Abstract
Background:
Chronic rhinosinusitis with nasal polyps is frequently managed with endoscopic sinus surgery (ESS). Prior studies describe individual clinical variables and eosinophil density measures as prognostic for polyp recurrence (PR). However, the relative prognostic significance of these have not been extensively investigated.
Objectives:
We sought to evaluate the impact of PR on measures of disease severity post-ESS and quantify the prognostic value of various clinical variables and biomarkers.
Methods:
Ninety-four patients with chronic rhinosinusitis with nasal polyps and prospectively biobanked polyp homogenates at the time of ESS were recruited 2 to 5 years post-ESS. Patients were evaluated with patient-reported outcome measures and endoscopic and radiographic scoring pre- and post-ESS. Biomarkers in polyp homogenates were measured with ELISA and Luminex. Relaxed least absolute shrinkage and selection operator regression optimized predictive clinical, biomarker, and combined models. Model performance was assessed using receiver-operating characteristic curve and random forest analysis.
Results:
PR was found in 39.4% of patients, despite significant improvements in modified Lund-Mackay (MLM) radiographic and 22-item Sinonasal Outcomes Test scores (both P < .0001). PR was significantly associated with worse post-ESS MLM, modified Lund-Kennedy, and 22-item Sinonasal Outcomes Test scores. Relaxed least absolute shrinkage and selection operator identified 2 clinical predictors (area under the curve = 0.79) and 3 biomarkers (area under the curve = 0.78) that were prognostic for PR. When combined, the model incorporating these pre-ESS factors: MLM, asthma, eosinophil cationic protein, anti-double-stranded DNA IgG, and IL-5 improved PR predictive accuracy to area under the curve of 0.89. Random forest analysis identified and validated each of the 5 variables as the strongest predictors of PR.
Conclusions:
PR had strong associations with patient-reported outcome measures, endoscopic and radiographic severity. A combined model comprised of eosinophil cationic protein, IL-5, pre-ESS MLM, asthma, and anti-double-stranded DNA IgG could accurately predict PR.
Keywords: Chronic rhinosinusitis with nasal polyps, polyp recurrence, clinical variables, biomarker, PROMs, relaxed LASSO, random forest
Chronic rhinosinusitis with nasal polyps (CRSwNP) is characterized by persistent inflammation of the nasal and paranasal sinus mucosa, which significantly impair quality of life. Endoscopic sinus surgery (ESS) is a well-established treatment for patients with CRSwNP who still need surgery despite medical management.1,2 Although ESS can remove polyps and inflamed mucosa, remit disease for some patients, and improve quality-of-life impairment, polyp recurrence (PR) is well described to frequently occur in CRSwNP. The biological and immunological risk factors that predispose patients to PR and the implications of PR on quality of life have not been adequately explored. In a meta-analysis, revision rates after ESS were between 14% and 24% for CRSwNP,3 and the frequency of PR has been reported as high as 40% after 18-months4 or 62% over 10 years in patients with uncontrolled severe CRSwNP.5,6
CRSwNP exhibits diverse inflammatory underpinnings that vary widely by geographic location, societal industrialization, as well as ethnicity.7,8 Type 2 inflammation characterized by the cytokines (IL-4, IL-5, and IL-13), IgE, and eosinophilic inflammation is the dominant form of inflammation found in the sinonasal tissue of CRSwNP in Western countries.9–12 Type 2 inflammation is also associated with increased frequency of asthma comorbidity and radiographic sinonasal disease severity. In contrast, type 1 and/or type 3 inflammation with low eosinophils is common in CRSwNP in Asian countries and is associated with elevated levels of non-type 2 cytokines like TNF-α and IL-17.13,14 Irrespective of the inflammatory endotype, ESS remains a common treatment modality for CRSwNP globally.15,16
Identification of patients for whom ESS is likely to fail has gained increased importance due to the availability of newly approved biologics for CRSwNP including omalizumab (targeting soluble IgE), mepolizumab (targeting soluble IL-5), and dupilumab (targeting the IL-4 receptor a),17–19 which medical guidelines currently recommend for treatment of post-ESS failures.20,21 While this area is under active investigation, factors identified by >1 independent research group as predictive of PR after ESS include sinonasal tissue or blood eosinophilia and presence of comorbid asthma.6,22–24 However, as many of these studies were carried out in Asia where CRSwNP pathogenesis differs substantially from the studies in Western countries, and Western studies are few and have primarily used tissue eosinophil density as a predictor, there exists a need for more rigorous studies.25
We had previously discovered that autoantibodies, particularly those against nuclear antigens such as anti-double-stranded DNA (anti-dsDNA), were increased in NPs obtained from patients at a revision surgery compared with those having primary surgery.26 In complementary lines of research, we have reported that the anti-dsDNA IgG is more frequently secreted by B cells expressing EBI2, a receptor that helps segregate B cells to extrafollicular regions in lymph nodes, which are also found at increased frequency in NP tissue.27,28 The autoreactivity to dsDNA in NP tissue appears to be part of a broader pattern of local tissue autoreactivity with concurrent autoantibodies detected to epithelial antigens and phospholipids as well as antibody-mediated complement activation. 26,29–31 However, to date, we lacked evidence that these autoantibody responses were pathogenic or had prognostic utility in CRSwNP.
To better characterize prognostic factors for ESS refractory CRSwNP, we undertook the present retrospective analysis of prospectively collected clinical data and specimens from patients undergoing ESS at Northwestern Memorial Hospital. To understand how PR relates to other post-ESS measures of disease severity in CRSwNP, we evaluated whether clinical variables and/or tissue biomarkers could solely or synergistically predict PR and investigated how autoreactivity in NP relates to type 2 inflammation. We also set out to establish the prognostic value of clinical variables and biomarkers in predicting polyp recurrence.
METHODS
Study population
Patients with CRSwNP who had an index ESS at Northwestern Memorial Hospital and had consented to collection of surgical samples in our research repository between 2012 and 2017 were prospectively invited to participate in a research evaluation 2 to 5 years post-ESS. Patients provided written informed consent to access collected tissue and clinical information, underwent a research-related examination that included a computed tomography scan, endoscopy, and administration of patient-reported outcome measures (PROMs). Patients who were pregnant, did not speak English, had fungal rhinosinusitis, or had revision ESS for any reason between their index surgery and research visit eligibility were excluded. Revision ESS was uncommon in this time frame (ie, ≤5 years), with only 2% of eligible patients found to have had revision surgery. This study is currently enrolling additional patients, and this article evaluates the first 94 patients enrolled. The study was approved by the Northwestern University Institutional Review Board.
