Abstract
Background:
Prenatal alcohol exposure (PAE), leading to fetal alcohol spectrum disorders (FASD), is a serious public health issue in the U.S. and globally. Diagnosis of FASD is crucial in obtaining appropriate care but is not always possible when PAE cannot be documented.
Methods:
Deciduous teeth from a child with known PAE and a child with known absence of PAE were analyzed using liquid chromatography-isotope dilution tandem mass spectrometry (LC-IDMS/MS) in a multiple-reaction monitoring mode for direct markers and LC-high resolution MS in positive and negative mode with hydrophilic interaction liquid chromatography and reverse phase chromatography, respectively, for indirect markers.
Results:
Direct markers of PAE (ethyl glucuronide and ethyl sulfate) were detected in pre- and postnatal dentine from a case tooth but not from a control tooth. Indirect biomarker analysis indicated a dysregulation of amino acids and an increase in cholesterol sulfate in the case compared to the control tooth.
Conclusions:
This proof-of-concept study demonstrates for the first time that direct biomarkers of prenatal alcohol exposure are detectable and measurable in deciduous teeth which begin forming in utero and are typically naturally shed between 5 and 12 years of age. Further examination of these novel biomarkers may allow diagnosis of FASD where documentation of PAE is otherwise unavailable. Furthermore, because teeth grow incrementally, defined growth zones can be sampled allowing for identification of gestational timing of PAE to help better understand mechanisms underlying alcohol’s disruption of perinatal development.
Keywords: alcohol, biomarkers, prenatal, fetal alcohol spectrum disorder (FASD)
Introduction
Alcohol (ethanol) has been recognized as a human teratogen for nearly 50 years. Prenatal alcohol exposure (PAE) results in diverse conditions that are collectively referred to as fetal alcohol spectrum disorders (FASD) (Bertrand et al., 2005). FASD is the leading known cause of developmental disabilities and represents a major, costly public health issue (S. Popova et al., 2012; Svetlana Popova et al., 2015). A recent national prevalence study estimated that conservatively 1 in 20, and possibly as many as 1 in 10, elementary school aged children in the U.S. may be affected by FASD (May et al., 2018).
Accurate diagnosis is critical to obtaining effective, timely treatment and support, optimizing the developmental trajectory of a child, and avoiding negative secondary outcomes (A. P. Streissguth et al., 2004; Chasnoff et al., 2015). When the characteristic facial dysmorphology of fetal alcohol syndrome (FAS) is not present, documentation of PAE is often required for diagnosis. Children without the cardinal facial features associated with FAS represent a far greater portion of those with FASD than children with cardinal features. Neurobehavioral deficits may be similar in severity regardless of dysmorphology and persist throughout the life of the person with FASD (Mattson et al., 2019; Mattson et al., 1998; Mattson et al., 2010). In addition, these children may be at higher risk for negative secondary disabilities due to missed diagnosis and failure to access appropriate treatment as well as lack of recognition of limitations by persons interacting with them (Ann P Streissguth et al., 2004).
Documentation of PAE occurs primarily by maternal self-report and, less frequently, by indirect evidence, e.g., collateral report, or court or medical records. There are situations where neither direct nor indirect evidence is available. Where information on PAE cannot be obtained and there is no facial dysmorphology, no FASD diagnosis can be made according to current diagnostic criteria (Hoyme et al., 2016). Consequently, children may fail to receive appropriate treatment and services. A sensitive, retrospective, objective marker of PAE may allow for an FASD diagnosis in situations where it might otherwise not be possible.
At present, it is difficult to clearly link the exact gestational timing and magnitude of PAE with specific outcomes as reliable information on quantity and frequency of PAE is lacking. This hampers research related to the etiology, pathology, and modifying factors of FASD, vulnerable periods of development, and potential interventions.
In addition to the direct and indirect methods of PAE detection mentioned above, in a minority of cases, biomarkers have been used to capture maternal exposure from a woman’s blood, urine, hair, or nails, or from the infant’s meconium captured at birth. Biomarkers currently in use include ethanol itself (EtOH), fatty acid ethyl esters (FAEEs), ethyl glucuronide (EtG), ethyl sulfate (EtS), and phosphatidylethanol (PEth) (Cabarcos et al., 2015). The amount of time following consumption of alcohol where markers may detect the exposure varies not only by marker and the maternal matrices sampled, but by amount consumed, genetics, and blood volume (Cabarcos et al., 2015). Markers measured in maternal blood, plasma, and urine reflect a window of hours or days (EtOH, EtG, EtS, FAEE) or, at best, 4–6 weeks (PEth) (Montag, 2016). To identify exposures throughout as much of the pregnancy as possible, even the most sensitive maternal biomarkers currently available are insufficient. Meconium and newborn hair may be able to detect heavy PAE over the last 16 weeks of pregnancy but there are barriers to obtaining these samples when they are available, and they are unlikely to be available at the time a child is likely to be recognized as having neurodevelopmental deficits or otherwise having potentially been affected by PAE. In addition to these limitations, there are less than optimal negative predictive values (NPV) and positive predictive values (PPV) for any one or combination of these biomarkers. As a result, none of the prenatal biomarkers presently available are sufficiently robust to be routinely employed in clinical practice. Importantly, to date, there has been no biomarker developed that is readily accessible, non-invasive, does not require maternal report, and can accurately reflect PAE that occurred many years previously. Biomarkers in deciduous teeth may be one way to approach this.
