Abstract
Background and Objectives:
Guidelines for stage II colon cancer recommend adjuvant chemotherapy (AC) only for tumors with high-risk features, but long-term outcomes data are mixed. We aimed to determine if AC was associated with a survival benefit in this population.
Methods:
Patients were identified from the National Cancer Database and included if they met the following criteria: diagnosis of stage II colon cancer, surgery, survival data, and complete data on 6 high-risk features. The cohort of 57,335 patients was stratified by receipt of AC. Sub-group analysis was performed on patients under 65 years with no comorbidities. Overall survival (OS) was the primary endpoint.
Results:
Increasing number of high-risk features was associated with significantly decreased median OS. AC was associated with significantly increased OS for patients with 0, 1, 2, and ≥3 high-risk features. On sub-group analysis, receipt of AC was associated with reduced risk of death (HR: 0.66, CI: 0.59–0.74). For patients in the sub-group who had a T4 tumor, AC was associated with increased OS (92.7 vs 83.6 months).
Conclusions:
AC should be considered for all younger, healthy patients with stage II colon cancer and may be associated with a survival benefit for patients with T4 disease.
Keywords: colon cancer, stage II, high-risk, adjuvant chemotherapy, NCDB
Introduction
Colorectal cancer remains the third most common malignancy and the second most common cause of cancer-related death worldwide.[1] Decades of research have led to advancements in understanding colorectal cancer pathophysiology resulting in better treatment options and screening strategies. As a result, in the United States, annual mortality for colorectal cancer has steadily declined. Globally, however, colorectal cancer continues to be a leading cause of cancer death.[2] In 2020, colorectal cancer reached 10% worldwide incidence and accounted for 9.4% of total cancer-related mortality.[3] Ninety percent of colorectal cancer is diagnosed in patients 50 years and older. However, more recent data indicates a rising incidence and mortality rate for patients under the age of 50 years.[4,5]
Treatment of colon cancer is guided by disease stage at presentation. Surgery with mesenteric lymphadenectomy is standard initial therapy for stages I-III. For patients with stage III colon cancer, regardless of micro-satellite stability status, large multi-institutional clinical trials have demonstrated a significant survival benefit with adjuvant chemotherapy (AC).[6–11] However, the relative benefit of AC for stage II disease is less obvious, partly because the historical cure rate has been reported as 70% to 80% after resection [12,13] and the 5-year overall survival (OS) for localized disease is approximately 91%.[14] Clinical trials have failed to show an OS benefit for stage II colon cancer patients who receive AC.[15–18] Furthermore, 10-year follow up from the MOSAIC trial did not find an OS benefit for patients with high-risk features, although they analyzed the data from the point of view of any high-risk feature and did not assess these individually.[19] After these trials, in 2009, National Comprehensive Cancer Network (NCCN) guidelines removed their recommendation for adjuvant chemotherapy for stage II colon cancer without high-risk features.[20]
Although recent retrospective outcomes-based literature from the National Cancer Database (NCDB) and other sources has investigated the advantage of AC in patients with high-risk stage II disease, the results have been mixed.[21–23] Additionally, these analyses have largely been done without incorporation of several high-risk variables (chiefly lymphovascular (LVI) or perineural invasion (PNI), which were incorporated in the NCDB in 2010), their follow-up was limited, or they did not account for non-disease-related confounding.[21,22,24,25] Clinical trials attempting to address this question have either not assessed high-risk features or not been designed to look at AC in only stage II disease with colon (not rectal) primary and high-risk features.[8,15–19,26] Here, we aim to analyze the effect of AC on stage II colon cancer assessing each of six high-risk clinicopathologic features while accounting for both age and comorbidities. The NCDB provides a national cohort with the length of follow-up required to answer this question.
Materials and Methods
The NCDB is jointly maintained by the American College of Surgeons and the American Cancer Society. Patients included in this database represent approximately 70% of new cancer diagnosis treated at the approximately 1,500 Commission on Cancer designated centers across the United States. This study was exempted from institutional review board approval given the de-identified nature of the data.
The schema for inclusion in this study is summarized in Figure 1. The 2004–2017 NCDB colon cancer participant user file (PUF) contains 986,839 cases, 644,097 of which are coded histology code 8140 (adenocarcinoma not otherwise specified). Patients with behavior code 3 (invasive), pathologic diagnostic confirmation codes 1–4, lack of prior cancer diagnosis, and tumors which were located from the cecum through the sigmoid colon were included. Appendiceal primary site was excluded. Next, patients with stage II disease were stringently selected by restaging all cases, first by excluding other than pathologically confirmed stage II disease, then by removing all remaining cases of in situ, T1, or T2 lesions, positive lymph nodes, or any indication of metastasis from all categories related to these features. Only patients who underwent resection and had follow-up data with known vital status were included; those with missing follow-up data or vital status were excluded. Patients who died within 30 days of surgery were removed. Finally, cases with missing data in any of the six high-risk categories described below were excluded. LVI and PNI were not recorded before 2010 in the NCDB, so only cases added to the database between 2010–2017 were included. The PUF was requested in 2020, and thus contained follow up data for the 57,335 patients included in the final cohort through that year.
Figure 1.
