Abstract
Background:
Epidemiological studies examining associations between traumatic brain injury (TBI) and Alzheimer’s Disease and Related Dementias (ADRD) have yielded conflicting results, which may be due methodological differences.
Objective:
To examine the relationship between the presence and severity of TBI and risk of ADRD using a population-based cohort with medical record abstraction for confirmation of TBI and ADRD.
Methods:
All TBI events among Olmsted County, Minnesota residents aged >40 years from 1985-1999 were confirmed by manual review and classified by severity. Each TBI case was randomly matched to 2 age-, sex-, and non-head injury population-based referents without TBI. For TBI events with non-head trauma the Trauma Mortality Prediction Model was applied to assign an overall measure of non-head injury severity and corresponding referents were matched on this variable. Medical records were manually abstracted to confirm ADRD diagnosis. Cox proportional hazards models examined the relationship between TBI and severity with risk of ADRD.
Results:
A total of 1,418 residents had a confirmed TBI (865 Possible, 450 Probable, and 103 Definite) and were matched to 2,836 referents. When combining all TBI severities, the risk of any ADRD was significantly higher for those with a confirmed TBI compared to referents (HR=1.32, 95% CI: 1.11, 1.58). Stratifying by TBI severity, Probable (HR=1.42, 95% CI: 1.05, 1.92) and Possible (HR=1.29, 95% CI: 1.02-1.62) TBI was associated with an increased risk of ADRD, but not Definite TBI (HR=1.22, 95% CI: 0.68, 2.18).
Conclusion:
Our analyses support including TBI as a potential risk factor for developing ADRD.
Keywords: Population, epidemiology, dementia, traumatic brain injury, Parkinson’s disease, Alzheimer’s disease
INTRODUCTION
Functionally limiting neurological impairment and societal costs related to traumatic brain injury (TBI) place a profound burden on individuals and their families in United States military and civilian communities [1]. Long-term functional limitations after TBI have been attributable to cognitive and neurobehavioral impairment in some individuals [2–4]. However, epidemiological studies examining associations between TBI and Alzheimer’s Disease and Related Dementias (ADRD) have yielded conflicting results. Some studies have reported an association between TBI and an increased risk for developing clinical or pathological ADRD [5–14]. However, others have not found an association [15–21]. There have also been conflicting reports of a differential risk of developing ADRD related to TBI severity [6, 13, 14].
These discrepant findings in associating TBI with ADRD may be due to methodological variations in defining TBI and ADRD, the TBI study population examined, consideration and classification of TBI severity, and the type of comparison group utilized [22]. Study populations have also varied widely, including at-risk groups (e.g., active-duty military personnel, veterans, or professional athletes, which are almost exclusively male) and community-based convenience samples, often not considering age at TBI. A limitation of some studies is their reliance on self- or proxy-report of TBI and injury severity [6, 7, 15, 20], which is hindered by recall bias and inaccurately assigning injury severity [23, 24]. Many studies have relied on TBI and ADRD case ascertainment using administrative datasets with diagnostic codes, patient registry data, or billing records, which lack accuracy [25, 26]. Given these limitations, we examined the relationship between the presence and severity of TBI and risk of ADRD using a population-based cohort of county residents. We used the Rochester Epidemiology Project (REP) medical records-linkage system to confirm all TBI cases among those aged 40 and older between 1985 and 1999, and to confirm subsequent ADRD outcomes through 2018 via medical record chart abstraction. We compared risk of ADRD to age- and sex-matched referents without a TBI, including matched non-head trauma, and assessed whether associations differed by sex.
MATERIALS AND METHODS
Participants and Study Design
The Rochester Epidemiology Project (REP) medical-records linkage system was used to identify all Olmsted County, Minnesota residents, aged 40 and older between 1985 and 1999 [27, 28]. Briefly, the REP links all medical records from all providers in Olmsted County (encompassing Mayo Clinic and Olmsted Medical Center including their affiliated hospitals and medical facilities) using a unit medical record system whereby all outpatients, inpatients, emergency room, and nursing home information is kept in the same unit record. The REP captures virtually all individuals who have resided in Olmsted County, MN at some time from 1966 to the present, regardless of age, sex, ethnicity, disease status, socio-economic status, or insurance status. Indeed, REP estimates of the Olmsted County population are 2-4% higher than those reported by the US Census [29]. The proportion of individuals by sex, age, and ethnicity in Olmsted County is representative of the state of Minnesota and the upper Midwest 5-state region. Incidence rates of many diseases in Olmsted County, including breast cancer, multiple myeloma and heart disease are similar to those observed in other US populations [29]. Because our goal was to estimate the long-term risk of ADRD, we limited the population to those who had at least 5 years of follow-up and those > 40 years of age at TBI, because the incidence of ADRD greatly increases with age. Thus, we identified all TBI cases for persons residing in Olmsted County between 1985 and 1999 who were aged 40 and over and followed residents for outcomes through 2018 via medical record review.
Standard protocol approvals, registration, and patient consent
The study was approved by the Institutional Review Boards of Mayo Clinic and Olmsted Medical Center. Written informed consent was not required for passive medical record review, but all participants had provided consent for the use of their medical records for research.
Identification of TBI and determination of TBI severity based on medical record review
The methods for identification of the TBI cohort have been described in detail [23, 30]. }Using the REP diagnostic index, a list of all potential residents in the population at any age was constructed with diagnostic codes suggestive of TBI for the period, January 1, 1985 to December 31, 1999 as previously described [23, 31]. The number of codes included in this list was extensive to obtain the greatest sensitivity for detecting TBI (see Supplementary Table 1). For each resident with a potential TBI code, nurse abstractors examined the written and electronic medical records to confirm the presence of TBI and classify its severity.