Tooth development begins in the fetus early in the second trimester. Enamel and dentine begin to mineralize and are deposited incrementally, like growth rings in a tree (Figure 1). Circulating substances are trapped as growth layers mineralize, creating an archive of exposures. At birth, an accentuated incremental growth layer is formed, known as the neonatal line. This accentuated layer provides a time stamp upon which the age of other growth layers can be determined. Unlike bone, teeth do not substantially remodel (Gulson & Gillings, 1997) and archived chemicals are preserved with remarkable stability in the hydroxyapatite matrix, with some proteins able to be extracted from teeth after thousands of years (Schmidt-Schultz & Schultz, 2007). The gradual, stable deposition and growth lines allow identification of specific prenatal and postnatal periods. Deciduous, or baby, teeth are naturally shed by children when they are between 5 and 12 years old; an age when a diagnosis of FASD may markedly improve a child’s developmental trajectory.
Figure 1. Key aspects of tooth development.
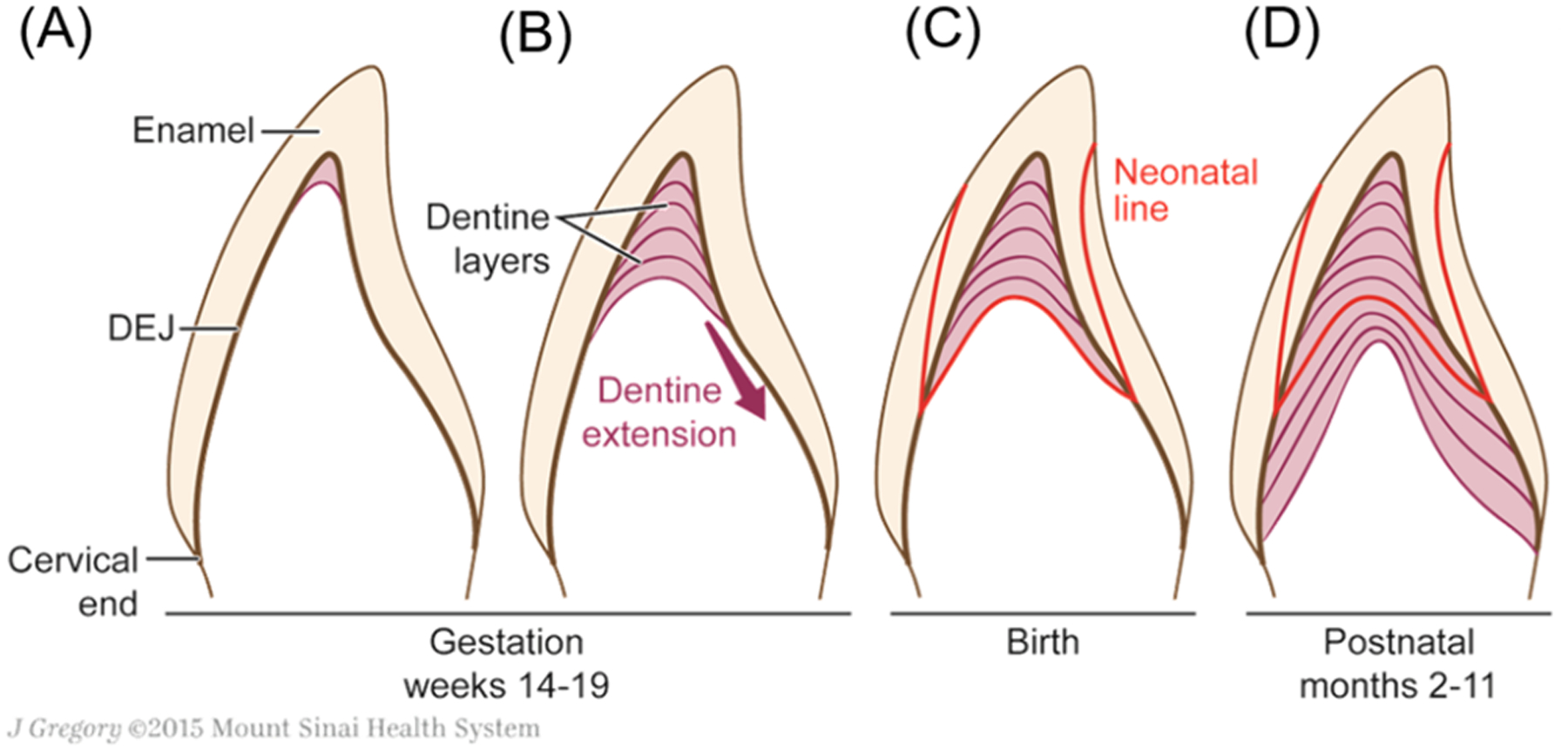
Tooth mineralization begins early in the second trimester, with completion of the enamel covered crown occurring 3–12 months after birth, depending on the tooth type. The neonatal line, a histological landmark visible in teeth, can be used to determine the timing of growth layers throughout the crown (Nina Sabel et al., 2008). Reprinted with permission (Morishita & Arora, 2017).