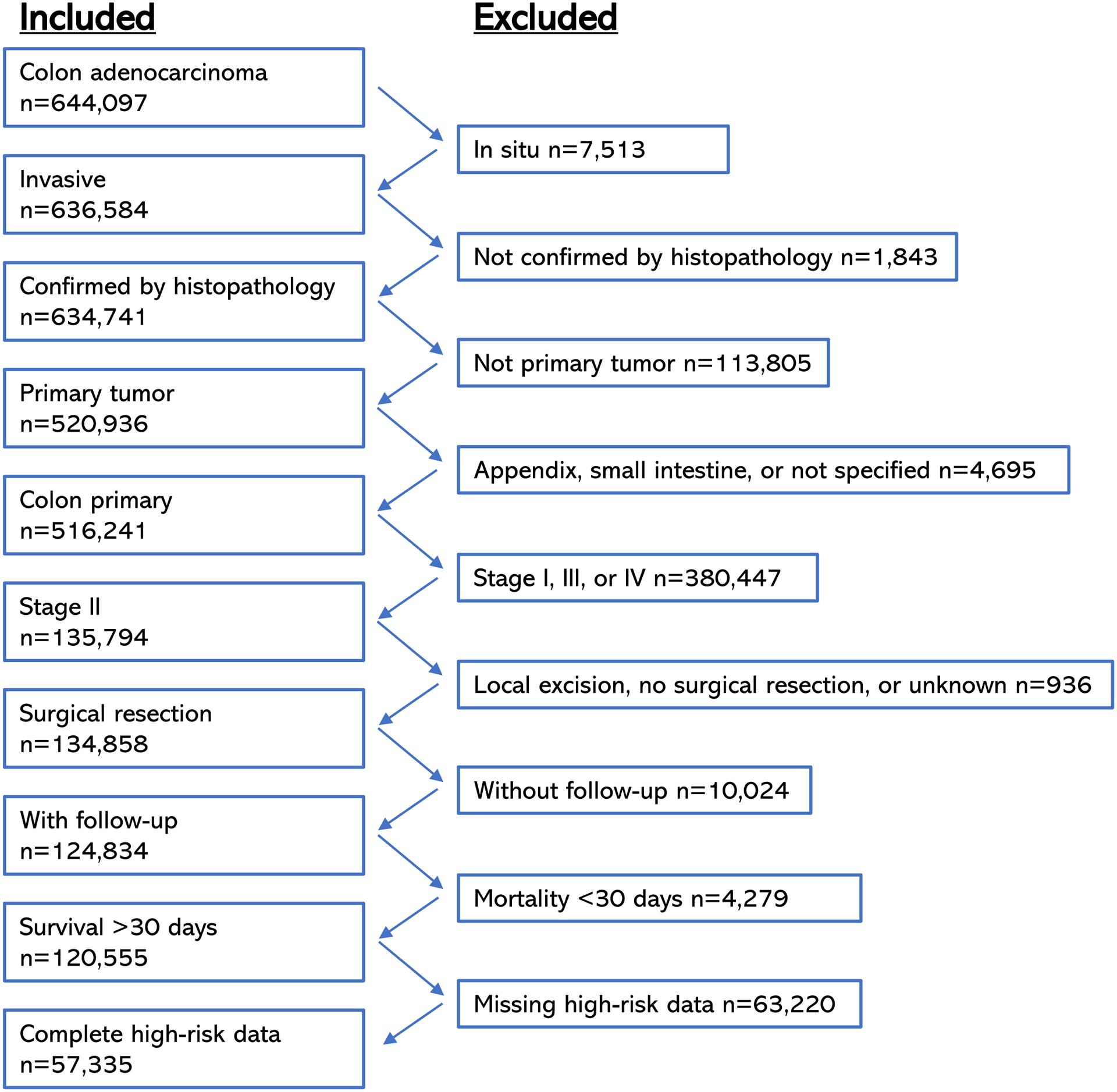
Inclusion and exclusion criteria for the cohort of patients with Stage II colon cancer.
Patients with LVI, PNI, positive resection margin, histologic grade 3 (poorly differentiated) or 4 (undifferentiated), inadequate lymph node harvest (fewer than 12 nodes evaluated), or a tumor stage 4 (T4) lesion, alone or in any combination, were considered high-risk in line with NCCN guidelines. Given the limitations of the NCDB data, clinical high-risk features such as obstruction or perforation were unable to be assessed. These patients were stratified into groups – one, two, or at least three high-risk features – and compared by group as well as each feature individually to those with no high-risk features and stratified by receipt of AC. Among the whole cohort, the proportional hazard of each high-risk feature, Charlson-Deyo comorbidity score (CDCS), age over 65 years, and adjuvant chemotherapy was analyzed. Also, OS for each stratum of high-risk feature was compared by receipt of adjuvant chemotherapy.
A sub-group of patients under age 65 years with no reported comorbidities (CDCS 0) was performed to control for these non-disease related variables, which could impact OS. Within this group, the association of each high-risk feature and the administration of adjuvant chemotherapy on OS was analyzed. Also, OS for each high-risk feature stratified by receipt of adjuvant chemotherapy was calculated.
Nominal variables were expressed as absolute values and percentages and evaluated using the Chi-squared test. Univariable and multivariable Cox proportional hazards models were created with OS as the outcome. OS was modeled using the Kaplan-Meier method and compared using the log-rank test. Median OS could not be calculated for the AC group in certain instances because 50% of the cohort did not reach the endpoint; in these circumstances, mean OS is reported. Statistics and data analysis were done using SPSS Statistics for Windows version 28 (SPSS Inc., Chicago, IL, USA).
Results
Of the 57,335 patients in the final cohort, 9,268 (16.2%) received AC. The AC group was significantly younger and healthier than the no chemotherapy group (Table 1). The AC group also had a slightly higher proportion of men. There was a significantly greater proportion of every high-risk feature (T4, high grade, LVI, PNI, inadequate lymph node harvest, and positive surgical margin) in the AC group compared to the no chemotherapy group, indicating an association between having a high-risk feature and receiving chemotherapy. Characteristics of the cohort can be seen grouped by receipt of AC in Table 1 and grouped by number of high-risk features in the Supplemental Table.
