Abstract
Background
The DASH eating plan is an evidence-based treatment for hypertension; however, adherence to DASH is low. To improve adherence to the DASH among adults with hypertension, we designed Nourish, a two-arm, 12-month randomized controlled trial. The COVID-19 pandemic necessitated a change from in-person to remotely delivered visits, requiring substantial protocol modifications to measure blood pressure accurately and safely for secondary outcome data.
Purpose
The purpose of this paper is to describe the implementation of an at-home blood pressure measurement protocol for the Nourish trial.
Conclusion
Our investigator team and study staff developed and implemented a robust and feasible blood pressure measurement protocol to be executed within an at-home format.
Clinical Implications
The described blood pressure measurement protocol provides a framework for use in future clinical trials and clinical settings in which a remote visit is preferred or required.
Introduction
Over 45% of adults in the United States (U.S.) have hypertension (HTN).1 National treatment guidelines recommend lifestyle changes - including adopting a heart-healthy eating pattern - for adults with HTN to reduce the risk of adverse health outcomes.2 One efficacious lifestyle intervention for HTN is the Dietary Approaches to Stop Hypertension (DASH) eating plan.3,4 DASH recommends increasing the intake of fruits and vegetables, low-fat dairy, lean proteins, beans and nuts, and limiting red meat, sugar-sweetened beverages and other sweets. DASH is listed as a heart-healthy eating pattern for U.S. adults and is recommended for adults with HTN.2,5,6Although the effects of DASH on lowering blood pressure (BP) are well-documented, adherence to DASH at a population level remains poor.6,7
To address this gap, we designed Nourish, a two-arm, 12-month randomized controlled trial. Nourish tests the efficacy of using a commercially-available smartphone app, combined with evidence-based behavior change principles, to improve adherence to DASH (primary outcome) and to lower BP (secondary outcome) among adults with HTN.
The original data collection plan for Nourish included in-person visits throughout the 12-month study period. However, the onset of the COVID-19 pandemic occurred six months prior to the planned launch of trial recruitment and enrollment activities. Consequently, all in-person research activities were suspended at our institution, necessitating a change to a remotely-delivered protocol. Fortunately, the trial’s primary outcome, six-month change in DASH adherence, did not pose a challenge to collect remotely via the online Automated Self-Administered 24-hour Dietary Assessment Tool (ASA24®).8 However, the secondary outcome, six-month change in BP, required substantial protocol changes to yield robust and feasible data collection.
In response, we developed a detailed protocol to remotely collect BP measurements. The protocol was developed in consultation with the trial’s investigators, including experts in HTN treatment, digital health, and research design. The purpose of this paper is to describe the implementation of an at-home BP measurement protocol within the Nourish trial.
Methods
Design and Population
The trial’s design, intervention components and data collection protocol have been previously described.9 Briefly, we recruited and enrolled a total of 301 adults (≥18 years of age) in the U.S. with HTN, operationally defined by a systolic BP of 120–159 mmHg, diastolic BP of 80–99 mmHg,2 and/or currently taking BP-lowering medication. Other major inclusion criteria were: English fluency; smartphone ownership with data plan and active email address; and a BMI ≥ 18.5kg/m2. Participants were recruited primarily through online methods, including patient portal messaging, ResearchMatch, and social media. Following enrollment, participants were randomized to the digital health intervention or attention control arm. The primary outcome of the trial is six-month change in adherence to DASH, as measured by 24-hour ASA24® dietary recalls. The secondary outcome is six-month change in average BP, as measured during online study visits, described below. Initial approvals from the Duke Health Institutional Review Board were obtained in 2019.
Intervention
The Nourish intervention is delivered via a smartphone app and includes five evidence-based behavior change techniques:10 education and skills training;11 goal setting;12,13 self-monitoring;14 personalized feedback;13 and responsive health coaching.15,16 The details of each intervention component have been reported previously.9 Both treatment arms receive education and skills training regarding the study app and the DASH eating pattern and are encouraged to participate in dietary self-monitoring. However, only intervention arm participants receive goal setting, personalized text message feedback on their progress, and responsive health coaching.
