Abstract
Background:
Hypertension-related increased arterial stiffness predicts development of target organ damage (TOD) and cardiovascular disease (CVD. We hypothesized that BP-related increased arterial stiffness is present in youth with elevated BP and is associated with TOD.
Methods:
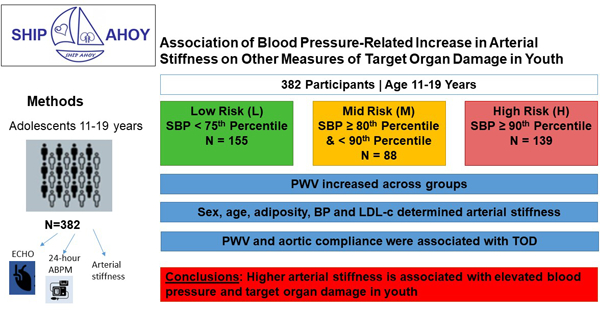
Participants were stratified by systolic blood pressure (SBP) into low- (L = SBP <75th percentile, N= 155), mid- (M = SBP ≥80th and <90th percentile, N= 88), and high-risk BP categories (H ≥ 90th percentile, N= 139), based on age-, sex- and height-specific pediatric BP cut-points. Clinic BP, 24-hour ambulatory blood pressure monitoring (ABPM), anthropometrics and laboratory data were obtained. Arterial stiffness measures included carotid-femoral pulse wave velocity (PWV), and aortic stiffness. Left ventricular mass index (LVMi), LV systolic and diastolic function and urine albumin/creatinine were collected. ANOVA with Bonferroni correction was used to evaluate differences in CV risk factors, PWV, and cardiac function across groups. General linear models were used to examine factors associated with arterial stiffness, and to determine if arterial stiffness is associated with TOD after accounting for blood pressure.
Results:
PWV increased across groups. Aortic distensibility, distensibility coefficient and compliance were greater in L than in M or H group. Significant determinants of arterial stiffness were sex, age, adiposity, BP and LDL-c. PWV and Aortic Compliance were significantly associated with TOD (systolic and diastolic cardiac function and urine albumin/creatinine ratio) after controlling for blood pressure.
Conclusions:
Higher arterial stiffness is associated with elevated BP and TOD in youth emphasizing the need for primary prevention of CVD.
Keywords: blood pressure, arterial stiffness, pediatrics, cardiac function, target organ damage, pulse wave velocity
Graphical Abstract
Introduction:
Cardiovascular disease (CVD) is the world’s leading cause of death.[1] Earlier onset of hypertension (HTN) is associated with greater risk for CVD,[2] which is concerning since the prevalence of elevated blood pressure (BP) in youth is near 15%.[3] Elevated BP and HTN lead to early vascular aging (EVA)[4, 5] which is associated with target organ damage (TOD) in adults[6] due to the transmission of high pressure pulsatile flow to delicate capillaries in important organs. Carotid-femoral pulse wave velocity (PWV), a measure of central arterial stiffness, is the gold standard assessment for EVA.[4] Measures of aortic stiffness and elasticity are additional measures that are also altered in pathophysiological conditions such as atherosclerosis, diabetes, and HTN.[7] Measurement of EVA is important as it is an independent predictor of CV events in adults.[8] Limited pediatric data show a similar relationship between EVA, BP and TOD but include few participants with elevated BP.[9, 10] We hypothesized that youth with elevated BP would have EVA[4] and that arterial stiffness would be related to TOD after controlling for BP.
Methods:
The study underwent institutional review board approval at each institution. Pparticipants and their parent/guardian provided written informed assent and consent. The data are available from the corresponding author upon reasonable request.
Population:
Participants in our multi-center study to evaluate the CV effects of elevated BP included 382 youth 60% male, 63% White and 16% Hispanic, age 11–19 years, mean = 15.6 ± 1.8 years, 35% of the participants were lean, 20% were overweight and 45% were obese [11]). Participants were stratified by systolic BP (SBP) into low-risk (L = SBP <75th percentile, N= 155), mid-risk (M = SBP ≥80th and <90th percentile, N= 88), and high-risk BP groups (H= SBP ≥ 90th percentile, N= 139) by the Fourth Report on High BP in Children,[12] since recruitment for the study started prior to release of the American Academy of Pediatrics Clinical Practice Guidelines.[13] For analyses, BP percentiles from the 2017 CPG on BP[13] were used. Exclusion criteria included current antihypertensive drug treatment or medications affecting BP, diabetes mellitus (type 1 or 2), chronic kidney disease, congenital heart disease, or secondary hypertension. Demographic, anthropometric data, vital signs, and lab values (fasting lipid panel, fasting glucose and insulin, creatinine, uric acid, C-reactive protein [CRP], urine Na/K ratio, urine albumin/creatinine ratio [ACR]) were obtained as previously described.[14]
Clinic BP Measurement:
Cuff size was based on arm circumference.[13] Blood pressures were obtained in the right arm by auscultation using an aneroid sphygmomanometer (Mabis MedicKit5; Mabis Healthcare, Waukegan, IL). Four BP measurements were obtained at 30-second intervals on each of 2 visits, with the average of the 2nd, 3rd, 4th and 6th, 7th, 8th BP measurements used.[15]
ABPM Measurement:
Ambulatory blood pressure was measured with the OnTrak 90227 (SpaceLabs, Snoqualmie, WA) according to pediatric ABPM guidelines.[16] ABP index was calculated as the mean measured BP divided by the 95th percentile from the pediatric normative data for sex and height.[17]
Measures of CV TOD:
Cardiac images[14] were read using Cardiology Analysis System (Digisonics, Houston, TX). LV mass was calculated using the Deveraux equation[18–20] and indexed (LVMI) to ht2.7[21]. Systolic function was evaluated with global longitudinal strain (GLS), strain rate, tine to peak longitudinal strain, time to peak longitudinal strain rate, LV ejection fraction (LVEF) and stroke volume (SV) (TOMTEC Corporation, Chicago, IL).[22] Cardiac output and systemic vascular resistance were calculated using standard formulas, with the assumption of 3 mmHg for CVP. Diastolic function was assessed using Doppler for mitral E/A and with tissue Doppler for average (septal/free wall) e’/a’ and E/e’.
