Abstract
The glycoprotein Ib–IX (GPIb-IX) complex mediates initial platelet adhesion to von Willebrand factor (VWF) immobilized on subendothelial matrix and endothelial surfaces, and transmits VWF binding-induced signals to stimulate platelet activation. GPIb-IX also functions as part of a mechanosensor to convert mechanical force received via VWF binding into intracellular signals, thereby greatly enhancing platelet activation. Thrombin binding to GPIb-IX initiates GPIb-IX signaling cooperatively with protease-activated receptors to synergistically stimulate the platelet response to low dose thrombin. GPIb-IX signaling may also occur following the binding of other GPIb-IX ligands such as leukocyte integrin αMβ2 and red cell-derived semaphorin 7A, contributing to thrombo-inflammation. GPIb-IX signaling requires the interaction between the cytoplasmic domains of GPIb-IX and 14-3-3 protein and is mediated through Src family kinases, the Rho family of small GTPases, phosphoinositide 3-kinase-Akt-cGMP-mitogen-activated protein kinase, and LIM kinase 1 signaling pathways, leading to calcium mobilization, integrin activation and granule secretion. This review summarizes the current understanding of GPIb-IX signaling.
Keywords: Glycoprotein Ib (GPIb), Platelet activation, Intracellular signaling, Thrombosis, von Willebrand factor, Integrin
Introduction
The heavily glycosylated platelet membrane glycoprotein Ib-IX complex (GPIb-IX), is composed of disulfide-linked glycoprotein (GP) Ibα and GPIbβ and the noncovalently bound GPIX.1, 2 This complex is also noncovalently associated with GPV, a negative regulator of GPIb-IX function.3, 4 Since the discovery that reduced expression of a major platelet glycoprotein (later known as GPIb) in Bernard-Soulier syndrome patients was responsible for their defective platelet adhesion to the subendothelium,5 it has been well-established that GPIb-IX is a major and von Willebrand factor (VWF) receptor important in platelet adhesion to the blood vessel wall upon vascular injury.6 The GPIb-IX-VWF interaction is particularly crucial in platelet adhesion and thrombus formation under high shear blood flow (such as in arteries and arterioles),although one should not overlook the importance of GPIb-IX in thrombus formation under low shear flow conditions as well.7, 8 Over the years, accumulating data have demonstrated that GPIb-IX not only mediates platelet adhesion, but also signaling leading to filopodia formation, granule secretion and importantly the activation of another major adhesion receptor, the integrin αIIbβ3 (GPIIb-IIIa).9, 10 The cooperative functions of both GPIb-IX and integrin αIIbβ3 are critical for stable platelet adhesion under flow, for hemostasis and thrombosis, and for the role of platelets in thrombo-inflammatory conditions.11, 12 GPIb-IX is not only a classic receptor in which ligand binding elicits signal transduction leading to platelet activation, but it also functions as part of a mechanosensor that, through the binding of VWF, converts mechanical force into chemical signals.13, 14 This force-enhanced signal transduction enables GPIb-IX to sense the levels and patterns of shear force to induce and regulate platelet responses while also allowing the shear-independent receptor functions of GPIb-IX to occur in response to different types of ligands. Thus GPIb-IX signaling is critical for rapid platelet adhesion and activation in flowing blood in response to different types of vascular insults including injury and inflammation. Despite its importance, many aspects of the mechanisms and pathways of GPIb-IX signaling are still unclear and remain a challenging opportunity for future research. This review briefly discusses the current understanding of GPIb-IX-mediated signal transduction.
GPIb-IX as a receptor for VWF and VWF-induced GPIb-IX signaling
In normal circulation, high affinity binding between plasma VWF and platelet GPIb-IX does not occur because the GPIbα binding site in the A1 domain of VWF is inactive or “hidden”.15–17 Upon vessel injury, VWF binds to the exposed subendothelial collagen, which induces a conformational change in VWF, revealing the “cryptic” binding site and triggering VWF-GPIb interaction.18 This process is facilitated by shear stress and can even be directly induced by very high shear force without VWF immobilization on the subendothelial matrix.18 VWF binding to GPIb exhibits rapid association and dissociation,19 and mediates fast and transient platelet adhesion to the blood vessel wall. The VWF binding function of GPIb-IX can also be regulated by protein-disulfide-isomerase-induced reduction of allosteric disulfide bonds in GPIbα20 and by signals from inside platelets.9, 12, 21 The GPIb-IX binding site for VWF is located within the N-terminal 45kDa region of the GPIbα extracellular domain containing 7 tandem leucine-rich repeats (LRRs).22 Crystal structure analyses suggest a curved half-opened “hand”-like structure in this region of GPIbα with the VWF A1 domain contact sites on the concaved “palm” and “thumb”.22, 23 Biophysical analyses suggest that the GPIb and VWF A1 domains form a “catch” bond24, 25 (also described as a “flex” bond26), which exhibits flow-enhanced adhesion and a pulling force-prolonged lifetime of the GPIbα bond with the A1 domain of VWF, much like pulling a weight on a hook.27 These characteristics explain why the VWF-GPIb-IX interaction is resistant to and even enhanced by shear force, and capable of catching platelets from fast flowing blood onto the vessel wall. However, it is important to note that, despite the shear-resistant nature of VWF binding to GPIb-IX, GPIb-IX-mediated platelet adhesion to VWF is unstable unless immediately followed by activation of the ligand-binding function of integrin αIIbβ3, which binds to VWF, fibrinogen or other integrin ligands to mediate stable platelet adhesion and spreading.9, 10, 15 This is evidenced by experiments showing that in the presence of integrin inhibitors (such as EDTA and RGDS), platelets only transiently adhere to VWF (rolling) under flow shear. In the absence of integrin inhibitors, however, platelets stably adhere to VWF in a GPIb-IX- and integrin-dependent manner.11, 28 There is increasingly strong evidence that VWF binding to GPIb-IX not only mediates initial transient platelet adhesion but also transduces signals activating integrin αIIbβ3.12, 29–32 VWF-induced GPIb-IX signaling also results in platelet shape changes (such as filopodia formation33) and secretion of granule contents,31 which likely facilitate stable platelet adhesion, platelet activation and thrombus formation.
Through the binding of VWF, mechanical force can induce conformational changes in GPIbα. Force-induced conformational changes were observed in two distinct regions of the GPIbα extracellular domain. The first is the ligand binding LRR domain, which was observed by single-molecule pulling experiments with a biomembrane force probe (BFP) and steered molecular dynamics simulations.32, 34 The LRR domain unfolding results in a more pronounced and longer-lasting catch bond with the VWF A1 domain,14 which may enhance shear resistance of the VWF-GPIb interaction and thus platelet adhesion in the presence of high shear flow.
The second force-induced conformational change was observed using optical tweezers13 and BFP14 in a juxtamembrane region of GPIbα termed as the mechanosensitive or mechanosensory domain (MSD).13, 35 The MSD can be unfolded upon the application of a pulling force, either through the bound VWF A1 domain or through an antibody bound to a site N-terminal to the MSD (Figure 1).13, 14 Importantly, MSD unfolding was highly correlated with strong α-type calcium signals, but if the MSD was not unfolded, only weak β-type or null calcium signals were observed.14 These findings built upon early studies into platelet mechanotransduction via GPIb, which identified calcium signaling as a critical intermediary in the platelet signaling cascades36, 37 and provides strong evidence supporting the hypothesis that MSD unfolding is the essential step for the mechanotransduction of force signals.38 This signaling results in activation of integrin αIIbβ3, converting the integrin from a low affinity state into an intermediate affinity state.32 Thus, the MSD is likely part of a sensory module required for converting mechanical force applied via the VWF-GPIb interaction into intracellular signals, inducing platelet activation and stable platelet adhesion. Interestingly, pulling force applied to GPIbα through the binding of the wild-type VWF A1 domain induces a strong calcium signal, whereas that with a mutant VWF A1 domain associated with type 2 von Willebrand diseases induces a weak calcium response. This difference correlates with the formation of a force-resistant catch bond between GPIbα and the wild type VWF A1 domain but only a weak slip bond between GPIbα and the mutant VWF-A1 domain.14 Furthermore, only a durable force, but not a short transient force, induces a strong elevation of intraplatelet calcium.14 These data suggest that GPIbα not only senses the strength and duration of pulling forces, but also differentiates the force and bond lifetime patterns.
Figure 1.
