Abstract
Genetic and early environmental differences including early health habits associate with future health. To provide insight on the causal nature of these associations, monozygotic (MZ) twin pairs discordant for health habits provide an interesting natural experiment. Twin pairs discordant for leisure‐time physical activity (LTPA) in early adult life is thus a powerful study design to investigate the associations between long‐term LTPA and indicators of health and wellbeing. We have identified 17 LTPA discordant twin pairs from two Finnish twin cohorts and summarize key findings of these studies in this paper. The carefully characterized rare long‐term LTPA discordant MZ twin pairs have participated in multi‐dimensional clinical examinations. Key findings highlight that compared with less active twins in such MZ twin pairs, the twins with higher long‐term LTPA have higher physical fitness, reduced body fat, reduced visceral fat, reduced liver fat, increased lumen diameters of conduit arteries to the lower limbs, increased bone mineral density in loaded bone areas, and an increased number of large high‐density lipoprotein particles. The findings increase our understanding on the possible site‐specific and system‐level effects of long‐term LTPA.
Keywords: body fat, exercise, genes, health, physical activity, twins
1. INTRODUCTION
Participation in leisure‐time physical activity (LTPA) has been shown to be associated with many indicators of good health. 1 However, based on observational studies, it is difficult to confirm the causality as many potential confounding factors including the genetic background influence these associations. 2 Further, in short‐term studies, reverse causality can bias results. It is possible that some genes may via the same mechanisms influence participation in physical activity, physical fitness, body composition, metabolism, and occurrence of chronic diseases (genetic pleiotropy). 3 , 4 Also, it is difficult to carry out high quality randomized controlled physical activity/exercise trials with long durations.
The genetic background of physical activity, physical fitness, and most of the non‐communicable chronic diseases is multifactorial. Monozygotic (MZ) twin pairs are usually reared together in a similar environment until early adulthood. As genetic and early environmental differences including early health habits may have an influence on future health, MZ twin pairs who become discordant for LTPA during adult life provide an interesting natural experiment to investigate the associations between LTPA and indicators of health and wellbeing. Also, twins in MZ twin pairs who are discordant for physical activity usually are concordant for many other health habits. 5 We have used this study design by comprehensively identifying LTPA discordant twin pairs from two Finnish twin cohorts (FinnTwin16 and older Finnish Twin Cohort). We summarize the key health‐related findings of these studies in this paper. The carefully characterized rare long‐term LTPA discordant MZ twin pairs have participated in multi‐dimensional clinical examinations with the aim to investigate the differences in physical fitness, body composition, vascular and metabolic risk factors, muscle and adipose tissue gene expressions, artery diameters, bone structure, as well as in brain structure and function. In particular, our short review presents findings which increase our understanding on the possible detailed site‐specific and system‐level effects of long‐term LTPA participation.
2. MATERIALS AND METHODS
2.1. Identification of the MZ twin pairs discordant for LTPA
The participants described here are from the Finnish population‐based twin cohorts: FinnTwin 16 Cohort 6 and the older Finnish Twin Cohort. 7 The pairs were comprehensively selected from the cohorts with a stepwise process using available questionnaire data and subsequent detailed telephone and face‐to‐face interviews (Figure 1). 8 , 9 See Appendix S1 for the detailed procedures to identify the long‐term LTPA discordant MZ twin pairs. The twin pairs of the TWINACTIVE study (five male and two female MZ pairs) had been strongly discordant for LTPA for 30+ years and those of FITFATTWIN study (10 male MZ pairs) for 3 years. The MZ pairs were interviewed in depth using the same structural interview protocol for physical activity, and the data were pooled from the LTPA‐discordant MZ pairs from both studies. The LTPA (including commuting activity) volume was 2.0 ± 1.8 MET‐hours per day for less active twins and 6.1 ± 3.7 for their more active co‐twins, the pairwise difference being 4.1 MET‐hours per day (95% CI 2.5–5.6; p < 0.0001) in the pooled data (see Table 1 for selected pooled data and Table S1 for selected data for TWINACTIVE and FITFATTWIN participants separately). The older TWINACTIVE pairs (mean age 62 years, range 50–74 years) represent pairs who were apparently healthy at baseline but may have developed some predisease/disease states during follow‐up prior to the clinical measurements. This may have an influence on the metabolic differences between the more and less active members of the MZ pairs in this older cohort. Among the younger FITFATTWIN participants (mean age 34 years, range 32–36 years), clinical disease was absent (and so does not have an influence on findings), thus difference in measured metabolic and clinical measures are showing more direct LTPA associations compared with those seen in older pairs. All the studies and their procedures that contributed to this summary review complied with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. All participating twins provided informed consent.