First morning urine albumin/creatinine (ACR) was obtained (microvascular dysfunction).[23, 24] Two individuals with extremely high ACR, had ACR set to missing.
Arterial Stiffness:
Pulse wave velocity:
Carotid-femoral Pulse wave velocity (PWV) was measured using a SphygmoCor CPV (AtCor Medical, Sydney, Australia) [25, 26]. The pulse transit time (PTT) is the difference in time between the peak of the R-wave (from ECG leads) to the foot of the femoral pressure wave (obtained with a tonometer) minus the R-wave to foot of the carotid pressure wave time and PWV is distance/PTT. PWV is highly reproducible with coefficient of variation of 7%.[27]
Aortic Stiffness:
Aortic stiffness was calculated using maximum diastolic and minimum systolic diameters of the ascending aorta 3–4 cm above the aortic valve in the parasternal long axis view.[28–31]
Aortic strain:
100(AoS – AoD)/AoD
Aortic distensibility:
(2 x Ao strain)/ (SBP – DBP)
Beta Stiffness index:
β = Ln(SBP/DBP)/Ao strain
Distensibility coefficient:
[2 x [(AoS – AoD)/AoD]/(SBP – DBP)]
Aortic compliance:
π[(AoS2-AoD2)/4(SBP-DBP)]
Peterson Elastic Modulus[32]:
Ep = (SBP-DBP)(AoD)/(AoS-AoD)
Statistical Analysis:
Statistical Analysis Software (SAS Institute Inc., version 9.4, Cary, North Carolina, USA) was used. Means/frequencies were obtained by BP group. Variance stabilizing procedures were employed as needed. Differences between groups were analyzed using analysis of variance with Bonferroni correction for multiple compaisons or chi square.
Independent determinants of arterial stiffness were determined from a full linear model (age, sex, race, ethnicity, waist/height ratio, LDL-C, TG/HDL ratio, HOMA-IR, Creatinine, Uric Acid, CRP, MAP, daytime Ambulatory SBP and DBP indices) reduced until all parameters remaining were significant. Additional models were constructed to determine if arterial stiffness remained a significant determinant of TOD after adjustments (same variables omitting laboratory values). Correlations were higher between arterial stiffness and BP and TOD than Cardiac Index and SVR so only arterial stiffness was modeled. Mediation analysis was performed to evaluate whether BP was mediating the relationship between arterial stiffness and TOD.
Results:
CV Risk Factors by BP group Tables 1 and 2) showed more adverse profile at higher BP. The majority of participants were overweight (20%) or obese (45%). There were more participants with obesity in the H group compared to L group. There were no differences in lipid between groups except HDL was higher in the L compared to H group and glucose, HOMA-IR, insulin and CRP were significantly lower in the L group compared to the H group. Uric acid was lower in the L compared to the H group (p<0.05). There were no significant differences found in the urine sodium/potassium ratio. Both daytime and nighttime ambulatory systolic and mean diastolic BP and indexed DBP increased across groups. There were no significant differences in systolic or diastolic dipping among groups.
Table 1.
Description of the study population (means, standard deviations or frequencies).
| Characteristics | Low (L) | Mid (M) | High (H) |
|---|---|---|---|
| N=155 | N=88 | N=139 | |
| Age (years)* | 15.6 ± 1.6 | 15.9 ± 1.8 | 15.3 ± 1.9 |
| Race (% non-White | 25.0 | 37.9 | 37.1 |
| Sex (% Male)† | 52.3 | 71.6 | 61.2 |
| Height (cm)* | 168.7 ± 9.1 | 171.8 ± 10.9 | 168.5 ± 9.9 |
| Weight (kg)‡ | 76.7 ± 23.3 | 85.6 ± 29.0 | 84.8 ± 25.0 |
| BMI (kg/m2)§ | 26.7 ± 7.0 | 28.7 ± 8.6 | 29.7 ± 7.9 |
| BMI percentile (%)§ | 78.0 ± 41.5 | 80.6 ± 20.9 | 86.4 ± 20.9 |
| Obese (%)§ | 36.1 | 46.6 | 54.7 |
| Waist (cm)§ | 86.1 ± 18.8 | 89.1 ± 19.5 | 93.5 ± 19.7 |
| Waist/height ratio|| | 0.5 ± 0.1 | 0.5 ± 0.1 | 0.6 ± 0.1 |
| Total cholesterol (mg/dl) | 152.0 ± 30.1 | 153.8 ±34.8 | 154.3 ± 33.2 |
| LDL (mg/dl) | 86.6 ± 26.9 | 90.7 ± 28.8 | 92.5 ± 28.4 |
| HDL (mg/dl)# | 47.4 ± 12.2 | 44.1 ± 11.5 | 43.3 ± 11.7 |
| Triglycerides (mg/dl) | 94.5 ± 62.2 | 99.4 ± 58.6 | 96.3 ± 48.8 |
| Glucose (mg/dl)§ | 87.9 ± 8.0 | 89.5 ± 10.4 | 90.8 ± 8.2 |
| Insulin (microIU/dl)§ | 17.9 ± 14.4 | 20.0 ± 14.4 | 23.8 ± 19.0 |
| HOMA_IR§ | 3.98 ± 3.46 | 4.47 ± 3.31 | 5.48 ± 4.78 |
| CRP (mg/dl)§ | 1.30 ± 1.69 | 1.39 ± 1.74 | 1.95 ± 2.16 |
| Creatinine (mg/dl)** | 0.72 ± 0.13 | 0.79 ± 0.18 | 0.72 ± 0.17 |
| Uric acid (mg/dl)§ | 5.41 ± 1.63 | 5.81 ± 1.40 | 6.04 ± 1.52 |
| Urine sodium/potassium ratio | 4.09 ± 3.01 | 4.19 ± 3.16 | 3.99 ± 2.64 |
Bonferroni adjusted P≤0.04 for
H<M
M<L
L<M&H
L< H
L&M<H
H<L
L&H<M
BMI, body mass index; CRP, C-reactive protein; DBP, diastolic blood pressure; HDL, high density lipoprotein; HR, heart rate; K1, Korotkoff sound 1; K5, Korotkoff sound 5; LDL, low density lipoprotein; MAP, mean arterial pressure; SBP, systolic blood pressure.
Table 2.