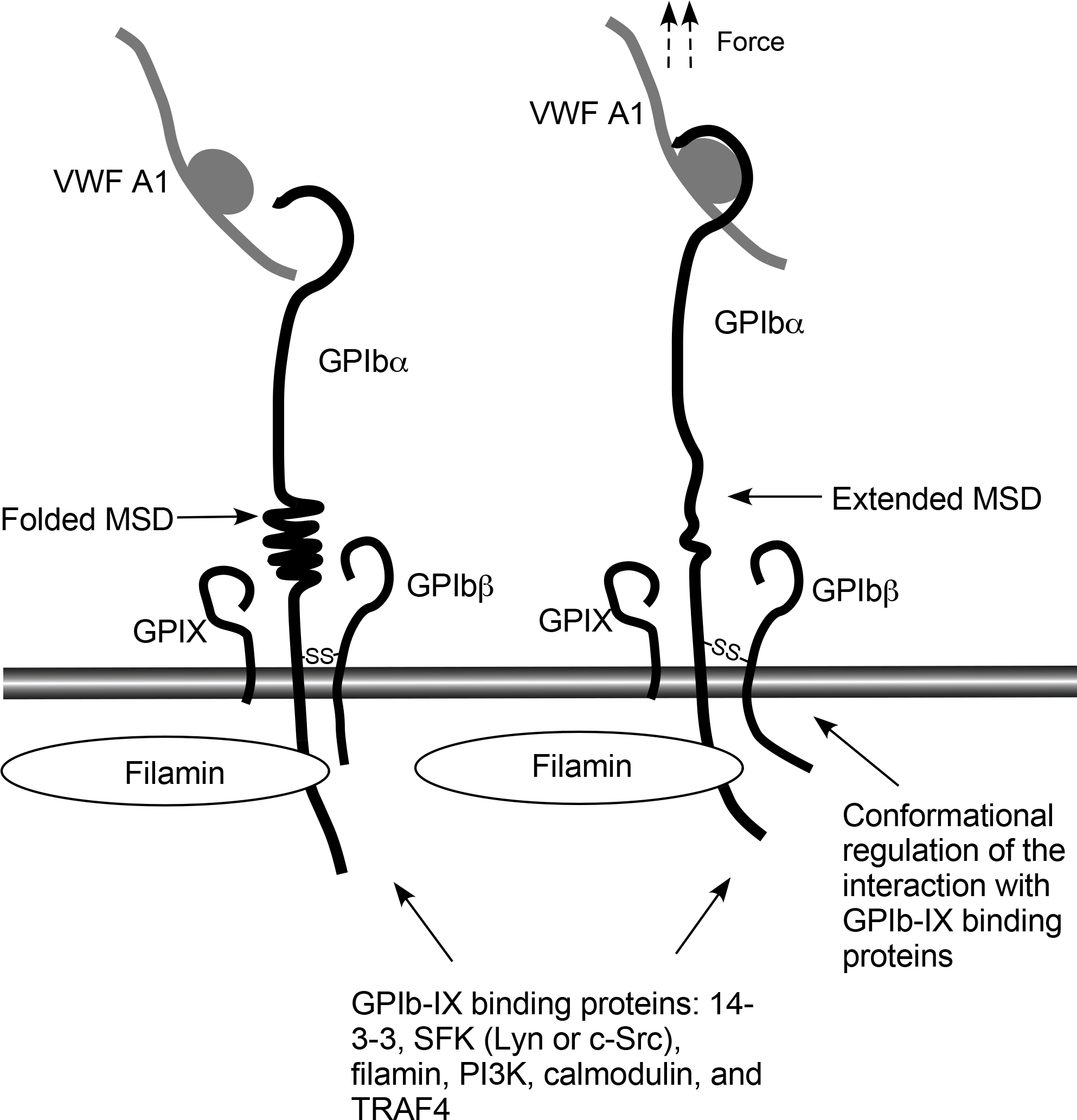
A schematic illustrating the shear-resistant and shear-responsive characteristics of GPIb-IX binding to VWF A1 domain. Left: GPIbα with folded mechanosensory domain (MSD) prior to VWF binding. Right: VWF binds to GPIbα via a shear force-resistant “catch bond”. Shear force, via GPIb-bound VWF, unfolds MSD of GPIbα, which subsequently changes the conformation of the transmembrane complex of GPIb-IX, resulting in cross-membrane signal transduction and regulating the interactions with proteins important for GPIb-IX-initiated intracellular signaling.
In addition to pulling force, MSD conformation can be affected by other factors. For example, removal of sialic acids leads to the unfolding of the O-glycosylated MSD in the absence of force, which is associated with platelet filopodia formation,39 suggesting possible alternative ways to transmit signals via the MSD. These data also suggest that glycosylation stabilizes the MSD in a folded conformation, making GPIb ready for the force-induced unfolding and signal transduction. It remains unclear whether and how ligand binding may affect MSD conformation in the absence of force. However, it is clear that the force-induced MSD unfolding is not the only mechanism for GPIb to transmit signals. VWF binding under static conditions is also capable of inducing GPIb signaling to activate integrin αIIbβ3 and integrin-dependent stable platelet adhesion and spreading on VWF,40 although this signal may not be sufficient to stimulate potent platelet aggregation without shear force. Additionally, the binding of other ligands such as thrombin to GPIbα can also transmit signals, which appears to be independent of shear force. Thus, GPIb-IX-dependent platelet adhesion on immobilized VWF and signal transduction can both occur under no or low shear conditions, but are greatly enhanced by high shear force. VWF-induced GPIb-IX signaling leading to integrin activation is independent of other platelet receptors,41 although GPIb-IX signaling cooperates with other agonist pathways to greatly promote platelet activation. The mechanisms of VWF-induced GPIb-IX transmembrane and intracellular signaling are likely shared by other GPIb-IX ligands and will be discussed in the following sections.
GPIb-IX as a thrombin receptor and thrombin-induced GPIb-IX signaling.
GPIb-IX has long been identified as a major high affinity thrombin-binding platelet membrane protein. The thrombin binding site is located in the C-terminal end of the 45 kDa N-terminal fragment of GPIbα surrounding 3 sulfated tyrosine residues (Y276DYY), near to but distinct from the site of VWF binding.12 GPIb-IX was convincingly shown to participate in low dose thrombin-induced platelet activation by multiple approaches.9 However, there have been controversies regarding whether GPIb serves as merely a docking site for thrombin to enhance thrombin cleavage of G protein-coupled protease-activated receptors (PARs)42 or if thrombin binding to GPIb-IX can also transmit signals directly into the cell to induce platelet activation as a classic receptor.4, 43 More recent data suggest a signaling-based cooperativity between GPIb-IX and PARs.44 Thrombin binding to GPIb likely induces GPIb-IX signaling, which greatly enhances platelet responses to PARs. Conversely, thrombin activation of the PAR signaling pathway is also crucial for inducing GPIb-IX-dependent activation of the Rac1-LIMK1 signaling pathway, which is essential for GPIb-IX-PAR cooperativity. Thus, at low concentrations of thrombin, both GPIb-IX signaling and PAR signaling are required for thrombin to induce platelet aggregation.44 A key distinction between thrombin and VWF-mediated GPIb-IX signaling is that thrombin-induced GPIb-IX signaling fails to elicit calcium elevation without the cooperativity of the PAR signaling pathway, whereas VWF induces calcium elevation on its own. The cooperativity between GPIb-IX signaling and PAR signaling is likely to be most important during thrombus formation in vivo under arterial flow, as thrombin concentrations at the site of limited arterial injury, particularly in the early phase of thrombus formation, are very low.44
Other GPIb-IX ligands and their roles in GPIb-IX signaling.
Although the primary ligands for the GPIb-IX receptor are VWF and thrombin, this multifunctional receptor also binds to a number of other proteins in the circulation that can be grouped into the following three categories. (1) Regulators of GPIb-IX ligand binding functions: High molecular weight kininogen (HK) negatively regulates thrombin binding to GPIbα and low dose- thrombin-induced platelet activation.45 Coagulation factor XII also binds GPIb-IX and partially displaces HK binding. However, only the activated form of FXII (FXIIa) has inhibitory effects on thrombin-induced platelet activation, even though the catalytic activity of FXIIa is not required.46 Protein-disulfide-isomerase (PDI) binds to GPIbα and catalyzes the reduction of disulfide bonds of Cys4-Cys17 and Cys209-Cys248, facilitating VWF binding to GPIb-IX, platelet-neutrophil interactions and vascular occlusion under thrombo-inflammatory conditions.20 (2) Counter receptors or ligands that facilitate GPIb-IX-dependent platelet adhesion to other cells: The GPIb-IX interaction with P-selectin was reportedly involved in platelet adhesion to vascular endothelial cells47 whereas GPIb-IX binding to Mac1 is important in platelet-leukocyte interactions leading to thrombosis and inflammation.48 Semaphorin 7A, a newly reported GPIb-IX ligand, also promotes neutrophil-platelet interactions particularly during myocardial ischemia-reperfusion injury.49 (3) Other GPIb-IX ligands facilitating platelet adhesion under high shear: Reelin forms a complex with the amyloid precursor protein (APP) and apolipoprotein E receptor 2 (ApoER2) 50 and these complexes participate in GPIb-IX-dependent platelet activation and thrombus formation under high shear.50 Thrombospondin-1 can also mediate platelet adhesion under high shear,51 however, the in vivo role of thrombospondin in thrombosis requires VWF,52 possibly due to the role of thrombospondin-1 in regulating VWF cleavage by ADAMTS13. It is not totally clear whether GPIb-IX signaling plays a role in the action of these GPIb-IX binding proteins. However, semaphorin 7A and reelin/APP/ApoER2 were suggested to stimulate platelet activation and enhance platelet thrombus formation under flow in a GPIb-IX-dependent manner.49, 50 Also, Mac1 expressed on leukocyte microparticles was suggested to activate platelets via interaction with GPIb.53 These data suggest that at least some of these GPIb-IX ligands induce GPIb-IX signaling, leading to platelet activation.
