Abstract
Primary tumors evolve metabolic mechanisms favoring glycolysis for ATP generation and antioxidant defenses. In contrast, metastatic cells frequently depend on mitochondrial respiration and oxidative phosphorylation (OxPhos). This reliance of metastatic cells on OxPhos can be exploited using drugs that target mitochondrial metabolism. Therefore, therapeutic agents that act via diverse mechanisms, including the activation of signaling pathways that promote the production of reactive oxygen species (ROS) and/or a reduction in antioxidant defenses may elevate oxidative stress and inhibit tumor cell survival. In this review, we will provide (1) a mechanistic analysis of function-selective extracellular signal-regulated kinase-1/2 (ERK1/2) inhibitors that inhibit cancer cells through enhanced ROS, (2) a review of the role of mitochondrial ATP synthase in redox regulation and drug resistance, (3) a rationale for inhibiting ERK signaling and mitochondrial OxPhos towards the therapeutic goal of reducing tumor metastasis and treatment resistance. Recent reports from our laboratories using metastatic melanoma and breast cancer models have shown the pre-clinical efficacy of novel and rationally designed therapeutic agents that target ERK1/2 signaling and mitochondrial ATP synthase, which modulate ROS events that may prevent or treat metastatic cancer. These findings and those of others suggest that targeting a tumor’s metabolic requirements and vulnerabilities may inhibit metastatic pathways and tumor growth. Approaches that exploit the ability of therapeutic agents to alter oxidative balance in tumor cells may be selective for cancer cells and may ultimately have an impact on clinical efficacy and safety. Elucidating the translational potential of metabolic targeting could lead to the discovery of new approaches for treatment of metastatic cancer.
Keywords: cancer metastasis, drug mechanisms, reactive oxygen species, mitochondria, targeting OxPhos, kinase signaling
INTRODUCTION
Cancer is the leading cause of death for men and women ages 40-79 and in 2022 an estimated 1,918,030 new cases of cancer and 609,360 cancer deaths are projected to occur in the United States1. Incidence for breast cancer in women has increased by about 0.5% per year from 2014-2018. There are also more than 3 million survivors in the U.S. with about 80% of them being post-menopausal2. Since breast cancer is a major public health issue, identifying new non-toxic metastasis-prevention agents would be highly significant. Melanoma of the skin is a highly metastatic cancer that is expected to affect almost 57,100 men and 42,000 women in 2022 accounting for 6% and 5% of the total cancer incidence, respectively. Metabolic changes in tumor cells result from onco/suppressor genetic events that activate specific signaling and transcriptional networks. Discovering the molecular connections between mitochondria and metastasis will determine whether inhibitors of mitochondrial OxPhos could have therapeutic impact for cancer treatment. Understanding how kinase signaling and metabolic pathways cooperate in promoting tumor progression will lead to discovery of novel combination approaches to increase efficacy and lower toxicity. Work in this area is significant and innovative because a focus on mechanisms of mitochondrial redox regulation, which include inhibition of ATP synthase, could reveal more effective therapeutic targets. This could eventually provide a rationale for translational and preclinical development of novel compounds to prevent or treat metastatic and drug-resistant tumors3-5. The goal of this review is to provide an overview of signaling and cancer metabolism pathways as they relate to metastatic cells and how metabolic regulation of glycolytic and mitochondrial OxPhos pathways play a role in survival and spread of metastatic cancers and their acquisition of treatment resistance. Mitochondrial generation of ATP and oxidative stress are two areas of therapeutic intervention. In particular, the targeting of kinase signaling pathways to increase reactive oxygen species (ROS) or directly targeting the production of ATP with ATP synthase inhibitors to generate ROS are two strategies with potential promise to alter cellular bioenergetics. Specific recent examples will be highlighted to show that inhibiting mitochondrial OxPhos and ERK1/2 signaling may increase ROS levels to compromise metastatic tumor cell survival.
Cancer treatments are changing the lives of people with primary breast cancer6-8. However, metastatic breast cancer is a continuing public health problem8,9. The difficulties in managing metastatic cancer and drug resistance are the motivation for designing new and effective treatments. Many hormone-responsive breast cancers are treatable with selective estrogen receptor modulators (SERMs) or aromatase inhibitors, which reduce estrogen levels. But treating metastatic breast cancer, especially triple-negative breast cancers (TNBC) that lack estrogen receptor, progesterone receptor, and HER2 expression, is currently less effective5,10. Approximately half of all melanomas express mutations in the BRAF gene, which leads to constitutive activation of the downstream MEK1/2 and ERK1/2 proteins that drive cancer cell survival. While targeted inhibition of mutated BRAF showed short term efficacy in treating late-stage metastatic melanoma, current clinical practice includes use of BRAF plus MEK1/2 inhibitors and/or immunotherapy approaches for late-stage metastatic melanoma11,12. For example, BRAF inhibitors – vemurafenib (Zelboraf), dabrafenib (Tafinlar) or encorafenib (Braftovi) and MAPK kinase (MEK) inhibitors – trametinib (Mekinist), cobimetinib (Cotellic) or binimetinib (Mektovi) are standard of care for treating advanced melanoma that has spread to other parts of the body11,12. In 2020, the FDA designated the ERK1/2 inhibitor ulixertinib available for the Expanded Access Program to treat cancers with aberrant ERK1/2 activity11,12. Trametinib is indicated in combination with dabrafenib after surgery as adjuvant therapy for stage III melanoma patients. Combination approaches may have several advantages, including reduced toxicity because of the use of lower individual drug levels and reduced risk of cancer recurrence and treatment resistance, resulting in more durable responses. While BRAF and MEK inhibitors transiently reduce metastatic burden and extend patient survival by a few months, most patients eventually develop acquired resistance to these therapies.
Evidence for reactivation of ERK1/2 in patients with resistance to BRAF and MEK inhibitors has promoted the development of the previously mentioned ulixertinib and other selective ERK1/2 inhibitors to improve therapeutic outcomes in melanoma or other cancers driven by constitutive ERK1/2 activation13. In addition, increased dependence on OxPhos is one reason for the development of acquired resistance to BRAF and MEK inhibitors14.
Over the last several years, a focus on the Warburg effect has diverted attention from the role of mitochondrial respiration in tumor progression. However, the premise and rationale for targeting metastatic cancer metabolism depends on the observations that metastatic tumors exhibit active mitochondrial OxPhos, which plays a central role in generation of ROS and cell death, survival, or metastasis15-18. Redox balance is regulated by the very high glucose uptake evident in tumor cells and the concomitant generation of TCA cycle metabolites that feed electrons into the mitochondrial electron transport chain (ETC). Primary cancers, including melanoma and breast cancers, are often treatable via a variety of interventions including surgery19. However, metastatic tumors, such as TNBC or melanoma with BRAF or NRAS mutations, are often resistant to treatment and may lack effective targeted therapies20. Metastatic cells in the circulation and cells homing to the metastatic site may depend on mitochondrial OxPhos for their energy needs5,10 because the ETC and mitochondrial adenosine triphosphate (ATP) synthase promote OxPhos and play a central role in regulation of cancer cell oxidative damage, growth, and survival21,22. Metastatic cells under hypoxic conditions exhibit functional OxPhos with variable glycolytic activity to promote a hybrid metabolic state15 that supports metastatic cell survival23. Additionally, kinase signaling pathways that include ERK1/2, phosphoinositide 3-kinase/protein kinase B (PI3K/Akt), and the Janus kinases (JAK) play a central role in linear (vertical) and crosstalk (horizontal)-dependent metabolic plasticity7. Active mitochondrial function and cell differentiation can reduce cell type evolution (heterogeneity), inhibit drug resistance, and prevent cancer initiation. However, in tumor cells mitochondrial reprogramming5,15,24-28 or constitutive activation of intracellular kinase signaling or both7,29-33 may promote metastatic progression. Therefore, inhibition of these pathways may restrict tumor cell proliferation or promote cell death through feedback inhibition of respiratory capacity, increased ROS, and lower glucose utilization. Our recent published data34-36 support the hypothesis that mitochondrial OxPhos and elevation of ROS are important drivers of oncogenesis. Partial inhibition of MAPK (ERK1/2) signaling or targeting mitochondrial ATP generation via ATP synthase targeting can disrupt the redox balance in tumor cells. Strategies to improve therapeutic efficacy by inhibiting glucose uptake, mitochondrial OxPhos, and anaerobic (glycolytic) metabolism may, therefore, be promising approaches to prevent or treat metastatic progression37,38.