Data Collection
Data collection visits for Nourish include survey measures and BP measurements; they occur at randomization (baseline) and two-, four-, six- and 12-months post-randomization. The baseline and follow-up visits last, on average, for 45 and 30 minutes, respectively. All Nourish visits occur via Zoom® videoconferencing. To ensure visit privacy and confidentiality, a Zoom® waiting room is enabled for all visits. Data are collected using REDCap®, a secure, online HIPAA-compliant data collection tool, and the Nourish app.
Remote BP Monitoring Equipment and Supplies
To accommodate remote data collection, we identified at-home digital BP monitors participants could operate during videoconference research visits. After investigating the available options for a BP monitor that was clinically-validated, affordable, and easy to use, we purchased the Omron® BP7100 digital upper arm monitor for all participants.
Participants were shipped a box of study supplies in advance of their Zoom® enrollment (randomization) visit. Participants received the Omron® BP monitor; 4 AA batteries for the monitor; and a flexible measuring tape to measure arm circumference.
Cost of Implementation
The cost of purchasing and shipping the necessary materials for the BP collection protocol was approximately $60 per participant. This includes $45 per participant for all materials ($37 of which was the cost of the BP monitor), plus an average shipping cost of approximately $15 per participant.
BP Measurement Procedures
Pre-procedure Preparation and Visit Scheduling
Prior to the randomization visit, participants are shown an information video made by the American Heart Association https://www.youtube.com/watch?v=rAwliNWe1bI&feature=youtu.be about how to take at-home BP measurements. The video link is also available within the Nourish smartphone app. Additionally, participants receive a printed copy of an American Heart Association infographic regarding proper at-home BP monitoring.17
In preparation for scheduling the randomization visit, participants are asked about their typical use of BP medications. Research staff aim to avoid scheduling the randomization and follow-up visits less than one hour after the time BP medications are typically taken by the participant. Prior to randomization visits, participants are emailed brief instructions for BP measurement procedures.
BP Measurement Technique
Before starting the BP measurement procedures, participants confirm BP can be assessed using their left arm (the preferred arm). If the participant cannot use their left arm due to their medical history – such as a left-side mastectomy or lymphadenectomy - they are instructed to use their right arm. For internal consistency of the BP measurements, the same arm and cuff placement (upper-arm or forearm) procedures established at the randomization visit are utilized at all follow-up visits, if possible.
Next, the appropriate cuff placement is determined. Participants wearing clothing covering their upper arm are instructed to roll up the sleeve. Next, participants are asked to stand; find the halfway point of the upper arm between the elbow and shoulder; and wrap the measuring tape around the arm at that location. Participants are then instructed to measure the circumference of that upper-arm mid-point, rounded to the nearest inch. If the measurement of the arm is ≤17” in circumference, an upper-arm BP measurement is used; if the left arm measurement is >17” a forearm measurement is used. A forearm measurement protocol, described below, was developed to ensure this intervention is accessible to all body shapes and sizes. Forearm measurements have been shown to be a valid way of measuring BP when the upper-arm circumference is greater than a large or extra-large sized cuff can accommodate.18–20 The 17” threshold was required due to the size limitations of the blood-pressure cuff included with the Omron(r) BP7100; it is the largest circumference offered for their validated at-home digital monitors.
The first BP measurement is taken during the randomization visit to establish trial eligibility and, for eligible participants, used as the baseline measure for the secondary outcome of six-month change in BP.
Staff-guided At-Home BP Measurement
Participants are requested to join videoconference visits with their cameras on so research staff can verify that BP procedures are correctly followed. Before proceeding with BP measurements, research staff confirm that participants have not consumed caffeine, smoked or exercised within the last 30 minutes, nor taken any BP-lowering medications within the previous 60 minutes. If any of these conditions apply, the visit is rescheduled for the earliest possible time after the required wait times have expired.