Mechanisms and pathways of GPIb-IX signal transduction.
The known binding sites for various GPIb-IX ligands are almost all located in the N-terminal region of GPIbα. Thus, the binding-initiated signals of these ligands are likely to be transmitted through the central stalk of GPIbα to reach the membrane spanning region of the GPIb-IX complex, possibly by the following mechanisms individually or in combination: (1) allostery by force-induced conformational change; unfolding of the MSD and other ectodomain conformational changes caused by force through VWF binding under shear is likely to be propagated across the membrane into the cytoplasmic domain of GPIb-IX.13, 14 (2) Ligand-binding induced receptor clustering; this possibility is suggested by the data that extracellular crosslinking of anti-GPIbα antibodies or induction of GPIb-IX clustering intracellularly caused similar GPIb-IX signaling to activate integrin αIIbβ3.54 (3) Force-independent conformational changes induced by ligand binding. The conformational changes in the ligand binding sites of GPIbα and the MSD are likely propagated to the membrane-spanning complex of GPIbα, GPIbβ and GPIX (Figure 1). This is likely required for transmembrane signal transmission, as different monoclonal antibodies recognizing epitopes in the extracellular domain of GPIbβ reportedly inhibited or stimulated GPIb-IX signaling.55, 56 The cytoplasmic domains of GPIbα and GPIbβ reportedly interact with several intracellular proteins (Figure 1), including Filamin A (actin-binding protein),57 14-3-3 proteins,58 PI3K,59, 60 Src family kinases (SFK),61 TNF receptor-associated factor 4 (TRAF4)62, 63 and calmodulin.64 Filamin A binding to the cytoplasmic domain of GPIbα serves as a major link between GPIb-IX and the actin cytoskeleton underlying the membrane (membrane skeleton) and thus functions as an important structural protein in shear-resistance and in maintaining membrane integrity and platelet shape.65 The Filamin A-GPIb-IX interaction was also suggested to regulate the binding of VWF multimers66 and shear-dependent GPIb-IX-mediated protein tyrosine phosphorylation.67 Furthermore, Filamin A binds to numerous intracellular signaling molecules including tyrosine kinase Syk and the small GTPases Cdc42 and RhoA.68 The Syk-Filamin interaction promotes ITAM signaling stimulated via GPVI and the C-type lectin-like receptor 2.69 Cdc42 plays a key role in GPIb-IX-stimulated filopodia formation,33 and both Cdc42 and RhoA regulate GPIb-IX-mediated transendothelial platelet biogenesis.33, 70
14-3-3 in GPIb-IX signaling.
The 14-3-3 proteins are a family of highly conserved dimeric 30 kDa proteins that bind to certain phosphorylated-serine containing peptide motifs and regulate phosphorylation-dependent protein-protein interactions.21 The ζ isoform of 14-3-3 was initially reported to bind to GPIb-IX.58 However, subsequent studies showed that GPIb interacts with all 6 14-3-3 isoforms expressed in platelets.71 Three reported 14-3-3 binding sites in the GPIbα cytoplasmic domain are the C-terminal binding site S602IRYSGHpSL610,72 the near C-terminal binding site L580VAGRRPpSALpS590,60, 73 and the central region R557GpSLP561 sequence.74, 75 There is also a reported binding site in GPIbβ at Arg164-Pro170.75 All binding sites are peptide motifs containing a key phosphorylated serine.
The binding of 14-3-3 to the GPIb-IX cytoplasmic domains plays a role in transmitting signals to regulate the extracellular ligand binding function of GPIb-IX. Phosphorylation at S166 in the 14-3-3 binding site of GPIbβ by protein kinase A reduces (but does not abolish) VWF binding to GPIb-IX.73, 76 Thus, mutating S166 of GPIbβ to alanine in a Chinese hamster ovary cell model enhanced VWF binding.72, 76 This enhancement was diminished by mutating/deleting the C-terminal 14-3-3 binding site of GPIbα.72, 76 Blocking 14-3-3 binding to GPIb-IX with a synthetic peptide (MPαC) derived from the GPIbα C-terminal 14-3-3 binding sequence inhibited ristocetin- and botrocetin-induced VWF binding, and also inhibited GPIb-IX-dependent transient platelet adhesion to VWF under shear.72 Based on these data, a “toggle switch” theory was proposed in which the interaction of 14-3-3 with its binding sites in GPIbα alone results in multimeric VWF binding to GPIb-IX. However, increased cAMP causes PKA activation-dependent 14-3-3 binding to both GPIbα and GPIbβ which negatively regulates VWF binding.9, 72 Interestingly, the central region 14-3-3 binding site as well as the near C-terminal 14-3-3 binding site of GPIbα (L580VAGRRPpSALpS590 and R557GpSLP561) overlap or partially overlap with two different GPIb-IX sequences necessary for interacting with filamin.73 Deleting the filamin binding sites together with all three 14-3-3 binding sites in GPIbα caused enhanced binding of VWF multimers (but not A1 domain) to GPIb-IX in the CHO cell expression model,66 leading to the hypothesis that 14-3-3 may regulate filamin A-GPIb interaction and thus the binding of VWF multimers to GPIb-IX.21 A recent study showed that deletion of the C-terminal 24 amino acid segment of GPIbα in mouse platelets caused increased platelet size but showed no significant effect on GPIb-IX/integrin αIIbβ3-dependent platelet adhesion to VWF under flow shear.77 It would be interesting to further investigate whether GPIb-IX-specific platelet rolling on VWF in the presence of integrin inhibitors are affected by this mutation. Also, as the C-terminal 24 amino acid residues of GPIbα partially overlap with one of the filamin binding sequences,73 it would be also interesting to know whether this mutant also partially affects filamin-GPIbα interaction, and thus platelet size and VWF binding function.
The binding of 14-3-3 to the GPIbα cytoplasmic domain is also required for the VWF-induced GPIb-IX signaling that leads to integrin activation.14, 21, 78 This is supported by finding that binding of the VWF A1 domain to platelet GPIb-IX together with a pulling force induces calcium elevation, which can be inhibited by MPαC peptide, an inhibitor of 14-3-3 binding to GPIbα.14 14-3-3 binding to GPIbα also mediates thrombin-induced GPIb-IX signaling (but not thrombin binding) that cooperates with PAR-mediated signals to induce platelet activation.44 Two of the 14-3-3 binding sites in GPIbα, one at the C-terminus and the other near the C-terminus, are both important for VWF/GPIb-IX-mediated integrin activation.14, 21, 78, 60, 77 Also, mouse platelets expressing GPIbα with a 6-residue deletion at the C-terminal 14-3-3 binding site showed impaired arterial thrombosis in the FeCl3-induced thrombosis model79 and impaired platelet-dependent tumor metastasis to the lung.79 A recent study using a deletion mutant of GPIbα lacking the C-terminal 24 amino acid residues showed it strongly reduced platelet thrombus formation on collagen under shear stress.77 The potent inhibitory effects of deletion of the GPIbα 14-3-3 binding sites on platelet adhesion and thrombus formation on collagen in a whole blood perfusion system combined with the knowledge that platelet adhesion/thrombus formation on the collagen-rich arterial sub-endothelium is dependent on VWF and GPIb 7 supports the importance of the 14-3-3-GPIb-IX interaction in VWF-induced GPIb-IX signaling. It also supports the potential role of GPIb-IX signaling in promoting collagen-mediated platelet activation as previously suggested.80 However, it remains unclear how 14-3-3 mediates or regulates GPIb-IX signaling leading to integrin activation.
The pathway of SFK, Rac1, phosphoinositide 3-kinase (PI3K), Akt, cGMP and mitogen-activated protein kinases (MAPK) in GPIb-IX signaling.
Besides 14-3-3, SFK provide the most proximal link between GPIb-IX and intracellular signaling pathways. SFK Lyn and c-Src are associated with GPIb-IX.61 SFK, particularly Lyn, are functionally required for signaling that results in VWF and low dose thrombin-induced platelet activation, and in GPIb-IX-initiated, integrin-dependent stable platelet adhesion to VWF under shear stress.81 One platelet pathway responsible for the requirement of Lyn is the Lyn-dependent phosphorylation and activation of guanine nucleotide exchange factor (GEF) Vav, which activates Rac1, a member of the Rho family of small GTPases.82 Activated Rac1 mediates activation of the PI3K pathway.82 Interestingly, PI3K was shown to interact with GPIbα59, 83 at a binding site in the C-terminal region of GPIbα proximal to the C-terminal 14-3-3 binding site,84 and is important in GPIb-IX signaling.28, 85 A major function of PI3K is to activate protein kinase Akt. Akt1 mediates the activation of the cGMP pathway,28 which activates the p38 and ERK mitogen-activated protein kinase (MAPK) pathway.86, 87 All these pathways are thus sequentially linked and lead to GPIb-IX-mediated platelet integrin activation and granule secretion (Figure 2).12, 85, 88 Activation of this pathway requires 14-3-3 binding to GPIb, as deletion of the C-terminal 14-3-3 binding site in GPIbα or use of the inhibitor peptide derived from the GPIbα C-terminal sequence inhibited VWF- or thrombin-induced GPIb-IX signaling, calcium elevation and platelet activation.44, 72 Another MAPK, ERK5, and associated casein kinase II, have also been suggested to phosphorylate and attenuate Phosphatase And Tensin Homolog (PTEN) activity, thus promoting the GPIb-IX-mediated activation of PI3K-Akt leading to platelet activation.89
Figure 2.