Table 1.
Characteristics of the cohort by receipt of AC.
| No Adjuvant Chemotherapy (n=46656) | Adjuvant Chemotherapy (n=9268) | Chi Square P | ||
|---|---|---|---|---|
| n (%) | ||||
| Age | <65 years | 15309 (32.8) | 6298 (68) | <0.001 |
| >65 years | 31347 (67.2) | 2970 (32) | ||
| Sex | Male | 22246 (47.7) | 4737 (51.1) | <0.001 |
| Female | 24410 (52.3) | 4531 (48.9) | ||
| Race | White | 38985 (83.6) | 7505 (81) | <0.001 |
| Black | 5349 (11.5) | 1274 (13.7) | ||
| Asian/Polynesian | 1463 (3.1) | 307 (3.3) | ||
| Other/Unknown | 859 (1.8) | 182 (2) | ||
| Hispanic | No | 42876 (94.6) | 8306 (92.3) | <0.001 |
| Yes | 2437 (5.4) | 695 (7.7) | ||
| Insurance Status | Insured | 44648 (96.8) | 8552 (93.5) | <0.001 |
| Uninsured | 1454 (3.2) | 593 (6.5) | ||
| CDC Score | 0 | 30268 (64.9) | 6993 (75.5) | <0.001 |
| 1 | 10990 (23.6) | 1772 (19.1) | ||
| 2 | 3531 (7.6) | 369 (4) | ||
| 3 | 1867 (4) | 134 (1.4) | ||
| Tumor Size | < 5cm | 20315 (43.5) | 3310 (35.7) | <0.001 |
| >= 5cm | 19684 (42.2) | 4696 (50.7) | ||
| Unknown | 6657 (14.3) | 1262 (13.6) | ||
| T Stage | T3 | 42003 (90.3) | 5840 (63.5) | <0.001 |
| T4A | 2811 (6) | 1783 (19.4) | ||
| T4B | 1722 (3.7) | 1571 (17.1) | ||
| Grade | Grade 1–2 | 39573 (84.8) | 7403 (79.9) | <0.001 |
| Grade 3–4 | 7083 (15.2) | 1865 (20.1) | ||
| Lymphovascular Invasion | No | 40647 (87.1) | 7228 (78) | <0.001 |
| Yes | 6009 (12.9) | 2040 (22) | ||
| Perineural Invasion | No | 43668 (93.6) | 8134 (87.8) | <0.001 |
| Yes | 2988 (6.4) | 1134 (12.2) | ||
| Lymph Node Harvest | >12 nodes | 43568 (93.4) | 8440 (91.1) | <0.001 |
| <12 nodes | 3088 (6.6) | 828 (8.9) | ||
| Margin | Negative | 45546 (97.6) | 8480 (91.5) | <0.001 |
| Positive | 1110 (2.4) | 788 (8.5) | ||
| Location | Right Colon | 24597 (54.2) | 3634 (40.4) | <0.001 |
| Transverse Colon | 5409 (11.9) | 966 (10.7) | ||
| Left Colon | 4690 (10.3) | 1216 (13.5) | ||
| Sigmoid Colon | 10693 (23.6) | 3177 (35.3) | ||
Decreased survival was associated with increasing number of high-risk features. Having no high-risk feature was associated with significantly longer OS (87.6 months) compared to those with one feature (82.8 months), two features (77.2 months), or three or more high-risk features (64.8 months) (Figure 2).
Figure 2.
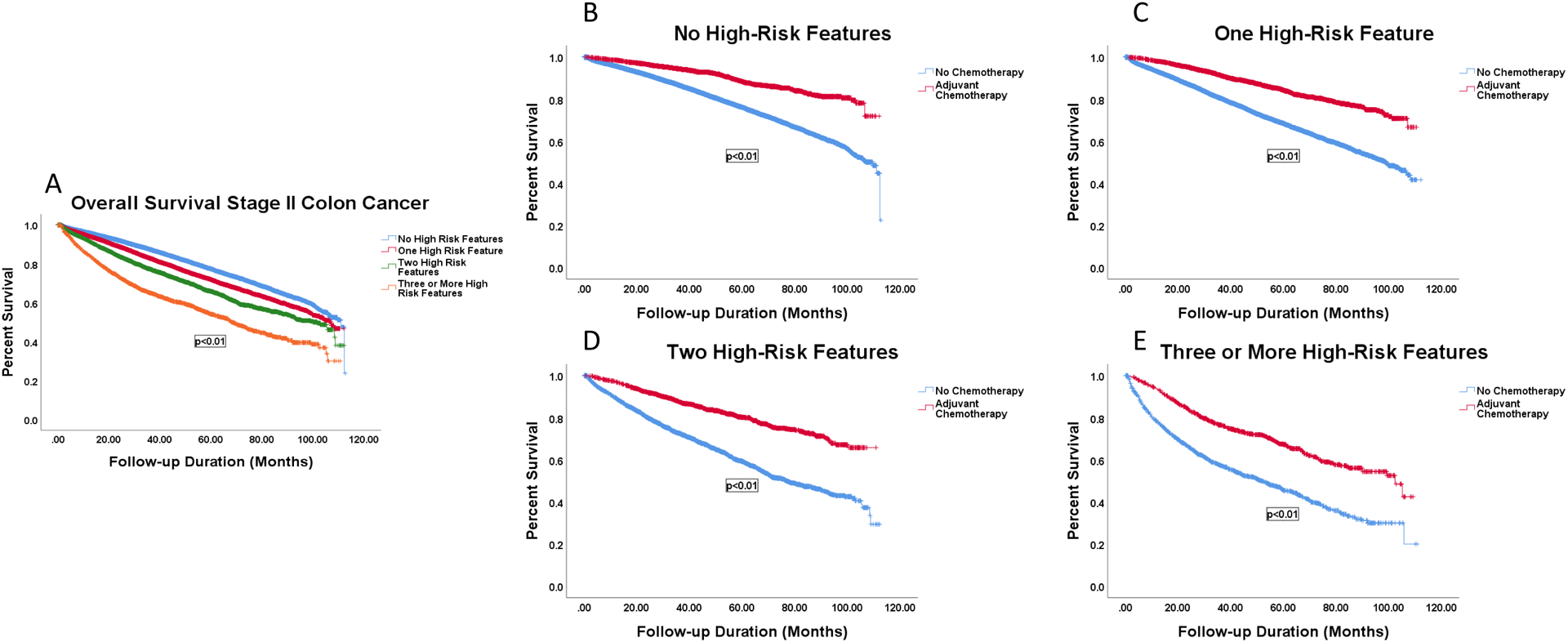
Survival curves by number of high-risk features stratified by receipt of AC. A: OS for patients with 0 (87.6 months), 1 (82.8 months, p<0.001), 2 (77.2 months, p<0.001), or 3 or more high-risk features (64.8, p<0.001). B: OS of patients with 0 high-risk features by receipt of AC, 86.2 vs 99.9 months, p<0.001. C: OS of patients with 1 high-risk feature by receipt of AC, 79.3 vs 94.4 months, p<0.001. D: OS of patients with 2 high-risk features by receipt of AC, 70.7 vs 90.6 months, p<0.001. E: OS of patients with 3 or more high-risk features by receipt of AC, 56.2 vs 77.0 months, p<0.001.