Teeth have been used for decades to measure cumulative exposure to metals such as lead, manganese, and other heavy metals. Organic chemicals have also been measured including antibiotics(Kanjanawattana et al., 2001; Schussl et al., 2014), recreational drugs(Pellegrini et al., 2006; Zeren et al., 2013; Cattaneo et al., 2003), and tobacco(Pascual et al., 2003; Garcia-Algar et al., 2003; Marchei et al., 2008) (reviewed in Andra et al.(Andra et al., 2015)). One alcohol biomarker has previously been measured in dental tissue: EtG was measured in whole pulverized permanent adult teeth and correlated with alcohol consumption derived from the Michigan Alcohol Screening Test (MAST) (Zeren et al., 2013). We have been developing methods that require much smaller amounts of tooth powder for organic chemical extraction that allow us to exploit the temporal information in teeth to provide timing of exposure markers measured (Andra, Austin, Wright, et al., 2015; Yu et al., 2021). In the present paper, this includes measurements at a temporal resolution to separate pre- and postnatal exposure. Our study provides the first evidence that biomarkers of alcohol exposure can be measured in deciduous (baby) teeth reflecting prenatal exposure.
Methods
This study utilized a case-control design. A deciduous tooth was analyzed from a child with known PAE (case) and compared to a deciduous tooth with known absence of PAE (unexposed control).
This study was approved by the University of California at San Diego (UCSD) Institutional Review Board.
Sample source & Recruitment
Two children 8–10 years of age, with and without an FASD diagnosis, and their primary guardians/parents were recruited by flyers posted on the FASD United Facebook page and by word of mouth. The parents/guardians contacted the study, were consented by telephone after which they were sent a packet with a parent consent form, a child assent form for children 7 years of age or older, a small envelope for tooth submission, a small gift for the child, and a stamped return envelope. As part of completing these forms, parents/guardians were asked to confirm the FASD diagnosis of the child. The information and samples were stored under unique project identification numbers with no personal identifiers. Samples were shipped to the Icahn School of Medicine at Mount Sinai for analysis.
Biomarker methods
Analysis of direct and indirect alcohol biomarkers were carried out at the Senator Frank R. Lautenberg Environmental Health Sciences Laboratory at the Icahn School of Medicine at Mount Sinai. Teeth were washed and sectioned in a vertical (labio-lingual/bucco-lingual) plane. To identify pre- and postnatal temporal developmental zones, the neonatal line was identified (Figure 1) and using a custom-built robot (Basque Engineering + Science Inc., MA, USA), dentine was micro-dissected from the pre- and postnatal regions of the tooth crown. Tooth powder mass collected ranged from 1.69 – 6.63 mg. Unlabeled ethyl-β-D-glucuronide (EtG) (product # E-016-1mL), ethyl sulfate (EtS) (product # E-064-1mL), and the corresponding labeled internal standard d5-EtG (isotopic purity: 98%, product # E-063-1mL) and d5-EtS (isotopic purity: 98%, product # E-066-1mL) were obtained from Cambridge Isotope Laboratories, Inc. (Andover, MA, USA). Organic chemicals were extracted using a one-step liquid-liquid extraction (LLE) with dichloromethane and diethyl ether (1:1, v/v). In brief, tooth powder aliquots were spiked with a labelled internal standards mixture to achieve a 10 ng/mL concentration in a 500 μL LLE extractant mixture volume. The tooth reagents mixture was sonicated for 2 hours at 25°C, extracted overnight at −20°C, centrifuged at 14000 rpm for 15 min., and the supernatant organic phase was collected and evaporated to dryness prior to reconstitution in 100 μL of acetonitrile and methanol (1:1, v/v). A two-pronged