Cardiovascular parameters stratified by BP group (means, standard deviations).
| Characteristics | Low (L) | Mid (M) | High (H) |
|---|---|---|---|
| N=155 | N=88 | N=139 | |
| K1 SBP (mmHg)* | 111.5 ± 9.8 | 126.0 ± 5.6 | 132.7 ± 7.2 |
| K4 DBP (mmHg)† | 74.3 ± 13.1 | 82.9 ± 12.8 | 86.3 ± 13.4 |
| K5 (mmHg)† | 67.2 ± 9.9 | 73.3 ± 8.1 | 75.4 ± 10.5 |
| MAP (mmHg)* | 81.9 ± 8.7 | 90.9 ± 5.9 | 94.5 ± 7.6 |
| SBP percentile (%)* | 50.6 ± 27.1 | 86.1 ± 7.6 | 95.3 ± 3.3 |
| DBP percentile (%)† | 53.2 ± 29.1 | 69.1 ± 23.7 | 75.3 ± 26.0 |
| HR (bpm)‡ | 72.0 ± 12.2 | 68.0 ± 11.6 | 73.6 ± 13.3 |
| Ambulatory Daytime SBP (mmHg)* | 117.9 ± 9.5 | 125.1 ± 9.0 | 130.5 ± 10.9 |
| Ambulatory Daytime SBP Index* | 0.88 ± 0.07 | 0.92 ± 0.07 | 0.97 ± 0.08 |
| Ambulatory Nighttime SBP (mmHg)* | 103.3 ± 9.2 | 110.0 ± 9.3 | 114.4 ± 10.6 |
| Ambulatory Nighttime SBP Index* | 0.88 ± 0.07 | 0.92 ± 0.08 | 0.97 ± 0.09 |
| Ambulatory Daytime DBP (mmHg)* | 69.0 ± 6.4 | 72.4 ± 6.8 | 75.0 ± 8.3 |
| Ambulatory Daytime DBP Index* | 0.84 ± 0.08 | 0.88 ± 0.08 | 0.91 ± 0.10 |
| Ambulatory Nighttime DBP (mmHg)* | 55.7 ± 5.7 | 58.1 ± 6.2 | 61.2 ± 8.6 |
| Ambulatory Nighttime DBP Index* | 0.84 ± 0.09 | 0.88 ± 0.10 | 0.92 ± 0.13 |
| SBP dipping (%) | 12.2 ± 6.1 | 11.9 ± 5.8 | 12.0 ± 5.9 |
| DBP dipping (%) | 19.0 ± 7.6 | 19.4 ± 7.8 | 18.1 ± 8.4 |
| Cardiac Output (ml/min)§, # | 3.2 ± 1.0 | 3.5 ± 1.3 | 3.9 ± 1.3 |
| Cardiac Index (L/min/m2) ||, # | 1.7 ± 0.5 | 1.8 ± 0.5 | 2.0 ± 0.6 |
| Systemic Vascular Resistance, metric (dynes*sec*cm−5) # | 2190.7 ± 738.3 | 2221.4 ± 780.0 | 2115.6 ± 749.4 |
| Systemic Vascular Resistance (Wood units) # | 27.4 ± 9.2 | 27.8 ± 9.7 | 26.4 ± 9.4 |
Bonferroni adjusted p ≤0.05 for
L<M<H
L<M&L
M<H
L<H
L&M<H
n is different for these parameters (L = 123, M = 78, H = 112) due to inability to make these measurements on the full cohort.
PWV was higher in H than in L group (L=4.83±0.69; M=5.08±0.76; H=5.35±0.92; p≤0.05) (Figure 1, Table 3) although values are within normal limits.[33] The prevalence of abnormal PWV in this entire cohort (defined as PWV ≥ 5.93 m/sec from healthy lean youth) was 10%.[33] with most in H group (L=6.5%; M=10.2%; H=13.7%). H had higher percentage of abnormal PWV than L (p = 0.039). PWV was higher in males versus females (5.16 versus 4.95 m/sec; P≤ 0.03). Aortic distensibility, distensibility coefficient, and aortic compliance were greater in L than in M or H (p≤0.05). There was no significant difference between groups in aortic strain, beta stiffness index or Peterson Elastic Modulus.
Figure 1.
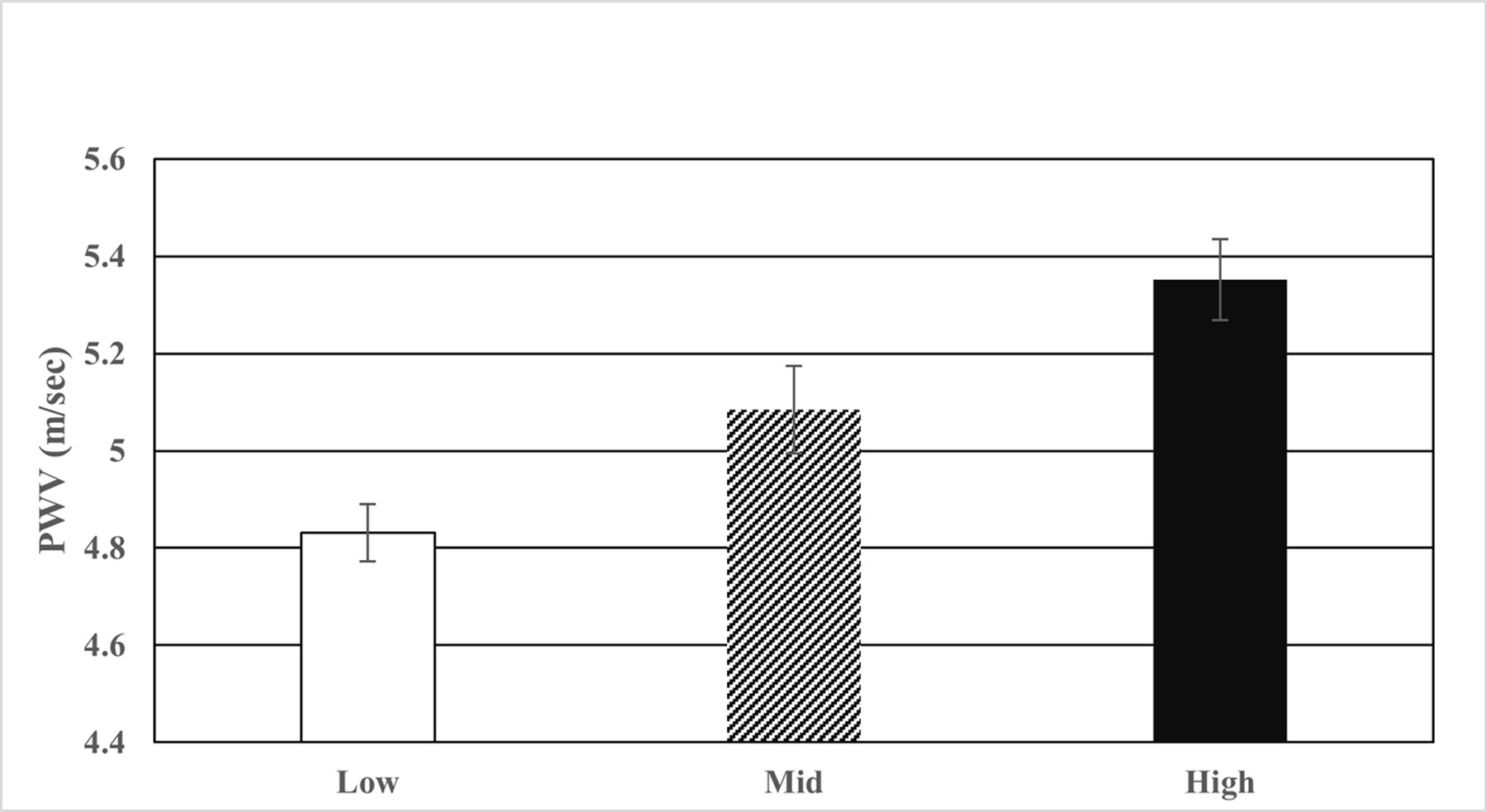
PWV by BP group (Bonferroni corrected P≤0.0001 for low < high).
