Abstract
Background:
Dysconnectivity theories, combined with advances in fundamental cognitive neuroscience, have led to increased interest in characterizing cerebellar abnormalities in psychosis. Smaller cerebellar grey matter volume has been found in schizophrenia-spectrum disorders. However, the course of these deficits across illness-stage, specificity to schizophrenia (versus psychosis more broadly), and relationship to clinical phenotypes, primarily cognitive impairment, remain unclear.
Methods:
The SUIT toolbox, a gold standard for analyzing human neuroimaging data of the cerebellum, was used to quantify cerebellar volumes and conduct voxel-based morphometry on structural magnetic resonance images obtained from 574 individuals (249 schizophrenia-spectrum, 108 bipolar with psychotic features, 217 non-psychiatric control). Analyses examining diagnosis (schizophrenia spectrum, bipolar disorder), illness-stage (early, chronic), and cognitive effects on cerebellum structure in psychosis were performed.
Results:
Cerebellar structure in psychosis did not differ significantly from healthy participants, regardless of diagnosis and illness-stage (effect sizes (ES)=0.01-0.14). In contrast, low premorbid cognitive functioning was associated with smaller whole and regional cerebellum volumes, including cognitive (lobules VI, VII, Crus I, fronto-parietal and attention networks) and motor (lobules I-IV, V, X, somatomotor network) regions in psychosis (ES=0.36-0.60). These effects were not present in psychosis cohorts with average estimated premorbid cognition.
Conclusion:
Cerebellar structural abnormalities in psychosis are related to lower premorbid cognitive functioning implicating early antecedents, atypical neurodevelopment, or both in cerebellar dysfunction. Future research focused on identifying the impact of early life risk factors for psychosis on the development of the cerebellum and cognition is warranted.
Keywords: cerebellum, psychosis, structural MRI, bipolar disorder, schizophrenia, heterogeneity
Introduction
The cerebellum has long been included in conceptualizations of the neural underpinnings of schizophrenia. Early theories, such as Stansky’s “intrapsychic ataxia,” suggest that psychosis results from a dyscoordination of cognitive processes, much like uncoordinated motor functions (ataxias)(1). Aligned with this thinking, modern dysconnectivity theories, including Andreasen’s cognitive dysmetria model, hypothesize that cerebellar abnormalities contribute to the mechanisms of psychosis(2, 3). Recent advances in cognitive neuroscience have provided evidence for a central and critical role of the cerebellum in various psychological processes(4–9), including cognitive and social processes, and the development of psychopathology(10, 11) that extend far beyond its traditionally circumscribed role in motor function. Indeed, “Little Brain” is a misnomer for the complex and fascinating cerebellum, a neural structure that while small in volume has been estimated to contain an upwards of 80% of the neurons in the human brain(12) and to cover 80% of the surface area of cerebral cortex(13).
Contemporary models emphasizing cerebellar dysfunction in psychosis are supported by human neuroimaging studies. Findings include smaller whole cerebellum volume, aberrant within-cerebellum and cerebellar-cerebral functional connectivity during rest and tasks, and lower activation during tasks(14–25). Neuroimaging findings are supported by neuropathological changes, including lower Purkinje cell density(26). Extensive work with cerebellar lesion patients shows region-specific cerebellar contributions to cognitive processes that overlap with deficits observed in psychosis(27). These advances are being applied to human neuroimaging studies, allowing for targeted analyses of anatomical lobules or functional divisions that may contribute to distinct aspects of psychopathology. A recent meta-analysis of 22 studies in first episode psychosis concluded that smaller cerebellar grey matter in schizophrenia is most prominent in lobules IV, V, and VII, as well as Crus I in individuals with schizophrenia (28). These lobules are significant for our understanding of psychosis, given their role in motor processes, which are widely observed as aberrant in psychosis, even preceding onset(29–31), and impaired cognitive processes(32–35). Similarly, a multi-site “mega analysis” by Moberget and colleagues(36), which included 983 individuals with schizophrenia and 1349 control participants from 12 sites, found smaller whole cerebellar grey matter volume (effect size (ES)=0.35) with the most robust effects in the posterior (“cognitive”) lobules and the fronto-parietal network (ES=0.16-0.40).
While past work has supported the finding of structural cerebellar abnormalities in schizophrenia, limitations in this body of knowledge remain. Small sample sizes combined with substantial heterogeneity in psychosis phenotypes have left studies underpowered to detect effects of interest. For example, even using optimized methods such as the Spatially Unbiased Infratentorial (SUIT) Toolbox, Moberget and colleagues(36) found that the effect sizes varied substantially by site (0.11-0.69), with only 5 out of the 12 included sites showing significant effect sizes. Consequently, three major gaps in our understanding of cerebellar structure in psychosis persist. First, studies have historically been limited to only those with a schizophrenia diagnosis and in a more chronic stage of illness, which leaves the specificity of diagnosis (schizophrenia or psychosis more broadly) and illness-stage (early vs. chronic) unclear. For example, some work suggests that smaller cerebellar grey matter volume is specific to schizophrenia (25), though others have documented similar impairments in bipolar patients(37) or reported opposite findings altogether in which bipolar and schizophrenia samples exhibit larger cerebellar volume compared to non-psychiatric control participants(38). Regarding illness-stage, findings are similarly mixed. Reports include both static abnormalities consistent with atypical early developmental processes (36) and progressive loss of total cerebellar grey matter volume, shown at 5- and 10-year intervals post first hospitalization, suggesting neuroprogression(23). Investigation of high risk groups suggests these differences precede the first episode, with smaller right cerebellum volume present only in individuals who convert to schizophrenia(39). Ultimately, to address questions regarding diagnostic and illness-stage specificity, studies have been underpowered, lacked appropriate comparison groups, or both.
Second, many studies take a whole brain approach, limiting conclusions about regional specificity. Unpredicted cerebellar differences are often reported in the context of broader cerebral findings (e.g., hippocampus, thalamus, prefrontal cortex, etc.), with cerebellum-specific studies only recently emerging. Historically, incidental cerebellar findings from whole-brain studies have resulted in unclear conclusions and subsequent work with ill-formed hypotheses on the role of the cerebellum in the development and maintenance of psychosis. Lobule-specific contributions to specific phenotypes also remain unclear outside of lesion studies. The question of regional specificity is critical, as targeted interventions are developed that rely on precise, mechanistic understanding of cerebellar circuits in disease phenomenology(40). Fortunately, advancements in cerebellar imaging, including higher resolution scanning and optimized processing tools, have equipped us to address the unique methodological challenges of cerebellar imaging(cf.(41)).
Third, it remains unclear how heterogeneous cerebellar findings in psychosis relate to differences in cognitive in psychosis. Cognitive ability is linked to differences in brain volume in psychosis (42), and thus may provide critical insights into observed structural heterogeneity in the cerebellum. Such a link would be unsurprising given prior work showing that higher cerebellar connectivity with fronto-parietal networks predicts better cognitive outcomes in schizophrenia patients(32). A preponderance of literature in psychosis points to structural changes in cognitive regions of cerebellum, including fronto-parietal networks. This question was not addressed in prior studies, including the study by Moberget and colleagues(36) that identified robust effects in this cerebellar network.
To address these knowledge gaps, the current study investigated cerebellar grey matter volume in a large cross-sectional cohort using gold-standard volumetric and voxel-based methods. We sought to determine the specificity of cerebellar deficits to schizophrenia (versus psychosis more broadly), the specificity to stage of illness (early vs. chronic), and association between cerebellar structure and cognitive impairment. Given prior work, we hypothesized that grey matter volume would be lower in the psychosis sample overall, with lobules I-IV and V and posterior lobule Crus I being disproportionately affected(28). We predicted that these abnormalities would be more pronounced in individuals with a schizophrenia-spectrum disorder (compared to bipolar disorder with psychotic features). We were agnostic to illness-stage given the lack of consistency in the literature. Finally, given the prominence of deficits in cerebellar cognitive lobules and networks in the literature, we predicted that individuals with impaired neuropsychological ability would show smaller cerebellar grey matter volumes, again with posterior lobules being more affected.
Methods
Study Participants and Procedures
All procedures were approved by the Vanderbilt University Institutional Review Board. Written, informed consent was provided by 643 individuals recruited for participation in one of three studies (CT00762866; R01MH070560; R01MH102266) conducted within the department of Psychiatry and Behavioral Sciences at Vanderbilt University Medical Center (VUMC). Inclusion and exclusion criteria are detailed in the Supplement (Note 1). Clinical participants were recruited from the Psychotic Disorders Program at VUMC. Diagnoses were confirmed using the Structured Clinical Interview for DSM-IV (SCID-IV;(43)). The clinical sample was composed of individuals in early (i.e., ≤2 years) or chronic (i.e., >2 years) stages of their illness (cf. (44)). Non-psychiatric control participants (hereto forward referred to as control participants) were recruited from Nashville and the surrounding area via advertisements. Current and past psychopathology was ruled out in the control group using the SCID-IV.