After these confirmation steps, research staff direct participants to be properly seated for BP measurement, with legs uncrossed, feet resting flat on the floor, and their back supported by a chair. Participants taking an upper-arm measurement are told to place the cuff at heart level and rest their arm on a table in front of them or on the arm of their chair, with the palm facing up. They are instructed to put the cuff on with the tubing facing the fingers of the hand, down the middle of the arm, along the brachial artery. Participants are told the pressure of the cuff should feel uniform across their arm. To test this, they are instructed to place one or two fingers under the cuff. All of the above procedures are followed for forearm measurements, except participants are directed to place the cuff at the largest part of their forearm, and to rest their arm with their hand raised toward their heart, with the tubing flipped and facing their shoulder, along the brachial artery.
Once these steps are complete, participants are given instructions to relax for five-minutes, while sitting quietly and refraining from talking, moving around, or reading. Participants are given the choice to have relaxing music played for them via the research staff’s computer or to sit in silence. Research staff turn off their camera and mute themselves during the relaxation period.
After five minutes, participants are asked to press start to take the first BP reading. They are instructed to take the second measurement after a one-minute rest period, and the third reading after the final one-minute rest period, for a total of three readings within the seven-minute period. Research staff keep track of the time and instruct participants when it is time to take a measurement. If participants experience any problems with using the monitor, such as receiving error messages, research staff utilize the Omron® product manual to assist them in real-time.
After each reading, once the monitor releases the pressure, participants are asked to hold up the BP monitor to the camera for the staff member to see the reading. The three systolic BP and diastolic BP measurements are recorded by the staff member within the participant record in REDCap. An average of the three readings is automatically calculated and displayed for trial eligibility and appropriate follow-up for elevated BP. An average of the second and third reading will be used for analyses (baseline and six-month BP data). The steps to these procedures are illustrated in Figure 1.
Figure 1.
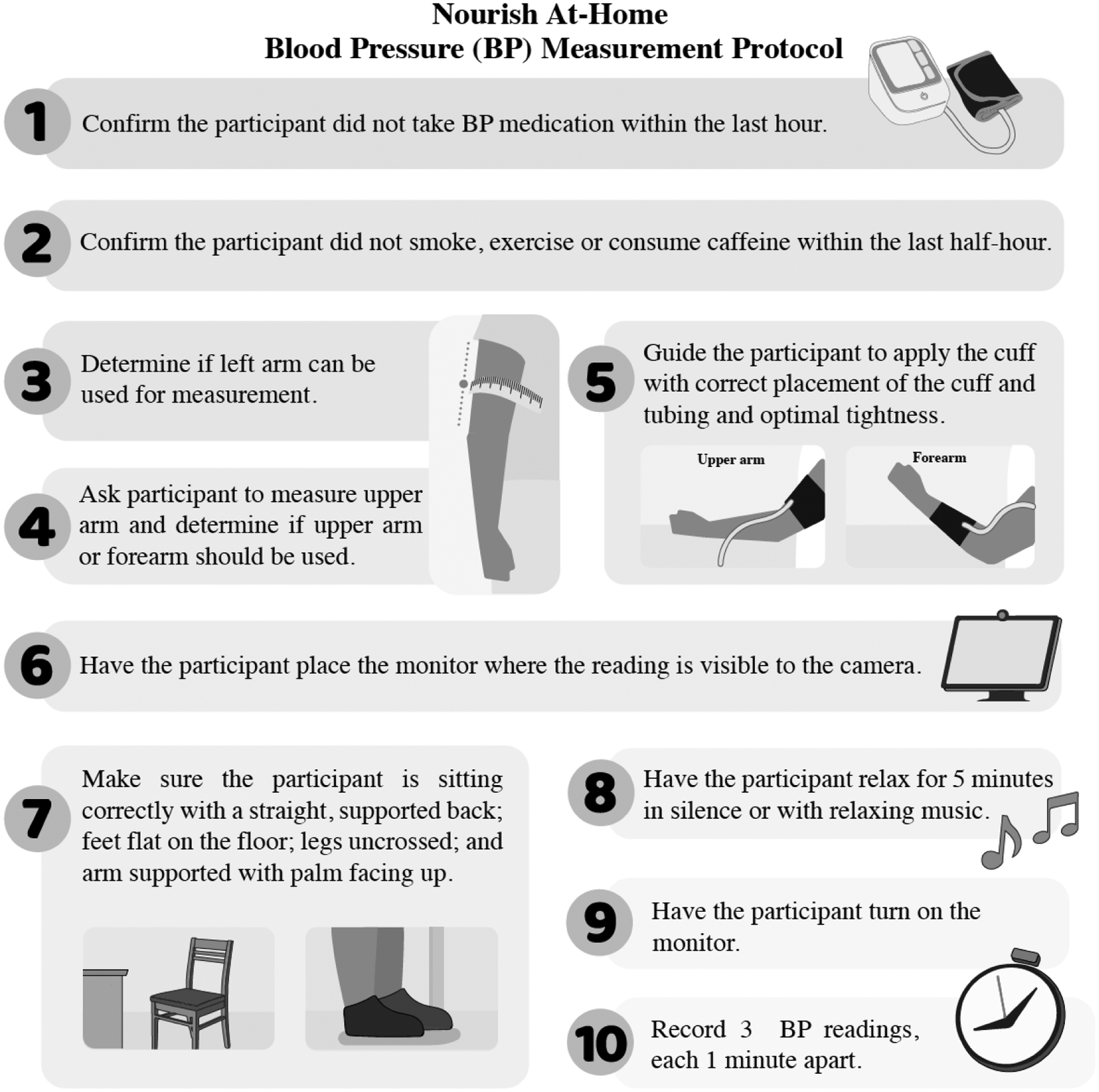
Nourish At-Home Blood Pressure (BP) Measurement Protocol
