Abstract
Cell-cell communications are central to a variety of physiological and pathological processes in multicellular organisms. Cells often rely on cellular protrusions to communicate with one another, which enable highly selective and efficient signaling within complex tissues. Owing to significant improvements in imaging techniques, identification of signaling protrusions has increased in recent years. These protrusions are structurally specialized for signaling and facilitate interactions between cells. Therefore, physical regulation of these structures must be key for the appropriate strength and pattern of signaling outcomes. However, the typical approaches for understanding signaling regulation tend to focus solely on changes in signaling molecules, such as gene expression, protein-protein interaction, and degradation. In this short review, we summarize the studies proposing the removal of different types of signaling protrusions—including cilia, neurites, MT (microtubule based)-nanotubes and microvilli—and discuss their mechanisms and significance in signaling regulation.
Keywords: Signaling protrusion, microtubule-based (MT)-nanotubes, cilia, neurite pruning, ectocytosis, trogocytosis, phagocytosis
Introduction
Signaling protrusions are specialized structures and the communication tools between cells. Therefore, if cells lose such structures, they cannot interact with other cells, just like if we lose our cell phones. Loss of signaling protrusions must greatly impact cells, which implies the possibility that cells may use the removal process to negatively regulate protrusion-mediated signaling. However, the detection of many types of protrusions is often difficult in vivo due to their highly fragile nature, and therefore the impact of structural regulation on signaling outcome is often overlooked by researchers.
Not all protrusions are too fragile to observe. Undoubtedly, the most prominent and robust example of protrusion-mediated signaling occurs in neurons forming synapses with other neurons or non-neuronal cells. Axons and dendrites are the signal sending and receiving structures, respectively, that permit a neuron to pass an electrical or chemical signal. Another example is cilia, which are often referred to as a cell’s “antenna” as they can sense extracellular stimuli or mechanical cues or work as special sites of receptor-ligand interaction (reviewed in [1, 2]). Defective removal of neuronal connections or cilia are both found to be the cause of signaling defects and are implicated in a number of human syndromes [3–7].
Other than neurons and cilia, mounting evidence suggests that protrusion-mediated signaling could be occurring in almost all cell types in a variety of contexts. These include morphogenesis during embryogenesis, immune functions, niche and stem cell interaction, and tumor progression [8–11]. Importantly, signaling protrusions are prevalent tools for cell-cell communication in complex tissue architecture. In the conventional model of paracrine signaling, signal-sending cells secrete ligands that can be received by essentially any cell located nearby. In complex tissue environments with many different cell types, protrusions can reach other cells to make direct “contact” to communicate privately and efficiently [9]. Moreover, protrusions even allow cells to communicate with distant cells contact-dependently [12].
To contribute to the efficiency of cell-cell communication, protrusions often have a specialized molecular composition for signaling. For example, the membrane of a cilia concentrates receptors and signaling molecules to locally initiate the signaling cascade. Thus, bringing the signaling factors in close proximity might be a strategy for efficient signaling activation (see [13] for a detailed review of structure-function relationships of cilia). Axons and dendrites in neurons concentrate ion channels, receptors, lipids, and cytoskeletal components for transport (see [14] for a recent review summarizing diverse structures of neurites). Cytonemes are actin-based signaling filopodia found in Drosophila wing imaginal disc cells. Cytonemes permit interaction of signaling proteins, such as ligands and receptors, between cells. Interestingly, cytonemes are induced/stabilized by the signaling molecules themselves and each cytoneme concentrates specific receptors to communicate with particular ligands [15].
The structural versatility of protrusions suited for signaling indicates that the loss of the protrusion should greatly impact the signaling outcome. Removal of protrusions or portions of protrusions must affect the turn-over time of signaling molecules, and thus must be an essential regulatory component of signaling outcomes. However, much remains unknown regarding the dynamics of these structures and how their structural changes influence signaling outcome.
1. Protrusions are sites of release of extracellular vesicles (EVs)
Apart from the increasing examples of signaling protrusions, the biology of extracellular vesicles (EVs) is rapidly growing as a field of interest. EVs are the generic term for the nanoscale membranous structures that are secreted or shed from many different types of cells and are abundant in body fluids. EVs found in body fluids are highly diverse and can be categorized based on their size and mode of biogenesis. Two types of EVs—exosomes (small EVs) and ectosomes (large EVs)—appear to be formed by distinct mechanisms [16–22]. Exosomes are produced in the endosomal pathway and are secreted by essentially any cell type [23, 24], ranging in size from 30–150 nm [25–28]. Ectosomes are formed by budding or scission of plasma membrane extensions and are generally larger than exosomes, ranging in size from 100–1000 nm [22, 25–28]. Ectosomes are membranous vesicles shed directly from portions of plasma membranes, a process often seen at the membrane of protrusions. This involves shedding of the entire lengths or portions of the protrusions and their release into the extracellular space as vesicles.
EVs can also be categorized based on their functions, as bioactive or non-bioactive vesicles. Increasing evidences of bioactive EVs have drawn researchers’ attention. Bioactive EVs participate in a diverse range of cellular events, and are considered to be a new mode of intercellular communication (reviewed in [29–31]). Moreover, EVs are studied not only for their biological functions, but also for their potential as clinical biomarkers and as cargo vehicles for the delivery of drugs or other components to specific cells for the treatment of diseases [32, 33]. Interestingly, structurally and functionally distinct types of cellular extensions such as cilia, axons, and other membrane protrusions are often found to release vesicles [34, 35], indicating the possibility that mechanisms of EV biogenesis, at least in some cases, could be related to the removal of protrusions or their components. Moreover, the requirement of common molecules, for example, tetraspanins [36], both for protrusion formation and EV biogenesis, suggests a strong link between the protrusion and EV biogenesis [36].
There are several classical examples of EV release from protrusions. Platelets are derived by shedding of plasma membrane protrusions of megakaryocytes [37]. Melanocytes release vesicles containing melanin (melanosomes) from filopodia to deliver melanin to keratinocytes [38, 39]. Cilia are also often found to produce EVs. One clear example of this was found in an unicellular organism, the green alga Chlamydomonas [40]. Ciliary EVs were shown to contain the peptide amidating enzyme peptidylglycine α-amidating monooxygenase (PAM), which converts prohormone into an active form. This EV transfer occurs between two mating algal types and is indispensable for successful mating [40]. Cilia-derived EVs have been also reported in mammals. Primary cilia in mammals are present in essentially all cell types, and are found to release EVs in addition to receiving EVs (reviewed in [41]), suggesting that ciliary EVs are a highly conserved mechanism and may be broadly utilized for different purposes.
How can protrusions do both jobs apparently independent each other, such as signaling and bioactive EV production, within a same structure? A single cilium seems to be able to release more than one type of EV. For example, EVs derived from the same cilia were shown to contain both bioactive and non-bioactive cargos. In mammalian cells, small EVs released from the base region of cilia activate Wnt signaling in target cells, whereas large EVs derived from a different portion (tips) of the same cilia, do not show any activity [42]. Similarly, in Caenorhabditis elegans (C. elegans), ciliated sensory neurons can shed EVs containing different components produced from distinct locations of the cilia (the tip or base) in response to a mating partner [43]. A more recent study in C. elegans suggests that EVs released specifically from the tip of the cilia are likely purposed for the “disposal” of ciliary materials [44]. In this case, EVs released from the ciliary tip are phagocytosed by the associated glial cells and a defect in this process results in local accumulation of ciliary proteins [44, 45]. These studies indicate that cargo sorting and the release of EVs are tightly regulated even within a protrusion. Cilia in different organisms (mammal and C. elegans) show a similar pattern of EV release, such that large ectosomes released from the tip are for disposal and small EVs (exosomes) are released from the base are for bioactivity. This may reflect a universal pattern of EV production in cilia or other protrusions as well (Figure 1). It is still unclear why these two positions of Cilia are used for different types of EV production.
Figure 1. Vesicles derived from different portions of cilia.

Large EVs (ectosomes) are typically shed from the tip region of cilia and phagocytosed, while small EVs (exosomes) are released from the base of cilia. Small EVs often exhibit bioactivity.