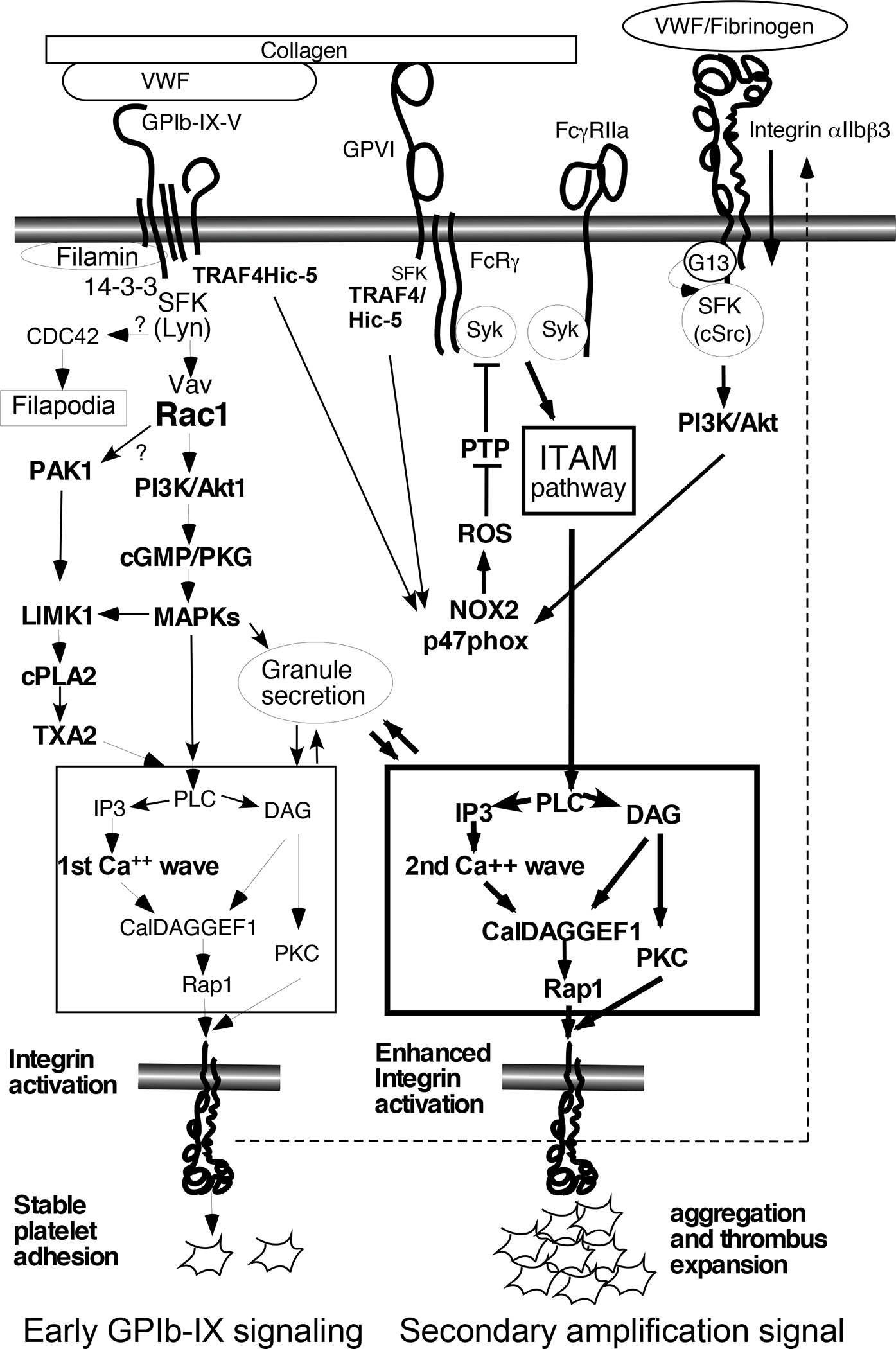
GPIb-IX-mediated early platelet activation pathways and secondary signal amplification mechanisms. In GPIb-IX-mediated early signaling, GPIb-IX ligation by VWF induces activation of Src family kinase (SFK) Lyn via a 14-3-3-dependent mechanism and a series of downstream signaling events as depicted in the figure, leading to activation of phospholipase C (PLC). A simplified schematic of the PLC-mediated common pathway leading to integrin αIIbβ3 activation and granule secretion is shown in the box with thin outlines: PLC cleaves membrane phospholipids to generate inositol 1,4,5 triphosphate (IP3) and diacylglycerol (DAG), which together activate calcium release and DAG-activated guanine nucleotide exchange factor 1 (CalDAGGEF1). CalDAGEF1 activates RAS-related protein 1 (Rap1), which induces talin binding to the cytoplasmic domain of integrin αIIbβ3 and integrin activation. DAG and/or calcium also activate protein kinase C (PKC) isoforms to stimulate granule secretion. Top Right: Activated integrin αIIbβ3 mediates stable platelet adhesion and initiates outside-in signaling, which, via a Gα13- and c-Src-dependent mechanism, activates NADPH oxidase 2 (NOX2) to generate reactive oxygen species (ROS) under shear stress. ROS inhibits protein tyrosine phosphatases (PTP), which are negative regulators of Spleen tyrosine kinase (Syk), facilitating Syk activation. Syk is activated by binding to immunoreceptor tyrosine-based activation motif (ITAM) in the cytoplasmic domain of GPVI-associated Fc receptor γ chain (FcRγ) or Fcγ receptor IIA (FcγRIIA). Activated Syk stimulates the ITAM signaling pathway (see ref12 for more details), to potently activate platelets via PLCγ, which, as depicted in the thick-outlined box, induces a 2nd wave of Ca++ release and robust platelet activation and thrombus formation. GPIbβ and GPVI also bind to TNF receptor associated factor 4 (TRAF4) in complex with Hydrogen peroxide-inducible clone 5 protein (Hic-5) and p47PHOX, a regulatory unit of NOX2, which then stimulates GPVI-mediated ITAM signaling.
Other abbreviations: GPIb, Glycoprotein Ib; VWF, von Willebrand factor; Rac1, Ras-related C3 botulinum toxin substrate 1; PAK1, P21-activated kinase 1; PI3K, phosphoinositide 3-kinase; cGMP, cyclic guanosine monophosphate; PKG, protein kinase G; MAPKs, mitogen-activated protein kinases; LIMK1, LIM domain kinase 1; cPLA2, cytosolic phospholipases A2; TXA2, thromboxane A2.
The role of cGMP in GPIb-IX-mediated platelet activation.
Both VWF and low dose thrombin induce elevation of intracellular cGMP (cyclic guanosine monophosphate), which activates the cGMP-dependent protein kinase (PKG).88 cGMP plays biphasic role in platelet activation: low concentrations of cGMP generated in the early phase of platelet activation, via PKG, promote integrin activation and granule secretion mediated by GPIb-IX and other receptors.88, 90 High concentrations of cGMP and cGMP generated at later phases of thrombus formation inhibit platelet activation and limit the growth of platelet thrombi88, 91 via PKG and PKA-dependent signaling pathways.12 The biphasic role of cGMP provides a potential explanation as to why GPIb-IX-mediated platelet activation is often seen as “measured” or “weak” and platelets adherent on the surface of a thrombus exposed to high shear appear less activated despite of clear evidence of integrin activation.
LIM kinase (LIMK) 1 and GPIb-IX signaling
LIMK1 is a protein kinase activated by the p21-activated kinase (PAK),92 and thus a downstream effector of Rac1 (also named p21). LIMK1 is expressed in platelets and promotes both VWF- and thrombin-induced GPIb-IX signaling and platelet activation.44, 93 This role of LIMK1 in platelet activation is selective for the GPIb-IX signaling pathway, and it in fact negatively regulates platelet activation induced by other platelet agonists such as PAR agonists and thromboxane A2.44, 93 This is in contrast to the ability of Lyn, Rac1, PI3K-Akt, cGMP and MAPK to stimulate the GPIb pathway as well as other agonist pathways. Interestingly, GPIb-IX-mediated LIMK1 activation appears to be dependent upon p38 and ERK MAPKs in addition to Rac1. Although it remains unclear how LIMK1 selectively mediates GPIb-IX-mediated platelet activation, LIMK1 is important in GPIb-IX-mediated phosphorylation of cytosolic phospholipase A2 (cPLA2), TXA2 generation and TXA2-dependent amplification of platelet activation.93 Thus, the LIMK1 pathway appears to serve as a GPIb-IX-selective mechanism for the activation of the TXA2 signaling pathway (Figure 2).12, 93
Calcium elevation, phospholipase C, and secondary amplification pathways.