Table 3.
Stiffness Parameters & Measures of TOD Parameters stratified by BP group (means, standard deviations).
| Characteristics | Low (L) | Mid (M) | High (H) |
|---|---|---|---|
| N=155 | N=88 | N=139 | |
| Pulse Wave Velocity (m/sec)* | 4.83 ± 0.69 | 5.08 ± 0.76 | 5.35 ± 0.92 |
| Aortic Strain (unitless) | 17.9 ± 7.9 | 18.1 ± 6.7 | 19.0 ± 7.7 |
| Beta Stiffness index (unitless) | 3.58 ± 2.30 | 3.60 ± 2.38 | 4.82 ± 11.45 |
| Peterson Elastic Modulus (mmHg) | 72.0 ± 41.5 | 81.5 ± 43.5 | 113.8 ± 237.4 |
| Aortic Distensibility (1/mmHg)† | 0.0084 ± 0.0039 | 0.0071 ± 0.0027 | 0.0069 ± 0.0030 |
| Distensibility Coefficient (1/mmHg)† | 0.02 ± 0.01 | 0.01 ± 0.01 | 0.01 ± 0.01 |
| Aortic Compliance (mm/mmHg)† | 0.030 ± 0.013 | 0.025 ± 0.011 | 0.023 ± 0.010 |
|
| |||
| LVM Index (g/m2.7)* | 31.5 ± 6.7 | 33.5 ± 6.8 | 33.5 ± 7.2 |
| Shortening Fraction (%) | 37.6 ± 4.4 | 36.7 ± 5.0 | 38.0 ± 5.0 |
| Ejection Fraction (%)‡ | 58.0 ± 7.2 | 55.4 ± 6.7 | 56.3 ± 6.7 |
| Peak Longitudinal Strain (%)§ | −20.9 ± 3.5 | −19.9 ± 3.2 | −20.1 ± 3.4 |
| Peak Longitudinal Strain Rate (/sec) | −1.03 ± 0.24 | −1.02 ± 0.24 | −1.03 ± 0.2 |
| Time to peak longitudinal strain (msec) | 37.2 ± 6.2 | 37.6 ± 6.1 | 38.8 ± 6.0 |
| Time to peak longitudinal strain rate (msec) | 22.9 ± 10.2 | 21.8 ± 9.5 | 22.8 ± 8.0 |
| e’/a’ ratio § | 2.47 ± 0.79 | 2.38 ± 0.62 | 2.27 ± 0.67 |
| E/A ratio § | 2.35 ± 0.70 | 2.24 ± 0.66 | 2.16 ± 0.63 |
| E/e’ ratio|| | 6.09 ± 1.38 | 5.88 ± 1.32 | 6.63 ± 1.60 |
| Urine Albumin/Creatinine ratio | 6.140 ± 7.500 | 6.040 ± 7.920 | 9.280 ± 20.250 |
Bonferroni adjusted P≤0.05 for
L<H
M&H< L
M<L
model p <0.07
L&M<H. Higher values indicate worsening arterial stiffness for the following parameters: Pulse Wave Velocity, Aortic Strain, Beta Stiffness index, Peterson Elastic Modulus. Lower values indicate worsening arterial stiffness for the following parameters: Aortic distensibility, Distensibility Coefficient, Aortic Compliance.
Left ventricular mass index was lower in L than in H group [LVMi (g/m2.7): L=31.5; M=33.5; H=33.5]. LVEF was higher in the L group versus M group (Table 3, all p≤ 0.05). There was no difference in other systolic function measures. E/e’ was higher in the H group, (worse diastolic function). Both E/A and e’/a’ trended lower (adverse) in H versus L (p =0.07). The prevalence of elevated ACR was low (3.9% overall) and did not differ among groups but there was higher ACR in H vs L and M groups combined (9.3 versus 6.1; p = 0.03).
Significant determinants of PWV (Table 4) were age, waist/height ratio, MAP and ABPM nighttime diastolic index (R2 = 0.26). Either clinic MAP and/or an ambulatory BP parameter was a major determinant of all measures of aortic stiffness. Male sex was a determinant for AD, Logβ stiffness index, DC and log[Peterson elastic pressure modulus]. LDL was associated with AS, DC, AC and log[Peterson elastic pressure modulus]. The amount of the variance in aortic stiffness explained by the models was low (R2: AS=0.07, AD=0.12, Logβ=0.12, DC=0.11, AC=0.12, log[Peterson]=0.09). After adjustments. AC (Table 5) remained a determinant of E/e’ and log[ACR]; PWV was a significant determinant of e’/a’, and T2PLS4c.