Certainly, not all EVs are bioactive. Indeed, EVs were initially described as a mean of disposal of unnecessary components from the cell [46]. However, because most studies of EVs have focused on their bioactivity, the impact of EV release on EV producing cells is less understood. Given that EVs are often released from protrusions utilized for signaling, it is worth revisiting the idea of disposal for signaling regulation.
2. Examples of protrusion removal via shedding
Shedding of portions or even the entirety of protrusions is observed in a variety of developmental and physiological processes, and many protrusions including neurons and cilia are removed from the cell rather than retracted or reabsorbed into the cell body. Often, removal occurs in an actively regulated manner via extrinsic or intrinsic factors. Moreover, the vesicles for disposal are often taken up or engulfed by neighboring cells for recycling of their contents, or by professional phagocytes for degradation (see Section 3 for more details). These processes are either “phagocytosis” or a mechanism called “trogocytosis” in which phagocytes directly contact a protrusion to ingest or “bite” small parts of cells, which are also released into extracellular spaces as EVs. Table1 summarizes examples of removal processes of different types of protrusions.
Table 1.
Example of removal of protrusions or their contents for the purpose of signaling regulation
| Type of protrusions | Cell type | Cargo | Fate of cargo | References |
|---|---|---|---|---|
| Cilia | Mammal, Photoreceptor | Opsins in outer membrane | Opsins taken up by RPE cells are recycled | [47–50] |
| Cilia | Mammal | G protein-coupled receptors (GPCRs) | Unknown | [51] |
| Cilia | Chlamydomonas reinhardtii | Vegetative lytic enzyme (VLE) | Release mitotic daughter cell from mother cell wall | [52] |
| Cilia | Chlamydomonas reinhardtii | peptidylglycine α-amidating monooxygen ase | Taken up by the other mating-type | [40] |
| Cilia | Chlamydomonas reinhardtii | membrane polypeptide, SAG1-C65 | Removed | [53] |
| Cilia | C. elegans, sensory neurons | Cargo required for mating behavior | Phagocytosed by the associated glial cells | [54] [45] |
| Filopodia | Mammal, melanocyte | Melanin in melanosomes | Taken up by keratinocytes | [55] |
| Microtubule-based (MT)-nanotubes | Drosophila, germline stem cells (GSCs) | Tkv receptor | Taken up by niche cells and degraded | [56] |
| Microvilli | Mammal, T-cells | T-cell receptor | Trapped on the surface of antigen presenting cells | [57] |
| Dendrites | Mammal, neurons | Neurites/synapses | Engulfed by microglia and degraded | [58] |
| Axons | Mammal, neurons | Neurites/synapses | Engulfed by astrocytes or microglia and degraded | [59] [60] |
2.1. Removal of portions or entire cilia
Cilia are specialized cellular projections found in a broad range of organisms, from unicellular organisms to humans. The structures of cilia are highly conserved, containing a nine-fold doublet microtubule bundle, called the axoneme, that extends from a mother centriole-derived basal body wrapped by a ciliary membrane. The primary cilium is non-motile and appears in most mammalian cell types. The primary cilium often functions as a specialized signaling center of cells; thus it has been deemed the cell’s antenna. Cilia detect various signals such as hedgehog, noncanonical Wnt, and GPCR-mediated signals including odorants and light [61, 62]. To execute their functions, the composition of cilia is specifically regulated apart from other regions of the cell. The ciliary membrane is concentrated with specialized lipids and proteins and separated from other cellular membranes via a diffusion barrier called the transition zone [63].
Given the function of cilia as the antennae, removal of the entirety or parts of cilia or contents should have a significant impact on rest of the cell. Shedding, ectocytosis or decapitation are the pathways for removing parts of cilia which have mainly been considered as a form of cellular disposal [64]. As a consequence of shedding or decapitation, the removed part of the cilium is released into the extracellular space [64]. Ciliary-derived vesicles have compositions different from the cilia from which they were derived. A subset of ciliary proteins including proteases, ESCRT proteins, small GTPases, and ubiquitinated proteins have been found to be enriched in cilia-derived EVs [65], indicating that cells sort components to be removed. The removal of ciliary components by EVs often regulates appropriate turnover time of proteins within cilia. For example, in the green alga Chlamydomonas, ectocytosis is required for the timely removal of ciliary components [52, 53]. Similarly, ectocytosis was reported to prevent excess accumulation of ciliary cargo in C. elegans sensory neurons [45]. Moreover, a study used real-time imaging of mammalian cells and showed that the GPCRs that localize on primary cilia are shed into vesicles from the tips of cilia upon signal activation [51] (Figure 2B). GPCRs are enriched in ectosomes and their removal is required for signal attenuation, indicating that signal-dependent ectocytosis regulates ciliary signaling [51] (Figure 2B). These examples suggest the universality of removal of ciliary components, which is strictly regulated in response to extrinsic cues.
Figure 2. Shedding of cilia regulates turnover of signaling molecules.

A) Shedding of outer segments of photoreceptors (purple cell). Outer segment discs contain rhodopsin, which belongs to the GPCR superfamily. Rhodopsins undergo both a conformational change (becoming opsins) and activation of the G protein upon receiving a photon, and need to be renewed/replaced daily. Part of the outer segment disk containing used opsins is shed, phagocytosed by neighboring retinal pigment epithelium (RPE) cells, transformed back to rhodopsins, and then transported into photoreceptor cells.
B) GPCRs (receptors) localize to cilia and accumulates in the tip portion. Upon stimulation by the hedgehog ligand, GPCRs that fail to be retrieved from the tip are removed by ectocytosis.
The “disposed” portion of cilia can also be recycled. A unique example of this is observed in the photoreceptor cells of mammalian retina (Figure 2A). Photoreceptor cells form specialized cilia (outer segment) that contain rhodopsin (the substance for photo-sensing), and the tip of each outer segment is released approximately 6 times per day to remove used opsins with oxidized retinols (reviewed in [50]). Released segments are then taken up by the neighboring retinal pigmented epithelium where the retinol is reduced and transported back to the photoreceptor cells to regenerate functional rhodopsin (Figure 2A).
In proliferating cells, extension and retraction of cilia occur in a cell cycle-dependent manner. Typically, the basal body derived from the mother centriole extends cilia in post-mitotic phases (G1 or G0 phases) of the cell cycle, then cilia dissociate prior to mitotic entry [66]. The exact timing of the ciliary cycle varies by cell type [66]. Two modes have been suggested for how cilia disappear during the cell cycle: resorption, in which the axoneme is depolymerized and ciliary contents are incorporated into the cell, and deciliation, in which the axoneme is excised near its base and the entire cilium is released into the extracellular space [67]. It is still unclear which proposed mechanism is true, or if it varies in different cases. A recent study used live imaging of cilia during serum-induced ciliary assembly and disassembly to determine the predominant mechanism of ciliary loss, finding that deciliation is more prevalent than resorption in mammalian cells [68].
A recent study demonstrated the decapitation of the ciliary tip appears to induce subsequent entire ciliary retraction in mouse embryonic fibroblasts. In this case, decapitation removes the tip region of ciliary components including the intraflagellar transport-B (IFT-B) complex which delivers α and β tubulin subunits towards the ciliary tip for elongation. Unexpectedly, this study showed that the loss of cilia is not just the consequence of routinely occurring cell cycle process, instead cilia removal and cell cycle regulation are mutually dependent processes [69].
2.2. Receptor removal from microtubule-based nanotubes
Another type of protrusion known to undergo the process of removal of its contents or portions is the MT-nanotube, which is spefically present on Drosophila germline stem cells (GSCs). In the Drosophila testis, 8–10 GSCs attach to the cluster of somatic cells, a major niche component called the hub (Figure 3) [70]. Hub cells secrete niche ligands, including Decapentaplegic (Dpp) (Figure 3). Dpp functions in the BMP signaling pathway and is required for GSC maintenance [71]. The receptor for Dpp, Thickveins (Tkv), localizes to the surface of MT-nanotubes, where Dpp-Tkv interaction and Tkv activation both occur. Therefore, MT-nanotubes promote Dpp-Tkv interaction and signal activation. However, at the same time, Dpp signal attenuation also occurs on MT-nanotubes [56].
Figure 3. Tkv removal from MT-nanotubes is required for signal adjustment.
