Abstract
Background
The HEART Pathway is a validated protocol for risk stratifying emergency department (ED) patients with possible acute coronary syndrome (ACS). Its performance in different age groups is unknown. The objective of this study is to evaluate its safety and effectiveness among older adults.
Methods
A pre‐planned subgroup analysis of the HEART Pathway implementation study was conducted. This prospective interrupted time series accrued adult ED patients with possible ACS who were without ST‐elevation across three US sites from 11/2013–01/2016. After implementation, providers prospectively used the HEART Pathway to stratify patients as low‐risk or non‐low‐risk. Patients were classified as older adults (≥65 years), middle‐aged (46–64 years), and young (21–45 years). Primary safety and effectiveness outcomes were 30‐day death or MI and hospitalization at 30 days, determined from health records, insurance claims, and death index data. Fisher's exact test compared low‐risk proportions between groups. Sensitivity for 30‐day death or MI and adjusted odds ratios (aORs) for hospitalization and objective cardiac testing were calculated.
Results
The HEART Pathway implementation study accrued 8474 patients, of which 26.9% (2281/8474) were older adults, 45.5% (3862/8474) middle‐aged, and 27.5% (2331/8474) were young. The HEART Pathway identified 7.4% (97/1303) of older adults, 32.0% (683/2131) of middle‐aged, and 51.4% (681/1326) of young patients as low‐risk (p < 0.001). The HEART Pathway was 98.8% (95% CI 97.1–100) sensitive for 30‐day death or MI among older adults. Following implementation, the rate of 30‐day hospitalization was similar among older adults (aOR 1.25, 95% CI 1.00–1.55) and cardiac testing increased (aOR 1.25, 95% CI 1.04–1.51).
Conclusion
The HEART Pathway identified fewer older adults as low‐risk and did not decrease hospitalizations in this age group.
Keywords: accelerated diagnostic protocol, acute coronary syndrome, geriatric, HEART Pathway, older adults
Key points
The safety and effectiveness of the HEART Pathway among older adults (≥65 years old) has not been previously investigated.
The HEART Pathway achieved high sensitivity (98.8%, 95% CI 97.1–100) for 30‐day death or MI among older adults.
Few older adults (7.4%) were classified as low‐risk, the rate of hospitalization was unchanged (aOR 1.25, 95% CI 1.00–1.55), and the rate of objective cardiac testing increased (aOR 1.25, 95% CI 1.04–1.51).
Why does this paper matter?
Use of the HEART Pathway in older adults is safe, but it increases cardiac testing and does not reduce hospitalizations.
INTRODUCTION
Each year 8–10 million patients visit an emergency department (ED) in the United States due to acute chest pain and are evaluated for acute coronary syndrome (ACS). 1 , 2 Although ACS occurs in <10% of ED patients with acute chest pain, nearly $10–13 billion is spent annually on comprehensive cardiovascular evaluations, which often include hospital admission, serial biomarkers, and objective cardiac testing . 3 , 4 , 5 , 6 , 7 Evaluating and risk stratifying patients for ACS is a high‐stakes and challenging process. 8 , 9 , 10 , 11 , 12 , 13 This is particularly challenging among older adults, because they are less likely to have classic ACS presentations. 8 , 14 , 15 , 16 To help with risk stratification, accelerated diagnostic protocols (ADPs), such as the History, Electrocardiogram, Age, Risk factors, and Troponin Pathway (HEART Pathway), were developed.
The safety and effectiveness of the HEART Pathway, which is widely used across the United States, are well demonstrated. 3 , 6 , 7 In prior studies, the HEART Pathway safely decreased the rate of hospitalizations, hospital length of stay, and cost among ED patients with acute chest pain. 1 , 2 , 3 , 4 , 5 Furthermore, the HEART Pathway has been shown to perform well among key subgroups, such as women and patients of non‐White race. 6 However, there is a lack of data evaluating whether the performance of the HEART Pathway differs by age group. Prior studies, which demonstrate that older adults are more likely to be hospitalized for chest pain, are at higher risk for developing ACS, and that those with ACS suffer worse outcomes, suggest that differences in HEART Pathway performance may occur with increasing age. 15 , 17 , 18 , 19 , 20 Thus, our objective was to compare the safety and effectiveness of the HEART Pathway among older adults, middle‐aged patients, and young patients presenting to the ED with acute chest pain.
METHODS
Study design
A pre‐planned secondary analysis of the HEART Pathway implementation study was conducted. The HEART Pathway implementation study is a prospective pre‐post interrupted time series study, which accrued ED patients with acute chest pain from 11/2013 to 01/2016. The Wake Forest University Health Sciences Institutional Review Board approved the study protocol and granted a waiver of informed consent. The study was registered with clinicaltrials.gov (NCT02056964). The methods are previously described. 3 , 7 , 21 The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines helped direct the research process. 22
Study setting and population
Eligible patients were accrued from EDs in three North Carolina (NC) hospitals: Wake Forest Baptist Medical Center (WFBMC), a tertiary‐care, academic hospital with approximately 114,000 annual encounters; Davie Medical Center (DMC), a community ED with approximately 12,000 annual encounters; and Lexington Medical Center (LMC), a community ED with approximately 37,000 annual encounters. Patients ≥21 years of age who presented with symptoms concerning for ACS were accrued. Patients with electrocardiogram (ECG) evidence of ST‐elevation myocardial infarction (STEMI) were excluded.
WFBMC and DMC accrued patients into the pre‐implementation cohort from 11/2013 to 10/2014 and the post‐implementation cohort from 02/2015 to 01/2016. A wash‐in period (11/2014–01/2015) allowed clinicians to train on the electronic health record (EHR) based HEART Pathway tool. LMC accrued patients into the pre‐implementation cohort from 01/2015 to 07/2015 and the post‐implementation cohort from 08/2015–01/2016 with a wash‐in period from 07/2015 to 08/2015. To prevent accruing repeat ED users in the pre‐implementation cohort, patients who visited the ED with possible ACS in the year prior to the study were excluded (N = 523). Patients who were transferred or who had encounters at multiple sites were classified based on their original ED encounter location.
For this analysis patients were classified by age group. Consistent with prior HEART Pathway studies, patients ≥65 years old were classified as older adults, 46–64 years old as middle‐aged, and 21–45 years old as young. 3 , 6 , 7 Age was determined at the time of index visit and was based on the patient's date of birth in the EHR.
Data collection
Index encounter data (from initial ED presentation to discharge from the ED, observation unit, or inpatient ward) were extracted from the health system's EHR data (Clarity‐Epic Systems Corporation, Verona, WI). Pre‐validated structured EHR variables or diagnoses and procedure codes (CPT, ICD9, and ICD10) were used to obtain demographics, past medical history, cardiovascular risk factors, comorbidities, troponin results, HEART Pathway assessments, disposition, diagnoses, and vital status. 23 , 24 , 25 , 26 , 27 Outcomes at 30 days were determined using EHR data for within‐network visits, insurance claims, and the North Carolina State Center for Health Statistics death index. Claims data were available on patients with Blue Cross Blue Shield (BCBS) of NC (the largest insurer in the state), MedCost, and NC Medicaid.
