Abstract
Background:
Thiopurines are an important class of immunosuppressants despite their risk for hematopoietic toxicity and narrow therapeutic indices. Benign neutropenia related to an ACKR1 variant (rs2814778-CC) is common among individuals of African ancestries.
Objective:
We tested whether rs2814778-CC was associated with azathioprine discontinuation attributed to hematopoietic toxicity and lower thiopurine dosing.
Design:
Retrospective cohort study.
Setting:
Two tertiary care centers.
Patients:
Thiopurines users with White or Black race.
Measurements:
Azathioprine discontinuation attributed to hematopoietic toxicity. Secondary outcomes included weight-adjusted last dose, white blood cell (WBC) and delta WBC counts.
Results:
The discontinuation rate of azathioprine attributed to hematopoietic toxicity was 3.92/100 person-years among patients with the CC genotype (n=101) and 1.34/100 person-years among patients without the CC genotype (n=1,365) [hazard ratio (HR)=2.92, 95%CI: 1.57–5.41, competing hazards model]. The risk remained significant when adjusted by race (HR=2.61, 1.01–6.71). The risk associated with race alone (HR=2.13, 1.21–3.75) was abrogated by adjustment for genotype (HR=1.13, 0.48–2.69). Lower dosing and other secondary outcomes, except delta WBC count, were significant among patients who did not discontinue for hematopoietic toxicity. Lower dosing was validated in an external cohort of 94 children of African ancestries prescribed the thiopurine 6-mercaptopurine (6-MP) for acute lymphoblastic leukemia (ALL). The CC genotype was independently associated with lower 6-MP intensity dosages relative to the target daily dose—75 mg/m2 (CC: median 0.83 interquartile range [0.70–0.94] vs TT or TC: 0.94 [0.73–1.13], P=0.013).
Limitations:
Unmeasured confounding. Data limited to tertiary centers.
Conclusion:
Patients with the CC genotype faced higher risk for discontinuation for attributed hematopoietic toxicity and lower doses. Genotype, even adjusted for race, is associated with those risks.
Primary Funding Source:
National Institutes of Health
The thiopurine azathioprine is a widely available, orally administered, and affordable immunosuppressant used globally for the treatment of a wide range of indications, including inflammatory conditions such as systemic lupus erythematosus (SLE), inflammatory bowel disease, and rheumatoid arthritis.(1) However, azathioprine has a narrow therapeutic index, and its use is often limited by side effects—primarily hematopoietic toxicity.(2–4) Genetic variants in thiopurine S-methyltransferase (TPMT) and nudix hydrolase 15 (NUDT15) more than double the risk for hematopoietic toxicity in patients taking azathioprine, but most patients who discontinue azathioprine do not carry these variants.(5–7)
Some individuals have lower neutrophil counts without increased risk for infection—a condition known as benign neutropenia.(8–10) One common form of benign neutropenia is due to a single nucleotide polymorphism (SNP), rs2814778, located in the promoter region of ACKR1 (also known as the Duffy antigen receptor for chemokines).(11, 12) This receptor is not only a chemokine binding protein, but also a blood group antigen expressed on erythrocytes and a receptor for Plasmodium vivax, a malaria parasite;(13) the homozygous variant rs2814778-CC prevents expression of ACKR1 on erythrocytes and confers resistance to P. vivax malaria.(14)
Individuals with the rs2814778-CC genotype have an ACKR1-null phenotype (i.e., Duffy-null). This phenotype is found in approximately half of U.S. patients with recent African ancestries, but it is rare in individuals of European ancestries;(11, 15) as such, average neutrophil counts are lower among patients with African ancestries. After observing that patients with EHR-reported Black race discontinued azathioprine for hematopoietic toxicity at a higher rate than patients with EHR-reported White race in data collection,(5) we hypothesized that the rs2814778-CC genotype was associated with a difference in thiopurine discontinuation and dose, independent of race, age, indication, institution, and TPMT and NUDT15 metabolizer status.
METHODS
This retrospective cohort study was conducted in BioVU, a clinical practice-based biobank at Vanderbilt University Medical Center. BioVU links de-identified electronic health records (EHR) with stored DNA samples(16) and access to demographic characteristics, clinical care notes, medical history, problem lists, medications, and diagnostic and procedure codes.(17) The study was reviewed by the Vanderbilt University Medical Center’s Institutional Review Board (IRB#180498) and determined to be non-human subjects research.
Study population:
The study population included 1,466 patients (Figure 1). We identified potential azathioprine users through natural language processing and review of clinical records. Using data gathered from this review (Supplement Methods 1), we applied the following inclusion criteria: (1) available DNA successfully genotyped using the Illumina Infinium® Expanded Multi-Ethnic Genotyping Array plus custom content platform (VUMC BioVU MEGAEX) that passed post-imputation quality control; (2) new azathioprine users; (3) azathioprine prescribed for inflammatory conditions including SLE, vasculitis, rheumatoid arthritis, other connective tissue disorders, or inflammatory bowel disease; and (4) EHR-reported race as Black or White. Exclusion criteria were: (1) record of previous treatment with a thiopurine in the EHR; and (2) previous medical history that could indicate potentially compromise genetic material (e.g., stem cell transplant).