TBI was defined as a traumatically induced injury that contributed to the physiological disruption of brain function. Evidence in the medical record used to determine physiological disruption included documentation of any of the following: concussion with loss of consciousness, post-traumatic amnesia, neurological signs of brain injury, and/or evidence of intracerebral, subdural, or epidural hematoma, cerebral or hemorrhagic contusion, or brain stem injury, penetrating brain injury, skull fracture, or post-concussive symptoms (dizziness, confusion, blurred vision, double vision, headache, nausea, or vomiting that lasted greater than 30 minutes and that were not attributable to preexisting or comorbid conditions). Information from all medical care settings was reviewed (e.g., hospital inpatient, hospital outpatient, emergency department, office visit, or nursing home). Detailed information available for review (electronically or via written medical health record) included medical history, all clinical assessments, consultation reports, surgical procedures, dismissal summaries, laboratory and radiology results, correspondence, death certificates, and autopsy reports. All open-text notes were examined for any indication of a relevant symptom. Individuals who did not seek medical attention specifically for either the event or for sequelae (i.e., injuries identified as part of the past medical history) were excluded because injury severity could not be confirmed. Injuries sustained before the index TBI are not known.
All incident and subsequent TBI events were characterized by mechanism of injury according to methods developed by the National Center for Injury Prevention and Control, Division of Injury Disability, Outcomes, and Programs (part of the Centers for Disease Control and Prevention) [32]. Incident events were assigned to one of the TBI severity classification categories using the classification system developed by Malec et al. [33], which emphasizes the use of positive evidence of brain injury obtained by reviewing clinical information contained within REP provider-linked medical records. This inclusive system classifies injury severity into ‘definite’ (consistent with moderate-severe), ‘probable’ (consistent with mild), and ‘possible’ (consistent with concussive) categories. Definite cases were those with evidence of either death due to this traumatic brain injury; loss of consciousness for at least 30 minutes; post-traumatic anterograde amnesia lasting at least 24 hours; a Glasgow Coma Scale full score in first 24 hours of <13 (and were not attributable to preexisting or comorbid conditions: blurred vision, confusion (mental state changes, dazed, dizziness, focal neurologic symptoms, headache, or nausea); or any of the following: intracerebral, subdural, or epidural hematoma; cerebral or hemorrhagic contusion; penetrating traumatic brain injury (dura penetrated); or subarachnoid hemorrhage. Probable cases lacked criteria for definite but had evidence of some loss of consciousness (momentary to <30 minutes); post-traumatic anterograde amnesia (momentary to <24 hours); or depressed, basilar, or linear skull fracture (dura intact). Possible (symptomatic) traumatic brain injury cases lacked criteria for either definite or probable but had evidence of any of the following symptoms that lasted ≥30 minutes and were not attributable to preexisting or comorbid conditions: blurred vision, confusion (mental state changes), dazed, dizziness, focal neurologic symptoms, headache, or nausea.
Selection of age- and sex-, and co-occurring non-head trauma matched referents
Date of first incident TBI was considered the index date for matching unexposed referents. Each person with a confirmed TBI was randomly matched to 2 sex-, age-, and co-occurring non-head trauma referents without TBI, who were residents of Olmsted County and seen in any Olmsted County medical facility in the year (+/−1) of the exposed individual’s TBI event. All referents had a thorough medical record review using the above-described resources to confirm the absence of a TBI in the medical record prior to the index date.
All confirmed TBI events were categorized as either having an isolated TBI or TBI associated with non-head traumatic injury [30]. For TBI events with non-head trauma, each of the accompanying non-head injuries was assigned a diagnosis code based on an empiric measure of injury severity. The Trauma Mortality Prediction Model (TMPM) was then applied to assign an overall measure of non-head injury severity to each individual case [34]. Referents matched to confirmed TBI events who experienced co-occurring non-head trauma were also required to have been hospitalized or seen in the emergency department for non-head traumatic injuries with a comparable TMPM score within ± 2 years of the exposed TBI index date.
Definition of ADRD outcomes
Previous studies have suggested moderate accuracy for detecting dementia using ICD codes and difficulty in accurately ascertaining dementia type [35–38]. Therefore, all charts from all residents with a confirmed TBI and their matched referents were manually abstracted by two of the authors (AWB, MMM). Although screening and diagnosis of dementia were not uniformly standardized across all providers in Olmsted County, the two reviewers manually reviewed the records in the same manner and discussed those that were ambiguous. A diagnosis of dementia was based on Diagnostic and Statistical Manual (DSM)-IV criteria [39]. Etiologic diagnoses for dementia cases were based on standard criteria for Alzheimer’s disease (AD) dementia [40], vascular dementia [41], Parkinson’s disease (PD) dementia (PDD) or dementia with Lewy bodies (DLB) [42], and frontotemporal dementia [43]. Medical record chart review using the REP has been found to have good sensitivity and specificity when compared to in-person research study visits with a physician, neuropsychologist, and nurse [38]. Because ADRD have a long preclinical phase, any case with a confirmed TBI event within 3 years of the approximate date of ADRD onset was excluded.
For a diagnosis of PD, previously specified criteria were utilized [44, 45]. This diagnostic criteria included the definition of parkinsonism as a syndrome and the definition of types of parkinsonism within the syndrome. Parkinsonism was defined as the presence of at least 2 of the following 4 cardinal signs: rest tremor, bradykinesia, rigidity, and impaired postural reflexes. PD was defined as parkinsonism with all 3 of the following: no other cause (e.g., repeated stroke with stepwise progression, repeated head injury, history of encephalitis, neuroleptic treatment ≤ 6 month before onset, hydrocephalus, brain tumor); no documentation of unresponsiveness to levodopa at doses of at least 1 g/d in combination with carbidopa (applicable only to patients who were treated); and no prominent or early (<1 y of onset) signs of more extensive nervous system involvement (e.g., dysautonomia) not explained otherwise [44]. The pathological validation of these clinical diagnoses by medical record chart review has been determined [46].
Other dementias/neurodegenerative diseases identified in the chart were also recorded including amyotrophic lateral sclerosis, alcohol dementia, corticobasal degeneration, dementia due to multiple sclerosis, multisystem atrophy, mixed dementia, normal pressure hydrocephalus with dementia, progressive primary aphasia, and progressive supranuclear palsy. Residents who had dementia but for which we were unable to determine etiology were defined as “Dementia NOS.”