HEART Pathway implementation
The HEART Pathway ADP was integrated into the EHR as an interactive clinical decision support (CDS) tool once the pre‐implementation period concluded. A best practice advisory (BPA) pop‐up alert occurred for patients with chest pain who had at least one troponin ordered. This BPA directed providers to complete a HEART Pathway assessment. Additionally, the HEART Pathway CDS was integrated into an EHR flowsheet, enabling providers to manually perform a HEART Pathway assessment (without the BPA). Thus, HEART Pathway assessments could be completed for patients with other signs or symptoms concerning for ACS (such as dyspnea, nausea, or radiating pain) or prior to ordering a troponin.
The HEART Pathway CDS integrated the History, ECG, Age, and Risk factor (HEAR) score and serial troponin measurements. Providers answered flowsheet questions for patients without STEMI, known coronary artery disease [CAD; defined as prior myocardial infarction (MI), prior coronary revascularization, or known coronary stenosis ≥70%], or new ischemic changes (new T‐wave inversions or ST‐segment depressions in contiguous leads) to calculate a HEAR score based off the HEART Pathway trial algorithm (Impathiq Inc., Raleigh, NC). 7 This HEAR score differs from the HEART score as the HEAR score use objective, binary questions instead of a subjective, gestalt‐based risk assessment. The HEART Pathway CDS also uses serial troponins at 0 and 3 h. Serum troponin was measured using the ADVIA Centaur platform TnI‐Ultra™ assay (Siemens, Munich, Germany) or the Access AccuTnI+3 assay (Beckman Coulter, Brea, CA).
Patients were classified as low‐risk or non‐low‐risk. Low‐risk patients had a non‐ischemic ECG, no known CAD, a HEAR score ≤3, and non‐elevated serial troponins and were deemed safe for discharge from the ED without objective cardiac testing (defined as stress testing, coronary computed tomography angiography [CCTA], or invasive coronary angiography). Non‐low‐risk patients had a HEAR score ≥4, an elevated troponin, known CAD, or ischemic ECG findings. Admission and/or objective cardiac testing were recommended for these patients. Figure S1 displays the HEART Pathway algorithm.
Outcomes
The primary safety outcome was the composite of all‐cause death or MI within 30 days of the index encounter. Coronary revascularization rate, a secondary endpoint, was defined as coronary artery bypass grafting, stent placement, or other percutaneous coronary intervention. Major adverse cardiac events (MACE) were evaluated as another secondary endpoint and defined as the composite of all‐cause death, MI, or revascularization. Acute MI and coronary revascularization were determined using diagnosis and procedure codes validated by prior cardiovascular trials. 23 , 24 , 25 , 26 , 27 Patients without 30‐day data from the EHR, insurers, or death index were considered free of 30‐day safety events for the primary analysis. 3 , 7 , 28 , 29 , 30
The primary effectiveness outcome was the rate of 30‐day hospitalization from the index encounter. Hospitalization included inpatient admission, transfer, and observation stay (including index observation unit care). Secondary outcomes included the proportion of patients receiving objective cardiac testing within 30 days of the index encounter and the early discharge rate, which was defined as the proportion of patients discharged from the ED without objective cardiac testing.
Statistical analysis
The statistical design of the HEART Pathway implementation study is previously described. 7 , 21 Patient characteristics were described by pre‐ and post‐implementation cohorts within each age group. Between age groups, categorical variables were compared with chi‐square tests and continuous variables were compared with Wilcoxon rank‐sum tests.
Unadjusted logistic regression was used to model the relationship between pre‐ and post‐implementation periods and study outcomes within each subgroup. These models were then adjusted for potential confounders, which were selected a priori: sex, race, ethnicity, insurance status, enrollment site, prior known CAD, diabetes, hypertension, hyperlipidemia, smoking, body mass index (BMI), and the presence of chest pain versus other symptoms concerning for ACS. To test for significant differences in implementation between age groups, logistic regression models were fit using the overall population, including age by implementation cohort (pre vs post) interaction terms. The same potential confounders were included in these models. BMI was missing for 2.9% of patients, so multivariate imputation, with replacement by predictive mean matching utilizing all predictors and outcome variables, was used to create 10 datasets with complete BMI data. No other covariates required imputation. Logistic models were fit for each imputed dataset and results averaged across sets. Adjusted odds ratios (aOR) and 95% confidence intervals (95% CIs) were derived for each outcome.
Post‐implementation, the percentage of patients identified as low‐risk and non‐low‐risk were calculated within each age group to determine the sensitivity, specificity, and positive and negative predictive values (PPV and NPV) of the HEART Pathway for all‐cause death or MI. Corresponding 95% exact binomial confidence intervals were computed. Positive and negative likelihood ratios were also computed along with 95% CIs that were calculated following the approach of Simel et al. 31 The proportion of safety events and effectiveness outcomes in the low‐risk post‐implementation cohort were compared between ages using Fisher's exact and chi‐square tests. Individual components of the HEART Pathway were also compared between age groups using chi‐square tests. Sensitivity analyses were conducted for older adults aged 65–75 and ≥75 years old.
RESULTS
Characteristics of the study subjects
The HEART Pathway implementation study accrued 8474 patients over 24 months. Figure S2 presents the participant flow diagram. The cohort was 53.6% (4541/8474) female, 34.0% (2884/8474) non‐White, and had a median age of 54 years (IQR 44–66). Older adults accounted for 26.9% (978 pre‐ and 1303 post‐implementation), middle‐aged patients 45.5% (1730 pre‐ and 2132 post‐implementation), and young patients 27.5% (1005 pre‐ and 1326 post‐implementation) of the sample. Table 1 summarizes cohort characteristics by age group. There was no statistically significant difference in the pre‐ and post‐implementation age group proportions (p = 0.25). At 30 days, death or MI occurred in 7.2% (611/8474) and revascularization without MI occurred in 1.0% (87/8474) of patients. The proportion of 30‐day death or MI was similar in the pre‐ and post‐implementation cohorts (p = 0.41). Table S1 describes the individual subcomponents of the HEART Pathway by age group.
TABLE 1.