CANCER METABOLISM IN METASTATIC CELLS
Tumor metabolic heterogeneity reflects the tissue of origin, oncogenic events that support specific survival or proliferation, and changes in the microenvironment of the tumor that promote an epithelial mesenchymal transition (EMT) and tumor progression39. A diversity of cells in the microenvironment of a tumor, including fibroblasts, tumor-associated macrophages, and adipocytes provide metabolic intermediates and nutrients that drive bioenergetics and escape from cell death signaling pathways. Because of the complexities that regulate metastatic spread of a tumor, there is high heterogeneity in drug response among different patients diagnosed with the same cancers. Understanding the metabolic mechanisms that regulate metastasis would, therefore, provide valuable information about precision medicine strategies to prevent or treat metastases. Glycolysis does play an important role in tumorigenesis because separately knocking down several glycolysis-regulating genes (HMMR, KIF20A, PGM2L1, and ANKZF1) resulted in less glucose consumption, less lactic acid production, and reduced cell migration and invasion of prostate cancer cells40. A risk score based on glycolysis-related genes may serve as an accurate prognostic marker for prostate cancer patients with biochemical recurrence. For these reasons, most research in recent years has focused on glycolytic pathways driving tumor growth (Warburg effect). However, there is still considerable interest in the “reverse Warburg” effect, which involves the capture of glycolytic lactate by cancer cells from adjacent stromal cells that is converted to pyruvate, which enters the TCA cycle and provides electrons for the mitochondrial ETC and ATP generation (OxPhos)15,39. Because of the complex tumor microenvironment, lactate can also be derived from fibroblasts, tumor cells or both.
Glycolysis-driven lactate dehydrogenase (LDH) can stimulate ROS production from mitochondria. In fact, the role of lactate in promoting metastasis is well-documented41-43. Lactate can regulate immunosuppression in the tumor microenvironment44 and lactate transporters like monocarboxylate transporter (MCT1-4) can promote MCT-dependent cancer metastasis45. One inhibitor of MCT1 (AZD3965) is currently under evaluation in clinical trials46. For these reasons, MCT1 inhibitors are currently in development to reduce lactate transport and enhance radiosensitivity47. Hexokinases (HKs), which mediate the utilization of glucose, are present in the cytoplasm where they phosphorylate intracellular glucose, the first rate-limiting step of glycolysis48,49. Of the four HK subtypes (encoded by different genes), HK1 is expressed in normal tissues while HK2 is highly expressed in cancer cells and facilitates chemoresistance and metastasis of hepatocellular, colorectal, lung, gastric, melanoma, and ovarian cancers50-57. HK2 can translocate from the cytoplasm to the outer mitochondrial membrane and interact with the voltage-dependent anion channel (VDAC) to promote resistance to chemotherapy by inhibiting apoptosis. Mitochondrial-associated HK2 (Mito-HK2) is also in close proximity to mitochondrial ATP production by ATP synthase and may prevent cell death by multiple mechanisms58. Mito-HK2 promotes survival by inhibiting mitochondrial permeabilization and the consequent release of pro-apoptotic cytochrome c59. The suppressive mechanism involved appears to include the inhibition of ROS accumulation51. Another anti-apoptotic mechanism includes HK2 inhibition of Bcl2/BAX association, which releases BAX from the mitochondria and promotes Bcl2 inhibition of apoptosis60. Mito-HK2 is also involved in regulating prostate cancer metabolic activities61,62. HK2, together with these interacting proteins, plays an important role in maintaining cancer cell malignancy and metastasis although no HK2 inhibitors have as yet shown efficacy in clinical trials.
Current translational medicine approaches targeting metabolism63, including mitochondrial metabolism, are beginning to have an impact on clinical therapeutics64-66. Recent findings indicate that glycolysis and mitochondrial OxPhos cooperate under hypoxic and nutrient-deprived selective pressures to promote drug resistance and fuel the metastatic potential of tumor cells and circulating metastatic cells that rely on mitochondrial OxPhos5,10. Therefore, mitochondria are an important therapeutic target in cancer24 because uncoupling mitochondrial membrane potential (MMP) from ATP production has been shown to inhibit glucose uptake, OxPhos, and also anaerobic (glycolytic) metabolism that support a metastatic phenotype24,37,38. A major regulator of mitochondrial respiration, or oxygen consumption rate (OCR), is the ATP synthase, which drives mitochondrial OxPhos in the presence or absence of glycolysis15,67. ATP synthase is also important in ATP production in tumor cells characterized by high glucose uptake under hypoxic conditions (a classic Warburg effect), suggesting cooperative tumorigenic effects of glycolysis and mitochondrial OxPhos15,23. In fact, a subgroup of breast cancer patients with significantly worse prognosis was identified on the basis of higher ATP synthase expression68. Drug-resistant metastatic tumors, such as triple negative breast cancers (TNBC), are often dependent on mitochondrial respiration (OxPhos) to generate energy and promote survival. Since there are no targeted therapies for TNBC and since most mitochondrial-targeting drugs exhibit substantial toxicity, there is a need to find new and safer therapeutic agents.
Mitochondrial dysfunction may be associated with increased cancer incidence and poor outcomes in select populations69. African American patients have higher cancer mortality rates and shorter survival times relative to European American patients70. Mitochondrial OxPhos and its dysfunction have been linked to racial disparity for cancer risk and survival of African American patients with prostate cancer71. A set of genes upregulated in several tumors was associated with enhanced OxPhos, transcription factors that promote mitochondrial biogenesis, and increased mitochondrial number in these patients72. By measuring mitochondrial content in patient tissues using a multi-cancer tissue microarray approach (TMA), OxPhos genes were found to be enriched in tumors from African American patients72. These tumors also exhibited enrichment for ERR1-PGC1α–mediated transcriptional programs that are associated with mitochondrial biogenesis. Changes in mitochondria may, therefore, be a distinguishing feature among tumors from African American patients and provide a rationale for repurposing mitochondrial inhibitors to treat these cancers73.
Mitochondrial ATP production has been a therapeutic target for cancer treatment for many years25. Cancer cells with high ATP levels are aggressive and exhibit multi-drug resistance, invasiveness, and spontaneous metastasis. Therefore, cancer cells exhibiting enhanced bioenergetics may resist environmental or chemotherapeutic pressure while simultaneously maintaining survival because of their increased ATP content, which allows for tumor recurrence and metastatic progression. Drug treatments that target mitochondrial ATP synthase and/or induce mitochondrial uncoupling (such as Bedaquiline or Niclosamide) have shown some promise in depleting cellular ATP and inhibiting metastasis25,74. New approaches to control cancer metabolism are also beginning to take into consideration the role of the tumor immune microenvironment in regulating metastasis75. As these cell-intrinsic metabolic pathways are defined, the role of the immune system in regulating metastatic metabolism76 and exploiting the metabolic vulnerabilities of normal and cancer cells to control metastasis may also be elucidated63.
MITOCHONDRIAL OXPHOS AND ATP SYNTHASE – IMPLICATIONS for METASTASIS
Production of ATP can promote tumor cell drug resistance and metastasis25, which has implications for therapeutic approaches that lower mitochondrial ATP levels. In fact, high levels of ATP in the extracellular milieu activates hypoxia-inducible factors, which are associated with cancer progression, metastasis, poor prognosis, and resistance to chemotherapy77. Inhibiting ATP synthase to reduce ATP levels can generate increased oxidative stress resulting from reduced antioxidant levels and lower rates of respiration (or oxygen consumption rates)25,35 and may have benefits for treating tumor growth and preventing metastasis. However, for mitochondrial OxPhos analysis in response to therapeutic drugs several caveats need to be considered78,79, including cellular energy availability, ATP production, glycolysis, extracellular acidification (ECAR) levels, energy partitioning, and mitochondrial dysfunction, which are essential in the design of anti-metastatic approaches for cancer therapy80. These considerations may be relevant since there are considerable differences in the response of primary and metastatic tumors to therapeutic drugs81. Inhibiting ATP synthesis and OxPhos may affect the survival of respiration-competent metastatic cancer cells25. We recently showed35 that targeting breast cancer metabolism with a novel inhibitor of mitochondrial ATP synthase may have therapeutic potential for breast cancer treatment by targeting mitochondrial OxPhos. Using a direct drug discovery approach and computer-assisted drug design (CADD), we identified novel small molecules that interfere with transcription factor Runx2 protein-DNA binding and transcriptional activity82. While normal epithelial cells were relatively resistant to these molecules, a lead compound (CADD522) inhibited breast cancer cell proliferation and tumorsphere formation and inhibited mitochondrial ATP synthase and respiration (oxygen consumption) while increasing ROS35. CADD522, was shown to not only reduce primary tumor growth in vivo but also to reduce experimental metastasis of tumor cells in the lungs of immune-compromised mice after tail-vein injection82. This compound lowered mitochondrial oxygen consumption rates (OCR) and ATP levels in human breast cancer cells. The inhibition of mitochondrial OxPhos resulted from the direct targeting of ATP synthase enzymatic activity. A key observation providing a clue to the mechanisms essential for these activities was the demonstration that the CADD522 compound inhibits mitochondrial respiration OCR35 and maximum respiratory capacity (MRC)83. Mitochondrial respiratory parameters in the future may be a more accurate depiction of metastatic capacity of tumor cells26,78,84,85. To measure ATP levels, MCF7 or MDA468 breast cancer cells were treated with CADD522 under conditions (presence of pyruvate or galactose) where cellular metabolism favors mitochondrial OxPhos-driven ATP production35. CADD522 lowered the levels of ATP under these conditions. Associated with this clear suppression of ATP production was the related increase in intracellular ROS levels. Results indicate that some of the ROS stimulated by CADD522 may be derived from mitochondria, which are implicated in tumor metastasis85.