Statistical analyses
Chi-square test for categorical variables and Wilcoxon rank sum tests for continuous variables were used to assess differences in characteristics between those with and without a confirmed TBI. Cox proportional hazards models were used to examine the relationship between TBI and any ADRD and type, with age as the timescale. The hazard ratio (HR) and 95% confidence intervals (CI) are reported for prediction of occurrence of ADRD for those with TBI compared to their matched referents. All individuals with confirmed TBI and all sex- and age-matched referents were followed in the REP census from the TBI event or corresponding index date until the earliest date of: a) the estimated onset of ADRD; b) the last medical visit in Olmsted County; c) December 31, 2018; or d) death. A plot of Schoenfeld residuals was utilized to confirm the proportional hazards assumption. If referents developed a confirmed TBI event after their index date, they were censored at that time. In addition, we assessed whether the associations between TBI and ADRD differed by sex or by age (continuous variable) by adding an interaction term to the model. Separate models were run to assess the associations between TBI and any ADRD, AD only, vascular dementia only, PD only, and PDD or DLB combined. Individuals with TBI were analyzed together and stratified by injury severity. All statistical tests were 2-sided and p-values less than 0.05 were considered statistically significant. Statistical analyses were performed using SAS version 9.4 (SAS Inc., Cary, NC).
RESULTS
The study flow and numbers are shown in Figure 1. Briefly, between January 1, 1985 and December 31, 1999, 45,791 Olmsted County residents with a diagnostic code indicative of a potential TBI were identified. Of these, 6,939 residents (15.2%) were aged 40 years and older at the time of the code and had at least 5 years of follow-up. After chart review, 1,418 (11.3%) were determined to have a confirmed TBI. These individuals were age- and sex-, and non-head trauma-matched to 2,836 residents that were confirmed to not have a TBI as of the index date of the those with TBI.
Fig. 1.
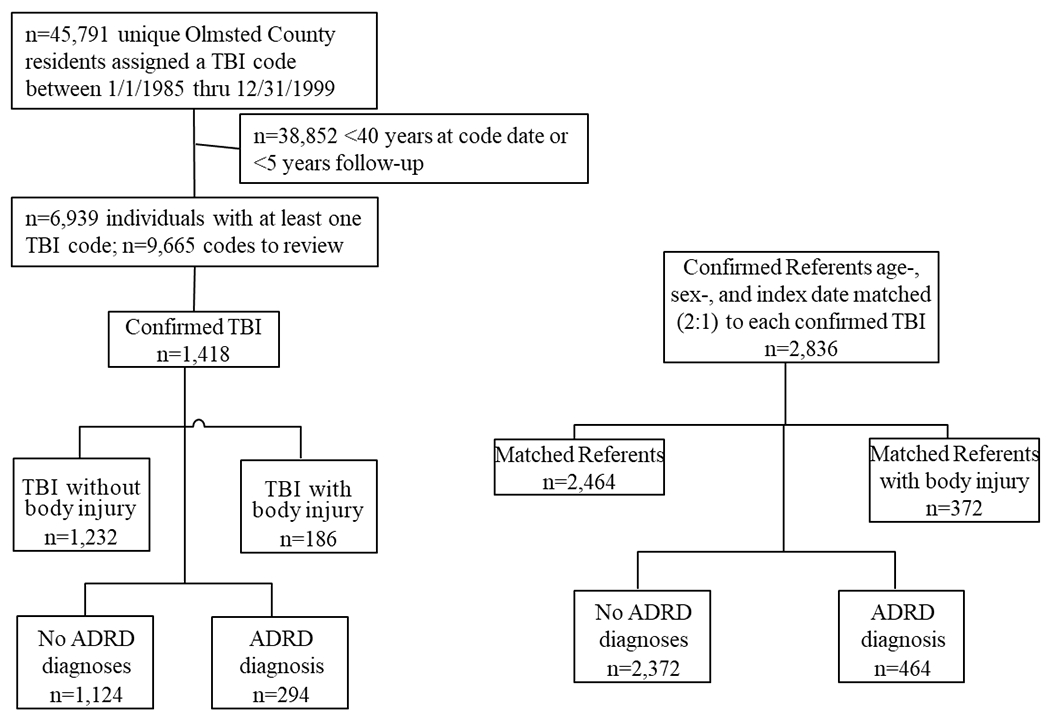
Study flow chart for identifying those with a confirmed traumatic brain injury and confirmed age- and sex-matched referents.
Participant characteristics by TBI status and incident ADRD are shown in Table 1. The median age at TBI was 55 years, 43% were male, and 13% had a co-occurring non-brain injury. Of the 1,418 TBI cases, 865 (61%) were Possible, 450 (32%) were Probable, and 103 (7%) were Definite. Compared to referents, those with TBI were more likely to develop any neurodegenerative disease (20.7% versus 16.4%, p = 0.0004). However, there were no differences in age at diagnosis or years from the index date to a neurodegenerative diagnosis between TBI cases and referents. Residents with a TBI, compared to referents, were more likely to develop AD dementia (12.9% versus 11.0%, p = 0.073), DLB (1.3% versus 0.7%, p = 0.056), PD (1.4% versus 1.0%, p = 0.264), and vascular dementia (5.6% versus 4.5%, p = 0.108) but the differences were not significant.
Table 1.