Collection of tissues
NPs were collected from each patient at the time of index ESS and were weighed and placed in PBS-Tween supplemented with a cocktail of protease inhibitors (Sigma-Aldrich, St Louis, Mo) at a 1:100 dilution. The tissue was homogenized with a Bullet Blender (Next Advance, Averill Park, NY) per the manufacturer’s instructions. The samples were then centrifuged at 4000 revolutions/min for 20 minutes at 4°C, after which the supernatants were subsequently aliquoted and frozen at − 80°C until further use. The total protein from the tissue homogenate was measured with a Bradford protein assay as described previously.29,32
Measurement of biomarkers
NP homogenate at a 1:50 dilution was used to measure eosinophil cationic protein (ECP) and anti-dsDNA IgG by ELISA (MBL International, Woburn, Mass, and ALPCO, Salem, NH, respectively) according to the manufacturer’s instructions. Another 18 inflammatory mediators selected to assay inflammation of several endotypes were measured by Luminex (MILLIPLEX MAP Hu- man High Sensitivity T-Cell Panel, Millipore Sigma, Burlington, Mass) including IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-13, IL-17, IL-21, IL-1β, IFN-γ, IFN-inducible T-cell α chemoattractant, TNF-α, GM-CSF, macro-phage inflammatory protein (MIP)-1α, MIP-1β, MIP-3α, and fractalkine. Lu-minex assays were performed at 1:2 dilution. All results were normalized to total tissue protein levels and reported as nanograms of ECP per milligram of total protein (ng/mg), picograms of inflammatory mediators per milligram of total protein (pg/mg), and international units of anti-dsDNA IgG per milli-gram of total protein (IU/mg). More detailed information on assay perfor-mance and sample measurement can be found in this article’s Online Repository available at www.jacionline.org. IL-2, IL-8, and IL-17 were ulti-mately not analyzed further as >60% of patients had levels out of the detect-able range (IL-2 and IL-17) or beyond the uppermost readings (IL-8). The remaining 17 tissue biomarkers were examined as described.
Clinical measures
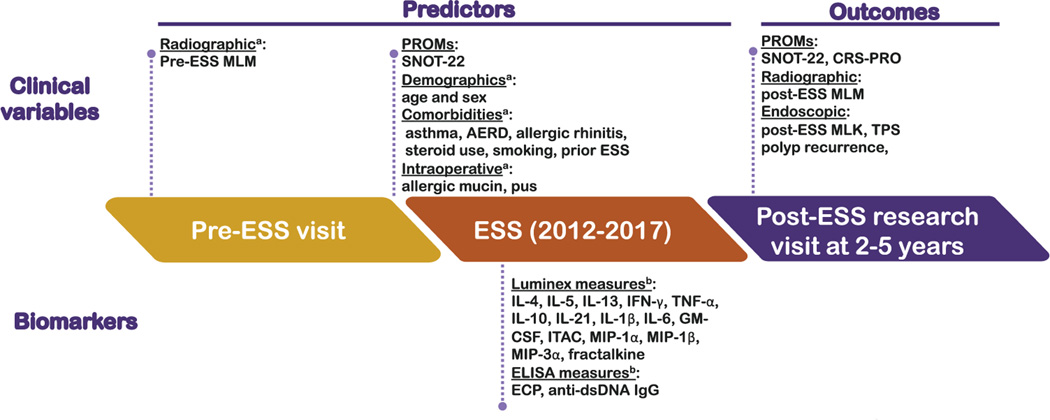
As illustrated in Fig 1, outcomes evaluated at the post-ESS assessments included endoscopic evaluation for PR, defined as the presence of any NPs on endoscopy, with further grading using the modified Lund-Kennedy (MLK) system, which also evaluates polyp size, edema, and discharge (range 0–12).33 For completeness, NPs were also evaluated using the total polyp score (TPS) (range 0–8), which has been used in clinical trials of CRSwNP. Radiographic severity was assessed on both pre- and post-ESS computed tomography imaging and scored using the modified Lund-Mackay score (MLM) (range 0–44).34 Post-ESS PROMs included both the 22-item Sinonasal Outcomes Test (SNOT-22) (range 0–110) and the 12-item Chronic Rhinosinusitis Patient-Reported Outcomes measure (CRS-PRO) (range 0–48),35–37 whereas only pre-ESS SNOT-22 scores were available as the CRS-PRO was only recently developed and validated. Higher scores for both the SNOT-22 and CRS-PRO indicate more severe symptoms. Patient questionnaires were completed via Research Electronic Data Capture (REDCap), a web-based data collection system for research studies at Feinberg School of Medicine.38 Patient comorbidities including aspirin exacerbated respiratory disease, asthma, allergic rhinitis, prior ESS, steroid use, as well as smoking at the time of ESS, were obtained by patient interview and verified by review of the patient’s medical record. The intraoperative findings of pus and allergic mucin were obtained from operative reports from the ESS. More detailed information can be found in this article’s Online Repository. In total, 11 clinical variables including 6 comorbidities, 2 intraoperative findings, pre-ESS MLM, age, and sex were analyzed in the prediction models.
FIG 1.
Study flowchart illustrating predictor and outcome variables collected, timing for collection, and onesanalyzed for predicting of PR. aClinical variables and bbiomarkervariables considered in the modelsfor prediction of PR. AERD, Aspirin exacerbated respiratory disease.
Statistical and machine learning analyses
Data analysis was conducted using R (version 4.05; R Foundation, Vienna, Austria) (dplyr, rpart, pROC, randomForest, ggplot2, and glmnet), and GraphPad Prism (version 9.0; GraphPad Software, La Jolla, Calif). Continuous variables were assessed for detectability, and those exceeding 60% detectability, which comprised the 17 biomarkers shown in Fig 1 and measures of radiographic, endoscopic, and PROMs scores, were assessed for normality. All biomarkers were log-transformed to be comparable and more stable in linear relationships with the target variable. Mann-Whitney U test was used for comparisons between continuous variables. Categorical variables were analyzed with Pearson chi-square test. The Wilcoxon matched-pairs signed-rank test was used to compare pre- and post-ESS measurements. Correlations between 2 variables were considered weak if between 0.25 and 0.39, moderate if between 0.40 to 0.69, and strong if ≥0.70.39,40 P < .05 was considered statistically significant.