CADD522 also inhibited ATP synthase enzymatic in a dose-dependent manner and interacted with specific ATP synthase subunits35, suggesting that lower ATP levels in response to CADD522 could be due to direct inhibition of mitochondrial ATP synthase activity, the central regulator of cell bioenergetic activity of the cell21,86. These results suggest that the effects of CADD522 involve its interaction with subunits of the F1-ATP synthase complex (Figure 1)86. Many metabolic poisons have been found to interact with mitochondrial ATP synthase and the enzyme complex has been the target of numerous approaches86 to inhibit cancer metabolism87. However, many of the approaches targeting the Fo-subunits of the ATP synthase (such as oligomycin) are too toxic for clinical use88,89. Whereas drugs that interact with and inhibit the F1-ATP synthase subunits (such as CADD522, metformin, resveratrol)86,90 have more favorable toxicity profiles and may be of particular interest in treating metastatic cancers. With regard to toxicity, the CADD522 has so far been shown to reduce tumor cell growth but not induce apoptosis35,82. In fact, normal epithelial cells and non-transformed breast epithelial (MCF10A) cells grown in 3D cultures were largely unaffected by CADD52235.
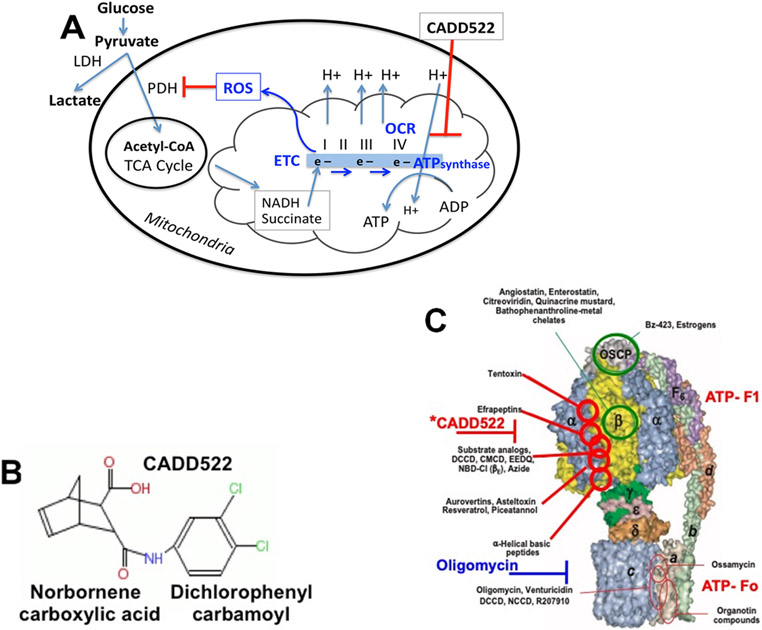
Figure 1. (A) ATP synthase, respiration (OCR), and ROS production.
ATP synthase plays a key role in mitochondrial ATP production, glucose oxidation, OCR, and ROS production. This regulation has implications for stem cell maintenance, drug resistance, and metastasis. We found a small molecule inhibitor of the Runx2 transcription factor (CADD522) that also inhibits ATP synthase leading to lower OCR and elevated ROS, inhibited PDH and TCA cycle glucose flux, and inhibited electron transport. (B) Lead Compound = fusion of groups found in nature: plant-derived core structures norbornene and dichlorophenyl identified by computer-assisted drug design (CADD). ETC, electron (e−) transport chain; OCR, oxygen consumption rate; TCA, tricarboxylic acid cycle; NADH, succinate, major electron donors; ETC Complex IV, electron acceptor (O2); PDH, pyruvate dehydrogenase. (C) Inhibitory sites of ATP synthase and putative (*) CADD522 interaction site. ATP synthase inhibitors act as scaffolds for new drug development because of their conserved function in multiple species (modified from Hong 2008)86. Some drugs (Oligomycin) inhibit ATP synthase activity (ATP production coupled to proton flux) because they block proton (H+) translocation across the mitochondrial inner membrane (Fo subunits). Rotation of the central stalk against the surrounding alpha(3)beta(3) F1-subunits leads to synthesis of ATP at three separate catalytic domains on the beta subunits. Many F1-complex-targeted drugs interact with alpha/beta sites. Cell-free mitochondrial lysates do not exhibit proton-coupled or Fo-dependent enzymatic activity, which resides on the F1 complex of subunits (the alpha/beta interaction sites) inhibited by CADD522.
Previous reports have shown that the general ROS scavenger N-acetyl cysteine (NAC) may provide protection against cytotoxic oxidants91,92 and inhibit apoptosis93 induced by ROS. Inhibition of ATP synthase by CADD522 enhanced ROS levels under conditions of oxidative stress and may be one explanation for its anti-tumor effects35,94. NAC was found to neutralize some of the CADD522-stimulated ROS in breast cancer cells and NAC attenuated the inhibitory effect of CADD522 on breast cancer cell growth. Expression of the catalytic subunit ß-F1-ATP synthase is tightly regulated by post-transcriptional signaling mechanisms that affect mRNA localization, stability, and translation95-99. CADD522 treated tumor cells may exhibit gene expression changes in response to this pro-oxidant molecule. Consistent with its effect on ATP synthase, the mRNA level of MT-ATP5B (mitochondrial ATP synthase F1 subunit beta) was markedly decreased by CADD522 treatment in breast cancer cells35 and it significantly inhibited expression of several mitochondrial biogenesis-related genes examined (PGC-1α, NRF-1, HRF2b, TFAM, TFB2M). Notably, the mRNA expression level of the key mitochondrial biogenesis-promoting transcription factor PGC-1α was almost completely suppressed by CADD52235. These changes in gene expression are commonly observed after treatment with drugs that target mitochondria in metastatic tumor cells100,101. This is also observed for mitochondrial targeted therapies to treat tubular cell acute kidney injury102, multi-targeted metabolic therapy for cancer that activates multi-gene pathways103, targeting mitochondrial malignancy and ROS generation for cancer therapy104, and targeting the redox imbalance in mitochondria to increase ROS to selectively inhibit cancer cells through altered gene expression promoting apoptosis, autophagy, and necrosis thus inhibiting their metastatic potential105. In summary, our data suggest that inhibition of mitochondrial ATP synthase and ROS generation are contributors to the effectiveness of CADD522 to delay tumor growth, metastasis, and cancer progression.
MAPK SIGNALING, REDOX REGULATION, AND THERAPEUTIC STRATEGIES
Mitogen-activated protein kinase (MAPK) family proteins, including ERK1/2, regulate and link intra- and extracellular signaling events to mediate changes in cellular gene expression, proliferation, and survival. Aberrant ERK1/2 activation in cancer cells is due to upstream mutations or overexpression of receptor tyrosine kinases, RAS G-proteins, and RAF kinases106-110. Mutations in MEK1, a direct activator of the ERK1/2 proteins, may also deregulate ERK1/2 activity111. In addition, an ERK2 mutation that changes a conserved glutamate to lysine (E322K) within a protein docking domain causes constitutive ERK2 activation and promotes cutaneous T-cell lymphoma progression112-114. Typically, anticancer drugs that target ERK1/2 signaling block the ATP binding or catalytic sites on receptor tyrosine kinases or serine/threonine kinases like BRAF and MEK1/2. These compounds are effective in blocking ERK1/2 signaling115-118. However, because ATP binding sites are conserved in many protein kinases, this strategy often results in off-target toxicity and the invariable development of acquired drug resistance119,120. Cellular ROS and oxidative stress play a confounding role in both promoting or inhibiting cancer cell proliferation, depending on the cellular context. There are several examples that demonstrate increased or decreased oxidative stress will sensitize cancer cells to growth inhibition and cell death85,121. Much research has supported the use of antioxidant strategies to protect against some cancers121. However, clinical trials have yet to show conclusive evidence for the beneficial effects of antioxidant supplements to reduce cancer risk122. In the context of the ERK1/2 pathway, antioxidants like NAC and vitamin E may even promote metastasis in lung cancer with KRAS mutations123. This mechanism is thought to occur through the stabilization of the transcription factor BACH1, which regulates the expression of genes involved in oxidative phosphorylation, EMT, and metastasis. In contrast, many anticancer drugs, including ERK1/2 pathway inhibitors, promote tumor cell death by increasing oxidative stress121. Melanocytes and melanoma cells are particularly sensitive to ROS and subtle changes in ROS levels can influence proliferation and survival of these cells124. Moreover, there is evidence to suggest that increased oxidative stress may inhibit melanoma cell metastasis92. Even if ROS elevation by individual therapeutic agents may not be sufficient to inhibit all cancer cells, enhanced oxidative stress may improve anticancer efficacy of combination therapies125. Recent data show that therapeutic approaches that increase ROS may benefit patients who have developed resistance to BRAF and MEK1/2 inhibitors126.