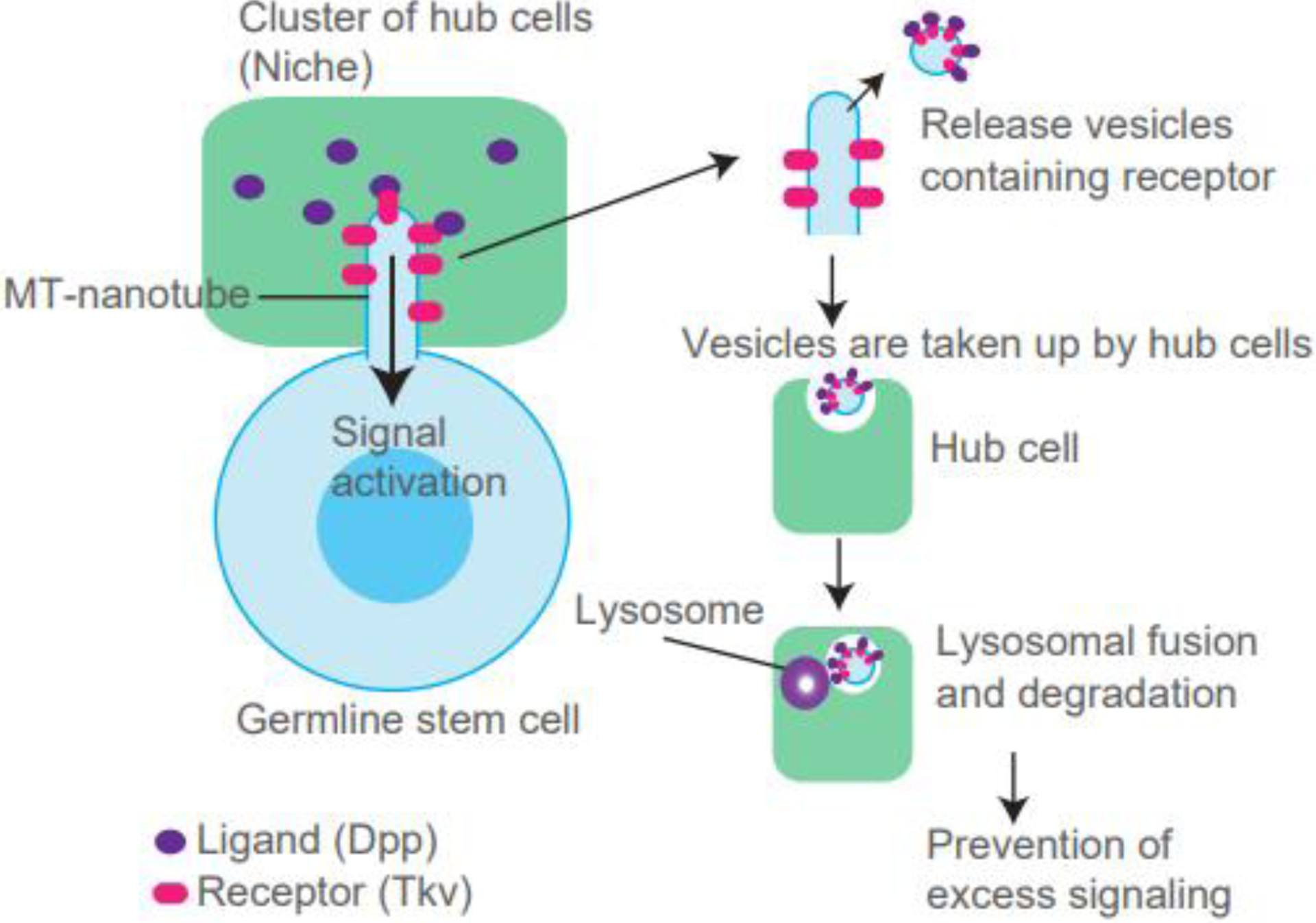
(Left) Microtubule (MT)-nanotube appears on the Drosophila GSC and promotes the reception of Dpp ligand secreted from the niche, a cluster of hub cells. (Right) Shedding of MT-nanotube membrane and potential engulfment by hub cells. Vesicles taken up are fused to lysosomes in hub cells and ultimately degraded, resulting in attenuation of the signal.
Tkv localized on MT-nanotubes is removed from the MT-nanotube and subsequently taken up by the adjacent niche cells where it is degraded. Expression of ubiquitination-defective Tkv, which cannot be removed from MT-nanotubes, overactivates downstream signaling, suggesting that Tkv removal is required for signal attenuation (Figure 3) [56]. MT-nanotubes share their molecular components with cilia, such as IFT-B [70], suggesting a possibility that Tkv removal may utilize a mechanism similar to ciliary ectocytosis. The mechanism of how Tkv is transported into hub cells to be degraded is still unknown. MT-nanotubes are stable structures during the entire cell cycle; however, they seem to disappear during mitosis, similar to cilia. GSCs are a constantly dividing population and the estimated cell cycle time is approximately 12 hours [72]. Therefore, the removal or resorption of the entire length of MT-nanotubes may occur at least once in 12 hours. It is still unclear whether Tkv is transported together with entire MT-nanotubes during the cell-cycle dependent removal process or if Tkv is specifically packaged in a vesicle and removed. Further studies using long-term live observation or ultrastructure analysis will be necessary to identify the mechanism of this phenomenon.
Moreover, it is still unclear why Tkv receptor must be removed in the hub cells, rather than in the GSC itself. When surface receptors are internalized, endosomes containing receptors can still signal into the cells until multivesicular body formation for subsequent lysosomal fusion [73]. Therefore, Tkv receptor release outside of the cell may be a safeguard to protect stem cells from excess activation of the niche signal. It will be interesting to investigate whether similar mechanisms are used for other stem cell systems.
2.3. Removal of microvilli
Microvilli are actin-dependent cellular membrane protrusions often described as thin and finger-like. They appear on a broad range of cell types including lymphocytes [74], intestinal epithelial cells [75], endothelial cells [76, 77], dendrites [78], neurons [79], and oocytes [80]. The function of microvilli varies depending on the cell type; for example, microvilli are well-known to facilitate absorption in the intestinal epithelium, while in lymphocytes they aid in adhesion [81, 82]. In the oocyte, it is suggested that microvilli are important for sperm binding and fusion [83–85]. The best-studied example of role of microvilli on signaling occurs at the immunological synapse formed between T-cells and antigen-presenting cells (APCs). T-cell receptors (TCRs) and co-receptors are highly clustered on the tips of microvilli, suggesting that microvilli play roles on signaling between T-cells and APCs [86–90]. A recent study demonstrated that the TCR containing vesicles are released from the tip of microvilli and localized on the surface of APCs [57], indicating the possibility that vesicle release from microvilli is for the purpose of signaling regulation.
Other than the example of T-cells, the roles of microvilli for cellular signaling are still unclear. However, studies over the past few decades have found that various receptors are enriched on microvilli surfaces, including insulin receptors [91], selectin [92]. Moreover, EVs have been reported to shed from microvilli. In mammalian cell culture, EVs containing the glycoprotein prominin-1 were reported to originate from epithelial cells’ microvilli [93], and the regulation of EV release from these microvilli was found to be dependent on prominin-1 binding to membrane cholesterol [94]. Additionally, it was recently reported in Drosophila that the microvilli of the wing imaginal disc epithelial cells also shed EVs which contain the morphogen Hedgehog [95] required for wing imaginal disc development. These indicate the possibility that EV shedding promotes remodeling of microvilli in order to regulate signaling in other cell types.
2.4. Removal of neuronal connections
Neurons initially extend an excess number of neurites to form multiple synapses from single cells. During development and the early years of life, neurons use a unique way to remove unnecessary neurite connections, which is called neurite pruning (reviewed in [96]). Pruning selectively removes axon or dendrite branches from neurons and is an essential process for establishment of the refined neural circuits [96]. Defective pruning is implicated in a variety of adolescent and adulthood neurological disorders, such as Alzheimer’s disease, multiple sclerosis, schizophrenia, and autism [3–6].
In past decades, studies in different systems have identified various extrinsic and intrinsic regulatory pathways for the determination of pruning, suggesting that the molecular mechanisms for each pruning event are unique [97]. It has been hypothesized that neural activity, both long-term potentiation (LTP) and long-term depression (LTD) are involved in the selection process to determine which connections are to be pruned [97]. During the dendrite pruning process, “thinning” at the proximal region of neurites is initiated first to trigger the subsequent pruning at distal portions of dendrites [98]. Local endocytosis is required for the dendrite thinning step at the defined region. During a later pruning step, the GTPases Rab5 and Dynamin are required for removal of chopped dendrites [98].