Pre‐ and post‐implementation cohort characteristics by age group
| Older adult (≥65 years) | Middle‐aged (46–64 years) | Young (21–45 years) | ||||
|---|---|---|---|---|---|---|
| N = 2281 | N = 3862 | N = 2331 | ||||
| Patient characteristics | Pre | Post | Pre | Post | Pre | Post |
| N = 978 | N = 1303 | N = 1730 | N = 2132 | N = 1005 | N = 1326 | |
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| Age—median (IQR) | 73.5 (11.8) | 73 (11.0) | 55 (9.0) | 54 (9.0) | 39 (10.0) | 39 (8.0) |
| Female | 542 (55.4) | 743 (57.0) | 886 (51.2) | 1121 (53.6) | 537 (53.4) | 712 (53.7) |
| Race | ||||||
| White | 779 (79.7) | 1006 (77.2) | 1098 (63.5) | 1331 (62.4) | 607 (60.4) | 796 (58.0) |
| Non‐White | 199 (20.3) | 297 (22.8) | 632 (36.5) | 801 (37.6) | 398 (39.6) | 557 (42.0) |
| Ethnicity | ||||||
| Hispanic or Latino | 16 (1.6) | 34 (2.6) | 57 (3.3) | 93 (4.4) | 61 (6.1) | 103 (7.8) |
| Site | ||||||
| WFBMC | 724 (74.0) | 1008 (77.4) | 1273 (73.6) | 1722 (80.8) | 723 (71.9) | 955 (72.0) |
| DMC | 86 (8.8) | 130 (10.0) | 186 (10.8) | 194 (9.1) | 124 (12.3) | 188 (14.2) |
| LMC | 168 (17.2) | 165 (12.7) | 271 (15.7) | 216 (10.1) | 158 (15.7) | 183 (13.8) |
| Insurance status | ||||||
| Blue cross | 154 (15.7) | 125 (9.6) | 442 (25.5) | 539 (25.3) | 194 (19.3) | 306 (23.1) |
| MedCost | 11 (1.1) | 13 (1.0) | 123 (7.1) | 167 (7.8) | 75 (7.5) | 106 (8.0) |
| Medicaid | 12 (1.2) | 18 (1.4) | 262 (15.1) | 378 (17.7) | 231 (23.0) | 291 (21.9) |
| Medicare | 741 (75.8) | 1088 (83.5) | 359 (20.8) | 423 (19.8) | 89 (8.9) | 106 (8.0) |
| Other insurance | 31 (3.2) | 38 (2.9) | 192 (11.1) | 244 (11.4) | 98 (9.8) | 131 (9.9) |
| Self‐pay | 29 (3.0) | 21 (1.6) | 352 (20.3) | 381 (17.9) | 318 (31.6) | 386 (29.1) |
| Risk factors | ||||||
| Prior CAD | 496 (50.7) | 647 (49.7) | 453 (26.2) | 529 (24.8) | 87 (8.7) | 104 (7.8) |
| Diabetes | 367 (37.5) | 485 (37.2) | 508 (29.4) | 615 (28.8) | 156 (15.5) | 190 (14.3) |
| Hyperlipidemia | 620 (63.4) | 858 (65.8) | 749 (43.3) | 900 (42.2) | 159 (15.8) | 235 (17.7) |
| Hypertension | 833 (85.2) | 1101 (84.5) | 1183 (68.4) | 1387 (65.1) | 390 (38.8) | 498 (37.6) |
| Smoking | 587 (60.0) | 731 (56.1) | 1146 (66.2) | 1346 (63.1) | 623 (62.0) | 801 (60.4) |
| BMI ≥30 kg/m2 | 332 (33.9) | 452 (34.7) | 845 (48.8) | 1065 (50.0) | 517 (51.4) | 678 (51.1) |
| PVD | 243 (24.8) | 358 (27.5) | 172 (9.9) | 220 (10.3) | 35 (3.5) | 57 (4.3) |
| Cerebrovascular disease | 248 (25.4) | 308 (23.6) | 166 (9.6) | 226 (10.6) | 42 (4.2) | 60 (4.5) |
| Comorbidities | ||||||
| COPD | 368 (37.6) | 526 (40.4) | 556 (32.1) | 689 (32.3) | 249 (24.8) | 328 (24.7) |
| Cancer | 314 (32.1) | 383 (29.4) | 209 (12.1) | 295 (13.8) | 47 (4.7) | 68 (5.1) |
| CKD | 229 (23.4) | 321 (24.6) | 140 (8.1) | 195 (9.1) | 47 (4.7) | 60 (4.5) |
Abbreviations: BMI, body mass index; CAD, coronary artery disease; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; DMC, Davie Medical Center; IQR, interquartile range; LMC, Lexington Medical Center; PVD, peripheral vascular disease; WFBMC, Wake Forest Baptist Medical Center.
Safety
The 30‐day death or MI rate before and after HEART Pathway implementation was similar among older adults (aOR 1.31, 95% CI 0.99–1.71), middle‐aged (aOR 1.23, 95% CI 0.94–1.61), and young patients (aOR 0.96, 95% CI 0.56–1.62) (Figure 1). The interaction terms between HEART Pathway implementation cohort and age group (older adults vs young and older adults vs middle‐aged) were not significant for 30‐day death or MI (p = 0.27 and 0.52, respectively). Table 2 details these aORs and interaction terms. The HEART Pathway identified 7.4% (97/1303) of older adults, 32.0% (683/2132) of middle‐aged, and 51.4% (681/1326) of young patients as low‐risk. Among low‐risk patients, the 30‐day death or MI rate was 2.1% (2/97, 95% CI 0.4–8.0) for older adults, 0.3% (2/683, 95% CI 0.1–1.2) for middle‐aged, and 0.3% (2/681, 95% CI 0.1–1.4) for young patients (p = 0.09) (Table 3). The two safety events among low‐risk older adults were non‐cardiac deaths: one from subarachnoid hemorrhage and the other after prolonged hospitalization for encephalopathy. Each low‐risk patient safety event is described in Table S2.
FIGURE 1.
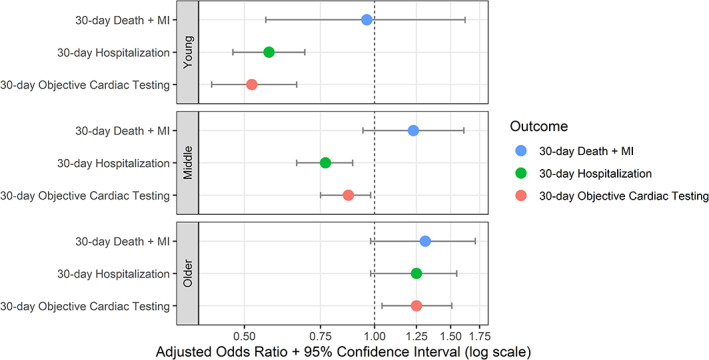
Pre‐ versus post‐implementation HEART Pathway outcomes by age group. MI, myocardial infarction
TABLE 2.