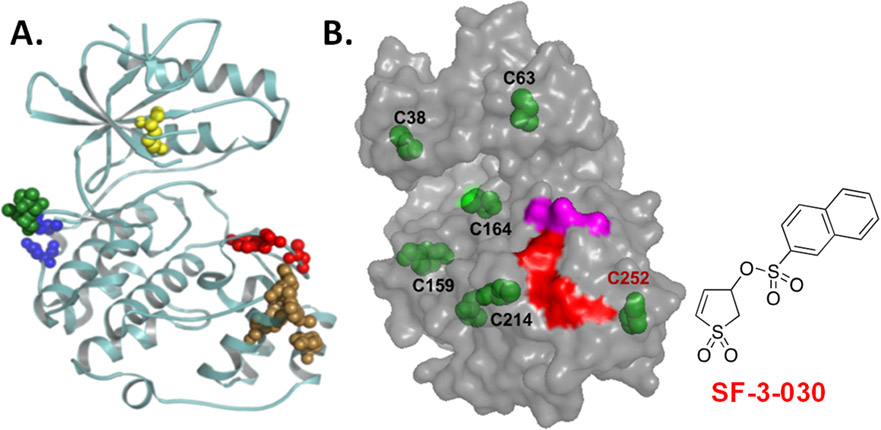
As an alternative to ATP/catalytic site inhibitors of protein kinases, we have used computer-aided drug design (CADD) to identify function-selective ERK1/2 inhibitors that disrupt ERK1/2 regulation of select substrate proteins127-130. Subsequent studies identified a novel compound, SF-3-030, that forms a covalent interaction with a cysteine residue near the F-recruitment site (FRS) on ERK1/2 that is distinct from the D-domain recruitment site (DRS) involved in interactions with MEK1/2 (Figure 2). The FRS on ERK2 facilitates interactions with substrates containing an F-site (FXF motif) that include the FOS family of transcription factors and c-Myc. Consistent with ATP competitive ERK1/2 inhibitors, SF-3-030 was more effective at inhibiting the proliferation of cancer cell lines containing activating mutations in the ERK1/2 pathway and it inhibited mutant BRAF expressing melanoma cell proliferation by increasing ROS production34.
Figure 2.
(A) Ribbon structure of ERK2 (PDB:4GT3) highlights substrate docking sites. The D-domain recruitment site (DRS) or F-recruitment site (FRS) are indicated by blue/green or brown spheres, respectively. The conserved lysine found in the ATP binding site and the activation site are the yellow and red spheres, respectively. Unlike ATP binding/catalytic site inhibitors that block all kinase activity, function-selective ERK1/2 inhibitors target a select group of substrates that interact with the DRS or FRS. (B) SF-3-030 forms a cysteine adduct near the FRS. Model of ERK2 shows the position of all cysteine residues (green). High-resolution LC-MS identified C252 (red font) to be the primary (90%) residue modified by SF-3-030. The adjacent FRS (red) and activating TXY motif (magenta) are shown for reference.
In support of its mechanism in disrupting ERK1/2 interactions at the FRS, SF-3-030 inhibited epidermal growth factor (EGF) or phorbol ester (PMA) induction of immediate early genes of the FOS family (c-Fos, FosB, and Fra-1) and c-Myc in HeLa cells130. In addition, SF-3-030 inhibited Activator Protein-1 (AP-1)-mediated transcription in HeLa cells treated with EGF or PMA and in A375 melanoma cells expressing a constitutively active BRAF (V600E) mutation34,130. AP-1 has been implicated in regulating the expression of genes that promote cell invasion and metastasis131. Comparing the effects SF-3-030 with ATP-competitive/catalytic site inhibitors of MEK1/2 and ERK1/2 in A375 cells containing activating BRAF and Ras mutations provided additional clues to the molecular mechanisms mediating the biological effects of SF-3-030. Transcriptome analysis by RNAseq comparing A375 cells treated with either SF-3-030 or the MEK inhibitor AZD6244 (positive control for ERK1/2 pathway inhibition) revealed overlap between SF-3-030 and AZD6244-regulated genes. A key finding was that both SF-3-030 and AZD6244 inhibited c-Myc levels at both the RNA and protein levels suggesting common effects of these compounds on ERK1/2-mediated signaling. In non-transformed airway smooth muscle cells, we have shown that SF-3-030 inhibits transforming growth factor-beta (TGFß) signaling132, which promotes cell invasion and metastasis133. Although SF-3-030 inhibits key regulators of cell invasion and metastasis, such as c-Myc, AP-1, and TGFß signaling, experimental evidence demonstrating this compound inhibits cancer cell metastasis in vivo has yet to be determined.
Since Myc proteins are key regulators of metabolic changes in cancer cells that support a metastatic phenotype134, we also determined whether SF-3-030 affected proteins that regulate cell metabolism. High-resolution liquid chromatography-tandem mass spectrometry was used to analyze protein changes in A375 cells in the first 12 hours after exposure to SF-3-030. Unique to SF-3-030 treated A375 cells was enhanced expression of markers of mitochondrial dysfunction and oxidative stress. In particular, SF-3-030 significantly increased the oxidative stress-induced growth inhibitor-1 (OSGIN1) protein, which is regulated by oxidized lipids and the transcription factor NRF2 to promote cytochrome c release and induce apoptosis135. These data indicate that SF-3-030 induced signaling events that reduce mitochondrial function and increase ROS. Another ROS responsive protein upregulated by SF-3-030 was the zinc finger transcription factor ZNF744, which has been reported to repress hepatocellular carcinoma cell invasion and metastasis136.
While ROS activation by SF-3-030 was consistent with elevated ROS in melanoma cells treated with ATP binding site and catalytic inhibitors of BRAF and MEK1/214,137,138, the rapid induction of NRF2 by SF-3-030 raised questions about the implications for cancer progression and metastasis. NRF2 competes with the previously mentioned BACH1 transcription factor to regulate genes involved in oxidative stress such as heme oxygenase-1 (HO-1)139. Oxidative stress displaces BACH1 repression to allow NRF2 to activate the HO-1 promoter. Consistent with the activation of NRF2, SF-3-030 is a potent inducer of HO-1. While HO-1 has been implicated in promoting cancer progression and metastasis, HO-1 may also have anti-metastatic functions140. The effects of SF-3-030 on HO-1 are consistent with the upregulation of HO-1 observed in melanoma cells that have developed acquired resistance to BRAF and MEK1/2 inhibitors36. SF-3-03 induction of NRF2 and HO-1 could suggest a protective response against oxidative stress that has been observed with other anticancer drugs and contributes to drug resistance141. However, the use of NRF2 inhibitors to block induction of HO-1 and other NRF2-regulated genes did not affect SF-3-030 inhibition of A375 cell proliferation. This finding suggests that SF-3-030–mediated inhibition of A375 melanoma cell proliferation and a possible metastatic phenotype is through a mechanism that is dependent on elevated ROS but not NRF2.
MAP kinases are essential intracellular signaling enzymes that mediate extracellular signals and receptor activation with gene expression changes that promote tumor growth and stress responses that lead to metastatic progression7. Inhibitors of kinases within this network have been extensively used in clinical settings13 and employ the use of maximum tolerated dose approaches to achieve maximum clinical benefits. Ironically, these approaches often do not yield durable responses due to the development of acquired drug resistance, unacceptable toxicities, and activation of alternative pathways, which may be difficult to target. One solution to overcome drug resistance may be to identify targets and combinations of drugs to inhibit multiple oncogenic pathways with lower drug concentrations that avoid the toxicity often associated with maximum tolerated dosing. Increased OxPhos is a common metabolic adaptation that supports drug resistance17,36,142. We have observed a number of protein changes in melanoma cells with acquired resistance to BRAF and MEK1/2 inhibitors, including increased protein levels of pyruvate kinase, mitochondrial pyruvate carrier 1, and mitochondrial pyruvate dehydrogenase subunits all of which support increased glycolytic ATP production and mitochondrial oxidative phosphorylation36. Other proteins that promote a metastatic phenotype and are upregulated in BRAF/MEK1/2 inhibitor resistant melanoma cells include matrix metalloproteinases (MMP1/3), tetraspanins (TSPAN3,6, 8,31), and caveolin1/2 isoforms36. Similarly, MMP inhibitors (TIMP1/3) were reduced in BRAF/MEK1/2 inhibitor resistant cells, which supports an environment conducive to ECM degradation, cell invasion, and metastasis. Moreover, the metastatic suppressor NDRG1 (N-MYC downstream regulated gene-1), which inhibits EMT and cell migration143 was downregulated in BRAF/MEK1/2 inhibitor-resistant melanoma cells. These findings support the link between inhibitors of the ERK1/2 pathway and MYC-mediated metabolic reprogramming involved in metastasis144 providing a rationale for further development of MYC selective inhibitors.