approach to biomarker development was employed: targeted analysis to detect direct biomarkers and untargeted analysis to identify indirect biomarkers. These biomarkers in neonatal tissues have been shown to correlate with maternal reports of alcohol consumption and measures in maternal tissues (Himes et al., 2015; Bakhireva et al., 2014; Cabarcos et al., 2015). Our second approach was to develop an untargeted analysis of prenatal tooth material to identify additional biomarkers of alcohol exposure. These included indirect biomarkers of prenatal alcohol exposure that reflect biological response to the toxic effects of alcohol to different organ systems. A combination of biomarkers may improve specificity and sensitivity of prenatal alcohol exposure determination (Montag, 2016; Bager et al., 2017;). For targeted analysis, extract aliquots were analyzed by liquid chromatography-isotope dilution tandem mass spectrometry (LC-IDMS/MS) in a multiple-reaction monitoring mode (MRM). A Shimadzu Nexera XR HPLC was used for the chromatographic separation of EtG and EtS on a Hypersil Gold AQ, 3 μm, 3.0 × 150 mm analytical column with 4.0 × 10 mm guard column (Thermo Scientific, Waltham, MA, USA). Mobile phase A consisted of 0.1% acetic acid in water, and mobile phase B was acetonitrile and methanol (1:1, v/v). A flow rate of 0.5 mL/min was used with the following gradient program: 0.0–1.0 min (5% B), 3.0 min (50% B), 3.0–5.0 min (50% B), 6.0 min (95% B), 6.0–7.0 min (95% B), 7.0–8.0 min (5% B), and 11.0 min (5% B). The injection volume was 20 μL. A Sciex 6500 triple quadrupole mass spectrometer equipped with electrospray ionization (ESI) source (SCIEX, Framingham, MA, USA) was operated in negative ionization mode for the detection and quantitation of EtG and EtS. Nitrogen was used as curtain and collision gas. Multiple-reaction monitoring mode (MRM) was used for data acquisition of each target analyte. Most prominent ion transition was used for quantitation and the most intense second ion transition was used for confirmation. The MRM transitions were 221→75, 85 for EtG, 226→75, 85 for d5-EtG, 125→80, 97 for EtS and 130→80, 98 for d5-EtS. For untargeted analysis, the same extract aliquots were analyzed by LC-high resolution MS in positive and negative mode with hydrophilic interaction liquid chromatography (HILIC) and reverse phase chromatography, respectively. Samples were analyzed in a randomized run order. Instrumental conditions are described in detail elsewhere (Niedzwiecki et al., 2021). All sample analyses included calibration standards for quantification of EtS and EtG and quality control samples (internal standards, blanks, pooled samples, replicates, etc.). Metabolites were identified based upon in-house database matching considering retention time, accurate mass, and MS/MS matching (when available) with analytical standards analyzed under the same conditions, providing the highest identification confidence level (Level 1 or 2) based on Metabolomics Standards Initiative criteria (Sumner et al., 2007).
Test methods did not include genetic analysis.
Results
One tooth from an individual diagnosed with FASD and one tooth collected from a control child were analyzed for biomarkers of PAE. The case tooth had documented PAE and a diagnosis of FAS.
The targeted approach detected fetal alcohol exposure biomarkers in the pre- (0.02 EtG, 0.007 EtS ng/mg) and post-natal dentine (0.05 EtG, 0.03 EtS ng/mg) collected from the case tooth (Figure 2). Neither biomarker was detected in the pre- or post-natal dentine of the control tooth. The limits of detection (LOD) were calculated as three times the standard deviation of ten replicate analyses of matrix tooth QC pool blank spiked with 0.1 ng/mL of EtG and EtS, and determined as 0.001 ng/mg for EtG and 0.005 ng/mg for EtS.
Figure 2. Chromatograms of Direct Biomarkers EtG and EtS in tooth powder.