Two hundred and seventeen control participants; 249 individuals diagnosed with schizophrenia-spectrum disorder including schizophrenia, schizophreniform, or schizoaffective disorder; and 108 individuals diagnosed with a bipolar disorder with psychotic features were selected for inclusion in the current study. Two individuals were further excluded due to not completing neuroimaging (no T1) for a final sample of 217 HC, 249 SZ (122 early, 127 chronic), and 108 BP participants (46 early, 62 chronic) (Table 1). No individuals were excluded due to missing cognitive data or poor imaging data or cerebellar segmentation.
Table 1.
Sample Demographics – Diagnostic Subgroups
| Schizophrenia Spectrum (SzS; n=249) | Bipolar w/Psychotic Features (BP; n=108) | Control (HC; n=217) | Statistics (F or χ2) | p-value (post-hoc) | |
|---|---|---|---|---|---|
|
|
|||||
| Sex (% male) | 70.3 | 50.0 | 61.3 | 13.77 | 0.001 |
|
| |||||
| Race (C/B/O) | 157/82/10 | 87/11/10 | 152/52/13 | 27.70 | <0.001 |
| Age (years ± SD) | 29.7 ± 11.2 | 31.2 ± 11.7 | 29.3 ± 10.1 | 1.21 | 0.30 |
|
| |||||
| Education (years) | |||||
| Personal | 13.2 ± 2.2 | 14.1 ± 1.9 | 15.2 ± 2.1 | 48.18 | <0.001 (HC >BP >SzS) |
| Parental | 14.4 ± 2.7 | 14.8 ± 2.2 | 14.5 ± 2.3 | 0.85 | 0.43 |
|
| |||||
| Handedness (%R) | 89.2 | 91.7 | 91.7 | 0.79 | 0.67 |
|
| |||||
| ICV (cm3) | 1536.7 ± 161.8 | 1561.4 ± 163.6 | 1537.3 ± 152.8 | 1.03 | 0.36 |
|
| |||||
| Illness-Stage | |||||
| Chronic/Early Stage | 127/122 | 61/47 | -- | -- | -- |
| Duration (months)† | 102.2 ± 133.9 | 86.3 ± 107.5 | -- | 1.17 | 0.24 |
| Age Onset (years) | 21.1 ± 5.3 | 24.1 ± 8.5 | -- | 3.37 | 0.001 (BP > SzS) |
|
| |||||
| Clinical Symptoms | |||||
| Positive (PANSS) | 18.0±8 | 15.1±9 | -- | 3.07 | 0.002 (SZ > BP) |
| Negative (PANSS) | 15.8±7 | 10.3±4 | -- | 9.58 | <0.001 (SZ > BP) |
| Mania (YMRS) | 4.9±7 | 8.0±12 | -- | −2.51 | 0.003 (BP > SzS) |
| Depression (HAMD) | 11.3±9 | 10.4±9 | 0.7±2 | 71.84 | <0.001 (SzS/BP > HC) |
|
| |||||
| WTAR (standardized) | 99.6 ± 15.8 | 106.7 ± 12.5 | 111.0 ± 10.8 | 41.51 | <0.001 (HC >BP >SzS) |
|
| |||||
| SCIP (z-score) | −1.10 ± 0.9 | −0.67 ± 0.9 | 0.09 ± 0.6 | 114.51 | <0.001 (HC >BP >SzS) |
|
| |||||
|
Cognitive Subgroups (Norm/Det/Comp) |
82/81/73 | 52/32/13 | -- | 14.29 | 0.001 |
WTAR, Wechsler Test of Adult Reading (48); SCIP, Screen for Cognitive Impairment in Psychiatry (49), ICV, transcranial volume as determined using the VBM toolbox (http://dbm.neuro.uni-jena.de/vbm/) for SPM (http://www.fil.ion.ucl.ac.uk/spm); for race, C=Caucasian, B=Black, and O=other race (e.g., Hispanic, Asian, Mixed-Race); PANSS, Positive and Negative Syndrome Scale (45); YMRS, Young Mania Rating Scale (46); HAMD, Hamilton Depression scale (47); Norm=neuropsychologically normal cognitive subgroup, Det=deteriorated cognitive subgroup; Comp=compromised cognitive subgroup. Italics indicate significant p-values.
Duration of illness was defined as the time at which an individual first met criteria for psychosis (based on extensive interview, review of medical records, and collateral reports) until the date of study enrollment.
For comprehensive demographic breakdown of the cognitive subgroups, please see Table 2.
Psychosis symptoms were measured using the Positive and Negative Syndrome Scale (PANSS)(45) and mood symptoms were measured using the Young Mania Rating Scale (YMRS)(46) and Hamilton Depression Rating Scale (HAMD)(47) for mania and depression, respectively. To assess estimated premorbid and current cognitive ability, the Wechsler Test of Adult Reading (WTAR)(48) and the Screen for Cognitive Impairment in Psychiatry (SCIP)(49) were administered, respectively (Supplementary Note 2).
Neuroimaging Data Acquisition and Pre-Processing
Image data storage and processing took place on the Vanderbilt University Institute of Imaging Science Center for Computational Imaging XNAT(50, 51). The processing pipelines were containerized using Singularity and were built at SingularityHub(52) (https://singularity-hub.org). Scanning was completed on one of two identical 3T Philips Intera Achieva scanners located at Vanderbilt University Institute of Imaging Sciences (VUIIS). Scanning parameters and preprocessing steps have been described previously (cf. (53) and Supplementary Note 3). Briefly, a high resolution T1-weighted anatomical image (32-channel head coil, 1 mm3 isotropic voxels, FOV=256 x 256 x 170 mm) was collected for each individual and visually inspected, blind to diagnosis status, for quality including head motion (e.g., blurring, ringing), brain artefacts, and incomplete coverage of the cerebellum.. No individuals meeting diagnostic criterial required exclusion following QA. Each study’s scanning parameters differed slightly (e.g., minor differences in TR/TE). Accordingly, ‘scan type’ was included as a covariate in all neuroimaging analyses.
Structural scans were segmented into grey, white, and cerebrospinal fluid (CSF) tissue classes using the Computational Anatomy Toolbox (CAT), version 12 (http://www.neuro.uni-jena.de/cat/) for Statistical Parametric Mapping (SPM), version 12 (http://www.fil.ion.ucl.ac.uk/spm). Total grey, white, and CSF volumes were calculated and summed to determine intracranial volume (ICV), which was used as a covariate in subsequent analyses. SPM generated ICV values were used in the current study for consistency, given that the cerebellar processing toolbox (see below) is embedded within the SPM package.
Cerebellar Optimization Using the Spatially Unbiased Infratentorial (SUIT) Toolbox
The Spatially Unbiased Infratentorial (SUIT; (54–56), https://github.com/baxpr/cersuit) toolbox was used to optimize cerebellar analyses. Using pre-processed images as described above, the cerebellum and brainstem were isolated from the whole brain. Cerebellum and brainstem were then segmented into grey matter, white matter, and CSF maps. These segmentation maps are normalized to a cerebellar (SUIT-space) template which is shown to optimize alignment procedures for the cerebellum beyond standard whole-brain processes(56). These normalized grey matter maps were (1) parcellated using the SUIT anatomical probabilistic atlas and SUIT Buckner-Yeo functional atlas (57), then resliced to native space for subsequent volumetric analyses or (2) modulated with a Jacobian transformation in preparation for voxel-based morphometry (VBM) analyses. All scans were visually inspected to assure proper isolation and segmentation; no subjects required exclusion following inspection.
Volumetric and Voxel-Wise Analyses
Grey Matter Volume.
The SUIT probabilistic atlas was used to define anatomical cerebellar lobules for a total of 28 hemispheric lobules. In native space, lobular grey matter volumes were extracted and analyzed as bilateral regions of interest (ROIs; 10 lobules) and one cerebellar vermis region (total of 11 ROIs). These ROIs were summed for a measure of total cerebellar grey matter volume. In addition to anatomical ROIs, the SUIT atlas was also used to estimate cerebellar grey matter volume in functional ROIs using the Buckner-Yeo atlas (7 functional networks(57)).
Voxel-Based Morphometry.
Volumetric analyses were followed by voxel-based analyses to investigate possible regional volume changes which might be missed by gross, whole-volume analyses. Nonlinear modulated whole cerebellar grey matter images were tested for homogeneity using CAT12’s automated quality check protocol, which checks image inhomogeneity defined as the mean correlation between gray matter volumes. Flagged images were visually inspected. Eighteen participants (15 schizophrenia-spectrum, 2 bipolar, and 1 control participants) were excluded from this analysis due to significant inhomogeneity. Outputs from the SUIT toolbox (individual grey matter maps modulated with a Jacobian transformation) were entered into an ANOVA using the CAT12 toolbox.