Axons are also subjected to elimination. One study demonstrated that axon pruning in neuromuscular junctions is mediated by shedding of membrane-bound remnant “axosomes”; which was shown with correlating light and serial electron microscopy [99]. Interestingly, axosomes are engulfed and subsequently eliminated by glial cells, reminiscent of the case of outer segment of photoreceptor and MT-nanotubes described above [99]. Microglia engulfment was also shown in presynaptic inputs via pruning of retinal ganglion cell axons [60]. Complement receptor 3(CR3)/C3 serves as the microglia-specific phagocytic signaling pathway and disruption of this pathway resulted in sustained deficits in synaptic connectivity [60].
Developmentally regulated pruning suggests the significance of removal of signaling structures for the purpose of terminating cell-cell interactions. Later, engulfment of neurites or synapses by microglia [58] or astrocytes [59] are both considered subtypes of trogocytosis (see section 3.5), implying the commonality of mechanisms among cilia, neurons, and MT-nanotubes.
3. Mechanisms of removal of protrusions
3.1. The role of the actomyosin cytoskeleton
Structurally, cilia are supported by a microtubule-based axoneme and originally thought to be devoid of F-actin [100]. However, recent studies have demonstrated that actin is required for ectocytosis of cilia, which shortens the cilia [51]. Activated GPCRs can be released from the tip of the cilia via signal-dependent ectocytosis, a process requiring actomyosin activity. Inhibition of actin has been shown to block ectocytosis and result in elongated cilia [51]. Decapitation of the tip of cilia is also induced by ciliary PI(4,5)P2-mediated F-Actin accumulation in ciliary lumen [69]. Consistently, previous work has established that the actin regulators RhoA, Rac, and Cdc42 are required for ectosome release from the plasma membrane [101], a process that is at least in part facilitated by cortical actomyosin contraction [102]. These findings not only demonstrate the crucial role for actomyosin contraction in ectocytosis, but also suggest a consensus in which actomyosin negatively regulates the length of cilia.
3.2. The endosomal sorting complexes required for transport (ESCRT) machinery
Although the exact mechanisms for ectocytosis remain elusive, findings from recent studies are shedding light on the process. The endosomal sorting complexes required for transport (ESCRT) machinery is indispensable for membrane remodeling [103–105]. ESCRT-III and Vps4-dependent shedding from the plasma membrane has been extensively studied in the context of viral budding and the process bears some resemblance to that of ectocytosis. In this mechanism, ubiquitination is a key step that is necessary for ESCRT machinery recruitment to the site of viral budding [106, 107]. Notably, ESCRT-III and Vps4 are recruited to the site of membrane budding just before virion release [108, 109], and these factors, along with the ESCRT-associated factor ALIX, are required for the budding and scission of the packaged virion from the plasma membrane [110]. The ESCRT-III complex forms spiral-like structures around the base of the “neck” of a budding membrane; it is thought that this spiral-like structure, formed by the subunit Snf7, is responsible for deforming the membrane around cargo to form a bud [111]. If ESCRT function is required for protrusion removal via promoting ectosome shedding, loss of ESCRT should cause defect of protrusion removal. Strikingly, a recent study using a combination of proteomic analysis and knockdown of ESCRT-related proteins in Chlamydomonas showed that the ESCRT proteins mediate ectosome release and thereby influence shortening of Cilia [112]. Similar mechanism may be used for removal of other protrusions.
3.3. Bardet-Biedl syndrome (BBS) proteins
Bardet-Biedl syndrome (BBS) are caused by the mutation of ciliopathy genes encoding BBS proteins [113]. BBS is characterized by many symptoms including male infertility, retinal degeneration, renal failure, and diabetes caused by the dysfunction of primary cilia [114, 115]. BBS components assemble a coat structure around vesicles [116], similar to COPI, COPII, and clathrin-coating, in an octameric complex referred to as the BBSome [117, 118]. The BBSome has been implicated in ciliary trafficking and is suggested to be required for transport of signaling receptors to ciliary membranes [116, 119, 120], and also regulate cilia disassembly [121, 122]. Studies have shown that BBS components negatively regulate ectocytosis in mammals and C. elegans [42] [123] where BBS proteins seem to regulate ciliary cargo retrieval from ciliary tip to cell body, indicating that BBS can act as a key factor that determines whether cargos will be removed into EV or retrieved into cells.
3.4. Tetraspanins
Tetraspanins form a transmembrane protein superfamily. They interact with a large variety of transmembrane and cytosolic signaling proteins to create membrane microdomains. Because they are abundantly found in the membrane of EVs, tetraspanins have been widely used as EV markers. Tetraspanin genes have been shown to regulate cargo sorting for EVs, and their function on ESCRT-independent endosomal sorting is essential for melanosome biogenesis and transfer [124, 125]. Intriguingly, tetraspanins are also enriched at various types of protrusions, including intestinal microvilli [126], oocyte microvilli [80, 127, 128], microprotrusions of platelets [129, 130], photoreceptor sensory cilia [131], and synapses [132], implicating the role of these protrusions as the platform of EV release. Moreover, studies have demonstrated that tetraspanins regulate a variety of signaling pathways, such as integrins, EGFR, TNF-α, c-Met, c-Kit and TGF-β [133]. This suggests that tetraspanins may be a key factor to determine the fate of signaling molecules by deciding whether to sort them into EVs for disposal. Although a number of signaling molecules have been identified to interact with tetraspanins, the mechanisms by which tetraspanins control signaling still remains largely elusive. Future studies, especially detailed structural analysis, will help us understand these mechanisms.
3.5. Final processes (eat-me signal and phagocytosis/trogocytosis)
What triggers ESCRT-III assembly to initiate ectocytosis from protrusions? The TAT-5 phospholipid flippase maintains the location of phosphatidylethanolamine (PE) on the inner leaflet of the plasma membrane. Absence of TAT-5 results in overproduction of EVs in early embryonic divisions of C. elegans [134, 135]. Flippases also translocate phosphatidylserine (PtdSer) from outer to inner leaflets of the plasma membrane. Opposing enzyme scramblases of the TMEM16 family of proteins function to shuffle PtdSer asymmetrically in a Ca2+- and caspase-dependent manner and often show a polarized localization in other cell types [136, 137]. These reports suggest that PE or PtdSer externalization may be upstream of ESCRT machinery. The engulfment of outer segments of retinal pigment epithelium occurs via recognizing PtdSer presented on the surface of outer segments by flippase, P4-ATPase, and Atp8a2 [138]. Surprisingly, the cargo opsin itself has also been reported to possess ATP-independent lipid flippase activity [139], indicating that certain cargos can promote their disposal or transport, and externalization of PtdSer was initially found in apoptotic cells as an “eat-me” signal for phagocytes [140]. This suggests the possibility that PtdSer externalization could be recognized by the same or similar phagocytic receptors present on phagocytes for clearance. It would be tempting to examine if PtdSer externalization is utilized for EV disposal. The membrane-flipping activity is known to be triggered by calcium influx [137]. However, whether PtdSer externalization is the common mechanism that triggers EV shedding across different cell types remains to be determined, and the entire mechanism of how PtdSer externalization is locally regulated is not fully understood (Figure 4).
Figure 4. Phagocytes can bite off protrusions (Trogocytosis).