Among patients with no high-risk feature, receipt of AC was associated with a mean OS survival benefit of 99.9 months vs 86.2 months in patients who did not receive AC (p<0.001). Receipt of AC was also associated with a mean OS survival benefit of 15 months, 20 months, and 21 months for those with zero, one, two, or three or more high-risk features, respectively (Figure 2).
Univariable proportional hazards analyses revealed associations between LVI, PNI, inadequate lymph node harvest, high-grade tumor, T4 stage, and positive resection margin with OS (Table 2). All six high-risk features were confirmed to be independently associated with OS on multivariable analysis, with T4B tumor stage demonstrating the largest impact among the cancer-specific high-risk features. Among the features not specific to colon cancer, age >65 years and CDCS ≥1 were associated with increased risk of death. Patients who received AC had a significant inverse correlation with risk of death on both univariable and multivariable analyses.
Table 2:
Univariable and multivariable Cox proportional hazards regression model of the entire cohort.
| Factor | Hazard Ratio (95% CI) | p Value | Hazard Ratio (95% CI) | p Value |
|---|---|---|---|---|
| Univariate | Multivariate | |||
| Lymphovascular Invasion | 1.270 (1.216–1.328) | <0.001 | 1.156 (1.102–1.211) | <0.001 |
| Perineural Invasion | 1.348 (1.271–1.429) | <0.001 | 1.281 (1.205–1.362) | <0.001 |
| Inadequate Lymph Node Harvest | 1.735 (1.647–1.828) | <0.001 | 1.643 (1.557–1.733) | <0.001 |
| High Grade (3–4) | 1.229 (1.178–1.283) | <0.001 | 1.105 (1.057–1.155) | <0.001 |
| T3 | -- | -- | -- | |
| T4A | 1.629 (1.544–1.718) | <0.001 | 1.670 (1.577–1.769) | <0.001 |
| T4B | 1.696 (1.596–1.802) | <0.001 | 2.049 (1.919–2.189) | <0.001 |
| Positive Resection Margin | 2.023 (1.884–2.171) | <0.001 | 1.659 (1.535–1.792) | <0.001 |
| Age >65 | 3.149 (3.016–3.287) | <0.001 | 2.741 (2.619–2.868) | <0.001 |
| CDCS 0 | -- | -- | ||
| CDCS 1 | 1.406 (1.352–1.462) | <0.001 | 1.255 (1.205–1.306) | <0.001 |
| CDCS 2 | 2.211 (2.094–2.335) | <0.001 | 1.840 (1.741–1.945) | <0.001 |
| CDCS 3 | 2.770 (2.582–2.973) | <0.001 | 2.244 (2.088–2.410) | <0.001 |
| Received Adjuvant Chemotherapy | 0.535 (0.507–0.564) | <0.001 | 0.570 (0.537–0.605) | <0.001 |
Given the significant impact of patient age over 65 years and CDCS of 1–3 and the likelihood that these variables were confounding the survival analysis, a sub-group analysis was performed by removing these patients (Table 3). Among younger, healthier patients (<65 years and CDCS 0, n=16,689), LVI, PNI, inadequate lymph node harvest, T4A/T4B lesion, and positive resection margin remained significantly correlated with increased risk of death on both univariable and multivariable analysis. High histologic grade was no longer associated with OS. Furthermore, receipt of AC was not associated with OS on univariable analysis but was independently associated with an OS benefit on multivariable analysis.