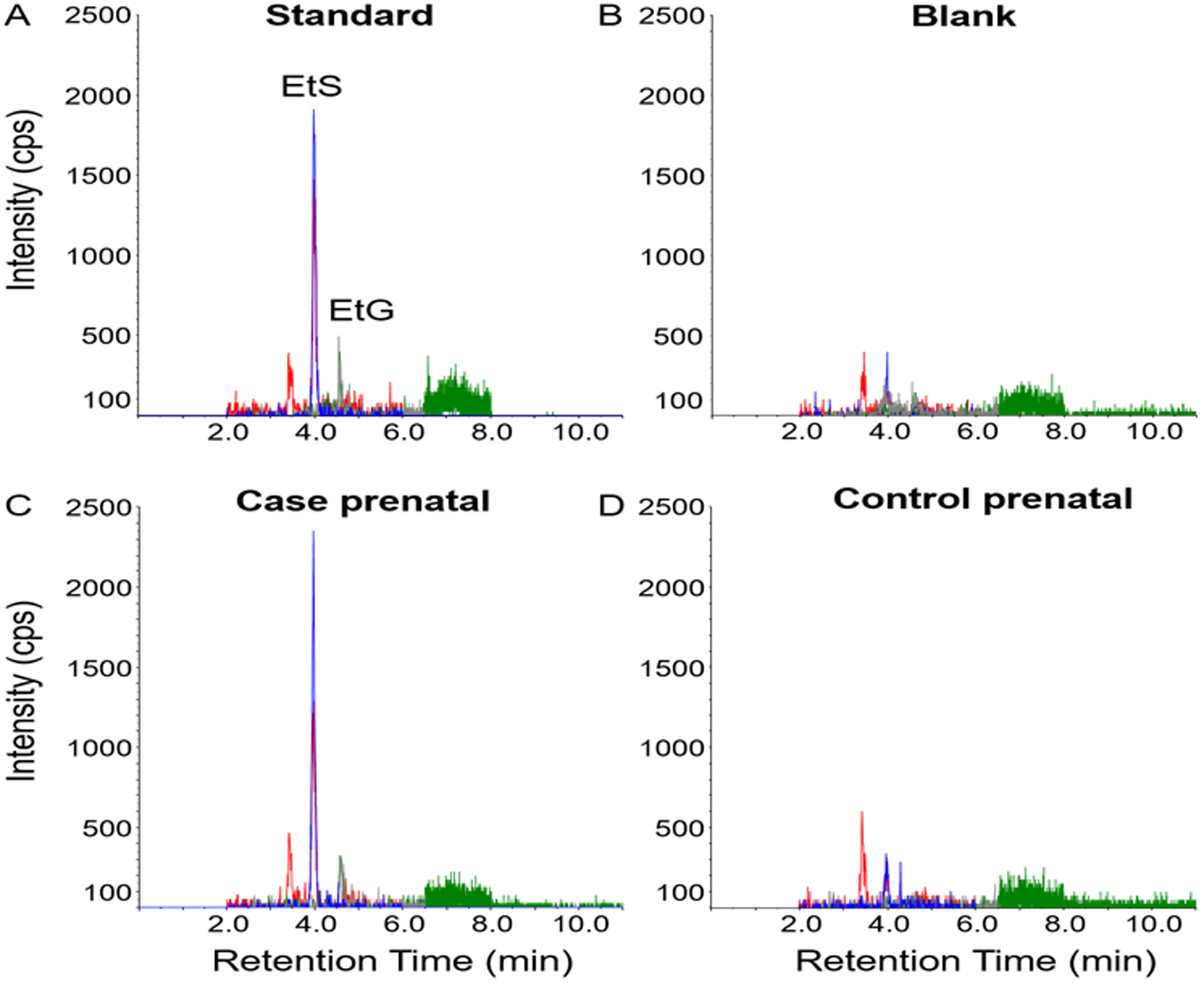
Extracted ion chromatograms from LC-IDMS/MS analysis of (A) EtG and EtS standard (0.1 ng/mL), (B) reagent blank, (C) extract fraction from prenatal dentine collected from case tooth, and (D) extract fraction from prenatal dentine from control tooth.
For the untargeted approach, results indicated a dysregulation of amino acids in the FASD tooth compared to the control, as well as an increase in cholesterol sulfate (Table 1). To assure the highest confidence, analysis was limited to metabolites identified from our in-house analytical standard library.
Table 1.
Untargeted analysis of Indirect Biomarkers of PAE.
| Compound | Exact Mass (Da) | Prenatal (case:control) |
Postnatal (case:control) |
|---|---|---|---|
| Inosine a | 268.0808 | 0.515 | 0.560 |
| Isoleucine a | 131.0946 | 2.64 | 3.65 |
| Tryptophan a | 204.0899 | 8.57 | 5.94 |
| Alanine a | 89.0477 | 1.93 | 2.02 |
| Serine a | 105.0426 | 4.36 | 3.81 |
| Histidine a | 155.0695 | 5.96 | 8.02 |
| Cholesterol sulfate b | 466.3117 | 26.6 | 8.88 |
Metabolite average intensity ratio in case vs control dentine fractions in duplicate injections.
HILIC positive mode,
reverse phase negative mode.
Discussion
The pilot work presented here demonstrates for the first time that it is possible to detect direct and indirect biomarkers of PAE from deciduous teeth. Detection of PAE using biomarkers in deciduous teeth will allow for diagnosis of FASD where this may otherwise have been impossible and, consequently, support access to appropriate treatment and interventions for affected children to optimize their developmental trajectories.
Similar to the only other study to measure direct biomarkers of alcohol exposure in dental tissue, pulverized permanent adult teeth (Zeren et al., 2013), we report that EtG can be measured in deciduous teeth. We also show that EtS, another direct biomarker of alcohol exposure can be measured. Our indirect biomarker data demonstrate the ability to measure potential tools for exploring the physiological response to alcohol exposure. Importantly, our method has high sensitivity enabling a much smaller amount of material, as low as 1.7 mg prenatal tooth powder, to be used compared with 50 mg powder collected from the whole tooth. Therefore, this technique can leverage the temporal information available in teeth to assign timing of pre- and postnatal exposure within deciduous teeth.