Several new anticancer targeted strategies that focus on prevention of drug resistance have been proposed7. The main hypothesis behind these approaches is that the use of specific drugs targeting cancer promoting genes may benefit from novel low-dose multi-drug combinations that target key kinase signaling networks and branch points, which are often the rate-determining steps in tumor progression. There are several aspects to these strategies that need to be evaluated including re-examining single agent approaches and choice of targets, vertical inhibition strategies (linear), horizontal inhibition strategies (crosstalk), low dose multi-drug strategies, and combination low-dose approaches. These studies should aim to use bioinformatics and mathematical modeling to identify novel combination approaches7. These considerations highlight several novel strategies that could be used for targeting interconnected oncogenic networks in metastatic cells with single or multiple targeting agents. Compensatory networks are activated when there is redundancy in oncogenic signaling and drug exposure is prolonged. For example, targeting only a single node with low-dose drug may avoid drug toxicity but may lead to reduced efficacy if oncogenic signaling is not completely blocked. On the other hand, targeting an individual node with high-dose drug could inhibit pathway activity with some efficacy but alternative pathways could be activated causing drug resistance7,25,145. Therefore, it would be of therapeutic benefit if the strategy of drug delivery is designed for the specific activating events present in a particular tumor and with the design of avoiding drug resistance7.
CONCLUSIONS
Chemotherapy improves overall survival of cancer patients and can be employed for the treatment of metastatic tumors but chemoresistance is an ongoing problem in achieving durable responses to therapy. Mitochondrial metabolism is an essential driver of tumor metastasis and chemoresistance in cancer. Many metabolic mechanisms of glycolysis-induced chemoresistance have been studied, including MAPK signaling pathways within the tumor microenvironment146-148. However, mitochondrial contributions to chemoresistance are also observed149. It is now becoming clearer that tumor cells respond to changes in ROS levels150,151. Early studies suggested that elevated ROS promoted tumorigenesis but there is no evidence that the use of antioxidants improves patient survival and, in some cases, antioxidant use may worsen outcomes92. In contrast, the use of prooxidants to further elevate ROS may improve therapeutic outcomes by inhibiting cancer cell metastasis152. One approach to increase ROS is through function-selective ERK1/2 inhibitors (such as SF-3-030)34 that target substrate binding sites and block some but not all enzyme activity. This is in contrast to current ERK1/2 (and most other kinase) inhibitors that target the ATP binding or catalytic sites and block all enzyme activity. Targeting ERK1/2 functions that selectively disrupt MYC and AP1-mediated transcription and cancer cell growth has the potential to reduce selective pressure that invariably drives acquired resistance to the current kinase inhibitors. For the ATP synthase inhibitor, CADD522, ROS is likely from mitochondrial sources since the drug directly inhibits ATP synthase, inhibits OCR, and increases ROS 35. Therefore, both SF-3-030 and CADD522 appear to disrupt mitochondrial function. Targeting these pathways with novel compounds that increase ROS to inhibit cancer cell proliferation and metastasis has the potential to mitigate acquired drug resistance. These findings are consistent with previous studies that implicate the ERK1/2 pathway in regulating OxPhos and the induction of ROS after treatment with inhibitors of BRAF and MEK1/214,137,138. In summary, we present evidence that selective targeting of unique ERK1/2 and ATP synthase mitochondrial functions increases ROS in tumor cells and may be a valid therapeutic approach to reduce cancer cell metastasis. Consideration of mitochondrial bioenergetics79 may, in future, lead to development of combination targeting approaches to overcome therapeutic limitations7. Such combination approaches should focus on the use of low dose, multidrug combination therapy to target key kinase signaling networks rather than individual kinases or signaling pathways, as a way of realizing the full potential of combination treatments.
ACKNOWLEDGEMENTS
The studies described in this review were supported by the University of Maryland Comprehensive Cancer Center and Cigarette Restitution Funds from the State of Maryland and the Veterans Administration (A.P., M.S.K) and Institute for Clinical & Translational Research, University of Maryland-Baltimore (P.S.).
Funding information
This work was supported, in part, by VA Merit Review funding (Award 1I01-BX004904-01) from the U.S. Department of Veterans Affairs, Biomedical Laboratory Research and Development (BLR&D) Service and the University of Maryland Marlene & Stewart Greenebaum Comprehensive Cancer Center, Cigarette Restitution Fund (CRF) (to A.P., M.S.K.)
National Institutes of Health National Cancer Institute [Grant R01-CA120215] (to P.S.)
University of Maryland School of Medicine, Anesthesiology Department (to B.M.P.)
Footnotes
CONFLICTS OF INTERESTS
The authors declare that there are no conflicts of interest.
The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
References
- 1.Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin 72, 7–33 (2022). [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD & Jemal A Cancer statistics, 2018. CA Cancer J Clin 68, 7–30 (2018). [DOI] [PubMed] [Google Scholar]
- 3.Tan J, Song M, Zhou M, et al. Antibiotic tigecycline enhances cisplatin activity against human hepatocellular carcinoma through inducing mitochondrial dysfunction and oxidative damage. Biochem Biophys Res Commun 483, 17–23 (2017). [DOI] [PubMed] [Google Scholar]
- 4.Zhang L, Xu L, Zhang F, et al. Doxycycline inhibits the cancer stem cell phenotype and epithelial-to-mesenchymal transition in breast cancer. Cell Cycle 16, 737–745 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caino MC & Altieri DC Molecular Pathways: Mitochondrial Reprogramming in Tumor Progression and Therapy. Clin Cancer Res 22, 540–545 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Waks AG & Winer EP Breast Cancer Treatment: A Review. JAMA 321, 288–300 (2019). [DOI] [PubMed] [Google Scholar]
- 7.Yesilkanal AE, Johnson GL, Ramos AF, et al. New strategies for targeting kinase networks in cancer. J Biol Chem 297, 101128 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kern R, Correa SC, Scandolara TB, et al. Current advances in the diagnosis and personalized treatment of breast cancer: lessons from tumor biology. Per Med 17, 399–420 (2020). [DOI] [PubMed] [Google Scholar]
- 9.Siegel RL, Miller KD & Jemal A Cancer statistics, 2020. CA Cancer J Clin 70, 7–30 (2020). [DOI] [PubMed] [Google Scholar]
- 10.Viale A, Corti D & Draetta GF Tumors and mitochondrial respiration: a neglected connection. Cancer Res 75, 3685–3686 (2015). [DOI] [PubMed] [Google Scholar]
- 11.Queirolo P & Spagnolo F BRAF plus MEK-targeted drugs: a new standard of treatment for BRAF-mutant advanced melanoma. Cancer Metastasis Rev 36, 35–42 (2017). [DOI] [PubMed] [Google Scholar]
- 12.Ralli M, Botticelli A, Visconti IC, et al. Immunotherapy in the Treatment of Metastatic Melanoma: Current Knowledge and Future Directions. J Immunol Res 2020, 9235638 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smalley I & Smalley KSM ERK Inhibition: A New Front in the War against MAPK Pathway-Driven Cancers? Cancer Discov 8, 140–142 (2018). [DOI] [PubMed] [Google Scholar]
- 14.Haq R, Shoag J, Andreu-Perez P, et al. Oncogenic BRAF regulates oxidative metabolism via PGC1alpha and MITF. Cancer Cell 23, 302–315 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jia D, Park JH, Jung KH, et al. Elucidating the Metabolic Plasticity of Cancer: Mitochondrial Reprogramming and Hybrid Metabolic States. Cells 7(2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu J, Tan M & Cai Q The Warburg effect in tumor progression: mitochondrial oxidative metabolism as an anti-metastasis mechanism. Cancer Lett 356, 156–164 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghosh P, Vidal C, Dey S, et al. Mitochondria Targeting as an Effective Strategy for Cancer Therapy. Int J Mol Sci 21(2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Denisenko TV, Gorbunova AS & Zhivotovsky B Mitochondrial Involvement in Migration, Invasion and Metastasis. Front Cell Dev Biol 7, 355 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peart O Breast intervention and breast cancer treatment options. Radiol Technol 86, 535M–558M; quiz 559-562 (2015). [PubMed] [Google Scholar]
- 20.Azim HA, Ghosn M, Oualla K, et al. Personalized treatment in metastatic triple-negative breast cancer: The outlook in 2020. Breast J 26, 69–80 (2020). [DOI] [PubMed] [Google Scholar]