Table 3:
Univariable and multivariable Cox proportional hazards regression model of patients under 65 years old with no comorbidities, n=16,689.
| Factor | Hazard Ratio (95% CI) | p Value | Hazard Ratio (95% CI) | p Value |
|---|---|---|---|---|
| Univariate | Multivariate | |||
| Lymphovascular Invasion | 1.346 (1.188–1.525) | <0.001 | 1.170 (1.021–1.340) | 0.023 |
| Perineural Invasion | 1.644 (1.418–1.907) | <0.001 | 1.429 (1.219–1.676) | <0.001 |
| Inadequate Lymph Node Harvest | 1.927 (1.652–2.248) | <0.001 | 1.929 (1.642–2.266) | <0.001 |
| High Grade (3–4) | 1.137 (0.996–1.299) | 0.058 | 1.080 (0.939–1.243) | 0.282 |
| T3 | -- | -- | ||
| T4A | 2.162 (1.879–2.487) | <0.001 | 2.171 (1.862–2.531 | <0.001 |
| T4B | 2.767 (2.422–3.162) | <0.001 | 2.768 (2.376–3.225) | <0.001 |
| Positive Resection Margin | 3.080 (2.614–3.628) | <0.001 | 2.150 (1.792–2.580) | <0.001 |
| Received Adjuvant Chemotherapy | 1.039 (0.937–1.152) | 0.473 | 0.657 (0.585–0.739) | <0.001 |
Finally, survival curves were created for each high-risk feature within the cohort of patients under 65 years with CDCS 0. Having a T4 lesion of either type was associated with increased OS after receipt of AC (T4A: 93.6 vs 85.4 months, p<0.001; T4B: 90.8 vs 80.4 months, p<0.001) (Figure 3). No other high-risk feature was significantly associated with increased OS with receipt of AC in the younger healthy population (data not shown).
Figure 3.
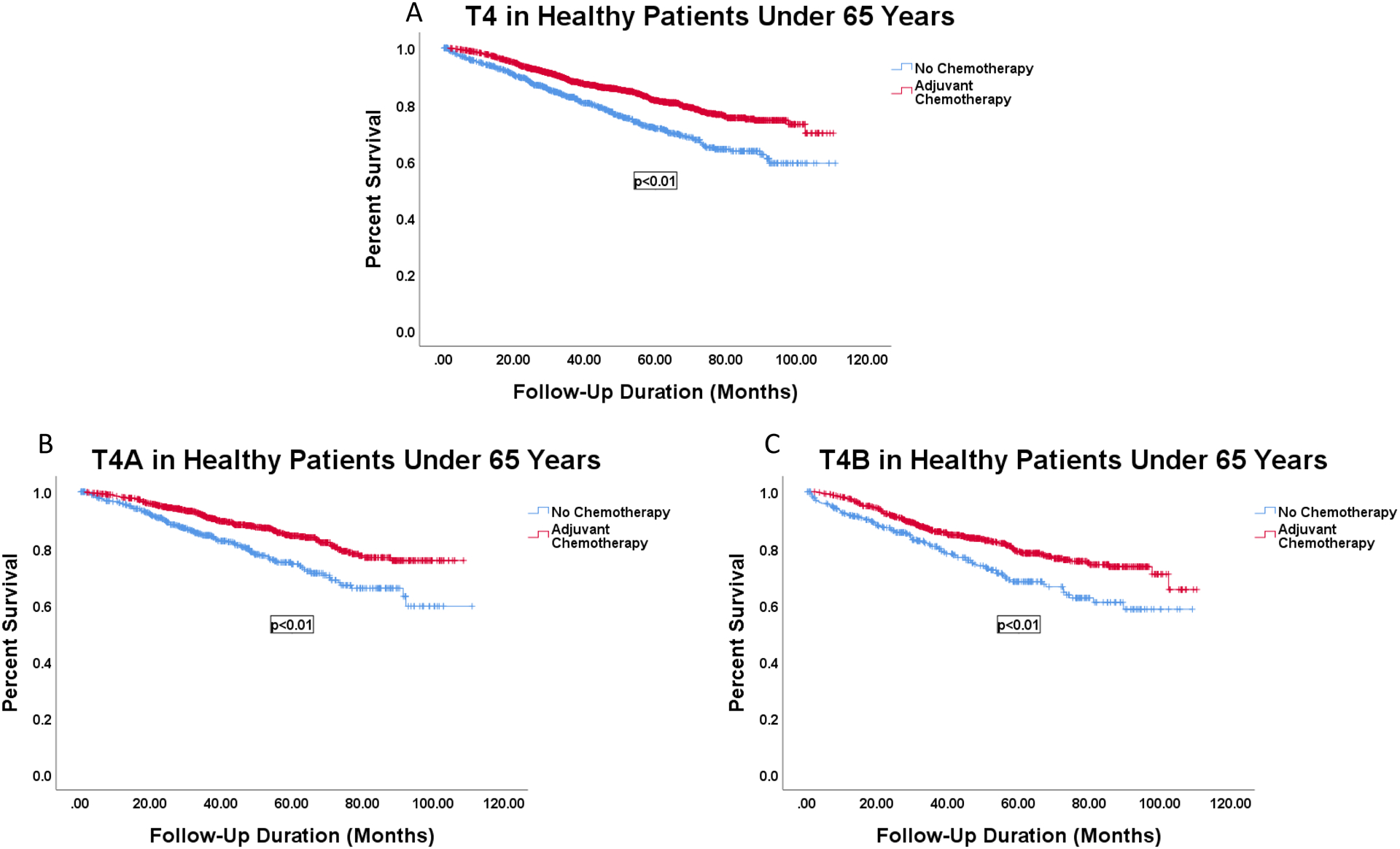
Survival curves of patients with T4 cancers among patients under 65 years old with no comorbidities by receipt of AC. A: Patients with any T4 lesion by receipt of AC, 83.6 vs 92.7 months, p<0.001. B: Patients with T4A lesions by receipt of AC, 85.4 vs 93.6 months, p<0.001. C: Patients with T4B lesions by receipt of AC, 80.4 vs 90.8 months, p<0.001.