Figure 1:
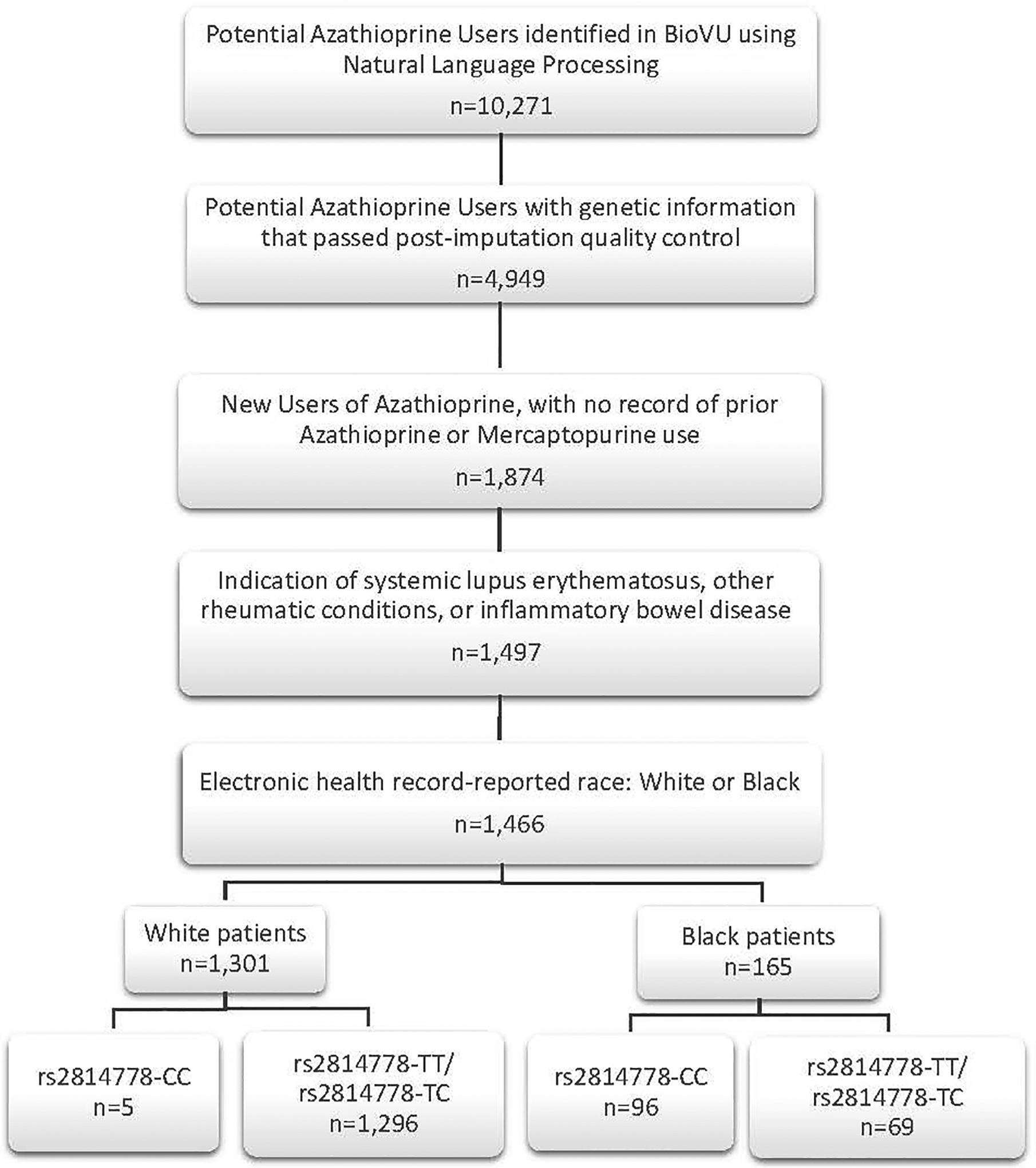
Inclusion Criteria in the VUMC Cohort
Genotyping and analysis of rs2814778:
The SNP associated with benign neutropenia— rs2814778—was directly genotyped as part of prior investigations on the VUMC BioVU MEGAEX platform. Standard quality control measures were applied (Supplement Methods 2).(5) Only the CC genotype for this SNP is associated with lower neutrophils; as such, we grouped individuals with TT and TC genotypes for comparison against patients with the CC genotype.
Follow-up:
Patients entered the cohort on the date of their first mention of azathioprine use in the EHR. We defined end of follow-up as the first of the following dates: (1) day of discontinuation; (2) last confirmed azathioprine prescription or mention of use + 90 days; (3) lost to follow-up; (4) day of death; or (5) end of the study—12/31/2018.
Outcomes:
We previously reviewed EHRs blinded to any genotype and recorded reasons (hematopoietic and non-hematopoietic) for azathioprine discontinuation (Supplement Methods 1).(5) The pre-specified primary study outcome was discontinuation of azathioprine attributed to hematopoietic toxicity, defined as leukopenia, neutropenia, thrombocytopenia, pancytopenia, and/or anemia, based on provider’s assessment. Patients fulfilled the outcome even if the review indicated additional reasons (e.g., fever), (Supplement Methods 1). Secondary outcomes were white blood cell (WBC) count and neutrophil count (NC) closest to end of follow-up (thirty days prior to last dose till three days subsequent), change in WBC count from initial dose to end of follow-up, and weight-adjusted last azathioprine dose. As a sensitivity analysis, we restricted WBC counts and NCs (stopping on last dose date).
Race:
We used EHR information to capture all clinical variables (Supplement Methods 1). To evaluate a real-world decision-making environment and account for potential social factors in our outcome, we used attribution of race in the clinical record, rather than a genetic determination of ancestry. Nevertheless, previous studies found that race and genetic ancestry have high concordance in BioVU, and we assessed the consistency of reported race and genetic ancestry (Supplement Methods 2).(18)
Other Covariates:
We also collected information about sex, age, initial daily dose of azathioprine, indication, calendar year of initial dose, baseline WBC count (closest measure to initial dose from 365 days prior to and including initial dose date), and TPMT (genetic variants, enzyme levels, or metabolites) testing before azathioprine exposure from the EHR (Supplement Methods 1). We generated calendar year tertiles and categorized indications as a binary variable (e.g., SLE or not). We assigned metabolizer status—normal, indeterminate, intermediate, or poor—for TPMT and NUDT15 based on the SNPs available after imputation and quality control (Supplement Methods 2; Supplement Table 1).(5) For statistical analyses, we designated patients’ overall TPMT/NUDT15 metabolizer status as the poorer of the two and further grouped patients as (1) poor/intermediate metabolizers or (2) normal/indeterminate metabolizers of azathioprine.
Statistical analyses:
We present demographic and clinical characteristics as numbers and percentages, dose and time-based variables as median and interquartile range (IQR), and other continuous variables as mean and standard deviation (SD). We used Fisher’s exact tests to compare categorical variables and Wilcoxon’s rank sum tests for continuous variables.
In the primary analysis, we estimated the incidence rates of azathioprine discontinuation attributed to hematopoietic toxicity between patients with and without the CC genotype. These rates were adjusted for competing risks for discontinuation.(19) Results were further adjusted for the following: (1) sex and age at initial dose; (2) sex, age at initial dose, and TPMT/NUDT15 metabolizer status group; and (3) sex, age at initial dose, TPMT/NUDT15 metabolizer status group, tertile of calendar year at initial dose, and indication of SLE (because SLE is frequently associated with leukopenia). As an alternative to potential overfitting, we calculated a propensity score using these variables; given the limited number of outcomes and relatively small exposure group,(20–23) we directly adjusted by the propensity score in Model 3. To assess balance, we calculated standardized mean differences after inverse probability treatment weighting. We also completed a sensitivity analysis using a linear predictor for our primary analysis Model 3.
These models were re-run adjusted for race to test whether race was acting as a confounder. Further, because the CC genotype is rare among individuals of predominantly European ancestries but common among individuals of predominantly African ancestries, several additional analyses addressed the relationship between race and genetic ancestry to rule out alternative potential explanations for the association. First, the association of another variant commonly associated with African ancestries—namely, rs334 (i.e., a variant that causes sickle-cell anemia)—was tested to determine if other alleles common in patients with African ancestries, but rare in patients with European ancestries, predicted azathioprine discontinuation for hematopoietic toxicity. Then, we addressed the possibility that socioeconomic factors or disparities in access to care based upon race propelled the differences we noted by genotype. If socioeconomic factors or disparities in access to care were playing a role in discontinuation attributed to hematopoietic toxicities, they likely would also contribute to discontinuation attributed to non-hematopoietic reasons. As such, we estimated the incidence rates and used Cox proportional-hazard models to compare the risk for azathioprine discontinuation due to hematopoietic toxicity and non-hematopoietic reasons by race. Finally, we repeated the primary analysis stratified by both genotype and race.