- 21.Martinez-Reyes I & Cuezva JM The H(+)-ATP synthase: a gate to ROS-mediated cell death or cell survival. Biochim Biophys Acta 1837, 1099–1112 (2014). [DOI] [PubMed] [Google Scholar]
- 22.Pelicano H, Zhang W, Liu J, et al. Mitochondrial dysfunction in some triple-negative breast cancer cell lines: role of mTOR pathway and therapeutic potential. Breast Cancer Res 16, 434 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huebbers CU, Adam AC, Preuss SF, et al. High glucose uptake unexpectedly is accompanied by high levels of the mitochondrial ss-F1-ATPase subunit in head and neck squamous cell carcinoma. Oncotarget 6, 36172–36184 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeBerardinis RJ & Chandel NS Fundamentals of cancer metabolism. Sci Adv 2, e1600200 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fiorillo M, Ozsvari B, Sotgia F, et al. High ATP Production Fuels Cancer Drug Resistance and Metastasis: Implications for Mitochondrial ATP Depletion Therapy. Front Oncol 11, 740720 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gandhi N & Das GM Metabolic Reprogramming in Breast Cancer and Its Therapeutic Implications. Cells 8(2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Santidrian AF, Matsuno-Yagi A, Ritland M, et al. Mitochondrial complex I activity and NAD+/NADH balance regulate breast cancer progression. J Clin Invest 123, 1068–1081 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pani G, Galeotti T & Chiarugi P Metastasis: cancer cell's escape from oxidative stress. Cancer Metastasis Rev 29, 351–378 (2010). [DOI] [PubMed] [Google Scholar]
- 29.Chang F, Steelman LS, Lee JT, et al. Signal transduction mediated by the Ras/Raf/MEK/ERK pathway from cytokine receptors to transcription factors: potential targeting for therapeutic intervention. Leukemia 17, 1263–1293 (2003). [DOI] [PubMed] [Google Scholar]
- 30.Webster MA, Hutchinson JN, Rauh MJ, et al. Requirement for both Shc and phosphatidylinositol 3' kinase signaling pathways in polyomavirus middle T-mediated mammary tumorigenesis. Mol Cell Biol 18, 2344–2359 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chae YC, Vaira V, Caino MC, et al. Mitochondrial Akt Regulation of Hypoxic Tumor Reprogramming. Cancer Cell 30, 257–272 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sharifi MN, Anandan A, Grogan P, et al. Therapy after cyclin-dependent kinase inhibition in metastatic hormone receptor-positive breast cancer: Resistance mechanisms and novel treatment strategies. Cancer 126, 3400–3416 (2020). [DOI] [PubMed] [Google Scholar]
- 33.Wang J, Rouse C, Jasper JS, et al. ABL kinases promote breast cancer osteolytic metastasis by modulating tumor-bone interactions through TAZ and STAT5 signaling. Sci Signal 9, ra12 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martinez R 3rd, Huang W, Samadani R, et al. Mechanistic Analysis of an Extracellular Signal-Regulated Kinase 2-Interacting Compound that Inhibits Mutant BRAF-Expressing Melanoma Cells by Inducing Oxidative Stress. J Pharmacol Exp Ther 376, 84–97 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim MS, Gernapudi R, Cedeno YC, et al. Targeting breast cancer metabolism with a novel inhibitor of mitochondrial ATP synthesis. Oncotarget 11, 3863–3885 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martinez R 3rd, Huang W, Buck H, et al. Proteomic Changes in the Monolayer and Spheroid Melanoma Cell Models of Acquired Resistance to BRAF and MEK1/2 Inhibitors. ACS Omega 7, 3293–3311 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frattini V, Pagnotta SM, Tala, et al. A metabolic function of FGFR3-TACC3 gene fusions in cancer. Nature 553, 222–227 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weinbach EC & Garbus J Mechanism of action of reagents that uncouple oxidative phosphorylation. Nature 221, 1016–1018 (1969). [DOI] [PubMed] [Google Scholar]
- 39.Patra S, Elahi N, Armorer A, et al. Mechanisms Governing Metabolic Heterogeneity in Breast Cancer and Other Tumors. Front Oncol 11, 700629 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guo K, Lai C, Shi J, et al. A Novel Risk Factor Model Based on Glycolysis-Associated Genes for Predicting the Prognosis of Patients With Prostate Cancer. Front Oncol 11, 605810 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dupuy F, Tabaries S, Andrzejewski S, et al. PDK1-Dependent Metabolic Reprogramming Dictates Metastatic Potential in Breast Cancer. Cell Metab (2015). [DOI] [PubMed] [Google Scholar]
- 42.Mookerjee SA, Goncalves RL, Gerencser AA, et al. The contributions of respiration and glycolysis to extracellular acid production. Biochim Biophys Acta 1847, 171–181 (2015). [DOI] [PubMed] [Google Scholar]
- 43.Liberti MV & Locasale JW The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem Sci 41, 211–218 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de la Cruz-Lopez KG, Castro-Munoz LJ, Reyes-Hernandez DO, et al. Lactate in the Regulation of Tumor Microenvironment and Therapeutic Approaches. Front Oncol 9, 1143 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Payen VL, Mina E, Van Hee VF, et al. Monocarboxylate transporters in cancer. Mol Metab 33, 48–66 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Silva A, Antunes B, Batista A, et al. In Vivo Anticancer Activity of AZD3965: A Systematic Review. Molecules 27, 181 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bola BM, Chadwick AL, Michopoulos F, et al. Inhibition of monocarboxylate transporter-1 (MCT1) by AZD3965 enhances radiosensitivity by reducing lactate transport. Mol Cancer Ther 13, 2805–2816 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Heneberg P Redox Regulation of Hexokinases. Antioxid Redox Signal 30, 415–442 (2019). [DOI] [PubMed] [Google Scholar]
- 49.Robey RB & Hay N Mitochondrial hexokinases, novel mediators of the antiapoptotic effects of growth factors and Akt. Oncogene 25, 4683–4696 (2006). [DOI] [PubMed] [Google Scholar]
- 50.Shi T, Ma Y, Cao L, et al. B7-H3 promotes aerobic glycolysis and chemoresistance in colorectal cancer cells by regulating HK2. Cell Death Dis 10, 308 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roberts DJ & Miyamoto S Hexokinase II integrates energy metabolism and cellular protection: Akting on mitochondria and TORCing to autophagy. Cell Death Differ 22, 248–257 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nordkap L, Priskorn L, Brauner EV, et al. Impact of psychological stress measured in three different scales on testis function: A cross-sectional study of 1362 young men. Andrology 8, 1674–1686 (2020). [DOI] [PubMed] [Google Scholar]
- 53.Reinsalu L, Puurand M, Chekulayev V, et al. Energy Metabolic Plasticity of Colorectal Cancer Cells as a Determinant of Tumor Growth and Metastasis. Front Oncol 11, 698951 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Han CY, Patten DA, Richardson RB, et al. Tumor metabolism regulating chemosensitivity in ovarian cancer. Genes Cancer 9, 155–175 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li XB, Gu JD & Zhou QH Review of aerobic glycolysis and its key enzymes - new targets for lung cancer therapy. Thorac Cancer 6, 17–24 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu J, Hu L, Wu F, et al. Poor prognosis of hexokinase 2 overexpression in solid tumors of digestive system: a meta-analysis. Oncotarget 8, 32332–32344 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.El Sayed SM, Mohamed WG, Seddik MA, et al. Safety and outcome of treatment of metastatic melanoma using 3-bromopyruvate: a concise literature review and case study. Chin J Cancer 33, 356–364 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bock FJ & Tait SWG Mitochondria as multifaceted regulators of cell death. Nat Rev Mol Cell Biol 21, 85–100 (2020). [DOI] [PubMed] [Google Scholar]
- 59.Vyssokikh MY & Brdiczka D The function of complexes between the outer mitochondrial membrane pore (VDAC) and the adenine nucleotide translocase in regulation of energy metabolism and apoptosis. Acta Biochim Pol 50, 389–404 (2003). [PubMed] [Google Scholar]
- 60.Lee HJ, Li CF, Ruan D, et al. Non-proteolytic ubiquitination of Hexokinase 2 by HectH9 controls tumor metabolism and cancer stem cell expansion. Nat Commun 10, 2625 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pecinova A, Alan L, Brazdova A, et al. Role of Mitochondrial Glycerol-3-Phosphate Dehydrogenase in Metabolic Adaptations of Prostate Cancer. Cells 9(2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shangguan X, He J, Ma Z, et al. SUMOylation controls the binding of hexokinase 2 to mitochondria and protects against prostate cancer tumorigenesis. Nat Commun 12, 1812 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stine ZE, Schug ZT, Salvino JM, et al. Targeting cancer metabolism in the era of precision oncology. Nat Rev Drug Discov 21, 141–162 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ozsvari B, Fiorillo M, Bonuccelli G, et al. Mitoriboscins: Mitochondrial-based therapeutics targeting cancer stem cells (CSCs), bacteria and pathogenic yeast. Oncotarget 8, 67457–67472 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Anastasiou D, Poulogiannis G, Asara JM, et al. Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to cellular antioxidant responses. Science 334, 1278–1283 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ashton TM, McKenna WG, Kunz-Schughart LA, et al. Oxidative Phosphorylation as an Emerging Target in Cancer Therapy. Clin Cancer Res 24, 2482–2490 (2018). [DOI] [PubMed] [Google Scholar]