Participant characteristics by TBI severity and incident ADRD
| TBI (n=1,418) | Referent (n=2,836) | p-value | |
|---|---|---|---|
| Male, n (%) | 611 (43.1) | 1,222 (43.1) | 1.000 |
| Mean age (range) | 55.0 (46.0,67.0) | 54.5 (46.0, 67.0) | 0.820 |
| Any incident neurodegenerative disease, n (%) | 294 (20.7) | 464 (16.4%) | 0.0004 |
| Mean age at diagnosis in years (range) | 83.5 (77.0, 89.0) | 85.0 (78.0, 90.0) | 0.112 |
| Mean years of follow-up (range) | 13.1 (6.8, 20.4) | 12.1 (7.2, 19.3) | 0.616 |
| Non-brain injury, n (%) | 186 (13%) | 373 (13%) | 1.000 |
| TBI severity | |||
| Definite, n (%) | 103 (7) | ||
| Probable | 450 (32) | ||
| Possible | 865 (61) | ||
| Dementia diagnosis* | |||
| Alzheimer’s disease, median IQR/n (%) | 183 (12.9) | 313 (11.0) | 0.073 |
| Dementia with Lewy bodies | 19 (1.3) | 21 (0.7) | 0.056 |
| Parkinson’s disease dementia | 20 (1.4) | 29 (1.0) | 0.264 |
| Vascular dementia | 80 (5.6) | 128 (4.5) | 0.108 |
Categories are not exclusive; some individuals had multiple dementia etiologies (e.g., Alzheimer’s disease and vascular dementia).
TBI, traumatic brain injury; ADRD, Alzheimer’s disease and related dementias; IQR, interquartile range.
The risk of developing any ADRD and type, by TBI severity, is shown in Table 2. When combining all TBI severities, the risk of any ADRD was significantly higher for those with a confirmed TBI compared to referents (HR = 1.32, 95% CI: 1.11, 1.58). Stratifying by TBI severity, Probable (HR = 1.42, 95% CI: 1.05, 1.92) and Possible (HR = 1.29, 95% CI: 1.02-1.62) TBI was associated with an increased risk, but not Definite TBI (HR = 1.22, 95% CI: 0.68, 2.18). Examining specific dementia types including AD dementia, vascular dementia, PD, or DLB, neither TBI exposure nor TBI severity was associated with any outcome.
Table 2.
Association between the presence and severity of a traumatic brain injury (TBI) and risk of Alzheimer’s disease and related disorders (ADRD)
| Outcome | Hazard Ratio | 95% CI | p-value |
|---|---|---|---|
| Any ADRD | |||
| All TBI | 1.32 | 1.11-1.58 | 0.002 |
| Definite | 1.22 | 0.68-2.18 | 0.510 |
| Probable | 1.42 | 1.05-1.92 | 0.024 |
| Possible | 1.29 | 1.02-1.62 | 0.033 |
| Alzheimer’s disease dementia | |||
| All TBI | 1.17 | 0.94-1.46 | 0.161 |
| Definite | 1.19 | 0.56-2.55 | 0.647 |
| Probable | 1.18 | 0.79-1.76 | 0.411 |
| Possible | 1.16 | 0.87-1.55 | 0.298 |
| Vascular dementia | |||
| All TBI | 1.26 | 0.92-1.72 | 0.145 |
| Definite | 0.97 | 0.40-2.36 | 0.940 |
| Probable | 1.42 | 0.82-2.46 | 0.215 |
| Possible | 1.25 | 0.82-1.90 | 0.293 |
| Parkinson’s disease* | |||
| All TBI | 1.31 | 0.71-2.43 | 0.390 |
| Lewy body dementia or Parkinson’s disease* | |||
| All TBI | 1.46 | 0.75-2.86 | 0.270 |
When examining Parkinson’s disease and Lewy body dementia and Parkinson’s disease dementia, there were too few outcomes to examine TBI severity.
TBI, traumatic brain injury; ADRD, Alzheimer’s disease and related dementias.
Figure 2 shows that the cumulative incidence of any ADRD was increased for those with TBI when TBI severity categories were combined. This significant difference persisted when Probable and Possible categories were analyzed separately but not for Definite injuries.
Fig. 2.
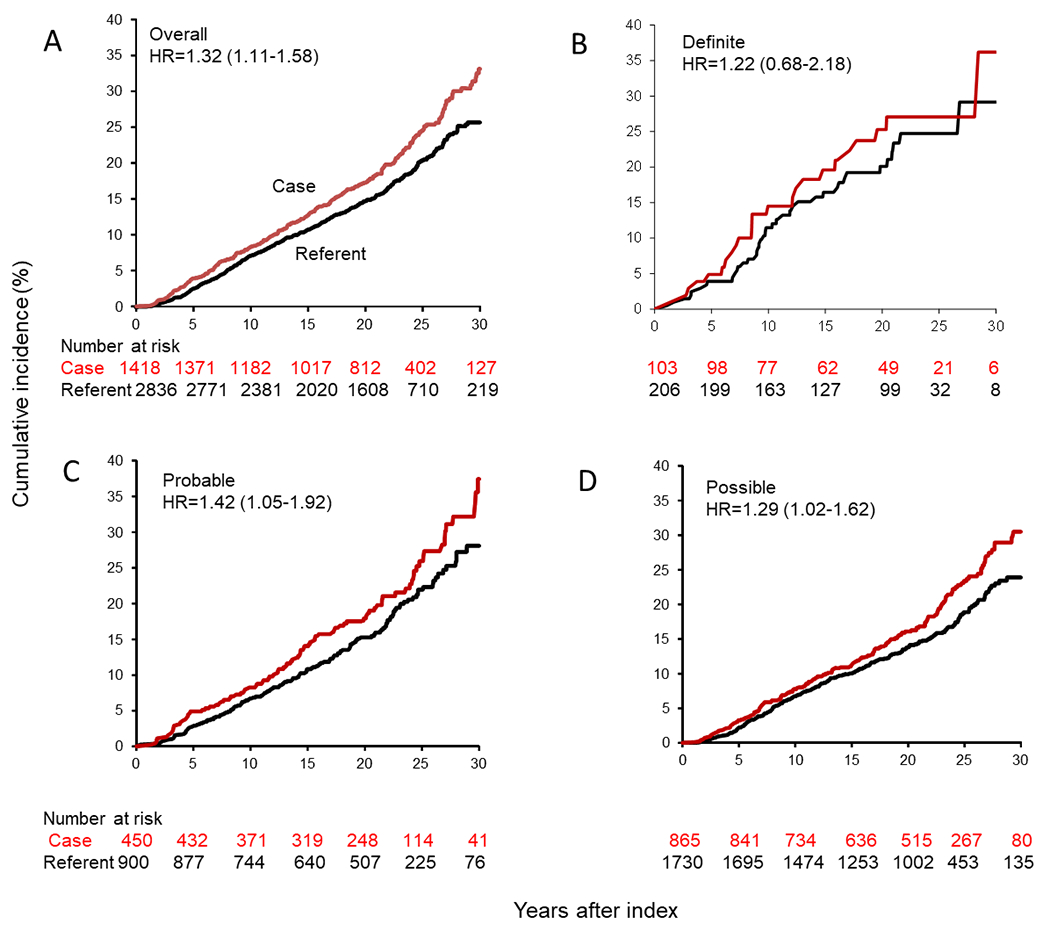
Cumulative incidence of Alzheimer’s disease and related dementias for Olmsted County residents with traumatic brain injury (TBI) or age- and sex-matched referents. A) Any TBI severity; B) Definite TBI; C) Probable TBI; D) Possible TBI.