GPIb-IX-mediated platelet activation signaling has two phases. The early phase signal induced by ligand binding to GPIb-IX activates the ligand binding function of integrin αIIbβ3. The late phase amplification signal, is mainly mediated by the integrin outside-in signaling pathway and integrin-stimulated ITAM signaling. These two phases are reflected in the intracellular calcium elevation induced by VWF binding to GPIb-IX. Under shear stress, platelet adhesion to VWF is associated with two major peaks of calcium elevation; a small peak associated with GPIb-IX early signaling followed by a late, but more robust integrin-dependent peak.36 Similar to other platelet agonist signaling pathways, GPIb-IX-mediated calcium elevation is mediated by IP3-dependent intracellular store release and requires phospholipase C (PLC), particularly PLCγ, which cleaves phospholipids to release IP3.12, 94 Whereas the exact pathway of GPIb-IX specific PLC activation is not completely clear, SFK are clearly required and several of the above-described pathways as well as secondary release of platelet agonists can lead to activation of various PLC isoforms. Also, phospholipase D1 reportedly promotes GPIb-IX-mediated SFK and PLCγ activation.95
The immunoreceptor tyrosine-based activation motifs (ITAMs) in the Fc receptors in human platelets, including Fc receptor γ chain (FcRγ) and Fcγ receptor IIA (FcγRIIA), stimulate Syk-dependent signaling leading to activation of PLCγ, calcium elevation and activation of protein kinase C, resulting in robust platelet activation.12 Because GPIb-IX can associate with FcγRIIA or FcRγ, it was hypothesized that GPIb-IX induces calcium elevation and platelet activation via the ITAM signaling pathway.9 This hypothesis is supported by evidence that the key enzymes of the ITAM pathway, such as Syk, are activated and important in promoting GPIb-IX-initiated platelet activation.96 However, FcRγ and FcγRIIA are not required for GPIb-IX-mediated calcium elevation and shape change.94 GPIb-IX-dependent platelet aggregation was reduced at low but not high concentrations of botrocetin in FcRγ-deficient mouse platelets.97 Another study demonstrated normal platelet aggregation of FcRγ-deficient mouse platelets in response to botrocetin in the presence of extracellular integrin ligand fibrinogen, although aggregation was inhibited in the absence of fibrinogen.98 This study also demonstrated that FcRγ was not required for integrin-independent TXA2 synthesis initiated by GPIb-IX signaling nor GPIb-IX-dependent integrin activation.98 Since mouse platelets do not express FcγRIIA, these data suggest that FcRγ and FcγRIIA are not required for GPIb-IX-mediated integrin activation and primary platelet aggregation, but may play a secondary secretion-dependent role in amplifying GPIb-IX-initiated platelet activation. Furthermore, the key ITAM kinase Syk is also dispensable for GPIb-IX-mediated integrin activation, GPIb-IX- and integrin-dependent stable platelet adhesion to VWF under shear and VWF/GPIb-IX-dependent platelet aggregation induced by ristocetin and botrocetin.28, 99 Thus, the ITAM pathway does not appear to be directly required for early phase GPIb-IX signaling leading to integrin activation.
It was recently shown that ligand binding to integrin αIIbβ3 transmits outside-in signaling to stimulate phosphorylation of p47phox, a regulatory component of NADPH oxidase 2 (NOX2), resulting in activation of NOX2.100 NOX2 catalyzes the generation of reactive oxygen species (ROS), which inhibit protein tyrosine phosphatases (PTP) leading to the activation of Syk and thus the ITAM pathway (Figure 2).100 Considering the importance of the Syk and ITAM pathway in greatly promoting platelet activation induced by integrin outside-in signaling, and the importance of integrin outside-in signaling in amplifying GPIb-IX-mediated platelet activation signals, we propose that integrin/ROS-dependent activation of the ITAM pathway plays a major role in GPIb-IX-induced second wave calcium elevation and amplification of platelet activation (Figure 2). Interestingly, GPIbβ and GPVI are reportedly associated with TRAF4/Hic-5/p47phox, and inhibitors of reactive oxygen species were shown to inhibit GPVI and low dose thrombin-induced, GPIb-IX-dependent platelet activation.62, 101 It is thus possible that GPIb-IX may also stimulate ROS generation independent of integrin outside-in signaling, and facilitate the ITAM signaling (Figure 2).
Conclusions
GPIb-IX not only mediates platelet adhesion under flow shear stress, but also acts as a mechanical/chemical receptor that receives and transmits signals from external mechanical force and/or ligand binding to initiate cellular responses. The molecular bases of these functions of GPIb-IX include the shear force-resistant adhesion bonding (catch bond), a mechanosensory domain of GPIbα and the intracellular interaction of the GPIb-IX complex with intracellular cytoskeletal and signaling molecules. Thus VWF binding to GPIb-IX induces a series of intracellular signaling events without requiring other agonist receptors. However, GPIb-IX also cooperates with thrombin and collagen receptors to enhance platelet response to these agonists. Increasing evidence suggests that GPIb-IX-mediated signaling includes two-phases as reflected by the two waves of calcium signals. The first phase signals via the 14-3-3 and SFK-initiated pathways and activates integrin αIIbβ3 to an intermediate state for ligand binding, which is essential for stable platelet adhesion. The second phase is mediated by integrin outside-in signaling, which shear force-dependently triggers ROS-mediated Syk activation and Syk-dependent ITAM signaling, leading to robust platelet activation and thrombus formation. Hence, an essential role of GPIb-IX signaling is to facilitate stable platelet adhesion to the exposed subendothelial matrix, the stimulated endothelium, leukocytes and other platelets under flow shear stress. This role is important not only for hemostasis and thrombosis, but also in the development of thrombo-inflammatory conditions, tumor metastasis and, in megakaryocytes, platelet biogenesis. Thus, targeting GPIb-IX signaling has the potential for treating thrombosis in stenotic arteries associated with stroke and heart attack, microvascular thrombosis such as in thrombotic thrombocytopenic purpura, and in thrombo-inflammatory conditions such as ischemia/reperfusion injury. Targeting GPIb-IX signaling may also have the potential to improve platelet preservation and transfusion, and in reducing tumor metastasis.
Acknowledgement
We thank Dr. Randal Skidgel for critical reading of this manuscript.
Disclosure Statement
The University of Illinois holds patents related to GPIb-IX and integrins. X.D. has equity interests in DuPage Medical Technology, Inc. which licenses the university patents. Other authors have no conflicts of interest to declare. This work was partially supported by National Heart, Lung and Blood Institute grants HL150797 (X.D), HL062350 (X.D.) and HL132019 (C.Z.).
References
- 1.Ware J Molecular analyses of the platelet glycoprotein Ib-IX-V receptor. Thromb Haemost 1998;79:466–478. [PubMed] [Google Scholar]
- 2.Quach ME, Li R. Structure-function of platelet glycoprotein Ib-IX. J Thromb Haemost 2020;18:3131–3141. Epub 2020/08/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramakrishnan V, Reeves PS, DeGuzman F, Deshpande U, Ministri-Madrid K, DuBridge RB, Phillips DR. Increased thrombin responsiveness in platelets from mice lacking glycoprotein V. 1999;96:13336–13341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramakrishnan V, DeGuzman F, Bao M, Hall SW, Leung LL, Phillips DR. A thrombin receptor function for platelet glycoprotein Ib-IX unmasked by cleavage of glycoprotein V. Proc Natl Acad Sci U S A 2001;98:1823–1828. Epub 2001/02/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caen JP, Nurden AT, Jeanneau C, Michel H, Tobelem G, Levy-Toledano S, Sultan Y, Valensi F, Bernard J. Bernard-Soulier syndrome: a new platelet glycoprotein abnormality. Its relationship with platelet adhesion to subendothelium and with the factor VIII von Willebrand protein. J Lab Clin Med 1976;87:586–596. Epub 1976/04/01. [PubMed] [Google Scholar]
