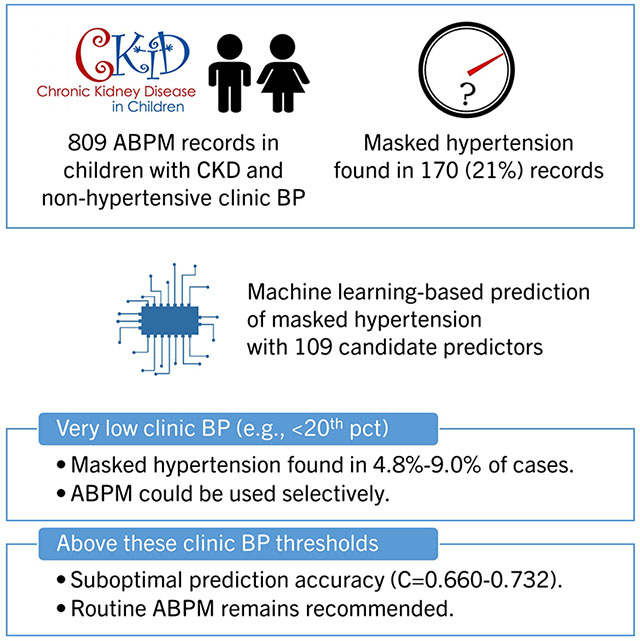
Abstract
Background:
Ambulatory blood pressure monitoring (ABPM) is routinely performed in children with chronic kidney disease (CKD) to identify masked hypertension, a risk factor for accelerated CKD progression. However, ABPM is burdensome, and developing an accurate prediction of masked hypertension may allow using ABPM selectively rather than routinely.
Methods:
To create a prediction model for masked hypertension using clinic blood pressure (BP) and other clinical characteristics, we analyzed 809 ABPM studies with non-hypertensive clinic BP among the participants of the Chronic Kidney Disease in Children study.
Results:
Masked hypertension was identified in 170 (21.0%) observations. We created prediction models for masked hypertension via gradient boosting, random forests, and logistic regression using 109 candidate predictors, and evaluated its performance using bootstrap validation. The models showed C-statistics from 0.660 (95%CI; 0.595-0.707) to 0.732 (95%CI; 0.695-0.786) and Brier scores from 0.148 (95%CI; 0.141-0.154) to 0.167 (95% CI; 0.152-0.183). Using the possible thresholds identified from this model, we stratified the dataset by clinic systolic/diastolic BP percentiles. The prevalence of masked hypertension was the lowest (4.8%) when clinic systolic/diastolic BP were both <20th percentile, and relatively low (9.0%) with clinic systolic BP<20th and diastolic BP<80th percentiles. Above these thresholds, the prevalence was higher with no discernable pattern.
Conclusions:
ABPM could be used selectively in those with low clinic BP, e.g., systolic BP<20th and diastolic BP<80th percentiles, although careful assessment is warranted as masked hypertension was not completely absent even in this subgroup. Above these clinic BP levels, routine ABPM remains recommended.
Keywords: masked hypertension, ambulatory blood pressure monitoring, chronic kidney disease, prediction
Graphical Abstract
INTRODUCTION
Masked hypertension (HTN), defined as normal clinic blood pressure (BP) but elevated ambulatory BP, is common in children with chronic kidney disease (CKD); up to half of children with CKD presenting non-hypertensive clinic BP may show elevated ambulatory levels (1–3). As HTN is a well-established risk factor for accelerated CKD progression in children and adolescents (4–6), routine 24-hour ambulatory blood pressure monitoring (ABPM) is recommended in this population (7,8). However, ABPM is expensive and often burdensome due to discomfort and long duration, which can adversely affect both patients’ willingness to undergo ABPM and the quality of the studies (1,8).
If the presence of masked HTN could be accurately predicted, ABPM could be selectively applied according to the predicted risk. Several clinical factors identified in previous studies might enable this prediction. Among those factors, clinic BP levels are perhaps the most comprehensively investigated for this purpose. Children and adolescents with very low clinic BP (<25th percentile) showed lower prevalence of masked HTN, whereas borderline high clinic BP has been commonly considered as a risk factor for masked HTN (1,2,9,10). Left ventricular hypertrophy has also been reported to be associated with masked HTN (3). However, it remains unclear whether these and other clinical factors could be integrated into a formal prediction model to quantify the risk of masked HTN with satisfactory prediction accuracy.
Creating a robust prediction model for masked HTN could be facilitated by using machine learning (ML) algorithms. ML can systematically and objectively analyze a large number of predictors with few statistical assumptions on the functional forms of the predictor-outcome association or the interactions between multiple predictors (11–13).
We hypothesized that, by applying robust ML algorithms on a large, high-dimensional dataset of children with CKD, one could create a prediction model for masked HTN that is sufficiently accurate to enable a more selective and targeted use of ABPM in this population. To test this hypothesis, we analyzed data from the Chronic Kidney Disease in Children (CKiD) Study, a multicenter cohort study of children with CKD in North America, via two approaches. First, we developed a ML prediction model based on 109 clinical factors and assessed its prediction performance. Second, we explored the feasibility of a simple, “bed-side” prediction by assessing the prevalence of masked HTN according to clinic systolic blood pressure (SBP) and diastolic blood pressure (DBP) percentiles using the thresholds identified from our ML-based prediction model.
METHODS
The Chronic Kidney Disease in Children Study
The CKiD Study is an ongoing prospective cohort study of children and adolescents with CKD from 63 sites across the United States and Canada. At enrollment, participants were ≤16 years with estimated glomerular filtration rate (eGFR) 30-90 ml/min per 1.73m2 and without a previous history of end-stage kidney disease. Enrollment occurred in 3 phases in 2005-2008 (n=586), 2011-2014 (n=305), and 2016-2020 (n=204). The CKiD Study conducts annual study visits of standardized protocols to collect longitudinal data focused on CKD progression and cardiovascular health, as well as growth and neurocognitive development. Full details on the study design have been described elsewhere (14). All study protocols were approved by local Institutional Review Boards, and informed consent and assent were obtained from participants and their guardians according to local requirements. Anonymized data have been made publicly available at the NIDDK Central Repository and can be accessed at https://repository.niddk.nih.gov/studies/ckid/.