We also performed several sensitivity analyses: (1) adjustment for baseline WBC counts; (2) restricted to adults (i.e., patients ≥18 years of age at initial dose); (3) stratified by TPMT/NUDT15 metabolizer status; (4) without competing risk; and (5) with only neutropenia, leukopenia, and pancytopenia included in the outcome (i.e., with anemia and thrombocytopenia included among competing risks).. Additionally, we used Wilcoxon rank-sum test to compare the secondary outcomes—weight-adjusted final dose, WBC counts and NC at last observation, and change in WBC counts from baseline to end of follow-up—among patients with and without the rs2814778-CC genotype. We stratified these analyses by whether patients discontinued for attributed hematopoietic toxicity.
All analyses were conducted using StataCorp’s Stata software version 16.0.(24)
Validation:
The validation cohort expanded our ability to examine the association between rs2814778-CC and clinical decision-making across a range of indications, treatment centers, and age groups; its internal uniformity allowed us to assess the potential impact of rs2814778 on drug metabolism. This cohort was comprised of 94 children of African ancestry with acute lymphoblastic leukemia (ALL) who were enrolled in the Children’s Oncology Group AALL03N1 trial (www.clinicaltrials.gov identifier NCT00268528)(25–27) and were normal TPMT and NUDT15 metabolizers. Azathioprine is a pro-drug of 6-mercaptopurine (6-MP);(28) in this trial, the planned target daily 6-MP protocol dose was 75 mg/m2, and the dose was adjusted based on bone marrow suppression or infections. We used the Wilcoxon-rank sum tests to compare dose intensity (i.e., the average ratio of actual 6-MP dose over planned per-protocol dose captured monthly over 6-months during maintenance therapy) between genotype groups. We also compared dose-adjusted metabolite levels—namely, thioguanine nucleotides (rTG) and 6-methylmercaptopurine nucleotides (MMPN) to address the possibility that differences in drug metabolism impacted outcomes. Genotypes for rs2814778 were directly interrogated using the Illumina Exome-12 BeadChip array.
Role of the funding source(s):
The funding source had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; nor in the decision to submit the paper for publication.
RESULTS
Cohort and follow-up:
The VUMC cohort included 1,466 new users of azathioprine with diagnoses of inflammatory conditions; their median follow-up was 20.7 [3.29–61.0] months. Patients had a mean age of 44.0±17.4 years, 985 (67.2%) were female, and 1,301 (88.7%) were White. EHR-reported race was consistent with genetic ancestry. In this cohort, 1,295 (99.5%) patients with White race had predominantly European ancestries, and 155 (93.9%) patients with Black race had predominantly African ancestries (P<0.001). Patients with the CC genotype (n=101) had a median follow-up of 21.6 months compared to 20.4 months for the 1,365 patients with the TT or TC genotype (P=0.56). Table 1 shows the baseline characteristics of patients included in the study by genotype. Patients with the CC genotype did not differ significantly in initial dose, calendar year of initial dose, or prior testing for TPMT. As anticipated, the CC genotype was very rare among patients with EHR-reported White race (n=5; 0.38%)(Supplement Table 2). Additionally, patients with the CC genotype had lower mean baseline WBC, younger age, higher prevalence of SLE, and lower prevalence of normal TPMT/NUDT15 metabolizer status compared to patients with the TT or TC genotype.
Table 1:
Patient Characteristics at Initial Azathioprine Dose by rs2814778 Genotype in the VUMC Cohort
| CC (n=101) | TT or TC (n=1365) | Standardized Mean Difference | |
|---|---|---|---|
| EHR-reported race, n (%) | |||
| White | 5 (5.0) | 1296 (95.0) | −4.13 |
| Black | 96 (95.0) | 69 (5.0) | |
| Sex, n (%) | |||
| Female | 75 (74.3) | 910 (66.7) | −0.17 |
| Male | 26 (25.7) | 455 (33.3) | |
| Age in years, mean±SD | 39.8±15.5 | 44.4±17.5 | 0.28 |
| Indications, n (%) | |||
| Systemic lupus erythematosus (SLE) | 26 (25.7) | 139 (10.2) | −0.41 |
| Inflammatory bowel disease (IBD) | 57 (56.5) | 755 (55.3) | −0.02 |
| Inflammatory condition other than SLE or IBD | 18 (17.8) | 471 (34.5) | 0.39 |
| Initial daily dose (mg/day), median [IQR] | 50 [50–100] | 50 [50–100] | 0.15 |
| Calendar year of initial dose, median [IQR] | 2011 [2007–2013] | 2010 [2008–2013] | −0.01 |
| Tested prior to initiation of azathioprine, n (%) | 53 (52.5) | 696 (51.0) | −0.03 |
| Overall TPMT/NUDT15 metabolizer status, n (%) | |||
| Normal | 87 (86.1) | 1257 (92.1) | 0.19 |
| Indeterminate | 3 (3.0) | 5 (0.4) | −0.20 |
| Intermediate | 10 (9.9) | 100 (7.3) | −0.09 |
| Poor | 1 (1.0) | 3 (0.2) | −0.10 |
| Baseline WBC (K/μL), mean±SD | 7.5±3.5* | 8.8±3.7† | 0.34 |
EHR=electronic health record, TPMT=thiopurine S-methyltransferase, NUDT15=nudix hydrolase 15, WBC=white blood cell count
n=92
n=1215
Azathioprine discontinuation attributed to hematopoietic toxicity by rs2814778 genotype:
The primary endpoint, discontinuation of azathioprine attributed to hematopoietic toxicity, occurred in 71 patients—12 patients with the CC genotype (3.92/100 person-years) and 59 patients with either the TT or TC genotype (1.34/100 person-years). In competing risk analysis, the risk for discontinuation attributed to hematopoietic toxicity was higher for patients with the CC genotype (HR=2.92, 95% CI:1.57–5.41, P=0.001), and this association remained significant in all adjusted models: Model 1 (aHR=3.05, 95% CI:1.65–5.62, P<0.001), Model 2 (aHR=2.89, 95%CI:1.56–5.30, P=0.001), and Model 3 (aHR=2.85, 95% CI:1.55–5.23, P=0.001), (Supplement Table 3). When we calculated standardized differences after inverse probability treatment weighting, all differences were <0.06, indicating good balance (Supplement Table 4);(20, 29) when we re-ran the propensity score adjusted model using a linear predictor as a sensitivity analysis, the results were consistent with our primary analysis adjusted Model 3 (HR=2.93, 95%CI: 1.60–5.37, p=0.001). A series of sensitivity analyses yielded similar results to our primary analysis (Supplement Table 3). Figure 2 shows the cumulative incidence of azathioprine discontinuation due to myelotoxicity over time by genotype group.