Discussion
This study demonstrated an association between improved OS and receipt of AC in all patients with stage II colon cancer. The analysis also showed this result is skewed by two major confounder categories: age and comorbidities. In the overall cohort, for patients with any number of high-risk features, including zero, receipt of AC was associated with an OS benefit. However, age and comorbidities carried the largest hazard for death on multivariable regression. Sub-group analysis of a cohort of young, healthy patients was performed to control for these characteristics independent of colon cancer biology which could affect survival. In this highly selected cohort, all the high-risk features except high-grade tumors were positively correlated with increased risk of death and receipt of AC was positively correlated with decreased risk of death on multivariable analysis. Also, in this selected cohort, of those features which showed an increased risk of death, only patients with T4 stage tumors were associated with improved OS after receiving AC.
Consensus guidelines from the NCCN and The American Society of Colon & Rectal Surgeons (ASCRS) currently do not recommend AC for low-risk stage II colon cancer.[27,28] NCCN states FOLFOX is “reasonable for stage II with multiple high-risk features”[28] and the recommendation from ASCRS for considering AC for stage II colon cancer is “weak” with the caveat that no benefit has been shown for low-risk disease.[27] The reason for the lack of clear guidance is a lack of clear evidence. There has never been a randomized controlled trial designed to assess OS in only stage II colon cancer looking at the relative risk of high-risk tumor features, or the relative benefit AC may provide. The inherent problem with a trial designed to assess OS in the stage II population is its overall good prognosis and the long-term follow-up which would be required to produce an answer. As such, there have been multiple retrospective studies using the NCDB and population-level data to try to answer this question, with mixed results.[21,22,25,29,30] In addition, as in this study, there are few studies that include both clinical and pathologic high-risk features.
Retrospective outcomes data which assess stage II cancer with high-risk features for disease recurrence have shown a benefit for patients who receive AC in certain circumstances.[21–23] An NCDB study evaluating high-risk stage II and stage III patients who followed NCCN guidelines for adjuvant chemotherapy showed a significant OS benefit.[23] In this study, high-risk features were defined by depth of invasion (T stage), histologic grade, margin status, and lymph node harvest. However, this study assessed patients who were diagnosed between 1998 and 2002, before either oxaliplatin was standard of care or other features now considered high-risk were collected in the NCDB. A later analysis of the California Cancer Registry identified high-risk features LVI, PNI, positive resection margin, grade 3 or 4, inadequate lymph node harvest, or T4 lesion.[22] This population-based study showed that patients with T4 disease benefit from AC, but the patient cohort was limited geographically (California) and the study follow-up was fewer than 5 years.[22] In 2016, a retrospective study of stage II colon cancer patients from the NCDB reported a statistically significant OS benefit with AC for all patients regardless of high-risk tumor pathologic features such as T4, poor differentiation, positive margins, or fewer than 12 lymph nodes evaluated.[21]
Clinical trial data evaluating AC for stage II colon cancer is also less than convincing. An older international trial, the IMPACT B2 trial assessed AC in Dukes’ stage B2 tumors did not demonstrate a survival benefit after adjuvant 5-fluorouracil versus patients treated with surgery alone.[15] The subsequent QUASAR trial assessed AC in colon or rectal cancer in a mostly stage II population, which failed to show an OS benefit on sub-group analysis of the stage II cohort with colonic primary although a small but significant decrease in the relative risk of recurrence at two years was found.[16] It must be recognized, however, that enrollment in these trials were conducted before the advent of platinum-based regimens as standard of care therapy. The MOSAIC trial followed these studies and assessed adjuvant fluorouracil and leucovorin with or without oxaliplatin in a stage II-III population. They did not find a survival benefit among patients with stage II disease on sub-group analysis but did among those with stage III disease.[17,19] Moreover, ten-year follow-up data assessed stage II disease with the high-risk features: T4 stage, perforation, obstruction, vascular invasion, poorly differentiated histology, and inadequate lymph node harvest, and did not find a survival benefit with the addition of oxaliplatin; however, these data were assessed from the point of view of having any high-risk feature. These results were largely confirmed by a parallel clinical trial by The National Surgical Adjuvant Breast and Bowel Project.[18]
Our analysis showed that after receiving AC, younger, healthy patients may experience the most benefit. Older patients with stage II colon cancer in the setting of multiple comorbidities may not experience improved survival with chemotherapy because of several factors, including that previous work has shown 5-year survival is around 91% if the disease is completely removed indicating there is a probability these patients succumb to something else first.[14] However, patients who do not receive an adequate lymph node harvest may be inappropriately down-staged and not receive AC when they should have, especially if they have concomitant LVI or PNI. Also, looking at this population separately may be more aligned with the reality of which patients currently receive AC, as a recent study of the NCDB showed that younger patients with stage II disease with high-risk features more frequently receive AC than do older patients,[31] and a study of Medicare beneficiaries showed that a younger age at diagnosis was associated with an increased likelihood of receipt of AC.[32]
Clarification of which patients with stage II colon cancer may benefit from AC is needed. As was done here, the assessment of a large national cohort analyzing the individual risk from each of these six high-risk features while controlling for age and comorbidities has not been done. After controlling for age and CDCS, a proportional regression model showed a significant positive correlation with risk of death for patients with all high-risk features except for grade. Within this sub-population, AC was not associated with decreased risk of death on univariable analysis but was on multivariable analysis. This difference indicates that receipt of AC does not improve survival for the entire group of young, healthy patients, but may when controlling for high-risk features. Within this group, having a T4 lesion carried the greatest risk, and indeed when this group received AC, there was a significant association with improved OS. This finding is in line with the prior work done by the California group[22] and two population-based international retrospective studies which also showed a survival benefit after AC in T4 disease.[33,34] By analyzing a younger cohort without major comorbid conditions as defined by CDCS, we attempted to isolate the effect of stage II disease on survival stratified by high-risk features and receipt of AC. In doing so we were able to show an association between AC and improved OS of 8 months and 10 months for all patients with stage II colon cancer with T4A and T4B lesions, respectively.