We next determined whether the association between the presence or severity of TBI and risk of any ADRD, or type, varied by sex. Including all TBI severities, men had a greater risk of any ADRD (HR = 1.59, 95% CI: 1.19, 2.12) compared to women (HR = 1.19, 95% CI: 0.95, 1.48). However, the interaction between TBI and sex was not significant for any ADRD (p = 0.119) or type (AD: p = 0.476; vascular dementia: p = 0.650; PD: p = 0.175; LBD: p = 0.470). We also did not find an interaction between age and any TBI or TBI severity for risk of any ADRD (all p>0.20).
DISCUSSION
This population-based analysis found that exposure to any severity of TBI at age 40 and older was associated with a 1.3-fold increased risk of ADRD compared to age-, sex-, and body-injury-matched population referents. However, when injury severity categories were analyzed separately, the risk for ADRD remained significant only for Possible and Probable TBI, but not Definite. In addition, we did not observe associations between TBI and specific dementia types including AD dementia, vascular dementia, FTD, PD, or LBD. We did not find that sex or age modified the association between TBI and risk of ADRD. Confirming TBI cases by medical record review, classifying TBI severity across its full spectrum, and accounting for non-head trauma enhanced the accuracy of these results.
It is somewhat difficult to directly compare the strength of the current results to previous studies because of differences in study designs, populations, and methods by which TBI and ADRD outcomes were ascertained [22]. Overall, we did observe that TBI was associated with an increased risk of any ADRD, which is similar to many other studies [5–14]. Previous population-based studies, most consistent with our study design, with follow-ups ranging from 5 to 25 years reported that TBI was associated with a 1.2 to 3.3-fold increased risk of dementia, with reduced strengths of association for the longer duration studies [11, 47–49]. Our estimate using medical record review instead of codes and about a 13-year follow-up is on the low-end of that range ( a hazard ratio of 1.32 for any TBI, 1.42 for Probable, and 1.29 for a Possible TBI) but consistent with those studies with over 10 years of follow-up. In comparison, the study with the greatest strength of an association between TBI and dementia had only 5 years and may be more affected by reverse causality [48].
The greater risk for developing ADRD after non-severe TBI found in the present analysis is consistent with a recent report from a large trauma registry sample of TBI cases identified by diagnostic coding and compared to traumatic orthopedic referents [13]. That study also found that moderate-severe TBI (consistent with our Definite classification) was associated with a HR of 1.8 for developing dementia, but the association was attenuated and no longer significant after adjustment for overall injury severity. This finding is consistent with our results of a non-significant HR for developing ADRD after Definite TBI, even after accounting for non-head trauma among those with TBI and their referents. In addition, Stopa et al. [13] found that TBI cases had higher 10-year mortality when compared to referents despite the brief follow-up time (less than 3 years). This is consistent with a previous report in our population that showed the increased risk for death after TBI existed only during the first 6 months after injury [30]. Together, these findings indicate that an increased risk for death following TBI does not appear to influence the relationship between TBI and ADRD, but that the association between TBI and mortality is likely due to injury-related factors [50].
Although we found an association between TBI and any ADRD, we did not find associations between TBI and specific dementia types including AD dementia, vascular dementia, DLB and PD. One explanation could be that we did not have enough outcome events. However, other studies with larger sample sizes and AD dementia outcomes have also found no associations [15, 51]. A neuropathological study from three cohorts found that TBI was associated with Lewy body pathology, but not with amyloid plaques or neurofibrillary tangles [51]. However, we also did not find an association between TBI and a clinical diagnosis of either DLB or PD. This is consistent with a recent study of 576 DLB participants from the National Alzheimer’s Coordinating Center which also did not find that a history of TBI was associated with DLB. Of note, however, the correspondence between clinical symptoms and neuropathology are not 1:1, and the etiologies of dementia in later-life are typically due to multiple underlying neuropathologies.
Most studies adjust for sex in models examining associations between TBI and ADRD, but few studies have assessed whether the relationship differs by sex. A recent study found that women were more vulnerable than men to persistent TBI-related cognitive and somatic symptoms [52] but the long-term risk of ADRD was not examined. However, a study of almost 1 million veterans reported a slightly, albeit significant, increased risk of dementia for men with TBI compared to women with TBI [53]. In the present study, 43% of the TBI cases were men. Consistent with the previous study [53], we found that men had a greater risk of ADRD than women. However, the difference was not statistically significant in the present study.
There are several strengths to this study. First, a major strength is our study design, with medical record review of both the TBI and ADRD outcome for confirmation. In addition, we were able to ascertain the severity of TBI and type of dementia. Second, for those with a confirmed TBI, who also had co-occurring body injury, we were able to match to confirmed referents without TBI but body injury. This is particularly important as non-head trauma may also increase the risk of ADRD. Last, we incorporated a population-based sample that captured all Olmsted County residents during the period of interest. Despite these strengths, there are also limitations. First, because we abstracted the medical records of those with at least one diagnostic code, we missed those who did not seek any medical care for the TBI and we did not have information on self-report. This would likely pertain to the mildest cases and could have artificially inflated hazard ratios for the probable and possible groups. Second, the number of the most severe (Definite) cases as well as individuals developing specific ADRD were relatively small, and this may have limited our power to observe an association. Third, while we matched participants with TBI and referents for non-head injury, there may be other medical or psychiatric co-morbidities that were unaccounted for that could have been more prevalent among those with TBI compared to referents. Last, because our sample only included those older than 40 years of age, results may not be generalizable to younger populations.