- 67.Viale A, Pettazzoni P, Lyssiotis CA, et al. Oncogene ablation-resistant pancreatic cancer cells depend on mitochondrial function. Nature 514, 628–632 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Isidoro A, Casado E, Redondo A, et al. Breast carcinomas fulfill the Warburg hypothesis and provide metabolic markers of cancer prognosis. Carcinogenesis 26, 2095–2104 (2005). [DOI] [PubMed] [Google Scholar]
- 69.Xiao J, Cohen P, Stern MC, et al. Mitochondrial biology and prostate cancer ethnic disparity. Carcinogenesis 39, 1311–1319 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Choudhury AR & Singh KK Mitochondrial determinants of cancer health disparities. Semin Cancer Biol 47, 125–146 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Beebe-Dimmer JL & Cooney KA Mitochondrial alterations may underlie race-specific differences in cancer risk and outcome. J Clin Invest 129, 2187–2188 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Piyarathna DWB, Balasubramanian A, Arnold JM, et al. ERR1 and PGC1alpha associated mitochondrial alterations correlate with pan-cancer disparity in African Americans. J Clin Invest 129, 2351–2356 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chaudhary AK, O'Malley J, Kumar S, et al. Mitochondrial dysfunction and prostate cancer racial disparities among American men. Front Biosci (Schol Ed) 9, 154–164 (2017). [DOI] [PubMed] [Google Scholar]
- 74.Chen W, Mook RA Jr., Premont RT, et al. Niclosamide: Beyond an antihelminthic drug. Cell Signal 41, 89–96 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dong Q, Nelson PJ & Zhao Y Editorial: Cancer Cell Metabolism and Immunomodulation in the Context of Tumor Metastasis. Front Oncol 11, 803213 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Reinfeld BI, Madden MZ, Wolf MM, et al. Cell-programmed nutrient partitioning in the tumour microenvironment. Nature 593, 282–288 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yang H, Geng YH, Wang P, et al. Extracellular ATP promotes breast cancer chemoresistance via HIF-1alpha signaling. Cell Death Dis 13, 199 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jaber SM, Yadava N & Polster BM Mapping mitochondrial respiratory chain deficiencies by respirometry: Beyond the Mito Stress Test. Exp Neurol 328, 113282 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schmidt CA, Fisher-Wellman KH & Neufer PD From OCR and ECAR to energy: Perspectives on the design and interpretation of bioenergetics studies. J Biol Chem 297, 101140 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sica V, Bravo-San Pedro JM, Stoll G, et al. Oxidative phosphorylation as a potential therapeutic target for cancer therapy. Int J Cancer 146, 10–17 (2020). [DOI] [PubMed] [Google Scholar]
- 81.Faubert B, Solmonson A & DeBerardinis RJ Metabolic reprogramming and cancer progression. Science 368(2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kim MS, Gernapudi R, Choi EY, et al. Characterization of CADD522, a small molecule that inhibits RUNX2-DNA binding and exhibits antitumor activity. Oncotarget 8, 70916–70940 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rose S, Frye RE, Slattery J, et al. Oxidative stress induces mitochondrial dysfunction in a subset of autism lymphoblastoid cell lines in a well-matched case control cohort. PLoS One 9, e85436 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Murphy MP How mitochondria produce reactive oxygen species. Biochem J 417, 1–13 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Galadari S, Rahman A, Pallichankandy S, et al. Reactive oxygen species and cancer paradox: To promote or to suppress? Free Radic Biol Med 104, 144–164 (2017). [DOI] [PubMed] [Google Scholar]
- 86.Hong S & Pedersen PL ATP synthase and the actions of inhibitors utilized to study its roles in human health, disease, and other scientific areas. Microbiol Mol Biol Rev 72, 590–641 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zheng J & Ramirez VD Inhibition of mitochondrial proton F0F1-ATPase/ATP synthase by polyphenolic phytochemicals. Br J Pharmacol 130, 1115–1123 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bonuccelli G, Peiris-Pages M, Ozsvari B, et al. Targeting cancer stem cell propagation with palbociclib, a CDK4/6 inhibitor: Telomerase drives tumor cell heterogeneity. Oncotarget 8, 9868–9884 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Patel BA, D'Amico TL & Blagg BSJ Natural products and other inhibitors of F1FO ATP synthase. Eur J Med Chem 207, 112779 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Salani B, Ravera S, Fabbi P, et al. Glibenclamide Mimics Metabolic Effects of Metformin in H9c2 Cells. Cell Physiol Biochem 43, 879–890 (2017). [DOI] [PubMed] [Google Scholar]
- 91.Lee YH, Kang BS & Bae YS Premature senescence in human breast cancer and colon cancer cells by tamoxifen-mediated reactive oxygen species generation. Life Sci 97, 116–122 (2014). [DOI] [PubMed] [Google Scholar]
- 92.Piskounova E, Agathocleous M, Murphy MM, et al. Oxidative stress inhibits distant metastasis by human melanoma cells. Nature 527, 186–191 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wu CA, Chao Y, Shiah SG, et al. Nutrient deprivation induces the Warburg effect through ROS/AMPK-dependent activation of pyruvate dehydrogenase kinase. Biochim Biophys Acta 1833, 1147–1156 (2013). [DOI] [PubMed] [Google Scholar]
- 94.Esparza-Molto PB & Cuezva JM The Role of Mitochondrial H(+)-ATP Synthase in Cancer. Front Oncol 8, 53 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ricart J, Izquierdo JM, Di Liegro CM, et al. Assembly of the ribonucleoprotein complex containing the mRNA of the beta-subunit of the mitochondrial H+-ATP synthase requires the participation of two distal cis-acting elements and a complex set of cellular trans-acting proteins. Biochem J 365, 417–428 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Margeot A, Blugeon C, Sylvestre J, et al. In Saccharomyces cerevisiae, ATP2 mRNA sorting to the vicinity of mitochondria is essential for respiratory function. EMBO J 21, 6893–6904 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.de Heredia ML, Izquierdo JM & Cuezva JM A conserved mechanism for controlling the translation of beta-F1-ATPase mRNA between the fetal liver and cancer cells. J Biol Chem 275, 7430–7437 (2000). [DOI] [PubMed] [Google Scholar]
- 98.Izquierdo JM & Cuezva JM Control of the translational efficiency of beta-F1-ATPase mRNA depends on the regulation of a protein that binds the 3' untranslated region of the mRNA. Mol Cell Biol 17, 5255–5268 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Corral-Debrinski M, Blugeon C & Jacq C In yeast, the 3' untranslated region or the presequence of ATM1 is required for the exclusive localization of its mRNA to the vicinity of mitochondria. Mol Cell Biol 20, 7881–7892 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rius-Perez S, Torres-Cuevas I, Millan I, et al. PGC-1alpha, Inflammation, and Oxidative Stress: An Integrative View in Metabolism. Oxid Med Cell Longev 2020, 1452696 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bost F & Kaminski L The metabolic modulator PGC-1alpha in cancer. Am J Cancer Res 9, 198–211 (2019). [PMC free article] [PubMed] [Google Scholar]
- 102.Tabara LC, Poveda J, Martin-Cleary C, et al. Mitochondria-targeted therapies for acute kidney injury. Expert Rev Mol Med 16, e13 (2014). [DOI] [PubMed] [Google Scholar]
- 103.Moreno-Sanchez R, Saavedra E, Rodriguez-Enriquez S, et al. Metabolic control analysis indicates a change of strategy in the treatment of cancer. Mitochondrion 10, 626–639 (2010). [DOI] [PubMed] [Google Scholar]
- 104.Ralph SJ, Rodriguez-Enriquez S, Neuzil J, et al. The causes of cancer revisited: "mitochondrial malignancy" and ROS-induced oncogenic transformation - why mitochondria are targets for cancer therapy. Mol Aspects Med 31, 145–170 (2010). [DOI] [PubMed] [Google Scholar]
- 105.Mani S, Swargiary G & Ralph SJ Targeting the redox imbalance in mitochondria: A novel mode for cancer therapy. Mitochondrion 62, 50–73 (2022). [DOI] [PubMed] [Google Scholar]
- 106.Reuter CW, Morgan MA & Bergmann L Targeting the Ras signaling pathway: a rational, mechanism-based treatment for hematologic malignancies? Blood 96, 1655–1669 (2000). [PubMed] [Google Scholar]