Figure 2:
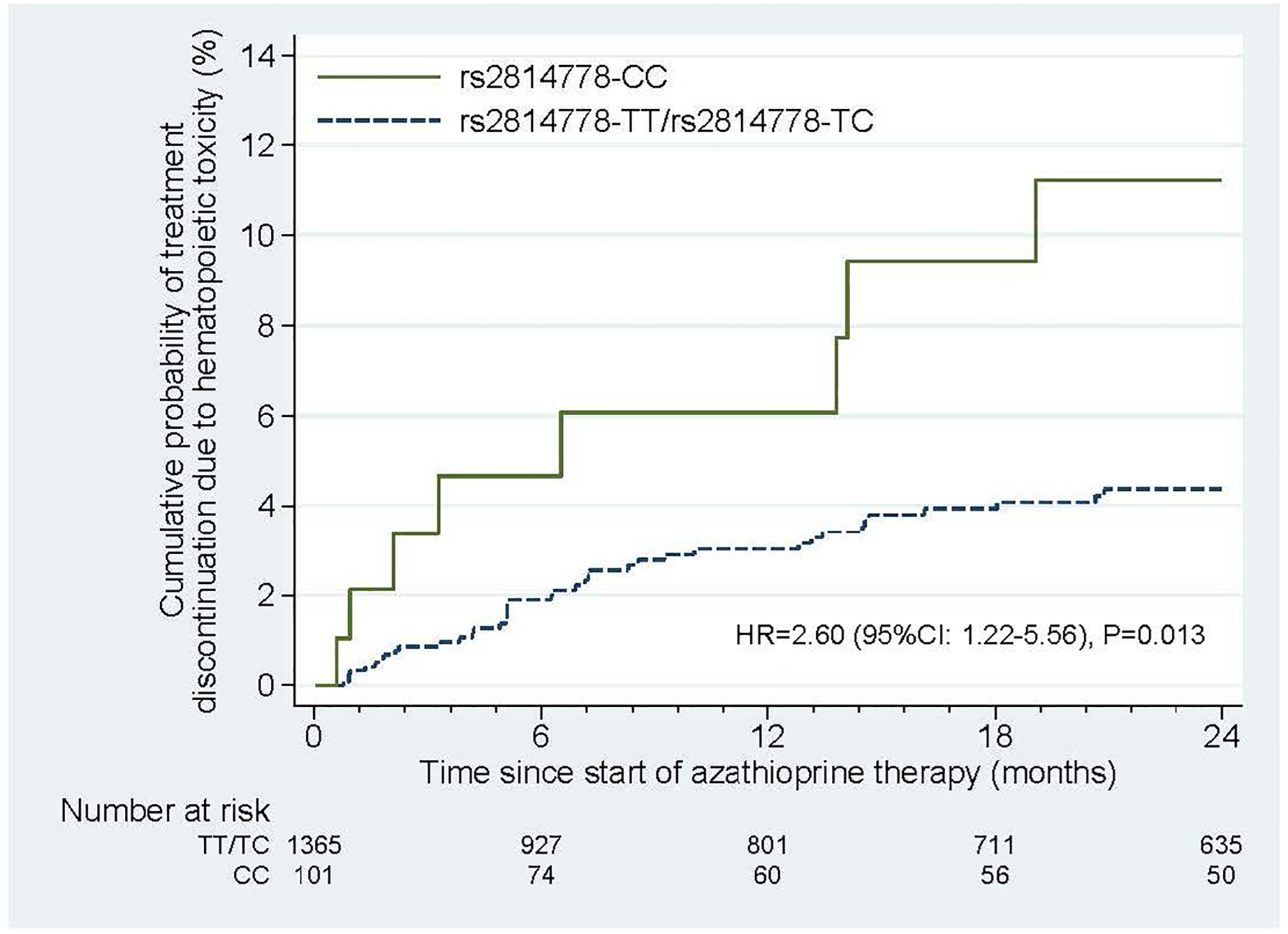
Probability of Azathioprine Discontinuation for Hematopoietic Toxicity in the VUMC Cohort by rs2814478 Genotype
HR=hazard ratio
Accounting for confounding by common ancestral genetic traits or EHR-reported race:
When the primary models were adjusted for race, the results suggested that race did not impact risk beyond its correlation with the genotype. Without adjusting for genotype, race had an association with discontinuation attributed to hematopoietic toxicity in competing risk analysis (HR=2.13, 95%CI: 1.21–3.75, P=0.009). However, that association was abrogated when race was adjusted by genotype (HR=1.13, 95%CI: 0.48–2.69, P=0.78), while the CC genotype remained significant when adjusted by race (HR=2.61, 95%CI: 1.01–6.71, P=0.047). Results were consistent after additional adjustments, when assessed by genetic ancestry, and when restricted to patients with Black race (Supplement Table 5).
Additional potential factors were addressed to assess whether race increased risk independent of the CC genotype, including (1) common ancestral traits, and (2) socioeconomic factors and/or access to care related to racial identification. First, an association analysis with the sickle cell anemia variant rs334 found no significant association between the minor allele and discontinuation attributed to hematopoietic toxicities (Supplement Table 5). With respect to the impact of racial identification, while associations for discontinuation attributed to hematopoietic toxicities remained significant by race before adjustment for genotype, non-hematologic toxicities were not (Supplement Figure 1; Supplement Table 3). Finally, the cohort was stratified by both race and genotype for association analysis to assess whether race was compounding the association detected for genotype. Patients with a CC genotype and EHR-reported White race were omitted from this analysis, because their numbers were very small (n=5) and most had predominantly African ancestries (n=4)(Supplement Table 2). Patients with the TT or TC genotype did not differ significantly in their associations by race. Relative to White patients with the TT or TC genotype, in competing risk analyses, Black patients with the TT or TC genotype had a HR=0.96 (95%CI: 0.30–2.99, P=0.94), while EHR-reported Black patients with the CC genotype had a HR=3.06 (95%CI: 1.65–5.70, P<0.001). This difference was consistent after adjustments (Supplement Table 5). Figure 3 shows the cumulative incidence of azathioprine discontinuation due to myelotoxicity over time by genotype group and EHR-reported race.
