Abstract
Background:
In the Catheter Ablation vs Antiarrhythmic Drug Therapy for Atrial Fibrillation (CABANA) trial, catheter ablation did not significantly reduce the primary endpoint of death, disabling stroke, serious bleeding, or cardiac arrest compared to drug therapy by intention-to-treat, but did improve quality of life (QOL) and freedom from AF recurrence. In the heart failure subgroup, ablation improved both survival and QOL. Cost effectiveness was a prespecified CABANA secondary endpoint.
Methods:
Medical resource use data were collected for all CABANA patients (n=2,204). Costs for hospital-based care were assigned using prospectively collected bills from US patients (N=1,171); physician and medication costs were assigned using the Medicare Fee Schedule and National Average Drug Acquisition Costs, respectively. Extrapolated life expectancies were estimated using age-based survival models. QOL adjustments were based on EQ-5D-based utilities measured during the trial. The primary outcome was the incremental cost-effectiveness ratio (ICER), comparing ablation to drug therapy based on intention-to-treat, and assessed from the US healthcare sector perspective.
Results:
Costs in the first 3 months averaged $20,794 ± SD 1,069 higher with ablation compared to drug therapy. The cumulative within-trial 5-year cost difference was $19,245 (95% CI $11,360 to $27,170) and the lifetime mean cost difference was $15,516 (95% CI −$2,963 to $35,512) higher with ablation compared to drug therapy. The drug therapy arm accrued an average of 12.5 life years (LYs) and 10.7 quality-adjusted life years (QALYs). For the ablation arm, the corresponding estimates were 12.6 LYs and 11.0 QALYs. The ICER was $57,893 per QALY gained, with 75% of bootstrap replications yielding an ICER≤$100,000 per QALY gained. With no QOL/utility adjustments, the ICER was $183,318 per LY gained.
Conclusion:
Catheter ablation of AF was economically attractive compared to drug therapy in the CABANA Trial overall at present benchmarks for healthcare value in the United States based on projected incremental QALYs but not LYs alone.
Keywords: Atrial Fibrillation, Anti-Arrhythmic Drug Therapy, Catheter Ablation, Pulmonary Vein Isolation, Health Economics
INTRODUCTION
Atrial fibrillation (AF) is associated with reduced life expectancy, has substantial adverse effects on quality of life (QOL) and significantly increases the costs of care.1–3 Catheter ablation of AF has been shown to produce a sustained reduction in AF recurrences and a durable improvement in quality of life relative to standard drug therapy.4, 5 In patients with heart failure, it may also enhance survival.6, 7 Yet catheter ablation is substantially more expensive, at least initially, than pharmacotherapy. What remains unclear is whether the incremental costs required to produce those added health benefits with ablation are in line with currently accepted benchmarks for good value in health care. In economic terms, if a quality adjusted life year (QALY) can be added by a novel therapy or strategy relative to control or usual care for acceptable additional costs (i.e., conventional thresholds for healthcare value less than $50,000 to $150,000 in the United States (US)), the therapy is “economically attractive.”8 At present, there are no large-scale empirical cost or cost-effectiveness studies of contemporary AF ablation procedures relative to drug therapy.
The Catheter Ablation vs Antiarrhythmic Drug Therapy for Atrial Fibrillation trial (CABANA), which is the largest randomized trial to date comparing catheter ablation with drug therapy in atrial fibrillation, found that the strategy of catheter ablation had a primary clinical event (composite of death, disabling stroke, serious bleeding or cardiac arrest) rate of 8.0% compared to 9.2% for drug therapy with standard rhythm and/or rate control drugs (hazard ratio 0.86, 95% confidence interval (CI) 0.65 to 1.15) when analyzed by intention-to-treat over a median follow-up of 48.5 months.9 There were significant gains in AF-specific quality of life, increased freedom from AF recurrence, and reductions in AF arrhythmic burden observed with ablation.4, 5 Prespecified subgroup analyses suggest that among patients with symptomatic heart failure (New York Heart Association (NYHA) class II or III), both survival and QOL were improved.7 Economic analyses were prospectively planned as part of the core CABANA research program.10
METHODS
Trial Design and Patient Population
The CABANA trial design and methods have been previously reported in detail.9, 10 CABANA enrolled participants who: (a) were aged 65 years and older, or younger than 65 years with 1 or more risk factors for stroke, (b) had electrocardiographic documentation of at least 2 episodes of paroxysmal AF or 1 episode of persistent AF in the 6 months prior to enrollment, and (c) were suitable candidates for catheter ablation or rhythm and/or rate control drug therapy. Participants from 126 centers across 10 countries were randomized to a strategy of catheter ablation or drug therapy alone. Trial enrollment occurred between November 2009 and April 2016 with follow up through December 2017. Approval of the appropriate institutional review board or ethics committee was obtained at all sites, and all patients provided written informed consent (ClinicalTrials.gov Identifier NCT00911508). Since this trial was funded by the US National Institutes of Health, the data underlying the analyses will be in the public domain within 2 years of the initial publication.
Overview of Economic Study
The CABANA economic study prospectively designated three major objectives: (1) collection of medical resource use data (all patients) and hospital cost data (US patients), (2) an intention-to-treat comparison of within-trial direct medical costs, and (3) a cost-effectiveness analysis conducted over a lifetime horizon from the perspective of the US healthcare system. The endpoints of the cost-effectiveness analysis included costs, life years (LYs), QALYs, and incremental cost-effectiveness ratios.
Within-Trial Resource Use Data Collection
Medical resource use was collected for all CABANA participants using electronic case report forms, including hospitalizations (with additional details on length of stay, days in the intensive care unit and total hospital days, reason for admission, and major procedures performed), selected outpatient care, and medication use. Extended care stays (i.e., skilled nursing, rehabilitation) were also recorded.
Within-Trial Cost Data Collection and Estimation
Hospital billing data (detailed, summary ledger, and UB-04 forms) were collected from participating sites in the US. For US patients with billing data (n=1,171 [95%] of 1,233 US patients), hospital-based costs were estimated from charges using departmental charge-to-cost conversion factors derived from each hospital’s annual Medicare Cost Report.11 Costs for encounters without bills were imputed using cost weights from multivariable generalized linear models with a gamma distribution and identity link developed from the collected billing data. Cost imputation models included age, reason for hospitalization, length of stay (ICU and non-ICU), coronary artery bypass grafting (CABG), percutaneous coronary intervention (PCI), catheter ablation, cardiovascular implantable electronic device, and baseline Canadian Cardiovascular Society AF symptom severity class.
Physician costs were estimated by mapping major cardiac procedures and other physician services as identified on the case report forms and hospital bills to Current Procedural Terminology codes in the 2018 Medicare national reimbursement schedule.12 For outpatient physician office visits, the economic analysis assumed annual cardiology follow-up visits with one additional 90-day visit following catheter ablation in the ablation arm.13 Antiarrhythmic and anticoagulation medication costs were assigned using the 2018 National Average Drug Acquisition Costs available from the Centers for Medicare and Medicaid Services, and additional outpatient costs for INR monitoring for patients on warfarin were included.14, 15
Resource use estimates and costs for specific time intervals were estimated using inverse probability weighting (IPW) to account for variable trial follow-up (median follow-up 48.5 months).16
Post-trial Projections
Health Care Costs
Health care costs beyond the study follow up were projected using IPW estimates based on cost data from follow-up years 3 through 5. This period was chosen because the cost differences between the ablation and drug groups were stable and therefore felt to reasonably represent longer term cost patterns.
Life Expectancy Estimation
To estimate lifetime survival curves for each patient, an age-based model was employed, which treats the hazard of death as a function of age (rather than time) and allows for the estimation of an entire survival distribution for the CABANA cohort.17–19 This approach avoids the use of parametric assumptions or simulation techniques and consists of two components: (1) estimation of within-trial survival using an age-based Cox proportional hazards regression model for left-truncated and right-censored data with adjustment for baseline clinical characteristics and treatment, and (2) lifetime survival projection beyond trial follow-up (after 5 years), with extrapolation based on trial experience after conditioning on 180-day survival. The CABANA cohort provided sufficient data to estimate survival curves up to 82 years of age. Beyond this age, 2017 US life tables were used to calibrate the remaining survival for each study patient.20 Within-trial and extrapolated lifetime survival probabilities were combined to create a complete survival curve (trial enrollment to death) for each trial patient. Life expectancies, calculated from the area under each patient-level survival curve, were averaged to obtain the mean predicted life expectancy in each intention-to-treat group (Figure S1).
To assess the fidelity of the age-based model to observed CABANA results, the model estimates of within-trial undiscounted life expectancy were compared to the empiric CABANA survival results summarized as restricted mean survival time by treatment.21 For the primary (base case) analysis, the age-based survival model was calibrated to attenuate any potential long-term differences in treatment effect of catheter ablation versus drug therapy by setting the hazard ratio for mortality (catheter ablation versus drug therapy) to 1.0 after 5 years.
Quality Adjusted Life Year Estimation
The CABANA trial collected the EQ-5D 3-level instrument via structured interviews at baseline, 3 months, 6 months, and then every 6 months until the end of study follow up.4, 10 EQ-5D responses were converted to a summary health index, which includes a scoring algorithm reflecting patient preferences (utility weights, with 1 representing perfect health and 0 representing death).22 The CABANA EQ-5D data, by intention to treat, have been previously reported.4 The mean utility difference between the ablation and drug groups over trial follow up was +0.020 (95% CI 0.010 to 0.031).4 Within trial life years were converted to QALYs using the empirical trial utility weight data. For the post-trial phase, the final 3 years of utility data were used to estimate a treatment group mean utility weight (Table S1). An additional annual age-related quality of life decrement of −0.00029 was applied to post-trial period (beyond 5 years).23
Statistical Analysis: General
All primary comparisons between treatment groups were performed according to the principle of intention-to-treat. Descriptive statistics included percentages for discrete variables, and medians (25th to 75th percentiles) and means ± standard deviation for continuous variables.