The results of our analysis in the context of existing literature support including TBI as a potential risk factor for developing neurodegenerative disease. However, disparate findings in our results and the literature, including counterintuitive associations favoring greater risk with diminishing injury severity, indicate that the assessment of TBI-associated risk will require greater clinical characterization to better understand this risk. Adverse health and psychosocial factors throughout the lifespan have been shown to moderate the risk of ADRD following TBI and there may be sex differences [54–56]. Additional research examining the relationship between TBI and ADRD is needed – and underway – particularly considering socioeconomic status, genomics and epigenetic exposures, neuropathology, race and ethnicity, and medical comorbidities.
Supplementary Material
ACKNOWLEDGMENTS
This work was funded by the Department of Defense, office of the Congressionally Directed Medical Research Programs, Peer Reviewed Alzheimer’s Research Program Convergence Science Research Award, AZ140069. This study used the resources of the Rochester Epidemiology Project (REP) medical records-linkage system, which is supported by the National Institute on Aging (NIA; AG 058738), by the Mayo Clinic Research Committee, and by fees paid annually by REP users. The content of this article is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health (NIH) or the Mayo Clinic.
Footnotes
CONFLICTS OF INTEREST
Dr. Mielke has consulted for Biogen, Brain Protection Company, and LabCorp. All other authors have no conflict of interest to report.
REFERENCES
- [1].Leibson CL, Brown AW, Hall Long K, Ransom JE, Mandrekar J, Osler TM, Malec JF (2012) Medical care costs associated with traumatic brain injury over the full spectrum of disease: a controlled population-based study. J Neurotrauma 29, 2038–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Gracia-Garcia P, Mielke MM, Rosenberg P, Bergey A, Rao V (2011) Personality changes in brain injury. J Neuropsychiatry Clin Neurosci 23, E14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ponsford JL, Downing MG, Olver J, Ponsford M, Acher R, Carty M, Spitz G (2014) Longitudinal follow-up of patients with traumatic brain injury: outcome at two, five, and ten years post-injury. J Neurotrauma 31, 64–77. [DOI] [PubMed] [Google Scholar]
- [4].Rao V, Bertrand M, Rosenberg P, Makley M, Schretlen DJ, Brandt J, Mielke MM (2010) Predictors of new-onset depression after mild traumatic brain injury. J Neuropsychiatry Clin Neurosci 22, 100–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Graves AB, White E, Koepsell TD, Reifler BV, van Belle G, Larson EB, Raskind M (1990) The association between head trauma and Alzheimer’s disease. Am J Epidemiol 131, 491–501. [DOI] [PubMed] [Google Scholar]
- [6].Mortimer JA, van Duijn CM, Chandra V, Fratiglioni L, Graves AB, Heyman A, Jorm AF, Kokmen E, Kondo K, Rocca WA, et al. (1991) Head trauma as a risk factor for Alzheimer’s disease: a collaborative re-analysis of case-control studies. EURODEM Risk Factors Research Group. Int J Epidemiol 20 Suppl 2, S28–35. [DOI] [PubMed] [Google Scholar]
- [7].O’Meara ES, Kukull WA, Sheppard L, Bowen JD, McCormick WC, Teri L, Pfanschmidt M, Thompson JD, Schellenberg GD, Larson EB (1997) Head injury and risk of Alzheimer’s disease by apolipoprotein E genotype. Am J Epidemiol 146, 373–384. [DOI] [PubMed] [Google Scholar]
- [8].Nemetz PN, Leibson C, Naessens JM, Beard M, Kokmen E, Annegers JF, Kurland LT (1999) Traumatic brain injury and time to onset of Alzheimer’s disease: a population-based study. Am J Epidemiol 149, 32–40. [DOI] [PubMed] [Google Scholar]
- [9].Guo Z, Cupples LA, Kurz A, Auerbach SH, Volicer L, Chui H, Green RC, Sadovnick AD, Duara R, DeCarli C, Johnson K, Go RC, Growdon JH, Haines JL, Kukull WA, Farrer LA (2000) Head injury and the risk of AD in the MIRAGE study. Neurology 54, 1316–1323. [DOI] [PubMed] [Google Scholar]
- [10].Barnes DE, Kaup A, Kirby KA, Byers AL, Diaz-Arrastia R, Yaffe K (2014) Traumatic brain injury and risk of dementia in older veterans. Neurology 83, 312–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Fann JR, Ribe AR, Pedersen HS, Fenger-Gron M, Christensen J, Benros ME, Vestergaard M (2018) Long-term risk of dementia among people with traumatic brain injury in Denmark: a population-based observational cohort study. Lancet Psychiatry 5, 424–431. [DOI] [PubMed] [Google Scholar]
- [12].Gardner RC, Burke JF, Nettiksimmons J, Kaup A, Barnes DE, Yaffe K (2014) Dementia risk after traumatic brain injury vs nonbrain trauma: the role of age and severity. JAMA Neurol 71, 1490–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Stopa BM, Tahir Z, Mezzalira E, Boaro A, Khawaja A, Grashow R, Zafonte RD, Smith TR, Gormley WB, Izzy S (2021) The impact of age and severity on dementia after traumatic brain injury: a comparison study. Neurosurgery 89, 810–818. [DOI] [PubMed] [Google Scholar]
- [14].Plassman BL, Havlik RJ, Steffens DC, Helms MJ, Newman TN, Drosdick D, Phillips C, Gau BA, Welsh-Bohmer KA, Burke JR, Guralnik JM, Breitner JC (2000) Documented head injury in early adulthood and risk of Alzheimer’s disease and other dementias. Neurology 55, 1158–1166. [DOI] [PubMed] [Google Scholar]
- [15].Mehta KM, Ott A, Kalmijn S, Slooter AJ, van Duijn CM, Hofman A, Breteler MM (1999) Head trauma and risk of dementia and Alzheimer’s disease: the Rotterdam Study. Neurology 53, 1959–1962. [DOI] [PubMed] [Google Scholar]