Clinic Blood Pressure
By protocol, clinic BP was measured at enrollment and then yearly at annual CKiD study visits. Measurements were obtained by auscultation using an aneroid sphygmomanometer (MedicKit 5, Mabis Healthcare; Waukegan, IL). BP was measured three times (at least 30 seconds apart) at each visit, and the mean value of the three was recorded as the clinic BP for that visit. For both systolic and diastolic BP, we considered raw reading, percentile, and range (the difference between the highest and lowest readings in the three measurements) as predictors. On an annual basis, study devices were calibrated, and coordinators were trained and/or recertified.
Ambulatory Blood Pressure Monitoring
ABPM was conducted at the second study visit (1 year after enrollment), and at every other annual visit thereafter. All ABPM studies were conducted with SpaceLabs 90217 monitors (SpaceLabs Healthcare, Issaquah, WA). Participants were instructed to wear the monitor continuously for 24 hours. Monitors measured BP every 20 minutes during the day and night. The participants self-recorded the time of sleeping and waking up. Data recorded in the device were extracted and processed at the central ABPM site for CKiD (University of Texas, Houston, PI: JA Samuels). ABPM studies were deemed acceptable for research and included in this analysis based upon the following criteria: (1) ≥21 hours of overall monitoring, (2) ≥18 hours with >1 valid BP measurement per hour, and (3) ≥1 valid BP measurement in ≥75% of wake hours and ≥75% of sleep hours. ABPM studies that failed to meet these criteria were followed by a repeat study (1).
Outcome Definitions
Since masked HTN is defined by non-hypertensive clinic BP with elevated ambulatory BP, we excluded CKiD person-visits in which the clinic BP had met the definition of stage 1 or stage 2 HTN according to the 2017 American Academy of Pediatrics guidelines (7). For children <13 years at the measurement, SBP/DBP≥95th percentile or ≥130/80 mmHg (whichever is lower) was considered hypertensive. For children ≥13 years, SBP/DBP≥130/80 mmHg was considered hypertensive. BP percentiles were determined for age, sex, and height according to the 2017 American Academy of Pediatrics guidelines (7).
We defined ambulatory HTN as mean systolic or diastolic ambulatory BP≥95th percentile during either the wake or sleep period according to the 2022 American Heart Association (AHA) statement for pediatric ABPM (15). BP load was not considered in the outcome definition (15). BP percentiles were determined for age and sex according to the data reported by the German Working Group on Pediatric Hypertension (16).
Statistical Analysis
We included all CKiD person-visits with non-hypertensive clinic BP and research quality ABPM data. We excluded person-visits in which the participants were <5 or >17 years since the ABPM percentile data was available for ages from 5 to 20 years, and the clinic BP percentile data was from 1 to 17 years.
Machine Learning-Based Prediction Model
We created “exploratory” prediction models for masked HTN using two ML algorithms, gradient boosting and random forests. Briefly, gradient boosting generates a sequence of parsimonious prediction models based on the residual error of the previous models (17), and random forests uses an ensemble of parsimonious prediction models generated through bootstrap aggregation (18). To optimize our models’ prediction performance, we conducted a random search of hyperparameters and selected the hyperparameter set that showed the highest area under the receiver operating characteristic curve (AUC) in a 5-fold cross validation (17), independently for gradient boosting and random forests.
Both models included 109 commonly measured predictors (Table S1), including clinic blood pressure (raw reading, percentile, and range; see above), demographic factors (age, sex, race, and ethnicity), CKD characteristics (duration of CKD at visit and primary diagnosis), socioeconomic factors (marital status of the parent[s], household income, and maternal education level), birth-related factors (gestational age at birth, birth weight, and small for gestational age), body size (height, weight, and body mass index), clinical laboratory variables (eGFR, serum creatinine, serum albumin, electrolytes, lipids, blood cell counts, hemoglobin, ferritin, transferrin), urine biomarkers (proteinuria, and protein and creatinine, as separate variables), echocardiographic findings (left ventricular mass [LVM], LVM percentile, and LVM index), medication and supplements (anti-hypertensive therapy, immunosuppressant therapy, steroids, antibiotics, growth hormone, vitamins, and nutritional supplements), and seasonality (day of the year).
To better illustrate the associations characterized by our prediction model, we further assessed the gradient boosting model using Shapley additive explanations (SHAP), a metric designed for interpreting model predictions (19). SHAP values represent a given predictor’s impact on the predicted outcome within each observation; in this study, SHAP values correspond to the impact of a given predictor on the predicted log odds of masked HTN in each person-visit. We used the SHAP values to assess the variable importance of each predictor, and visualize the association of a predictor with masked HTN (19).
Additionally, the large number of predictors (p=109) relative to the sample size (n=809) and the number of cases (n=170) could have introduced inefficiency to the ML algorithms (20,21). Therefore, we created more parsimonious, “final” models using gradient boosting, random forests, and logistic regression. Based on the variable importance from the exploratory gradient boosting model and the findings from the previous literature, we selected the following 9 predictors for the final models: clinic SBP percentile, clinic DBP percentile, clinic DBP range, LVM percentile, body mass index percentile, urine protein, mean corpuscular hemoglobin concentration, angiotensin II receptor blocker use, and day of the year.
To summarize, we created a total of 5 prediction models: “exploratory” gradient boosting and random forests with 109 predictors, and “final” gradient boosting, random forests, and logistic regression with 9 high-impact predictors.