Measurement of exposure magnitude as well as gestational and postnatal timing of exposures will enhance understanding of prenatal development and facilitate research into the mechanisms of PAE developmental effects. In the present paper, we document the presence of biomarkers in both prenatal and postnatal dentine. Postnatal alcohol exposure generally occurs via breastfeeding but may occur by other means such as medications. It has been associated with negative child outcomes(May et al., 2016). Because developmental processes often occur sequentially with few opportunities to revisit processes disrupted by exposures, the gestational timing of exposure is critical. Time-sensitive windows of some exposures are well delineated. For example, the teratogenicity of thalidomide occurs between 20 and 36 days after fertilization (Vargesson, 2015). Other critical periods of development, such as those for specific neurobehavioral effects, are more difficult to study. FASD is principally a brain-based disorder and the brain develops throughout pregnancy and continues to develop postnatally. In the prenatal period, the second and third trimesters are primarily devoted to brain development and growth. The disruption of development by PAE may prove to be uniquely complex as it may occur by direct and indirect mechanisms, at myriad timepoints during the entire pregnancy. Our technique that retains fetal exposure timing while providing both exposure measures (Figure 1) as well as biological response measures (Table 1) will help unravel these complex mechanisms.
Strengths of this feasibility analysis include the known PAE status of the “case” and known lack of PAE of the “control” teeth with clear, consistent signals detected in the case tooth. The ability to measure both direct and indirect biomarkers using the same samples is a strength that may contribute to a greater understanding of the interplay between exposures and metabolic networks.
Limitations of this approach to detection of PAE is the inability to identify alcohol exposure that occurs in the first trimester of pregnancy. While these pilot data are limited to analysis of only two deciduous teeth, in view of the urgency of disseminating the finding that it is possible to detect PAE in deciduous teeth, this data is submitted for publication. The sample size is being augmented with recruitment from ongoing studies conducted within the Collaborative Initiative on Fetal Alcohol Spectrum Disorders (CIFASD) (www.cifasd.org) where participants have well-characterized FASD diagnoses and neurodevelopmental outcome measures. The goal of this work will be to determine the sensitivity and specificity of these direct and indirect biomarkers and to assess associations among the magnitude and gestational timing of PAE with neurobehavioral deficits.
Future directions include work currently underway to further validate these novel biomarkers, increase sensitivity to detect lower levels of PAE, associate detected exposures with outcomes, and explore potential interactions with co-exposures. Co-exposures of interest at this time include cannabis, tobacco, and opioids.
Novel, sensitive, quantitative, unbiased, non-invasively obtained biomarkers of PAE may be helpful in obtaining diagnoses of FASD where otherwise not possible and in fine-tuning clinical treatment paradigms. They may furthermore enhance understanding of FASD through applications in research.
Conclusions
These preliminary results demonstrate the feasibility of measuring biomarkers of PAE from naturally shed primary teeth. Direct biomarkers measured the presence and absence of PAE in tooth samples. Indirect biomarker analysis supported dysregulation of amino acids and an increase in cholesterol sulfate with PAE compared to the absence of PAE.
Acknowledgements
The authors thank the participants, primarily from FASD United which was previously called NOFAS and from the UCSD FASD Registry, who generously and enthusiastically provided samples. The authors would also like to thank Georgia Dolios, Ravi Jagani, Divya Pulivarthi and Jyoti Chumber for technical laboratory support. All or part of this work was done in conjunction with the Collaborative Initiative on Fetal Alcohol Spectrum Disorders (CIFASD), which is funded by grants from the National Institute on Alcohol Abuse and Alcoholism (NIAAA). Additional information about CIFASD can be found at www.cifasd.org. This work was supported by the National Institutes of Health from grants awarded by the National Institute on Alcohol Abuse and Alcoholism (UH2AA029062 ACM, CA), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (R00HD087523, CA) and the National Institute of Environmental Health Sciences (P30ES023515, CA, SSA, LMP, MA; R01ES031117, LMP; R21ES030882, LMP, SSA; U2CES030859, MA, LMP, CA; R35ES030435, R01ES026033, MA).