Safety and effectiveness events by age group in the pre‐ and post‐implementation cohorts
| Older adult (≥65 years) | Middle‐aged (46–64 years) | Young (21–45 years) | Interaction | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age X Implementation Cohort (Baseline: Older adult) | |||||||||||
| Pre | Post | aOR a | Pre | Post | aOR a | Pre | Post | aOR a | Young * Implementation Cohort | Middle‐aged * Implementation Cohort | |
| N = 978 (%) | N = 1303 (%) | N = 1730 (%) | N = 2132 (%) | N = 1005 (%) | N = 1326 (%) | p‐value | p‐value | ||||
| Safety | |||||||||||
| Index | |||||||||||
| Death b | 4 (0.4) | 9 (0.7) | 1.69 (0.52–5.52) | 2 (0.1) | 5 (0.2) | 2.03 (0.39–10.48) | 1 (0.1) | 1 (0.1) | 0.76 (0.05–12.2) | 0.60 | 0.86 |
| MI | 85 (8.7) | 140 (10.7) | 1.42 (1.05–1.92) | 98 (5.7) | 138 (6.5) | 1.38 (1.04–1.84) | 28 (2.8) | 36 (2.7) | 1.05 (0.60–1.83) | 0.25 | 0.63 |
| Revascularization b | 41 (4.2) | 68 (5.2) | 1.26 (0.85–1.87) | 71 (4.1) | 75 (3.5) | 0.85 (0.61–1.19) | 7 (0.7) | 11 (0.8) | 1.19 (0.46–3.09) | 0.92 | 0.14 |
| Death + MI | 88 (9.0) | 147 (11.3) | 1.45 (1.08–1.95) | 100 (5.8) | 141 (6.6) | 1.38 (1.04–1.84) | 29 (2.9) | 37 (2.8) | 1.03 (0.60–1.77) | 0.21 | 0.57 |
| MACE (Death + MI + Revasc) | 100 (10.2) | 164 (12.6) | 1.43 (1.08–1.90) | 126 (7.3) | 153 (7.2) | 1.14 (0.87–1.49) | 31 (3.1) | 38 (2.9) | 1.00 (0.58–1.70) | 0.18 | 0.15 |
| 30‐Day follow‐up | |||||||||||
| Death b | 25 (2.6) | 20 (1.5) | 0.59 (0.33–1.08) | 9 (0.5) | 3 (0.1) | 0.27 (0.07–1.00) | 3 (0.3) | 1 (0.1) | 0.25 (0.03–2.43) | 0.47 | 0.28 |
| MI c | 7 (0.7) | 12 (0.9) | 1.32 (0.52–3.36) | 10 (0.6) | 11 (0.5) | 0.90 (0.38–2.13) | 1 (0.1) | 6 (0.5) | 4.59 (0.55–38.23) | 0.29 | 0.56 |
| Revascularization d | 7 (0.7) | 18 (1.4) | 2.00 (0.83–4.83) | 16 (0.9) | 15 (0.7) | 0.77 (0.38–1.57) | 2 (0.2) | 5 (0.4) | 1.90 (0.37–9.84) | 0.96 | 0.10 |
| Death + MI c | 29 (3.0) | 31 (2.4) | 0.85 (0.51–1.43) | 18 (1.0) | 13 (0.6) | 0.60 (0.29–1.24) | 3 (0.3) | 7 (0.5) | 1.85 (0.48–7.23) | 0.29 | 0.46 |
| MACE (Death + MI + Revasc) c | 35 (3.6) | 32 (3.3) | 0.99 (0.63–1.57) | 13 (0.6) | 24 (1.1) | 0.72 (0.41–1.25) | 5 (0.5) | 10 (0.8) | 1.52 (0.51–4.49) | 0.44 | 0.36 |
| 30‐Day (Index + Follow‐up) | |||||||||||
| Death b | 29 (3.0) | 29 (2.2) | 0.75 (0.44–1.26) | 11 (0.6) | 8 (0.4) | 0.59 (0.24–1.47) | 4 (0.4) | 2 (0.2) | 0.38 (0.07–2.07) | 0.46 | 0.66 |
| MI | 88 (9.0) | 144 (11.1) | 1.42 (1.06–1.91) | 106 (6.1) | 143 (6.7) | 1.32 (1.00–1.75) | 29 (2.9) | 37 (2.8) | 1.04 (0.60–1.80) | 0.23 | 0.47 |
| Revascularization d | 48 (4.9) | 85 (6.5) | 1.38 (0.96–1.99) | 86 (5.0) | 89 (4.2) | 0.84 (0.62–1.14) | 9 (0.9) | 16 (1.2) | 1.36 (0.60–3.08) | 0.97 | 0.04 |
| Death + MI | 110 (11.2) | 166 (12.7) | 1.31 (0.99–1.71) | 116 (6.7) | 148 (6.9) | 1.23 (0.94–1.61) | 32 (3.2) | 39 (2.9) | 0.96 (0.56–1.62) | 0.27 | 0.52 |
| MACE (Death + MI + Revasc) | 124 (12.7) | 189 (14.5) | 1.33 (1.02–1.72) | 144 (8.3) | 164 (7.7) | 1.06 (0.82–1.37) | 35 (3.5) | 42 (3.2) | 0.95 (0.57–1.58) | 0.22 | 0.13 |
| Effectiveness | |||||||||||
| Index | |||||||||||
| Hospitalization | 737 (75.4) | 1012 (77.7) | 1.20 (0.96–1.48) | 1102 (63.7) | 1208 (56.7) | 0.78 (0.67–0.90) | 329 (39.0) | 362 (27.3) | 0.58 (0.47–0.70) | <0.0001 | 0.0002 |
| Objective Cardiac Testing | 290 (29.7) | 444 (34.1) | 1.29 (1.07–1.56) | 636 (36.8) | 697 (32.7) | 0.86 (0.74–0.99) | 219 (21.8) | 166 (12.5) | 0.50 (0.40–0.64) | <0.001 | <0.001 |
| Early Discharge | 220 (22.5) | 268 (20.6) | 0.84 (0.67–1.05) | 580 (33.5) | 860 (40.3) | 1.28 (1.10–1.49) | 590 (58.7) | 918 (69.2) | 1.61 (1.33–1.95) | <0.001 | 0.0004 |
| 30‐Day (Index ± Follow‐up) | |||||||||||
| Hospitalization | 752 (76.9) | 1035 (79.4) | 1.25 (1.00–1.55) | 1127 (65.1) | 1235 (57.9) | 0.77 (0.66–0.89) | 409 (40.7) | 379 (28.6) | 0.57 (0.47–0.69) | <0.001 | <0.001 |
| Objective Cardiac Testing | 330 (33.7) | 487 (37.4) | 1.25 (1.04–1.51) | 699 (40.4) | 771 (36.2) | 0.87 (0.75–1.00) | 252 (25.1) | 204 (15.4) | 0.52 (0.42–0.66) | <0.001 | 0.0003 |
Note: Bold font denotes findings of statistical significance.
Abbreviations: aOR, adjusted odds ratio; MI, myocardial infarction.
Models adjusted for sex, race, ethnicity, BMI, ED location, insurance status, smoking, history of CAD, diabetes, hyperlipidemia, hypertension, and the presence of chest pain versus other symptoms concerning for acute coronary syndrome.
Index death, index revascularization, and 30‐day death are unadjusted given the small number of events.
Adjusted for sex, race, and ED location given small number of events.
30‐Day revascularization is only adjusted for sex given the small number of events.
TABLE 3.