- [16].Launer LJ, Andersen K, Dewey ME, Letenneur L, Ott A, Amaducci LA, Brayne C, Copeland JR, Dartigues JF, Kragh-Sorensen P, Lobo A, Martinez-Lage JM, Stijnen T, Hofman A (1999) Rates and risk factors for dementia and Alzheimer’s disease: results from EURODEM pooled analyses. EURODEM Incidence Research Group and Work Groups. European Studies of Dementia. Neurology 52, 78–84. [DOI] [PubMed] [Google Scholar]
- [17].Katzman R, Aronson M, Fuld P, Kawas C, Brown T, Morgenstern H, Frishman W, Gidez L, Eder H, Ooi WL (1989) Development of dementing illnesses in an 80-year-old volunteer cohort. Ann Neurol 25, 317–324. [DOI] [PubMed] [Google Scholar]
- [18].Dams-O’Connor K, Gibbons LE, Bowen JD, McCurry SM, Larson EB, Crane PK (2013) Risk for late-life re-injury, dementia and death among individuals with traumatic brain injury: a population-based study. J Neurol Neurosurg Psychiatry 84, 177–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Savica R, Parisi JE, Wold LE, Josephs KA, Ahlskog JE (2012) High school football and risk of neurodegeneration: a community-based study. Mayo Clin Proc 87, 335–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Mielke MM, Savica R, Wiste HJ, Weigand SD, Vemuri P, Knopman DS, Lowe VJ, Roberts RO, Machulda MM, Geda YE, Petersen RC, Jack CR Jr. (2014) Head trauma and in vivo measures of amyloid and neurodegeneration in a population-based study. Neurology 82, 70–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Barnes DE, Byers AL, Gardner RC, Seal KH, Boscardin WJ, Yaffe K (2018) Association of mild traumatic brain injury with and without loss of consciousness with dementia in US military veterans. JAMA Neurol 75, 1055–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Brett BL, Gardner RC, Godbout J, Dams-O’Connor K, Keene CD (2022) Traumatic brain injury and risk of neurodegenerative disorder. Biol Psychiatry 91, 498–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Leibson CL, Brown AW, Ransom JE, Diehl NN, Perkins PK, Mandrekar J, Malec JF (2011) Incidence of traumatic brain injury across the full disease spectrum: a population-based medical record review study. Epidemiology 22, 836–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Sherer M, Sander AM, Nick TG, Melguizo MS, Tulsky DS, Kisala P, Hanks R, Novack TA (2015) Key dimensions of impairment, self-report, and environmental supports in persons with traumatic brain injury. Rehabil Psychol 60, 138–146. [DOI] [PubMed] [Google Scholar]
- [25].Bazarian JJ, Veazie P, Mookerjee S, Lerner EB (2006) Accuracy of mild traumatic brain injury case ascertainment using ICD-9 codes. Acad Emerg Med 13, 31–38. [DOI] [PubMed] [Google Scholar]
- [26].Marceaux JC, Soble JR, O’Rourke JJF, Swan AA, Wells M, Amuan M, Sagiraju HKR, Eapen BC, Pugh MJ (2020) Validity of early-onset dementia diagnoses in VA electronic medical record administrative data. Clin Neuropsychol 34, 1175–1189. [DOI] [PubMed] [Google Scholar]
- [27].Rocca WA, Yawn BP, St. Sauver JL, Grossardt BR, Melton LJ 3rd (2012) History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc 87, 1202–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].St. Sauver JL, Grossardt BR, Yawn BP, Melton LJ 3rd, Rocca WA (2011) Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester Epidemiology Project. Am J Epidemiol 173, 1059–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].St. Sauver JL, Grossardt BR, Leibson CL, Yawn BP, Melton LJ 3rd, Rocca WA (2012) Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clin Proc 87, 151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Brown AW, Leibson CL, Mandrekar J, Ransom JE, Malec JF (2014) Long-term survival after traumatic brain injury: a population-based analysis controlled for nonhead trauma. J Head Trauma Rehabil 29, E1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Faul M, Xu L, Wald MM, Coronado VG (2010) Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations and Deaths 2002–2006, Centers for Disease Control and Prevention, National Center for Injury Prevention and Control. Atlanta, GA. [Google Scholar]
- [32].Thurman DJ, Sniezek JE, Johnson D, Greenspan A, Smith SM (1995) Guidelines for Surveillance of Central Nervous System Injury, US Department of Health and Human Services, Public Health Service, Centers for Disease Control, Atlanta, GA. [Google Scholar]
- [33].Malec JF, Brown AW, Leibson CL, Flaada JT, Mandrekar JN, Diehl NN, Perkins PK (2007) The Mayo classification system for traumatic brain injury severity. J Neurotrauma 24, 1417–1424. [DOI] [PubMed] [Google Scholar]
- [34].Glance LG, Osler TM, Mukamel DB, Meredith W, Wagner J, Dick AW (2009) TMPM-ICD9: a trauma mortality prediction model based on ICD-9-CM codes. Ann Surg 249, 1032–1039. [DOI] [PubMed] [Google Scholar]
- [35].Peterson BJ, Rocca WA, Bower JH, Savica R, Mielke MM (2020) Identifying incident Parkinson’s disease using administrative diagnostic codes: a validation study. Clin Park Relat Disord 3, 100061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Li L, Binney LE, Luengo-Fernandez R, Silver LE, Rothwell PM, Oxford Vascular S (2020) Temporal trends in the accuracy of hospital diagnostic coding for identifying acute stroke: a population-based study. Eur Stroke J 5, 26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ponjoan A, Garre-Olmo J, Blanch J, Fages E, Alves-Cabratosa L, Marti-Lluch R, Comas-Cufi M, Parramon D, Garcia-Gil M, Ramos R (2019) How well can electronic health records from primary care identify Alzheimer’s disease cases? Clin Epidemiol 11, 509–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Knopman DS, Petersen RC, Rocca WA, Larson EB, Ganguli M (2011) Passive case-finding for Alzheimer’s disease and dementia in two U.S. communities. Alzheimers Dement 7, 53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].American Psychiatric Association (1994) Diagnostic and Statistical Manual of Mental Disorders (DSM-IV), American Psychiatric Association, Washington, DC. [Google Scholar]