Statistical Analyses
Volumetric group differences in whole cerebellar volume and lobular volume were investigated using univariate ANOVAs with age, sex, ICV, and scan type (to account for the three individual studies detailed above) entered as covariates. Post-hoc comparisons were used to examine group-specific effects. Comparisons were Bonferroni-corrected to p<0.0026 (0.05/19 ROIs including 11 lobular ROIs, 7 functional ROIs, and whole cerebellum).
Voxel-based analyses were performed using separate, independent one-way ANOVAs with preplanned between group t-tests to examine diagnosis, illness-stage, and cognitive subgroups including covariates for sex, age, ICV, and scan type. For diagnosis groups the control participants were compared to all psychosis patients and each diagnostic group (psychosis spectrum and bipolar) and, the psychosis spectrum and bipolar groups were compared. For illness-stage, t-tests were set up to compare the control group to each illness-stage (early, chronic). Finally, cognitive subgroups were compared. Individuals with psychosis were assigned to one of three cognitive subgroups: neuropsychologically normal, deteriorated, and compromised, based on their Wechsler Test of Adult Reading (WTAR(48)), Screen for Cognitive Impairment in Psychiatry (SCIP(49)), and discrepancy between their WTAR and SCIP (cf. (42); Supplementary Note 4 & Table 1). Briefly, neuropsychologically normal individuals were characterized by estimated premorbid and current cognitive ability in the normative range. The deteriorated group also had normative estimated premorbid ability, but their current ability is in the impaired range. The compromised group is notable for impaired estimated premorbid ability, with impaired current ability. T-tests were established comparing the control group to each cognitive group (neuropsychologically normal, deteriorated, compromised) and the neuropsychologically normal group to each cognitively impaired group (deteriorated, compromised). All independent samples t-tests were thresholded at cluster-level pFWE<0.05 for voxel-wise cluster-defining threshold p=0.001 (uncorrected).
Symptom and Medication Correlates.
To determine whether medication dose or symptoms were driving group differences, cerebellar volumes (whole, lobular, functional ROIs) were correlated with chlorpromazine equivalent (CPZ) values; PANSS positive, negative, and general scores; YMRS scores; and HAMD scores. Partial correlations were computed for these variables to control for age, sex, ICV, and scan type.
Results
Diagnosis and Illness-stage Effects
Whole Cerebellar Grey Matter Volume.
No differences were observed between individuals with psychosis and the control group (Cohen’s d Effect Size (ES)=−0.06, negative indicates smaller in psychosis sample) (Fig. 1A & Supplemental Figure 1). No differences were observed when the psychosis sample was divided into schizophrenia spectrum and bipolar disorder with psychotic features; neither group differed from the control group (ES=−0.08 to 0.00). Similarly, chronic- and early-stage groups did not differ from the control group in whole cerebellar grey matter volume (ES=−0.08 to 0.00). No significant diagnosis by illness-stage interaction effects were present.
Figure 1. Diagnosis-Based Findings.
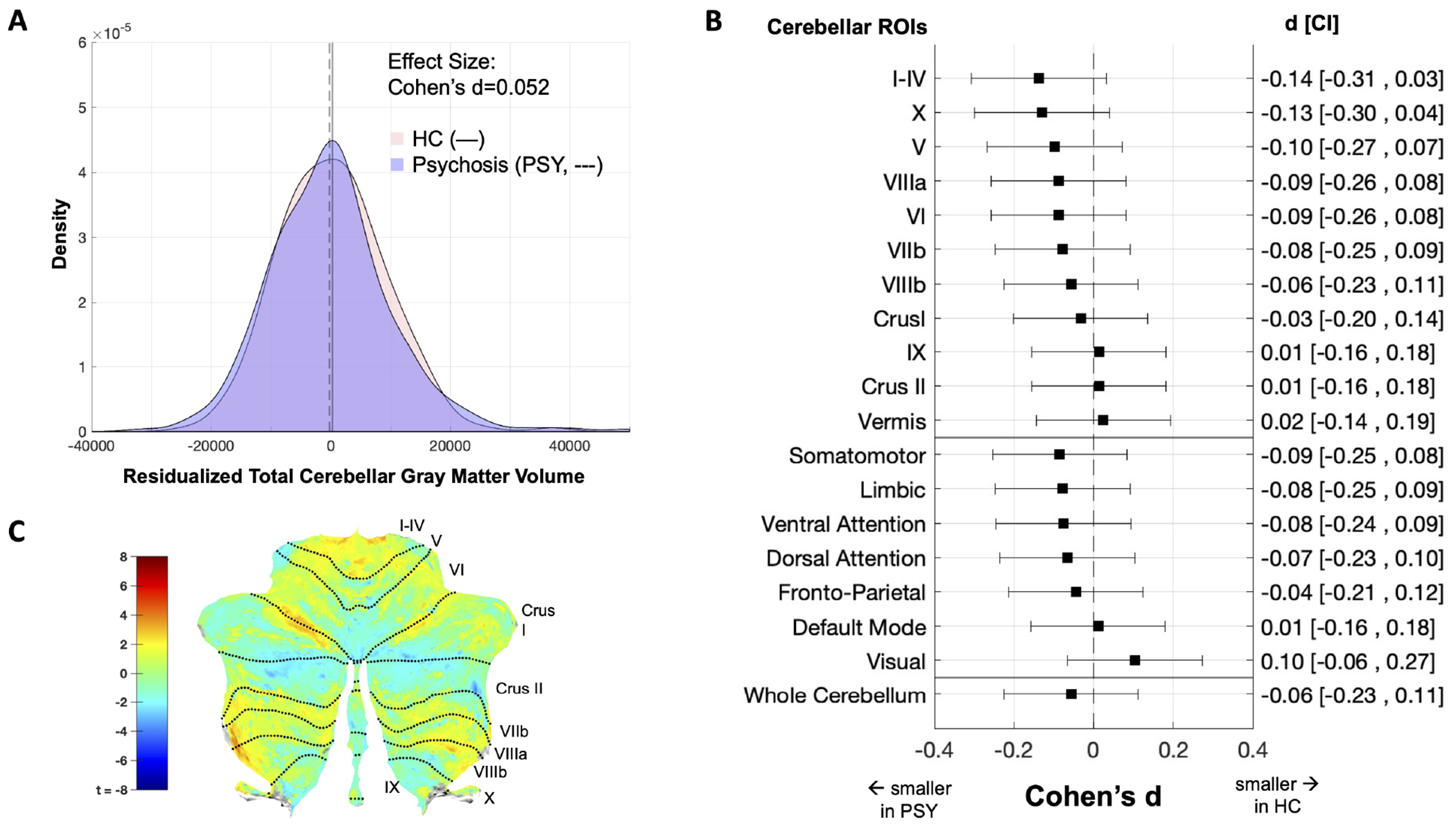
Effects from diagnosis-based comparisons. (A) unstandardized, residualized whole cerebellar grey matter volume using sex and age as covariates. HC = control, PSY = psychosis. (B) cerebellar volume for 10 bilateral lobules, cerebellar vermis, and 7 functional networks ((57). Left axis indicates the ROI, right axis displays the Cohen’s d effect size with confidence interval (see Supplementary Note X for calculation) based on the univariate test comparing all psychosis patients with the control group, using sex, age, ICV, and scan type as covariates. Effect sizes <0 indicate that the region is smaller in the psychosis group. (C) VBM results plotted on a cerebellar flatmap (55) as the uncorrected t-map of the comparison of all psychosis patients and healthy controls. No significant group differences were observed in whole cerebellar volume (A), ROI volume (B), or voxel-based regional (C).
ROI-Based Grey Matter Volume.
Cerebellar lobular grey matter estimates were consistent with previous reports (cf.(36); Supplementary Fig. 2). For both anatomical and functional ROIs, no significant group differences were observed in comparisons between control participants and the full psychosis sample (ES=−0.14 to 0.10; Fig. 1B); control and schizophrenia-spectrum patients or bipolar groups (ES=−0.20 to 0.17), between control and first episode or chronic illness-stage groups (ES=−0.19 to 0.12); and there was no significant diagnosis by illness-stage interaction.
Voxel Based Morphometry.
No clusters passed correction for any of the planned contrasts for diagnosis and illness-stage (Fig. 1C, Supplementary Fig. 3 & 4).
Cognitive Subgroup Effects
Consistent with prior reports (42, 58) 40% of the psychosis cohort group was classified as cognitively normal, while the remaining 60% were classified as cognitively deteriorated (34%) and cognitively compromised (26%). In terms of demographics, the cognitive subgroups were similar in age, though the compromised group had the lowest personal and parental educational attainment and was more symptomatic per PANSS (Table 2). Intracranial volume (ICV) was similar across groups (Table 2, Supplementary Fig. 5), consistent with prior work, including an earlier analysis of a subset of these data (42) as well as an extension of this work by an independent group(59).
Table 2.