Low‐risk patient 30‐day safety and effectiveness events in the post‐implementation cohort
| Older adult (≥65 years) | Middle‐aged (46–64 years) | Young (21–45 years) | |
|---|---|---|---|
| N = 97 (%) | N = 683 (%) | N = 681 (%) | |
| n (%, 95% CI) | n (%, 95% CI) | n (%, 95% CI) | |
| Safety | |||
| Death | 2 (2.1, 0.4–8.0) | 1 (0.1, 0–0.9) | 1 (0.1, 0–1.0) |
| MI | 0 (0, 0–4.8) | 1 (0.1, 0–0.9) | 1 (0.1, 0–1.0) |
| Revascularization | 0 (0, 0–4.8) | 1 (0.1, 0–0.9) | 1 (0.1, 0–1.0) |
| Death + MI | 2 (2.1, 0.4–8.0) | 2 (0.3, 0.1–1.2) | 2 (0.3, 0.1–1.4) |
| MACE (Death + MI + Revasc) | 2 (2.1, 0.4–8.0) | 3 (0.4, 0.1–1.4) | 2 (0.3, 0.1–1.4) |
| Effectiveness | |||
| Hospitalization | 29 (29.9, 21.2–40.2) | 136 (19.9, 17.0–23.1) | 103 (15.1, 12.6–18.1) |
| Objective cardiac testing | 15 (15.5, 9.2–24.5) | 87 (12.7, 10.4–15.5) | 54 (7.9, 6.0–10.3) |
| Early discharge | 72 (74.2, 64.2–82.3) | 554 (81.1, 77.9–83.9) | 577 (84.7, 81.8–87.3) |
Abbreviation: MI, myocardial infarction.
The HEART Pathway achieved high sensitivity for 30‐day death or MI across all groups. Among older adults, it was 98.8% (95% CI 97.1–100) sensitive. By comparison, the sensitivity among middle‐aged patients was 98.6% (95% CI 96.7–100) (p = 1.0) and 94.9% (95% CI 88.0–100) among young patients (p = 0.17). Test characteristics of the HEART Pathway for each age group are summarized in Table 4.
TABLE 4.
HEART Pathway test characteristics of low‐risk and high‐risk assessments for the outcome of all‐cause death or MI at 30 days
| Age group | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) | +LR (95% CI) | −LR (95% CI) |
|---|---|---|---|---|---|---|
| Older adult (≥65 years) | 98.8 (97.1–100) | 85.4 (83.2–87.6) | 46.7 (40.7–52.6) | 97.9 (95.1–100) | 5.26 (4.43–6.25) | 0.13 (0.03–0.52) |
| Middle‐aged (46–64 years) | 98.6 (96.7–100) | 92.0 (90.7–93.2) | 44.0 (37.9–50.2) | 99.7 (99.3–100) | 9.43 (7.85–11.33) | 0.04 (0.01–0.14) |
| Young (21–45 years) | 94.9 (88.0–100) | 94.1 (92.5–95.6) | 35.0 (24.7–45.2) | 99.7 (99.3–100) | 13.18 (9.68–17.95) | 0.09 (0.02–0.33) |
Abbreviations: LR, likelihood ratio; NPV, negative predictive value; PPV, positive predictive value.
Effectiveness
After HEART Pathway implementation, 79.4% (1035/1303) of older adults were hospitalized between the index visit and 30‐day follow‐up, compared to 76.9% (752/978) pre‐implementation, an absolute increase of 2.5%; aOR 1.25 (95% CI 1.00–1.55). Among middle‐aged patients, 57.9% (1235/2132) were hospitalized post‐implementation versus 65.1% (1127/1730) pre‐implementation, an absolute decrease of 7.2%; aOR 0.77 (95% CI 0.66–0.89). Post‐implementation, 28.6% (379/1326) of young patients were hospitalized compared to 40.7% (409/1005) pre‐implementation, an absolute decrease of 12.1%; aOR 0.57 (95% CI 0.47–0.69) (Figure 1). Interaction term testing between HEART Pathway implementation and age group for 30‐day hospitalization was significant between older adults versus young (p < 0.001) and older adults versus middle‐aged (p < 0.001). Table 2 provides the aORs and Table S3 provides the absolute difference in percent for each outcome.
Objective cardiac testing at 30 days occurred in 37.4% (487/1303) of older adults post‐implementation compared to 33.7% (330/978) pre‐implementation, an absolute increase of 3.6%; aOR 1.25 (95% CI 1.04–1.51). Among middle‐aged patients, 36.2% (771/2132) received cardiac testing by 30 days post‐implementation versus 40.4% (699/1730) pre‐implementation, an absolute decrease of 4.2%, aOR 0.87 (95% CI 0.75–1.00). With young patients, 15.4% (204/1326) received cardiac testing by 30 days post‐implementation versus 25.1% (252/1005) pre‐implementation, an absolute decrease of 9.7%; aOR 0.52 (95% CI 0.42–0.66) (Figure 1). Interaction terms between HEART Pathway implementation and 30‐day cardiac testing acquisition were significant between older adults versus young (p < 0.001) and older adults versus middle‐aged (p < 0.001) groups.
Among older adults, early discharge occurred in 20.6% (268/1303) post‐implementation versus 22.5% (220/978) pre‐implementation, an absolute decrease of 1.9%; aOR 0.84 (95% CI 0.67–1.05). Early discharge occurred in 40.3% (860/2132) of middle‐aged patients post‐implementation versus 33.5% (580/1730) pre‐implementation, an absolute increase of 6.8%; aOR 1.28 (95% CI 1.10–1.49). Among young patients, early discharge occurred in 69.2% (918/1326) post‐implementation versus 58.7% (590/1005) pre‐implementation, an absolute increase of 10.5%; aOR 1.61 (95% CI 1.33–1.95). Interaction term testing between HEART Pathway implementation and early ED discharge was significant between the older adults versus young (p < 0.001) and older adults versus middle‐aged (p < 0.001) groups. A comparison of post‐implementation 30‐day effectiveness outcomes by age group is provided in Table S4. Sensitivity analyses examining pre‐ versus post‐implementation outcomes among subgroups of older patients 65–75 and ≥75 years old did not meaningfully change results (Tables S5 and S6).
DISCUSSION
This subgroup analysis of the HEART Pathway implementation study contributes to the chest pain risk stratification literature by demonstrating the safety of the HEART Pathway among older adults. The HEART Pathway remained highly sensitive for 30‐day death or MI among older adults. While 30‐day hospitalization and objective cardiac testing decreased among young and middle‐aged patients, hospitalization trended up in older adults and cardiac testing increased. The early discharge rate of older adults remained low before and after HEART Pathway implementation.
Among low‐risk older adults, there were only two 30‐day adverse events. On further review, both deaths were from non‐cardiac conditions (subarachnoid hemorrhage and encephalopathy) that would not be expected to be detected by the HEART Pathway. Our a priori primary safety endpoint included all‐cause death, so we report sensitivity and NPV based on missing these two events. All‐cause mortality was chosen for this trial because our outcome ascertainment relied on EHR and claims data, which can make determining cause of death difficult. However, if we had used an endpoint of 30‐day cardiac death or MI, then both these patients would not have been considered misses and the sensitivity and NPV of the HEART Pathway among older adults would have climbed to 100%.