The relaxed least absolute shrinkage and selection operator (LASSO) is a regularized regression algorithm commonly used to select variables that have the strongest relationship with the outcome of interest (determined to be PR). Compared with other methods like stepwise regression models, it has the advantages of variable selection and optimization of the number of predictive variables, resulting in a parsimonious model that utilizes the most important set of predictors.41,42 The performance of relaxed LASSO is controlled by 2 parameters, λ and γ. The parameter λ ≥0 impacts the number of variables selected by LASSO by shrinking relevant coefficient estimates to 0. Larger λ results in greater shrinkage and fewer variables being identified as important. Once variables have been selected, γ ϵ [0,1] reverses shrinkage on the coefficient estimates of selected variables to reduce bias; γ = 0 removes all shrinkage, while γ = 1 removes no shrinkage. The optimal λ and γ were obtained from 10-fold cross-validation to avoid model overfitting. We used relaxed LASSO algorithms to build prediction models using (1) clinical variables measured at the time of ESS, (2) biomarkers from NP homogenates, and (3) both clinical variables and biomarkers. We compared the model performance of clinical measures with biomarkers and further evaluated whether the combination of both enhanced prediction accuracy. The discriminability of the model was assessed by logistic regression (LR), the receiver-operating characteristic (ROC) curves, and the corresponding area under the curve (AUC). The optimal cutoff value was determined by using Youden index, a measure of overall prognostic effectiveness. Akaike information criterion (AIC) was also used to evaluate the quality of models. In a separate machine learning analysis, random forests (RFs) were used as an independent analytic method to validate relaxed LASSO-based variable identification and to quantify the prognostic importance of variables.43
RESULTS
Baseline characteristics and patient data
The study recruited a total of 94 patients with CRSwNP who had ESS between 2012 and 2017 with an average of 34.9 ± 6 11.3 months elapsing after ESS. In this time frame, a total of 382 patients had ESS for CRSwNP with biobanked tissue and were eligible for this study; however, the coronavirus disease 2019 pandemic severely impacted willingness for patients to come in for research visits. The analysis carried out thus represents variables accrued prior to the onset of the pandemic. Forty-two women (41.5%) and 58 men (58.5%) participated; the average age was 49.5 years. Clinical characteristics, intraoperative, pre- and post-ESS MLM, MLK, SNOT-22, CRS-PRO, and TPS are summarized in Table I. Over 90% of these patients had complete [T1] debridement of NPs and inflammatory tissue via a bilateral maxillary antrostomy, ethmoidectomy, sphenoidectomy, and frontal sinusotomy, reflecting the severity of the inflammation in this patient population. For non-normal continuous variables, median and interquartile range were presented. For categorical variables, frequency counts and percentages were presented. There were no significant differences noted between patients with PR and non-PR for the frequency of patient-reported post-ESS medication use (see Table E1 in this article’s Online Repository at www.jacionline.org)
TABLE I.
Basel ine clinical characteristics in the study
| Patient demographics and clinical characteristics | n (%) | Median (IQR) | |
|---|---|---|---|
|
| |||
| Demographic | |||
| Sex | Female | 39 (41.5) | |
| Male | 55 (58.5) | ||
| Age (y) | 49.5 (41.8–60) | ||
| Comorbidity | |||
| AERD | No AERD | 73 (77.7) | |
| AERD | 21 (22.3) | ||
| Asthma | No or prior diagnosis | 39 (41.5) | |
| Active | 55 (58.5) | ||
| Allergic rhinitis | Absence | 39 (41.5) | |
| Presence | 55 (58.5) | ||
| Prior ESS | Primary | 50 (53.2) | |
| Revision | 44 (46.8) | ||
| Steroid use | No | 41 (43.6) | |
| Nasal and/or inhaled only | 25 (26.6) | ||
| Oral steroid with or without nasal/inhaled | 28 (29.8) | ||
| Smoking | Never smoker | 72 (76.6) | |
| Current or prior smoker | 22 (23.4) | ||
| Intraoperative findings | |||
| Pus | Absence | 76 (80.9) | |
| Presence | 18 (19.1) | ||
| Allergic mucin | Absence | 66 (70.2) | |
| Presence | 28 (29.8) | ||
| PROMs | |||
| SNOT-22 | Pre-ESS | 22.5 (0–46.0) | |
| Post-ESS | 19.0 (7.8–40.1) | ||
| CRS-PRO | Pre-ESS | NA | |
| Post-ESS | 15.0 (6.0–26.3) | ||
| Radiographic score | |||
| MLM | Pre-ESS | 28 (20.0–36.0) | |
| Post-ESS | 7.5 (3.0–20.0) | ||
| Endoscopic score | |||
| MLK | Pre-ESS | NA | |
| Post-ESS | 2 (0–6.0) | ||
| PR | 37 (39.4) | ||
| TPS in PR | 2.0 (1.0–3.0) | ||
| Tissue biomarkers (pg/mg) | |||
| IL-4 | 7.3 (3.0–14.1) | ||
| IL-5 | 4.0 (1.1–10.0) | ||
| IL-6 | 7.6 (2.9–21.9) | ||
| IL-13 | 10.6 (3.3–22.9) | ||
| IL-10 | 1.0 (0.6–2.7) | ||
| IL-21 | 1.3 (0.5–2.6) | ||
| ITAC | 29.8 (15.4–51.0) | ||
| TNF-α | 0.9 (0.4–2.5) | ||
| GM-CSF | 0.4 (0.2–3.1) | ||
| Fractalkine | 5S0.0 (233.1–1246) | ||
| IFN-γ | 0.7 (0.3–3.7) | ||
| MIP-1α | 17.9 (10.0–42.6) | ||
| MIP-1β | 35.4 (18.8–72.0) | ||
| MIP-3α | 5.3 (2.3–14.3) | ||
| IL-1β | 0.5 (0.2–1.2) | ||
| Anti-dsDNA IgG (IU/mg) | 337.0 (158.0–712.1) | ||
| ECP (ng/mg) | 924.5 (395.3–2375) | ||
Demographics, inflammatory mediators, clinical variables, and PROMs measured in the study (N = 94).
AERD, Aspirin exacerbated respiratory disease; IQR, interquartile range; NA, not available.
Changes in radiographic severity and PROMs post-ESS
Among the various clinical and patient-reported metrics, pre- and post-ESS MLM radiographic scores were available for all patients, while SNOT-22 were available for 59% of patients pre-ESS (n = 57) and for all patients post-ESS (n = 94). The MLM scores improved by 73.2% from a median of 28.0 to 7.5 following ESS (P < .0001) (Fig 2, A). The SNOT-22 scores were improved by 58.1% from a median of 43.0 to 18.0 following ESS (n = 57, P < .0001) (Fig 2, B). Unlike multiple prior studies describing weak or no correlations between radiographic outcomes and PROMs, we found a moderate positive correlation between post-ESS MLM and post-SNOT-22 scores (R = 0.40, P < .01) (Fig 2, C), although pre-ESS MLM and SNOT-22 scores did not, albeit with a smaller subset (see Fig E1, A in this article’s Online Repository at www.jacionline.org).
FIG 2.
Evaluation of disease severity at pre- and post-ESS time points. A, Comparisons between matched pairs of pre- and post-ESS radiographic severity measured by MLM. B, Comparisons between matched pairs of pre- and post-ESS levels of SNOT-22. Wilcoxon matched-pairs signed-rank test was used for the comparisons. C, Correlation between post-ESS MLM and SNOT-22 was analyzed by Spearman correlation analysis. **P< .01 and ****P< .0001. Median of the measurements were indicated as red and blue lines for pre- and post-ESS, respectively.