Sample Demographics – Cognitive Subgroups
| Control (n=217) | NP Normal (n=134) | Impaired |
Statistics (F or χ2) | p-value (post hoc) † | ||
|---|---|---|---|---|---|---|
| Deteriorated (n=113) | Compromised (n=86) | |||||
|
|
||||||
| Sex (% male) | 61.3 | 66.4 | 63.7 | 65.1 | 1.046 | 0.790 |
|
| ||||||
| Race (C/B/O) | 152/52/13 | 105/25/4 | 90/18/5 | 38/42/6 | 38.845 | <0.001 |
| Age (years ± SD) | 29.27 ± 10.13 | 28.70 ± 10.47 | 30.10 ± 11.50 | 31.51 ± 12.40 | 1.354 | 0.256 |
|
| ||||||
| Education (years) | ||||||
| Personal | 15.21 ± 2.10 | 14.33 ± 2.11 | 13.41 ± 2.12 | 12.33 ± 1.62 | 44.757 | <0.001 (HC > NP Norm > Det > Comp) |
| Parental | 14.48 ± 2.35 | 15.26 ± 2.46 | 14.96 ± 2.64 | 12.89 ± 1.98 | 16.698 | <0.001 (NP Norm > HC/Det > Comp) |
|
| ||||||
| Handedness (%R) | 91.7 | 87.3 | 91.2 | 91.9 | 2.198 | 0.532 |
|
| ||||||
| ICV (cm3) | 1537.33 ± 152.79 | 1540.36 ± 155.00 | 1557.26 ± 166.09 | 1536.72 ± 167.05 | 0.449 | 0.718 |
|
| ||||||
| Diagnostic Groups | ||||||
| SzS (Chronic:FE) | -- | 82 (35:27) | 81 (41:40) | 73 (44:29) | 4.784 | 0.091 |
| BP (Chronic:FE) | -- | 52 (25:27) | 32 (21:11) | 13 (9:4) | 4.663 | 0.324 |
| Age Onset (years) | -- | 22.78 ± 6.20 | 21.77 ± 6.40 | 21.05 ± 7.03 | 1.943 | 0.145 |
|
| ||||||
| Clinical Symptoms | ||||||
| Positive (PANSS) | -- | 15.93 ± 8.45 | 17.55 ± 8.81 | 18.12 ± 7.31 | 3.358 | 0.019 ‡ |
| Negative (PANSS) | -- | 12.36 ± 5.45 | 13.64 ± 5.85 | 17.36 ± 8.14 | 12.558 | <0.001 (Comp > NP Norm / Det) |
| Mania (YMRS) | -- | 4.40 ± 1.03 | 3.92 ± 0.95 | 3.47 ± 1.03 | 12.147 | <0.001 (NP Norm > Det > Comp) |
| Depression (HAMD) | 0.71 ± 1.52 | 9.82 ± 8.53 | 11.79 ± 8.44 | 11.26 ± 8.93 | 48.381 | <0.001 (HC < NP Norm / Det / Comp) |
|
| ||||||
| WTAR (standardized) | 111.01 ± 10.84 | 110.11 ± 9.31 | 108.52 ± 8.46 | 81.45 ± 7.91 | 218.029 | <0.001 (Comp < NP Norm / Det / HC) |
|
| ||||||
| SCIP (z-score) | 0.09 ± 0.63 | −0.10 ± 0.54 | −1.43 ± 0.57 | −1.66 ± 0.93 | 240.530 | <0.001 (HC > NP Norm > Det > Comp) |
WTAR, Wechsler Test of Adult Reading (1); SCIP, Screen for Cognitive Impairment in Psychiatry (3), TIV, transcranial volume as determined using the VBM toolbox (http://dbm.neuro.uni-jena.de/vbm/) for SPM (http://www.fil.ion.ucl.ac.uk/spm); for race, C=Caucasian, B=Black, and O=other race (e.g., Hispanic, Asian, Mixed-Race); PANSS, Positive and Negative Syndrome Scale (11); YMRS, Young Mania Rating Scale(12); HAMD, Hamilton Depression scale(13); Szs, schizophrenia spectrum; BP, bipolar with psychotic features; NP Normal/NP Norm, neuropsychologically normal; Comp, compromised; Det, deteriorated; HC/Control, healthy control; FE, first-episode/early stage psychosis. Italics indicate significant p-values.
Whole Cerebellar Grey Matter Volume.
A main effect of cognitive group was observed (F(2,542)=6.998, p<0.001, ES=−0.53). Bonferroni-corrected comparisons showed smaller volume in the compromised group compared to the control (p=0.002, ES=−0.47), but not in the neuropsychologically normal (p=1.00, ES=−0.05) or deteriorated (p=1.00, ES=−0.14) groups (Fig. 2B).
Figure 2. Cognitive Subgroups Findings.
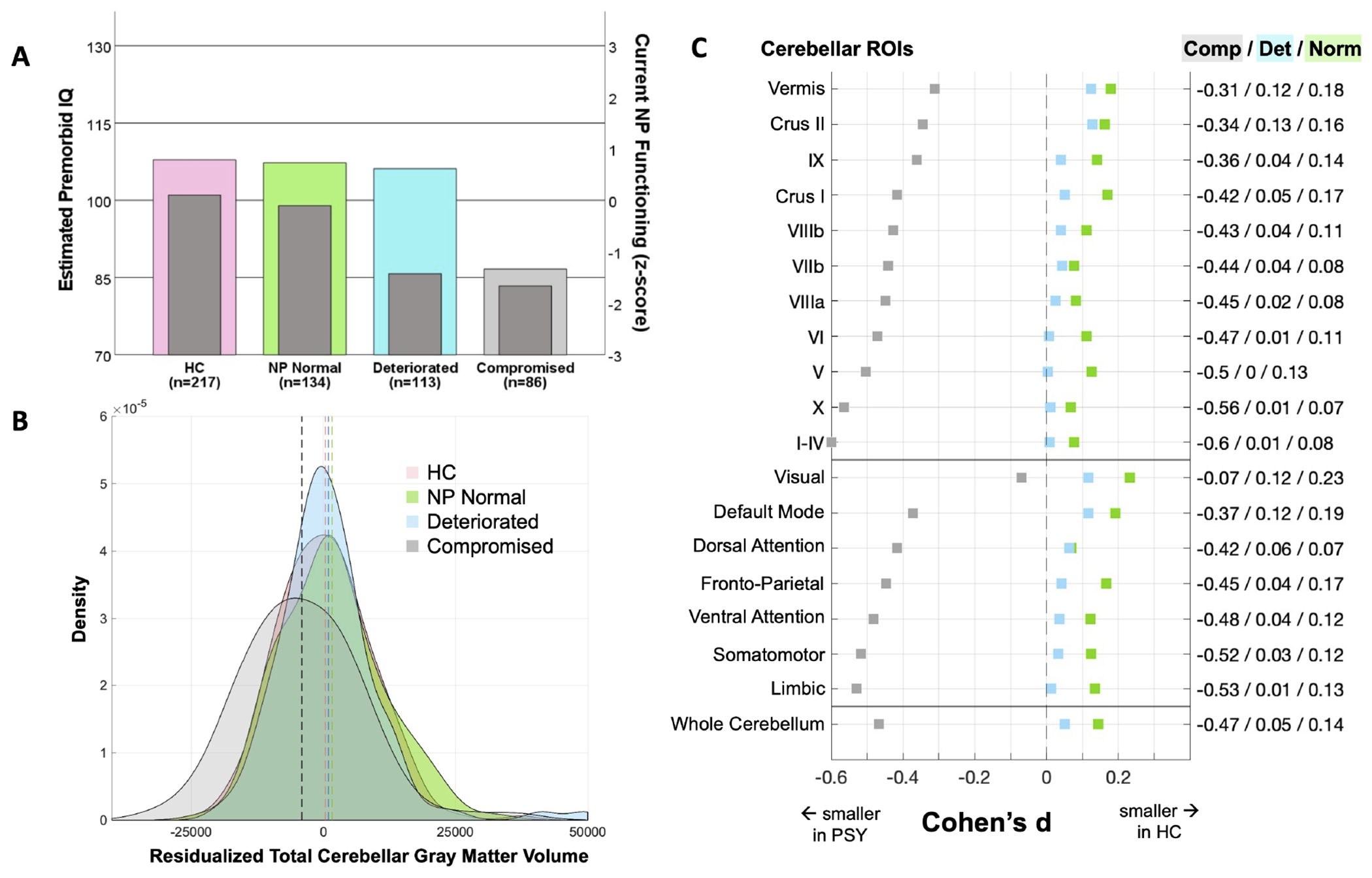
Three psychosis cognitive subgroups were generated (A) according to Woodward & Heckers (42) using estimated premorbid IQ (left axis, colored bars) and current neuropsychological functioning (right axis, dark grey bars). (B) unstandardized, residualized whole cerebellar grey matter volume using sex and age as covariates indicated that the compromised (comp, light grey) group had significantly smaller volume compared to the healthy control (HC, pink), neuropsychologically normal (NP Normal/Norm, green), and deteriorated (Det, blue) groups. (C) ROI analyses showed significant effects for the compromised group only. All effects plotted are for the indicated group compared to the HC group; right axis displays the corresponding effect size. Figure depicts effect sizes for cerebellar volume of 10 bilateral lobules, cerebellar vermis, and 7 functional networks (57). based on the univariate test using sex, age, ICV, and scan type as covariates. Effect sizes <0 indicate that the region is smaller in the psychosis cognitive subgroup. For subgroup effects with associated confidence intervals, see Supplementary Figs. 6–8.