Few older adults were identified as low‐risk by the HEART Pathway. With the HEAR score, older adults automatically receive 2 points for age. In addition, the prevalence of cardiac risk factors, such as hypertension, hyperlipidemia, and diabetes is high among older adults. 2 , 32 , 33 , 34 Thus, most older adults receive at least 1 point for risk factors. Because of this, it is easy for older adults to receive at least 4 points, making them non‐low‐risk.
While the HEART Pathway decreased hospitalization and objective cardiac testing and increased early discharge in the young and middle‐aged cohorts, its impact on these outcomes differed in older adults. The HEART Pathway increased objective cardiac testing and had a trend toward increased hospitalizations among older adults. These age differences were observed in both unadjusted and adjusted analyses. Furthermore, our multivariable models demonstrated significant interaction terms for age by HEART Pathway implementation for hospitalization, early discharge, and objective cardiac testing. These differences were likely driven by providers following HEART Pathway care recommendations, which suggest observation or admission for further cardiac testing, including serial troponins and objective cardiac testing, in non‐low‐risk patients. This increase in testing among older adults following HEART Pathway implementation was the likely driver of more index MI diagnoses in the post‐implementation group.
This is the first analysis to test whether the performance of an ADP for patients with possible ACS differs by age. In particular, the utility of tools like the HEART Pathway have not been assessed in older adults. Tools used for other conditions have been shown to perform differently in older adults. For example, Wells Criteria for pulmonary embolism is less sensitive in the older adult population. 35 Thus, it was essential to test for age differences in the test performance of the HEART Pathway for key safety outcomes. This study adds to the literature by demonstrating that the HEART Pathway maintains high sensitivity among older adults. However, the performance of other chest pain tools, such as Emergency Department Assessment of Chest Pain Score ADP, the Troponin‐only Manchester Acute Coronary Syndromes (T‐MACS) decision aid, the High‐STEACS pathway, and the European Society of Cardiology (ESC) 0/1‐hour algorithm, is unknown in this age group. 30 , 36 , 37 , 38 , 39 , 40 The T‐MACS, High‐STEACS, and the ESC 0/1‐hour algorithm do not account for age in their risk assessments, so their performance in older adults may be quite different. Given the increased uncertainty when assessing older adults with chest pain, the increased morbidity among this age group, and the heterogenous approach in existing risk stratification methods, dedicated and comparative research for determining best practices in this group is needed.
Although the HEART Pathway recommends that most older adults be admitted, shared decision making can still play an important role in guiding disposition. 41 , 42 , 43 Among older adults in the post‐implementation cohort, less than 2.5% experienced non‐index death or MI during the 30‐day follow‐up period. While this is above the 1% miss rate that most emergency providers accept, some older adults may find this level of risk acceptable and prefer discharge with outpatient management. 11 By equipping patients with knowledge regarding their level of risk, emergency providers can engage in informed shared decision making with their older adult patients.
This analysis has limitations. Secular trends and provider maturation effects are potential threats to the validity of our results. However, event rates were fairly consistent over time. Although this study accrued patients from three diverse sites in our region, the results may not be generalizable elsewhere. Despite the size of the study, only 97 older adults were considered low‐risk, thereby increasing imprecision. Among older adults, more than 90% were insured with Medicare or a Blue Cross advantage plan whereas more young and middle‐aged patients were uninsured. It is possible that insurance status influenced cardiac testing and hospitalization among older adults. However, the multivariable models adjusted for insurance status. Additionally, the HEART Pathway includes age as a scoring component. While differences in performance among age cohorts was anticipated, this study aimed to report on the magnitude of differences. Using the EHR to collect events may have decreased event detection compared to traditional methods of follow‐up. However, supplementing the EHR data with death index and claims data identified only 16 additional 30‐day safety events. Furthermore, high sensitivity troponin assays were not used during the study period.
This novel analysis of age group differences in a chest pain ADP shows that the HEART Pathway has high sensitivity for 30‐day death or MI among older adults. However, use of the HEART Pathway in older adults increased objective cardiac testing and did not reduce hospitalizations. These findings differ from young and middle‐aged patients, who had decreased objective cardiac testing and hospitalization rates following HEART Pathway implementation. In total, these data indicate that the HEART Pathway can be used to safely risk stratify patients across the age spectrum of adult ED patients with acute chest pain, but that its efficiency gains from reduced hospitalizations and objective cardiac testing are limited to young and middle‐aged patients.
CONFLICT OF INTEREST
Ms. Paradee and Dr. O'Neill have no conflicts. Dr. Ashburn receives funding from NHLBI (T32HL076132). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Dr. Snavely receives funding from Abbott and HRSA (1H2ARH399760100). Dr. Stopyra receives research funding from NCATS/NIH (KL2TR001421), HRSA (H2ARH399760100), and Roche Diagnostics. Dr. Mahler receives funding/support from Roche Diagnostics, Abbott Laboratories, Ortho Clinical Diagnostics, Siemens, Grifols, Pathfast, Quidel, Genetesis, Cytovale, and HRSA (1H2ARH399760100). He is a consultant for Roche, Quidel, Abbott, Genetesis, Inflammatix, Radiometer, and Amgen and the Chief Medical Officer for Impathiq Inc.
AUTHOR CONTRIBUTIONS
Nicklaus P. Ashburn, Jason P. Stopyra, and Simon A. Mahler conceived the study idea. Anna C. Snavely and Brennan E. Paradee coordinated data management and performed the statistical analyses. Nicklaus P. Ashburn drafted the manuscript. All authors contributed to the manuscript and substantially to its revision. Nicklaus P. Ashburn takes responsibility for the manuscript as a whole.
SPONSOR'S ROLE
This project was funded by the Donaghue Foundation and the Association of American Medical Colleges. The funding sources had no role in the design or conduct of this investigation.
Supporting information
Figure S1 The HEART Pathway algorithm.
Figure S2. The participant flow diagram.
Table S1. Individual components of the HEART Pathway in the post‐implementation cohort by age for patients with a complete HEART Pathway assessment.
Table S2. Safety events at 30‐days among low‐risk patients by age group.
Table S3. Absolute percentage difference in safety and effectiveness outcomes pre‐ versus post‐implementation by age group.
Table S4. Comparison of outcomes between age groups post‐implementation at 30 days.
Table S5. Sensitivity analysis evaluating adjusted odds ratios among subgroups of older adults 65–75 and ≥75 years old.
Table S6. Sensitivity analysis evaluating HEART Pathway test characteristics for the outcome of 30‐day all‐cause death or MI among subgroups of older adults 65–75 and ≥75 years old.
ACKNOWLEDGMENTS
The authors acknowledge the support of the Wake Forest Clinical and Translational Science Institute for assisting with data extraction, study design, and project management.