Associations between PR and other post-ESS disease severity end points
In examining post-ESS outcomes, we found on endoscopy during the research visit that 39.4% of patients with CRSwNP had PR (PR+). Not surprisingly, PR was associated with worse outcomes than non-PR (PR−), given SNOT-22 and CRS-PRO scores were 1.9-fold (PR−: median = 15.0; PR+: median = 29.0) and 2.2-fold (PR−: median = 10.0; PR+: median = 22.0) higher, respectively (both P < .01) (Fig 3). Additionally, in patients with PR, post-ESS MLK endoscopic score was dramatically higher in the PR group than in the non-PR group (PR−: median = 0.0; PR+: median = 6.0), and MLM radiographic score was 4-fold (PR−: median = 5.0; PR+: median = 20.0) higher (both P < .0001). Patients with PR had a median TPS of 2.0 (not shown). Given that PR exhibited important associations with PROMs, radiographic, endoscopic scores, and TPS, we employed PR as our primary outcome measure for further analyses.
FIG 3.
Associations between PR and post-ESS clinical outcomes. Post-ESS SNOT-22, CRS-PRO, MLK, and MLM were compared between non-PR (PR-, n = 57) and PR (PR+, n = 37). Mann-Whitney U test was used for the comparisons. **P< .01 and ****P< .0001. Median with interquartile range of the measurements are indicated as blue and red lines for PR- and PR+, respectively.
We noted during the comparison of pre-ESS SNOT-22 and MLM in PR+ and PR− groups that there were no significant differences in SNOT-22 (Fig E1, B), but MLM was significantly higher in the PR+ compared with in the PR− group (Fig E1, C).
Prediction model with pre-ESS clinical variables
To investigate the predictive values of clinical variables in determining PR, a LASSO regression, which aided variable selection and optimization, was applied to the 11 clinical variables. With the optimal λ and γ, 2 predictive factors of PR were selected by the algorithm: pre-ESS MLM and asthma (Fig 4, A). The regression coefficients of each variable were ascertained by the multivariate LR analysis (Table II). In the clinical LR [T2] model, the model with pre-ESS MLM and asthma were further adjusted for age and sex resulting in an AUC of 0.79 and AIC of 108.7 (Fig 4, B) (Table II). We noted that aspirin exacerbated respiratory disease was not selected by the relaxed LASSO model, despite separate analysis showing univariate LR association with PR (data not shown). This omission may be attributable to the strong predictive value of pre-ESS MLM and asthma, both of which are also known to be strongly associated with aspirin exacerbated respiratory disease status.
FIG 4.
Feature selections for prediction of PR using relaxed LASSO and the prediction efficacy assessed using ROC curves. LASSO coefficient plot showing the relationship between the number of computed variables and goodness of fit (binomial deviance). A, For LASSO models considering only clinical variables, using 2 variables (log [λ] = −2): pre-ESS MLM and asthma optimized goodness of fit across a range of λ and γ penalties, whereas permitting consideration of all 11 clinical variables (log [λ] = −7) decreased goodness of fit. B, ROC curves for predicting PR using the LASSO identified clinical variables had an AUC of 0.79. C, For LASSO models considering only biomarker variables, 3 variables—ECP, dsDNA-IgG, and IL-5—were selected with optimal λ and γ penalty measures (log [λ] = −2), whereas increasing the number of considered variables to all 17 measured biomarkers (log [λ] = −8) decreased goodness of fit. D, ROC curves for predicting PR using the LASSO identified biomarker variables had an AUC of 0.78. E, For LASSO models considering both clinical and biomarker variables, 5 variables (log [λ] = −2) — pre-ESS MLM, asthma, ECP, dsDNA-IgG, and IL-5—were selected with optimal λ and γ penalty measures. F, ROC curves for predicting PR in the combined model with AUC of 0.89.
TABLE II.
Multivariate logistic regression analyses of the predictors in CRSwNP
| Analysis methods | Estimate | SE | Z value | P value | AUC | AIC |
|---|---|---|---|---|---|---|
|
| ||||||
| Multivariate analysis | ||||||
| Clinical prediction model | 0.79 | 108.7 | ||||
| Pre-ESS MLM | 0.07 | 0.03 | 2.46 | <.05 | ||
| Asthma | 1.80 | 0.60 | 3.15 | <.01 | ||
| Biomarker prediction model | 0.78 | 113.6 | ||||
| Log (ECP) | 0.72 | 0.23 | 3.10 | <.01 | ||
| Log (anti-dsDNA IgG) | 0.44 | 0.27 | 1.63 | <.05 | ||
| Log (IL-5) | 0.17 | 0.19 | 0.93 | <.05 | ||
| Combined prediction model | ||||||
| Pre-ESS MLM | 0.08 | 0.03 | 2.29 | <.05 | 0.89 | 94.1 |
| Asthma | 2.36 | 0.74 | 3.20 | <.05 | ||
| Log (ECP) | 0.44 | 0.27 | 1.60 | <.05 | ||
| Log (anti-dsDNA IgG) | 0.59 | 0.33 | 1.80 | <.05 | ||
| Log (IL-5) | 0.54 | 0.25 | 2.16 | <.05 | ||
Multivariate models were adjusted for age and sex.
Prediction model with tissue biomarkers
Given that no comprehensive analyses of tissue biomarkers have been performed to predict PR in Western patients with CRSwNP, we investigated whether our array of inflammatory biomarkers could predict PR in our patient population. First, to evaluate the intercorrelation and organization of biomarkers studied, the various inflammatory mediators measured on pre-ESS tissue were analyzed by a Spearman correlation matrix and visualized using a heatmap and hierarchical clustering (see Fig E2 in this article’s Online Repository at www.jacionline.org). We found the inflammatory mediators naturally grouped into 3 clus-ters, clusters 1 and 2 were composed of type 1 cytokines and proinflammatory chemokines such as TNF-α, IFN-γ, and GM-CSF; cluster 2 made up only of IL-6 and MIP-1α/β; and cluster 3 containing anti-dsDNA IgG, and ECP along with type 2 cytokines IL-4, IL-5, and IL-13. We noted that while anti-dsDNA IgG and ECP were not significantly correlated with each other, they both fell into the type 2 cluster and had significant correlation with IL-5 and IL-13.
The ability of the 17 biomarker predictors to predict PR was assessed using a relaxed LASSO regression, which was especially helpful in the setting of collinear variables described in the matrix. The LASSO coefficient profiles of biomarkers and the shrinkage from 17 to 0 are demonstrated in Fig 4, C. With the optimal λ and γ, 3 predictive factors of PR were selected by the algorithm: log (ECP), log (anti-dsDNA IgG), and log (IL-5).
Multivariate LR analysis ascertained the regression coefficients of LASSO-selected variables, showing that log-transformed ECP, anti-dsDNA IgG, and IL-5 could significantly predict PR (P < .01, P < .05, and P < .05, respectively) (Table II), further adjustment for age and sex predicted PR with an AUC of 0.78 and AIC of 113.6 (Fig 4, D).