ROI-Based Grey Matter Volume.
Significant differences were present in 9 of 10 cerebellar lobules (lobules I-IV, V, VI, VIIb, VIIIa, VIIIb, X, Crus I, and Crus II) and 5 of 7 networks (DAN, VAN, limbic, FPN, and DMN), with post hoc comparisons revealing these differences were driven by smaller volume in the compromised group (ES=−0.60 to −0.34, Fig. 2C, Supplementary Fig. 8) compared to all other groups (control, neuropsychologically normal, and deteriorated, ES=0.00 to 0.20, Supplementary Fig. 6–8).
Voxel Based Morphometry.
VBM results were consistent with lobular and functional ROI analyses; the compromised group showed smaller regional volumes compared to all other groups (Fig. 3, Table 3). No clusters survived correction for contrasts in the other direction (e.g., compromised > neuropsychologically normal; Supplementary Fig. 9).
Figure 3. Cognitive Subgroups Voxel-Based Morphometry Analysis.
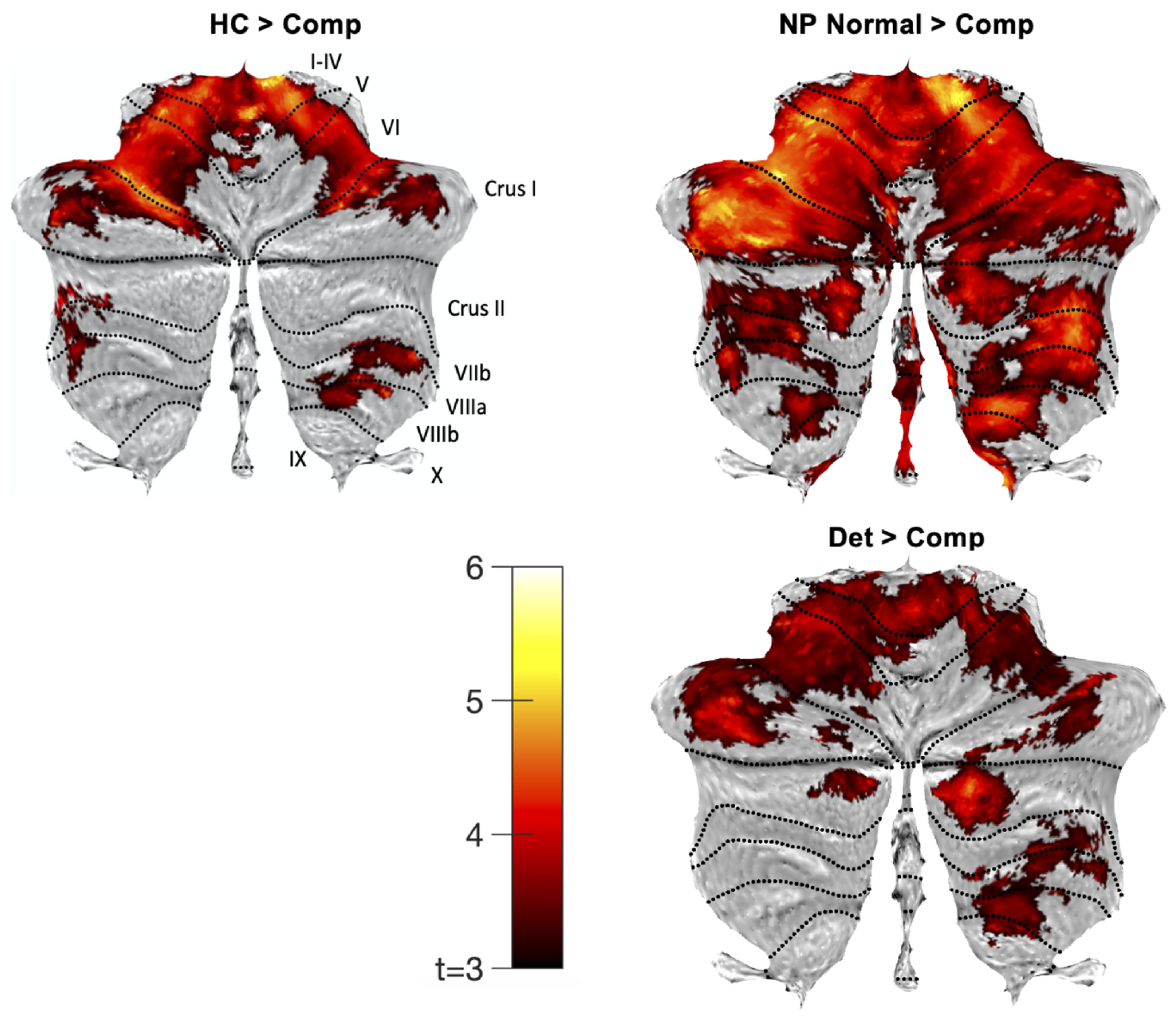
Cerebellar flatmaps (57) indicating significant effects of comparisons between the compromised (Comp) psychosis subgroup and other cognitive subgroups (control, HC; neuropsychologically normal, NP Normal; and deteriorated, Det), thresholded at cluster-level pFWE < 0.05 for voxel-wise cluster-defining threshold p = 0.001 (uncorrected).
Table 3.
Cognitive Subgroups VBM Analysis Findings
| Contrast | Cluster size (voxels) | MNI coordinates | t-value | p-value † | Region‡ | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| HC > Impaired | 639 | 9 | −39 | −21 | 5.64 | <0.001 | Right I-IV |
| 711 | −25 | −60 | −32 | 5.46 | <0.001 | Left VI | |
| 234 | 24 | −68 | −34 | 4.54 | 0.016 | Right VI | |
| 178 | 3 | −52 | −23 | 4.34 | 0.043 | Right I-IV | |
| 323 | −11 | −37 | −19 | 4.14 | 0.004 | Left I-IV | |
|
| |||||||
| HC > Compromised | 17198 | 9 | −47 | −23 | 5.66 | <0.001 | Right I-IV |
| 1295 | 25 | −48 | −49 | 4.42 | <0.001 | Right VIIIb | |
| 799 | 48 | −57 | −28 | 4.20 | <0.001 | Right Crus I | |
| 245 | −37 | −47 | −44 | 4.10 | 0.006 | Left Crus II | |
|
| |||||||
| NP Normal > Impaired | 2636 | −28 | −56 | −33 | 5.31 | <0.001 | Left VI |
| 1910 | 14 | −42 | −27 | 4.83 | <0.001 | Right I-IV | |
| 1998 | −37 | −74 | −24 | 4.80 | <0.001 | Left Crus I | |
| 440 | 10 | −51 | −37 | 4.74 | <0.001 | Right IX | |
| 799 | 42 | −58 | −54 | 4.21 | <0.001 | Right VIIb | |
| 475 | 5 | −61 | −52 | 4.17 | <0.001 | Right IX | |
| 663 | 36 | −54 | −21 | 3.86 | <0.001 | Right VI | |
|
| |||||||
| NP Normal > Compromised | 49315 | −28 | −56 | −33 | 5.86 | <0.001 | Left VI |
|
| |||||||
| Deteriorated > Compromised | 16559 | 17 | −85 | −36 | 4.86 | <0.001 | Left Crus II |
| 152 | −16 | −77 | −31 | 4.30 | 0.047 | Left Crus I | |
| 2335 | 14 | −60 | −57 | 4.15 | <0.001 | Right VIIIb | |
| 447 | −8 | −86 | −32 | 4.14 | <0.001 | Left Crus II | |
HC = control group; Impaired = cognitively compromised and cognitively deteriorated subgroups; NP Normal = neuropsychologically normal cognitive group
thresholded at cluster-level pFWE < 0.05 for voxel-wise cluster-defining threshold p = 0.001 (uncorrected)
region is determined by the location of peak activation
Symptom and Medication Correlates
Positive and general PANSS scores correlated weakly with several ROI volumes; however, none survived correction for multiple comparisons (Supplementary Note 7). No significant correlations were observed between volumes and PANSS negative scores, chlorpromazine equivalents, HAMD, or YMRS scores.