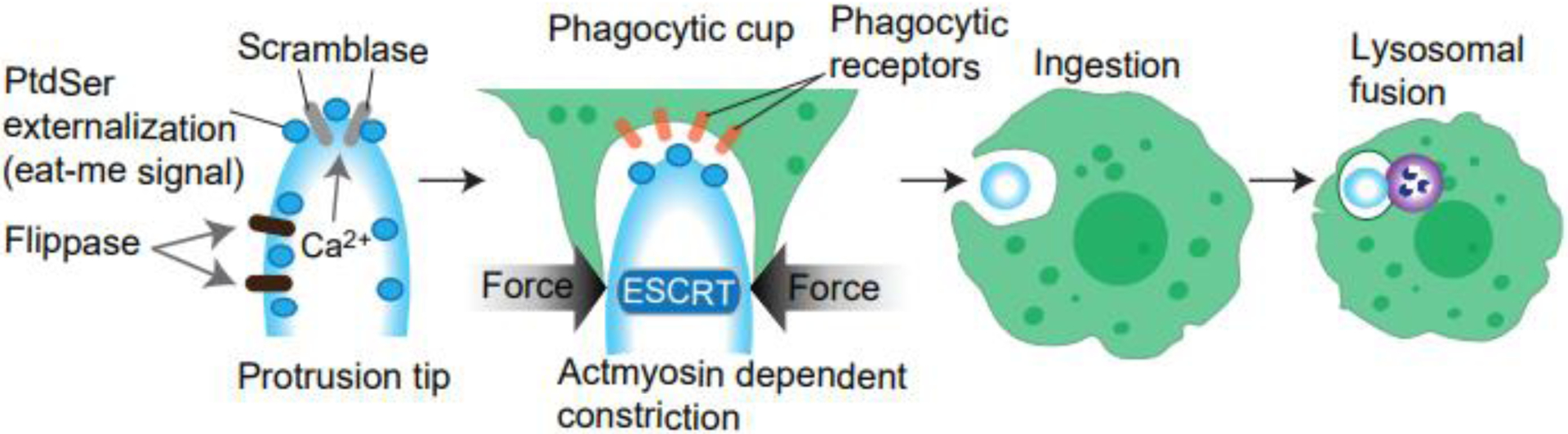
(Left) PtdSer normally localizes to the inner leaflet of the plasma membranes via the function of flippase. Scramblase is activated by Ca2+ and scrambles PtdSer localization. (Middle) Externally localized PtdSer is recognized by phagocytic receptors, and these receptors recruit actomyosin to the phagocytic cups. Phagocytic cups generate constriction forces to “bite” up the tip of the protrusion (Right). Protrusions are cell-autonomously shed via activating ESCRT. These mechanisms were both reported in the engulfment of photoreceptor outer segment, and thus may occur simultaneously (see text).
The activation of phagocytic receptors in the phagocytic retinal epithelia leads to myosin recruitment in the phagocytic cup and is required for successful engulfment [141]. This “bite” mechanism is closely related to the mechanism referred to as “trogocytosis” (Figure 4). Trogocytosis has been actively studied in immune cell interactions at immunological synapses [142]. Trogocytosis is similar to phagocytosis but involves only eating parts of cells. Although this process can ultimately kill the target cells [143], in many cases the removal occurs only partially and, interestingly, cellular protrusions are often targeted by this ingestion [143]. A study using time-lapse imaging revealed that trogocytosis is a rapid process, immediately triggered when phagocytes meet filopodia [58]. Increasing evidences suggest that trogocytosis may be broadly utilized even outside of immune responses [144]. Defining further molecular mechanism of this process is a fascinating future study.
4. Unsolved questions
The reason why EV release preferentially occurs from protrusions is not clear. Protrusions may act to compartmentalize cargos away from their cell body for further sorting into EVs. Or they may be structurally suitable to sense extracellular stimuli which drive release or shedding of vesicles. Another possibility is that protrusion formation itself utilizes similar molecular components for exocytosis or ectocytosis, and thus cells have evolved to use the same components for bioactive EV production. The usage of abundant surface area and curvature of plasma membranes on protrusions should be suitable to produce EVs in an energy efficient manner. It is currently unknown if there is any qualitative/quantitative difference between protrusion-derived EV and cellular membrane-derived EV.
Another intriguing question is how protrusions and their contents are determined to be removed or not. During development, different cells may stop or start their interaction or change their interacting partner. Therefore, the remodeling of protrusions needs to be regulated developmentally via extrinsic and/or intrinsic factors. During the remodeling of neural networks, the neuronal activity itself seems to be a primary factor for determination of the fate of connections [97]. It is tempting to speculate whether there is a universal determination mechanism for the formation or removal of signaling protrusions.
In contrast to occasional removal, daily turnover of protrusion contents occurs through constant removal, which appears to be essential for adjusting signaling at the physiological level. Several examples suggest or support this. SAG1-containing cargo moves unidirectionally on the cilia and never enters the cell body [53]. MT-nanotubes carry Tkv receptor into niche cells and Tkv is never observed in the cell body unless trafficking is suppressed [56]. In this way, niche signal reception is tightly limited to stem cell populations by limiting the Tkv receptor to be inherited into differentiating daughter cells during cell division [56]. Removal of protein at a certain rate and location may contribute to the specificity and selectivity of protrusion-mediated signaling.
Do protrusions use any common mechanism for disposal processes? Indeed, many mechanistic and molecular commonalities have been found in different types of protrusions. For example, removal of signaling components is dependent on signal activation itself [51]. Ubiquitinated proteins are often targeted for the removal process, and the cargo sorting processes share similar molecular machinery. More and more studies using proteomics approach aiming to determine composition of EVs and protrusions have identified interesting similarity among different systems. Ultimately, an “eat-me” signal exposed on the surface of protrusions may be a common mechanism for either shedding or targeted by “biting” through trogocytosis. Further studies will be necessary to identify how protrusions or their components are targeted by these common mechanisms to be disposed from cells.
Conclusion and remarks
Signaling protrusions contribute to special temporal regulation of cell-cell communication over a variety of cell types. Studies described in this review suggest that the removal of protrusions occurs constantly in order to maintain physiological turnover of proteins or in response to developmental or environmental signals, and dysregulation of the removal process greatly impacts signaling outcome. Studying regulation of protrusions has been challenging due to technical limitations. Future studies utilizing new techniques such as super-resolution live imaging, cryo-correlative light and electron microscopy (Cryo-CLEM) or high sensitive proteomics approaches may greatly contribute to this field. Identifying common molecular pathways and comprehensively examining the impact of manipulation of these pathways on various cellular protrusions may greatly facilitate our understanding of signaling regulation in vitro and in vivo.
Acknowledgements
We thank Inaba lab members for valuable discussions. Christopher Bonin, Geneva R Hargis for manuscript editing. This research is supported by 1R35GM128678 from National Institute for General Medical Sciences and start-up funds from UConn Health (to M.I.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Financial Interest Statement
The authors have no competing financial interests to declare.