Figure 3:
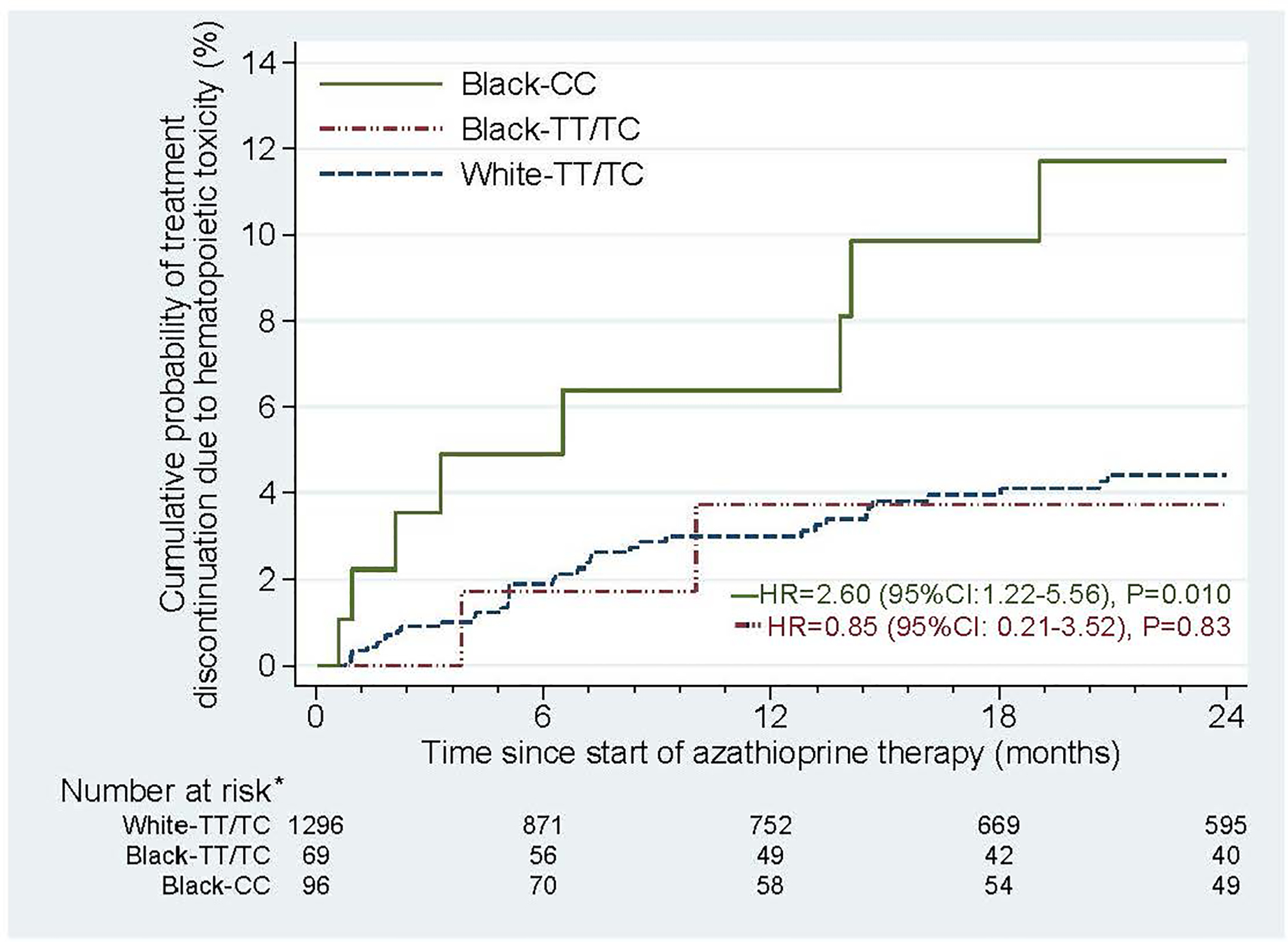
Probability of Azathioprine Discontinuation for Hematopoietic Toxicity in the VUMC Cohort by EHR-Reported Race and rs281447S Genotype
EHR=electronic health record, HR=hazard ratio
*White-CC patients excluded due to small numbers (n=5)
Secondary outcomes:
Secondary outcomes included available last WBC count, last NC, change in WBC count from baseline to end of follow-up, and weight-adjusted final dose. Among patients discontinued for attributed hematopoietic toxicity (n=71), the last WBC count, last NC, and weight-adjusted final dose did not differ significantly between genotypes (Table 2; Supplement Table 6). Among patients who did not discontinue for attributed hematopoietic toxicity (n=1,294), last WBC count and NC were significantly lower for patients with the CC genotype compared to patients with the TT or TC genotype. Moreover, the weight-adjusted final dose was 1.05 [0.65–1.49] mg/kg/day for patients with the CC genotype versus 1.27 [0.78–1.89] mg/kg/day for patients with the TT or TC genotype (P=0.009). This difference remained significant when restricted to normal TPMT/NUDT15 metabolizers (1.02 [0.65–1.47] versus 1.31 [0.79–1.90] mg/kg/day, P=0.005). There was no significant difference in the change in WBC count from baseline to end of follow-up among patients in either group.
Table 2:
Last WBC Count, Last NC, Delta WBC Count, and Weight-Adjusted Last Dose by rs2814778 Genotype, Stratified by Discontinuation Attributed to Hematopoietic Toxicity
| Discontinued for Hematopoietic Toxicity | Did not Discontinue for Hematopoietic Toxicity | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CC (n=12) | TT or TC (n=59) | CC (n=89) | TT or TC (n=1,306) | |||||||
| n | median [IQR] | n | median [IQR] | P-value | n | median [IQR] | n | median [IQR] | P-value | |
| Available Last WBC Count (× 109 cells/L) | 10 | 2.8 [1.8–3.4] | 46 | 3.2 [2.4–4.4] | 0.29 | 51 | 5.9 [4.8–7.9] | 709 | 6.9 [5.4–9.2] | 0.014 |
| Available Last NC (× 109 cells/L) | 5 | 1.4 [1.1–2.3] | 26 | 2.4 [1.5–3.2] | 0.21 | 30 | 3.2 [2.7–5.1] | 357 | 4.6 [3.4–6.2] | 0.002 |
| Delta WBC Count (× 109 cells/L): Available Baseline to Available Last | 10 | −2.8 [−4.4–0.4] | 43 | −4.2 [−6.8– −1.7] | 0.125 | 51 | −0.8 [−4.1–1.1] | 643 | −0.9 [−3.1–0.7] | 0.80 |
| Available Weight-Adjusted Last Dose (mg/kg/day) | 12 | 1.3 [1.1–2.0] | 57 | 1.5 [0.8–2.0] | 0.81 | 85 | 1.1 [0.7–1.5] | 1,231 | 1.3 [0.8–1.9] | 0.009 |
| Available Weight-Adjusted Last Dose (mg/kg/day), Normal TPMT/NUDT15 metabolizers only | 9 | 1.3 [1.2–1.7] | 48 | 1.7 [0.9–2.0] | 0.80 | 76 | 1.0 [0.7–1.5] | 1,135 | 1.3 [0.8–1.9] | 0.005 |
WBC=white blood cell count, NC=neutrophil count, TPMT=thiopurine S-methyltransferase, NUDT15=nudix hydrolase 15
Validation cohort:
Because the CC genotype is so rare among individuals of predominantly European ancestries and in order to avoid the potential impact of disparate TPMT/NUDT15 metabolizer status, the validation cohort was limited to patients of African ancestries with normal TPMT/NUDT15 metabolizer status. Consistent with dosing results in the main VUMC cohort (for patients who were not discontinued for hematopoietic toxicity), children with African ancestry who carried the CC genotype (n=67) tolerated lower 6-MP dose intensity compared to children who carried the TT or TC genotypes (n=27): 0.83 [0.70–0.94] vs 0.94 [0.73–1.13], P=0.013 (Figure 4). In contrast, dose-adjusted metabolite levels did not differ significantly between the two groups (Supplement Figure 2).