Discussion
While the cerebellum has been identified as a key node in dysconnectivity theories of schizophrenia, the specificity of these deficits to psychosis-spectrum diagnostic groups, specificity to illness-stage, and contributions of cognitive function to heterogeneity has been unclear. The current study used a large cross-sectional dataset to localize cerebellar deficits among these groups. Moreover, this study aimed to parse some of the heterogeneity present throughout the literature by investigating cerebellar structural changes within psychosis cognitive subgroups, given findings indicating robust deficits of cerebellar cognitive (posterior) regions in psychotic disorders(30, 32).
Differences in cerebellar structure, including grey matter volume (Fig. 1A and 1B) and voxel-based morphometry (Fig. 1C), were not present when looking at diagnostic groups (schizophrenia-spectrum, bipolar with psychotic features) or illness-stage (early, chronic). This was in stark contrast to findings by Moberget and colleagues(36) that individuals with a schizophrenia diagnosis show smaller cerebellar volume in posterior (cognitive) lobules and functional networks. One major difference is that the current study used ICV calculated by CAT12, compared to Moberget and colleagues’ study(36) which used eTIV estimated in Freesurfer. Here, this was done to maintain consistency in analysis packages given that SUIT and CAT12 both utilize SPM. Although it is unclear why ICV and eTIV values are poorly correlated (Supplementary Note 5 & Fig. 12), confidence in the current findings in enhanced by observations of a similar trend in effects when using eTIV (Freesurfer), though marginally weaker than effects from ICV (CAT12) (Supplementary Fig. 13–15). In addition, the current work is highly powered (96.4%) to replicate findings by Moberget and colleagues(36). Given the robust findings in the current work, it is possible that the effects are largely driven by heterogeneity across samples. Moberget and colleagues reported large effect sizes for the pooled sample (Moberget et al. ES=0.35; current study ES=0.08), though there was substantial variability across the 14 sites(36) with only 5 sites showing significant effects. It is likely that cognitive impairment contributes to this heterogeneity and may explain the inconsistent findings across studies. Aligned with this hypothesis, Moberget’s study highlighted the robustness of effects in cognitive cerebellar lobules(36) and prior work has shown that cognitive performance is associated with cerebellar findings in psychosis samples(37).
To better elucidate these effects, the current work used cognitive ability to define psychosis types. Individuals with a compromised neuropsychological profile show markedly smaller cerebellar volume compared to all other groups (whole cerebellum ES=−0.47; Fig. 2B and 2C). Deficits were pronounced in cognitive lobules, with moderate effect sizes in lobule VI (ES=−0.47), Crus I (ES=−0.42), and the fronto-parietal (ES=−0.45) and attention (ES=−0.42 to −0.48) networks. (Fig. 2C & 3). The compromised group is distinguished from the other cognitive subgroups (neuropsychologically normal and deteriorated) by a low estimated premorbid IQ (Fig. 2A). This suggests an early developmental process contributing to these anatomical deficits that is supported by prior work. In a neurodevelopmental sample, cerebellar structural features spanning anterior and posterior lobules were shown to be predictive of general cognitive function in youth aged 8-23 (33), with more circumscribed cerebellar deficits (lobule VI and Crus I) relating to psychotic-like experiences and symptoms. A developmental model is also supported by fundamental impairments in the motor system. Studies rating childhood home movies of individuals who then go on to develop psychosis have noted motor impairments present early in development(29, 30, 60). These motor system phenotypes are squarely in line with the current observations of cerebellar abnormalities. In fact, the current study identified the strongest effects in motor ROIs, including lobules I-IV (ES=−0.60), lobule V (ES=−0.50), lobule X (ES=−0.56), and the somatomotor network (ES=−0.52). These motor regions tend to develop earlier in life, quickly reaching their peak by birth to young childhood, compared to cognitive regions that slowly continue to develop into mid adulthood(61–63). Taken together with our findings, this work provides evidence for a formative role of early developmental processes, cognition, and cerebellar development in psychosis, though causal mechanisms remain unclear.
The current work has several limitations. First, alcohol and cannabis use were not included in our models. Both substances have a high density of receptors in the cerebellum and alter cerebellar volume with chronic use(64–67). Prior work in a subset of the current sample has shown that a lifetime history of cannabis or alcohol abuse or dependence does not significantly influence grey matter estimates, including in cerebellum(68). Moberget and colleagues(36) did report on harmful alcohol use. In analysis of a subset of their sample with no alcohol use, effect sizes increased marginally. Accordingly, this suggests that if harmful alcohol consumption did impact our findings, then the current work is likely underestimating the effects in our sample. Future work should seek to better characterize these effects using high resolution data on dose (frequency, volume, potency) and timing of use to clarify brain effects, including social determinants of substance use on cerebellar structure.
Second, the current work was restricted to structure and did not investigate cerebellar function. The cerebellum alone may contribute to some low-order cognitive processes(69), performing timing, prediction, and model updating(70). Stemming from these basic processes, a broader role for the cerebellum in higher-order processes lies in its ability to coordinate and regulate (through timing, predicting, and modeling) the cerebral regions that more directly perform high-order computations and outputs. For example, a compelling and growing literature in rodent models has shown that Purkinje cell firing rates in the Crus I region of the cerebellum modulate neural oscillations within hippocampus and PFC(71). In humans, non-invasive stimulation of the cerebellum has been shown to increase theta oscillations in frontal regions(72). In psychosis specifically, transcranial magnetic stimulation of the cerebellum has shown promise in reducing negative symptoms(40, 73). It remains unclear how anatomical changes reported here relate to cerebello-cerebral connectivity changes and associated symptom and behavior profiles.
Third, the lack of longitudinal data hindered a more nuanced investigation of the progressive nature of cerebellar aberrations in psychosis. Cross-sectionally, this study did not show effects of illness-stage. Prior work in samples with a longer course of illness have identified a degenerative effect on the cerebellum in schizophrenia(23). Investigating these questions in longitudinal samples that can track individual changes in cerebellum will be helpful to the field. Moreover, future work may seek to better characterize the cognitive subgroups, including the neuropsychologically normal and deteriorated groups. In the current study, the cognitively compromised group exhibited lower parental education compared to the other groups. An individual’s cognitive ability is highly related to parental cognitive ability and is also associated with critical social determinants, including socioeconomic status and education. While race was included in our estimates of cognitive ability, future work should use large scale, developmental datasets to parse the roles and interactions of education, socioeconomic status, race, and related social determinants in cognitive and cerebellar development, broadly and within the context of psychosis(cf. (74, 75)). Uncovering such risk and protective factors in neurodevelopment, as well as the association with onset and maintenance of psychosis will be critical for the development of targeted, large-scale prevention and intervention strategies and will require longitudinal designs.
Conclusions
The current study suggests that cerebellar aberrations are not tied to a specific diagnosis or the stage of illness alone. Rather, this work confirms the substantial heterogeneity within psychosis broadly and suggests that developmental factors, indexed here by premorbid cognitive disturbances, are key contributors to the cerebellar aberrations observed in psychosis. Future work would benefit from further parsing this heterogeneity and identifying risk and protective factors to aberrant cerebellar development. Moreover, the field will benefit from a clearer understanding of how specific cerebellar deficits contribute to key phenotypic profiles within psychosis and related disorders.
Supplementary Material
KEY RESOURCE TABLE
| Resource Type | Specific Reagent or Resource | Source or Reference | Identifiers | Additional Information |
|---|---|---|---|---|
| Add additional rows as needed for each resource type | Include species and sex when applicable. | Include name of manufacturer, company, repository, individual, or research lab. Include PMID or DOI for references; use “this paper” if new. | Include catalog numbers, stock numbers, database IDs or accession numbers, and/or RRIDs. RRIDs are highly encouraged; search for RRIDs at https://scicrunch.org/resources. | Include any additional information or notes if necessary. |
| Other | 3T Philips Intera Achieva Scanner | Philips Healthcare, Inc. | T1 Structural Image | |
| Software; Algorithm | Freesurfer 6 | PMID: 9931268; PMID: 11832223 | Pre-Processing; Cerebral Volumes | |
| Software; Algorithm | Statistical Parametric Mapping (SPM) | Wellcome Centre for Human Neuroimaging (https://www.fil.ion.ucl.ac.uk/spm/) | Volumetric and VBM Analyses | |
| Software; Algorithm | Computational Anatomy Toolbox v12 (Cat12 Toolbox) for SPM | Structural Brain Mapping Group (http://www.neuro.uni-jena.de/cat/) | VBM Analysis | |
| Software; Algorithm | Spatially Unbiased Intfratentorial (SUIT) Toolbox for SPM | PMID: 16904911; PMID: 26230510; PMID: 19457380; Diedrichsen Lab (https://www.diedrichsenlab.org/imaging/suit.htm) | Cerebellum-specific isolation and segmentation | |
| Software; Algorithm | MATLAB v2019a | Mathworks | Statistics and Figures | |
| Software; Algorithm | SPSS v28 | IBM | Statistics |
Acknowledgments
This work was supported by NIMH grants R01 MH102266 (NDW) and R01 MH070560 (SH), the Charlotte and Donald Test Fund, and the Vanderbilt Institute for Clinical and Translational Research (through grant 1-UL-1-TR000445 from the National Center for Research Resources/NIH). This work was conducted in part using the resources of the Center for Computational Imaging at Vanderbilt University Institute of Imaging Science, and the Advanced Computing Center for Research and Education at Vanderbilt University, Nashville, TN.