This analysis also showed that among cancers with high-risk features, patients who had a T4 lesion received AC most commonly. Of patients who received AC, 36.2% had a T4 lesion – a substantially higher proportion as compared to patients with LVI (22.0%), high-grade tumors (20.1%), PNI (12.2%), inadequate lymph node harvest (8.9%), and positive resection margin (8.5%). Patients with three or more high-risk features received AC more often (39.1%) compared to those with zero, one, or two features (Supplemental Table). T4 tumors (and LVI) are more frequently associated with a greater number of high-risk features compared to the others. These data may be indicative of national practice patterns and reveal a common conception among oncologists that patients with more high-risk features may experience greater benefit from AC, especially if they have a T4 lesion as well as perhaps LVI, which may be considered a precursor to lymph node dissemination.
Limitations are those inherent to large national cohort retrospective databases studies. Specific comorbidities such as heart disease, smoking history, and diabetes are not included in the NCDB and could add to confounding if distribution is skewed. Also, data capture is imperfect. For instance, patients who are coded as receiving chemotherapy very likely did, but there are also likely several patients who did receive chemotherapy but outside of a Commission on Cancer center who were therefore not coded that way. Furthermore, this study is retrospective, unmatched, and not randomized, which in addition to decreasing the impact of unknown confounding variables, would have addressed information, treatment, and selection biases, which are certainly unaccounted for in our study. However, the size of the dataset and final cohort mitigate many of these limitations, especially potential confounding from non-coded comorbidities and information bias. Selection bias is mitigated by our sub-group analysis. Finally, the NCDB does not contain data on recurrence-free survival, so this could not be analyzed.
Conclusions
Younger patients with low comorbidity burden who are diagnosed with stage II colon cancer may experience a survival benefit from AC. Of all the pathologic high-risk features analyzed, T4 stage was the most highly associated factor with OS benefit. AC should be considered for patients with T4 disease and/or multiple high-risk features.
Supplementary Material
Supplemental Table. Characteristics of the cohort stratified by number of high-risk features.
Synopsis:
Optimal treatment for stage II colon cancer with high-risk features is unclear. The National Cancer Database provides a large, national cohort with long term follow up. Among these patients, those who are younger and healthier may benefit from adjuvant chemotherapy, which is associated with improved overall survival in patients with T4 disease.
Funding:
This research was partially supported by the Intramural Research Program of the National Cancer Institute.
Data Sharing and Accessibility Statement:
The findings of this study are supported by data that are available from the National Cancer Database. Restrictions apply to the availability of data from this source which were used under license for the purposes of this analysis.
References
- 1.Xi Y, Xu P. Global colorectal cancer burden in 2020 and projections to 2040. Transl Oncol. 2021;14(10):101174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–249. [DOI] [PubMed] [Google Scholar]
- 3.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. International journal of cancer. 2015;136(5):E359–E386. [DOI] [PubMed] [Google Scholar]
- 4.Siegel RL, Jakubowski CD, Fedewa SA, Davis A, Azad NS. Colorectal Cancer in the Young: Epidemiology, Prevention, Management. Am Soc Clin Oncol Educ Book. 2020;40:1–14. [DOI] [PubMed] [Google Scholar]
- 5.Siegel RL, Miller KD, Goding Sauer A, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70(3):145–164. [DOI] [PubMed] [Google Scholar]
- 6.Shah MA, Renfro LA, Allegra CJ, et al. Impact of Patient Factors on Recurrence Risk and Time Dependency of Oxaliplatin Benefit in Patients With Colon Cancer: Analysis From Modern-Era Adjuvant Studies in the Adjuvant Colon Cancer End Points (ACCENT) Database. J Clin Oncol. 2016;34(8):843–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanoff HK, Carpenter WR, Martin CF, et al. Comparative effectiveness of oxaliplatin vs non-oxaliplatin-containing adjuvant chemotherapy for stage III colon cancer. J Natl Cancer Inst. 2012;104(3):211–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmoll HJ, Twelves C, Sun W, et al. Effect of adjuvant capecitabine or fluorouracil, with or without oxaliplatin, on survival outcomes in stage III colon cancer and the effect of oxaliplatin on post-relapse survival: a pooled analysis of individual patient data from four randomised controlled trials. Lancet Oncol. 2014;15(13):1481–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tougeron D, Mouillet G, Trouilloud I, et al. Efficacy of Adjuvant Chemotherapy in Colon Cancer With Microsatellite Instability: A Large Multicenter AGEO Study. J Natl Cancer Inst. 2016;108(7). [DOI] [PubMed] [Google Scholar]
- 10.Figueredo A, Charette ML, Maroun J, Brouwers MC, Zuraw L. Adjuvant therapy for stage II colon cancer: a systematic review from the Cancer Care Ontario Program in evidence-based care’s gastrointestinal cancer disease site group. J Clin Oncol. 2004;22(16):3395–3407. [DOI] [PubMed] [Google Scholar]
- 11.Vogel JD, Felder SI, Bhama AR, et al. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Management of Colon Cancer. Dis Colon Rectum. 2022;65(2):148–177. [DOI] [PubMed] [Google Scholar]
- 12.Dotan E, Cohen SJ. Challenges in the management of stage II colon cancer. Semin Oncol. 2011;38(4):511–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moertel CG, Fleming TR, Macdonald JS, et al. Intergroup study of fluorouracil plus levamisole as adjuvant therapy for stage II/Dukes’ B2 colon cancer. J Clin Oncol. 1995;13(12):2936–2943. [DOI] [PubMed] [Google Scholar]
- 14.Institute NC. Surveillance, Epidemiology, and End Results Program. 2011–2017.