If a participant’s average SBP is less than 90 mm hg or above 160 mmHg or if their average DBP is above 100 mmHg (described in Table 1), a second set of three BP readings is taken after a minimum of 30 minutes. Participants are encouraged to rest during this time, which may improve measurement stability in participants for whom the standard 5- minute rest period was insufficient, such as participants with white coat syndrome.21,22 A second set of measurements also allows research staff to confirm there were no procedural errors that may have biased the first set of measurements.
Table 1.
Safety and eligibility protocol responses to levels of average blood pressure after 1 or 2 sets of measurements.
| Average of 1st set of 3 measurements | Average of 2nd set of 3 measurements | Participant reports symptoms | Advise participant to seek medical attention… | Baseline eligibility status | AE |
|---|---|---|---|---|---|
| SBP < 90 and DBP < 80 | SBP < 90 and DBP < 80 | Yes | Immediately | Eligibleb | * |
| No | Within 1 week | Eligibleb | * | ||
| 90 ≤ SBP < 120 and DBP < 80 | — | — | Eligibleb | ||
| 120 ≤ SBP < 140 or 80 ≤ DBP < 90 | — | — | Eligible | ||
| 140 ≤ SBP < 160 or 90 ≤ DBP < 100 | Yes | Within 3 months | Eligible | ||
| No | Within 3 months | Eligible | |||
| 160 ≤ SBP < 180 or 100 ≤ DBP < 110 | Yes | Immediatelya | Ineligible | * | |
| No | Within 1 montha | Ineligible | |||
| 180 ≤ SBP or 110 ≤ DBP | Yes | Immediatelya | Ineligible | * | |
| No | Within 1 weeka | Ineligible | * | ||
| 90 ≤ SBP < 120 and DBP < 80 | — | — | — | Eligibleb | |
| 120 ≤ SBP < 140 or 80 ≤ DBP < 90 | — | — | — | Eligible | |
| 140 ≤ SBP < 160 or 90 ≤ DBP < 100 | — | — | — | Eligible | |
| 160 ≤ SBP < 180 or 100 ≤ DBP < 110 | SBP < 90 and DBP < 80 | Yes | Immediatelya | Eligibleb | * |
| No | Within 1 weeka | Eligibleb | * | ||
| 90 ≤ SBP < 120 and DBP < 80 | — | — | Eligibleb | ||
| 120 ≤ SBP < 140 or 80 ≤ DBP < 90 | — | — | Eligible | ||
| 140 ≤ SBP < 160 or 90 ≤ DBP < 100 | Yes | Within 3 months | Eligible | ||
| No | Within 3 months | Eligible | |||
| 160 ≤ SBP < 180 or 100 ≤ DBP < 110 | Yes | Immediately | Ineligible | * | |
| No | Within 1 month | Ineligible | |||
| 180 ≤ SBP or 110 ≤ DBP | Yes | Immediately | Ineligible | * | |
| No | Within 1 week | Ineligible | * | ||
| 180 ≤ SBP or 110 ≤ DBP | SBP < 90 and DBP < 80 | Yes | Immediatelya | Eligibleb | * |
| No | Within 1 weeka | Eligibleb | * | ||
| 90 ≤ SBP < 120 and DBP < 80 | — | — | Eligibleb | ||
| 120 ≤ SBP < 140 or 80 ≤ DBP < 90 | — | — | Eligible | ||
| 140 ≤ SBP < 160 or 90 ≤ DBP < 100 | Yes | Within 3 months | Eligible | ||
| No | Within 3 months | Eligible | |||
| 160 ≤ SBP < 180 or 100 ≤ DBP < 110 | Yes | Immediately | Ineligible | * | |
| No | Within 1 month | Ineligible | |||
| 180 ≤ SBP or 110 ≤ DBP | Yes | Immediately | Ineligible | * | |
| No | Within 1 week | Ineligible | * |
Abbreviations: SBP, systolic blood pressure; DBP, diastolic blood pressure; AE, adverse event.
As dramatic swings in average blood pressure are unlikely in a short time, extra care should first be taken to troubleshoot the equipment and to confirm there is no user error, including taking an additional set of measurements.