The authors wish to thank patients and their families for participation in this study. This work was conducted in part using the resources of the Advanced Computing Center for Research and Education at Vanderbilt University, Nashville, TN. We also acknowledge the contributions of the members of the Psychiatric Neuroimaging Program in the Vanderbilt University Medical Center Department of Psychiatry & Behavioral Sciences who collected the data used in this study, especially Kristan Armstrong, Molly Boyce, Erin Brosey, Victoria Fox, and Yasmeen Iqbal Neal.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors have no conflicts of interest or biomedical financial disclosures to report.
References
- 1.Stransky E (1904): Zur Lehre der dementia praecox. Zbl Nervenheilk. 27:1–19. [Google Scholar]
- 2.Andreasen NC, Pierson R (2008): The role of the cerebellum in schizophrenia. Biol Psychiatry. 64:81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andreasen NC, Nopoulos P, O’Leary DS, Miller DD, Wassink T, Flaum M (1999): Defining the phenotype of schizophrenia: cognitive dysmetria and its neural mechanisms. Biol Psychiatry. 46:908–920. [DOI] [PubMed] [Google Scholar]
- 4.Moberget T, Ivry RB (2016): Cerebellar contributions to motor control and language comprehension: searching for common computational principles. Ann N Y Acad Sci. 1369:154–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buckner RL (2013): The cerebellum and cognitive function: 25 years of insight from anatomy and neuroimaging. Neuron. 80:807–815. [DOI] [PubMed] [Google Scholar]
- 6.Sokolov AA (2018): The Cerebellum in Social Cognition. Frontiers in Cellular Neuroscience. 12.29440991 [Google Scholar]
- 7.Van Overwalle F, Manto M, Cattaneo Z, Clausi S, Ferrari C, Gabrieli JDE, et al. (2020): Consensus Paper: Cerebellum and Social Cognition. Cerebellum. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Timmann D, Drepper J, Frings M, Maschke M, Richter S, Gerwig M, et al. (2010): The human cerebellum contributes to motor, emotional and cognitive associative learning. A review. Cortex. 46:845–857. [DOI] [PubMed] [Google Scholar]
- 9.Schmahmann JD (2019): The cerebellum and cognition. Neurosci Lett. 688:62–75. [DOI] [PubMed] [Google Scholar]
- 10.Wang SS, Kloth AD, Badura A (2014): The cerebellum, sensitive periods, and autism. Neuron. 83:518–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.ten Donkelaar HJ, Lammens M, Wesseling P, Thijssen HO, Renier WO (2003): Development and developmental disorders of the human cerebellum. J Neurol. 250:1025–1036. [DOI] [PubMed] [Google Scholar]
- 12.Herculano-Houzel S (2011): Not all brains are made the same: new views on brain scaling in evolution. Brain Behav Evol. 78:22–36. [DOI] [PubMed] [Google Scholar]
- 13.Sereno MI, Diedrichsen J, Tachrount M, Testa-Silva G, d’Arceuil H, De Zeeuw C (2020): The human cerebellum has almost 80% of the surface area of the neocortex. Proc Natl Acad Sci U S A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhuo C, Wang C, Wang L, Guo X, Xu Q, Liu Y, et al. (2018): Altered resting-state functional connectivity of the cerebellum in schizophrenia. Brain Imaging Behav. 12:383–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim D-J, Moussa-Tooks AB, Bolbecker AR, Apthorp D, Newman SD, O’Donnell BF, et al. (2020): Cerebellar-cortical dysconnectivity in resting-state associated with sensorimotor tasks in schizophrenia. Human Brain Mapping. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He H, Luo C, Luo Y, Duan M, Yi Q, Biswal BB, et al. (2019): Reduction in gray matter of cerebellum in schizophrenia and its influence on static and dynamic connectivity. Human brain mapping. 40:517–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo W, Liu F, Chen J, Wu R, Zhang Z, Yu M, et al. (2015): Resting-state cerebellar-cerebral networks are differently affected in first-episode, drug-naive schizophrenia patients and unaffected siblings. Sci Rep. 5:17275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu H, Fan G, Xu K, Wang F (2011): Changes in cerebellar functional connectivity and anatomical connectivity in schizophrenia: a combined resting-state functional MRI and diffusion tensor imaging study. J Magn Reson Imaging. 34:1430–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Collin G, Hulshoff Pol HE, Haijma SV, Cahn W, Kahn RS, van den Heuvel MP (2011): Impaired cerebellar functional connectivity in schizophrenia patients and their healthy siblings. Front Psychiatry. 2:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moussa-Tooks AB, Kim D-J, Bartolomeo LA, Purcell JR, Bolbecker AR, Newman SD, et al. (2018): Impaired Effective Connectivity During a Cerebellar-Mediated Sensorimotor Synchronization Task in Schizophrenia. Schizophrenia Bulletin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lundin NB, Kim D-J, Tullar RL, Moussa-Tooks AB, Kent JS, Newman SD, et al. (2021):Cerebellar Activation Deficits in Schizophrenia During an Eyeblink Conditioning Task. Schizophrenia Bulletin Open. 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bernard JA, Mittal VA (2015): Dysfunctional Activation of the Cerebellum in Schizophrenia: A Functional Neuroimaging Meta-Analysis. Clin Psychol Sci. 3:545–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pantelis C, Yücel M, Wood SJ, Velakoulis D, Sun D, Berger G, et al. (2005): Structural brain imaging evidence for multiple pathological processes at different stages of brain development in schizophrenia. Schizophrenia bulletin. 31:672–696. [DOI] [PubMed] [Google Scholar]
- 24.Arango C, Moreno C, Martinez S, Parellada M, Desco M, Moreno D, et al. (2008): Longitudinal brain changes in early-onset psychosis. Schizophr Bull. 34:341–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laidi C, d’Albis MA, Wessa M, Linke J, Phillips ML, Delavest M, et al. (2015): Cerebellar volume in schizophrenia and bipolar I disorder with and without psychotic features. Acta Psychiatrica Scandinavica. 131:223–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mavroudis IA, Petrides F, Manani M, Chatzinikolaou F, Ciobica AS, Padurariu M, et al. (2017): Purkinje cells pathology in schizophrenia. A morphometric approach. Rom J Morphol Embryol. 58:419–424. [PubMed] [Google Scholar]
- 27.Stoodley CJ, MacMore JP, Makris N, Sherman JC, Schmahmann JD (2016): Location of lesion determines motor vs. cognitive consequences in patients with cerebellar stroke. Neuroimage Clin. 12:765–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ding Y, Ou Y, Pan P, Shan X, Chen J, Liu F, et al. (2019): Cerebellar structural and functional abnormalities in first-episode and drug-naive patients with schizophrenia: a meta-analysis. Psychiatry Research: Neuroimaging. 283:24–33. [DOI] [PubMed] [Google Scholar]
- 29.Mittal VA, Walker EF (2009): Movement abnormalities: a putative biomarker of risk for psychosis. The handbook of neuropsychiatric biomarkers, endophenotypes and genes: Springer, pp 239–258. [Google Scholar]
- 30.Ridler K, Veijola JM, Tanskanen P, Miettunen J, Chitnis X, Suckling J, et al. (2006): Fronto-cerebellar systems are associated with infant motor and adult executive functions in healthy adults but not in schizophrenia. Proc Natl Acad Sci U S A. 103:15651–15656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hirjak D, Wolf RC, Kubera KM, Stieltjes B, Maier-Hein KH, Thomann PA (2015): Neurological soft signs in recent-onset schizophrenia: Focus on the cerebellum. Prog Neuropsychopharmacol Biol Psychiatry. 60:18–25. [DOI] [PubMed] [Google Scholar]
- 32.Repovs G, Csernansky JG, Barch DM (2011): Brain network connectivity in individuals with schizophrenia and their siblings. Biol Psychiatry. 69:967–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moberget T, Alnaes D, Kaufmann T, Doan NT, Cordova-Palomera A, Norbom LB, et al. (2019): Cerebellar Gray Matter Volume Is Associated With Cognitive Function and Psychopathology in Adolescence. Biol Psychiatry. [DOI] [PubMed] [Google Scholar]
- 34.Kim T, Lee KH, Oh H, Lee TY, Cho KIK, Lee J, et al. (2018): Cerebellar Structural Abnormalities Associated With Cognitive Function in Patients With First-Episode Psychosis. Front Psychiatry. 9:286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dean DJ, Bernard JA, Orr JM, Pelletier-Baldelli A, Gupta T, Carol EE, et al. (2014): Cerebellar Morphology and Procedural Learning Impairment in Neuroleptic-Naive Youth at Ultrahigh Risk of Psychosis. Clin Psychol Sci. 2:152–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moberget T, Doan NT, Alnaes D, Kaufmann T, Cordova-Palomera A, Lagerberg TV, et al. (2018): Cerebellar volume and cerebellocerebral structural covariance in schizophrenia: a multisite megaanalysis of 983 patients and 1349 healthy controls. Mol Psychiatry. 23:1512–1520. [DOI] [PubMed] [Google Scholar]