FIGURE 1.
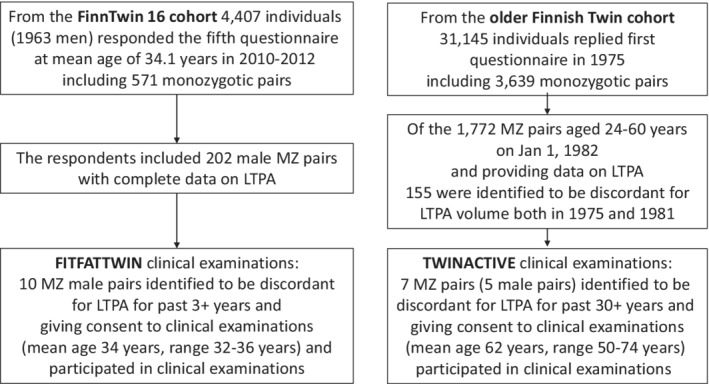
Identification of the rare leisure‐time physical activity (LTPA) discordant monozygotic (MZ) twin pairs from the population‐based twin cohorts
TABLE 1.
Selected data from monozygotic twin pairs discordant for leisure time physical activity habits pooled from TWINACTIVE and FITFATTWIN studies
| Less active co‐twins (N = 17) | More active co‐twins (N = 17) | Intrapair difference Mean (95% CI) | |
|---|---|---|---|
| Mean ± SD | |||
| VO2peak, ml/kg/min | 33.0 ± 6.6 | 38.6 ± 7.6 | 5.6 (3.3–8.0) |
| Past 12‐month LTPA, LTMET‐hours/day | 2.0 ± 1.8 | 6.1 ± 3.7 | 4.1 (2.5–5.6) |
| Waist circumference, cm | 92.3 ± 9.0 | 88.4 ± 7.8 | −3.9 (−6.9 to −0.8) |
| Fat percent, % | 22.6 ± 6.0 | 19.7 ± 4.7 | −3.0 (−5.6 to −0.6) |
| Visceral adipose tissue area, cm2 | 144 ± 54 | 107 ± 55 | −37 (−61 to −13) |
| Liver fat index, MRI signal intensity | 15.1 ± 16.6 | 6.8 ± 5.9 | −8.3 (−15.4 to −1.1) |
| ApoB:ApoA1 ratio | 0.61 ± 0.13 | 0.53 ± 0.03 | −0.08 (−0.13 to −0.02) |
| HDL cholesterol, mmol/L | 1.43 ± 0.10 | 1.54 ± 0.11 | 0.11 (0.01–0.22) |
| HDL2 cholesterol, mmol/L | 0.93 ± 0.35 | 1.05 ± 0.42 | 0.12 (0.02–0.22) |
| HDL diameter, nm | 9.72 ± 0.23 | 9.83 ± 0.24 | 0.11 (0.05–0.16) |
| Very large HDL particles, μmol/L | 0.21 ± 0.11 | 0.27 ± 0.16 | 0.07 (0.02–0.11) |
| Large HDL particles, μmol/L | 0.80 ± 0.43 | 0.98 ± 0.50 | 0.17 (0.08–0.27) |
| Medium HDL particles, μmol/L | 1.82 ± 0.47 | 1.82 ± 0.43 | −0.00 (−0.13 to 0.12) |
| Small HDL particles, μmol/L | 4.64 ± 0.36 | 4.55 ± 0.39 | −0.09 (−0.25 to 0.06) |
Abbreviations: ApoA1, apolipoprotein A1; ApoB, apolipoprotein B; CI, confidence interval; HDL, high density lipoprotein; LTMET‐hours/day, leisure‐time metabolic equivalent for physical activity during leisure‐time and the commute to and from work indicating daily leisure‐time physical activity volume; MRI, magnetic resonance imaging.