References
- 1.Anvarian Z, et al. , Cellular signalling by primary cilia in development, organ function and disease. Nat Rev Nephrol, 2019. 15(4): p. 199–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Satir P and Christensen ST, Overview of structure and function of mammalian cilia. Annu Rev Physiol, 2007. 69: p. 377–400. [DOI] [PubMed] [Google Scholar]
- 3.Geloso MC and D’Ambrosi N, Microglial Pruning: Relevance for Synaptic Dysfunction in Multiple Sclerosis and Related Experimental Models. Cells, 2021. 10(3): p. 686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brucato FH and Benjamin DE, Synaptic Pruning in Alzheimer’s Disease: Role of the Complement System. Global journal of medical research, 2020. 20(6): p. 10.34257/gjmrfvol20is6pg1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keshavan MS, Anderson S, and Pettergrew JW, Is Schizophrenia due to excessive synaptic pruning in the prefrontal cortex? The Feinberg hypothesis revisited. Journal of Psychiatric Research, 1994. 28(3): p. 239–265. [DOI] [PubMed] [Google Scholar]
- 6.Kim HJ, et al. , Deficient autophagy in microglia impairs synaptic pruning and causes social behavioral defects. Molecular Psychiatry, 2017. 22(11): p. 1576–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Broekhuis JR, Leong WY, and Jansen G, Chapter Three - Regulation of Cilium Length and Intraflagellar Transport, in International Review of Cell and Molecular Biology, Jeon KW, Editor. 2013, Academic Press. p. 101–138. [DOI] [PubMed] [Google Scholar]
- 8.Yamashita YM, Inaba M, and Buszczak M, Specialized Intercellular Communications via Cytonemes and Nanotubes. Annu Rev Cell Dev Biol, 2018. 34: p. 59–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buszczak M, Inaba M, and Yamashita YM, Signaling by Cellular Protrusions: Keeping the Conversation Private. 2016, Elsevier Ltd. p. 526–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tamzalit F, et al. , Interfacial actin protrusions mechanically enhance killing by cytotoxic T cells. Science immunology, 2019. 4(33): p. eaav5445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roehlecke C and Schmidt MHH, Tunneling Nanotubes and Tumor Microtubes in Cancer. Cancers, 2020. 12(4): p. 857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kornberg TB, Cytonemes and the dispersion of morphogens. Wiley interdisciplinary reviews. Developmental biology, 2014. 3(6): p. 445–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia G 3rd, Raleigh DR, and Reiter JF, How the Ciliary Membrane Is Organized Inside-Out to Communicate Outside-In. Current biology : CB, 2018. 28(8): p. R421–R434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goaillard J-M, et al. , Diversity of Axonal and Dendritic Contributions to Neuronal Output. Frontiers in cellular neuroscience, 2020. 13: p. 570–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roy S, et al. , Cytoneme-mediated contact-dependent transport of the Drosophila decapentaplegic signaling protein. Science (New York, N.Y.), 2014. 343(6173): p. 1244624–1244624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Théry C, et al. , Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles, 2018. 7(1): p. 1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.E.L.A. S, et al. , Extracellular vesicles: biology and emerging therapeutic opportunities. at Rev Drug Discov, 2013. 12(5): p. 347–57. [DOI] [PubMed] [Google Scholar]
- 18.Thompson AG, et al. , Extracellular vesicles in neurodegenerative disease - pathogenesis to biomarkers. Nat Rev Neurol, 2016. 12(6): p. 346–57. [DOI] [PubMed] [Google Scholar]
- 19.van Niel G, D’Angelo G, and Raposo G, Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol, 2018. 19(4): p. 213–228. [DOI] [PubMed] [Google Scholar]
- 20.Mathieu M, et al. , Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol, 2019. 21(1): p. 9–17. [DOI] [PubMed] [Google Scholar]
- 21.Théry C, Ostrowski M, and Segura E, Membrane vesicles as conveyors of immune responses. Nat Rev Immunol, 2009. 9(8): p. 581–93. [DOI] [PubMed] [Google Scholar]
- 22.Yáñez-Mó M, et al. , Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles, 2015. 4: p. 27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kalluri R and LeBleu VS, The biology, function, and biomedical applications of exosomes. Science, 2020. 367(6478). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hessvik NP and Llorente A, Current knowledge on exosome biogenesis and release. Cell Mol Life Sci, 2018. 75(2): p. 193–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zaborowski MP, et al. , Extracellular Vesicles: Composition, Biological Relevance, and Methods of Study. Bioscience, 2015. 65(8): p. 783–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Borges FT, Reis LA, and Schor N, Extracellular vesicles: structure, function, and potential clinical uses in renal diseases. Braz J Med Biol Res, 2013. 46(10): p. 824–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bebelman MP, et al. , Biogenesis and function of extracellular vesicles in cancer. Pharmacol Ther, 2018. 188: p. 1–11. [DOI] [PubMed] [Google Scholar]
- 28.Raposo G and Stoorvogel W, Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol, 2013. 200(4): p. 373–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simeone P, et al. , Extracellular Vesicles as Signaling Mediators and Disease Biomarkers across Biological Barriers. Int J Mol Sci, 2020. 21(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Margolis L and Sadovsky Y, The biology of extracellular vesicles: The known unknowns. PLOS Biology, 2019. 17(7): p. e3000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Niel G, D’Angelo G, and Raposo G, Shedding light on the cell biology of extracellular vesicles. Nature Reviews Molecular Cell Biology, 2018. 19(4): p. 213–228. [DOI] [PubMed] [Google Scholar]
- 32.Kosaka N, et al. , Exploiting the message from cancer: the diagnostic value of extracellular vesicles for clinical applications. Experimental & Molecular Medicine, 2019. 51(3): p. 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herrmann IK, Wood MJA, and Fuhrmann G, Extracellular vesicles as a next-generation drug delivery platform. Nature Nanotechnology, 2021. 16(7): p. 748–759. [DOI] [PubMed] [Google Scholar]
- 34.Rilla K, Diverse plasma membrane protrusions act as platforms for extracellular vesicle shedding. J Extracell Vesicles, 2021. 10(11): p. e12148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mathieu M, et al. , Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nature Cell Biology, 2019. 21(1): p. 9–17. [DOI] [PubMed] [Google Scholar]
- 36.Bari R, et al. , Tetraspanins regulate the protrusive activities of cell membrane. Biochemical and biophysical research communications, 2011. 415(4): p. 619–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Andrews RK and Gardiner EE, Basic mechanisms of platelet receptor shedding. Platelets, 2017. 28(4): p. 319–324. [DOI] [PubMed] [Google Scholar]
- 38.Wu XS, et al. , Melanoregulin regulates a shedding mechanism that drives melanosome transfer from melanocytes to keratinocytes. Proc Natl Acad Sci U S A, 2012. 109(31): p. E2101–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ando H, et al. , Melanosomes are transferred from melanocytes to keratinocytes through the processes of packaging, release, uptake, and dispersion. J Invest Dermatol, 2012. 132(4): p. 1222–9. [DOI] [PubMed] [Google Scholar]
- 40.Luxmi R, et al. , Cilia-based peptidergic signaling. PLoS Biol, 2019. 17(12): p. e3000566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ikegami K and Ijaz F, Current understandings of the relationship between extracellular vesicles and cilia. The Journal of Biochemistry, 2021. 169(2): p. 139–145. [DOI] [PubMed] [Google Scholar]
- 42.Volz A-K, et al. , Bardet-Biedl syndrome proteins modulate the release of bioactive extracellular vesicles. Nature Communications, 2021. 12(1): p. 5671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang J, et al. , Sensory cilia act as a specialized venue for regulated extracellular vesicle biogenesis and signaling. Curr Biol, 2021. 31(17): p. 3943–3951.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang W, et al. , Intraflagellar Transport Proteins as Regulators of Primary Cilia Length. Front Cell Dev Biol, 2021. 9: p. 661350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Razzauti A and Laurent P, Ectocytosis prevents accumulation of ciliary cargo in C. elegans sensory neurons. Elife, 2021. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johnstone RM, et al. , Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem, 1987. 262(19): p. 9412–20. [PubMed] [Google Scholar]
- 47.Basinger S, Hoffman R, and Matthes M, Photoreceptor shedding is initiated by light in the frog retina. Science, 1976. 194(4269): p. 1074–6. [DOI] [PubMed] [Google Scholar]