Prediction model combined with biomarkers and clinical variables
A natural extension of the clinical- and biomarker-perspective analyses described is to combine these variables into a single model. To this end, relaxed LASSO regression with all 28 variables including clinical variables and biomarkers was performed (Fig 4, E). With optimal λ and γ, 5 predictive variables of PR were selected: log (ECP), log (anti-dsDNA IgG), log (IL-5), pre-ESS MLM, and asthma. In the model adjusted for age and sex: logit (PR) = −10.6 + 0.4 log (ECP) + 0.6 log (anti-dsDNA IgG) 1 0.5 log (IL-5) 1 0.1 pre-ESS MLM 1 2.4 asthma 1 0.5 sex - 0.04 age. It had an improved AUC to 0.89 and AIC to 94.1 over the clinical- and biomarker-only models (Fig 4, F). According to the Youden index, the optimal cutoff value was 0.35 (You- den index = 0.69, sensitivity = 89%; specificity = 80%). The model containing the aforementioned variables is detailed in Table II. Diagnosis of the regression model was demonstrated by quantile-quantile plots and Cook distance (see Fig E3 in this article’s Online Repository at www.jacionline.org). These effect plots, illustrating the predicted probabilities as a marginal function of each variable, validated the linear trends between the selected variables and our target PR (see Fig E4 in this article’s Online Repository at www.jacionline.org).
Use of machine learning to evaluate the probability and predictive accuracy of the model
An RF analysis was performed to further quantify the relative predictive power of each predictor variable for PR. The importance of each variable in the RF was reflected by the Gini coefficient in the Gini plot. A higher mean decrease in the Gini plot indicates greater variable importance. The Gini plots showed that log (ECP), pre-ESS MLM, asthma, log (IL-5), log (anti-dsDNA IgG), and log (IL-13) were the main predictors for PR, ranked by the largest to smallest mean decrease in the Gini coefficient (Fig 5). RF reported here offered consistent prognostic results to previous LASSO models with the same variables being selected and ranked as the topmost important predictors. We noted that IL-5 and IL-13 were highly correlated in tissue (R = 0.72, P < .0001) and IL-13 had predictive potential as demonstrated by RF models, but when considered together with IL-5, it was out-selected in the LASSO model, which penalized collinearity, and IL-5 likely gave slightly better performance then IL-13 for predicting PR.
FIG 5.
RF analysis of predictors for PR in patients with CRSwNP. Variable importance was determined by the percentage increase in prediction error when that specific variable was excluded as compared to the model with all variables. The importance was demonstrated by Gini coefficient in Gini plots. The mean decrease in the Gini coefficient demonstrated the most important variables in the combined prediction model. ★The 6 most important variables for PR prediction are log (ECP), pre-ESS MLM, asthma, log (IL-5), log (anti-dsDNA IgG), and log (IL-13).
DISCUSSION
In this study we performed a comprehensive analysis of clinical variables and inflammatory biomarkers measured in NP tissue obtained during ESS and identified predictors that predicted PR among patients with CRSwNP 2 to 5 years after surgery. We found that PR, being a clinically relevant measure of disease severity, was associated with higher concurrent radiographic (MLM), endoscopic (MLK and TPS), and PROMs scores. Furthermore, using relaxed LASSO analysis, an unbiased variable selection process, we identified both clinical predictors (pre-ESS MLM and asthma) and biomarker predictors (log [ECP], log [anti-dsDNA IgG], and log [IL-5]) of PR. Using clinical- or biomarker-only predictors resulted in models of equivalent predictive power. However, when integrating both types of predictors, the model with the 5 optimal variables improved the predictive accuracy to 0.89. Additionally, RF analysis quantified the prognostic value of each variable and consistently identified the same 5 variables as the strongest predictors of PR.
A challenge for studying CRS outcomes has been that because no singular end point has been found to characterize success after treatment, both the recent European Position Statement on Rhinosinusitis and International Consensus statements recommend consideration of PROMs, endoscopic, and/or radiographic variables.2,44 Similar to prior studies, we found that PROMs and the presence of polyps were significantly improved following ESS,2,4 and our study constitutes the first confirmation that radiographic severity was also improved with MLM scores being less than half of their pre-ESS levels even 3 years post-ESS. Of the 39% of patients that experienced PR, their polyp size had a median TPS of 2.0, suggesting that most cases of PR were relatively minor. Nonetheless, PR had strong implications on worsened post-ESS PROMs and endoscopic and radiographic outcomes and thus was our primary outcome variable. Interestingly, despite reports that radiographic severity is poorly correlated with PROMs,45 we found that in the post-ESS setting it was moderately well correlated.
Although the importance of type 2 inflammation to CRSwNP pathogenesis has been demonstrated through successful clinical trials of several pharmacologic agents, the ability of type 2 biomarkers to serve as prognostic factors after ESS has only begun to be investigated. Most prior studies evaluated the influence of surrogate markers of type 2 inflammation like asthma, tissue eosinophil density or the presence of eosinophilic mucin.5,16,46–48 Although we do not use tissue eosinophil counts in our study, we previously demonstrated that ECP levels are strongly correlated with tissue eosinophil density.49 Our study replicated the findings of some of these studies and validated asthma, eosinophil density, and radiographic severity as independent prognostic factors for PR. In this study, we additionally compared the relative prognostic importance of a broad spectrum of non-type 2 biomarkers with type 2 inflammatory mediators including the critical cytokines IL-4, IL-5, IL-13, and ECP, which we utilized as a surrogate for tissue eosinophil density. We noted that all of the identified prognostic biomarkers were affiliated with the type 2 cluster of biomarkers in our multivariate models (LASSO, LR, and RF). IL-5 and ECP were identified as having independent prognostic value for PR prediction. The type 2 cytokine IL-5 outcompeted IL-13 in the LASSO model; this was likely due to the collinearity, even though both had predictive potentials as demonstrated by RF models. Few prior studies have been able to compare the relative predictive ability of these critical aspects of type 2 inflammation. Van Zele et al23 had demonstrated that IL-5 and ECP had univariate prognostic importance in a small case-control study. In contrast, 3 very recent studies from China had conflicting results.50–52 In 2 studies that used CRSwNP alone or mixed with CRSsNP, tissue cytokines did not predict CRS status after surgery and all the identified variables were clinical variables.50,51 In contrast, a separate study of patients with CRSwNP found that IL-5 and IL-13 were predictive of “uncontrolled CRS” on univariate analysis but were not independently predictive compared to a remarkably predictive biomarker cystatin-SN.52 We noted that CRSwNP in Chinese patients is significantly less eosinophilic than CRSwNP in Western patients is and has larger proportions of type 1 and 3 inflammations;7 consequently, direct comparability of ESS outcomes in Chinese patients with our study may not be appropriate.