- 6.Clemetson KJ, Clemetson JM. Molecular abnormalities in Glanzmann’s thrombasthenia, Bernard-Soulier syndrome, and platelet-type von Willebrand’s disease. Curr Opin Hematol 1994;1:388–393. Epub 1994/09/01. [PubMed] [Google Scholar]
- 7.Sakariassen KS, Nievelstein PF, Coller BS, Sixma JJ. The role of platelet membrane glycoproteins Ib and IIb-IIIa in platelet adherence to human artery subendothelium. Br J Haematol 1986;63:681–691. [DOI] [PubMed] [Google Scholar]
- 8.Cosemans JM, Schols SE, Stefanini L, de Witt S, Feijge MA, Hamulyak K, Deckmyn H, Bergmeier W, Heemskerk JW. Key role of glycoprotein Ib/V/IX and von Willebrand factor in platelet activation-dependent fibrin formation at low shear flow. Blood 2011;117:651–660. Epub 2010/11/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Du X Signaling and regulation of the platelet glycoprotein Ib-IX-V complex. Curr Opin Hematol 2007;14:262–269. Epub 2007/04/07. [DOI] [PubMed] [Google Scholar]
- 10.Li Z, Delaney MK, O’Brien KA, Du X. Signaling during platelet adhesion and activation. Arterioscler Thromb Vasc Biol 2010;30:2341–2349. Epub 2010/11/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Savage B, Almus-Jacobs F, Ruggeri ZM. Specific synergy of multiple substrate-receptor interactions in platelet thrombus formation under flow. Cell 1998;94:657–666. [DOI] [PubMed] [Google Scholar]
- 12.Estevez B, Du X. New Concepts and Mechanisms of Platelet Activation Signaling. Physiology (Bethesda) 2017;32:162–177. Epub 2017/02/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang W, Deng W, Zhou L, Xu Y, Yang W, Liang X, Wang Y, Kulman JD, Zhang XF, Li R. Identification of a juxtamembrane mechanosensitive domain in the platelet mechanosensor glycoprotein Ib-IX complex. Blood 2015;125:562–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ju L, Chen Y, Xue L, Du X, Zhu C. Cooperative unfolding of distinctive mechanoreceptor domains transduces force into signals. eLife 2016;5:e15447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruggeri ZM, Mendolicchio GL. Adhesion mechanisms in platelet function. Circ Res 2007;100:1673–1685. Epub 2007/06/23. [DOI] [PubMed] [Google Scholar]
- 16.Springer TA. von Willebrand factor, Jedi knight of the bloodstream. Blood 2014;124:1412–1425. Epub 2014/06/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Z, Lin J, Sulchek T, Cruz MA, Wu J, Dong JF, Zhu C. Domain-specific mechanical modulation of VWF-ADAMTS13 interaction. Mol Biol Cell 2019;30:1920–1929. Epub 2019/05/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fu H, Jiang Y, Yang D, Scheiflinger F, Wong WP, Springer TA. Flow-induced elongation of von Willebrand factor precedes tension-dependent activation. Nat Commun 2017;8:324. Epub 2017/08/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doggett TA, Girdhar G, Lawshe A, Schmidtke DW, Laurenzi IJ, Diamond SL, Diacovo TG. Selectin-like kinetics and biomechanics promote rapid platelet adhesion in flow: the GPIb(alpha)-vWF tether bond. Biophys J 2002;83:194–205. Epub 2002/06/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li J, Kim K, Jeong SY, Chiu J, Xiong B, Petukhov PA, Dai X, Li X, Andrews RK, Du X, et al. Platelet Protein Disulfide Isomerase Promotes Glycoprotein Ibalpha-Mediated Platelet-Neutrophil Interactions Under Thromboinflammatory Conditions. Circulation 2019;139:1300–1319. Epub 2018/12/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Y, Ruggeri ZM, Du X. 14-3-3 proteins in platelet biology and glycoprotein Ib-IX signaling. Blood 2018;131:2436–2448. Epub 2018/04/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uff S, Clemetson JM, Harrison T, Clemetson KJ, Emsley J. Crystal structure of the platelet glycoprotein Ib(alpha) N-terminal domain reveals an unmasking mechanism for receptor activation. J Biol Chem 2002;277:35657–35663. Epub 2002/06/28. [DOI] [PubMed] [Google Scholar]
- 23.Huizinga EG, Tsuji S, Romijn RA, Schiphorst ME, de Groot PG, Sixma JJ, Gros P. Structures of glycoprotein Ibalpha and its complex with von Willebrand factor A1 domain. Science 2002;297:1176–1179. Epub 2002/08/17. [DOI] [PubMed] [Google Scholar]
- 24.Yago T, Lou J, Wu T, Yang J, Miner JJ, Coburn L, López JA, Cruz MA, Dong JF, McIntire LV, et al. Platelet glycoprotein Ibalpha forms catch bonds with human WT vWF but not with type 2B von Willebrand disease vWF. J Clin Invest 2008;118:3195–3207. Epub 2008/08/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ju L, Dong JF, Cruz MA, Zhu C. The N-terminal flanking region of the A1 domain regulates the force-dependent binding of von Willebrand factor to platelet glycoprotein Ibα. J Biol Chem 2013;288:32289–32301. Epub 2013/09/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim J, Zhang CZ, Zhang X, Springer TA. A mechanically stabilized receptor-ligand flex-bond important in the vasculature. Nature 2010;466:992–995. Epub 2010/08/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McEver RP, Zhu C. Rolling cell adhesion. Annu Rev Cell Dev Biol 2010;26:363–396. Epub 2009/07/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yin H, Stojanovic A, Hay N, Du X. The role of Akt in the signaling pathway of the glycoprotein Ib-IX induced platelet activation. Blood 2008;111:658–665. Epub 2007/10/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Marco L, Girolami A, Russell S, Ruggeri ZM. Interaction of asialo von Willebrand factor with glycoprotein Ib induces fibrinogen binding to the glycoprotein IIb/IIIa complex and mediates platelet aggregation. J Clin Invest 1985;75:1198–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gralnick HR, Williams SB, Coller BS. Asialo von Willebrand factor interactions with platelets. Interdependence of glycoproteins Ib and IIb/IIIa for binding and aggregation. J Clin Invest 1985;75:19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kroll MH, Harris TS, Moake JL, Handin RI, Schafer AI. von Willebrand factor binding to platelet GpIb initiates signals for platelet activation. J Clin Invest 1991;88:1568–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Y, Ju LA, Zhou F, Liao J, Xue L, Su QP, Jin D, Yuan Y, Lu H, Jackson SP, et al. An integrin α(IIb)β(3) intermediate affinity state mediates biomechanical platelet aggregation. Nat Mater 2019;18:760–769. Epub 2019/03/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pleines I, Eckly A, Elvers M, Hagedorn I, Eliautou S, Bender M, Wu X, Lanza F, Gachet C, Brakebusch C, et al. Multiple alterations of platelet functions dominated by increased secretion in mice lacking Cdc42 in platelets. Blood 2010;115:3364–3373. Epub 2010/02/09. [DOI] [PubMed] [Google Scholar]
- 34.Ju L, Lou J, Chen Y, Li Z, Zhu C. Force-Induced Unfolding of Leucine-Rich Repeats of Glycoprotein Ibα Strengthens Ligand Interaction. Biophys J 2015;109:1781–1784. Epub 2015/11/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang XF, Zhang W, Quach ME, Deng W, Li R. Force-Regulated Refolding of the Mechanosensory Domain in the Platelet Glycoprotein Ib-IX Complex. Biophys J 2019;116:1960–1969. Epub 2019/04/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mazzucato M, Pradella P, Cozzi MR, De Marco L, Ruggeri ZM. Sequential cytoplasmic calcium signals in a 2-stage platelet activation process induced by the glycoprotein Ibalpha mechanoreceptor. Blood 2002;100:2793–2800. Epub 2002/09/28. [DOI] [PubMed] [Google Scholar]
- 37.Nesbitt WS, Kulkarni S, Giuliano S, Goncalves I, Dopheide SM, Yap CL, Harper IS, Salem HH, Jackson SP. Distinct glycoprotein Ib/V/IX and integrin alpha IIbbeta 3-dependent calcium signals cooperatively regulate platelet adhesion under flow. J Biol Chem 2002;277:2965–2972. Epub 2001/11/20. [DOI] [PubMed] [Google Scholar]
- 38.Deng W, Xu Y, Chen W, Paul DS, Syed AK, Dragovich MA, Liang X, Zakas P, Berndt MC, Di Paola J, et al. Platelet clearance via shear-induced unfolding of a membrane mechanoreceptor. Nat Commun 2016;7:12863. Epub 2016/09/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Y, Chen W, Zhang W, Lee-Sundlov MM, Casari C, Berndt MC, Lanza F, Bergmeier W, Hoffmeister KM, Zhang XF, et al. Desialylation of O-glycans on glycoprotein Ibalpha drives receptor signaling and platelet clearance. Haematologica 2021;106:220–229. Epub 2020/01/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yap CL, Hughan SC, Cranmer SL, Nesbitt WS, Rooney MM, Giuliano S, Kulkarni S, Dopheide SM, Yuan Y, Salem HH, et al. Synergistic adhesive interactions and signaling mechanisms operating between platelet glycoprotein Ib/IX and integrin alpha IIbbeta 3. Studies in human platelets and transfected Chinese hamster ovary cells. J Biol Chem 2000;275:41377–41388. Epub 2000/09/01. [DOI] [PubMed] [Google Scholar]