Table 4:
Determinants of Arterial Stiffness
| PWV | Aortic strain | Aortic distensibility | Log Beta Stiffness Index | Distensibility Coefficient | Aortic Compliance | Log Peterson elastic pressure modulus | |
|---|---|---|---|---|---|---|---|
| Intercept | −0.5 | 24.6 | 0.01 | 1.8 | 0.03 | 0.05 | 3.7 |
| Age | 0.07 | 0.004 | |||||
| Sex (male) | 0.002 | −0.1 | 0.003 | −0.1 | |||
| Waist/height | 2.06 | ||||||
| LDL | −0.03 | −0.00002 | −0.00005 | 0.002 | |||
| logCRP | −0.0004 | 0.05 | |||||
| Creatinine | 0.5 | ||||||
| Mean Arterial Pressure | 0.01 | −0.02 | 0.0002 | −0.008 | |||
| ABP daytime SBP index | −0.02 | −0.05 | 1.4 | ||||
| ABP daytime DBP index | 2.6 | 0.8 | |||||
| ABP nighttime SBP index | 16.0 | −0.007 | |||||
| ABP nighttime DBP index | −20.7 | ||||||
| R2 | 0.26 | 0.07 | 0.12 | 0.12 | 0.11 | 0.12 | 0.09 |
Full model included: age, race, sex, waist-to-height ratio, LDL, Triglyceride, HDL, HOMA-IR, Creatinine, Urine acid, CRP, MAP, HR, Ambulatory SBP Index Daytime, Ambulatory DBP Index Daytime. All model p ≤0.0001 and all parameter estimates ≤0.05.
Table 5:
Association of Different Measures of Arterial Stiffness with Left Ventricular and Microvascular Dysfunction
| E/e’ | e’/a’ | Time to Peak Longitudinal strain | Log Urine Albumin/ Creatinine | |
|---|---|---|---|---|
| Pulse Wave Velocity (femoral) | −0.05 | 0.91 | ||
| Aortic compliance | 0.02 | −13.09 | ||
| Intercept | 3.04 | 1.96 | 12.82 | −5.54 |
| Age | −0.14 | |||
| Sex (male) | 0.08 | 1.83 | 0.23 | |
| Waist/height | 2.54 | −0.85 | −2.22 | |
| Mean Arterial Pressure | 0.08 | |||
| ABP daytime SBP index | 6.89 | 0.48 | 0.81 | |
| ABP daytime DBP index | −3.04 | −0.71 | ||
| Heart rate | −0.01 | 0.15 | 0.01 | |
| R2 | 0.2 | 0.26 | 0.17 | 0.11 |
Full model included: age, race, sex, waist-to-height ratio, HR, Ambulatory SBP Index Daytime, Ambulatory DBP Index Daytime and one of the arterial stiffness variables. All model p ≤0.0001 and all parameter estimates ≤0.05. Increase in E/e’ or decrease in e’/a’ is consistent with worsening diastolic function. Increase in strain indicates worse systolic function.
In mediation analyses BP was either not significant in a model relating arterial stiffness to TOD (time to peak strain, Ualb/cr ratio), or did not change the beta estimate for arterial stiffness and (e’/a’) indicating no mediation by BP. However BP was significant for the model of E/e’ and the beta estimate for aortic compliance was reduced substantially indicating the BP mediated the relationship between AC and E/e’(data not shown).
Discussion:
We show that youth with elevated BP had adverse CV risk profile and increased arterial stiffness and BP (clinic or ambulatory BP) is a significant predictor of arterial stiffness. In turn, arterial stiffness predicts BP-related TOD (cardiac structure and function, microvascular dysfunction. Our data are the first to show that clinic BP and out of office BP related increases in arterial stiffness may be associated with cardiac TOD in youth. Longitudinal studies are needed to determine the time course for development of elevated BP and increased arterial stiffness and to make inferences on causality.
The association between elevated BP and arterial stiffness is well documented in adults.[5, 9, 34–44] Gedikli et al. demonstrated that arterial stiffness (measured by aortic PWV and AIx) was significantly higher in a group of prehypertensive adults compared to normotensive controls.[40] Similarly, in a longitudinal study of 777 adults followed over a 25 year period, participants with either prehypertension (SBP 120–139 mmHg) or hypertension (SBP > 140 mmHg) were found to have a steeper rate of PWV increase over the study period compared to normotensive controls.[38] In addition to clinic blood pressures, elevated BP measurements on ABPM were also found to correlate with PWV.[43, 45]
Similar findings have been noted in pediatric populations.[34, 46–54] In a study of 1171 children in Switzerland (average age of 7 years), participants with elevated BP (BP 90–95th %tile) or hypertension had higher PWV compared to their normotensive peers (PWV 4.44 vs 4.56 vs 4.29).[46] In 501 Spanish youth, a graded increase in PWV was present across BP strata.[55] A recent systematic review of carotid-femoral pulse wave velocity (PWV) in youth showed that cardiometabolic risk factors were positively associated with PWV, including positive associations with BP, impaired glucose metabolism, and metabolic syndrome.[56] Our study is the largest in the U.S. to show similar increase in PWV across BP groups, suggesting that elevation in BP prior to a clinical diagnosis of HTN is associated with vascular impairment even in youth.
Few studies have evaluated ABPM data and arterial stiffness in youth. Although Stabouli et al. found a significant correlation between PWV and many ABPM parameters, including mean BP, BP load, and variability, on analysis of covariance, only weighted 24-hr SBP variability and daytime SBP variability independently predicted PWV.[47] In contrast, our study found that only clinic MAP and ABPM nighttime diastolic index were independently associated with PWV. This difference may in part be due to differences in the population studied. While Stabouli’s population consisted of a random sample of younger school aged children (average age 10 years), our study population primarily consisted of adolescents with increased BMI. Our study uniquely evaluated multiple aspects of arterial stiffness in youth and demonstrated that youth with normal BP have lower arterial stiffness by aortic distensibility, distensibility coefficient and aortic compliance compared to youth with elevated BP
The association between arterial stiffness and TOD has not been well explored. A study of 338 young adults found a linear relationship between 4-chamber global longitudinal strain and lower brachial distensibility, suggesting increased arterial stiffness was associated with subclinical decline in systolic function.[57] In a study of adolescents and young adults with systemic lupus erythematosus (SLE), Chow et al. demonstrated that carotid arterial stiffness was a significant independent determinant of LV mass, early diastolic myocardial tissue velocity, and systolic strain rate of LV free wall.[58] Another pediatric study evaluated lean, obese and diabetic youth and found LVMi to be independently associated with higher global stiffness index calculated from 5 measurements of carotid artery stiffness, augmentation index, branchial distensibility and pulse wave velocity.[10] This study further demonstrates the association between arterial stiffness and CV TOD that persists after adjustment for BP, including a reduction in subclinical systolic and diastolic function and microvascular dysfunction (increased Ualb/cr ratio). However, our mediation analyses suggest that BP does mediate the relationship between aortic compliance and diastolic function.