Within-trial medical resource use as observed within the first 5 years of trial follow-up was summarized by treatment group. Incident rate ratios (IRRs) were used to compare the all-cause hospitalization rates and ablation-related hospitalization rates by treatment group using generalized linear models specified with a negative binomial distribution and log link, where treatment was included as the independent variable. Resource use and costs were compared between treatments for specific time intervals of interest (90 days, 91 days-1year, 1–5 years). Uncertainty in the incremental cost-effectiveness ratio (ICER) estimates was quantified using non-parametric bootstrap techniques.24, 25 Confidence intervals were calculated from 5,000 bootstrap replications with replacement using percentile-based interpretation. Analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, North Carolina).
Base Case Cost-Effectiveness Analyses
Incremental cost-effectiveness ratios were calculated as the ablation minus drug therapy difference in mean lifetime cost divided by the ablation-drug therapy difference in mean quality-adjusted life expectancy. The “base case” or primary analysis assumed the following: no incremental survival benefit after 5 years, preservation of quality of life/utility differences achieved in last 3 years of empirical CABANA follow-up, and rates of antiarrhythmic and anticoagulant use at end of trial maintained over lifetime. Cost-effectiveness was displayed on the incremental cost-effectiveness plane and summarized using cost-effectiveness acceptability curves.26 Costs were valued in 2018 US dollars and adjusted using the National Health Expenditures Personal Health Care Index or the Centers for Medicare & Medicaid Services inpatient market basket update, as appropriate.27 A 3% discount rate was applied to all future costs and benefits.28
To aid in interpretation, $100,000 per QALY gained was used as the dividing line between economically attractive and unattractive.29 Additionally, the value taxonomy proposed by American College of Cardiology and American Heart Association was considered: high-value represents either cost-savings or an ICER <$50,000 per QALY gained; intermediate value is represented by ICERs between $50,000 to <$150,000 per QALY gained; and low value is described by ICERs ≥$150,000 per QALY gained.8
Cost Effectiveness Sensitivity Analyses
Our primary method of addressing the uncertainty associated with our estimates of LYs, QALYs, costs, and ICERs was to use bootstrap replications with replacement, as described above. To supplement these uncertainty analyses and define the influence of key assumptions made in our base case analysis on the resulting cost-effectiveness estimates, several deterministic sensitivity analyses were performed. To explore the effect of assuming that the utility differences observed in the later years of the CABANA follow-up would persist indefinitely in follow-up, one sensitivity analysis assumed no QOL/utility treatment differences beyond the observed 5-year trial follow up period. Other sensitivity analyses included varying the cost of the catheter ablation, the time horizon used to estimate cost effectiveness, the discount rate, and assumptions related to the long-term use and cost of anticoagulation therapy. To examine the potential influence of crossovers on the cost-effectiveness estimates, an additional sensitivity analysis was conducted based on the previously reported per-protocol treatment clinical outcome comparisons, which demonstrated a larger effect size on mortality for ablation (HR 0.69, 95% CI 0.47–1.01) relative to the ITT analysis (HR for mortality 0.85, 95% CI 0.60 – 1.21).9 The per-protocol catheter ablation group included patients randomized to catheter ablation who received an ablation within the 6-month time window following randomization, and censored drug group patients who received ablation at time of ablation.
Subgroup Analysis
CABANA prespecified a subgroup analysis in heart failure patients, defined as NYHA Class II or III recorded in the baseline case report form. The clinical and QOL outcomes from the 778 patients in this subgroup have been reported.7 The cost effectiveness of ablation versus drug therapy for the subgroup was also pre-specified and calculated using the same methods and assumptions described for the base-case analysis.
RESULTS
Study Cohort
As previously reported, the 2,204 participants enrolled in CABANA had a median age of 68 years, 37% were female, and 10% identified as an ethnic or racial minority.9 Paroxysmal AF was present in 43% at time of enrollment, with the remainder having persistent or long-standing persistent AF; 26% had diabetes; 10% had a history of prior stroke or transient ischemic attack; 15% had a history of heart failure; and the median CHA2DS2-VASc score was 3.
Within-Trial Resource Use
During CABANA follow up (median 48.5 months), there were more all-cause hospitalizations per patient in the ablation group compared to drug therapy (ablation: 2.59 hospitalizations; drug: 2.16 hospitalizations; difference 0.43; IRR 1.20, 95% CI 1.12–1.30; p<0.001) (Table 1). The difference was largely driven by ablation-related hospitalizations (1.08 ablation versus 0.30 drug therapy; difference 0.78; IRR 3.54, 95% CI 3.14–4.00; p<0.001), which included initial and repeat ablation procedures. There was a lower rate of hospitalizations for non-ablation arrhythmia management (such as drug initiation or dose titration) in the ablation group relative to drug therapy (difference −0.40). Hospitalization rates for heart failure, other cardiovascular (such as myocardial infarction, primary percutaneous coronary intervention, transient ischemic attack or ischemic stroke) and non-cardiovascular causes did not differ between treatment groups.
Table 1.
Within Trial Resource Use (Overall and Stratified by Follow up period)
| Resource Use* | Catheter Ablation (N=1108) |
Drug Therapy (N=1096) |
Difference (ablation – drug) |
|---|---|---|---|
| Total Within Trial | |||
| Hospitalizations | 2.59 (2.25) | 2.16 (2.27) | 0.43 |
| First Ablation | 0.88 (0.33) | 0.23 (0.42) | 0.65 |
| Repeat / Redo Ablation | 0.20 (0.49) | 0.07 (0.31) | 0.13 |
| Non-Ablation Arrhythmia Management | 0.55 (1.01) | 0.95 (1.17) | −0.40 |
| Heart Failure | 0.09 (0.45) | 0.08 (0.41) | 0.01 |
| Other Cardiovascular | 0.24 (0.69) | 0.24 (0.64) | 0.00 |
| Non-Cardiovascular | 0.63 (1.18) | 0.58 (1.16) | 0.05 |
| Time in Hospital, days | 10.56 (16.55) | 8.98 (15.10) | 1.58 |
| ICU / CCU | 0.83 (3.15) | 0.84 (3.46) | −0.01 |
| Non ICU / CCU | 9.73 (15.70) | 8.14 (14.34) | 1.59 |
|
| |||
| Within First 90 days by Intention to Treat | |||
| Hospitalizations | 1.06 (0.68) | 0.63 (0.73) | 0.43 |
| First Ablation | 0.78 (0.41) | 0.03 (0.17) | 0.75 |
| Repeat / Redo Ablation | 0.01 (0.09) | 0.00 (0.00) | 0.01 |
| Non-Ablation Arrhythmia Management | 0.15 (0.41) | 0.51 (0.62) | −0.36 |
| Heart Failure | 0.02 (0.14) | 0.02 (0.14) | 0.00 |
| Other Cardiovascular | 0.04 (0.21) | 0.03 (0.22) | 0.01 |
| Non-Cardiovascular | 0.06 (0.24) | 0.04 (0.23) | 0.02 |
| Time in Hospital, days | 3.77 (5.15) | 2.59 (4.56) | 1.18 |
| ICU / CCU | 0.25 (1.01) | 0.29 (1.57) | −0.04 |
| Non-ICU / CCU | 3.52 (4.99) | 2.30 (4.18) | 1.22 |
|
| |||
| Between 90 to 365 days by Intention to Treat | |||
| Hospitalizations | 0.45 (0.83) | 0.45 (0.83) | 0.00 |
| First Ablation | 0.08 (0.27) | 0.09 (0.29) | −0.01 |
| Repeat / Redo Ablation | 0.07 (0.27) | 0.01 (0.11) | 0.06 |
| Non-Ablation Arrhythmia Management | 0.12 (0.41) | 0.15 (0.45) | −0.03 |
| Heart Failure | 0.02 (0.15) | 0.02 (0.22) | 0.00 |
| Other Cardiovascular | 0.05 (0.23) | 0.07 (0.33) | −0.02 |
| Non-Cardiovascular | 0.11 (0.36) | 0.11 (0.42) | 0.00 |
| Time in Hospital, days | 1.73 (5.86) | 1.66 (4.29) | 0.07 |
| ICU / CCU | 0.17 (2.10) | 0.18 (1.22) | −0.01 |
| Non-ICU / CCU | 1.57 (4.90) | 1.48 (3.94) | 0.09 |
|
| |||
| Between 365 days to 5-years by Intention to Treat | |||
| Hospitalizations | 1.09 (1.77) | 1.08 (1.67) | 0.01 |
| First Ablation | 0.02 (0.13) | 0.12 (0.32) | −0.10 |
| Repeat/Redo Ablation | 0.13 (0.37) | 0.06 (0.28) | 0.07 |
| Non-Ablation Arrhythmia Management | 0.27 (0.68) | 0.29 (0.74) | −0.02 |
| Heart Failure | 0.06 (0.36) | 0.04 (0.26) | 0.02 |
| Other Cardiovascular | 0.15 (0.60) | 0.14 (0.45) | 0.01 |
| Non-Cardiovascular | 0.46 (1.05) | 0.43 (0.95) | 0.03 |
| Time in Hospital, days | 5.06 (12.26) | 4.73 (11.90) | 0.33 |
| ICU / CCU | 0.41 (1.98) | 0.37 (2.51) | 0.04 |
| Non-ICU / CCU | 4.65 (11.74) | 4.36 (11.21) | 0.29 |
Medical resource use is reported as mean (standard deviation) from observed data through 5 years of CABANA trial follow up. This table excludes ablations that did not have a corresponding hospitalization documented on the study case report form. Abbreviations: CCU – coronary care unit; ICU – intensive care unit.
Differences in all-cause and arrhythmia-related hospitalization rates were primarily observed within the first 90 days of trial enrollment (Table 1). After 90 days, there was no significant difference between the intention-to-treat groups in hospitalization rates.
Within-Trial Outcomes
Costs
The average cumulative 5-year within-trial costs (adjusted for variable follow-up), including costs for hospitalization, extended care, inpatient and outpatient procedures, physician fees, and medications, were $75,381 in the ablation group and $56,137 in the drug therapy group (mean difference $19,245, 95% CI $11,360 to $27,170) (Table 2). The greater costs in the catheter ablation group were driven by costs due to hospitalization and hospital-based procedures.
