BA, Turrigiano GG, Sengupta P, Ciliary neuropeptidergic signaling dynamically regulates excitatory synapses in postnatal neocortical pyramidal neurons, eLife 10 (2021), e65427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Varoqueaux F, Williams EA, Grandemange S, Truscello L, Kamm K, Schierwater B, Jekely G, Fasshauer D, High cell diversity and complex peptidergic signaling underlie placozoan behavior, Curr. Biol 28 (2018), 3495–3501. [DOI] [PubMed] [Google Scholar]
- [107].Taghert PH, Nitabach MN, Peptide neuromodulation in invertebrate model systems, Neuron 76 (2012), 82–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Elphick MR, Thorndyke MC, Molecular characterisation of SALMFamide neuropeptides in sea urchins, J. Exp. Biol 208 (2005), 4273–4282. [DOI] [PubMed] [Google Scholar]
- [109].Violette A, Biass D, Dutertre S, Koua D, Piquemal D, Pierrat F, Stöcklin R, Favreau P, Large-scale discovery of conopeptides and conoproteins in the injectable venom of a fish-hunting cone snail using a combined proteomic and transcriptomic approach, J. Proteomics 75 (2012), 5215–5225. [DOI] [PubMed] [Google Scholar]
- [110].Palma MS, Hymenoptera venom peptides. In:Kastin A (Ed.), Handbook of Biologically Active Peptides, second ed., Academic Press, San Diego, USA, (2013), 416–422. [Google Scholar]
- [111].Delgado-Prudencio G, Possani LD, Becerril B, Ortiz E, The dual α-amidation system in scorpion venom glands, Toxins (Basel) 11 (2019), 425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Robinson SD, Mueller A, Clayton D, Starobova H, Hamilton BR, Payne RJ, Vetter I, King GF, Undheim EAB, A comprehensive portrait of the venom of the giant red bull ant, Myrmecia gulosa, reveals a hyperdiverse hymenopteran toxin gene family, Sci. Adv 4 (2018), eaau4640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Kumar D, Mains RE, Eipper BA, King SM, Ciliary and cytoskeletal functions of an ancient monooxygenase essential for bioactive amidated peptide synthesis, Cell. Mol. Life Sci 76 (2019), 2329–2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Rompolas P, Patel-King RS, King SM, An outer arm dynein conformational switch is required for metachronal synchrony of motile cilia in planaria, Mol. Biol. Cell 21 (2010), 3669–3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Czyzyk TA, Ning Y, Hsu MS, Peng B, Mains RE, Eipper BA, Pintar JE, Deletion of peptide amidation enzymatic activity leads to edema and embryonic lethality in the mouse, Dev. Biol 287 (2005), 301–313. [DOI] [PubMed] [Google Scholar]
- [116].Lee MT, Bonneau AR, Takacs CM, Bazzini AA, DiVito KR, Fleming ES, Giraldez AJ, Nanog, Pou5f1 and SoxB1 activate zygotic gene expression during the maternal-to-zygotic transition, Nature 503 (2013), 360–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Luxmi R, Blaby-Haas C, Kumar D, Rauniyar N, King SM, Mains RE, Eipper BA, Proteases shape the Chlamydomonas secretome: comparison to classical neuropeptide processing machinery, Proteomes 6 (2018), 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Back N, Kanerva K, Kurutihalli V, Yanik A, Ikonen E, Mains RE, Eipper BA, The endocytic pathways of a secretory granule membrane protein in HEK293 cells: PAM and EGF traverse a dynamic multivesicular body network together, Eur. J. Cell Biol 96 (2017), 407–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Carlson K, Pomerantz SC, Vafa O, Naso M, Strohl W, Mains RE, Eipper BA, Optimizing production of Fc-amidated peptides by Chinese hamster ovary cells, BMC Biotechnol 15 (2015), 95–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Bonnemaison M, Bäck N, Lin Y, Bonifacino JS, Mains R, Eipper B, AP-1A controls secretory granule biogenesis and trafficking of membrane secretory granule proteins, Traffic 15 (2014), 1099–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Gonzalez-Begne M, Lu B, Han X, Hagen FK, Hand AR, Melvin JE, Yates JR, Proteomic analysis of human parotid gland exosomes by multidimensional protein identification technology (MudPIT), J. Proteome Res 8 (2009), 1304–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Principe S, Jones EE, Kim Y, Sinha A, Nyalwidhe JO, Brooks J, Semmes OJ, Troyer DA, Lance RS, Kislinger T, Drake RR, In-depth proteomic analyses of exosomes isolated from expressed prostatic secretions in urine, Proteomics 13 (2013), 1667–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Wang Z, Hill S, Luther JM, Hachey DL, Schey KL, Proteomic analysis of urine exosomes by multidimensional protein identification technology (MudPIT), Proteomics 12 (2012), 329–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Soni H, Bode J, Nguyen CDL, Puccio L, Neßling M, Piro RM, Bub J, Phillips E, Ahrends R, Eipper BA, Tews B, Goidts V, PERK-mediated expression of peptidylglycine α-amidating monooxygenase supports angiogenesis in glioblastoma, Oncogenesis 9 (2020), 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Shimizu T, Yoshino, Ken-ichi and Suzuki, Norio Identification and characterization of putative receptors for sperm-activating peptide I (SAP-I) in spermatozoa of the sea urchin Hemicentrotus pulcherrimus, Dev. Growth Diff 36 (1994), 209–221. [DOI] [PubMed] [Google Scholar]
- [126].Elrick MM, Samson WK, Corbett JA, Salvatori AS, Stein LM, Kolar GR, Naatz A, Yosten GL, Neuronostatin acts via GPR107 to increase cAMP-independent PKA phosphorylation and proglucagon mRNA accumulation in pancreatic alpha-cells, Am. J. Physiol. Regul. Integr. Comp. Physiol 310 (2015), R143–R155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Lee CS, Ahn W, Choi YE, The G-protein alpha-subunit gene CGA1 is involved in regulation of resistance to heat and osmotic stress in Chlamydomonas reinhardtii, Cell. Mol. Biol 63 (2017), 29–39. [DOI] [PubMed] [Google Scholar]
- [128].Urano D, Jones AM, “Round up the usual suspects”: a comment on nonexistent plant G protein-coupled receptors, Plant Physiol 161 (2013), 1097–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Kennedy MJ, Ehlers MD, Mechanisms and function of dendritic exocytosis, Neuron 69 (2011), 856–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Jin H, White SR, Shida T, Schulz S, Aguiar M, Gygi SP, Bazan JF, Nachury MV, The conserved Bardet-Biedl syndrome proteins assemble a coat that traffics membrane proteins to cilia, Cell 141 (2010), 1208–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Schou KB, Pedersen LB, Christensen ST, Ins and outs of GPCR signaling in primary cilia, EMBO Rep 16 (2015), 1099–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]