Model validation
We evaluated the prediction performance of the models using the 0.632+ bootstrap validation method with 100 repetitions (22,23). We assessed both discrimination and calibration. Discrimination represents the model’s ability to correctly assign higher predicted risk to those who had developed the outcome, and calibration represents the bias between the predicted risk and the true risk (24). We assessed AUC, Brier score, and calibration curve of the models. AUC can range from 0.5 to 1, with higher values indicating superior discrimination. The Brier score, analogous to the mean squared error, can range from 0 to 1, with lower values indicating superior calibration. We also examined the scaled Brier score, which represents the relative reduction in Brier score achieved with the prediction model compared to a “null” model. The calibration curve was assessed in two steps as per the hierarchical approach proposed by Van Calster and colleagues (25,26). First, we fit a logistic regression between the observed outcome versus the predicted probability (converted to log odds) to assess the overall calibration. Using this approach, a prediction model with perfect calibration would appear as the identity line (y=x) with an intercept of 0 and a slope of 1 from the logistic regression. The intercept and slope may represent systematic overfitting or underfitting and/or overestimation or underestimation of risk (25). Specifically, the intercept can be interpreted as the bias between the observed proportion and the predicted probability among cases with predicted probability of 0.5, and the slope can be interpreted as the correspondence between the observed proportion and the predicted probability. Then, we repeated the analysis using the locally-estimated scatterplot smoothing (LOESS) method to graphically examine the calibration at different risk levels.
Prevalence of Masked Hypertension by Clinic Blood Pressure
We examined the exploratory gradient boosting model using SHAP values and identified potential thresholds for clinic SBP and DBP percentiles around which the risk of masked HTN may notably differ. Based on these findings, we stratified the entire study population by the quantiles of clinic SBP and DBP percentiles (<20, 20-40, 40-60, 60-80, and ≥80). We calculated the prevalence of mashed HTN within each combination of the quintiles and visualized the results on a 5x5 heat map. All analyses were performed using R version 4.0.3.
RESULTS
Study Population
Table 1 presents the descriptive statistics of demographic and clinical characteristics of the training and validation set, stratified by ABPM results. Among all 809 cases included in our study, masked HTN was observed in 170 (21.0%). Compared to cases with normal ambulatory BP, those with masked HTN had a slightly higher clinic BP (median SBP percentile, 53 vs. 40; and median DBP percentile, 59 vs. 48) and a smaller body size (median height percentile, 27 vs. 33; and median weight percentile, 42 vs. 51), and were less likely to report concurrent use of angiotensin II receptor blockers (42.4% vs. 58.4%), annual household income >$75,000 (32.9% vs. 40.0%), and maternal education level of college or greater (30.5% vs. 39.3%).
Table 1. Population Characteristics.
Continuous variables are shown in median [interquartile range] and categorical variables are in n (%).
| Characteristic | Masked hypertension (n=170) | Normal ambulatory blood pressure (n=639) |
|---|---|---|
| Age, y | 13 [10-16] | 14 [11-16] |
| Male sex | 93 (54.7%) | 368 (57.6%) |
| Race | ||
| White | 121 (71.2%) | 483 (75.6%) |
| African American | 19 (11.2%) | 49 (7.7%) |
| Others | 30 (17.6%) | 107 (16.7%) |
| Hispanic/Latino | 12 (7.1%) | 92 (14.4%) |
| Clinic systolic blood pressure, pct | 53 [34-70] | 40 [20-59] |
| Clinic diastolic blood pressure, pct | 59 [39-82] | 48 [25-68] |
| Height, pct | 27 [7-50] | 33 [13-61] |
| Weight, pct | 42 [12-78] | 51 [23-81] |
| Body mass index, pct | 51 [23-83] | 61 [32-86] |
| Low birth weight | 27 (17.1%) | 113 (18.6%) |
| Duration of CKD, y | 12 [9-15] | 12 [9-15] |
| Glomerular CKD | 33 (19.4%) | 136 (21.3%) |
| estimated GFR, mL/min/1.73 m2 | 49 [36-62] | 50 [37-64] |
| Serum sodium, mmol/L | 139 [138-141] | 139 [138-141] |
| Serum potassium, mmol/L | 4 [4-5] | 4 [4-5] |
| Total cholesterol, mg/dL | 167 [146-187] | 163 [142-187] |
| Triglycerides, mg/dL | 106 [77-158] | 101 [72-150] |
| LDL, mg/dL | 90 [73-105] | 88 [71-109] |
| Hemoglobin, pct | 22 [3-63] | 18 [1-57] |
| Left ventricular mass, pct | 42 [15-77] | 37 [14-66] |
| Medications | ||
| ACE inhibitor | 113 (66.5%) | 436 (68.2%) |
| ARB | 72 (42.4%) | 373 (58.4%) |
| Diuretics | 17 (10.0%) | 48 (7.5%) |
| Corticosteroids | 16 (9.4%) | 45 (7.0%) |
| Annual household income | ||
| ≤$36,000 | 64 (38.3%) | 182 (29.7%) |
| $36,001-75,000 | 48 (28.7%) | 185 (30.2%) |
| >$75,000 | 55 (32.9%) | 245 (40.0%) |
| Maternal education | ||
| Highschool | 59 (36.0%) | 200 (32.5%) |
| Some college | 55 (33.5%) | 173 (28.1%) |
| College and higher | 50 (30.5%) | 242 (39.3%) |
Abbreviations: Pct, percentile; CKD, chronic kidney disease; LDL, low density lipoprotein; ACE, angiotensin converting enzyme; and ARB, angiotensin II receptor blocker.