Figure 4:
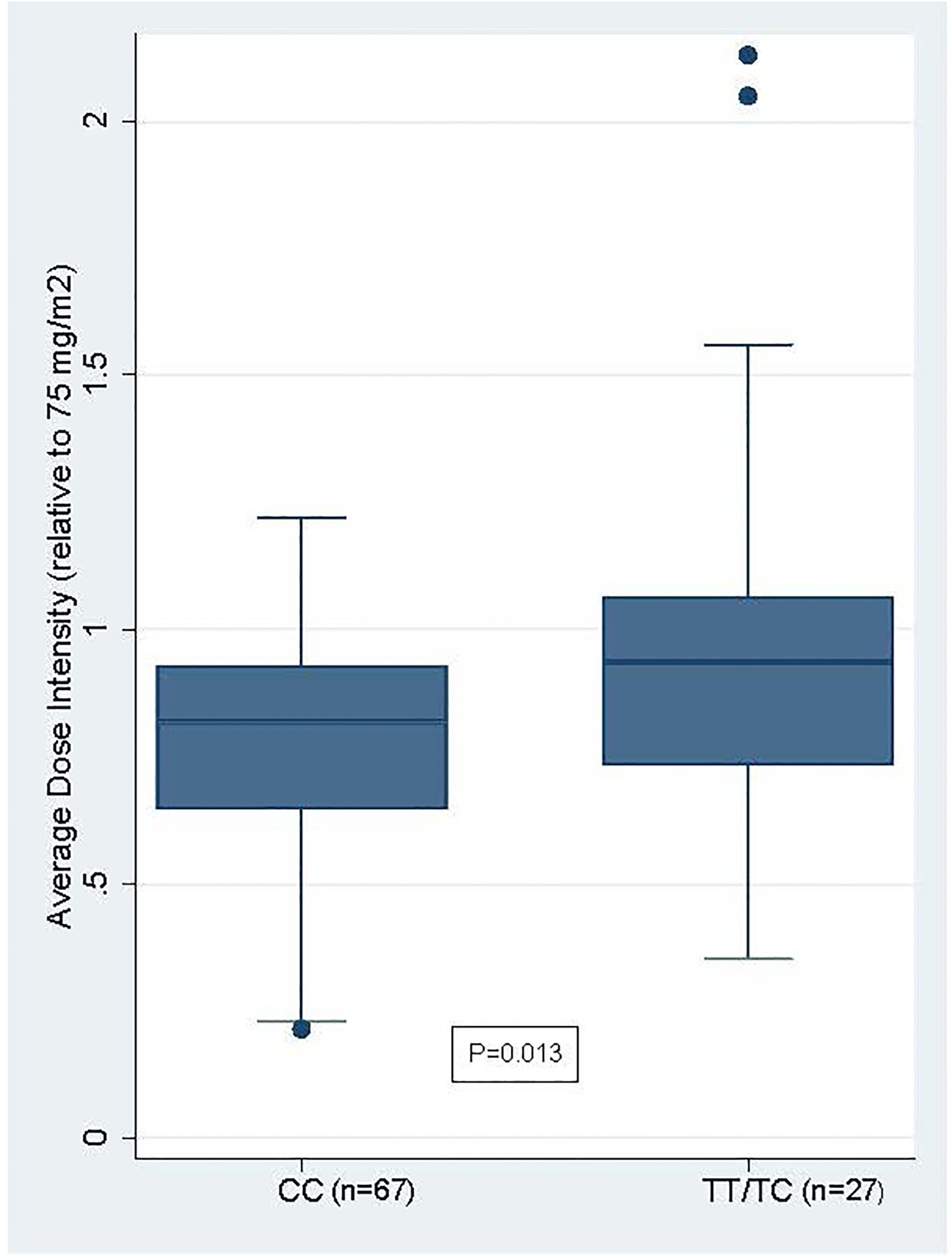
Average Dose Intensity for 6-MP by rs2814478 Genotype in the Validation Cohort
6-MP=6-mercaptopurine
DISCUSSION
In this retrospective cohort study of new thiopurine users, the ACKR1 variant rs2814778-CC was associated with a difference in thiopurine discontinuation and dose, independent of race, age, indication, institution, and TPMT and NUDT15 metabolizer status. In the primary cohort, we sought to identify the patterns of discontinuation and dose reduction by genotype and to verify that they were evident external to race across a range of indications, ages, and metabolizer statuses. We found that patients with the rs2814778-CC genotype discontinued azathioprine due to attributed hematopoietic toxicity at an almost 3-fold higher rate compared to patients who carried the TT or TC genotype. Multiple sensitivity analyses supported the robustness of these findings. In the validation cohort, we further expanded the scope of indications and ages addressed by this study to confirm a broader pattern of dose-reduction by genotype; moreover, the restricted characteristics of this cohort (i.e., race, indication, age group, and TPMT metabolizer phenotype) allowed us to examine whether drug metabolism as measured by metabolites—not available in the primary cohort—were differential by genotype in an otherwise largely uniform cohort. In both the primary and validation cohorts we found that patients with the CC genotype had lower dosing, but patients in the validation cohort had no difference in metabolites by genotype.
Racial minority populations experience health and healthcare disparities that arise from multiple interactive factors, including social and biological determinants.(30) Increasing evidence supports the notion that a failure to account for biologic determinants, such as the CC genotype, has altered clinical practice adversely in other situations (e.g., unnecessary bone marrow biopsies or under-prescription of clozapine).(31, 32) Cell count monitoring (e.g., WBC, ANC) is a standard part of care in the use of thiopurines, meant to guide clinical decision making regarding the safety of continued use and dose. Given that standards are primarily derived from patients with European ancestry, results that fall below these “safe” thresholds routinely lead to discontinuation or dose reduction. For patients with the rs2814778-CC genotype, a genotype found in the majority of Black patients in the United States (and likewise 58.1% of patients with reported Black race in the primary cohort), this means that WBC results that fall within the usual observed range for a Duffy-null phenotype could trigger clinical decisions that would not be made if WBC results fell within the usual observed range for patients without a Duffy-null phenotype.(33, 34)
In the past, some researchers have argued that race could be used to predict genetic traits, and from these traits, clinical outcomes. However, this notion has been challenged, favoring the evaluation of a patient’s individual circumstances.(35) Based on our results as well as currently available information for the rs2814778-CC genotype, we recommend testing for the Duffy-null phenotype be considered if leukopenia is detected while a patient is on azathioprine or prior to azathioprine initiation. Duffy antigen phenotyping is more widely available and returns reliable results more quickly than genotyping.(33, 36) If the Duffy-null phenotype is present, an absolute neutrophil count below 1.5 × 109 cells/L is typical, and this count is not necessarily associated with adverse events;(33, 37) as such, treatment modification (i.e., discontinuation or dose reduction) is not automatically indicated if the threshold remains within the typical range for patients with the Duffy-null phenotype. This recommendation to test all patients, rather than patients with Black race only, is grounded in several concerns: first, the genotype appears among other racial/ethnic groups;(38) second, there is a possibility of inconsistent racial identification and genetic ancestry; and third, making this recommendation only for patients with Black race risks the possibility of reducing genetic diversity to a uniform racial characteristic.