Ashburn NP, Snavely AC, Paradee BE, O'Neill JC, Stopyra JP, Mahler SA. Age differences in the safety and effectiveness of the HEART Pathway accelerated diagnostic protocol for acute chest pain. J Am Geriatr Soc. 2022;70(8):2246‐2257. doi: 10.1111/jgs.17777
Clinical Trial Registration: clinicaltrials.gov identifier: NCT02056964.
Previous Presentation: Preliminary data were presented at the Society for Academic Emergency Medicine's annual conference in 2020.
Funding information Association of American Medical Colleges; Patrick and Catherine Weldon Donaghue Medical Research Foundation
REFERENCES
- 1. Owens PL, Barrett ML, Gibson TB, Andrews RM, Weinick RM, Mutter RL. Emergency department care in the United States: a profile of national data sources. Ann Emerg Med. 2010;56(2):150‐165. doi: 10.1016/j.annemergmed.2009.11.022 [DOI] [PubMed] [Google Scholar]
- 2. Benjamin EJ, Muntner P, Alonso A, et al. Heart Disease and Stroke Statistics‐2019 update: a report from the American Heart Association. Circulation. 2019;139(10):e56‐e528. doi: 10.1161/CIR.0000000000000659 [DOI] [PubMed] [Google Scholar]
- 3. Mahler SA, Riley RF, Hiestand BC, et al. The HEART Pathway randomized trial: identifying emergency department patients with acute chest pain for early discharge. Circ Cardiovasc Qual Outcomes. 2015;8(2):195‐203. doi: 10.1161/CIRCOUTCOMES.114.001384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hermann LK, Weingart SD, Duvall WL, Henzlova MJ. The limited utility of routine cardiac stress testing in emergency department chest pain patients younger than 40 years. Ann Emerg Med. 2009;54(1):12‐16. doi: 10.1016/j.annemergmed.2009.01.006 [DOI] [PubMed] [Google Scholar]
- 5. Torio CM, Moore BJ. National inpatient hospital costs: the most expensive conditions by Payer, 2013: Statistical Brief #204. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Agency for Healthcare Research and Quality (US); 2016. Accessed October 25, 2021. https://www.ncbi.nlm.nih.gov/pubmed/27359025 [PubMed] [Google Scholar]
- 6. Stopyra JP, Riley RF, Hiestand BC, et al. The HEART Pathway randomized controlled trial one‐year outcomes. Acad Emerg Med. 2019;26(1):41‐50. doi: 10.1111/acem.13504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mahler SA, Lenoir KM, Wells BJ, et al. Safely identifying emergency department patients with acute chest pain for early discharge. Circulation. 2018;138(22):2456‐2468. doi: 10.1161/circulationaha.118.036528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dezman ZD, Mattu A, Body R. Utility of the history and physical examination in the detection of acute coronary syndromes in emergency department patients. West J Emerg Med. 2017;18(4):752‐760. doi: 10.5811/westjem.2017.3.32666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mokhtari A, Dryver E, Söderholm M, Ekelund U. Diagnostic values of chest pain history, ECG, troponin and clinical gestalt in patients with chest pain and potential acute coronary syndrome assessed in the emergency department. Springerplus. 2015;4:219. doi: 10.1186/s40064-015-0992-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pope JH, Aufderheide TP, Ruthazer R, et al. Missed diagnoses of acute cardiac ischemia in the emergency department. N Engl J Med. 2000;342(16):1163‐1170. doi: 10.1056/NEJM200004203421603 [DOI] [PubMed] [Google Scholar]
- 11. Than M, Herbert M, Flaws D, et al. What is an acceptable risk of major adverse cardiac event in chest pain patients soon after discharge from the emergency department?: a clinical survey. Int J Cardiol. 2013;166(3):752‐754. doi: 10.1016/j.ijcard.2012.09.171 [DOI] [PubMed] [Google Scholar]
- 12. Brown TW, McCarthy ML, Kelen GD, Levy F. An epidemiologic study of closed emergency department malpractice claims in a national database of physician malpractice insurers. Acad Emerg Med. 2010;17(5):553‐560. doi: 10.1111/j.1553-2712.2010.00729.x [DOI] [PubMed] [Google Scholar]
- 13. Rusnak RA, Stair TO, Hansen K, Fastow JS. Litigation against the emergency physician: common features in cases of missed myocardial infarction. Ann Emerg Med. 1989;18(10):1029‐1034. doi: 10.1016/s0196-0644(89)80924-2 [DOI] [PubMed] [Google Scholar]
- 14. Solomon CG, Lee TH, Cook EF, et al. Comparison of clinical presentation of acute myocardial infarction in patients older than 65 years of age to younger patients: the Multicenter chest pain study experience. Am J Cardiol. 1989;63(12):772‐776. doi: 10.1016/0002-9149(89)90040-4 [DOI] [PubMed] [Google Scholar]
- 15. Dai X, Busby‐Whitehead J, Alexander KP. Acute coronary syndrome in the older adults. J Geriatr Cardiol. 2016;13(2):101‐108. doi: 10.11909/j.issn.1671-5411.2016.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Grosmaitre P, Le Vavasseur O, Yachouh E, et al. Significance of atypical symptoms for the diagnosis and management of myocardial infarction in elderly patients admitted to emergency departments. Arch Cardiovasc Dis. 2013;106(11):586‐592. doi: 10.1016/j.acvd.2013.04.010 [DOI] [PubMed] [Google Scholar]
- 17. Zaman MJ, Stirling S, Shepstone L, et al. The association between older age and receipt of care and outcomes in patients with acute coronary syndromes: a cohort study of the myocardial ischaemia National Audit Project (MINAP). Eur Heart J. 2014;35(23):1551‐1558. doi: 10.1093/eurheartj/ehu039 [DOI] [PubMed] [Google Scholar]
- 18. Avezum A, Makdisse M, Spencer F, et al. Impact of age on management and outcome of acute coronary syndrome: observations from the Global Registry of Acute Coronary events (GRACE). Am Heart J. 2005;149(1):67‐73. doi: 10.1016/j.ahj.2004.06.003 [DOI] [PubMed] [Google Scholar]
- 19. Rosengren A, Wallentin L, Simoons M, et al. Age, clinical presentation, and outcome of acute coronary syndromes in the Euroheart acute coronary syndrome survey. Eur Heart J. 2006;27(7):789‐795. doi: 10.1093/eurheartj/ehi774 [DOI] [PubMed] [Google Scholar]
- 20. Alexander KP, Newby LK, Cannon CP, et al. Acute coronary care in the elderly, part I: non‐ST‐segment‐elevation acute coronary syndromes: a scientific statement for healthcare professionals from the American Heart Association Council on Clinical Cardiology: in collaboration with the Society of Geriatric Cardiology. Circulation. 2007;115(19):2549‐2569. doi: 10.1161/CIRCULATIONAHA.107.182615 [DOI] [PubMed] [Google Scholar]