Participants with SBP < 120 and DBP < 80 are eligible if they are taking medication to control their blood pressure or self-report having a diagnosis of hypertension.
Indicates an adverse event would be documented. Follow-up with participant would determine if the event should be escalated to a serious adverse event.
Safety Protocol for Elevated BP
Research staff follow detailed safety procedures to address low or elevated BP measurements obtained during study visits, as summarized in Table 1. The REDCap® project used for data documentation is programmed to flag elevated or low BPs and instruct research staff members on the appropriate next steps, including a list of symptoms to identify a potential hypertensive or hypotensive emergency; recommendations for participants to seek follow-up clinical care, based on BP levels and symptomatology; and the handling of adverse event review and reporting as necessary. The automated programming of these procedures helps maintain intervention fidelity, participant safety, and the consistency of the responses from research staff.
Discussion
The onset of the COVID-19 pandemic necessitated innovative modifications to allow the continuation of clinical trials, including our HTN management trial. As done in our previous studies, we intended to measure BP during in-person study visits.23,24 This was not feasible in Nourish, as research activities switched to remote procedures in mid-2020. We thus developed and implemented an at-home BP measurement protocol. To-date, we have conducted 996 remote research visits that included BP measurements. Although we developed this protocol to adapt to the challenges of conducting clinical research during COVID-19, opportunities to use a remote protocol beyond the pandemic exist for research and clinical practice.
One benefit to adjusting our protocol to be fully remote was that it opened recruitment to individuals living throughout the U.S., thus increasing the representativeness of our participant sample. Our randomized participants reside in 40 U.S. states. Further, it increased the flexibility for research staff to meet with participants during evening and weekend hours and decreased barriers to participating in the trial, such as traveling to the research site several times. As such, it allowed us to reach participants with competing demands, including inflexible work schedules, caregiving responsibilities, and limited access to research sites due to proximity or lack of access to reliable transportation. Although we are not able to capture the true effect of these modifications on our enrollment, we believe they increased our ability to recruit and retain participants who otherwise would not be able to participate in a study like Nourish.