The selection of the twin pairs to each sub‐study and their results have been previously published and the used measurement methods described in detail in the references given for each outcome. The pooled results given in Table 1 are previously unpublished.
To test our hypotheses, data analyses were carried out as pairwise analyses comparing inactive versus active members of twin pairs discordant for LTPA. The normality of the variables was assessed by the Shapiro–Wilk test. In the pairwise comparison, student's paired t‐test was used for normally distributed variables and the Wilcoxon matched‐pair signed rank test for non‐normally distributed variables. The 95% confidence intervals (CI) were calculated for the absolute mean differences between the inactive versus active co‐twins. The symmetry test was used for categorical variables. In the numeric results, in our results section or the new result shown in Table 1 adjustment for multiple testing is not used, but in the original papers, we have used more sophisticated statistical analyses or given corrected statistical thresholds for data‐driven analyses with a higher number of variables, such as circulating biomarker, 10 gene expression, 11 and brain related analyses. 9 , 12 , 13 , 14 In this short review, concerning the numeric results of these analyses, we refer to those given in the original papers.
3. RESULTS
Main findings on the MZ pairs discordant for LTPA.
3.1. Fitness and heart function
According to pairwise analyses, aerobic fitness (measured by a maximal exercise test using bicycle ergometer 8 , 9 ) was higher in the more active twins compared with their less active co‐twins (for pooled results see Table 1 and for the results of younger and older pairs separately, see Table S1).
3.2. Body weight and fat accumulation
In both the FITFATTWIN and TWINACTIVE studies, body weight (pooled difference between active and inactive members 1.8 kg (95% CI −1.8 to 5.5; p = 0.31) did not differ between the members of the pairs as strongly as body fat composition (Table 1 and Table S1). 15 , 16 When data were pooled from these two studies, among the 17 MZ pairs waist circumference, body fat percent, liver fat content, and in particular visceral fat was lower in active compared with inactive twins (Table 1).
3.3. Risk factors/circulating biomarkers measured from venous blood samples
Various associations in circulating biomarkers with high LTPA were seen in the TWINACTIVE study. 10 In the pooled data from TWINACTIVE and FITFATTWIN studies, we saw that the more active twins had lower ApoB:ApoA1 –ratios (difference − 0.079, 95% CI −0.133 to −0.024; p = 0.0077), higher HDL2 cholesterol levels (difference 0.118 mmol/L, 95% CI 0.017–0.218; p = 0.0247), and higher HDL particle diameters (difference 0.108 nm, 95% CI 0.054–0.162; p = 0.0007) (Table 1) compared with the less active twins.