- 41.Kasirer-Friede A, Cozzi MR, Mazzucato M, De Marco L, Ruggeri ZM, Shattil SJ. Signaling through GP Ib-IX-V activates alpha IIb beta 3 independently of other receptors. Blood 2004;103:3403–3411. [DOI] [PubMed] [Google Scholar]
- 42.De Candia E, Hall SW, Rutella S, Landolfi R, Andrews RK, De Cristofaro R. Binding of thrombin to glycoprotein Ib accelerates the hydrolysis of Par-1 on intact platelets. J Biol Chem 2001;276:4692–4698. Epub 2000/11/21. [DOI] [PubMed] [Google Scholar]
- 43.Adam F, Guillin MC, Jandrot-Perrus M. Glycoprotein Ib-mediated platelet activation. A signalling pathway triggered by thrombin. Eur J Biochem 2003;270:2959–2970. Epub 2003/07/09. [DOI] [PubMed] [Google Scholar]
- 44.Estevez B, Kim K, Delaney MK, Stojanovic-Terpo A, Shen B, Ruan C, Cho J, Ruggeri ZM, Du X. Signaling-mediated cooperativity between glycoprotein Ib-IX and protease-activated receptors in thrombin-induced platelet activation. Blood 2016;127:626–636. Epub 2015/11/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bradford HN, Dela Cadena RA, Kunapuli SP, Dong JF, Lopez JA, Colman RW. Human kininogens regulate thrombin binding to platelets through the glycoprotein Ib-IX-V complex. Blood 1997;90:1508–1515. Epub 1997/08/15. [PubMed] [Google Scholar]
- 46.Bradford HN, Pixley RA, Colman RW. Human factor XII binding to the glycoprotein Ib-IX-V complex inhibits thrombin-induced platelet aggregation. J Biol Chem 2000;275:22756–22763. Epub 2000/05/10. [DOI] [PubMed] [Google Scholar]
- 47.Romo GM, Dong JF, Schade AJ, Gardiner EE, Kansas GS, Li CQ, McIntire LV, Berndt MC, López JA. The glycoprotein Ib-IX-V complex is a platelet counterreceptor for P-selectin. J Exp Med 1999;190:803–814. Epub 1999/09/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang Y, Gao H, Shi C, Erhardt PW, Pavlovsky A, Soloviev D A, Bledzka K, Ustinov V, Zhu L, Qin J, et al. Leukocyte integrin Mac-1 regulates thrombosis via interaction with platelet GPIbα. Nature Communications 2017;8:15559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Köhler D, Granja T, Volz J, Koeppen M, Langer HF, Hansmann G, Legchenko E, Geisler T, Bakchoul T, Eggstein C, et al. Red blood cell-derived semaphorin 7A promotes thrombo-inflammation in myocardial ischemia-reperfusion injury through platelet GPIb. Nature Communications 2020;11:1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gowert NS, Kruger I, Klier M, Donner L, Kipkeew F, Gliem M, Bradshaw NJ, Lutz D, Kober S, Langer H, et al. Loss of Reelin protects mice against arterial thrombosis by impairing integrin activation and thrombus formation under high shear conditions. Cell Signal 2017;40:210–221. Epub 2017/09/26. [DOI] [PubMed] [Google Scholar]
- 51.Jurk K, Clemetson KJ, de Groot PG, Brodde MF, Steiner M, Savion N, Varon D, Sixma JJ, Van Aken H, Kehrel BE. Thrombospondin-1 mediates platelet adhesion at high shear via glycoprotein Ib (GPIb): an alternative/backup mechanism to von Willebrand factor. FASEB J 2003;17:1490–1492. Epub 2003/06/26. [DOI] [PubMed] [Google Scholar]
- 52.Prakash P, Kulkarni PP, Chauhan AK. Thrombospondin 1 requires von Willebrand factor to modulate arterial thrombosis in mice. Blood 2015;125:399–406. Epub 2014/10/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pluskota E, Woody NM, Szpak D, Ballantyne CM, Soloviev DA, Simon DI, Plow EF. Expression, activation, and function of integrin alphaMbeta2 (Mac-1) on neutrophil-derived microparticles. Blood 2008;112:2327–2335. Epub 2008/05/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kasirer-Friede A, Ware J, Leng L, Marchese P, Ruggeri ZM, Shattil SJ. Lateral clustering of platelet GP Ib-IX complexes leads to up-regulation of the adhesive function of integrin alpha IIbbeta 3. J Biol Chem 2002;277:11949–11956. Epub 12002 Jan 11925. [DOI] [PubMed] [Google Scholar]
- 55.Perrault C, Moog S, Rubinstein E, Santer M, Baas MJ, de la Salle C, Ravanat C, Dambach J, Freund M, Santoso S, et al. A novel monoclonal antibody against the extracellular domain of GPIbbeta modulates vWF mediated platelet adhesion. Thromb Haemost 2001;86:1238–1248. Epub 2002/01/31. [PubMed] [Google Scholar]
- 56.Quach ME, Chen W, Wang Y, Deckmyn H, Lanza F, Nieswandt B, Li R. Differential regulation of the platelet GPIb-IX complex by anti-GPIbbeta antibodies. J Thromb Haemost 2021;19:2044–2055. Epub 2021/04/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Andrews RK, Fox JE. Interaction of purified actin-binding protein with the platelet membrane glycoprotein Ib-IX complex. J Biol Chem 1991;266:7144–7147. [PubMed] [Google Scholar]
- 58.Du X, Harris SJ, Tetaz TJ, Ginsberg MH, Berndt MC. Association of a phospholipase A2 (14-3-3 protein) with the platelet glycoprotein Ib-IX complex. J Biol Chem 1994;269:18287–18290. Epub 1994/07/15. [PubMed] [Google Scholar]
- 59.Mu FT, Andrews RK, Arthur JF, Munday AD, Cranmer SL, Jackson SP, Stomski FC, Lopez AF, Berndt MC. A functional 14-3-3zeta-independent association of PI3-kinase with glycoprotein Ib alpha, the major ligand-binding subunit of the platelet glycoprotein Ib-IX-V complex. Blood 2008;111:4580–4587. Epub 2008/02/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mangin P, David T, Lavaud V, Cranmer SL, Pikovski I, Jackson SP, Berndt MC, Cazenave J-P, Gachet C, Lanza Fo. Identification of a novel 14-3-3ζ binding site within the cytoplasmic tail of platelet glycoprotein Ibα. Blood 2004;104:420–427. [DOI] [PubMed] [Google Scholar]
- 61.Wu Y, Asazuma N, Satoh K, Yatomi Y, Takafuta T, Berndt MC, Ozaki Y. Interaction between von Willebrand factor and glycoprotein Ib activates Src kinase in human platelets: role of phosphoinositide 3-kinase. Blood 2003;101:3469–3476. Epub 2002/10/24. [DOI] [PubMed] [Google Scholar]
- 62.Arthur JF, Shen Y, Gardiner EE, Coleman L, Murphy D, Kenny D, Andrews RK, Berndt MC. TNF receptor-associated factor 4 (TRAF4) is a novel binding partner of glycoprotein Ib and glycoprotein VI in human platelets. J Thromb Haemost 2011;9:163–172. Epub 2010/10/16. [DOI] [PubMed] [Google Scholar]
- 63.Kim CM, Son YJ, Kim S, Kim SY, Park HH. Molecular basis for unique specificity of human TRAF4 for platelets GPIbbeta and GPVI. Proc Natl Acad Sci U S A 2017;114:11422–11427. Epub 2017/10/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Andrews RK, Munday AD, Mitchell CA, Berndt MC. Interaction of calmodulin with the cytoplasmic domain of the platelet membrane glycoprotein Ib-IX-V complex. Blood 2001;98:681–687. Epub 2001/07/27. [DOI] [PubMed] [Google Scholar]
- 65.Kanaji T, Ware J, Okamura T, Newman PJ. GPIbα regulates platelet size by controlling the subcellular localization of filamin. Blood 2012;119:2906–2913. Epub 2011/12/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Englund GD, Bodnar RJ, Li Z, Ruggeri ZM, Du X. Regulation of von Willebrand factor binding to the platelet glycoprotein Ib-IX by a membrane skeleton-dependent inside-out signal. J Biol Chem 2001;276:16952–16959. Epub 2001/03/30. [DOI] [PubMed] [Google Scholar]
- 67.Feng S, Resendiz JC, Lu X, Kroll MH. Filamin A binding to the cytoplasmic tail of glycoprotein Ibalpha regulates von Willebrand factor-induced platelet activation. Blood 2003;102:2122–2129. Epub 2003/06/07. [DOI] [PubMed] [Google Scholar]
- 68.Rosa JP, Raslova H, Bryckaert M. Filamin A: key actor in platelet biology. Blood 2019;134:1279–1288. Epub 2019/09/01. [DOI] [PubMed] [Google Scholar]
- 69.Falet H, Pollitt AY, Begonja AJ, Weber SE, Duerschmied D, Wagner DD, Watson SP, Hartwig JH. A novel interaction between FlnA and Syk regulates platelet ITAM-mediated receptor signaling and function. J Exp Med 2010;207:1967–1979. Epub 2010/08/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dutting S, Gaits-Iacovoni F, Stegner D, Popp M, Antkowiak A, van Eeuwijk JMM, Nurden P, Stritt S, Heib T, Aurbach K, et al. A Cdc42/RhoA regulatory circuit downstream of glycoprotein Ib guides transendothelial platelet biogenesis. Nat Commun 2017;8:15838. Epub 2017/06/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mangin PH, Receveur N, Wurtz V, David T, Gachet C, Lanza F. Identification of five novel 14-3-3 isoforms interacting with the GPIb-IX complex in platelets. J Thromb Haemost 2009;7:1550–1555. Epub 2009/06/30. [DOI] [PubMed] [Google Scholar]