This study was confined to data from tertiary care centers, thus potentially pre-selecting for access to care and/or socioeconomic factors. While we did not have access to direct measures of socioeconomic status (e.g., income, education level), one would anticipate that if socioeconomic factors were a driving factor in the observed differences, discontinuation for non-hematopoietic reasons would also be differential between races; however, our results showed no significant difference, again suggesting that this genotype, rather than other measured and unmeasured potential confounders, has the dominant role. Another limitation was that we relied upon clinical decision-making as recorded in the EHR, rather than specific lab results to determine the primary outcome. As clinicians monitor their patients, a pattern of results (e.g., a downward trajectory in WBC levels) may lead to discontinuation or suboptimal dosing prior to achieving a pre-specified level of toxicity; however, and consistent with our primary analysis, we observed a similar threshold for final weight-adjusted dose, last WBC count, and last NC in patients with and without the CC genotype among those discontinued for attributed hematopoietic toxicity. Moreover, for patients who did not discontinue azathioprine for hematopoietic toxicity, last WBC count, last NC, and most importantly, final weight-adjusted dose were significantly lower for patients with the CC genotype. Additionally, genotype status was not known to the treating providers. Nevertheless, the results were robust across multiple sensitivity analyses and consistent with findings in a validation cohort of children with ALL; in both cohorts, patients with the CC genotype received lower-intensity doses of thiopurines if they were not discontinued for hematopoietic toxicity (both had a ratio of approximately 0.88). Further, in the validation cohort, the dose-adjusted 6-MP metabolite concentrations were similar by rs2814778 genotype, which suggests that the association between the CC genotype and azathioprine dose or discontinuation for attributed hematopoietic toxicity is unrelated to the pharmacokinetics of thiopurines. Finally, the validation cohort primarily confirmed the findings of our secondary outcome. However, the breadth of the primary and validation cohorts affirms the likelihood that this phenomenon is driven by perceptions of risk largely unrelated to disease characteristics or drug metabolism.
In summary, patients with the CC genotype faced higher risk for azathioprine discontinuation attributed to hematopoietic toxicity and lower doses of thiopurines. Genotype, even adjusted for race, is associated with those risks. As such, the next steps for research will be to determine if specific lower WBC counts with the rs2814778-CC genotype impart greater risks for infection, and if not, whether different WBC thresholds are appropriate for discontinuation or dose reduction. A second important question to address will be whether patients with the rs2814778-CC genotype taking thiopurines suffer adverse consequences resulting from undertreatment of disease.
Supplementary Material
ACKNOWLEDGEMENTS:
We thank Ms. Annette Oeser and Ms. Meghan Corriere for insightful comments about this project. No compensation was received for their contributions.
Funding/Support:
This study was supported by grants R01GM126535, R01GM109145, R35GM131770, and by the Rheumatology Research Foundation K-supplement and R01 bridge awards. Dr. Chung also was supported by grant R01AR073764. Dr. Mosley was supported by grant R01GM130791. Dr. Cox was supported by grants P50MD017347 and U54MD010722. Dr. J. Yang was supported by grant R35GM141947. The dataset(s) used for the analyses described were obtained from Vanderbilt University Medical Center’s BioVU Vanderbilt University Medical Center’s BioVU projects are supported by numerous sources: institutional funding, private agencies, and federal grants. These include the NIH funded Shared Instrumentation Grant S10OD017985 and S10RR025141; CTSA grants UL1TR002243, UL1TR000445, and UL1RR024975. Genomic data are also supported by investigator-led projects that include U01HG004798, R01NS032830, RC2GM092618, P50GM115305, U01HG006378, U19HL065962, R01HD074711, R35GM141947; and additional funding sources available at https://victr.vumc.org/biovu-funding/.
Role of the Funder/Sponsor:
The funding sources had no role in the collection, analysis, or interpretation of data, writing of the manuscript, or decision to submit for publication. All drafts of the manuscript were prepared by the authors. All authors approved the final submitted version.
Conflict of Interest Disclosures:
The authors do not have any conflicts of interest related to this work to report. J. Yang receives additional funding from Takeda Pharmaceutical Company.
REFERENCES
- 1.Organization WH. WHO Model List of Essential Medicines 2019. [July 28, 2021]. 21st:[Available from: https://www.who.int/publications/i/item/WHOMVPEMPIAU2019.06.
- 2.Zabala W, Cruz R, Barreiro-de Acosta M, M. C, Panes J, Echarri A, et al. New genetic associations in thiopurine-related bone marrow toxicity among inflammatory bowel disease patients. Pharmacogenomics. 2013;14(6):631–40. [DOI] [PubMed] [Google Scholar]
- 3.Matimba A, Li F, Livshits A, Cartwright CS, Scully S, Fridley BL, et al. Thiopurine pharmacogenomics: association of SNPs with clinical response and functional validation of candidate genes. Pharmacogenomics. 2014;15(4):433–47. doi: 10.2217/pgs.13.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Toruner M, Loftus EV Jr., Harmsen WS, Zinsmeister AR, Orenstein R, Sandborn WJ, et al. Risk factors for opportunistic infections in patients with inflammatory bowel disease. Gastroenterology. 2008;134(4):929–36. doi: 10.1053/j.gastro.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 5.Dickson AL, Daniel LL, Zanussi J, Plummer WD, Wei WQ, Liu G, et al. TPMT and NUDT15 variants predict discontinuation of azathioprine for myelotoxicity in patients with inflammatory disease: real-world clinical results. Clin Pharmacol Ther. 2021. Epub 2021/09/29. doi: 10.1002/cpt.2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roberts RL, Barclay ML. Update on thiopurine pharmacogenetics in inflammatory bowel disease. Pharmacogenomics. 2015;16(8):891–903. [DOI] [PubMed] [Google Scholar]
- 7.Broekman M, Coenen MJH, Wanten GJ, van Marrewijk CJ, Klungel OH, Verbeek ALM, et al. Risk factors for thiopurine-induced myelosuppression and infections in inflammatory bowel disease patients with a normal TPMT genotype. Aliment Pharmacol Ther. 2017;46(10):953–63. doi: 10.1111/apt.14323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bain B, Seed M, Godsland I. Normal values for peripheral blood white cell counts in women of four different ethnic origins. J Clin Pathol. 1984;37(2):188–93. Epub 1984/02/01. doi: 10.1136/jcp.37.2.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shaper AG, Lewis P. Genetic neutropenia in people of African origin. Lancet. 1971;2(7732):1021–3. Epub 1971/11/01. doi: 10.1016/s0140-6736(71)90335-7. [DOI] [PubMed] [Google Scholar]