3.4. Muscle and adipose tissue gene expressions
In the TWINACTIVE study, muscle biopsies were obtained from the twin pairs. Gene expression in skeletal muscle of the central pathways of energy metabolism, especially of genes related to the processes of oxidative phosphorylation were up‐regulated among the more physically active twins. 11 Interestingly, the upregulation of skeletal muscle oxidative phosphorylation gene set expression correlated with the number of large HDL particles (r = 0.75; p = 0.0003), but not with the number of small HDL particles. 11 In both skeletal muscle and subcutaneous fat tissue samples, the up‐regulated pathways among the active twins compared with their inactive co‐twins included branched‐chain amino acid (BCAA) degradation. 11
3.5. Arteries to lower limbs
The TWINACTIVE study used contrast‐enhanced magnetic resonance angiography to measure the diameters of aorta, iliac, and femoral arteries. Compared with less active co‐twins, the active twins had larger lumen diameters in these arteries supplying blood to lower limbs; lumen diameter difference of upper abdominal aorta 1.8 mm (95% CI 0.3–2.7; 0.025), distal aorta 2.8 mm (95% CI 1.5–4.0; p = 0.002), of right common iliac artery 2.6 mm (95% CI 0.8–4.0; p = 0.016) and of right femoral artery 1.3 mm (95% CI 0.6–2.0; p = 0.005). 17 A similar difference was not seen in the size of carotid arteries. 17
3.6. Tibial bones
The TWINACTIVE study using peripheral quantitative computer tomography showed that compared with less active twins of these MZ twin pairs, the more active ones had thicker tibial cortical bone in antero‐posterior direction the difference in the mid‐cortical bone cross‐sectional area being 40 mm2 (95% CI 19–61; p = 003) and higher trabecular bone density in the distal tibia the difference in the total volumetric bone mineral density of the trabecular bone of the distal tibia being 26 mg/cm3 (95% CI 0–53; p = 0.050). 18 There were no clear differences in the external dimensions of the bones. 18
3.7. Brain structure and functions
The FITFATTWIN study showed that total‐brain white matter, gray matter (GM), and total intracranial volumes did not differ between the more and less active twins when analyzed with magnetic resonance imaging whole‐brain voxel‐based morphometry. However, compared with the less active twins, the more active co‐twins showed larger striatal and non‐dominant inferior frontal gyrus GM volumes. 9 Also, there was regional differentiation in GM volumes between more and less active co‐twins suggesting higher GM volume in the left hippocampus in more active co‐twins. 12 Additional comparisons showed differing automatic deviance‐detection processes in brain regions involved with sensorimotor, visual, and memory functions in electrophysiological studies between the more and less active co‐twins. 13 , 14
For summary of the main results, see Figure 2. More data on the findings are also available in nine PhD theses (see links to the texts in Appendix S1).
FIGURE 2.
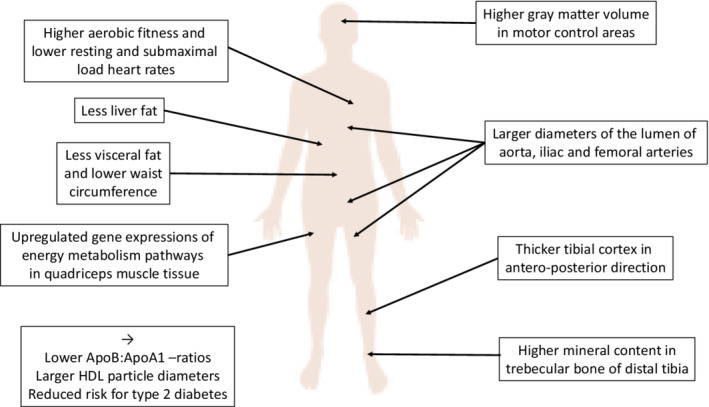
Key health‐related findings showing how more physically active twins of monozygotic twin pairs differ from their less active co‐twins
4. DISCUSSION
4.1. Comments on the main findings
We found expected differences between the more and less active members of the MZ twin pairs in physical fitness. Given the clear differences in LTPA levels, the observation that dietary energy intake tended to be higher in more physically active members, indicates that different LTPA levels are the most plausible explanation for the observed body composition differences, 16 , 19 the findings also being in line with data from randomized controlled trials. 20 The concentrations of the smallest HDL particles has been reported to be negatively associated with the concentrations of the large HDL particles, and unlike large HDL particles, the concentrations of the small HDL particles associate similarly with cardio‐metabolic risk as the concentrations of apolipoprotein‐B containing particles. 21 It is to note that the HDL particle size has been shown to be associated with its functions, and the association with the increased number of large HDL particles may be related to their other signal carrier function supporting high use of oxygen and high energy metabolism rather than only reverse cholesterol transport. 22 , 23 , 24 The high correlation between oxidative phosphorylation gene set expression in skeletal muscle and the number of large HDL particles support the idea that the functions of HDL particles include associations with exercise related oxygen use in skeletal muscles. 23 The observed up‐regulation of BCAA degradation 11 pathway among the active twins compared with their inactive co‐twins is likely an indicator of increased BCAA degradation in healthy mitochondria to uphold lipid oxidation. 25 So, serum BCAA levels correlate with low LTPA and high body fat as has been shown earlier by Felig et al. 26 and by Pietiläinen et al. 27 among MZ twin pairs discordant for BMI.
