Abstract
Bacterial vaginosis (BV) is the most common genital infection in women and is associated with an increased risk of sexually transmitted infections and HIV. This study uses a syndemic approach to evaluate factors associated with BV. Non-pregnant, HIV-negative, sexually active cis-gender women aged 18–45 years living in Miami, Florida were recruited from Nov.2018- Jun.2021. Participants completed a sociodemographic and behavioral questionnaire along with gynecological examinations. BV was diagnosed by Amsel criteria and confirmed by a Nugent score ≥4. A syndemic score was calculated as the sum of factors associated with BV. The association between syndemic score and BV was assessed using logistic regression. Of 166 women included, 60.2% had BV. Race, ethnicity, education, vaginal sex, recent cannabis use, and reasons for intravaginal practices were included in the syndemic score. Higher odds of BV were found in women with a score of ≥3 compared to women with a score of 0/1. A higher syndemic score was associated with increased odds of having BV. Multilevel interventions to decrease BV are needed to decrease women’s risk of acquiring HIV.
Keywords: Bacterial Vaginosis, Syndemic, HIV, Sexually Transmitted Infections
Resumen
La vaginosis bacteriana (VB) es la infección genital más común en mujeres y está asociada con un mayor riesgo de enfermedades de transmisión sexual (ETS) y de VIH. Este estudio utilizó un enfoque sindémico para evaluar factores asociados con VB. Entre noviembre del 2018 y junio del 2021, se reclutaron mujeres cisgénero de entre 18–45 años, que no estuvieran embarazadas, que fueran VIH negativas y sexualmente activas, y que vivieran en Miami, Florida. Las participantes completaron un cuestionario sociodemográfico y de comportamiento junto con un examen ginecológico. Se diagnosticó VB empleando los criterios de Amsel y se confirmó empleando el criterio de Nugent con una puntuación ≥4. La puntuación sindémica fue calculada como la suma de factores asociados con VB utilizando. La asociación entre la puntuación sindémica con VB se evaluó mediate una regresión logística. De 166 mujeres incluidas, 60.2% fueron diagnosticadas con VB. Los factores incluidos en la puntuación sindémica fueron la raza, etnia, educación, sexo vaginal, consumo reciente de cannabis, y el uso de prácticas intravaginales. Se encontraron mayores probabilidades de VB en mujeres con una puntuación ≥3 en comparación con aquellas con una puntuación de 0/1. Una puntuación sindémica alta se asoció con una mayor probabilidad de tener VB. Son necesarias intervenciones multinivel para disminuir la VB y disminuir el riesgo de que las mujeres contraigan ETS y VIH.
Introduction
BV is the most common genital infection among women (1, 2). It is characterized by a vaginal microbiome that has deviated from the normal flora, lacking Lactobacillus and having an abundance of Gardnerella species (3). If symptomatic, BV may present with abnormal discharge, itchiness, and malodor (4, 5). Further gynecological and obstetric complications from BV include preterm births, pelvic inflammatory diseases, and importantly, increased susceptibility to acquiring sexually transmitted infections (STI) (6) and HIV (3). However, the factors implicated on the etiology of BV are still unknown.
BV has been also associated with intravaginal practices (IVP) and the use of products that alter the vaginal compartment (7, 8). IVP refer to a variety of behaviors to maintain a vagina that women perceive as hygienic, healthy, or pleasing to sexual partners (9). IVP include cleansing, douching, or the insertion of products intravaginally. These practices are especially widespread among women from Sub-Saharan African cultures and ethnic minorities in the United States (1, 10–17). In addition to an increased risk for BV, IVP can also increase the risk for STI and HIV due to the disruption of intravaginal mucosal integrity.
Furthermore, having new or multiple sexual partners, identifying as Black or African-American, and having low income, have also been strongly associated with BV among women in the US (18–21). Therefore, investigating additive relationships between risk behaviors, sociocultural factors, and biological factors allows for a syndemic approach to assess multilevel predictors of BV (22, 23). A “syndemic” is defined as multiple interacting conditions that synergistically fuel each other resulting in increased risk for disease (24). The syndemic model is an analytic approach to understand how a combination of risk factors interact to exacerbate the burden of negative health outcomes in the context of disease clusters (24, 25). Understanding risk factors for BV, could guide interventions to better protect women from BV and future co-occurring incidence of HIV.