Table 2.
Within Trial Costs
| Cumulative 5-Year Within Trial Costs† |
Catheter Ablation (N=1108) |
Drug Therapy (N=1096) |
Difference (ablation – drug) |
|---|---|---|---|
| Hospitalizations | 62,214 (57,773, 66,706) | 41,622 (37,842, 45,747) | 20,552 (13,782, 27,292) |
| Ablation Hospitalizations | 35,102 (33,030, 37,130) | 11,857 (10,306, 13,509) | 23,246 (20,289, 26,085) |
| Emergency Department | 568 (447, 697) | 623 (477, 789) | −56 (−265, 143) |
| Extended Care Stays | 944 (549, 1434) | 912 (439, 1542) | 32 (−700, 707) |
| Physician Fees* | 3572 (3360, 3793) | 3415 (3196, 3633) | 158 (−214, 533) |
| Medications | 8107 (7433, 8796) | 9524 (8775, 10,292) | −1417 (−2537, −263) |
| Anticoagulation | 7561 (6910, 8225) | 8375 (7670, 9103) | −814 (−1882, 269) |
| Antiarrhythmic Drugs | 546 (495, 601) | 1149 (1071, 1227) | −603 (−706, −497) |
| Total Costs | 75,381 (70,395, 80,462) | 56,137 (51,768, 60,723) | 19,245 (11,360, 27,170) |
Excluding physician fees for inpatient care, which are included in hospitalization costs
Costs were adjusted for variable follow-up inverse probability weighting and are reported as mean (95% confidence intervals) in US dollars. Confidence intervals were estimated using a bootstrap.
The mean cost of catheter ablation per procedure pooled over both treatment groups was $26,656 ± 9,123. Patients in the ablation group averaged $35,102 in ablation-related hospitalization costs with the corresponding costs in the drug arm of $11,857 (difference, $23,246; 95% CI $20,289 to $26,085) (Table 2). Average medication costs were lower in the ablation group (difference −$1,417, 95% CI −$2,537 to −$263), attributable to lower cumulative antiarrhythmic drug costs at 5 years ($546 for ablation versus $1,149 for drug therapy; difference −$603, 95% CI −$706 to −$497) and lower anticoagulation costs ($7,561 for ablation versus $8,375 for drug therapy; difference −$814, 95% CI −$1,882 to $269). There were no significant differences in within-trial cumulative costs for extended stay facilities or non-hospital physician fees.
When the average cost difference between treatment groups was examined by follow-up interval, ablation was more expensive than drug therapy by $20,794 within the first 3 months (Figure S2). The difference in interval costs narrowed to $616 between 3 to 12 months, and remained non-substantial for remaining CABANA empiric follow up to 5 years. The narrowing in mean interval costs beyond 3 months was due to costs associated with crossover ablation in the drug therapy group.
Life Expectancy
Model-based 5-year life expectancy estimates agreed very closely with empirical 5-year restricted mean survival time estimates (Table S2). The difference in life expectancies (ablation versus drug) in the age-based model was identical to the empiric estimate at the end of 5-year trial follow up.
Projected Outcomes
Lifetime Costs
After discounting, the lifetime mean costs were $151,877 for the ablation group and $136,361 for the drug therapy group (Table 3). Relative to the drug therapy arm, the lifetime incremental cost for the ablation arm was $15,516 (95% CI −$2,963 to $35,512).
Table 3.
Cumulative Costs, Life Expectancy and Cost-Effectiveness by Intention-to-Treat (Base Case) and by Per-Protocol Analysis
| Scenario | Catheter Ablation* | Drug Therapy* | Difference* | Incremental Cost Effectiveness Ratio | Probability of Cost-Effectiveness |
||
|---|---|---|---|---|---|---|---|