The finding of differences in the aorta and conduit arteries to lower limbs but not similarly in carotid arteries suggest location‐specific effects to arteries supplying the muscles used most during exercising. Also, the bone related findings were site‐specific according to loading unlike in case of the effects of postmenopausal hormone‐replacement therapy. 28 There were no clear differences in the external dimensions of the bones which are likely to change more during growth. 18 Also, the brain‐related findings provide evidence for site‐specific structural modulation in healthy young adult brain likely associated with long‐term LTPA.
All in all, our MZ twin pair studies show associations between high level of LTPA and beneficial changes in many disease risk factors, however, it is to note that our studies with the whole older Finnish Twin Cohort has not shown that high LTPA is associated with reduced risk of death when using the co‐twin control design among MZ twin pairs. 29 , 30 , 31 In particular, at older ages occurrence of diseases contributes to the physical activity discordances. 32
4.2. Strengths and limitations of the co‐twin control study design
As a result of the match for age, sex, gene sequence, and a close match for intrauterine and childhood environment, the MZ co‐twin control study represents a unique study design to investigate the health effects of long‐term physical activity controlling for genetic and familial factors. However, it is uncommon that co‐twins of a MZ twin pair have persistently different activity levels, and it was therefore difficult to find large number of MZ twin pairs significantly discordant for long‐term physical activity. This limitation itself speaks for a genetic and other familial basis for lifetime activity patterns. Consequently, the number of LTPA discordant MZ pairs remained small in both studies. We included extensive retrospective questionnaire‐based follow‐ups of LTPA habits combined with detailed structured interviews in our studies. However, it is to note that our findings are not necessarily generalizable to other populations as the heritability of physical activity behavior depends on both the definition and country of origin. 33 Although our data relies on self‐reported measures of physical activity, taking into account that we had a multi‐step procedure using multiple repeated questionnaire and interview methods to document the LTPA levels (see Appendix S1), our data provide reliable estimates of long‐term LTPA differences between the co‐twins of the MZ twin pairs. 34 It is to note that other health habits and socioeconomic status of the members of MZ pairs are more similar than between randomly selected individuals. There were no significant differences in marital status, alcohol use, smoking habits, or work‐related physical activity between inactive and active co‐twins in our studies. 8 , 9 Participants of the FITFATTWIN study had somewhat lower BMI and mean physical activity level but otherwise rather similar subject characteristics compared with that of the other men in the cohort. 9 The generalizability of the results to women needs further research. Based on the previous studies, twins seem not to differ from the general population on many traits, behavior, and diseases. 35 , 36 Because the number of subjects in both studies remained quite low, we were able to carry out in depth clinical examinations, which usually are not included in large population studies.