With 85% of the U.S population owning a smartphone and 77% having home broadband,25 it is becoming increasingly feasible for research teams to consider remote recruitment and data collection procedures. Our team chose to make all intervention components accessible via smartphone to facilitate research with traditionally hard-to-reach populations, including individuals with lower income and lower education whom are more likely to be smartphone-dependent for internet access.23 These implications extend to clinical settings, where remote protocols offer the opportunity for telehealth visits or at-home monitoring to replace or complement in-person clinic visits.
Our team also designed Nourish to be weight-inclusive and accessible for participants in a variety of body sizes.26 Given this priority, we did not exclude individuals in larger body sizes. Our team, however, had difficulty developing a BP measurement protocol that aligned with this approach. Specifically, we faced two challenges: (1) finding a monitor with the capability to measure BP from participants with a variety of arm sizes; and (2) identifying best practices for BP measurement when the arm circumference of the participant exceeded the recommended size for the cuff that came with the monitor. It was critical for our study to meet these two criteria with a single monitor, as our final eligibility criteria was based on BP readings and we needed to send the monitor and cuff prior to knowing a person’s arm circumference. Unfortunately, the majority of validated at-home BP monitors were not weight-inclusive to larger arm circumferences and as such, would yield inaccurate and potentially uncomfortable BP measurements for participants with larger arms. The available models with larger-sized cuffs often utilized wrist monitors or lacked clinical validation information. Although wrist measurements are preferred over forearm measurements when upper-arm measurements are not possible, we were unable to use wrist monitors due to not knowing participant arm circumference prior to the enrollment visit.27 Given these limitations, we chose a monitor with a flexible cuff size and developed an adaptive protocol that included forearm measurements, when necessary. In future trials, we would consider requiring an arm circumference measurement prior to enrollment.
There are also challenges to conducting remote visits that our team had not faced during in-person data collection visits. Specifically, the biggest challenge is related to the use of new BP devices and videoconference technology. The BP devices described within this protocol have a series of possible errors that occur during BP measurements. Although an error code is often displayed when a reading is incomplete, troubleshooting over videoconferencing, rather than in-person, can be difficult. The most common error codes are often from user error. For example, the tube being detached from the base of the monitor; the participant moving their arm too much, clothing being in the way of the cuff; and/or batteries being too low. We have mitigated the latter error by sending a pack of batteries prior to enrollment and before the final 12-month visit. Many of our participants also need assistance using Zoom videoconferencing or experience unstable internet connection in remote locations. This leads to retries of BP measurements, as well as lengthy research visits and the necessity of being flexible and adaptive to unique challenges for each participant.
There are limitations to this protocol that should be considered. First, although participants are asked to be on-camera for visits and each step of the protocol is verbally confirmed, research staff are often limited to observing the participant’s upper body. As a result, research staff are unable to observe if the participant’s feet remain on the floor with legs remain uncrossed for the duration of the protocol. To mitigate protocol deviations, research staff remind the participants of the proper techniques before each BP measurement session begins, as described above. Second, we were unable to complete a verification step of our procedures against clinic-based standards implemented in previous trials prior to implementation. Understanding this was a limitation we would face due to the COVID-19 pandemic, we developed our protocol in accordance with the ACC and AHA guideline recommendations2 and included several steps to improve the reliability of our measurements. We also selected a device that has been previously validated and meets the criteria to be included on the US Blood Pressure Validated Device Listing.28 Third, because the visits were conducted via Zoom(r), we are unable to standardize or control the environment in which BP is taken at each visit. We do, however, instruct the participants to be in a location where they can rest their arm at heart level and recommend that participants find a quiet, private space to complete their visits. Last, these procedures were developed for the BP monitor and cuff used in Nourish and may require modifications in future studies if different equipment is utilized.
Conclusion
The COVID-19 pandemic necessitated innovative modifications for research and clinical workflows to continue in a safe and effective manner. Our investigator team and study staff responded by developing and implementing study procures that could be implemented in a remote and at-home format. The BP measurement protocol described in this paper provides a framework that can be used in future clinical trials or clinical settings in which a remote visit is preferred or required.