| $50,000 | $100,000 | $150,000 | |||||
| Intention-To-Treat Analysis (Base Case) | |||||||
| Lifetime Costs, USD | 151,877 (133,394 – 157,359) |
136,361 (116,183 – 142,242) |
15,516 (−2,963 – 35,512) |
-- | -- | -- | -- |
| QALYs | 11.01 (10.82 – 11.20) |
10.74 (10.55 – 10.93) |
0.27 (0.07 – 0.47) |
$57,893 per QALY | 41% | 75% | 88% |
| LYs | 12.63 (12.41 – 12.85) |
12.54 (12.32 – 12.76) |
0.08 (−0.14 – 0.32) |
$183,318 per LY | 17% | 33% | 45% |
|
| |||||||
| Per-Protocol Analysis | |||||||
| Lifetime Costs, USD | 148,105 (129,978 – 154,137) |
106,667 (87,237 – 113,009) |
41,438 (22,830 – 60,759) |
-- | -- | -- | -- |
| QALYs | 10.70 (10.50 – 10.90) |
10.13 (9.95 – 10.31) |
0.57 (0.30 – 0.84) |
$72,835 per QALY | 12% | 81% | 97% |
| LYs | 12.24 (12.01 – 12.47) |
11.95 (11.73 – 12.16) |
0.29 (−0.02 – 0.61) |
$141,832 per LY | 1% | 24% | 52% |
Estimates expressed as mean (95% confidence interval).
Abbreviations: LY – life year; QALY – quality-adjusted life year; USD – United States dollar.
Life Expectancy
Projected life expectancy from time of study randomization was similar between the ablation and drug therapy groups (16.7 versus 16.6 years; difference 0.09, 95% CI −0.28 to 0.48). After discounting at 3%, projected life expectancy with ablation was 0.08 LYs (95% CI −0.14 to 0.32) greater than drug therapy (12.63 versus 12.54 years) (Table 3). Under base case assumptions, discounted QALYs were significantly greater for the ablation group compared to drug therapy (11.0 versus 10.7 QALYs; difference 0.27, 95% CI 0.07 to 0.47).
Cost-Effectiveness Base Case Results
Under base case assumptions, the ICER for ablation compared to drug therapy was $57,893 per QALY gained (Table 3), with an 75% likelihood of meeting a $100,000 per QALY gained willingness-to-pay threshold (Figure 1, Figure 2A). If the analysis used only the difference in life years with no QOL/utility adjustments, the ICER was $183,318 per LY gained.
Figure 1.
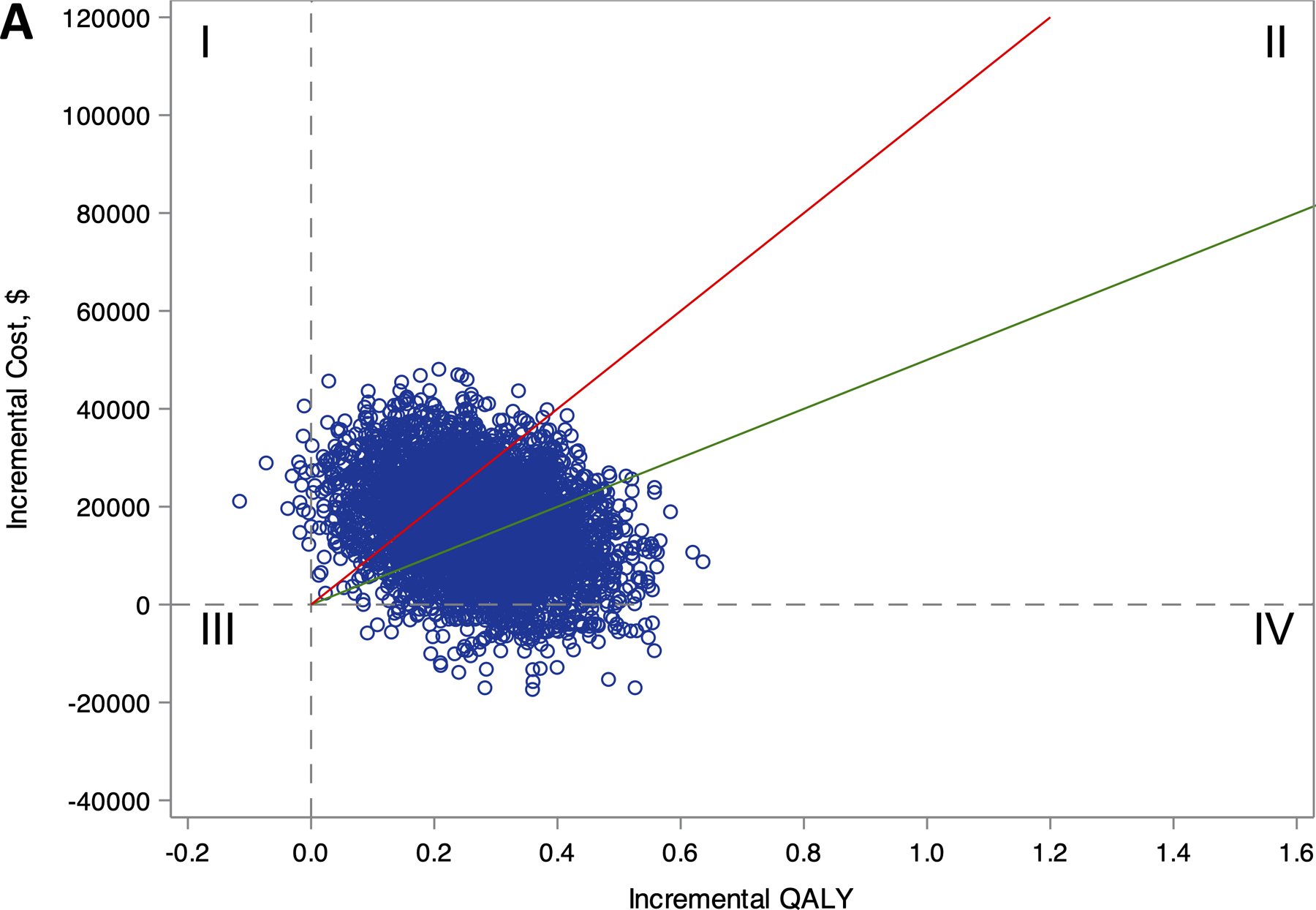
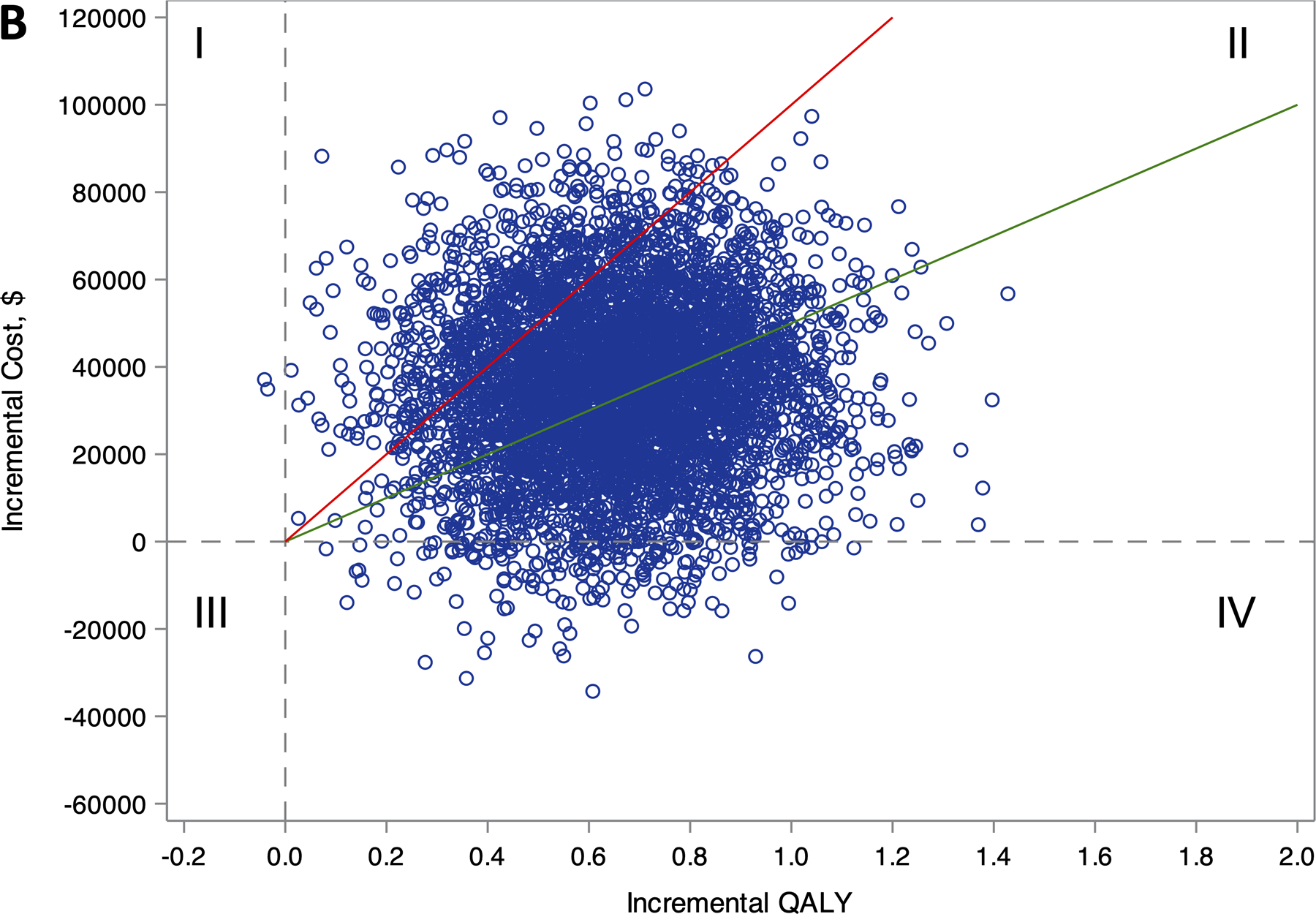
Distribution of lifetime incremental costs and effectiveness comparing ablation to drug therapy for (A) the entire CABANA cohort (N=2204), and (B) the heart failure subgroup with NYHA class ≥II symptoms (N=778).
Estimates of incremental costs and quality-adjusted life years (QALYs) are shown (1 blue circle for each of 5000 bootstrap samples). Quadrant I represent scenarios where ablation is more costly and less effective, Quadrant II represents scenarios where ablation is more costly and effective, Quadrant III represents scenarios where ablation is less costly and less effective, and Quadrant IV represents scenarios where ablation is less costly and more effective. The willingness-to-pay thresholds of $50,000 and $100,000 per QALY gained are represented as the slope of the green and red lines, respectively. Scenarios that fall below these willingness-to-pay-thresholds are considered economically attractive.
Figure 2.
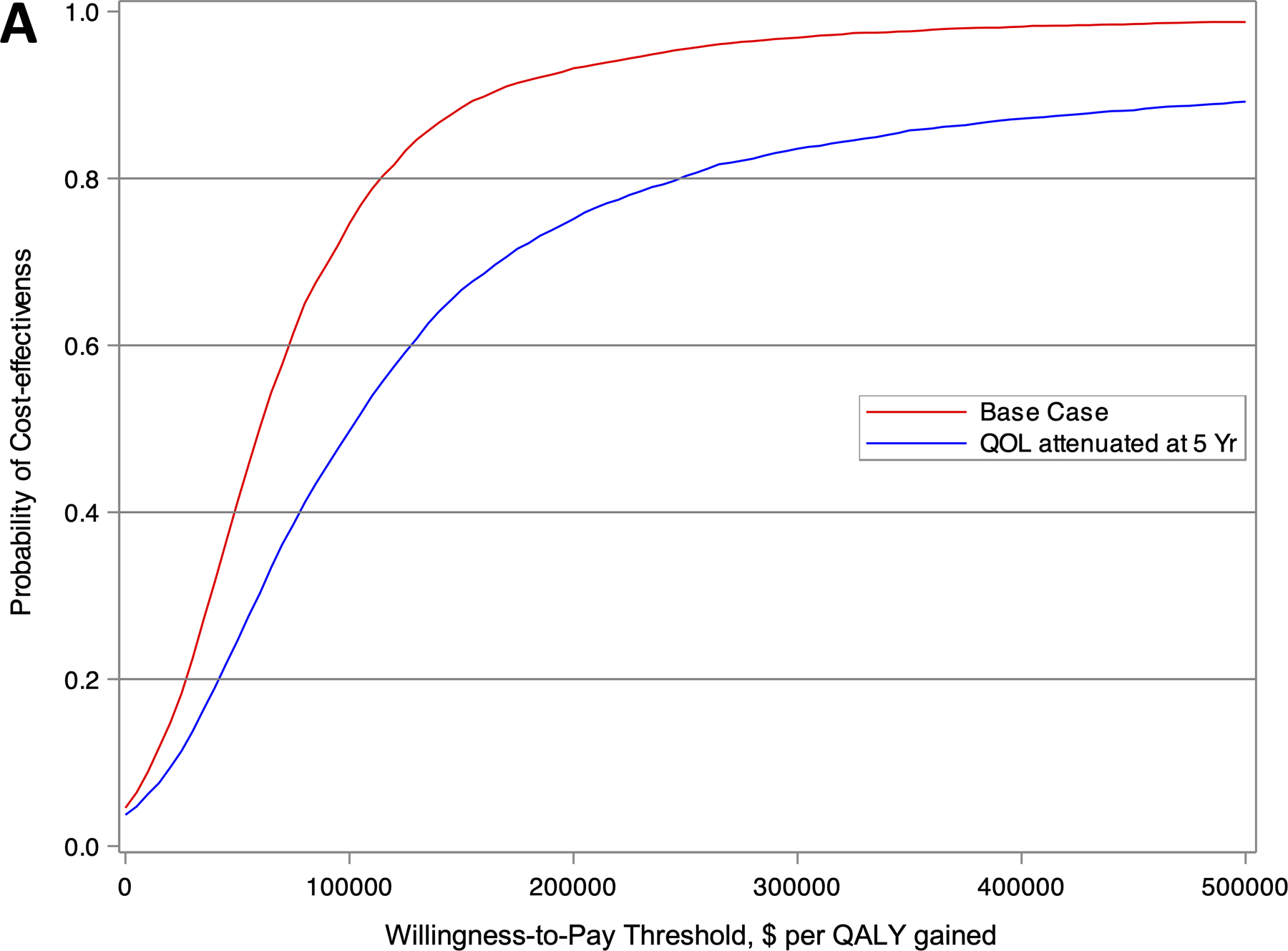
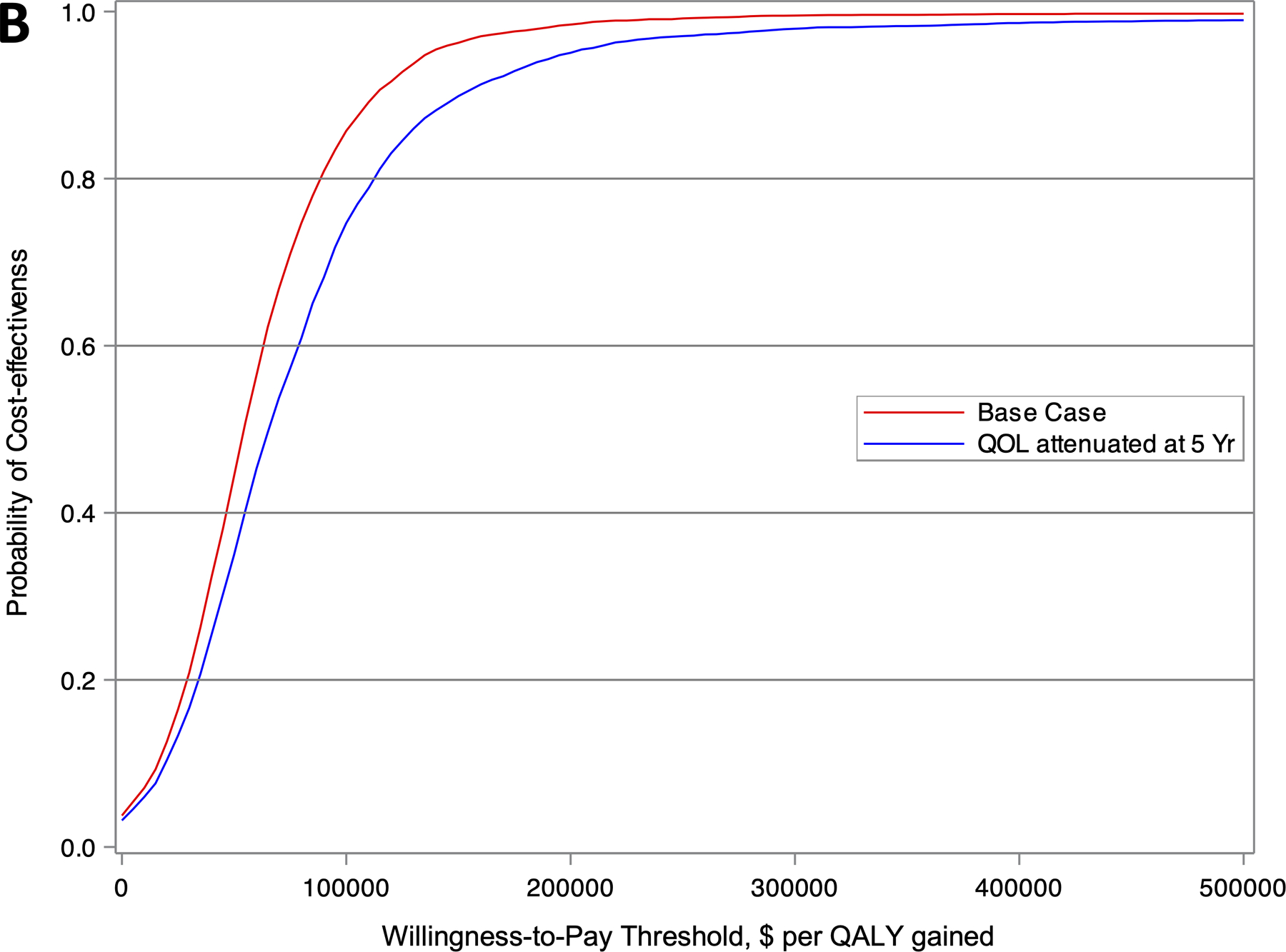
Cost-effectiveness acceptability curve showing the probability of a strategy being cost-effective over a range of willingness-to-pay (WTP) thresholds for (A) the entire CABANA cohort (N=2204), and (B) the heart failure subgroup with NYHA class ≥II symptoms (N=778). The red and blue lines represent the base case and sensitivity analysis with attenuation in quality of life after 5 years, respectively.