4.3. Reasons for the LTPA discordances
In the small number of LTPA discordant, MZ pairs statistically significant reasons for the discordances could not be identified. However, it is to note that when motives for LTPA were measured in the larger twin cohorts, the motivational factors for LTPA that differed significantly between the more physically active twins and their less physically active co‐twins in both the cohorts were mastery (improve skills/get better at an activity), physical fitness (be physically fit), and psychological state (improve psychological well‐being). 37 , 38 In young adulthood, family and work‐related commitments obviously influenced the occurrence of LTPA discordances between the members of the twin pairs. 9 Interestingly, however, the barriers to exercise training (including e.g., lack of time or lack of facilities for exercise) did not differ between the less and more active twins at an older age. 37
4.4. Perspective
Studying rare monozygotic twin pairs who are discordant for LTPA is an interesting study design as it is practically impossible to conduct very long‐term controlled exercise interventions. As many of the associations between LTPA and health‐related outcomes were seen in the pairwise analyses among MZ twin pairs, our study findings support the existence of causal relationships. Expectedly, the pairwise differences were larger among the members of the older twin pairs with longer and stronger differences in LTPA, see Table S1. The present data from LTPA discordant MZ twin pairs are in agreement with the data from RCTs 39 showing that exercise interventions improve fitness, body composition, and selected cardiometabolic disease risk factors. In the future, collaborative studies using different twin cohorts with higher number of long‐term LTPA discordant twin pairs are warranted to add to the current knowledge.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
Supporting information
Appendix S1 Supporting Information
ACKNOWLEDGEMENT
We thank the participants of the reported twin studies and the collaborating authors of different specific research reports. Main funding of the substudies: TWINACTIVE study: The Finnish Ministry of Education and Culture (grant to UMK), Academy of Finland (grant to UMK), Finnish Cultural Foundation (grant to TL), Juho Vainio Foundation (grants to UMK, TL and SA) and Yrjö Jahnnsson Foundation (grant to SA). FITFATTWIN study: The Finnish Ministry of Education and Culture (UMK), META‐PREDICT (within the European Union Seventh Framework Program, HEALTH‐F2‐2012‐277936 to UMK), Juho Vainio Foundation (MR), and Finnish Cultural Foundation (MR). MOBILETWIN study: The Finnish Ministry of Education and Culture (grant OKM/56/626/2013 to UMK). The Finnish Twin Cohort Study has been supported by multiple grants over the past 45 years, including grants from the Academy of Finland (to JK) and NIH (to JK and Richard J Rose for FinnTwin16). The current grant (#336823) from the Academy of Finland supporting JK is specifically acknowledged. MAK is supported by a research grant from the Sigrid Juselius Foundation, Finland.
Kujala UM, Leskinen T, Rottensteiner M, et al. Physical activity and health: Findings from Finnish monozygotic twin pairs discordant for physical activity. Scand J Med Sci Sports. 2022;32:1316‐1323. doi: 10.1111/sms.14205
Section III: Health, Disease & Physical Activity (Short review article)
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. 2018 Physical Activity Guidelines Advisory Committee . 2018 Physical Activity Guidelines Advisory Committee Scientific Report. U.S. Department of Health and Human Services; 2018. [Google Scholar]
- 2. Kujala UM. Is physical activity a cause of longevity? It's not as straightforward as some would believe. A Critical Analysis. Br J Sports Med. 2018;52:914‐918. [DOI] [PubMed] [Google Scholar]
- 3. Kujala U. Physical activity, genes, and lifetime predisposition to chronic disease. Eur Rev Aging Phys Act. 2011;8:31‐36. [Google Scholar]