- 107.Shapiro P Ras-MAP kinase signaling pathways and control of cell proliferation: relevance to cancer therapy. Crit Rev Clin Lab Sci 39, 285–330 (2002). [DOI] [PubMed] [Google Scholar]
- 108.Bennasroune A, Gardin A, Aunis D, et al. Tyrosine kinase receptors as attractive targets of cancer therapy. Crit Rev Oncol Hematol 50, 23–38 (2004). [DOI] [PubMed] [Google Scholar]
- 109.Arora A & Scholar EM Role of tyrosine kinase inhibitors in cancer therapy. J Pharmacol Exp Ther 315, 971–979 (2005). [DOI] [PubMed] [Google Scholar]
- 110.Mendelsohn J & Baselga J Epidermal growth factor receptor targeting in cancer. Semin Oncol 33, 369–385 (2006). [DOI] [PubMed] [Google Scholar]
- 111.Emery CM, Vijayendran KG, Zipser MC, et al. MEK1 mutations confer resistance to MEK and B-RAF inhibition. Proc Natl Acad Sci U S A 106, 20411–20416 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Arvind R, Shimamoto H, Momose F, et al. A mutation in the common docking domain of ERK2 in a human cancer cell line, which was associated with its constitutive phosphorylation. Int J Oncol 27, 1499–1504 (2005). [PubMed] [Google Scholar]
- 113.Mahalingam M, Arvind R, Ida H, et al. ERK2 CD domain mutation from a human cancer cell line enhanced anchorage-independent cell growth and abnormality in Drosophila. Oncol Rep 20, 957–962 (2008). [PubMed] [Google Scholar]
- 114.da Silva Almeida AC, Abate F, Khiabanian H, et al. The mutational landscape of cutaneous T cell lymphoma and Sezary syndrome. Nat Genet 47, 1465–1470 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Flaherty KT & McArthur G BRAF, a target in melanoma: implications for solid tumor drug development. Cancer 116, 4902–4913 (2010). [DOI] [PubMed] [Google Scholar]
- 116.Zhong H & Bowen JP Recent advances in small molecule inhibitors of VEGFR and EGFR signaling pathways. Curr Top Med Chem 11, 1571–1590 (2011). [DOI] [PubMed] [Google Scholar]
- 117.Wu P, Nielsen TE & Clausen MH FDA-approved small-molecule kinase inhibitors. Trends Pharmacol Sci 36, 422–439 (2015). [DOI] [PubMed] [Google Scholar]
- 118.Wu P, Nielsen TE & Clausen MH Small-molecule kinase inhibitors: an analysis of FDA-approved drugs. Drug Discov Today 21, 5–10 (2016). [DOI] [PubMed] [Google Scholar]
- 119.Facchinetti F, Lacroix L, Mezquita L, et al. Molecular mechanisms of resistance to BRAF and MEK inhibitors in BRAF(V600E) non-small cell lung cancer. Eur J Cancer 132, 211–223 (2020). [DOI] [PubMed] [Google Scholar]
- 120.Kakadia S, Yarlagadda N, Awad R, et al. Mechanisms of resistance to BRAF and MEK inhibitors and clinical update of US Food and Drug Administration-approved targeted therapy in advanced melanoma. Onco Targets Ther 11, 7095–7107 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Trachootham D, Alexandre J & Huang P Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov 8, 579–591 (2009). [DOI] [PubMed] [Google Scholar]
- 122.Goodman M, Bostick RM, Kucuk O, et al. Clinical trials of antioxidants as cancer prevention agents: past, present, and future. Free Radic Biol Med 51, 1068–1084 (2011). [DOI] [PubMed] [Google Scholar]
- 123.Wiel C, Le Gal K, Ibrahim MX, et al. BACH1 Stabilization by Antioxidants Stimulates Lung Cancer Metastasis. Cell 178, 330–345 e322 (2019). [DOI] [PubMed] [Google Scholar]
- 124.Meierjohann S Oxidative stress in melanocyte senescence and melanoma transformation. Eur J Cell Biol 93, 36–41 (2014). [DOI] [PubMed] [Google Scholar]
- 125.Adams DJ, Boskovic ZV, Theriault JR, et al. Discovery of small-molecule enhancers of reactive oxygen species that are nontoxic or cause genotype-selective cell death. ACS Chem Biol 8, 923–929 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang J, Yao Z, Jonsson P, et al. A Secondary Mutation in BRAF Confers Resistance to RAF Inhibition in a BRAF(V600E)-Mutant Brain Tumor. Cancer Discov 8, 1130–1141 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hancock CN, Macias A, Lee EK, et al. Identification of novel extracellular signal-regulated kinase docking domain inhibitors. J Med Chem 48, 4586–4595 (2005). [DOI] [PubMed] [Google Scholar]
- 128.Chen F, Hancock CN, Macias AT, et al. Characterization of ATP-independent ERK inhibitors identified through in silico analysis of the active ERK2 structure. Bioorg Med Chem Lett 16, 6281–6287 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Boston SR, Deshmukh R, Strome S, et al. Characterization of ERK docking domain inhibitors that induce apoptosis by targeting Rsk-1 and caspase-9. BMC Cancer 11, 7 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Samadani R, Zhang J, Brophy A, et al. Small-molecule inhibitors of ERK-mediated immediate early gene expression and proliferation of melanoma cells expressing mutated BRaf. Biochem J 467, 425–438 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ozanne BW, Spence HJ, McGarry LC, et al. Transcription factors control invasion: AP-1 the first among equals. Oncogene 26, 1–10 (2007). [DOI] [PubMed] [Google Scholar]
- 132.Defnet AE, Huang W, Polischak S, et al. Effects of ATP-competitive and function-selective ERK inhibitors on airway smooth muscle cell proliferation. FASEB J 33, 10833–10843 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Padua D & Massague J Roles of TGFbeta in metastasis. Cell Res 19, 89–102 (2009). [DOI] [PubMed] [Google Scholar]
- 134.Dong Y, Tu R, Liu H, et al. Regulation of cancer cell metabolism: oncogenic MYC in the driver's seat. Signal Transduct Target Ther 5, 124 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Li R, Chen W, Yanes R, et al. OKL38 is an oxidative stress response gene stimulated by oxidized phospholipids. J Lipid Res 48, 709–715 (2007). [DOI] [PubMed] [Google Scholar]
- 136.Guan C, He L, Chang Z, et al. Correction: ZNF774 is a potent suppressor of hepatocarcinogenesis through dampening the NOTCH2 signaling. Oncogene 39, 2844 (2020). [DOI] [PubMed] [Google Scholar]
- 137.Cesi G, Walbrecq G, Zimmer A, et al. ROS production induced by BRAF inhibitor treatment rewires metabolic processes affecting cell growth of melanoma cells. Mol Cancer 16, 102 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yuan L, Mishra R, Patel H, et al. BRAF Mutant Melanoma Adjusts to BRAF/MEK Inhibitors via Dependence on Increased Antioxidant SOD2 and Increased Reactive Oxygen Species Levels. Cancers (Basel) 12, 1661 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Padilla J & Lee J A Novel Therapeutic Target, BACH1, Regulates Cancer Metabolism. Cells 10, 634 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Luu Hoang KN, Anstee JE & Arnold JN The Diverse Roles of Heme Oxygenase-1 in Tumor Progression. Front Immunol 12, 658315 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Telkoparan-Akillilar P, Suzen S & Saso L Pharmacological Applications of Nrf2 Inhibitors as Potential Antineoplastic Drugs. Int J Mol Sci 20, 2025 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Barbato A, Scandura G, Puglisi F, et al. Mitochondrial Bioenergetics at the Onset of Drug Resistance in Hematological Malignancies: An Overview. Front Oncol 10, 604143 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Park KC, Paluncic J, Kovacevic Z, et al. Pharmacological targeting and the diverse functions of the metastasis suppressor, NDRG1, in cancer. Free Radic Biol Med 157, 154–175 (2020). [DOI] [PubMed] [Google Scholar]
- 144.Lee GY, Chun YS, Shin HW, et al. Potential role of the N-MYC downstream-regulated gene family in reprogramming cancer metabolism under hypoxia. Oncotarget 7, 57442–57451 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Krol M, Pawlowski KM, Majchrzak K, et al. Why chemotherapy can fail? Pol J Vet Sci 13, 399–406 (2010). [PubMed] [Google Scholar]
- 146.Asl ER, Amini M, Najafi S, et al. Interplay between MAPK/ERK signaling pathway and MicroRNAs: A crucial mechanism regulating cancer cell metabolism and tumor progression. Life Sci 278, 119499 (2021). [DOI] [PubMed] [Google Scholar]
- 147.Morris EJ, Jha S, Restaino CR, et al. Discovery of a novel ERK inhibitor with activity in models of acquired resistance to BRAF and MEK inhibitors. Cancer Discov 3, 742–750 (2013). [DOI] [PubMed] [Google Scholar]
- 148.Weigelt B, Lo AT, Park CC, et al. HER2 signaling pathway activation and response of breast cancer cells to HER2-targeting agents is dependent strongly on the 3D microenvironment. Breast Cancer Res Treat 122, 35–43 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Liu C, Jin Y & Fan Z The Mechanism of Warburg Effect-Induced Chemoresistance in Cancer. Front Oncol 11, 698023 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hayes JD, Dinkova-Kostova AT & Tew KD Oxidative Stress in Cancer. Cancer Cell 38, 167–197 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kennel KB & Greten FR Immune cell - produced ROS and their impact on tumor growth and metastasis. Redox Biol 42, 101891 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Tasdogan A, Ubellacker JM & Morrison SJ Redox Regulation in Cancer Cells during Metastasis. Cancer Discov 11, 2682–2692 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.