Acknowledgements:
This trial is funded by the National Heart, Lung and Blood Institute at the National Institutes of Health (R01HL146768). The funder had no role in study design, data collection, data analysis and interpretation of data, in the writing of the report, and in the decision to submit this article for publication.
Footnotes
Conflicts of interest: The authors have no conflicts of interests to declare.
Trial Number: NCT0387576
References
- 1.Ostchega Y, Fryar CD, Nwankwo T, Nguyen DT. Hypertension Prevalence Among Adults Aged 18 and Over: United States, 2017–2018. NCHS Data Brief. 2020;(364):1–8. [PubMed] [Google Scholar]
- 2.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task. Hypertens (Dallas, Tex 1979). 2018;71(6):1269–1324. doi: 10.1161/HYP.0000000000000066 [DOI] [PubMed] [Google Scholar]
- 3.Svetkey LP, Simons-Morton D, Vollmer WM, et al. Effects of dietary patterns on blood pressure: subgroup analysis of the Dietary Approaches to Stop Hypertension (DASH) randomized clinical trial. Arch Intern Med. 1999;159(3):285–293. doi: 10.1001/archinte.159.3.285 [DOI] [PubMed] [Google Scholar]
- 4.Appel LJ, Moore TJ, Obarzanek E, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med. 1997;336(16):1117–1124. doi: 10.1056/NEJM199704173361601 [DOI] [PubMed] [Google Scholar]
- 5.National Heart L and BI. DASH Eating Plan. https://www.nhlbi.nih.gov/health-topics/dash-eating-plan. Accessed October 19, 2021.
- 6.U.S. Department of Health and Human Services and U.S. Department of Agriculture. Dietary Guidelines for Americans: 2015–2020.; 2015. doi: 10.1097/nt.0b013e31826c50af [DOI]
- 7.Kwan MW-M, Wong MC-S, Wang HH-X, et al. Compliance with the Dietary Approaches to Stop Hypertension (DASH) diet: a systematic review. PLoS One. 2013;8(10):e78412. doi: 10.1371/journal.pone.0078412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Subar AF, Kirkpatrick SI, Mittl B, et al. The Automated Self-Administered 24-hour dietary recall (ASA24): a resource for researchers, clinicians, and educators from the National Cancer Institute. J Acad Nutr Diet. 2012;112(8):1134–1137. doi: 10.1016/j.jand.2012.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller HN, Berger MB, Askew S, et al. The Nourish Protocol: A digital health randomized controlled trial to promote the DASH eating pattern among adults with hypertension. Contemp Clin Trials. 2021;109:106539. doi: 10.1016/j.cct.2021.106539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Michie S, Wood CE, Johnston M, Abraham C, Francis JJ, Hardeman W. Behaviour change techniques: the development and evaluation of a taxonomic method for reporting and describing behaviour change interventions (a suite of five studies involving consensus methods, randomised controlled trials and analysis of qualitative d. Health Technol Assess. 2015;19(99):1–188. doi: 10.3310/hta19990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Appel LJ, Champagne CM, Harsha DW, et al. Effects of comprehensive lifestyle modification on blood pressure control: main results of the PREMIER clinical trial. JAMA. 2003;289:2083–2093. doi: 10.1001/jama.289.16.2083 [DOI] [PubMed] [Google Scholar]
- 12.Samdal GB, Eide GE, Barth T, Williams G, Meland E. Effective behaviour change techniques for physical activity and healthy eating in overweight and obese adults; systematic review and meta-regression analyses. Int J Behav Nutr Phys Act. 2017;14(1):42. doi: 10.1186/s12966-017-0494-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lara J, Evans EH, O’Brien N, et al. Association of behaviour change techniques with effectiveness of dietary interventions among adults of retirement age: a systematic review and meta-analysis of randomised controlled trials. BMC Med. 2014;12:177. doi: 10.1186/s12916-014-0177-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fakih El Khoury C, Karavetian M, Halfens RJG, Crutzen R, Khoja L, Schols JMGA. The Effects of Dietary Mobile Apps on Nutritional Outcomes in Adults with Chronic Diseases: A Systematic Review and Meta-Analysis. J Acad Nutr Diet. 2019;119(4):626–651. doi: 10.1016/j.jand.2018.11.010 [DOI] [PubMed] [Google Scholar]
- 15.Olsen JM, Nesbitt BJ. Health coaching to improve healthy lifestyle behaviors: an integrative review. Am J Health Promot. 2010;25(1):e1–e12. doi: 10.4278/ajhp.090313-LIT-101 [DOI] [PubMed] [Google Scholar]
- 16.Kivelä K, Elo S, Kyngäs H, Kääriäinen M. The effects of health coaching on adult patients with chronic diseases: a systematic review. Patient Educ Couns. 2014;97(2):147–157. doi: 10.1016/j.pec.2014.07.026 [DOI] [PubMed] [Google Scholar]
- 17.American Heart Association. Blood Pressure Measurement Instructions.; 2018. https://www.heart.org/-/media/files/health-topics/high-blood-pressure/how_to_measure_your_blood_pressure_letter_size.pdf?la=en.