- 4. Sillanpää E, Palviainen T, Ripatti S, Kujala UM, Kaprio J. Polygenic score for physical activity is associated with multiple common diseases. Med Sci Sports Exerc. 2022;54(2):280‐287. doi: 10.1249/MSS.0000000000002788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kaprio J, Koskenvuo M, Sarna S. Cigarette smoking, use of alcohol, and leisure‐time physical activity among same‐sexed adult male twins. Prog Clin Biol Res. 1981;69 Pt C:37‐46. [PubMed] [Google Scholar]
- 6. Kaidesoja M, Aaltonen S, Bogl L, Heikkilä K, Kaartinen S, et al. FinnTwin 16: a longitudinal study from age 16 of a population‐based Finnish twin cohort. Twin Res Hum Genetics. 2019;22:530‐539. [DOI] [PubMed] [Google Scholar]
- 7. Kaprio J, Bollepalli S, Buchwald J, Iso‐Markku P, Korhonen T, et al. The older Finnish twin cohort – 45 years of follow‐up. Twin res Hum Genet 2019;22:240–254. [DOI] [PubMed] [Google Scholar]
- 8. Leskinen T, Waller K, Mutikainen S, et al. Effects of 32‐year leisure time physical activity discordance in twin pairs on health (TWINACTIVE study): aims, design and results for physical fitness. Twin Res Human Genetics. 2009;12:108‐117. [DOI] [PubMed] [Google Scholar]
- 9. Rottensteiner M, Leskinen T, Niskanen E, Aaltonen S, Mutikainen S, et al. Physical activity, fitness, glucose homeostasis, and brain morphology in twins. Med Sci Sports Exerc. 2015;47:509‐518. [DOI] [PubMed] [Google Scholar]
- 10. Kujala UM, Mäkinen V‐P, Heinonen I, et al. Long‐term leisure‐time physical activity and serum metabolome. Circulation. 2013;127:340‐348. [DOI] [PubMed] [Google Scholar]
- 11. Leskinen T, Rinnankoski‐Tuikka R, Rintala M, et al. Differences in muscle and adipose tissue gene expression and cardio‐metabolic risk factors in the members of physical activity discordant twin pairs. PLoS one. 2010;5:e12609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tarkka IM, Hautasaari P, Pesonen H, et al. Long‐term physical activity may modify brain structure and function: studies in young healthy twins. J Phys Act Health. 2019;16:637‐643. [DOI] [PubMed] [Google Scholar]
- 13. Hautasaari P, Savic AM, Loberg O, Niskanen E, Kaprio J, et al. Somatosensory brain function and gray matter regional volumes differ according to exercise history: evidence from monozygotic twins. Brain Topogr. 2017;30:77‐86. [DOI] [PubMed] [Google Scholar]
- 14. Pesonen H, Savic AM, Kujala UM, Tarkka IM. Long‐term physical activity modifies automatic visual processing. Int J Sport Exerc Psychol. 2019;17:275‐284. [Google Scholar]
- 15. Leskinen T, Sipilä S, Alen M, et al. Leisure time physical activity and high‐risk fat: a longitudinal population‐based twin study. Int J Obesity. 2009;33:1211‐1218. [DOI] [PubMed] [Google Scholar]
- 16. Rottensteiner M, Leskinen T, Järvelä‐Reijonen E, et al. Leisure‐time physical activity and intra‐abdominal fat in young adulthood: a monozygotic co‐twin control study. Obesity. 2016;24:1185‐1191. [DOI] [PubMed] [Google Scholar]
- 17. Leskinen T, Usenius J‐P, Alen M, Kainulainen H, Kaprio J, Kujala UM. Leisure time physical activity and artery lumen diameters: a monozygotic co‐twin control study. Scand J Med Sci Sports. 2011;21:e208‐e214. [DOI] [PubMed] [Google Scholar]
- 18. Ma H, Leskinen T, Alen M, et al. Long‐term leisure time physical activity and properties of bone: a twin study. J Bone Mineral Res. 2009;24:1427‐1433. [DOI] [PubMed] [Google Scholar]
- 19. Rintala M, Lyytikäinen A, Leskinen T, et al. Leisure time physical activity and nutrition: a twin study. Public Health Nutr. 2011;14:846‐852. [DOI] [PubMed] [Google Scholar]
- 20. Slentz CA, Aiken LB, Houmard JA, et al. Inactivity, exercise, and visceral fat. STRRIDE: a randomized, controlled study of exercise intensity and amount. J Appl Physiol. 2005;99:1613‐1618. [DOI] [PubMed] [Google Scholar]