- 37.Wolfers T, Doan NT, Kaufmann T, Alnaes D, Moberget T, Agartz I, et al. (2018): Mapping the Heterogeneous Phenotype of Schizophrenia and Bipolar Disorder Using Normative Models. JAMA Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Watson DR, Anderson JM, Bai F, Barrett SL, McGinnity TM, Mulholland CC, et al. (2012): A voxel based morphometry study investigating brain structural changes in first episode psychosis. Behav Brain Res. 227:91–99. [DOI] [PubMed] [Google Scholar]
- 39.Borgwardt S, McGuire P, Fusar-Poli P (2011): Gray matters!--mapping the transition to psychosis. Schizophr Res. 133:63–67. [DOI] [PubMed] [Google Scholar]
- 40.Brady RO Jr., Gonsalvez I, Lee I, Ongur D, Seidman LJ, Schmahmann JD, et al. (2019): Cerebellar-Prefrontal Network Connectivity and Negative Symptoms in Schizophrenia. Am J Psychiatry. appiajp201818040429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bernard JA, Mittal VA (2014): Cerebellar-motor dysfunction in schizophrenia and psychosis-risk: the importance of regional cerebellar analysis approaches. Front Psychiatry. 5:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Woodward ND, Heckers S (2015): Brain Structure in Neuropsychologically Defined Subgroups of Schizophrenia and Psychotic Bipolar Disorder. Schizophr Bull. 41:1349–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.First M, Spitzer R, Gibbon M, Williams J (2002): Structured clinical interview for DSM-IV-TR axis I disorders, research version, patient edition (SCID-I/P). New York: Biometrics Research. [Google Scholar]
- 44.Birchwood M, Todd P, Jackson C (1998): Early intervention in psychosis: the critical period hypothesis. The British journal of psychiatry. 172:53–59. [PubMed] [Google Scholar]
- 45.Kay SR, Fiszbein A, Opler LA (1987): The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 13:261–276. [DOI] [PubMed] [Google Scholar]
- 46.Young RC, Biggs JT, Ziegler VE, Meyer DA (1978): A rating scale for mania: reliability, validity and sensitivity. The British journal of psychiatry. 133:429–435. [DOI] [PubMed] [Google Scholar]
- 47.Hamilton M (1960): The Hamilton Depression Scale—accelerator or break on antidepressant drug discovery. Psychiatry. 23:56–62. [DOI] [PubMed] [Google Scholar]
- 48.Wechsler D (2001): Wechsler Test of Adult Reading: WTAR. Psychological Corporation. [Google Scholar]
- 49.Purdon SE (2005): The screen for cognitive impairment in psychiatry (SCIP): administration manual and normative data. Edmonton, Alberta: PNL Inc. [Google Scholar]
- 50.Huo Y, Blaber J, Damon SM, Boyd BD, Bao S, Parvathaneni P, et al. (2018): Towards Portable Large-Scale Image Processing with High-Performance Computing. J Digit Imaging. 31:304–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Harrigan RL, Yvernault BC, Boyd BD, Damon SM, Gibney KD, Conrad BN, et al. (2016): Vanderbilt University Institute of Imaging Science Center for Computational Imaging XNAT: A multimodal data archive and processing environment. Neuroimage. 124:1097–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sochat VV, Prybol CJ, Kurtzer GM (2017): Enhancing reproducibility in scientific computing: Metrics and registry for Singularity containers. PLoS One. 12:e0188511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sheffield JM, Rogers BP, Blackford JU, Heckers S, Woodward ND (2020): Insula functional connectivity in schizophrenia. Schizophr Res. 220:69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Diedrichsen J (2006): A spatially unbiased atlas template of the human cerebellum. Neuroimage. 33:127–138. [DOI] [PubMed] [Google Scholar]
- 55.Diedrichsen J, Zotow E (2015): Surface-Based Display of Volume-Averaged Cerebellar Imaging Data. PLoS One. 10:e0133402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Diedrichsen J, Balsters JH, Flavell J, Cussans E, Ramnani N (2009): A probabilistic MR atlas of the human cerebellum. Neuroimage. 46:39–46. [DOI] [PubMed] [Google Scholar]
- 57.Buckner RL, Krienen FM, Castellanos A, Diaz JC, Yeo BT (2011): The organization of the human cerebellum estimated by intrinsic functional connectivity. J Neurophysiol. 106:2322–2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weickert TW, Goldberg TE, Gold JM, Bigelow LB, Egan MF, Weinberger DR (2000): Cognitive impairments in patients with schizophrenia displaying preserved and compromised intellect. Archives of general psychiatry. 57:907–913. [DOI] [PubMed] [Google Scholar]
- 59.Czepielewski LS, Wang L, Gama CS, Barch DM (2016): The Relationship of Intellectual Functioning and Cognitive Performance to Brain Structure in Schizophrenia. Schizophrenia Bulletin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mittal VA, Walker EF (2007): Movement abnormalities predict conversion to Axis I psychosis among prodromal adolescents. J Abnorm Psychol. 116:796–803. [DOI] [PubMed] [Google Scholar]
- 61.Tiemeier H, Lenroot RK, Greenstein DK, Tran L, Pierson R, Giedd JN (2010): Cerebellum development during childhood and adolescence: a longitudinal morphometric MRI study. Neuroimage. 49:63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang VY, Zoghbi HY (2001): Genetic regulation of cerebellar development. Nature Reviews Neuroscience. 2:484–491. [DOI] [PubMed] [Google Scholar]
- 63.Romero JE, Coupe P, Lanuza E, Catheline G, Manjón JV (2021): Toward a unified analysis of cerebellum maturation and aging across the entire lifespan: A MRI analysis. Human Brain Mapping. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moulton EA, Elman I, Becerra LR, Goldstein RZ, Borsook D (2014): The cerebellum and addiction: insights gained from neuroimaging research. Addiction biology. 19:317–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lisdahl KM, Thayer R, Squeglia LM, McQueeny TM, Tapert SF (2013): Recent binge drinking predicts smaller cerebellar volumes in adolescents. Psychiatry Research: Neuroimaging. 211:17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Medina KL, Nagel BJ, Tapert SF (2010): Abnormal cerebellar morphometry in abstinent adolescent marijuana users. Psychiatry Research: Neuroimaging. 182:152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Varnäs K, Okugawa G, Hammarberg A, Nesvåg R, Rimol LM, Franck J, et al. (2007): Cerebellar volumes in men with schizophrenia and alcohol dependence. Psychiatry and clinical neurosciences. 61:326–329. [DOI] [PubMed] [Google Scholar]
- 68.Quinn M, McHugo M, Armstrong K, Woodward N, Blackford J, Heckers S (2018): Impact of substance use disorder on gray matter volume in schizophrenia. Psychiatry Res Neuroimaging. 280:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schmahmann JD, Guell X, Stoodley CJ, Halko MA (2019): The Theory and Neuroscience of Cerebellar Cognition. Annu Rev Neurosci. 42:337–364. [DOI] [PubMed] [Google Scholar]
- 70.Moberget T, Ivry RB (2019): Prediction, Psychosis and the Cerebellum. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McAfee SS, Liu Y, Sillitoe RV, Heck DH (2019): Cerebellar Lobulus Simplex and Crus I Differentially Represent Phase and Phase Difference of Prefrontal Cortical and Hippocampal Oscillations. Cell Rep. 27:2328-2334 e2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Singh A, Trapp NT, De Corte B, Cao S, Kingyon J, Boes AD, et al. (2019): Cerebellar Theta Frequency Transcranial Pulsed Stimulation Increases Frontal Theta Oscillations in Patients with Schizophrenia. Cerebellum. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhu L, Zhang W, Zhu Y, Mu X, Zhang Q, Wang Y, et al. (2021): Cerebellar Theta Burst Stimulation for the Treatment of Negative Symptoms of Schizophrenia: A Multicenter, Double-blind, Randomized Controlled Trial. Psychiatry Research. [DOI] [PubMed] [Google Scholar]
- 74.Assari S, Boyce S (2021): Race, Socioeconomic Status, and Cerebellum Cortex Fractional Anisotropy in Pre-Adolescents. Adolescents. 1:70–94. [DOI] [PubMed] [Google Scholar]
- 75.Anglin DM, Ereshefsky S, Klaunig MJ, Bridgwater MA, Niendam TA, Ellman LM, et al. (2021): From Womb to Neighborhood: A Racial Analysis of Social Determinants of Psychosis in the United States. Am J Psychiatry.appiajp202020071091. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