Per-Protocol Analysis
Among patients included in the per-protocol analysis (N=2,066), the incremental lifetime costs were $41,438 greater in the ablation group relative to the drug therapy group. Patients in the catheter ablation group gained 0.29 additional LYs and 0.57 additional QALYs compared to those in the drug therapy group. The ICER was $72,835 per QALY gained, with an 81% likelihood of meeting a $100,000 per QALY gained willingness-to-pay threshold.
Additional Sensitivity Analyses
Additional sensitivity analyses are summarized in Table 4. As there were limited data to extrapolate the long-term effect of catheter ablation on quality of life, a sensitivity analysis was conducted where attenuated the effect of ablation on quality of life beyond the empiric follow-up of CABANA. Specifically, the utility weights of the ablation and drug therapy groups were set to equivalence at 5 years. With this adjustment to the base case, the ICER for ablation versus drug therapy was $93,877 per QALY gained.
Table 4.
Cumulative Costs, Life Expectancy and Cost Effectiveness by Scenario (Intention-to-Treat)
| Scenario | Catheter Ablation | Drug Therapy | Difference | Incremental Cost-Effectiveness Ratio | Probability of Cost-Effectiveness | ||
|---|---|---|---|---|---|---|---|
| $50,000 | $100,000 | $150,000 | |||||
| Attenuated QoL Benefit† | |||||||
| QALYs (base costs) | 10.97 (10.64 – 11.36) |
10.81 (10.46 – 11.10) |
0.17 (−0.24 – 0.50) |
$93,877 per QALY | 25% | 50% | 67% |
|
| |||||||
| 5-Year Time Horizon | |||||||
| Total Costs, USD | 75,381 (70,413 – 80,449) |
56,137 (51,818 – 60,791) |
19,245 (11,376 – 26,924) |
-- | |||
| QALYs | 3.99 (3.97 – 4.00) |
3.87 (3.86 – 3.88) |
0.12 (0.10 – 0.13) |
$165,991 per QALY | 0% | 3% | 34% |
|
| |||||||
| 10-Year Time Horizon | |||||||
| Total Costs | 107,604 (96,975 – 113,012) |
89,707 (79,021 – 94,705) |
17,897 (5,471 – 30,872) |
-- | |||
| QALYs | 6.96 (6.91 – 7.01) |
6.75 (6.70 – 6.80) |
0.21 (0.14 – 0.28) |
$85,117 per QALY | 13% | 66% | 95% |
|
| |||||||
| 50% Catheter Ablation Cost (base QALYs) | |||||||
| Total Costs | 129,080 (111,814 – 133,852) |
123,242 (103,972 – 127,691) |
5,838 (−10,449 – 24,347) |
$21,783 per QALY | 71% | 91% | 96% |
|
| |||||||
| 75% Catheter Ablation Cost (base QALYs) | |||||||
| Total Costs | 140,497 (122,746 – 145,437) |
129,802 (110,234 – 135,350) |
10,677 (−6,502 – 29,431) |
$39,838 per QALY | 56% | 84% | 93% |
|
| |||||||
| 125% Catheter Ablation Cost (base QALYs) | |||||||
| Total Costs | 163,276 (142,921 – 169,378) |
142,921 (122,186 – 149,153) |
20,335 (1,653 – 40,727) |
$75,947 per QALY | 26% | 63% | 83% |
|
| |||||||
| 150% Catheter Ablation Cost (base QALYs) | |||||||
| Total Costs | 174,675 (155,186 – 181,057) |
149,480 (127,883 – 156,276) |
25,194 (5,262 – 46,272) |
$94,002 per QALY | 16% | 52% | 75% |
|
| |||||||
| Excluding anticoagulation costs (base QALYs)‡ | |||||||
| Total Costs | 125,402 (113,912 – 137,269) |
107,041 (94,391 – 119,969) |
18,361 (769 – 36,174) |
$68,505 per QALY | 33% | 71% | 87% |
|
| |||||||
| 0% Discount Rate | |||||||
| Total Costs | 190,213 (165,338 – 196,596) |
176,832 (148,367 – 183,678) |
13,381 (−10,210 – 39,810) |
-- | |||
| QALYs | 14.53 (14.20 – 14.89) |
14.22 (13.89 – 14.54) |
0.31 (−0.01 – 0.63) |
$42,573 per QALY | 52% | 77% | 86% |
|
| |||||||
| 5% Discount Rate | |||||||
| Total Costs | 134,667 (119,703 – 139,856) |
118,218 (101,420 – 122,834) |
16,449 (1,337 – 32,985) |
-- | |||
| QALYs | 9.43 (9.30 – 9.56) |
9.19 (9.05 – 9.32) |
0.24 (0.09 – 0.39) |
$68,000 per QALY | 30% | 71% | 89% |
Attenuated QOL benefit – This sensitivity analysis assumes a conservative scenario where the catheter ablation group does not gain any quality-of-life benefits beyond the end of the trial (5-years). Similar to the base case, this analysis did not allow the catheter ablation group to accumulate additional life years compared to the drug group since the within-trial analysis does not support a mortality benefit with ablation by intention-to-treat. That is, the hazard ratio of mortality (catheter ablation versus drug therapy) is set to 1.0 beyond 5 years.
Due to the clinical uncertainty of anticoagulation following successful catheter ablation, we conducted a sensitivity analysis excluding anticoagulation costs. This conservative scenario removes long-term cost offsets from potential differences in anticoagulation between treatment groups.
Abbreviations: LY – life year; QALY – quality-adjusted life year; QoL – quality of life; USD – United States dollar.
The influence of the catheter ablation procedure costs on the estimated ICER was assessed (Figure S3). When the cost per ablation was varied between 50% and 150% of the base case value, the ICER range ranged from $21,783 to $94,002 per QALY gained (Table 4). As there was uncertainty in clinical practice patterns regarding anticoagulation following ablation, a scenario analysis excluding anticoagulation cost offsets between the ablation and drug group was performed. In this scenario, the estimated ICER was $68,505 per QALY gained.
The time horizon of the analysis also had an influential effect on the results. When the time horizon was limited to 5-years, the ICER was $165,991 per QALY gained. When the time horizon was extended to 10 years, the ICER fell to $85,117 per QALY gained, well below the $100,000 per QALY gained threshold.
Heart Failure Subgroup
Among the subgroup of patients with NYHA class ≥II at baseline (N=778), those randomized to catheter ablation had an average of 9.96 QALYs (95% CI 9.66 to 10.26) and 11.89 LYs (95% CI 11.53 to 12.25) with a lifetime total cost of $182,463 (95% CI $151,803 to $203,144) (Table 5). Patients in the drug therapy group accrued an average of 9.31 QALYs (95% CI 8.99 to 9.64) and 11.36 LYs (95% CI 10.97 to 11.75) with a lifetime cost of $147,204 (95% CI $116,990 to $166,117). The incremental cost for ablation was $35,259 and the incremental benefits were 0.65 QALYs and 0.53 LYs. The incremental cost-effectiveness ratio for catheter ablation compared to drug therapy was $54,135 per QALY gained, with an 86% likelihood of meeting a $100,000 per QALY gained willingness-to-pay threshold (Figure 2B). Without QOL/utility adjustments, the ICER was $70,907 per LY gained, with a 73% likelihood of meeting a $100,000 per QALY gained threshold.
Table 5.