- 21. Ala‐Korpela M, Zhao S, Järvelin MR, Mäkinen VP, Ohukainen P. Apt interpretation of comprehensive lipoprotein data in largescale epidemiology: disclosure of fundamental structural and metabolic relationships. Int J Epidemiol. 2021;51:996‐1011. doi: 10.1093/ije/dyab156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wilkins JT, Seckler HS. HDL modification: recent developments and their relevance to atherosclerotic cardiovascular disease. Curr Opin Lipidol. 2019;30:24‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lehti M, Donelan E, Abplanalp W, Al‐Massadi O, Habegger KM, et al. High‐density lipoprotein maintains skeletal muscle function by modulating cellular respiration in mice. Circulation. 2013;128:2364‐2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vickers KC, Michell DL. HDL‐small RNA export, transport, and functional delivery in atherosclerosis. Curr Atheroscler Rep. 2021;23:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kainulainen H, Hulmi JJ, Kujala UM. Potential role of branched‐chain amino acid catabolism in regulating fat oxidation. Exerc Sport Sci Rev. 2013;41:194‐200. [DOI] [PubMed] [Google Scholar]
- 26. Felig P, Marliss E, Cahill GF Jr. Plasma amino acid levels and insulin secretion in obesity. New Engl J Med. 1969;281:811‐816. [DOI] [PubMed] [Google Scholar]
- 27. Pietiläinen KH, Naukkarinen J, Rissanen A, et al. Global transcript profiles of fat in monozygotic twins discordant for BMI: pathways behind acquired obesity. PLoS Med. 2008;5:e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mikkola TM, Heinonen A, Kovanen V, et al. Influence of long‐term postmenopausal hormone‐replacement therapy on estimated structural bone strength: a study in discordant monozygotic twins. J Bone Miner Res. 2011;26:546‐552. [DOI] [PubMed] [Google Scholar]
- 29. Kujala UM, Kaprio J, Sarna S, Koskenvuo M. Relationship of leisure‐time physical activity and mortality: the Finnish Twin Cohort. JAMA. 1998;279:440‐444. [DOI] [PubMed] [Google Scholar]
- 30. Kujala UM, Kaprio J, Koskenvuo M. Modifiable risk factors as predictors of all‐cause mortality: the roles of genetics and childhood environment. Am J Epidemiol. 2002;156:985‐993. [DOI] [PubMed] [Google Scholar]
- 31. Karvinen S, Waller K, Silvennoinen M, et al. Physical activity in adulthood: genes and mortality. Sci Rep. 2015;5:18259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kujala UM, Hautasaari P, Vähä‐Ypyä H, et al. Chronic diseases and objectively measured physical activity among aged individuals ‐ A cross‐sectional twin cohort study. Ann Med. 2019;51:78‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Duncan GE, Goldberg J, Noonan C, Moudon AV, Hurvitz P, Buchwald D. Unique environmental effects on physical activity participation: a twin study. PLoS ONE. 2008;3:e2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Waller K, Kaprio J, Kujala UM. Associations between long‐term physical activity, waist circumference and weight gain: a 30‐year longitudinal twin study. Int J Obesity. 2008;32:353‐361. [DOI] [PubMed] [Google Scholar]
- 35. Evans DM, Martin NG. The Validity of Twin Studies. GeneScreen. 2000;1:77‐79. [Google Scholar]
- 36. Barnes JC, Boutwell BB. A demonstration of the generalizability of twin‐based research on antisocial behavior. Behav Genet. 2013;43:120‐131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Aaltonen S, Leskinen T, Morris T, et al. Motives and barriers to physical activity in twin pairs discordant for leisure time physical activity for 30 years. Int J Sports Med. 2012;33:157‐163. [DOI] [PubMed] [Google Scholar]
- 38. Aaltonen S, Rottensteiner M, Kaprio J, Kujala UM. Motives for physical activity among active and inactive persons in their mid‐30s. Scand J Med Sci Sports. 2014;24:727‐735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kujala UM. Summary of the effects of exercise therapy in non‐communicable diseases: clinically relevant evidence from meta‐analyses of randomized controlled trials. MedRxiv. 2021. doi: 10.1101/2021.02.11.21251608 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supporting Information
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