Role of Individual Predictors
Table 2 shows the variable importance of the predictors from the final gradient boosting model. The relative contribution of the predictors to the model’s ability to predict masked HTN was evaluated using variable importance, which is expressed as a percent. Among the 9 predictors included in this model, clinic SBP percentile had the highest (14.1%) variable importance, followed by day of the year (13.5%), clinic DBP percentile (12.9%), and urine protein (12.3%). The associations of these predictors with masked HTN are presented in Figure S1. For SBP/DBP and LVM, the percentile values showed higher importance than the raw values. Additionally, we estimated the association of clinic SBP and DBP percentiles with masked HTN after controlling for other predictors using SHAP values (Figure 1). Overall, the predicted odds of masked HTN increased notably when clinic SBP exceeded the 20th percentile or DBP exceeded the 80th percentile, although it was low and relatively uniform below those thresholds. Among cases with clinic SBP≥20th percentile and DBP<80th percentile, there were no discernable pattern between clinic BP percentiles and the predicted odds of masked HTN.
Table 2. Variable Importance of the Predictors included in the Final Gradient Boosting Model.
Variable importance was evaluated using the mean absolute Shapley additive explanations (SHAP) values, obtained from the “final” gradient boosting model (See Table 3) and transformed to a relative scale with a total of 100%.
| Rank | Feature | Importance |
|---|---|---|
| 1 | Clinic systolic blood pressure percentile | 14.1% |
| 2 | Day of the year | 13.5% |
| 3 | Clinic diastolic blood pressure percentile | 12.9% |
| 4 | Urine protein | 12.3% |
| 5 | Left ventricular mass percentile | 11.8% |
| 6 | Body mass index percentile | 11.1% |
| 7 | Mean corpuscular hemoglobin concentration | 9.2% |
| 8 | Angiotensin II receptor blocker use | 8.5% |
| 9 | Clinic diastolic blood pressure range* | 6.5% |
Clinic blood pressure was measured three times consecutively. Range represents the difference between the highest and the lowest measurements.
Figure 1. Relative Odds of Masked Hypertension by Clinic Systolic and Diastolic Blood Pressure Percentiles.
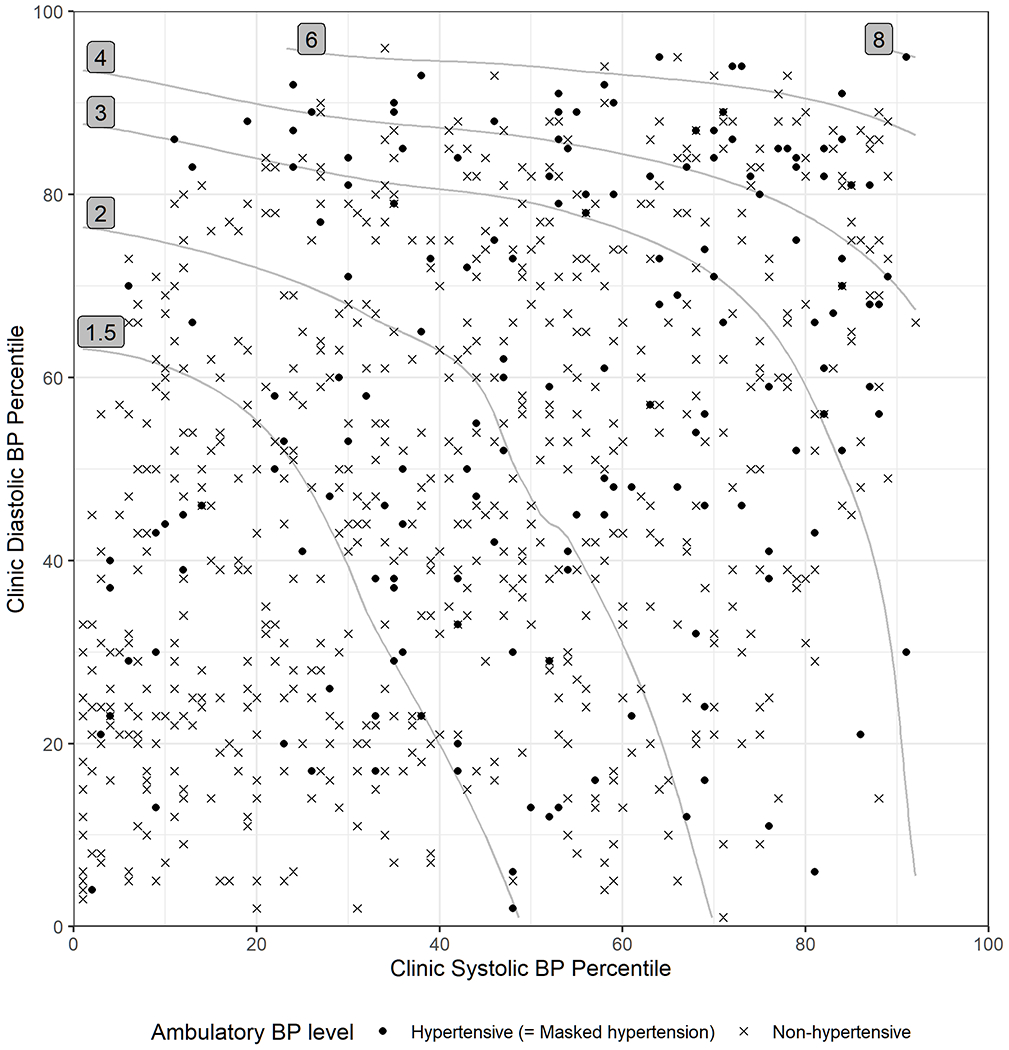
The association of clinic systolic and diastolic BP percentiles with masked hypertension was characterized using the exploratory gradient boosting model and represented in relative odds compared to the lowest risk level. The raw data were overlaid as dots or crosses according to the observed ambulatory BP outcome. Abbreviations: BP, blood pressure.