A major motivation for our study was to understand the prognostic implications of elevated anti-dsDNA IgG autoantibodies that we had previously identified in NPs. We have accumulated evidence of highly aberrant local B-cell activation in CRSwNP with evidence for increased plasmablasts and extrafollicular responses promoting autoreactivity; previously studies revealed evidence that anti-dsDNA autoantibodies are associated with worse cross-sectional clinical severity.26–28 In the present study, we sought to understand the prognostic value of anti-dsDNA IgG and its relationship with type 2 inflammation and found that although anti-dsDNA IgG did not correlate with ECP, it however moderately (R = 0.4 and R = 0.5, both P < .0001, respectively) but significantly correlated with IL-13 and IL-5 and resided in the type 2 cluster of inflammatory mediators. As a prognostic biomarker, multivariate LR and RF models further selected anti-dsDNA IgG as an independent predictor for PR. These results echoed prior descriptions of autoimmunity that occurred in some patients with severe airway type 2 inflammation evidenced by highly elevated IL-5 and IL-13 among patients with sputum autoantibodies and severe asthma.53 The clinical implications of these findings are that the development of autoimmune responses in CRSwNP tissue confers independent risk for future persistence and recurrence of disease after ESS. Possible reasons for why this may occur, while still conjectural at this stage, include direct tissue damage from antibody-mediated complement activation or indirectly through the ability of some autoantibodies (eg, anti-phospholipid antibodies) to alter local propensity for coagulation, which forms the substance of NPs. In CRSwNP, we believe the results of this study provide evidence that anti-dsDNA IgG may improve prognostic value after ESS similar to the case with SLE, where serum anti-dsDNA antibodies have great utility in predicting lupus nephritis.54,55 Furthermore, extrapolation of the clinical findings of this article would suggest that in addition to targeting type 2 inflammation, identifying mechanisms to inhibit these local autoimmune responses may be important for the treatment of refractory CRSwNP.
In our statistical and machine learning analyses, we found that models combining clinical variables and biomarkers outperformed models that only used either one. We believe the identified radiographic severity measured via MLM score plus asthma status may together more fully reflect the extent of sinonasal disease and respiratory system involvement than was captured by the biomarkers measured from NP homogenates. NP tissue in patients with CRSwNP who are candidates for ESS are generally abundant and conceivably obtainable prior to ESS via biopsy. Nonetheless, these results suggest that tissue inflammation needs to be considered together with individual patient characteristics for optimal prognostic accuracy.
Admittedly, this study has several limitations. First, although all eligible patients with prospectively biobanked tissue were invited to attend post-ESS follow-up within a fixed time period, this was not necessarily the earliest time that PR developed, and discovery of PR was retrospective. Consequently, a study of post-ESS time to PR analysis could not be performed and the study design can best be described as a retrospective analysis of prospective data. Second, not all patients eligible for the study, in part due to the beginning of the coronavirus disease 2019 pandemic during the study enrollment, were able to participate in the research visit and a small number of eligible patients (2%) had undergone revision surgery between the time of biobanking and enrollment eligibility and were excluded. These effects may bias our study toward recruitment of patients of a certain outcome although we point out that study invitations were done independent of outcome or clinical follow-up. Third, although patients received continued medical management by their treating physicians and these may have affected outcomes, no obvious differences were discovered between those with PR and those without. Fourth, validation with more patients is required to confirm the generalizability of the model we established. Nonetheless, we were reassured that despite relatively modest sample size, we were able to validate the prognostic clinical variables previously identified by other groups.22,46,56 Finally, 3 of the inflammatory mediators, IL-2, IL-8, and IL-17 were frequently out of the linear range of the assays and were excluded. Consequently, we were not able to analyze the influence of type 3-related mediators such as IL-17 on post-ESS outcomes. We do note the type 3 CRSwNP in our prior analysis is rare in Western populations and constitutes only 18% of CRSwNP samples.57
CONCLUSIONS
Our results indicate that PR serves as an emblematic indicator for CRSwNP severity, and it has significant associations with other major outcomes, including PROMs and endoscopic and radiographic severities. Using a combined set of clinical and biological variables in the final LASSO model, we were able to predict PR and severity with high predictive accuracy (AUC = 0.89). Our findings indicate that patients with high ECP, antidsDNA IgG, IL-5, pre-ESS MLM, and asthma before surgery are at highest risk for recurrent polyposis post-ESS. Patients with high levels of these biomarkers and disease characteristics may derive the greatest benefit from adjunctive therapies to prevent subsequent PR.
Supplementary Material
Clinical implications:
PR after sinus surgery for CRSwNP had strong associations with worse clinical and patient-reported outcomes. Three biomarkers augmented 2 clinical variables to accurately predict PR.
Acknowledgments
This work was supported by the National Institutes of Health (grants R01 AI134952 and R01 DC016645) and the Chronic Rhinosinusitis Integrative Studies Program 2 (CRISP2) (grant P01,AI145818).
D. B. Conley has received consulting fees for Intersect ENT and XORAN. K. C. Welch has received consultant fees from Baxter, OptiNose, and Acclarent. R.C. Kern is a consultant for Lyra Therapeutics, GlaxoSmithKline, Sanofi/Regeneron, and Novartis/Genentech. A. T. Peters has received personal fees from AstraZeneca and GlaxoSmithKline and personal fees and grants from Sanofi Regeneron, Merck, and OptiNose. A. Kato has received a consultant fee from Astellas Pharma and a gift for his research from Lyra Therapeutics. W.W. Stevens has served on advisory boards for GlaxoSmithKline, Genentech, and Bristol Meyers Squibb. R. P. Schleimer has received personal fees from Intersect ENT, Merck, GlaxoSmithKline, Sanofi, AstraZeneca/Medimmune, Genentech, Actobio Therapeutics, Lyra Therapeutics, Astellas Pharma Inc, and Otsuka Inc; and has Siglec-8- and Siglec-8 ligand-related patents licensed by Johns Hopkins to Allakos Inc. B. K. Tan has received personal fees from Sanofi Regeneron/Genzyme.
Abbreviations used
- AIC
Akaike information criterion
- anti-dsDNA
Anti-double-stranded DNA
- AUC
Area under the curve
- CRS-PRO
12-Item Chronic Rhinosinusitis Patient-Reported Outcomes measure
- CRSwNP
Chronic rhinosinusitis with nasal polyps
- ECP
Eosinophil cationic protein
- ESS
Endoscopic sinus surgery
- LASSO
Least absolute shrinkage and selection operator
- LR
Logistic regression
- MIP
Macrophage inflammatory protein
- MLK
Modified Lund-Kennedy endoscopic score
- MLM
Modified Lund-Mackay radiographic score
- NP
Nasal polyp
- PR
Polyp recurrence
- PROMs
Patient-reported outcome measures
- RF
Random forest
- ROC
Receiver-operating characteristic
- SNOT−22
22-Item Sinonasal Outcomes Test
- TPS
Total polyp score
Footnotes
Disclosure of potential conflict of interest:
The rest of the authors declare that they have no relevant conflicts of interest.