- 18.Leblanc M-É, Croteau S, Ferland A, et al. Blood pressure assessment in severe obesity: validation of a forearm approach. Obesity (Silver Spring). 2013;21(12):E533–41. doi: 10.1002/oby.20458 [DOI] [PubMed] [Google Scholar]
- 19.Leblanc M-È, Cloutier L, Poirier P. Sensitivity, specificity, and predictive values of a forearm blood pressure measurement method in severe obesity. Blood Press Monit. 2015;20(2):79–82. doi: 10.1097/MBP.0000000000000097 [DOI] [PubMed] [Google Scholar]
- 20.Leblanc M-È, Auclair A, Leclerc J, et al. Blood Pressure Measurement in Severely Obese Patients: Validation of the Forearm Approach in Different Arm Positions. Am J Hypertens. 2019;32(2):175–185. doi: 10.1093/ajh/hpy152 [DOI] [PubMed] [Google Scholar]
- 21.Scherpbier-de Haan N, van der Wel M, Schoenmakers G, et al. Thirty-minute compared to standardised office blood pressure measurement in general practice. Br J Gen Pract J R Coll Gen Pract. 2011;61(590):e590–7. doi: 10.3399/bjgp11X593875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mahe G, Comets E, Nouni A, et al. A minimal resting time of 25 min is needed before measuring stabilized blood pressure in subjects addressed for vascular investigations. Sci Rep. 2017;7(1):12893. doi: 10.1038/s41598-017-12775-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steinberg DM, Kay MC, Svetkey LP, et al. Feasibility of a Digital Health Intervention to Improve Diet Quality Among Women With High Blood Pressure: Randomized Controlled Feasibility Trial. JMIR mHealth uHealth. 2020;8(12):e17536. doi: 10.2196/17536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Foley P, Steinberg D, Levine E, et al. Track: A randomized controlled trial of a digital health obesity treatment intervention for medically vulnerable primary care patients. Contemp Clin Trials. 2016;48:12–20. doi: 10.1016/j.cct.2016.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pew Research Center. Mobile Fact Sheet. https://www.pewresearch.org/internet/fact-sheet/mobile/. Published 2021. Accessed June 21, 2021.
- 26.Tylka TL, Annunziato RA, Burgard D, et al. The weight-inclusive versus weight-normative approach to health: evaluating the evidence for prioritizing well-being over weight loss. J Obes. 2014;2014:983495. doi: 10.1155/2014/983495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muntner P, Shimbo D, Carey RM, et al. Measurement of Blood Pressure in Humans: A Scientific Statement From the American Heart Association. Hypertens (Dallas, Tex 1979). 2019;73(5):e35–e66. doi: 10.1161/HYP.0000000000000087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.US Blood Pressure Validated Device Listing. https://www.validatebp.org. Accessed October 19, 2021.