Prediction Performance
Table 3 summarizes the models’ prediction performance. AUC ranged from 0.660 (95% CI; 0.595, 0.707) in the exploratory gradient boosting model to 0.732 (95% CI; 0.695, 0.786) in the final random forests. Brier score ranged from 0.148 (95% CI; 0.141, 0.154) in the logistic regression to 0.167 (95% CI; 0.152, 0.183) in the exploratory gradient boosting, which respectively represented 10.8% and 1.7% relative reduction compared to the null model. The ML models showed similar receiver operating characteristic (ROC) curves, whereas the logistic regression showed more homogeneous ROC curves across the bootstrap iterations (Figure S2).
Table 3. Comparison of Prediction Performance among Models.
“Exploratory” models used all 109 predictors. “Final” models used 9 predictors (Table 2), which were selected based on previous literature and the variable importance from the exploratory gradient boosting model. Discrimination and calibration of these models were assessed via AUC and Brier score, respectively, using the 0.632+ bootstrap validation method. The scaled Brier score represents the relative reduction in Brier score achieved with a prediction model compared to a “null” model.
| Model | AUC | Brier Score | Scaled Brier Score |
|---|---|---|---|
| Gradient Boosting | |||
| Exploratory | 0.660 (0.595, 0.707) | 0.167 (0.152, 0.183) | 1.7% (−5.7%, 6.7%) |
| Final | 0.704 (0.648, 0.761) | 0.159 (0.145, 0.176) | 4.6% (−3.2%, 11.0%) |
| Random Forests | |||
| Exploratory | 0.693 (0.640, 0.757) | 0.157 (0.141, 0.176) | 6.6% (3.9%, 10.2%) |
| Final | 0.732 (0.695, 0.786) | 0.151 (0.133, 0.168) | 8.3% (2.1%, 14.0%) |
| Logistic Regression | |||
| Final | 0.717 (0.699, 0.732) | 0.148 (0.141, 0.154) | 10.8% (8.9%, 12.5%) |
Abbreviations: AUC, area under receiver operating characteristics curve.
The calibration curve analysis showed mixed results. The logistic regression showed the most favorable metrics, with an intercept of 0.002 (95% CI; −0.154, 0.154) and a slope of 0.898 (95% CI; 0.725, 1.096), which did not significantly deviate from the ideal values of 0 and 1, respectively. The ML-based prediction models showed slightly worse calibration (Table S2). The LOESS calibration curves corroborate these assessments (Figure S2). Of note, at mild and moderate risk levels (e.g., predicted probability <0.4), the calibration curves were close to the identity line, indicating a relatively acceptable calibration in these risk levels. However, at higher risk levels, some of the bootstrapped curves showed severe departures from the identity line, possibly due to the small sample size at this risk level.
Prevalence of Masked Hypertension by Clinic Blood Pressure
Based on the findings above (Figure 1), we divided our cohort into a low-risk group (clinic SBP<20th percentile and DBP<80th percentile) versus a high-risk group (clinic SBP≥20th percentile or DBP≥80th percentile). The low-risk group was substantially less likely to have masked HTN compared to the high-risk group (9.0% vs. 23.8%).
When stratified into quintiles of clinic SBP and DBP percentiles (Figure 2), masked HTN was the least common (4.8%) among cases in which clinic SBP and DBP were both <20th percentile. Masked HTN was also relatively less common (7.4%-13.2%; Figure 2) in other strata with clinic SBP<20th percentile and DBP<80th percentile, and in the stratum with clinic SBP 20th-40th percentile and DBP<20th percentile. Among cases in which clinic SBP and DBP were both between 20th and 80th percentiles, the observed prevalence ranged from 12.9% and 31.2% without a discernable pattern. Among cases in which either clinic SBP or DBP was ≥80th percentile, the observed prevalence was slightly higher than the rest of the cases.
Figure 2. Prevalence of Masked Hypertension by Clinic Systolic and Diastolic Blood Pressure Percentiles.
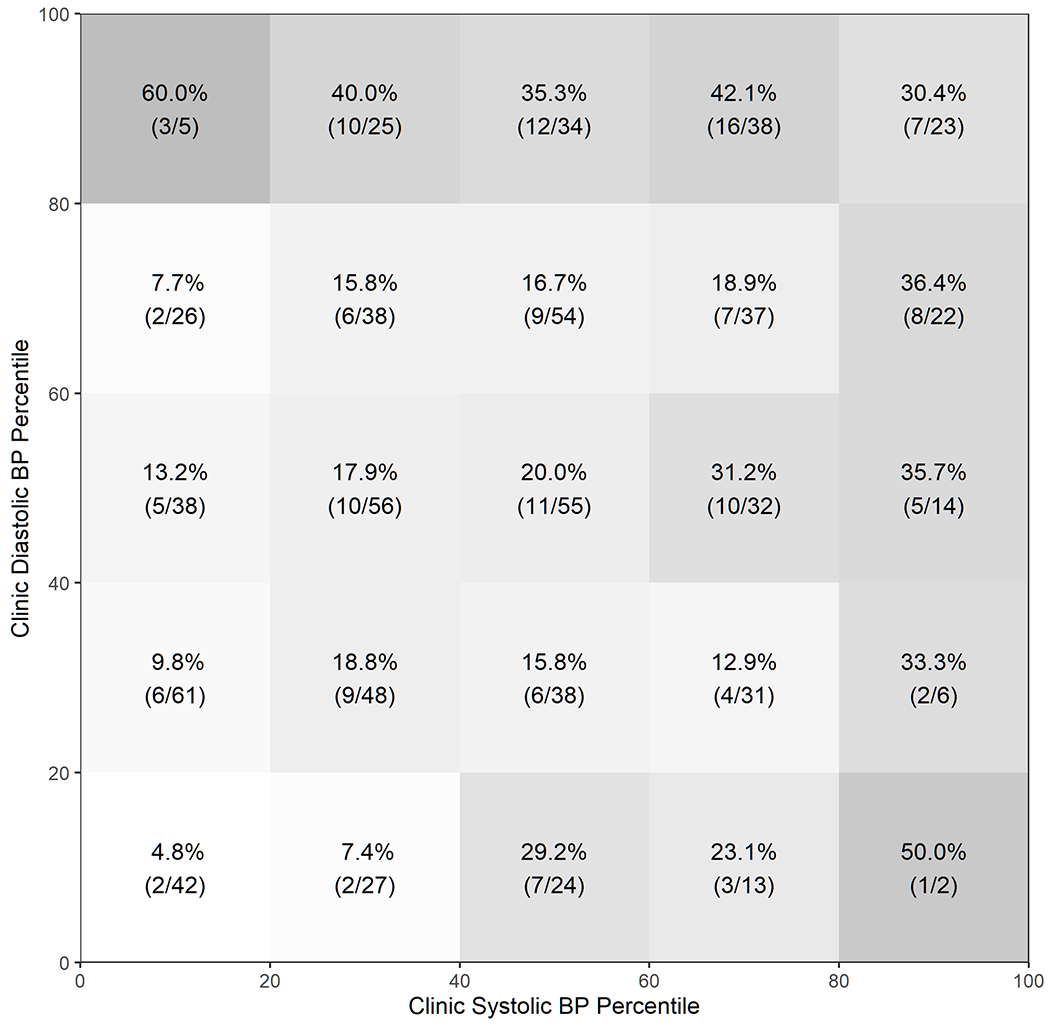
Parentheses represent (masked hypertension / all cases).