- 10.Lakhotia R, Aggarwal A, Link ME, Rodgers GP, Hsieh MM. Natural history of benign ethnic neutropenia in individuals of African ancestry. Blood Cells Mol Dis. 2019;77:12–6. Epub 2019/03/26. doi: 10.1016/j.bcmd.2019.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reich D, Nalls MA, Kao WH, Akylbekova EL, Tandon A, Patterson N, et al. Reduced neutrophil count in people of African descent is due to a regulatory variant in the Duffy antigen receptor for chemokines gene. PLoS Genet. 2009;5(1):e1000360. Epub 2009/01/31. doi: 10.1371/journal.pgen.1000360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nalls MA, Wilson JG, Patterson NJ, Tandon A, Zmuda JM, Huntsman S, et al. Admixture mapping of white cell count: genetic locus responsible for lower white blood cell count in the Health ABC and Jackson Heart studies. Am J Hum Genet. 2008;82(1):81–7. Epub 2008/01/09. doi: 10.1016/j.ajhg.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rappoport N, Simon AJ, Amariglio N, Rechavi G. The Duffy antigen receptor for chemokines, ACKR1,- ‘Jeanne DARC’ of benign neutropenia. Br J Haematol. 2019;184(4):497–507. Epub 2018/12/29. doi: 10.1111/bjh.15730. [DOI] [PubMed] [Google Scholar]
- 14.Horuk R The Duffy Antigen Receptor for Chemokines DARC/ACKR1. Frontiers in Immunology. 2015;6(279). doi: 10.3389/fimmu.2015.00279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grann VR, Ziv E, Cecil K, Neugut AI, Wei Y, Jacobson JS, et al. Duffy (Fy), DARC, and neutropenia among women from the United States, Europe and the Caribbean. Br J Haematol. 2008;143(2):288–93. doi: 10.1111/j.1365-2141.2008.07335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roden DM, Pulley JM, Basford MA, Bernard GR, Clayton EW, Balser JR, et al. Development of a large-scale de-identified DNA biobank to enable personalized medicine. Clin Pharmacol Ther. 2008;84(3):362–9. doi: 10.1038/clpt.2008.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wei WQ, Denny JC. Extracting research-quality phenotypes from electronic health records to support precision medicine. Genome Med. 2015;7(1):41. doi: 10.1186/s13073-015-0166-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dumitrescu L, Ritchie MD, Brown-Gentry K, Pulley JM, Basford M, Denny JC, et al. Assessing the accuracy of observer-reported ancestry in a biorepository linked to electronic medical records. Genetics In Medicine. 2010;12(10):648–50. doi: 10.1097/GIM.0b013e3181efe2df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association. 1999;94(446):496–509. doi: 10.1080/01621459.1999.10474144. [DOI] [Google Scholar]
- 20.Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res. 2011;46(3):399–424. Epub 2011/06/08. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raad H, Cornelius V, Chan S, Williamson E, Cro S. An evaluation of inverse probability weighting using the propensity score for baseline covariate adjustment in smaller population randomised controlled trials with a continuous outcome. BMC Medical Research Methodology. 2020;20(1):70. doi: 10.1186/s12874-020-00947-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elze MC, Gregson J, Baber U, Williamson E, Sartori S, Mehran R, et al. Comparison of Propensity Score Methods and Covariate Adjustment: Evaluation in 4 Cardiovascular Studies. J Am Coll Cardiol. 2017;69(3):345–57. Epub 2017/01/21. doi: 10.1016/j.jacc.2016.10.060. [DOI] [PubMed] [Google Scholar]
- 23.Chesnaye NC, Stel VS, Tripepi G, Dekker FW, Fu EL, Zoccali C, et al. An introduction to inverse probability of treatment weighting in observational research. Clinical Kidney Journal. 2021;15(1):14–20. doi: 10.1093/ckj/sfab158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.StataCorp. Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC; 2019. [Google Scholar]
- 25.Bhatia S, Landier W, Hageman L, Kim H, Chen Y, Crews KR, et al. 6MP adherence in a multiracial cohort of children with acute lymphoblastic leukemia: a Children’s Oncology Group study. Blood. 2014;124(15):2345–53. Epub 2014/05/16. doi: 10.1182/blood-2014-01-552166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang JJ, Landier W, Yang W, Liu C, Hageman L, Cheng C, et al. Inherited NUDT15 variant is a genetic determinant of mercaptopurine intolerance in children with acute lymphoblastic leukemia. J Clin Oncol. 2015;33(11):1235–42. doi: 10.1200/JCO.2014.59.4671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Study to Assess Compliance With Long-Term Mercaptopurine Treatment in Young Patients With Acute Lymphoblastic Leukemia in Remission [Internet]. [cited October 15, 2021]. Available from: https://clinicaltrials.gov/ct2/show/NCT00268528.
- 28.FDA. Azathioprine package insert [cited 2021 September 1, 2021]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/016324s034s035lbl.pdf.
- 29.Haukoos JS, Lewis RJ. The Propensity Score. Jama. 2015;314(15):1637–8. Epub 2015/10/27. doi: 10.1001/jama.2015.13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Serchen J, Doherty R, Atiq O, Hilden D. A Comprehensive Policy Framework to Understand and Address Disparities and Discrimination in Health and Health Care: A Policy Paper From the American College of Physicians. Ann Intern Med. 2021;174(4):529–32. Epub 2021/01/12. doi: 10.7326/m20-7219. [DOI] [PubMed] [Google Scholar]
- 31.Van Driest SL, Abul-Husn NS, Glessner JT, Bastarache L, Nirenberg S, Schildcrout JS, et al. Association Between a Common, Benign Genotype and Unnecessary Bone Marrow Biopsies Among African American Patients. JAMA Internal Medicine. 2021. doi: 10.1001/jamainternmed.2021.3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Legge SE, Pardiñas AF, Helthuis M, Jansen JA, Jollie K, Knapper S, et al. A genome-wide association study in individuals of African ancestry reveals the importance of the Duffy-null genotype in the assessment of clozapine-related neutropenia. Mol Psychiatry. 2019;24(3):328–37. Epub 2019/01/17. doi: 10.1038/s41380-018-0335-7. [DOI] [PubMed] [Google Scholar]
- 33.Merz LE, Achebe M. When non-Whiteness becomes a condition. Blood. 2021;137(1):13–5. Epub 2020/11/13. doi: 10.1182/blood.2020008600. [DOI] [PubMed] [Google Scholar]
- 34.Deyrup A, Graves JL, Jr. Racial Biology and Medical Misconceptions. N Engl J Med. 2022;386(6):501–3. Epub 2022/02/05. doi: 10.1056/NEJMp2116224. [DOI] [PubMed] [Google Scholar]
- 35.Barr DA. The practitioner’s dilemma: can we use a patient’s race to predict genetics, ancestry, and the expected outcomes of treatment? Ann Intern Med. 2005;143(11):809–15. Epub 2005/12/07. doi: 10.7326/0003-4819-143-11-200512060-00009. [DOI] [PubMed] [Google Scholar]
- 36.Chang EY, Tormey CA, Siddon AJ, Rahmani M, Wong EY. Duffy Antigen Phenotyping Is a Useful and Clinically Available Test for Benign Ethnic Neutropenia. Blood. 2018;132:2546. doi: 10.1182/blood-2018-99-117178.30352784 [DOI] [Google Scholar]
- 37.Legge SE, Christensen RH, Petersen L, Pardiñas AF, Bracher-Smith M, Knapper S, et al. The Duffy-null genotype and risk of infection. Human Molecular Genetics. 2020;29(20):3341–9. doi: 10.1093/hmg/ddaa208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paz Z, Nalls M, Ziv E. The genetics of benign neutropenia. Israel Medical Association Journal. 2011;13(10):625–9. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