REFERENCES
- 1.Rudmik L, Soler ZM, Hopkins C, Schlosser RJ, Peters A, White AA, et al. Defining appropriateness criteria for endoscopic sinus surgery during management of uncomplicated adult chronic rhinosinusitis: a RAND/UCLA appropriateness study. Rhinology 2016;54:117–28. [DOI] [PubMed] [Google Scholar]
- 2.Orlandi RR, Kingdom TT, Smith TL, Bleier B, DeConde A, Luong AU, et al. International consensus statement on allergy and rhinology: rhinosinusitis 2021. Int Forum Allergy Rhinol 2021;11:21S–7S9. [DOI] [PubMed] [Google Scholar]
- 3.Loftus CA, Soler ZM, Koochakzadeh S, Desiato VM, Yoo F, Nguyen SA, et al. Revision surgery rates in chronic rhinosinusitis with nasal polyps: meta-analysis of risk factors. Int Forum Allergy Rhinol 2020;10:199–207. [DOI] [PubMed] [Google Scholar]
- 4.DeConde AS, Mace JC, Levy JM, Rudmik L, Alt JA, Smith TL. Prevalence of polyp recurrence after endoscopic sinus surgery for chronic rhinosinusitis with nasal polyposis. Laryngoscope 2017;127:550–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vlaminck S, Vauterin T, Hellings PW, Jorissen M, Acke F, Van Cauwenberge P, et al. The importance of local eosinophilia in the surgical outcome of chronic rhinosinusitis: a S-year prospective observational study. Am J Rhinol Allergy 2014; 28:260–4. [DOI] [PubMed] [Google Scholar]
- 6.Vlaminck S, Acke F, Prokopakis E, Speleman K, Kawauchi H, van Cutsem JC, et al. Surgery in nasal polyp patients: outcome after a minimum observation of 10 years. Am J Rhinol Allergy 2021;S5:449–57. [DOI] [PubMed] [Google Scholar]
- 7.Wang X, Zhang N, Bo M, Holtappels G, Zheng M, Lou H, et al. Diversity of T(H) cytokine profiles in patients with chronic rhinosinusitis: a multicenter study in Europe, Asia, and Oceania. J Allergy Clin Immunol 2016;1S8:1S44–5S. [DOI] [PubMed] [Google Scholar]
- 8.Tan BK, Klingler AI, Poposki JA, Stevens WW, Peters AT, Suh LA, et al. Heterogeneous inflammatory patterns in chronic rhinosinusitis without nasal polyps in Chicago, Illinois. J Allergy Clin Immunol 2017;1S9:699–70Se7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bachert C, Marple B, Hosemann W, Cavaliere C, Wen W, Zhang N. Endotypes of chronic rhinosinusitis with nasal polyps: pathology and possible therapeutic implications. J Allergy Clin Immunol Pract 2020;8:1514–9. [DOI] [PubMed] [Google Scholar]
- 10.Tomassen P, Vandeplas G, Van Zele T, Cardell LO, Arebro J, Olze H, et al. Inflammatory endotypes of chronic rhinosinusitis based on cluster analysis of biomarkers. J Allergy Clin Immunol 2016;1S7:1449–56.e4. [DOI] [PubMed] [Google Scholar]
- 11.Kato A. Immunopathology of chronic rhinosinusitis. Allergol Int 2015;64:121–S0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bochner BS, Stevens WW. Biology and function of eosinophils in chronic rhinosinusitis with or without nasal polyps. Allergy Asthma Immunol Res 2021;13:8–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Y, Gevaert E, Lou H, Wang X, Zhang L, Bachert C, et al. Chronic rhinosinusitis in Asia. J Allergy Clin Immunol 2017;140:1230–9. [DOI] [PubMed] [Google Scholar]
- 14.Bachert C, Marple B, Schlosser RJ, Hopkins C, Schleimer RP, Lambrecht BN, et al. Adult chronic rhinosinusitis. Nat Rev Dis Primers 2020;6:86. [DOI] [PubMed] [Google Scholar]
- 15.Han JK. Subclassification of chronic rhinosinusitis. Laryngoscope 2013; 123(suppl 2):S15–27. [DOI] [PubMed] [Google Scholar]
- 16.Kountakis SE, Arango P, Bradley D, Wade ZK, Borish L. Molecular and cellular staging for the severity of chronic rhinosinusitis. Laryngoscope 2004;114: 1895–905. [DOI] [PubMed] [Google Scholar]
- 17.Gevaert P, Calus L, Van Zele T, Blomme K, De Ruyck N, Bauters W, et al. Omalizumab is effective in allergic and nonallergic patients with nasal polyps and asthma.. J Allergy Clin Immunol 2013;131:110–116e1. [DOI] [PubMed] [Google Scholar]
- 18.Bachert C, Mannent L, Naclerio RM, Mullol J, Ferguson BJ, Gevaert P, et al. Effect of subcutaneous dupilumab on nasal polyp burden in patients with chronic sinusitis and nasal polyposis: a randomized clinical trial. JAMA 2016;315:469–79. [DOI] [PubMed] [Google Scholar]
- 19.Bachert C, Sousa AR, Lund VJ, Scadding GK, Gevaert P, Nasser S, et al. Reduced need for surgery in severe nasal polyposis with mepolizumab: randomized trial. J Allergy Clin Immunol 2017;140:1024–31.e14. [DOI] [PubMed] [Google Scholar]
- 20.Fokkens WJ, Lund V, Bachert C, Mullol J, Bjermer L, Bousquet J, et al. EUFOREA consensus on biologics for CRSwNP with or without asthma. Allergy 2019;74:2312–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han JK, Bosso JV, Cho SH, Franzese C, Lam K, Lane AP, et al. Multidisciplinary consensus on a stepwise treatment algorithm for management of chronic rhinosinusitis with nasal polyps. Int Forum Allergy Rhinol 2021;11:1407–16. [DOI] [PubMed] [Google Scholar]
- 22.Tokunaga T, Sakashita M, Haruna T, Asaka D, Takeno S, Ikeda H, et al. Novel scoring system and algorithm for classifying chronic rhinosinusitis: the JESREC Study. Allergy 2015;70:995–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Zele T, Holtappels G, Gevaert P, Bachert C. Differences in initial immunoprofiles between recurrent and nonrecurrent chronic rhinosinusitis with nasal polyps. Am J Rhinol Allergy 2014;28:192–8. [DOI] [PubMed] [Google Scholar]
- 24.Du K, Zheng M, Zhao Y, Jiao C, Xu W, Hao Y, et al. A nomogram combing peripheral parameters for estimation of CRSwNP recurrence. Am J Rhinol Allergy 2021;35:578–86. [DOI] [PubMed] [Google Scholar]
- 25.Fujieda S, Matsune S, Takeno S, Ohta N, Asako M, Bachert C, et al. Dupilumab efficacy in chronic rhinosinusitis with nasal polyps from SINUS-52 is unaffected by eosinophilic status. Allergy 2022;77:186–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tan BK, Li QZ, Suh L, Kato A, Conley DB, Chandra RK, et al. Evidence for intranasal antinuclear autoantibodies in patients with chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol 2011;128:1198–206.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feldman S, Kasjanski R, Poposki J, Hernandez D, Chen JN, Norton JE, et al. Chronic airway inflammation provides a unique environment for B cell activation and antibody production. Clin Exp Allergy 2017;47:457–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.BaiJ Hulse KE, Huang JH Schleimer RP, Tan BK. Anti-dsDNA specific antibody secreting cells are increased in both frequency and abundance in chronic rhinosinusitis with nasal polyps. J Immunol 2020;204 (abstr). [Google Scholar]