Perspectives
These data demonstrate that cardiovascular risk profile and arterial stiffness worsens the higher the blood pressure category in adolescents without known preexisting conditions. Our data are the first to show that clinic BP and out of office BP related increases in arterial stiffness may be associated with cardiac TOD in youth. Longitudinal studies are needed to determine the time course for development of elevated BP and increased arterial stiffness and to make inferences on causality.
Limitations:
Due to the cross-sectional design of our study, causality and overall timeline for the development of TOD in our population cannot be determined. It is possible that some participants with WCH were included in the H group, however, in our modeling we included ABPM variables to correct for this. Whether a BP related increase in arterial stiffness or other factors (obesity related insulin resistance) cause increased stiffness resulting in elevated BP cannot be determined in our cross-sectional design. Our participants were oversampled toward the higher BP distribution (> 80%ile) and this may reduce generalizability to other populations. Finally, our study utilized a tonometric device for measuring PWV which, although is the gold standard, is not equivalent to oscillometric measures of PWV[59, 60] used in other studies.
Conclusion:
Youth with elevated BP have higher arterial stiffness which is associated with other preclinical measures of TOD. Early intervention in youth with high BP should be prioritized to prevent early cardiovascular disease.
Supplementary Material
Perspectives:
In the largest multi-center study of the effect of blood pressure on target organ damage in youth, we report the effect of elevated blood pressure on arterial stiffness. We also confirm the relationship between stiffer vessels and adverse cardiac structural and functional changes.
Pathophysiological Novelty and Relevance.
What is new?
Clinic BP and out of office BP related increases in arterial stiffness may be associated with cardiac TOD in youth
What is relevant?
Youth with elevated BP have higher arterial stiffness which is associated with other preclinical measures of TOD
Clinical/Pathophysiological Implications?
Early intervention in youth with high BP should be prioritized to prevent early cardiovascular disease.
Source of Funding:
American Heart Association (AHA) grant 15SFRN2368.
Abbreviations
- ABP
Ambulatory blood pressure
- ABPM
Ambulatory blood pressure monitor
- AC
Aortic compliance
- ACR
urine albumin/creatinine ratio
- ANOVA
Analysis of variance
- AoD
Aortic diameter in diastole
- AoS
Aortic diameter in systole
- AS
Aortic stiffness
- BP
Blood pressure
- CDC
Center for Disease Control
- CPG
Clinical practice guidelines
- CVD
Cardiovascular disease
- CVP
Central venous pressure
- DC
Distensibility coefficient
- DBP
Diastolic blood pressure
- EVA
Early vascular aging
- GLS
Global longitudinal strain
- HTN
Hypertension
- LV
Left ventricle
- LVEF
Left ventricular ejection fraction
- LVMi
Left ventricular mass index
- LVSF
Left ventricular shortening fraction
- MAP
Mean arterial pressure
- NHANES
National Health and Nutrition Examination Survey
- PP
Pulse pressure
- PWV
Pulse wave velocity
- SBP
Systolic Blood Pressure
- SV
Stroke volume
- TDI
Tissue doppler imaging
- TOD
Target Organ Damage
Footnotes
| Full name | Degree | Affiliation | Any COI? | Disclosures | |
|---|---|---|---|---|---|
| Shalayna Woodley | Cincinnati Children’s Hospital & University of Cincinnati | Shalayna.woodley@cchmc.org | Yes | AHA-moderate support | |
| Richard Becker | MD | University of Cincinnati | BECKERRC@ucmail.uc.edu | Yes | AHA -moderate support |
| Stephen R Daniels | MD, PhD | Children’s Hospital Colorado | Stephen.Daniels@childrenscolorado.org | No | None |
| Bonita E Falkner | MD | Thomas Jefferson University | bonita.falkner@jefferson.edu | No | None |
| Michael Ferguson | MD | Boston Children’s Hospital | Michael.Ferguson@childrens.harvard.edu | No | None |
| Joseph T Flynn | MD, MS | Seattle Children’s Hospital | joseph.flynn@seattlechildrens.org | Yes | AHA -moderate support; |
| Jessica Haley | MD | Rady Children’s Hospital | Jhaley1@rchsd.org | No | None |
| Coral Hanevold | MD | Seattle Children’s Hospital | coral.hanevold@seattlechildrens.org | Yes | AHA minor support |
| Stephen R Hooper | PhD | University of North Carolina | stephen_hooper@med.unc.edu | Yes | AHA minor support |
| Julie R. Ingelfinger | MD | MassGeneral Hospital for Children at MGH Hospital | jingelfinger@nejm.org | No | None |
| Philip R Khoury | PhD | Cincinnati Children’s Hospital & University of Cincinnati | Phil.khoury@cchmc.org | No | None |
| Marc B Lande | MD, MPH | University of Rochester Medical Center | Marc_Lande@URMC.Rochester.edu | Yes | AHA minor support |
| Lisa J Martin | PhD | Cincinnati Children’s Hospital & University of Cincinnati | Lisa.Martin@cchmc.org | Yes | AHA minor support |
| Kevin Meyers | MD | Children’s Hospital of Philadelphia | meyersk@email.chop.edu | Yes | AHA minor support |
| Mark Mitsnefes | MD, MS | Cincinnati Children’s Hospital & University of Cincinnati | mark.mitsnefes@cchmc.org | Yes | AHA -moderate support |
| Bernard Rosner | PhD | Harvard University | bernard.rosner@channing.harvard.edu | Yes | AHA minor support |
| Joshua Samuels | MD | University of Texas Health Sciences Center | Joshua.A.Samuels@uth.tmc.edu | Yes | AHA minor support |
| Elaine M Urbina | MD, MS | Cincinnati Children’s Hospital & University of Cincinnati | elaine.urbina@cchmc.org | No | AHA -moderate support |
References
- 1.Roth GA, et al. , Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J Am Coll Cardiol, 2020. 76(25): p. 2982–3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang C, et al. , Association of age of onset of hypertension with cardiovascular diseases and mortality. JACC 2020. 75(23): p. 2921–2930. [DOI] [PubMed] [Google Scholar]