DISCUSSION
In our study of 809 children and adolescents with CKD and non-hypertensive clinic BP levels, higher clinic BP levels and other several clinical factors were predictive of masked HTN. However, despite several meaningful trends observed in the prediction models, the overall accuracy was not remarkably high even after assessing 109 clinical factors using multiple robust prediction algorithms. On one hand, using the potential thresholds identified using our prediction model, we found that masked HTN was less common among cases with clinic SBP<20th percentile and DBP<80th percentile compared to the rest of the study population (9.0% vs 23.8%). These thresholds may serve as simple, although not precise, criteria to evaluate the necessity for ABPM on an individual basis. On the other hand, above these thresholds, the lack of a clear risk stratification supports the current recommendation for routine ABPM in children with CKD over its potential alternative, a selective use of ABPM based on the patient’s predicted risk of masked HTN.
At the individual clinical factor level, our findings correspond with previous studies that have identified risk factors for masked HTN in children with CKD. Clinic BP readings are perhaps the most studied and used predictor of masked HTN. The AHA statement on ABPM in children and adolescents has suggested that elevated clinic BP, especially when within 20% of the 95th percentile, may serve as a possible indicator of masked HTN (8–10). Similarly, a more recent analysis of the CKiD data found a very low prevalence of elevated ambulatory BP levels (2%-7%) among children with clinic BP below the 25th percentile (2). These findings were replicated in our graphical analyses (Figures 1 and 2), and clinic SBP and DBP percentiles were highly informative predictors in our full prediction model (Table 2). Another previous analysis of the CKiD data found an association of left ventricular hypertrophy with masked HTN (3). Our analysis also identified LVM percentile as one of the informative predictors (Table 2). Lastly, our models suggested day of the year as one of the influential predictors; specifically, masked HTN was observed more commonly during colder seasons (Figure S1). These findings are consistent with previous studies that reported associations of winter season and low ambient temperature with BP variability and masked HTN (27–30).
Our prediction performance is generally comparable with those from previous studies in children and adolescents. A cross-sectional study of healthy adolescents (11-19 years old) who underwent ABPM as part of a study of BP levels and cardiovascular risk in youth (n=247) reported sensitivity and specificity of 0.868 and 0.574 using clinic SBP over the 85th percentile as the threshold (31). Another study of 55 obese children reported sensitivity and specificity of 0.67 and 0.78 using clinic SBP over the 93rd percentile as the threshold (32). The prediction performance reported in these studies are generally similar to our prediction performance estimates, especially considering that our study population may have a more complicated pathophysiology due to CKD and concomitant use of angiotensin converting enzyme inhibitors (ACEi) and angiotensin II receptor blockers (ARB). Additionally, we evaluated the prediction performance in a dedicated validation procedure, a practice that typically yields a more robust and conservative estimate of the prediction performance (22,23).
Our findings have two implications. First, when clinic SBP and DBP are both low, the risk of masked HTN appears to be low, although not zero. The observed prevalence in our population was substantially lower among cases with clinic SBP<20th and DBP<80th percentiles (9.0%), and even lower among cases with clinic SBP<20th and DBP<20th percentiles (4.8%), compared to the overall prevalence of 21.0%. ABPM would still be beneficial below these thresholds, but to a smaller extent. The benefits and burdens of ABPM could be considered on an individual basis below these thresholds. However, above these thresholds, neither our prediction model nor our clinic BP stratification analysis provided an effective risk stratification that may justify a selective use of ABPM. In these cases, there appears to be no viable alternative to the currently recommended practice of routine ABPM.
There are several limitations to this study. First, by using additional predictors or alternative analytical methods, one might achieve sufficiently high prediction performance to support a selective use of ABPM in all children with CKD. There still exists a possibility that an alternative statistical method would yield superior prediction performance than what we observed. However, considering the robustness of the ML algorithms we used in this analysis, we hypothesize that the prediction performance observed in our study was likely limited by unmeasured variables rather than the analytic techniques. Although we assessed 109 clinical factors that represent a wide spectrum of commonly available clinical information, masked HTN appears to be mostly determined by unmeasured factors. Second, some subclinical and non-clinical factors that may influence ABPM results or BP patterns were not available for analysis in our data. Additional information regarding clinic BP (e.g., time of the day or time since the last meal) or ambulatory BP (e.g., physical activity or mental stress during the day) may improve the performance of the prediction models (33,34). Particularly, the impact of seasonal variation on masked HTN deserves further exploration. Several factors like ambient temperature, school seasons, and holidays may explain this observation; more detailed circumstantial data may be needed.