- 48.Kwon W and Freeman SA, Phagocytosis by the Retinal Pigment Epithelium: Recognition, Resolution, Recycling. Front Immunol, 2020. 11: p. 604205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Salinas RY, et al. , Photoreceptor discs form through peripherin-dependent suppression of ciliary ectosome release. J Cell Biol, 2017. 216(5): p. 1489–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kevany BM and Palczewski K, Phagocytosis of retinal rod and cone photoreceptors. Physiology (Bethesda), 2010. 25(1): p. 8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nager AR, et al. , An Actin Network Dispatches Ciliary GPCRs into Extracellular Vesicles to Modulate Signaling. Cell, 2017. 168(1–2): p. 252–263.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wood CR, et al. , The cilium secretes bioactive ectosomes. Curr Biol, 2013. 23(10): p. 906–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cao M, et al. , Uni-directional ciliary membrane protein trafficking by a cytoplasmic retrograde IFT motor and ciliary ectosome shedding. Elife, 2015. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang J, et al. , C. elegans ciliated sensory neurons release extracellular vesicles that function in animal communication. Curr Biol, 2014. 24(5): p. 519–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Scott G, et al. , Filopodia are conduits for melanosome transfer to keratinocytes. J Cell Sci, 2002. 115(Pt 7): p. 1441–51. [DOI] [PubMed] [Google Scholar]
- 56.Ladyzhets S, et al. , Self-limiting stem-cell niche signaling through degradation of a stem-cell receptor. PLoS Biol, 2020. 18(12): p. e3001003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim H-R, et al. , T cell microvilli constitute immunological synaptosomes that carry messages to antigen-presenting cells. Nature Communications, 2018. 9(1): p. 3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weinhard L, et al. , Microglia remodel synapses by presynaptic trogocytosis and spine head filopodia induction. Nat Commun, 2018. 9(1): p. 1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nguyen JV, et al. , Myelination transition zone astrocytes are constitutively phagocytic and have synuclein dependent reactivity in glaucoma. Proc Natl Acad Sci U S A, 2011. 108(3): p. 1176–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schafer DP, et al. , Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron, 2012. 74(4): p. 691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wheway G, Nazlamova L, and Hancock JT, Signaling through the Primary Cilium. Front Cell Dev Biol, 2018. 6: p. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mykytyn K and Askwith C, G-Protein-Coupled Receptor Signaling in Cilia. Cold Spring Harb Perspect Biol, 2017. 9(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Garcia-Gonzalo FR, et al. , A transition zone complex regulates mammalian ciliogenesis and ciliary membrane composition. Nature genetics, 2011. 43(8): p. 776–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pan BT, et al. , Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. J Cell Biol, 1985. 101(3): p. 942–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Long H, et al. , Comparative Analysis of Ciliary Membranes and Ectosomes. Curr Biol, 2016. 26(24): p. 3327–3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Plotnikova OV, Pugacheva EN, and Golemis EA, Primary cilia and the cell cycle. Methods in cell biology, 2009. 94: p. 137–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liang Y, et al. , Mechanism of ciliary disassembly. Cell Mol Life Sci, 2016. 73(9): p. 1787–02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mirvis M, et al. , Primary cilium loss in mammalian cells occurs predominantly by whole-cilium shedding. PLoS biology, 2019. 17(7): p. e3000381–e3000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Phua SC, et al. , Dynamic Remodeling of Membrane Composition Drives Cell Cycle through Primary Cilia Excision. Cell, 2017. 168(1–2): p. 264–279.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Inaba M, Buszczak M, and Yamashita YM, Nanotubes mediate niche-stem-cell signalling in the Drosophila testis. Nature, 2015. 523(7560): p. 329–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kawase E, et al. , Gbb/Bmp signaling is essential for maintaining germline stem cells and for repressing bam transcription in the Drosophila testis. Development (Cambridge, England), 2004. 131(6): p. 1365–75. [DOI] [PubMed] [Google Scholar]
- 72.Gadre P, et al. , The rates of stem cell division determine the cell cycle lengths of its lineage. iScience, 2021. 24(11): p. 103232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Piper RC and Katzmann DJ, Biogenesis and function of multivesicular bodies. Annual review of cell and developmental biology, 2007. 23: p. 519–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Alexander E, Sanders S, and Braylan R, Purported difference between human T-and B-cell surface morphology is an artefact. Nature, 1976. 261(5557): p. 239–41. [DOI] [PubMed] [Google Scholar]
- 75.Sauvanet C, et al. , Structure, regulation, and functional diversity of microvilli on the apical domain of epithelial cells. Annu Rev Cell Dev Biol, 2015. 31: p. 593–621. [DOI] [PubMed] [Google Scholar]
- 76.Gabbiani G and Majno G, Endothelial microvilli in the vessels of the rat gasserian ganglion and testis. Z Zellforsch Mikrosk Anat, 1969. 97(1): p. 111–7. [DOI] [PubMed] [Google Scholar]
- 77.Fujimoto S, Yamamoto K, and Takeshige Y, Electron microscopy of endothelial microvilli of large arteries. Anat Rec, 1975. 183(2): p. 259–65. [DOI] [PubMed] [Google Scholar]
- 78.Fisher PJ, et al. , Dendritic cell microvilli: a novel membrane structure associated with the multifocal synapse and T-cell clustering. Blood, 2008. 112(13): p. 5037–45. [DOI] [PubMed] [Google Scholar]
- 79.Elsaesser R and Paysan J, The sense of smell, its signalling pathways, and the dichotomy of cilia and microvilli in olfactory sensory cells. BMC Neurosci, 2007. 8 Suppl 3(Suppl 3): p. S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Runge KE, et al. , Oocyte CD9 is enriched on the microvillar membrane and required for normal microvillar shape and distribution. Dev Biol, 2007. 304(1): p. 317–25. [DOI] [PubMed] [Google Scholar]
- 81.Ivetič A, et al. , Mutagenesis of the Ezrin-Radixin-Moesin Binding Domain of L-selectin Tail Affects Shedding, Microvillar Positioning, and Leukocyte Tethering*. Journal of Biological Chemistry, 2004. 279(32): p. 33263–33272. [DOI] [PubMed] [Google Scholar]
- 82.von Andrian UH, et al. , A central role for microvillous receptor presentation in leukocyte adhesion under flow. Cell, 1995. 82(6): p. 989–999. [DOI] [PubMed] [Google Scholar]
- 83.Yanagimachi R, Chapter 4 Sperm-Egg Association in Mammals, in Current Topics in Developmental Biology, Moscona AA and Monroy A, Editors. 1978, Academic Press. p. 83–105. [PubMed] [Google Scholar]
- 84.Shalgi R and Phillips DM, Mechanics of in vitro fertilization in the hamster. Biol Reprod, 1980. 23(2): p. 433–44. [DOI] [PubMed] [Google Scholar]
- 85.Yanagimachi R and Noda YD, Ultrastructural changes in the hamster sperm head during fertilization. Journal of Ultrastructure Research, 1970. 31(5): p. 465–485. [DOI] [PubMed] [Google Scholar]
- 86.Singer II, et al. , CCR5, CXCR4, and CD4 are clustered and closely apposed on microvilli of human macrophages and T cells. J Virol, 2001. 75(8): p. 3779–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jung Y, et al. , Three-dimensional localization of T-cell receptors in relation to microvilli using a combination of superresolution microscopies. Proc Natl Acad Sci U S A, 2016. 113(40): p. E5916–e5924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim HR, et al. , T cell microvilli constitute immunological synaptosomes that carry messages to antigen-presenting cells. Nat Commun, 2018. 9(1): p. 3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ghosh S, et al. , ERM-Dependent Assembly of T Cell Receptor Signaling and Co-stimulatory Molecules on Microvilli prior to Activation. Cell Rep, 2020. 30(10): p. 3434–3447.e6. [DOI] [PubMed] [Google Scholar]
- 90.Cai E, et al. , Visualizing dynamic microvillar search and stabilization during ligand detection by T cells. Science, 2017. 356(6338): p. eaal3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Carpentier JL, et al. , Surface redistribution of 125I-insulin in cultured human lymphocytes. J Cell Biol, 1981. 91(1): p. 17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hasslen SR, et al. , Spatial distribution of L-selectin (CD62L) on human lymphocytes and transfected murine L1–2 cells. Histochem J, 1995. 27(7): p. 547–54. [PubMed] [Google Scholar]
- 93.Marzesco AM, et al. , Release of extracellular membrane particles carrying the stem cell marker prominin-1 (CD133) from neural progenitors and other epithelial cells. J Cell Sci, 2005. 118(Pt 13): p. 2849–58. [DOI] [PubMed] [Google Scholar]
- 94.Marzesco AM, et al. , Release of extracellular membrane vesicles from microvilli of epithelial cells is enhanced by depleting membrane cholesterol. FEBS Lett, 2009. 583(5): p. 897–902. [DOI] [PubMed] [Google Scholar]
- 95.Hurbain I, et al. , Microvilli-derived extracellular vesicles carry Hedgehog morphogenic signals for Drosophila wing imaginal disc development. Current Biology, 2022. 32(2): p. 361–373.e6. [DOI] [PubMed] [Google Scholar]