- 15.Efficacy of adjuvant fluorouracil and folinic acid in B2 colon cancer. International Multicentre Pooled Analysis of B2 Colon Cancer Trials (IMPACT B2) Investigators. J Clin Oncol. 1999;17(5):1356–1363. [PubMed] [Google Scholar]
- 16.Quasar Collaborative G, Gray R, Barnwell J, et al. Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. Lancet. 2007;370(9604):2020–2029. [DOI] [PubMed] [Google Scholar]
- 17.Andre T, Boni C, Mounedji-Boudiaf L, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350(23):2343–2351. [DOI] [PubMed] [Google Scholar]
- 18.Kuebler JP, Wieand HS, O’Connell MJ, et al. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: results from NSABP C-07. J Clin Oncol. 2007;25(16):2198–2204. [DOI] [PubMed] [Google Scholar]
- 19.Andre T, de Gramont A, Vernerey D, et al. Adjuvant Fluorouracil, Leucovorin, and Oxaliplatin in Stage II to III Colon Cancer: Updated 10-Year Survival and Outcomes According to BRAF Mutation and Mismatch Repair Status of the MOSAIC Study. J Clin Oncol. 2015;33(35):4176–4187. [DOI] [PubMed] [Google Scholar]
- 20.Engstrom PF. NCCN clinical practire guidelines in oncology: colon cancer. 2009;Version 3.2009. [DOI] [PubMed]
- 21.Casadaban L, Rauscher G, Aklilu M, Villenes D, Freels S, Maker AV. Adjuvant chemotherapy is associated with improved survival in patients with stage II colon cancer. Cancer. 2016;122(21):3277–3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Babcock BD, Aljehani MA, Jabo B, et al. High-Risk Stage II Colon Cancer: Not All Risks Are Created Equal. Ann Surg Oncol. 2018;25(7):1980–1985. [DOI] [PubMed] [Google Scholar]
- 23.Boland GM, Chang GJ, Haynes AB, et al. Association between adherence to National Comprehensive Cancer Network treatment guidelines and improved survival in patients with colon cancer. Cancer. 2013;119(8):1593–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Connor ES, Greenblatt DY, LoConte NK, et al. Adjuvant chemotherapy for stage II colon cancer with poor prognostic features. J Clin Oncol. 2011;29(25):3381–3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Achilli P, Crippa J, Grass F, et al. Survival impact of adjuvant chemotherapy in patients with stage IIA colon cancer: Analysis of the National Cancer Database. Int J Cancer. 2021;148(1):161–169. [DOI] [PubMed] [Google Scholar]
- 26.Gill S, Loprinzi CL, Sargent DJ, et al. Pooled analysis of fluorouracil-based adjuvant therapy for stage II and III colon cancer: who benefits and by how much? J Clin Oncol. 2004;22(10):1797–1806. [DOI] [PubMed] [Google Scholar]
- 27.Vogel JD, Eskicioglu C, Weiser MR, Feingold DL, Steele SR. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Treatment of Colon Cancer. Dis Colon Rectum. 2017;60(10):999–1017. [DOI] [PubMed] [Google Scholar]
- 28.Benson AB VA, Al-Hawary MM, et al. NCCN clinical practire guidelines in oncology: colon cancer. 2021;Version 3.2021.
- 29.Spindler BA, Bergquist JR, Thiels CA, et al. Incorporation of CEA Improves Risk Stratification in Stage II Colon Cancer. J Gastrointest Surg. 2017;21(5):770–777. [DOI] [PubMed] [Google Scholar]
- 30.Reif de Paula T, Gorroochurn P, Simon HL, Haas EM, Keller DS. A national evaluation of the use and survival impact of adjuvant chemotherapy in Stage II colon cancer from the national cancer database. Colorectal Dis. 2022;24(1):40–49. [DOI] [PubMed] [Google Scholar]
- 31.Hagerty BL, Aversa JG, Dominguez DA, et al. Age Determines Adjuvant Chemotherapy Use in Resected Stage II Colon Cancer. Dis Colon Rectum. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schrag D, Rifas-Shiman S, Saltz L, Bach PB, Begg CB. Adjuvant chemotherapy use for Medicare beneficiaries with stage II colon cancer. J Clin Oncol. 2002;20(19):3999–4005. [DOI] [PubMed] [Google Scholar]
- 33.Kumar A, Kennecke HF, Renouf DJ, et al. Adjuvant chemotherapy use and outcomes of patients with high-risk versus low-risk stage II colon cancer. Cancer. 2015;121(4):527–534. [DOI] [PubMed] [Google Scholar]
- 34.Teufel A, Gerken M, Hartl J, et al. Benefit of adjuvant chemotherapy in patients with T4 UICC II colon cancer. BMC Cancer. 2015;15:419. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table. Characteristics of the cohort stratified by number of high-risk features.
Data Availability Statement
The findings of this study are supported by data that are available from the National Cancer Database. Restrictions apply to the availability of data from this source which were used under license for the purposes of this analysis.