Supplementary Material
PATHOPHYSIOLOGIC NOVELTY AND SIGNIFICANCE.
What is New?
A machine learning algorithm to predict masked hypertension identified SBP<20th and DBP<80th percentile as possible thresholds below which masked hypertension was relatively uncommon.
What is Relevant?
The importance of detecting and managing masked hypertension often collides with the burdens of conducting ambulatory blood pressure monitoring (ABPM) in children. A risk stratification may enable a selective application of ABPM.
What are the Pathophysiological Implications?
Our results support that ABPM might be performed selectively, rather than routinely, in children with CKD presenting sufficiently low clinic blood pressure. Above those clinic blood pressure levels, the current recommendation practice of routine ABPM assessment remains a more prudent strategy in pediatric CKD management.
PERSPECTIVES.
Our prediction model to identify masked HTN in children with CKD presenting with non-hypertensive clinic BP revealed some meaningful trends, while it did not achieve remarkably high accuracy. On one hand, our model showed that masked HTN was notably less common in cases with clinic SBP<20th and DBP<80th percentiles. These thresholds might be used to assess the necessity of ABPM on an individual basis. On the other hand, above these clinic BP thresholds, our analyses did not yield a sufficient risk stratification that may support a selective use of ABPM and underscore the need to identify more variables that may be useful for prediction of masked hypertension. Taken together, our findings suggest that a selective use of ABPM based on the individual risk profile could be carefully considered in children with CKD when their clinic BP levels are sufficiently low. However, among those with clinic BP above those levels, the currently recommended practice of routine ABPM is a more prudent strategy because neither clinic BP nor other clinical information could predict the risk of masked HTN accurately.
ACKNOWLEDGEMENTS
Data in this manuscript were collected by the Chronic Kidney Disease in children prospective cohort study (CKiD) with clinical coordinating centers (Principal Investigators) at Children’s Mercy Hospital and University of Missouri Kansas City (Bradley Warady) and Children’s Hospital of Philadelphia (Susan Furth), Central Biochemistry Laboratory (George Schwartz) at University of Rochester Medical Center, and data coordinating center (Alvaro Muñoz and Derek Ng) at Johns Hopkins Bloomberg School of Public Health. (Collaborator list: https://statepi.jhsph.edu/ckid/site-investigators/)
Funding:
The CKiD Study is supported by grants from National Institute of Diabetes and Digestive and Kidney Diseases, with additional funding from Eunice Kennedy Shriver National Institute of Child Health and Human Development, and National Heart, Lung, and Blood Institute (U01DK066143, U01DK066174, U24DK082194, U24DK066116).
ABBREVIATIONS
- CKiD
The Chronic Kidney Disease in Children study
- LOESS
Locally-estimated scatterplot smoothing
- LVM
Left ventricular mass
- ML
Machine learning
- SHAP
Shapley additive explanations
Footnotes
Conflicts of Interest: None.
Availability of Data and Material: Data for this analysis can be obtained from the National Institute of Diabetes and Digestive and Kidney Diseases Central Repository.
REFERENCES
- 1.Samuels J, Ng D, Flynn JT, Mitsnefes M, Poffenbarger T, Warady BA, et al. Ambulatory blood pressure patterns in children with chronic kidney disease. Hypertension 2012;60(1):43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mitsnefes MM, Pierce C, Flynn J, Samuels J, Dionne J, Furth S, et al. Can office blood pressure readings predict masked hypertension? Pediatr Nephrol 2016;31(1):163–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mitsnefes M, Flynn J, Cohn S, Samuels J, Blydt-Hansen T, Saland J, et al. Masked hypertension associates with left ventricular hypertrophy in children with CKD. J Am Soc Nephrol 2010;21(1):137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The ESCAPE Trial Group. Strict Blood-Pressure Control and Progression of Renal Failure in Children. N Engl J Med 2009;361(17):1639–1650. [DOI] [PubMed] [Google Scholar]
- 5.Mitsnefes M, Ho P-L, McEnery PT. Hypertension and progression of chronic renal insufficiency in children: a report of the North American Pediatric Renal Transplant Cooperative Study (NAPRTCS). J Am Soc Nephrol 2003;14(10):2618–2622. [DOI] [PubMed] [Google Scholar]
- 6.Warady BA, Abraham AG, Schwartz GJ, Wong CS, Muñoz A, Betoko A, et al. Predictors of Rapid Progression of Glomerular and Nonglomerular Kidney Disease in Children and Adolescents: The Chronic Kidney Disease in Children (CKiD) Cohort. American Journal of Kidney Diseases 2015;65(6):878–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flynn JT, Kaelber DC, Baker-Smith CM, Blowey D, Carroll AE, Daniels SR, et al. Clinical Practice Guideline for Screening and Management of High Blood Pressure in Children and Adolescents. Pediatrics 2017;140(3). [DOI] [PubMed] [Google Scholar]
- 8.Flynn JT, Daniels SR, Hayman LL, Maahs DM, McCrindle BW, Mitsnefes M, et al. Update: Ambulatory Blood Pressure Monitoring in Children and Adolescents: A Scientific Statement From the American Heart Association. Hypertension 2014;63(5):1116–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graves JW, Althaf MM. Utility of ambulatory blood pressure monitoring in children and adolescents. Pediatr Nephrol 2006;21(11):1640–1652. [DOI] [PubMed] [Google Scholar]