- 21. Mahler SA, Burke GL, Duncan PW, et al. HEART Pathway accelerated diagnostic protocol implementation: prospective pre‐post interrupted time series design and methods. JMIR Res Protoc. 2016;5(1):e10. doi: 10.2196/resprot.4802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. The EQUATOR Network. Accessed October 25, 2021. http://www.equator-network.org/reporting-guidelines/strobe/
- 23. Culler SD, Kugelmass AD, Brown PP, Reynolds MR, Simon AW. Trends in coronary revascularization procedures among Medicare beneficiaries between 2008 and 2012. Circulation. 2015;131(4):362‐370; discussion 370. doi: 10.1161/CIRCULATIONAHA.114.012485 [DOI] [PubMed] [Google Scholar]
- 24. Riley RF, Don CW, Powell W, Maynard C, Dean LS. Trends in coronary revascularization in the United States from 2001 to 2009: recent declines in percutaneous coronary intervention volumes. Circ Cardiovasc Qual Outcomes. 2011;4(2):193‐197. doi: 10.1161/CIRCOUTCOMES.110.958744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. So L, Evans D, Quan H. ICD‐10 coding algorithms for defining comorbidities of acute myocardial infarction. BMC Health Serv Res. 2006;6:161. doi: 10.1186/1472-6963-6-161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tonelli M, Wiebe N, Fortin M, et al. Methods for identifying 30 chronic conditions: application to administrative data. BMC Med Inform Decis Mak. 2015;15:31. doi: 10.1186/s12911-015-0155-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD‐9‐CM and ICD‐10 administrative data. Med Care. 2005;43(11):1130‐1139. doi: 10.1097/01.mlr.0000182534.19832.83 [DOI] [PubMed] [Google Scholar]
- 28. Mahler SA, Hiestand BC, Goff DC Jr, Hoekstra JW, Miller CD. Can the HEART score safely reduce stress testing and cardiac imaging in patients at low risk for major adverse cardiac events? Crit Pathw Cardiol. 2011;10(3):128‐133. doi: 10.1097/HPC.0b013e3182315a85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mahler SA, Miller CD, Hollander JE, et al. Identifying patients for early discharge: performance of decision rules among patients with acute chest pain. Int J Cardiol. 2013;168(2):795‐802. doi: 10.1016/j.ijcard.2012.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hess EP, Brison RJ, Perry JJ, et al. Development of a clinical prediction rule for 30‐day cardiac events in emergency department patients with chest pain and possible acute coronary syndrome. Ann Emerg Med. 2012;59(2):115‐25.e1. doi: 10.1016/j.annemergmed.2011.07.026 [DOI] [PubMed] [Google Scholar]
- 31. Simel DL, Samsa GP, Matchar DB. Likelihood ratios with confidence: sample size estimation for diagnostic test studies. J Clin Epidemiol. 1991;44(8):763‐770. doi: 10.1016/0895-4356(91)90128-v [DOI] [PubMed] [Google Scholar]
- 32. Kannel WB, McGee DL. Diabetes and cardiovascular disease: the Framingham study. Jama. 1979;241(19):2035‐2038. doi: 10.1001/jama.1979.03290450033020 [DOI] [PubMed] [Google Scholar]
- 33. Vokonas PS, Kannel WB, Cupples LA. Epidemiology and risk of hypertension in the elderly: the Framingham study. J Hypertens Suppl. 1988;6(1):S3‐S9. Accessed October 25, 2021. https://www.ncbi.nlm.nih.gov/pubmed/3216240 [PubMed] [Google Scholar]
- 34. Rodgers JL, Jones J, Bolleddu SI, et al. Cardiovascular risks associated with gender and aging. J Cardiovasc Dev Dis. 2019;6(2):19. doi: 10.3390/jcdd6020019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schouten HJ, Geersing G‐J, Oudega R, van Delden JJM, Moons KGM, Koek HL. Accuracy of the Wells clinical prediction rule for pulmonary embolism in older ambulatory adults. J Am Geriatr Soc. 2014;62(11):2136‐2141. doi: 10.1111/jgs.13080 [DOI] [PubMed] [Google Scholar]
- 36. Christenson J, Innes G, McKnight D, et al. A clinical prediction rule for early discharge of patients with chest pain. Ann Emerg Med. 2006;47(1):1‐10. doi: 10.1016/j.annemergmed.2005.08.007 [DOI] [PubMed] [Google Scholar]
- 37. Scheuermeyer FX, Wong H, Yu E, et al. Development and validation of a prediction rule for early discharge of low‐risk emergency department patients with potential ischemic chest pain. Cjem. 2014;16(2):106‐119. doi: 10.2310/8000.2013.130938 [DOI] [PubMed] [Google Scholar]
- 38. Than M, Flaws D, Sanders S, et al. Development and validation of the emergency department assessment of chest pain score and 2 h accelerated diagnostic protocol. Emerg Med Australas. 2014;26(1):34‐44. doi: 10.1111/1742-6723.12164 [DOI] [PubMed] [Google Scholar]
- 39. Chapman AR, Fujisawa T, Lee KK, et al. Novel high‐sensitivity cardiac troponin I assay in patients with suspected acute coronary syndrome. Heart. 2019;105(8):616‐622. doi: 10.1136/heartjnl-2018-314093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shah ASV, Anand A, Strachan FE, et al. High‐sensitivity troponin in the evaluation of patients with suspected acute coronary syndrome: a stepped‐wedge, cluster‐randomised controlled trial. Lancet. 2018;392(10151):919‐928. doi: 10.1016/S0140-6736(18)31923-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Probst MA, Noseworthy PA, Brito JP, Hess EP. Shared decision‐making as the future of emergency cardiology. Can J Cardiol. 2018;34(2):117‐124. doi: 10.1016/j.cjca.2017.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schaffer JT, Hess EP, Hollander JE, et al. Impact of a shared decision making intervention on health care utilization: a secondary analysis of the chest pain choice Multicenter randomized trial. Acad Emerg Med. 2018;25(3):293‐300. doi: 10.1111/acem.13355 [DOI] [PubMed] [Google Scholar]
- 43. Gafni‐Pappas G, DeMeester SD, Boyd MA, et al. The HAS‐choice study: utilizing the HEART score, an ADP, and shared decision‐making to decrease admissions in chest pain patients. Am J Emerg Med. 2018;36(10):1825‐1831. doi: 10.1016/j.ajem.2018.02.005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 The HEART Pathway algorithm.
Figure S2. The participant flow diagram.
Table S1. Individual components of the HEART Pathway in the post‐implementation cohort by age for patients with a complete HEART Pathway assessment.
Table S2. Safety events at 30‐days among low‐risk patients by age group.
Table S3. Absolute percentage difference in safety and effectiveness outcomes pre‐ versus post‐implementation by age group.
Table S4. Comparison of outcomes between age groups post‐implementation at 30 days.
Table S5. Sensitivity analysis evaluating adjusted odds ratios among subgroups of older adults 65–75 and ≥75 years old.
Table S6. Sensitivity analysis evaluating HEART Pathway test characteristics for the outcome of 30‐day all‐cause death or MI among subgroups of older adults 65–75 and ≥75 years old.