Economic Outcomes of the Heart Failure Subgroup
| Heart Failure Subgroup | Catheter Ablation | Drug Therapy | Difference | Incremental Cost Effectiveness Ratio | Probability of Cost-Effectiveness |
||
|---|---|---|---|---|---|---|---|
| $50,000 | $100,000 | $150,000 | |||||
| Base Case | |||||||
| Lifetime Costs, USD | 182,463 (151,803, 203,144) |
147,204 (116,990, 166,117) |
35,259 (−2,463, 74,666) |
-- | |||
| QALYs | 9.96 (9.66, 10.26) |
9.31 (8.99, 9.64) |
0.65 (0.26, 1.05) |
$54,135 per QALY | 44% | 85% | 96% |
| LYs | 11.89 (11.53, 12.25) |
11.36 (10.97, 11.75) |
0.53 (0.06, 1.01) |
$70,907 per LY | 35% | 72% | 86% |
|
| |||||||
| Attenuated QoL Benefit † | |||||||
| QALYs | 9.91 (9.61, 10.21) |
9.36 (9.04, 9.69) |
0.54 (0.15, 0.95) |
$64,720 per QALY | 35% | 75% | 90% |
Attenuated QOL benefit – This sensitivity analysis assumes a conservative scenario where the catheter ablation group does not gain any quality-of-life benefits beyond the end of the trial (5-years). Similar to the base case, this analysis did not allow the catheter ablation group to accumulate additional life years compared to the drug group since the within-trial analysis does not support a mortality benefit with ablation by intention-to-treat. That is, the hazard ratio of mortality (catheter ablation versus drug therapy) is set to 1.0 beyond 5 years.
Abbreviations: LY – life year; QALY – quality-adjusted life year; QoL – quality of life; USD – United States dollar.
DISCUSSION
Results from our analysis provide randomized trial-based evidence that choosing ablation rather than drug therapy alone in symptomatic AF patients produces extra quality adjusted life years at a cost that meets current criteria for good value in health care. Three aspects of our analysis results are particularly noteworthy. First, ablation does not appear to make AF a less expensive condition to manage over the long term. The narrowing of cost difference between treatment groups over the first year of follow-up (from a mean difference of $20,794 at 3 months to a difference of $616 at 12 months) was due primarily to drug therapy patients crossing over to ablation therapy. After the first year, costs in the two arms did not differ out to the end of trial follow-up. Second, while patients undergoing ablation had a consistently lower mortality rate than patients treated with drug therapy, the average effect size by ITT was modest (absolute risk reduction of 0.9%). Extrapolating these effects to a lifetime horizon (estimated difference of 0.08 discounted LYs), these incremental benefits were insufficient for ablation to meet conventional criteria for economic attractiveness (ICER of $183,318 per LY). Finally, the case for ablation being cost effective in the base case analysis (ICER of $57,893 per QALY gained) rests primarily upon the assumption underlying the validity of the QALY construct, namely that: a) eligible patients with AF would be willing to trade additional survival for improved AF-related QOL; and that: b) the EQ-5D health utility index data collected on CABANA patients fairly and accurately captures the terms of this trade-off.30
As noted above, the mean cumulative costs were higher in the ablation group compared to drug group, driven by higher hospitalization costs from the initial ablation procedure and redo procedures and mitigated somewhat by crossover procedures in the drug therapy arm. Most procedure-based therapies are not cost neutral or cost saving, but they may be cost effective depending on the efficiency of the relationship between the production of extra units of health benefits and the extra costs/investments needed to produce those units. In the base case analysis, catheter ablation produced an average of +0.27 additional QALYs per patient. The mean cost to produce those extra QALYs was $15,516 per patient. Taking the ratio of these two measures yields the base case incremental cost effectiveness ratio of $57,893 per QALY gained. Bootstrap uncertainty analysis for this result indicated that 75% of CABANA Trial replications yielded an ICER below the US societal benchmark of $100,000 per QALY. The base case results were not substantially affected by cost assumptions, variations in the quality-of-life adjustment, or discount rates. Notably, these results were robust when varying ablation costs; catheter ablation remained cost-effective when increasing the cost per ablation procedure by 50%.
In CABANA, 9.2% of patients randomized to ablation did not receive the procedure, while 27.5% of patients randomized to drug therapy crossed over to ablation during follow-up.9 To examine the effects of these crossovers on the base case results, a post-hoc analysis was conducted using a previously defined 6 month per protocol treatment assignment definition. In the per protocol analysis, incremental costs for the ablation arm were ~2.7 times higher ($41,438 per-protocol versus $15,516 intention-to-treat) since there were no procedural costs accrued in the drug therapy group from crossover procedures. However, since incremental benefits were also larger (~2.1 times increase in incremental QALYs produced), the cost per each additional QALY in the per protocol analysis met conventional thresholds for economic attractiveness in the US ($72,835 per QALY, 81% of bootstrap replications below $100,000 per QALY).
There are several prior model-based cost-effectiveness analyses conducted over a decade ago that assess the value of catheter ablation relative to drug therapy in patients with AF.31, 32 However, these prior model-based analyses projected health benefits based on the prevailing assumption at the time that successful rhythm control provided protection for recurrent ischemic stroke.33 In these prior economic studies,31, 32 this critical assumption likely overestimated the benefit of catheter ablation due to differences in stroke and mortality that favoured ablation over medical therapy. Indeed, our contemporary understanding of catheter ablation is that it is not a curative therapy, but rather a means to reduce symptoms, decrease AF recurrence, and improve quality-of-life.34 With more contemporary clinical assumptions, the present study results are consistent with prior model-based economic evaluations conducted from the perspective of the US and United Kingdom, where the estimated ICERs met country-specific thresholds for value.35–38 Notably, these model-based cost-effectiveness analyses were limited by the small sample size and short-term follow-up of the external studies used to inform the model inputs of ablation effectiveness. In contrast to model-based economic evaluations, a strength of the present analysis is the use of the prospective trial experience to support extrapolation of costs and life expectancy for each individual patient. While country-specific cost-effectiveness analyses are required to properly account for regional differences in the delivery, availability and cost of healthcare resources,39 the present analysis may help provide insights regarding which factors may influence the cost-effectiveness of catheter ablation in other regions. For example, the study identified the index procedural cost as an important determinant of overall cost-effectiveness. If ablation procedures are less expensive relative to other medical services in other countries compared to the US, the overall value proposition of catheter ablation would likely improve, which may be the case in the United Kingdom, Canada and Australia.40–42 Such an improvement would be particularly relevant in countries with lower thresholds for cost-effectiveness. Additionally, two non-pecuniary factors, QOL and coexisting heart failure, were key contributors to the cost-effectiveness of ablation, independent of choice of unit costs.
Concordant with our evolving understanding of the benefit of catheter ablation in patients with concomitant AF and HF,6, 7, 43 the value of ablation was particularly attractive in the subgroup of CABANA patients with NYHA class II or greater HF, where each additional QALY could be attained for $54,135 in lifetime costs. Similar to the base case, there were greater quality-of-life gains in the catheter ablation group compared to drug therapy. However, ablation also conferred added life expectancy, which contributed to the improved value proposition in the HF subgroup. Among CABANA patients with NYHA class >II heart failure, the catheter ablation group had a 43% relative reduction in all-cause mortality (hazard ratio, 0.57 [95% CI, 0.33–0.96]) compared with drug therapy alone.7
This subgroup analysis kept the same assumptions as the base case by attenuating treatment benefit beyond 5-years. However, in ablation trials with relatively long follow up, such as CABANA and CASTLE AF (Catheter Ablation versus Standard conventional Treatment in patients with Left ventricular dysfunction and Atrial Fibrillation), there was a reduction in AF arrhythmic burden with catheter ablation that persisted to the end of trial follow up.5, 6 Assuming that a sustained reduction in AF arrhythmic burden is associated with a reduced risk of mortality and hospitalization,44 this analysis may represent a conservative estimate of cost-effectiveness because it is possible that the mortality benefits of ablation persist beyond 5 years.
The estimate of cost-effectiveness in the CABANA HF subgroup is consistent with published model-based economic evaluations of AF ablation in the HF population.41,42,45 These prior economic analyses evaluated a population consisting of patients with HF with reduced ejection fraction. In the present study, CABANA HF patients primarily had preserved EF and less than 8% had an LVEF < 35% when echocardiographic data were available.7 Thus, this HF subgroup analysis extends the evidence for the value of ablation to HF patients with preserved EF.
Limitations
Several caveats should be considered when interpreting the present study. First, the analysis covers a lifetime horizon, as is standard practice in medical economic analyses, but we are unable to empirically validate the incremental clinical benefits of catheter ablation beyond the 5-year CABANA follow-up. Using sensitivity analyses, the present study was able to explore the consequences on ICER estimates of varying the amount of post-trial extrapolation of treatment-related survival and QOL included. In a sensitivity analysis where quality of life gains were attenuated beyond empiric trial follow up (at 5 years), the ICER was $93,877 per QALY gained, which still meets conventional thresholds for good value in health care within the US. This analysis may be considered conservative, as there is no clinical reason to expect an abrupt disappearance of demonstrated treatment differences at trial closeout. Second, the treatment groups were not masked, which raises the possibility of some overestimation of QOL benefits attributed to a placebo effect in the ablation group. The patterns of benefit seen in the QOL benefits, with sustained effects over years of follow-up and greater absolute benefit in patients with greater AF-related impairment, argue against a placebo effect as the best explanation for observed QOL differences in CABANA.4 Additionally, recent studies have demonstrated that decreased AF arrhythmic burden is associated with greater improvements in quality-of-life following catheter ablation.46,47 This observed relationship decreases the likelihood of a substantial placebo effect. Third, CABANA was an international trial and the economic analysis assumed that economic cost weights derived from US patients could be used to estimate costs for all the non-US patients in the trial. Analyses by region did not identify any geographic differences in overall resource utilization. Using all the trial data in such situations with a uniform set of cost weights provides the best precision for cost estimation and ensures that the numerator and denominator of the ICER are obtained from the same patient cohort.48 Finally, the HF subgroup in CABANA was defined phenotypically using NYHA class reported on the baseline case report form. CABANA did not require imaging or biomarker data at baseline and did not collect information about possible prior HF hospital-based care. The importance of validating the clinical findings in this subgroup has been previously noted.7 By extension, the present economic analysis results in this subgroup presume that the clinical effectiveness estimates are correct and can be replicated.