- 29.Kato A, Peters A, Suh L, Carter R, Harris KE, Chandra R, et al. Evidence of a role for B cell-activating factor of the TNF family in the pathogenesis of chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol 2008;121:1385–92, 92e1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jeffe JS, Seshadri S, Hamill KJ, Huang JH, Carter R, Suh L, et al. A role for anti-BP180 autoantibodies in chronic rhinosinusitis. Laryngoscope 2013;123:2104–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Roey GA, Vanison CC, Wu J, Huang JH, Suh LA, Carter RG, et al. Classical complement pathway activation in the nasal tissue of patients with chronic rhinosinusitis. J Allergy Clin Immunol 2017;140:89–100.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Min JY, Ocampo CJ, Stevens WW, Price CPE, Thompson CF, Homma T, et al. Proton pump inhibitors decrease eotaxin-3/CCL26 expression in patients with chronic rhinosinusitis with nasal polyps: possible role of the nongastric H,K-ATPase. J Allergy Clin Immunol 2017;139:130–41.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Psaltis AJ, Li G, Vaezeafshar R, Cho KS, Hwang PH. Modification of the LundKennedy endoscopic scoring system improves its reliability and correlation with patient-reported outcome measures. Laryngoscope 2014;124:2216–23. [DOI] [PubMed] [Google Scholar]
- 34.Okushi T, Nakayama T, Morimoto S, Arai C, Omura K, Asaka D, et al. A modified Lund-Mackay system for radiological evaluation of chronic rhinosinusitis. Auris Nasus Larynx 2013;40:548–53. [DOI] [PubMed] [Google Scholar]
- 35.Hopkins C, Gillett S, Slack R, Lund VJ, Browne JP. Psychometric validity of the 22-item Sinonasal Outcome Test. Clin Otolaryngol 2009;34:447–54. [DOI] [PubMed] [Google Scholar]
- 36.Ghadersohi S, Price CPE, Jensen SE, Beaumont JL, Kern RC, Conley DB, et al. Development and preliminary validation of a new patient-reported outcome measure for chronic rhinosinusitis (CRS-PRO). J Allergy Clin Immunol Pract 2020; 8:2341–50.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin KA, Price CPE, Huang JH, Ghadersohi S, Cella D, Kern RC, et al. Responsiveness and convergent validity of the chronic rhinosinusitis patient-reported outcome (CRS-PRO) measure in CRS patients undergoing endoscopic sinus surgery. Int Forum Allergy Rhinol 2021;11:1308–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Akoglu H. User’s guide to correlation coefficients. Turk J Emerg Med 2018;18: 91–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schober P, Boer C, Schwarte LA. Correlation coefficients: appropriate use and interpretation. Anesth Analg 2018;126:1763–8. [DOI] [PubMed] [Google Scholar]
- 41.Tibshiranit R. Regression shrinkage and selection via the lasso. J Royal Stat Soc 1996;58:267–88. [Google Scholar]
- 42.Meinshausen N. Relaxed lasso. 2006. [Google Scholar]
- 43.Breiman L. Random forests. Mach Learn 2001;45:5–32. [Google Scholar]
- 44.Fokkens WJ, Lund VJ, Hopkins C, Hellings PW, Kern R, Reitsma S, et al. European position paper on rhinosinusitis and nasal polyps 2020. Rhinology 2020; 58(suppl S29):1–464. [DOI] [PubMed] [Google Scholar]
- 45.Hopkins C, Browne JP, Slack R, Lund V, Brown P. The Lund-Mackay staging system for chronic rhinosinusitis: how is it used and what does it predict? Otolaryngol Head Neck Surg 2007;137:555–61. [DOI] [PubMed] [Google Scholar]
- 46.Nakayama T, Yoshikawa M, Asaka D, Okushi T, Matsuwaki Y, Otori N, et al. Mucosal eosinophilia and recurrence of nasal polyps—new classification of chronic rhinosinusitis. Rhinology 2011;49:392–6. [DOI] [PubMed] [Google Scholar]
- 47.McHugh T, Snidvongs K, Xie M, Banglawala S, Sommer D. High tissue eosinophilia as a marker to predict recurrence for eosinophilic chronic rhinosinusitis: a systematic review and meta-analysis. Int Forum Allergy Rhinol 2018;8: 1421–9. [DOI] [PubMed] [Google Scholar]
- 48.Lou H, Meng Y, Piao Y, Zhang N, Bachert C, Wang C, et al. Cellular phenotyping of chronic rhinosinusitis with nasal polyps. Rhinology 2016;54:150–9. [DOI] [PubMed] [Google Scholar]
- 49.Stevens WW, Ocampo CJ, Berdnikovs S, Sakashita M, Mahdavinia M, Suh L, et al. Cytokines in chronic rhinosinusitis. role in eosinophilia and aspirin-exacerbated respiratory disease. Am J Respir Crit Care Med 2015;192:682–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pan L, Liao B, Guo CL, Liu JX, Wang H, Long XB, et al. Inflammatory features and predictors for postsurgical outcomes in patients with nasal polyps stratified by local and systemic eosinophilia. Int Forum Allergy Rhinol 2021;11: 846–56. [DOI] [PubMed] [Google Scholar]
- 51.Guo CL, Liao B, Liu JX, Pan L, Liu Z. Predicting difficult-to-treat chronic rhinosinusitis by noninvasive biological markers. Rhinology 2021;59:81–90. [DOI] [PubMed] [Google Scholar]
- 52.Wu D, Yan B, Wang Y, Wang C, Zhang L. Prognostic and pharmacologic value of cystatin SN for chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol 2021;148:450–60. [DOI] [PubMed] [Google Scholar]
- 53.Mukherjee M, Bulir DC, Radford K, Kjarsgaard M, Huang CM, Jacobsen EA, et al. Sputum autoantibodies in patients with severe eosinophilic asthma. J Allergy Clin Immunol 2018;141:1269–79. [DOI] [PubMed] [Google Scholar]
- 54.Rekvig OP. Anti-dsDNA antibodies as a classification criterion and a diagnostic marker for systemic lupus erythematosus: critical remarks. Clin Exp Immunol 2015;179:5–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pisetsky DS, Lipsky PE. New insights into the role of antinuclear antibodies in systemic lupus erythematosus. Nat Rev Rheumatol 2020;16:565–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sella GCP, Tamashiro E, Sella JA, Aragon DC, Mendonca TN, Arruda LKP, et al. Asthma is the dominant factor for recurrence in chronic rhinosinusitis. J Allergy Clin Immunol Pract 2020;8:302–9. [DOI] [PubMed] [Google Scholar]
- 57.Stevens WW, Peters AT, Tan BK, Klingler AI, Poposki JA, Hulse KE, et al. Associations between inflammatory endotypes and clinical presentations in chronic rhinosinusitis. J Allergy Clin Immunol Pract 2019;7:2812–20.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.