- 3.Jackson SL, et al. , Hypertension Among Youths - United States, 2001–2016. MMWR Morb Mortal Wkly Rep, 2018. 67(27): p. 758–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kucharska-Newton AM, Stoner L, and Meyer ML, Determinants of Vascular Age: An Epidemiological Perspective. Clin Chem, 2019. 65(1): p. 108–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benetos A, et al. , Determinants of accelerated progression of arterial stiffness in normotensive subjects and in treated hypertensive subjects over a 6-year period. Circulation, 2002. 105(10): p. 1202–7. [DOI] [PubMed] [Google Scholar]
- 6.Roman MJ, et al. , Parallel cardiac and vascular adaptation in hypertension. Circulation, 1992. 86(6): p. 1909–18. [DOI] [PubMed] [Google Scholar]
- 7.Bader H, Importance of the gerontology of elastic arteries in the development of essential hypertension. Clin Physiol Biochem, 1983. 1(1): p. 36–56. [PubMed] [Google Scholar]
- 8.Mitchell GF, et al. , Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation, 2010. 121(4): p. 505–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Urbina EM, et al. , Cardiac and vascular consequences of pre-hypertension in youth. Journal of Clinical Hypertension, 2011. 13(5): p. 332–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Urbina EM, et al. , Relationship between elevated arterial stiffness and increased left ventricular mass in adolescents and young adults. J Pediatr, 2011. 158(5): p. 715–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuczmarski RJ, et al. , CDC growth charts: United States. Adv Data, 2000(314): p. 1–27. [PubMed] [Google Scholar]
- 12.National High Blood Pressure Education Program Working Group on High Blood Pressure in, C. and Adolescents, The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics, 2004. 114(2 Suppl 4th Report): p. 555–76. [PubMed] [Google Scholar]
- 13.Flynn JT, et al. , Clinical Practice Guideline for Screening and Management of High Blood Pressure in Children and Adolescents. Pediatrics, 2017. 140(3): p. 1–72. [DOI] [PubMed] [Google Scholar]
- 14.Urbina EM, et al. , Association of Blood Pressure Level With Left Ventricular Mass in Adolescents. Hypertension, 2019. 74(3): p. 590–596. [DOI] [PubMed] [Google Scholar]
- 15.Mendizabal B, et al. , SHIP-AHOY (Study of High Blood Pressure in Pediatrics: Adult Hypertension Onset in Youth). Hypertension, 2018. 72(3): p. 625–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamdani G, et al. , Prediction of Ambulatory Hypertension Based on Clinic Blood Pressure Percentile in Adolescents. Hypertension, 2018. 72(4): p. 955–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wuhl E, et al. , Distribution of 24-h ambulatory blood pressure in children: normalized reference values and role of body dimensions. J Hypertens, 2002. 20(10): p. 1995–2007. [DOI] [PubMed] [Google Scholar]
- 18.Devereux RB, et al. , Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol, 1986. 57(6): p. 450–8. [DOI] [PubMed] [Google Scholar]
- 19.Gottdiener JS, et al. , American Society of Echocardiography recommendations for use of echocardiography in clinical trials. J Am Soc Echocardiogr, 2004. 17(10): p. 1086–119. [DOI] [PubMed] [Google Scholar]
- 20.Lang RM, et al. , Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr, 2015. 28(1): p. 1–39 e14. [DOI] [PubMed] [Google Scholar]
- 21.de Simone G, et al. , Effect of growth on variability of left ventricular mass: Assessment of allometric signals in adults and children and their capacity to predict cardiovascular risk. Journal of the American College of Cardiology, 1995. 25(5): p. 1056–1062. [DOI] [PubMed] [Google Scholar]
- 22.Dandel M, et al. , Strain and strain rate imaging by echocardiography - basic concepts and clinical applicability. Curr Cardiol Rev, 2009. 5(2): p. 133–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Futrakul N, Sridama V, and Futrakul P, Microalbuminuria--a biomarker of renal microvascular disease. Ren Fail, 2009. 31(2): p. 140–3. [DOI] [PubMed] [Google Scholar]
- 24.Chapter 1: Definition and classification of CKD. Kidney Int Suppl (2011), 2013. 3(1): p. 19–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Urbina EM, et al. , Noninvasive assessment of subclinical atherosclerosis in children and adolescents: recommendations for standard assessment for clinical research: a scientific statement from the American Heart Association. Hypertension, 2009. 54(5): p. 919–50. [DOI] [PubMed] [Google Scholar]
- 26.Townsend RR, et al. , Recommendations for Improving and Standardizing Vascular Research on Arterial Stiffness: A Scientific Statement From the American Heart Association. Hypertension, 2015. 66(3): p. 698–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Urbina EM, et al. , Burden of Cardiovascular Risk Factors Over Time and Arterial Stiffness in Youth With Type 1 Diabetes Mellitus: The SEARCH for Diabetes in Youth Study. J Am Heart Assoc, 2019. 8(13): p. e010150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nemes A, et al. , Echocardiographic evaluation and clinical implications of aortic stiffness and coronary flow reserve and their relation. Clin Cardiol, 2008. 31(7): p. 304–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nistri S, et al. , Aortic elasticity and size in bicuspid aortic valve syndrome. Eur Heart J, 2008. 29(4): p. 472–9. [DOI] [PubMed] [Google Scholar]
- 30.Cho JY and Kim KH, Evaluation of Arterial Stiffness by Echocardiography: Methodological Aspects. Chonnam Med J, 2016. 52(2): p. 101–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nichols WW ORM, McDonald’s blood flow in arteries : theoretical, experimental, and clinical principles Sixth Editiion ed, ed. Nichols WW ORM, Vlachopoulos C 2011, Boca Raton, FL: CRC Press. 755. [Google Scholar]
- 32.O’Rourke MF, et al. , Clinical applications of arterial stiffness; definitions and reference values. Am J Hypertens, 2002. 15(5): p. 426–44. [DOI] [PubMed] [Google Scholar]