Reference List
- Andra SS, Austin C and Arora M (2015) ‘Tooth matrix analysis for biomonitoring of organic chemical exposure: Current status, challenges, and opportunities.’ Environmental Research, 142, 10//, pp. 387–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bager H, Christensen LP, Husby S and Bjerregaard L (2017) ‘Biomarkers for the Detection of Prenatal Alcohol Exposure: A Review.’ Alcoholism: Clinical and Experimental Research, 41(2) pp. 251–261. [DOI] [PubMed] [Google Scholar]
- Bakhireva LN, Leeman L, Savich RD, Cano S, Gutierrez H, Savage DD and Rayburn WF (2014) ‘The validity of phosphatidylethanol in dried blood spots of newborns for the identification of prenatal alcohol exposure.’ Alcohol Clin Exp Res, 38(4), Apr, pp. 1078–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand J, Floyd RL and Weber MK (2005) Mortality and Morbidity Weekly Report, 54(RR 11) p. 1. [PubMed] [Google Scholar]
- Cabarcos P, Álvarez I, Tabernero MJ and Bermejo AM (2015) ‘Determination of direct alcohol markers: a review.’ Analytical and bioanalytical chemistry, 407(17) pp. 4907–4925. [DOI] [PubMed] [Google Scholar]
- Cattaneo C, Gigli F, Lodi F and Grandi M (2003) ‘The detection of morphine and codeine in human teeth: an aid in the identification and study of human skeletal remains.’ Journal of Forensic Odonto-Stomatology, 21(1), Jun, pp. 1–5. [PubMed] [Google Scholar]
- Chasnoff IJ, Wells AM and King L (2015) ‘Misdiagnosis and missed diagnoses in foster and adopted children with prenatal alcohol exposure.’ Pediatrics, 135(2) pp. 264–270. [DOI] [PubMed] [Google Scholar]
- Garcia-Algar O, Vall O, Segura J, Pascual JA, Diaz D, Mutnoz L, Zuccaro P, Pacifici R and Pichini S (2003) ‘Nicotine concentrations in deciduous teeth and cumulative exposure to tobacco smoke during childhood.’ The Journal of the American Medical Association, 290(2), July 9, 2003, pp. 196–197. [DOI] [PubMed] [Google Scholar]
- Gulson BL and Gillings BR (1997) ‘Lead exchange in teeth and bone--a pilot study using stable lead isotopes.’ Environmental Health Perspectives, 105(8) pp. 820–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himes SK, Dukes KA, Tripp T, Petersen JM, Raffo C, Burd L, Odendaal H, Elliott AJ, Hereld D, Signore C, Willinger M and Huestis MA (2015) ‘Clinical sensitivity and specificity of meconium fatty acid ethyl ester, ethyl glucuronide, and ethyl sulfate for detecting maternal drinking during pregnancy.’ Clin Chem, 61(3), Mar, pp. 523–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyme HE, Kalberg WO, Elliott AJ, Blankenship J, Buckley D, Marais A-S, Manning MA, Robinson LK, Adam MP and Abdul-Rahman O (2016) ‘Updated clinical guidelines for diagnosing fetal alcohol spectrum disorders.’ Pediatrics, 138(2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanjanawattana S, Mangkornkarn C, Wilairat P and Vongsavan N (2001) ‘Determination of lidocaine in dental pulp by high-performance liquid chromatography.’ J Endod, 27(1), Jan, pp. 31–35. [DOI] [PubMed] [Google Scholar]
- Marchei E, Joya X, Garcia-Algar O, Vall O, Pacifici R and Pichini S (2008) ‘Ultrasensitive detection of nicotine and cotinine in teeth by high-performance liquid chromatography/tandem mass spectrometry.’ Rapid Communications in Mass Spectrometry, 22(16), Aug, pp. 2609–2612. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Bernes GA and Doyle LR (2019) ‘Fetal alcohol spectrum disorders: a review of the neurobehavioral deficits associated with prenatal alcohol exposure.’ Alcoholism: Clinical and Experimental Research, 43(6) pp. 1046–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Riley EP, Gramling L, Delis DC and Jones KL (1998) ‘Neuropsychological comparison of alcohol-exposed children with or without physical features of fetal alcohol syndrome.’ Neuropsychology, 12(1) p. 146. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Roesch SC, Fagerlund Å, Autti-Rämö I, Jones KL, May PA, Adnams CM, Konovalova V, Riley EP and CIFASD (2010) ‘Toward a neurobehavioral profile of fetal alcohol spectrum disorders.’ Alcoholism: Clinical and Experimental Research, 34(9) pp. 1640–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Hasken JM, Blankenship J, Marais A-S, Joubert B, Cloete M, de Vries MM, Barnard R, Botha I and Roux S (2016) ‘Breastfeeding and maternal alcohol use: Prevalence and effects on child outcomes and fetal alcohol spectrum disorders.’ Reproductive Toxicology, 63 pp. 13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Chambers CD, Kalberg WO, Zellner J, Feldman H, Buckley D, Kopald D, Hasken JM, Xu R and Honerkamp-Smith G (2018) ‘Prevalence of fetal alcohol spectrum disorders in 4 US communities.’ JAMA, 319(5) pp. 474–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montag AC (2016) ‘Fetal alcohol-spectrum disorders: Identifying at-risk mothers.’ International Journal of Women’s Health, 8 p. 311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishita H and Arora M (2017) ‘Tooth-matrix biomarkers to reconstruct critical periods of brain plasticity.’ Trends in neurosciences, 40(1) pp. 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedzwiecki MM, Eggers S, Joshi A, Dolios G, Cantoral A, Lamadrid-Figueroa H, Amarasiriwardena C, Téllez-Rojo MM, Wright RO and Petrick L (2021) ‘Lead exposure and serum metabolite profiles in pregnant women in Mexico City.’ Environmental Health, 20(1), 2021/12/10, p. 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual JA, Diaz D, Segura J, Garcia-Algar O, Vall O, Zuccaro P, Pacifici R and Pichini S (2003) ‘A simple and reliable method for the determination of nicotine and cotinine in teeth by gas chromatography/mass spectrometry.’ Rapid Communications in Mass Spectrometry, 17(24) pp. 2853–2855. [DOI] [PubMed] [Google Scholar]
- Pellegrini M, Casá A, Marchei E, Pacifici R, Mayné R, Barbero V, Garcia-Algar O and Pichini S (2006) ‘Development and validation of a gas chromatography-mass spectrometry assay for opiates and cocaine in human teeth.’ Journal of Pharmaceutical and Biomedical Analysis, 40(3) pp. 662–668. [DOI] [PubMed] [Google Scholar]
- Popova S, Lange S, Burd L and Rehm J (2012) ‘Health care burden and cost associated with fetal alcohol syndome: Based on official Canadian data.’ PLoS One, 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popova S, Lange S, Burd L and Rehm J (2015) ‘The economic burden of fetal alcohol spectrum disorder in Canada in 2013.’ Alcohol and Alcoholism, 51(3) pp. 367–375. [DOI] [PubMed] [Google Scholar]
- Sabel N, Johansson C, Kühnisch J, Robertson A, Steiniger F, Norén JG, Klingberg G and Nietzsche S (2008) ‘Neonatal lines in the enamel of primary teeth—A morphological and scanning electron microscopic investigation.’ Archives of Oral Biology, 53(10), 2008/10/01/, pp. 954–963. [DOI] [PubMed] [Google Scholar]
- Schmidt-Schultz TH and Schultz M (2007) ‘Well preserved non-collagenous extracellular matrix proteins in ancient human bone and teeth.’ International Journal of Osteoarchaeology, 17(1) pp. 91–99. [Google Scholar]
- Schussl Y, Pelz K, Kempf J and Otten JE (2014) ‘Concentrations of amoxicillin and clindamycin in teeth following a single dose of oral medication.’ Clin Oral Investig, 18(1), Jan, pp. 35–40. [DOI] [PubMed] [Google Scholar]
- Streissguth AP, Bookstein FL, Barr HM, Sampson PD, O’Malley K and Young JK (2004) ‘Risk factors for adverse life outcomes in fetal alcohol syndrome and fetal alcohol effects.’ J Dev Behav Pediatr, 25(4), Aug, pp. 228–238. [DOI] [PubMed] [Google Scholar]
- Streissguth AP, Bookstein FL, Barr HM, Sampson PD, O’MALLEY K and Young JK (2004) ‘Risk factors for adverse life outcomes in fetal alcohol syndrome and fetal alcohol effects.’ Journal of Developmental & Behavioral Pediatrics, 25(4) pp. 228–238. [DOI] [PubMed] [Google Scholar]
- Sumner LW, Amberg A, Barrett D, Beale MH, Beger R, Daykin CA, Fan TWM, Fiehn O, Goodacre R, Griffin JL, Hankemeier T, Hardy N, Harnly J, Higashi R, Kopka J, Lane AN, Lindon JC, Marriott P, Nicholls AW, Reily MD, Thaden JJ and Viant MR (2007) ‘Proposed minimum reporting standards for chemical analysis Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI).’ Metabolomics: Official journal of the Metabolomic Society, 3(3) pp. 211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargesson N (2015) ‘Thalidomide-induced teratogenesis: History and mechanisms.’ Birth Defects Research Part C: Embryo Today: Reviews, 105(2) pp. 140–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M, Tu P, Dolios G, Dassanayake PS, Volk H, Newschaffer C, Fallin MD, Croen L, Lyall K, Schmidt R, Hertz-Piccioto I, Austin C, Arora M and Petrick LM (2021) ‘Tooth biomarkers to characterize the temporal dynamics of the fetal and early-life exposome.’ Environ Int, 157, Dec, p. 106849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeren C, Keten A, Çelik S, Damlar İ, Daglıoglu N, Çeliker A and Karaarslan B (2013) ‘Demonstration of ethyl glucuronide in dental tissue samples by liquid chromatography/electro-spray tandem mass spectrometry.’ Journal of forensic and legal medicine, 20(6) pp. 706–710. [DOI] [PubMed] [Google Scholar]