- 96.Schuldiner O and Yaron A, Mechanisms of developmental neurite pruning. Cellular and molecular life sciences : CMLS, 2015. 72(1): p. 101–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vanderhaeghen P and Cheng H-J, Guidance molecules in axon pruning and cell death. Cold Spring Harbor perspectives in biology, 2010. 2(6): p. a001859–a001859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kanamori T, et al. , Local endocytosis triggers dendritic thinning and pruning in Drosophila sensory neurons. Nat Commun, 2015. 6: p. 6515. [DOI] [PubMed] [Google Scholar]
- 99.Bishop DL, et al. , Axon branch removal at developing synapses by axosome shedding. Neuron, 2004. 44(4): p. 651–61. [DOI] [PubMed] [Google Scholar]
- 100.Francis SS, et al. , A hierarchy of signals regulates entry of membrane proteins into the ciliary membrane domain in epithelial cells. Journal of Cell Biology, 2011. 193(1): p. 219–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Antonyak MA, Wilson KF, and Cerione RA, R(h)oads to microvesicles. Small GTPases, 2012. 3(4): p. 219–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Betapudi V, et al. , Anti-β2GPI antibodies stimulate endothelial cell microparticle release via a nonmuscle myosin II motor protein-dependent pathway. Blood, 2013. 122(23): p. 3808–3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hurley JH, ESCRTs are everywhere. Embo j, 2015. 34(19): p. 2398–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Booth AM, et al. , Exosomes and HIV Gag bud from endosome-like domains of the T cell plasma membrane. J Cell Biol, 2006. 172(6): p. 923–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Colombo M, et al. , Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J Cell Sci, 2013. 126(Pt 24): p. 5553–65. [DOI] [PubMed] [Google Scholar]
- 106.Patnaik A, Chau V, and Wills JW, Ubiquitin is part of the retrovirus budding machinery. Proc Natl Acad Sci U S A, 2000. 97(24): p. 13069–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Strack B, et al. , A role for ubiquitin ligase recruitment in retrovirus release. Proc Natl Acad Sci U S A, 2000. 97(24): p. 13063–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Baumgärtel V, et al. , Live-cell visualization of dynamics of HIV budding site interactions with an ESCRT component. Nat Cell Biol, 2011. 13(4): p. 469–74. [DOI] [PubMed] [Google Scholar]
- 109.Jouvenet N, et al. , Dynamics of ESCRT protein recruitment during retroviral assembly. Nat Cell Biol, 2011. 13(4): p. 394–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Langelier C, et al. , Human ESCRT-II complex and its role in human immunodeficiency virus type 1 release. Journal of virology, 2006. 80(19): p. 9465–9480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chiaruttini N, et al. , Relaxation of Loaded ESCRT-III Spiral Springs Drives Membrane Deformation. Cell, 2015. 163(4): p. 866–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Long H, et al. , Comparative Analysis of Ciliary Membranes and Ectosomes. Current Biology, 2016. 26(24): p. 3327–3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Forsythe E and Beales PL, Bardet-Biedl syndrome. European journal of human genetics : EJHG, 2013. 21(1): p. 8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zaghloul NA and Katsanis N, Mechanistic insights into Bardet-Biedl syndrome, a model ciliopathy. J Clin Invest, 2009. 119(3): p. 428–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Daniels AB, et al. , Genotype-phenotype correlations in Bardet-Biedl syndrome. Arch Ophthalmol, 2012. 130(7): p. 901–7. [DOI] [PubMed] [Google Scholar]
- 116.Jin H, et al. , The conserved Bardet-Biedl syndrome proteins assemble a coat that traffics membrane proteins to cilia. Cell, 2010. 141(7): p. 1208–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nachury MV, et al. , A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell, 2007. 129(6): p. 1201–13. [DOI] [PubMed] [Google Scholar]
- 118.Loktev AV, et al. , A BBSome subunit links ciliogenesis, microtubule stability, and acetylation. Dev Cell, 2008. 15(6): p. 854–65. [DOI] [PubMed] [Google Scholar]
- 119.Lechtreck KF, et al. , Cycling of the signaling protein phospholipase D through cilia requires the BBSome only for the export phase. J Cell Biol, 2013. 201(2): p. 249–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.McIntyre JC, Hege MM, and Berbari NF, Trafficking of ciliary G protein-coupled receptors. Methods Cell Biol, 2016. 132: p. 35–54. [DOI] [PubMed] [Google Scholar]
- 121.Patnaik SR, et al. , Bardet-Biedl Syndrome proteins regulate cilia disassembly during tissue maturation. Cell Mol Life Sci, 2019. 76(4): p. 757–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hernandez-Hernandez V, et al. , Bardet-Biedl syndrome proteins control the cilia length through regulation of actin polymerization. Hum Mol Genet, 2013. 22(19): p. 3858–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Akella JS, et al. , Ciliary Rab28 and the BBSome negatively regulate extracellular vesicle shedding. eLife, 2020. 9: p. e50580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Theos AC, et al. , A lumenal domain-dependent pathway for sorting to intralumenal vesicles of multivesicular endosomes involved in organelle morphogenesis. Dev Cell, 2006. 10(3): p. 343–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.van Niel G, et al. , The tetraspanin CD63 regulates ESCRT-independent and -dependent endosomal sorting during melanogenesis. Dev Cell, 2011. 21(4): p. 708–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.McConnell RE, et al. , The enterocyte microvillus is a vesicle-generating organelle. J Cell Biol, 2009. 185(7): p. 1285–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kaji K, et al. , The gamete fusion process is defective in eggs of Cd9-deficient mice. Nat Genet, 2000. 24(3): p. 279–82. [DOI] [PubMed] [Google Scholar]
- 128.Miyado K, et al. , The fusing ability of sperm is bestowed by CD9-containing vesicles released from eggs in mice. Proc Natl Acad Sci U S A, 2008. 105(35): p. 12921–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Israels SJ and McMillan-Ward EM, Platelet tetraspanin complexes and their association with lipid rafts. Thromb Haemost, 2007. 98(5): p. 1081–7. [PubMed] [Google Scholar]
- 130.Brisson C, et al. , Co-localization of CD9 and GPIIb-IIIa (alpha IIb beta 3 integrin) on activated platelet pseudopods and alpha-granule membranes. Histochem J, 1997. 29(2): p. 153–65. [DOI] [PubMed] [Google Scholar]
- 131.Khattree N, Ritter LM, and Goldberg AFX, Membrane curvature generation by a C-terminal amphipathic helix in peripherin-2/rds, a tetraspanin required for photoreceptor sensory cilium morphogenesis. Journal of Cell Science, 2013. 126(20): p. 4659–4670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Murru L, et al. , Tetraspanins shape the synapse. Mol Cell Neurosci, 2018. 91: p. 76–81. [DOI] [PubMed] [Google Scholar]
- 133.Termini CM and Gillette JM, Tetraspanins Function as Regulators of Cellular Signaling. Frontiers in Cell and Developmental Biology, 2017. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Beer KB, et al. , Extracellular vesicle budding is inhibited by redundant regulators of TAT-5 flippase localization and phospholipid asymmetry. Proc Natl Acad Sci U S A, 2018. 15(6): p. E1127–e1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wehman AM, et al. , The P4-ATPase TAT-5 inhibits the budding of extracellular vesicles in C. elegans embryos. Curr Biol, 2011. 21(23): p. 1951–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Stöhr H, et al. , TMEM16B, a novel protein with calcium-dependent chloride channel activity, associates with a presynaptic protein complex in photoreceptor terminals. J Neurosci, 2009. 29(21): p. 6809–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Suzuki J, et al. , Calcium-dependent phospholipid scrambling by TMEM16F. Nature, 2010. 468(7325): p. 834–8. [DOI] [PubMed] [Google Scholar]
- 138.Coleman JA, Kwok MC, and Molday RS, Localization, purification, and functional reconstitution of the P4-ATPase Atp8a2, a phosphatidylserine flippase in photoreceptor disc membranes. J Biol Chem, 2009. 284(47): p. 32670–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Menon I, et al. , Opsin is a phospholipid flippase. Curr Biol, 2011. 21(2): p. 149–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Segawa K and Nagata S, An Apoptotic ‘Eat Me’ Signal: Phosphatidylserine Exposure. Trends Cell Biol, 2015. 25(11): p. 639–650. [DOI] [PubMed] [Google Scholar]
- 141.Strick DJ, Feng W, and Vollrath D, Mertk drives myosin II redistribution during retinal pigment epithelial phagocytosis. Invest Ophthalmol Vis Sci, 2009. 50(5): p. 2427–35. [DOI] [PubMed] [Google Scholar]
- 142.Batista FD, Iber D, and Neuberger MS, B cells acquire antigen from target cells after synapse formation. Nature, 2001. 411(6836): p. 489–94. [DOI] [PubMed] [Google Scholar]
- 143.Joly E and Hudrisier D, What is trogocytosis and what is its purpose? Nature Immunology, 2003. 4(9): p. 815–815. [DOI] [PubMed] [Google Scholar]
- 144.Bettadapur A, et al. , Biting Off What Can Be Chewed: Trogocytosis in Health, Infection, and Disease. Infection and Immunity. 88(7): p. e00930–19. [DOI] [PMC free article] [PubMed] [Google Scholar]