CONCLUSIONS
Among patients with symptomatic atrial fibrillation enrolled in CABANA, catheter ablation met criteria for economic attractiveness relative to drug therapy both overall, based primarily on incremental quality of life benefits, and in the heart failure subgroup, based on both survival and quality of life benefits.
Supplementary Material
CLINICAL PERSPECTIVE.
What is New?
The CABANA trial found that catheter ablation did not significantly reduce the primary endpoint of death, disabling stroke, serious bleeding, or cardiac arrest compared to drug therapy by intention-to-treat, but improved quality of life and freedom from AF recurrence.
The economic implications have not been previously described.
In this trial-based economic evaluation, catheter ablation was estimated to cost $57,893 per quality-adjusted life-year gained compared to drug therapy in the overall cohort (driven by improvement in quality of life), and $54,135 per QALY gained in the heart failure subgroup (driven by both gains in quality of life and survival).
What are the Clinical Implications?
In CABANA-eligible patients with symptomatic atrial fibrillation, catheter ablation increases the quality-adjusted life expectancy compared to drug therapy alone for an increased cost within present benchmarks for good value in healthcare within the United States.
These findings provide additional economic support for the use of catheter ablation in patients with atrial fibrillation and the subgroup of patients with concomitant heart failure in CABANA.
ACKNOWLEDGEMENTS
We are indebted to the CABANA patients for agreeing to participate in this trial. The authors are also indebted to the local site investigators and study coordinators who enrolled, cared for, and followed the CABANA patients.
SOURCES OF FUNDING
This study was supported by the National Institutes of Health ([NIH] grants U01HL89709, U01HL089786, U01HL089907, and U01HL089645), St. Jude Medical Foundation and Corporation, Biosense Webster Inc, Medtronic, Inc, and Boston Scientific Corporation.
Abbreviations:
- AAD
antiarrhythmic drug
- AF
atrial fibrillation
- CABANA
Catheter Ablation vs Antiarrhythmic Drug Therapy for Atrial Fibrillation trial
- CASTLE
Catheter Ablation versus Standard conventional Treatment in patients with Left ventricular dysfunction and Atrial Fibrillation trial
- CI
confidence interval
- HF
heart failure
- ICER
incremental cost effectiveness ratio
- IPW
inverse probability weighting
- IRR
incident rate ratio
- LY
life year
- NYHA
New York Heart Association
- QALY
quality adjusted life year
- QOL
quality of life
Footnotes
DISCLOSURES
Dr. Chew is supported by a Canadian Institutes of Health Research Banting Fellowship and an Arthur JE Child Cardiology Fellowship outside the submitted work.
Dr. Cowper reports research grant support from Mayo Clinic and NIH/NHLBI for the submitted work, and research grant support from Merck, Eli Lilly, Novartis, AstraZeneca, Bristol Myers Squibb, AGA Medical, Tenax Therapeutics, GE Healthcare and Gilead outside the submitted work.
Dr. Piccini is supported by R01HL128595 from the National Heart, Lung and Blood Institute and receives grants for clinical research from Abbott, American Heart Association, Association for the Advancement of Medical Instrumentation, Bayer, Boston Scientific, NIH, and Philips; consulting from Abbott, Abbvie, Ablacon, Altathera, ARCA Biopharma, Biotronik, Boston Scientific, Bristol Myers Squibb, LivaNova, Medtronic, Milestone, ElectroPhysiology Frontiers, Pfizer, Sanofi, Philips, and Up-to-Date outside the submitted work.
Dr. Poole reports research grants paid directly to the University of Washington from Biotonik, Kestra Medical and AtriCure.
Ms. Monahan reports grants from NIH/NHLBI, St. Jude Foundation and Corporation, Biosense Webster, Inc., Medtronic, Inc, and Boston Scientific Corp, during the conduct of the study; consulting without compensation from Biosense Webster, Inc; and personal fees from Thermedical outside the submitted work.
Dr. Al-Khalidi reports grants from the NIH/NHLBI and Mayo Clinic during the conduct of the study.
Dr. Bahnson reports grants from the NIH/NHLBI and Mayo Clinic during the conduct of the study; grants from St. Jude Medical, Inc, Abbott Medical, Biosense Webster Inc, Johnson & Johnson, NIH, and Boston Scientific Corp.
Dr. Lee reports grants from the NIH/NHLBI and Mayo Clinic, as well as Data Safety and Monitoring Board service on studies funded by Astra-Zeneca, Medtronic, Merck, Amgen, and the Cardiovascular Research Foundation during the conduct of the study.
Dr. Packer in the past 12 months has provided consulting services for Abbott, AtriFix, Biosense Webster, Inc., Cardio Syntax, EBAmed, Johnson & Johnson, MediaSphere Medical, LLC, MedLumics, Medtronic, NeuCures, St. Jude Medical, Siemens, Spectrum Dynamics, Centrix, and Thermedical. Dr. Packer received no personal compensation for these consulting activities, unless noted. Dr. Packer receives research funding from the Abbott, Biosense Webster, Boston Scientific/EPT, CardioInsight, EBAmed, Medtronic, Inc, Siemens, St. Jude Medical, Inc, Thermedical, Inc., NIH, Robertson Foundation, Vital Project Funds, Inc., Mr. and Mrs. J. Michael Cook/Fund. Mayo Clinic and Dr. Packer have a financial interest in Analyze-AVW technology that may have been used to analyze some of the heart images in this research. In accordance with the Bayh-Dole Act, this technology has been licensed to commercial entities, and both Mayo Clinic and Dr. Packer have received royalties greater than $10,000, the federal threshold for significant financial interest. In addition, Mayo Clinic holds an equity position in the company to which the AVW technology has been licensed. Dr. Packer and Mayo Clinic jointly have equity in a privately held company, EBAmed. Royalties from Wiley & Sons, Oxford, and St. Jude Medical.
Dr. Mark reports grants from NIH/NHLBI and Mayo Clinic during the conduct of the study; grants from Merck and HeartFlow; and personal fees from Novartis outside the submitted work.
The remaining authors report no conflicts.
REFERENCES
- 1.Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB and Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation 1998;98:946–952. [DOI] [PubMed] [Google Scholar]
- 2.Dorian P, Paquette M, Newman D, Green M, Connolly SJ, Talajic M and Roy D. Quality of life improves with treatment in the Canadian Trial of Atrial Fibrillation. Am Heart J 2002;143:984–990. [DOI] [PubMed] [Google Scholar]
- 3.Kim MH, Johnston SS, Chu BC, Dalal MR and Schulman KL. Estimation of total incremental health care costs in patients with atrial fibrillation in the United States. Circ Cardiovasc Qual Outcomes 2011;4:313–320. [DOI] [PubMed] [Google Scholar]
- 4.Mark DB, Anstrom KJ, Sheng S, Piccini JP, Baloch KN, Monahan KH, Daniels MR, Bahnson TD, Poole JE, Rosenberg Y, et al. Effect of Catheter Ablation vs Medical Therapy on Quality of Life Among Patients With Atrial Fibrillation: The CABANA Randomized Clinical Trial. JAMA 2019;321:1275–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poole JE, Bahnson TD, Monahan KH, Johnson G, Rostami H, Silverstein AP, Al-Khalidi HR, Rosenberg Y, Mark DB, Lee KL, et al. Recurrence of Atrial Fibrillation After Catheter Ablation or Antiarrhythmic Drug Therapy in the CABANA Trial. J Am Coll Cardiol 2020;75:3105–3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marrouche NF, Brachmann J, Andresen D, Siebels J, Boersma L, Jordaens L, Merkely B, Pokushalov E, Sanders P, Proff J, et al. Catheter Ablation for Atrial Fibrillation with Heart Failure. The New England Journal of Medicine 2018;378:417–427. [DOI] [PubMed] [Google Scholar]
- 7.Packer DL, Piccini JP, Monahan KH, Al-Khalidi HR, Silverstein AP, Noseworthy PA, Poole JE, Bahnson TD, Lee KL, Mark DB. Ablation Versus Drug Therapy for Atrial Fibrillation in Heart Failure: Results From the CABANA Trial. Circulation 2021;143:1377–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson JL, Heidenreich PA, Barnett PG, Creager MA, Fonarow GC, Gibbons RJ, Halperin JL, Hlatky MA, Jacobs AK, Mark DB, et al. ACC/AHA statement on cost/value methodology in clinical practice guidelines and performance measures: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and Task Force on Practice Guidelines. Circulation 2014;129:2329–2345 [DOI] [PubMed] [Google Scholar]
- 9.Packer DL, Mark DB, Robb RA, Monahan KH, Bahnson TD, Poole JE, Noseworthy PA, Rosenberg YD, Jeffries N, Mitchell LB, et al. Effect of Catheter Ablation vs Antiarrhythmic Drug Therapy on Mortality, Stroke, Bleeding, and Cardiac Arrest Among Patients With Atrial Fibrillation: The CABANA Randomized Clinical Trial. JAMA 2019;321:1261–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Packer DL, Mark DB, Robb RA, Monahan KH, Bahnson TD, Moretz K, Poole JE, Mascette A, Rosenberg Y, Jeffries N, et al. Catheter Ablation versus Antiarrhythmic Drug Therapy for Atrial Fibrillation (CABANA) Trial: Study Rationale and Design. Am Heart J 2018;199:192–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mark DB and Hlatky MA. Medical economics and the assessment of value in cardiovascular medicine: Part I. Circulation 2002;106:516–520. [DOI] [PubMed] [Google Scholar]
- 12.US Centers for Medicare and Medicare Services. Overview of the Medicare Physician Fee Schedule https://www.cms.gov/medicare/physician-fee-schedule/search/overview [accessed November 22, 2019].