- 10.Urbina E, Alpert B, Flynn J, Hayman L, Harshfield GA, Jacobson M, et al. Ambulatory blood pressure monitoring in children and adolescents: recommendations for standard assessment: a scientific statement from the American Heart Association Atherosclerosis, Hypertension, and Obesity in Youth Committee of the council on cardiovascular disease in the young and the council for high blood pressure research. Hypertension 2008;52(3):433–451. [DOI] [PubMed] [Google Scholar]
- 11.Foster JC, Liu D, Albert PS, Liu A. Identifying subgroups of enhanced predictive accuracy from longitudinal biomarker data by using tree-based approaches: applications to fetal growth. Journal of the Royal Statistical Society: Series A (Statistics in Society) 2017;180(1):247–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rajkomar A, Dean J, Kohane I. Machine Learning in Medicine. N Engl J Med 2019;380(14):1347–1358. [DOI] [PubMed] [Google Scholar]
- 13.Bae S, Massie AB, Caffo BS, Jackson KR, Segev DL. Machine learning to predict transplant outcomes: helpful or hype? A national cohort study. Transpl Int 2020;33(11):1472–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furth SL, Cole SR, Moxey-Mims M, Kaskel F, Mak R, Schwartz G, et al. Design and methods of the Chronic Kidney Disease in Children (CKiD) prospective cohort study. Clin J Am Soc Nephrol 2006;1(5):1006–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flynn JT, Urbina EM, Brady TM, Baker-Smith C, Daniels SR, Hayman LL, et al. Ambulatory Blood Pressure Monitoring in Children and Adolescents: 2022 Update: A Scientific Statement From the American Heart Association. Hypertension 2022;79(7). [DOI] [PubMed] [Google Scholar]
- 16.Wühl E, Witte K, Soergel M, Mehls O, Schaefer F, German Working Group on Pediatric Hypertension. Distribution of 24-h ambulatory blood pressure in children: normalized reference values and role of body dimensions. J Hypertens 2002;20(10):1995–2007. [DOI] [PubMed] [Google Scholar]
- 17.Chen T, Guestrin C. XGBoost: A Scalable Tree Boosting System. In: Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining - KDD ’16. San Francisco, California, USA: ACM Press; 2016:785–794. [Google Scholar]
- 18.Breiman L Random Forests. Mach Learn 2001;45(1):5–32. [Google Scholar]
- 19.Lundberg SM, Lee S-I. A Unified Approach to Interpreting Model Predictions. Advances in Neural Information Processing Systems 2017;30:4765–4774. [Google Scholar]
- 20.Bertsimas D, Dunn J. Optimal classification trees. Mach Learn 2017;106(7):1039–1082. [Google Scholar]
- 21.Ruggieri S Complete Search for Feature Selection in Decision Trees. Journal of Machine Learning Research 2019;20(104):1–34. [Google Scholar]
- 22.Efron B, Tibshirani R. Improvements on Cross-Validation: The .632+ Bootstrap Method. Journal of the American Statistical Association 1997;92(438):548. [Google Scholar]
- 23.Steyerberg EW, Harrell FE, Borsboom GJ, Eijkemans MJ, Vergouwe Y, Habbema JD. Internal validation of predictive models: efficiency of some procedures for logistic regression analysis. J Clin Epidemiol 2001;54(8):774–781. [DOI] [PubMed] [Google Scholar]
- 24.Alba AC, Agoritsas T, Walsh M, Hanna S, Iorio A, Devereaux PJ, et al. Discrimination and Calibration of Clinical Prediction Models: Users’ Guides to the Medical Literature. JAMA 2017;318(14):1377. [DOI] [PubMed] [Google Scholar]
- 25.Van Calster B, Nieboer D, Vergouwe Y, De Cock B, Pencina MJ, Steyerberg EW. A calibration hierarchy for risk models was defined: from utopia to empirical data. Journal of Clinical Epidemiology 2016;74:167–176. [DOI] [PubMed] [Google Scholar]
- 26.Van Calster B, McLernon DJ, van Smeden M, Wynants L, Steyerberg EW, Topic Group ‘Evaluating diagnostic tests and prediction models’ of the STRATOS initiative. Calibration: the Achilles heel of predictive analytics. BMC Med 2019;17(1):230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jehn M, Appel LJ, Sacks FM, Miller ER, DASH Collaborative Research Group. The effect of ambient temperature and barometric pressure on ambulatory blood pressure variability. Am J Hypertens 2002;15(11):941–945. [DOI] [PubMed] [Google Scholar]
- 28.Alpérovitch A, Lacombe J-M, Hanon O, Dartigues J-F, Ritchie K, Ducimetière P, et al. Relationship between blood pressure and outdoor temperature in a large sample of elderly individuals: the Three-City study. Arch Intern Med 2009;169(1):75–80. [DOI] [PubMed] [Google Scholar]
- 29.Iimuro S, Imai E, Watanabe T, Nitta K, Akizawa T, Matsuo S, et al. Clinical Correlates of Ambulatory BP Monitoring among Patients with CKD. CJASN 2013;8(5):721–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ziegelasch N, Vogel M, Siekmeyer W, Billing H, Dähnert I, Kiess W. Seasonal variation of blood pressure in children. Pediatr Nephrol 2021;36(8):2257–2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hamdani G, Flynn JT, Becker RC, Daniels SR, Falkner B, Hanevold CD, et al. Prediction of Ambulatory Hypertension Based on Clinic Blood Pressure Percentile in Adolescents. Hypertension 2018;72(4):955–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bhatt GC, Pakhare AP, Gogia P, Jain S, Gupta N, Goel SK, et al. Predictive Model for Ambulatory Hypertension Based on Office Blood Pressure in Obese Children. Front Pediatr 2020;8:232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Franklin SS, O’Brien E, Thijs L, Asayama K, Staessen JA. Masked Hypertension: A Phenomenon of Measurement. Hypertension 2015;65(1):16–20. [DOI] [PubMed] [Google Scholar]
- 34.Pickering TG, Davidson K, Gerin W, Schwartz JE. Masked Hypertension. Hypertension 2002;40(6):795–796. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