- 72.Dai K, Bodnar R, Berndt MC, Du X. A critical role for 14-3-3zeta protein in regulating the VWF binding function of platelet glycoprotein Ib-IX and its therapeutic implications. Blood 2005;106:1975–1981. Epub 2005/06/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Feng S, Christodoulides N, Resendiz JC, Berndt MC, Kroll MH. Cytoplasmic domains of GpIbalpha and GpIbbeta regulate 14-3-3zeta binding to GpIb/IX/V. Blood 2000;95:551–557. Epub 2000/01/11. [PubMed] [Google Scholar]
- 74.Yuan Y, Zhang W, Yan R, Liao Y, Zhao L, Ruan C, Du X, Dai K. Identification of a novel 14-3-3zeta binding site within the cytoplasmic domain of platelet glycoprotein Ibalpha that plays a key role in regulating the von Willebrand factor binding function of glycoprotein Ib-IX. Circ Res 2009;105:1177–1185. Epub 2009/10/31. [DOI] [PubMed] [Google Scholar]
- 75.Andrews RK, Harris SJ, McNally T, Berndt MC. Binding of purified 14-3-3 zeta signaling protein to discrete amino acid sequences within the cytoplasmic domain of the platelet membrane glycoprotein Ib-IX-V complex. Biochemistry 1998;37:638–647. Epub 1998/02/21. [DOI] [PubMed] [Google Scholar]
- 76.Bodnar RJ, Xi X, Li Z, Berndt MC, Du X. Regulation of Glycoprotein Ib-IX-von Willebrand Factor Interaction by cAMP-dependent Protein Kinase-mediated Phosphorylation at Ser 166 of Glycoprotein Ibbeta. J Biol Chem 2002;277:47080–47087. [DOI] [PubMed] [Google Scholar]
- 77.Constantinescu-Bercu A, Wang YA, Woollard KJ, Mangin P, Vanhoorelbeke K, Crawley JT, Salles C II. The GPIbalpha intracellular tail - role in transducing VWF- and Collagen/GPVI-mediated signaling. Haematologica 2021. Epub 2021/06/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gu M, Xi X, Englund GD, Berndt MC, Du X. Analysis of the roles of 14-3-3 in the platelet glycoprotein Ib-IX-mediated activation of integrin alpha(IIb)beta(3) using a reconstituted mammalian cell expression model. J Cell Biol 1999;147:1085–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jain S, Zuka M, Liu J, Russell S, Dent J, Guerrero JA, Forsyth J, Maruszak B, Gartner TK, Felding-Habermann B, et al. Platelet glycoprotein Ib alpha supports experimental lung metastasis. Proc Natl Acad Sci U S A 2007;104:9024–9028. Epub 2007/05/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ruan CG, Du XP, Xi XD, Castaldi PA, Berndt MC. A murine antiglycoprotein Ib complex monoclonal antibody, SZ 2, inhibits platelet aggregation induced by both ristocetin and collagen. Blood 1987;69:570–577. [PubMed] [Google Scholar]
- 81.Yin H, Liu J, Li Z, Berndt MC, Lowell CA, Du X. Src family tyrosine kinase Lyn mediates VWF/GPIb-IX-induced platelet activation via the cGMP signaling pathway. Blood 2008;112:1139–1146. Epub 2008/06/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Delaney MK, Liu J, Zheng Y, Berndt MC, Du X. The role of Rac1 in glycoprotein Ib-IX-mediated signal transduction and integrin activation. Arterioscler Thromb Vasc Biol 2012;32:2761–2768. Epub 2012/09/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Munday AD, Berndt MC, Mitchell CA. Phosphoinositide 3-kinase forms a complex with platelet membrane glycoprotein Ib-IX-V complex and 14-3-3zeta. Blood 2000;96:577–584. Epub 2000/07/11. [PubMed] [Google Scholar]
- 84.Mu FT, Cranmer SL, Andrews RK, Berndt MC. Functional association of phosphoinositide-3-kinase with platelet glycoprotein Ibalpha, the major ligand-binding subunit of the glycoprotein Ib-IX-V complex. J Thromb Haemost 2010;8:324–330. Epub 2009/10/31. [DOI] [PubMed] [Google Scholar]
- 85.Yap CL, Anderson KE, Hughan SC, Dopheide SM, Salem HH, Jackson SP. Essential role for phosphoinositide 3-kinase in shear-dependent signaling between platelet glycoprotein Ib/V/IX and integrin alpha(IIb)beta(3). Blood 2002;99:151–158. Epub 2002/01/05. [DOI] [PubMed] [Google Scholar]
- 86.Li Z, Zhang G, Feil R, Han J, Du X. Sequential activation of p38 and ERK pathways by cGMP-dependent protein kinase leading to activation of the platelet integrin alphaIIb beta3. Blood 2006;107:965–972. Epub 2005/10/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li Z, Xi X, Du X. A mitogen-activated protein kinase-dependent signaling pathway in the activation of platelet integrin alpha IIbbeta3. J Biol Chem 2001;276:42226–42232. Epub 2001/08/28. [DOI] [PubMed] [Google Scholar]
- 88.Li Z, Xi X, Gu M, Feil R, Ye RD, Eigenthaler M, Hofmann F, Du X. A stimulatory role for cGMP-dependent protein kinase in platelet activation. Cell 2003;112:77–86. [DOI] [PubMed] [Google Scholar]
- 89.Cheng Z, Gao W, Fan X, Chen X, Mei H, Liu J, Luo X, Hu Y. Extracellular signal-regulated kinase 5 associates with casein kinase II to regulate GPIb-IX-mediated platelet activation via the PTEN/PI3K/Akt pathway. J Thromb Haemost 2017;15:1679–1688. Epub 2017/06/13. [DOI] [PubMed] [Google Scholar]
- 90.Li Z, Zhang G, Marjanovic JA, Ruan C, Du X. A platelet secretion pathway mediated by cGMP-dependent protein kinase. J Biol Chem 2004;279:42469–42475. [DOI] [PubMed] [Google Scholar]
- 91.Wen L, Feil S, Wolters M, Thunemann M, Regler F, Schmidt K, Friebe A, Olbrich M, Langer H, Gawaz M, et al. Publisher Correction: A shear-dependent NO-cGMP-cGKI cascade in platelets acts as an auto-regulatory brake of thrombosis. Nat Commun 2018;9:4969. Epub 2018/11/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Edwards DC, Sanders LC, Bokoch GM, Gill GN. Activation of LIM-kinase by Pak1 couples Rac/Cdc42 GTPase signalling to actin cytoskeletal dynamics. Nat Cell Biol 1999;1:253–259. Epub 1999/11/13. [DOI] [PubMed] [Google Scholar]
- 93.Estevez B, Stojanovic-Terpo A, Delaney MK, O’Brien KA, Berndt MC, Ruan C, Du X. LIM kinase-1 selectively promotes glycoprotein Ib-IX-mediated TXA2 synthesis, platelet activation, and thrombosis. Blood 2013;121:4586–4594. Epub 2013/04/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mangin P, Yuan Y, Goncalves I, Eckly A, Freund M, Cazenave JP, Gachet C, Jackson SP, Lanza F. Signaling role for phospholipase C gamma 2 in platelet glycoprotein Ib alpha calcium flux and cytoskeletal reorganization. Involvement of a pathway distinct from FcR gamma chain and Fc gamma RIIA. J Biol Chem 2003;278:32880–32891. Epub 2003/06/19. [DOI] [PubMed] [Google Scholar]
- 95.Klier M, Gowert NS, Jäckel S, Reinhardt C, Elvers M. Phospholipase D1 is a regulator of platelet-mediated inflammation. Cell Signal 2017;38:171–181. Epub 2017/07/18. [DOI] [PubMed] [Google Scholar]
- 96.Falati S, Edmead CE, Poole AW. Glycoprotein Ib-V-IX, a receptor for von Willebrand factor, couples physically and functionally to the Fc receptor gamma-chain, Fyn, and Lyn to activate human platelets. Blood 1999;94:1648–1656. Epub 1999/09/09. [PubMed] [Google Scholar]
- 97.Wu Y, Suzuki-Inoue K, Satoh K, Asazuma N, Yatomi Y, Berndt MC, Ozaki Y. Role of Fc receptor gamma-chain in platelet glycoprotein Ib-mediated signaling. Blood 2001;97:3836–3845. Epub 2001/06/05. [DOI] [PubMed] [Google Scholar]
- 98.Liu J, Pestina TI, Berndt MC, Jackson CW, Gartner TK. Botrocetin/VWF-induced signaling through GPIb-IX-V produces TxA2 in an αIIbβ3- and aggregation-independent manner. Blood 2005;106:2750–2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Badolia R, Kostyak JC, Dangelmaier C, Kunapuli SP. Syk Activity Is Dispensable for Platelet GP1b-IX-V Signaling. Int J Mol Sci 2017;18. Epub 2017/06/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xu Z, Liang Y, Delaney MK, Zhang Y, Kim K, Li J, Bai Y, Cho J, Ushio-Fukai M, Cheng N, et al. Shear and Integrin Outside-In Signaling Activate NADPH-Oxidase 2 to Promote Platelet Activation. Arterioscler Thromb Vasc Biol 2021;41:1638–1653. Epub 2021/03/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Carrim N, Arthur JF, Hamilton JR, Gardiner EE, Andrews RK, Moran N, Berndt MC, Metharom P. Thrombin-induced reactive oxygen species generation in platelets: A novel role for protease-activated receptor 4 and GPIbalpha. Redox Biol 2015;6:640–647. Epub 2015/11/17. [DOI] [PMC free article] [PubMed] [Google Scholar]