- 13.Machlin SR and Mitchell EM. Expenses for Office-Based Physician Visits by Specialty and Insurance Type, 2016 Statistical Brief #517 October 2018. Agency for Healthcare Research and Quality, Rockville, MD. http://www.meps.ahrq.gov/mepsweb/data_files/publications/st517/stat517.pdf [PubMed] [Google Scholar]
- 14.Centers for Medicare and Medicaid. National Average Drug Acquisition Cost 2018 https://www.medicaid.gov/medicaid/prescription-drugs/pharmacy-pricing/index.html [accessed November 22, 2019].
- 15.Bobade RA, Helmers RA, Jaeger TM, Odell LJ, Haas DA and Kaplan RS. Time-driven activity-based cost analysis for outpatient anticoagulation therapy: direct costs in a primary care setting with optimal performance. J Med Econ 2019;22:471–477. [DOI] [PubMed] [Google Scholar]
- 16.Bang H and Tsiatis AA. Estimating medical costs with censored data. Biometrika 2000;87:329–343. [Google Scholar]
- 17.Cowper PA, Sheng S, Lopes RD, Anstrom KJ, Stafford JA, Davidson-Ray L, Al-Khatib SM, Ansell J, Dorian P, Husted S, et al. Economic Analysis of Apixaban Therapy for Patients With Atrial Fibrillation From a US Perspective: Results From the ARISTOTLE Randomized Clinical Trial. JAMA Cardiol 2017;2:525–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mark DB, Nelson CL, Anstrom KJ, Al-Khatib SM, Tsiatis AA, Cowper PA, Clapp-Channing NE, Davidson-Ray L, Poole JE, Johnson G, et al. Cost-effectiveness of defibrillator therapy or amiodarone in chronic stable heart failure: results from the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT). Circulation 2006;114:135–142. [DOI] [PubMed] [Google Scholar]
- 19.Nelson CL, Sun JL, Tsiatis AA and Mark DB. Empirical estimation of life expectancy from large clinical trials: use of left-truncated, right-censored survival analysis methodology. Stat Med 2008;27:5525–5555. [DOI] [PubMed] [Google Scholar]
- 20.Arias E and Xu J. United States Life Tables, 2017. National Vital Statistics Report; vol 68 no 7 2019;68:1–65. [PubMed] [Google Scholar]
- 21.Royston P and Parmar MK. Restricted mean survival time: an alternative to the hazard ratio for the design and analysis of randomized trials with a time-to-event outcome. BMC Med Res Methodol 2013;13:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shaw JW, Johnson JA and Coons SJ. US valuation of the EQ-5D health states: development and testing of the D1 valuation model. Med Care 2005;43:203–220. [DOI] [PubMed] [Google Scholar]
- 23.Sullivan PW, Lawrence WF and Ghushchyan V. A national catalog of preference-based scores for chronic conditions in the United States. Med Care 2005;43:736–749. [DOI] [PubMed] [Google Scholar]
- 24.O'Brien BJ and Briggs AH. Analysis of uncertainty in health care cost-effectiveness studies: an introduction to statistical issues and methods. Stat Methods Med Res 2002;11:455–468. [DOI] [PubMed] [Google Scholar]
- 25.Efron B, Tibshirani RJ An introduction to the bootstrap New York: Chapman & Hall/CRC; 1993. [Google Scholar]
- 26.Willan AR and Briggs AH. Statistical analysis of cost-effectiveness data Chichester, West Sussex, England: John Wiley & Sons, Ltd.; 2006. [Google Scholar]
- 27.Dunn A, Grosse SD and Zuvekas SH. Adjusting Health Expenditures for Inflation: A Review of Measures for Health Services Research in the United States. Health Serv Res 2018;53:175–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sanders GD, Neumann PJ, Basu A, Brock DW, Feeny D, Krahn M, Kuntz KM, Meltzer DO, Owens DK, Prosser LA, et al. Recommendations for Conduct, Methodological Practices, and Reporting of Cost-effectiveness Analyses: Second Panel on Cost-Effectiveness in Health and Medicine. JAMA 2016;316:1093–1103. [DOI] [PubMed] [Google Scholar]
- 29.Vanness DJ, Lomas J and Ahn H. A Health Opportunity Cost Threshold for Cost-Effectiveness Analysis in the United States. Ann Intern Med 2021;174:25–32. [DOI] [PubMed] [Google Scholar]
- 30.Rand LZ and Kesselheim AS. Controversy Over Using Quality-Adjusted Life-Years In Cost-Effectiveness Analyses: A Systematic Literature Review: Systematic literature review examines the controversy over the use of quality-adjusted life-year in cost-effectiveness analyses. Health Affairs 2021;40:1402–1410. [DOI] [PubMed] [Google Scholar]
- 31.Blackhouse G, Assasi N, Xie F, Gaebel K, Campbell K, Healey JS, O'Reilly D and Goeree R. Cost-effectiveness of catheter ablation for rhythm control of atrial fibrillation. Int J Vasc Med 2013;2013:262809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chan PS, Vijan S, Morady F and Oral H. Cost-effectiveness of radiofrequency catheter ablation for atrial fibrillation. J Am Coll Cardiol 2006;47:2513–2520. [DOI] [PubMed] [Google Scholar]
- 33.Falk RH. Rate control is preferable to rhythm control in the majority of patients with atrial fibrillation. Circulation 2005;111:3141–3150. [DOI] [PubMed] [Google Scholar]
- 34.January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC Jr., Ellinor PT, Ezekowitz MD, Field ME, Furie KL, et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation 2019;140:e125–e151 [DOI] [PubMed] [Google Scholar]
- 35.Ollendorf D, Silverstein M and Bobo T. Management options for atrial fibrillation. US Institute for Clinical and Economic Review 2010. Available from: https://icer.org/wp-content/uploads/2020/10/Atrial-Fibrillation-Final-09-24-10.pdf
- 36.Perry M, Kemmis Betty S, Downes N, Andrews N, Mackenzie S. Atrial fibrillation: diagnosis and management-summary of NICE guidance. BMJ 2021;373:n1150. [DOI] [PubMed] [Google Scholar]
- 37.McKenna C, Palmer S, Rodgers M, Chambers D, Hawkins N, Golder S, Van Hout S, Pepper C, Todd D and Woolacott N. Cost-effectiveness of radiofrequency catheter ablation for the treatment of atrial fibrillation in the United Kingdom. Heart 2009;95:542–549. [DOI] [PubMed] [Google Scholar]
- 38.Reynolds MR, Zimetbaum P, Josephson ME, Ellis E, Danilov T and Cohen DJ. Cost-effectiveness of radiofrequency catheter ablation compared with antiarrhythmic drug therapy for paroxysmal atrial fibrillation. Circulation Arrhythmia and Electrophysiology 2009;2:362–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Drummond M, Barbieri M, Cook J, Glick HA, Lis J, Malik F, Reed SD, Rutten F, Sculpher M and Severens J. Transferability of economic evaluations across jurisdictions: ISPOR Good Research Practices Task Force report. Value Health 2009;12:409–418. [DOI] [PubMed] [Google Scholar]
- 40.National Institute for Health and Care Excellence. Atrial fibrillation: diagnosis and management (Cost-effectiveness analysis J3: Ablation) https://www.nice.org.uk/guidance/ng196/evidence/j3-ablation-costeffectiveness-analysis-pdf-326949243734 [accessed November 15, 2021] [PubMed]
- 41.Lau D, Sandhu RK, Andrade JG, Ezekowitz J, So H, and Klarenbach S. Cost‐Utility of Catheter Ablation for Atrial Fibrillation in Patients with Heart Failure: An Economic Evaluation. Journal of the American Heart Association 2021;10:e019599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gao L, Moodie M. Modelling the lifetime cost-effectiveness of catheter ablation for atrial fibrillation with heart failure. BMJ open 2019;9:e031033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Turagam MK, Garg J, Whang W, Sartori S, Koruth JS, Miller MA, Langan N, Sofi A, Gomes A, Choudry S, et al. Catheter Ablation of Atrial Fibrillation in Patients With Heart Failure: A Meta-analysis of Randomized Controlled Trials. Ann Intern Med 2019;170:41–50. [DOI] [PubMed] [Google Scholar]
- 44.Brachmann J, Sohns C, Andresen D, Siebels J, Sehner S, Boersma L, Merkely B, Pokushalov E, Sanders P, Schunkert H, et al. Atrial Fibrillation Burden and Clinical Outcomes in Heart Failure: The CASTLE-AF Trial. JACC Clin Electrophysiol 2021;7:594–603. [DOI] [PubMed] [Google Scholar]
- 45.Chew DS, Loring Z, Anand J, Fudim M, Lowenstern A, Rymer JA, Weimer K, Atwater BD, DeVore AD, Exner DV, et al. Economic Evaluation of Catheter Ablation of Atrial Fibrillation in Patients with Heart Failure With Reduced Ejection Fraction. Circ Cardiovasc Qual Outcomes 2020;13:e007094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Samuel M, Khairy P, Champagne J, Deyell MW, Macle L, Leong-Sit P, Novak P, Badra-Verdu M, Sapp J, Tardif JC, et al. Association of Atrial Fibrillation Burden With Health-Related Quality of Life After Atrial Fibrillation Ablation: Substudy of the Cryoballoon vs Contact-Force Atrial Fibrillation Ablation (CIRCA-DOSE) Randomized Clinical Trial. JAMA Cardiology 2021;6(11):1324–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Terricabras M, Mantovan R, Jiang CY, Betts TR, Chen J, Deisenhofer I, Macle L, Morillo CA, Haverkamp W, Weerasooriya R, et al. Association between quality of life and procedural outcome after catheter ablation for atrial fibrillation: a secondary analysis of a randomized clinical trial. JAMA network open 2020;3:e2025473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ramsey S, Willke R, Briggs A, Brown R, Buxton M, Chawla A, Cook J, Glick H, Liljas B, Petitti D, et al. Good research practices for cost-effectiveness analysis alongside clinical trials: the ISPOR RCT-CEA Task Force report. Value Health 2005;8:521–533. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