- [40].McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM (1984) Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 34, 939–944. [DOI] [PubMed] [Google Scholar]
- [41].Roman GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JC, Garcia JH, Amaducci L, Orgogozo JM, Brun A, Hofman A, et al. (1993) Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology 43, 250–260. [DOI] [PubMed] [Google Scholar]
- [42].McKeith IG, Dickson DW, Lowe J, Emre M, O’Brien JT, Feldman H, Cummings J, Duda JE, Lippa C, Perry EK, Aarsland D, Arai H, Ballard CG, Boeve B, Burn DJ, Costa D, Del Ser T, Dubois B, Galasko D, Gauthier S, Goetz CG, Gomez-Tortosa E, Halliday G, Hansen LA, Hardy J, Iwatsubo T, Kalaria RN, Kaufer D, Kenny RA, Korczyn A, Kosaka K, Lee VM, Lees A, Litvan I, Londos E, Lopez OL, Minoshima S, Mizuno Y, Molina JA, Mukaetova-Ladinska EB, Pasquier F, Perry RH, Schulz JB, Trojanowski JQ, Yamada M (2005) Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 65, 1863–1872. [DOI] [PubMed] [Google Scholar]
- [43].Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, Freedman M, Kertesz A, Robert PH, Albert M, Boone K, Miller BL, Cummings J, Benson DF (1998) Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 51, 1546–1554. [DOI] [PubMed] [Google Scholar]
- [44].Bower JH, Maraganore DM, McDonnell SK, Rocca WA (2000) Influence of strict, intermediate, and broad diagnostic criteria on the age- and sex-specific incidence of Parkinson’s disease. Mov Disord 15, 819–825. [DOI] [PubMed] [Google Scholar]
- [45].McKeith IG (2006) Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the Consortium on DLB International Workshop. J Alzheimers Dis 9, 417–423. [DOI] [PubMed] [Google Scholar]
- [46].Savica R, Grossardt BR, Bower JH, Ahlskog JE, Rocca WA (2013) Incidence and pathology of synucleinopathies and tauopathies related to parkinsonism. JAMA Neurol 70, 859–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Nordstrom A, Nordstrom P (2018) Traumatic brain injury and the risk of dementia diagnosis: a nationwide cohort study. PLoS Med 15, e1002496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Lee YK, Hou SW, Lee CC, Hsu CY, Huang YS, Su YC (2013) Increased risk of dementia in patients with mild traumatic brain injury: a nationwide cohort study. PLoS One 8, e62422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Morissette MP, Prior HJ, Tate RB, Wade J, Leiter JRS (2020) Associations between concussion and risk of diagnosis of psychological and neurological disorders: a retrospective population-based cohort study. Fam Med Community Health 8, e000390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Esterov D, Bellamkonda E, Mandrekar J, Ransom JE, Brown AW (2021) Cause of death after traumatic brain injury: a population-based health record review analysis referenced for nonhead trauma. Neuroepidemiology 55, 180–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Crane PK, Gibbons LE, Dams-O’Connor K, Trittschuh E, Leverenz JB, Keene CD, Sonnen J, Montine TJ, Bennett DA, Leurgans S, Schneider JA, Larson EB (2016) Association of traumatic brain injury with late-life neurodegenerative conditions and neuropathologic findings. JAMA Neurol 73, 1062–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Levin HS, Temkin NR, Barber J, Nelson LD, Robertson C, Brennan J, Stein MB, Yue JK, Giacino JT, McCrea MA, Diaz-Arrastia R, Mukherjee P, Okonkwo DO, Boase K, Markowitz AJ, Bodien Y, Taylor S, Vassar MJ, Manley GT, Investigators T-T, Adeoye O, Badjatia N, Bullock MR, Chesnut R, Corrigan JD, Crawford K, Dikmen S, Duhaime AC, Ellenbogen R, Feeser VR, Ferguson AR, Foreman B, Gardner R, Gaudette E, Gonzalez L, Gopinath S, Gullapalli R, Hemphill JC, Hotz G, Jain S, Keene CD, Korley FK, Kramer J, Kreitzer N, Lindsell C, Machamer J, Madden C, Martin A, McAllister T, Merchant R, Nolan A, Ngwenya LB, Noel F, Palacios E, Puccio A, Rabinowitz M, Rosand J, Sander A, Satris G, Schnyer D, Seabury S, Sun X, Toga A, Valadka A, Wang K, Yuh E, Zafonte R (2021) Association of sex and age with mild traumatic brain injury-related symptoms: a TRACK-TBI study. JAMA Netw Open 4, e213046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Kornblith E, Peltz CB, Xia F, Plassman B, Novakovic-Apopain T, Yaffe K (2020) Sex, race, and risk of dementia diagnosis after traumatic brain injury among older veterans. Neurology 95, e1768–e1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Gerring JP, Wade S (2012) The essential role of psychosocial risk and protective factors in pediatric traumatic brain injury research. J Neurotrauma 29, 621–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Sayed N, Culver C, Dams-O’Connor K, Hammond F, Diaz-Arrastia R (2013) Clinical phenotype of dementia after traumatic brain injury. J Neurotrauma 30, 1117–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Nordstrom P, Michaelsson K, Gustafson Y, Nordstrom A (2014) Traumatic brain injury and young onset dementia: a nationwide cohort study. Ann Neurol 75, 374–381. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