- 33.Urbina EM, Kimball TR, Khoury PR, Daniels SR, Dolan LM, Increased arterial stiffness is found in adolescents with obesity or obesity-related type 2 diabetes mellitus. J Hypertens, 2010. 28(8): p. 1692–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Urbina EM, et al. , Cardiac and vascular consequences of pre-hypertension in youth. J Clin Hypertens (Greenwich), 2011. 13(5): p. 332–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kudo U, et al. , Influence of obesity on blood pressure and arterial stiffness in the early teens. Obes Res Clin Pract, 2013. 7(3): p. e211–7. [DOI] [PubMed] [Google Scholar]
- 36.Whelton PK, et al. , 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension, 2018. 71(6): p. e13–e115. [DOI] [PubMed] [Google Scholar]
- 37.Liang X, et al. , Determinants of pulse wave velocity trajectories from youth to young adulthood: the Georgia Stress and Heart Study. J Hypertens, 2019. 37(3): p. 563–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alghatrif M, et al. , Longitudinal trajectories of arterial stiffness and the role of blood pressure: the Baltimore longitudinal study of aging. Hypertension, 2013. 62(5): p. 934–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Amar J, et al. , Arterial stiffness and cardiovascular risk factors in a population-based study. J Hypertens, 2001. 19(3): p. 381–7. [DOI] [PubMed] [Google Scholar]
- 40.Gedikli O, et al. , Effects of prehypertension on arterial stiffness and wave reflections. Clin Exp Hypertens, 2010. 32(2): p. 84–9. [DOI] [PubMed] [Google Scholar]
- 41.Cozma A, et al. , Determining Factors of Arterial Stiffness in Subjects with Metabolic Syndrome. Metab Syndr Relat Disord, 2018. 16(9): p. 490–496. [DOI] [PubMed] [Google Scholar]
- 42.Cecelja M and Chowienczyk P, Dissociation of aortic pulse wave velocity with risk factors for cardiovascular disease other than hypertension: a systematic review. Hypertension, 2009. 54(6): p. 1328–36. [DOI] [PubMed] [Google Scholar]
- 43.Rosa J, et al. , Relationship between clinical, 24-hour, average day-time and night-time blood pressure and measures of arterial stiffness in essential hypertension. Physiol Res, 2008. 57(2): p. 303–6. [DOI] [PubMed] [Google Scholar]
- 44.Coutinho T, et al. , Arterial stiffness is associated with increase in blood pressure over time in treated hypertensives. J Am Soc Hypertens, 2014. 8(6): p. 414–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kotsis V, et al. , Arterial stiffness and 24 h ambulatory blood pressure monitoring in young healthy volunteers: the early vascular ageing Aristotle University Thessaloniki Study (EVA-ARIS Study). Atherosclerosis, 2011. 219(1): p. 194–9. [DOI] [PubMed] [Google Scholar]
- 46.Kochli S, et al. , Obesity, High Blood Pressure, and Physical Activity Determine Vascular Phenotype in Young Children. Hypertension, 2019. 73(1): p. 153–161. [DOI] [PubMed] [Google Scholar]
- 47.Stabouli S, et al. , Arterial stiffness and SBP variability in children and adolescents. J Hypertens, 2015. 33(1): p. 88–95. [DOI] [PubMed] [Google Scholar]
- 48.Batista MS, et al. , Factors associated with arterial stiffness in children aged 9–10 years. Rev Saude Publica, 2015. 49: p. 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kulsum-Mecci N, et al. , Effects of Obesity and Hypertension on Pulse Wave Velocity in Children. J Clin Hypertens (Greenwich), 2017. 19(3): p. 221–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McCloskey K, et al. , The effect of known cardiovascular risk factors on carotid-femoral pulse wave velocity in school-aged children: a population based twin study. J Dev Orig Health Dis, 2014. 5(4): p. 307–13. [DOI] [PubMed] [Google Scholar]
- 51.Fujiwara H, et al. , Arterial stiffness in junior high school students: Longitudinal observations. Pediatr Int, 2018. 60(2): p. 127–135. [DOI] [PubMed] [Google Scholar]
- 52.Im JA, et al. , Association between brachial-ankle pulse wave velocity and cardiovascular risk factors in healthy adolescents. J Pediatr, 2007. 150(3): p. 247–51. [DOI] [PubMed] [Google Scholar]
- 53.Niboshi A, et al. , Characteristics of brachial-ankle pulse wave velocity in Japanese children. Eur J Pediatr, 2006. 165(9): p. 625–9. [DOI] [PubMed] [Google Scholar]
- 54.Tokgoz ST, et al. , The evaluation of arterial stiffness of essential hypertension and white coat hypertension in children: a case-control study. Cardiol Young, 2018. 28(3): p. 403–408. [DOI] [PubMed] [Google Scholar]
- 55.Lurbe E, et al. , Blood pressure and obesity exert independent influences on pulse wave velocity in youth. Hypertension, 2012. 60(2): p. 550–5. [DOI] [PubMed] [Google Scholar]
- 56.Stoner L, Kucharska-Newton A, and Meyer ML, Cardiometabolic Health and Carotid-Femoral Pulse Wave Velocity in Children: A Systematic Review and Meta-Regression. J Pediatr, 2020. 218: p. 98–105 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mehta S, et al. , Arterial Thickness and Stiffness Are Independently Associated with Left Ventricular Strain. J Am Soc Echocardiogr, 2018. 31(1): p. 99–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chow PC, et al. , Relation of arterial stiffness to left ventricular structure and function in adolescents and young adults with pediatric-onset systemic lupus erythematosus. J Rheumatol, 2007. 34(6): p. 1345–52. [PubMed] [Google Scholar]
- 59.Zinoveev A, et al. , Aortic pressure and forward and backward wave components in children, adolescents and young-adults: Agreement between brachial oscillometry, radial and carotid tonometry data and analysis of factors associated with their differences. PLoS One, 2019. 14(12): p. e0226709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zocalo Y, et al. , Forward and Backward Aortic Components and Reflection Indexes in Children and Adolescents: Determinants and Role in High Pressure States. Curr Hypertens Rev, 2018. 14(2): p. 137–153. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