Florida is the epicenter of the HIV epidemic, and women living in Miami are among those at highest risk for HIV in the US (26, 27). Currently, limited information is known about how the combination of demographic and behavioral factors contributes to BV among women in the US. Consequently, this study aims at exploring the factors associated with BV in a diverse population of women using a syndemic approach.
Methods
Between November 2018 and June 2021, participants were recruited from the community using flyers, word of mouth, and through the Center for AIDS Research (CFAR) and the Center for HIV Research and Mental Health (CHARM) located in Miami, Florida. Cis-gender women, aged 18–45 years, who were HIV-negative and sexually active within the previous three months were invited to participate. Women who were pregnant; immunosuppressed; with an intrauterine device; had a history of cervical surgical treatment; antibiotic use; or diagnosed with STI within two months prior to being screened were excluded from the study. Participants were recruited as part of a longitudinal study evaluating factors associated with recurrent BV utilizing a multidisciplinary approach. Results reported in this study are findings from participants who completed the baseline visit. The study was approved by the University of Miami Institutional Review Board (IRB# 20180758) and informed consent was obtained before completion of study assessments. Human subjects research was conducted conforming to the University of Miami’s ethical standards and the Helsinki Declaration of 1975, revised in 2013.
Measures
Participants were screened and asked to complete an in-person/electronic/telephone questionnaire prior to an in-person visit at the Infectious Diseases Research Unit at the University of Miami. The questionnaire collected information on sociodemographics, sexual behavior, substance use, and IVP. These included age; race (Black or African-American, White, and Other); ethnic origin (Hispanic, Non-Hispanic, and Other); education (less than high school - 0 to 11 grade, high school, or more than high school - college, some college, or graduate school); average monthly income (<$1,000, $1,000-$3,000, >$3,000); number of sexual partners in the past month; number of sexual encounters; frequency of condom use; type of sex (vaginal sex only or vaginal and oral sex); cigarette smoking; alcohol intake; drug use (marijuana/cannabis, cocaine); frequency of alcohol and drug use (for cannabis and cocaine use - in the past year, month, week, day or today; for alcohol use - less than a drink per month, less than 7 drinks a week, or daily); use of any IVP (e.g., intravaginal douches, soap, water, or other products) and reasons for IVP (hygiene “to clean myself”, partner preferences “to please my partner”, or health “to prevent infections such as STI”).
During the in-person visit, participants underwent HIV and pregnancy testing to confirm eligibility, and an ensuing gynecological examination was conducted to assess for BV. During the gynecological examination, a bivalve speculum previously moisturized with sterile normal saline was introduced in the vagina to retract the vaginal wall. Sterile swabs were used to assess Amsel criteria and Nugent scoring (28, 29). Identification of Amsel criteria required measuring the vaginal pH, performing a Whiff test, and placing a drop of vaginal fluid on a grease free glass slide for clue cells detection. For Nugent scoring, the vaginal discharge was also smeared on clean glass slides, air dried, and transported to a microbiology laboratory for Gram’s staining and review by trained laboratory technicians. An episode of BV was diagnosed if a participant met three out of four Amsel criteria (presence of discharge; pH >4.5; ≥20% of clue cells; positive whiff test) and confirmed by a Nugent score ≥4 (29, 30). Women diagnosed with symptomatic BV were provided with same-day standard of care treatment with metronidazole as per CDC guidelines (500 mg PO bid for 7 days) (31, 32).
Statistical Analysis
Descriptive analyses were conducted to examine the distribution of variables. Demographic and behavioral variables were operationalized as categorical factors; in multiple separate Chi-square and Fisher’s exact test analyses to test for significant differences, and the distribution of participants in each category was compared between those with and without BV. T-tests or Mann-Whitney tests were used for continuous comparison of groups.
Six factors were determined to be associated with BV via Fisher’s exact test and Chi-square analyses; and were evaluated for appropriateness to include in a syndemic conditions summary score: (1) Race, (2) Ethnicity, (3) Education, (4) Vaginal Sex, (5) Recent Cannabis Use, (6) Reasons for IVP. Levels associated with significant elevations in risk for BV were assigned a point in our final scoring. For example, one point each was assigned for identifying as Black, non-Hispanic, not completing high school, reporting vaginal sex in the previous month, reporting recent (less than one week) cannabis use, and use of IVP for hygiene purposes. The number of syndemic factors were calculated as a sum score (33–37). Cumulative syndemic scores were operationalized as ordinal variables. Due to small number of individuals reporting 0 or 6 syndemic factors, those with 0 or 1 syndemic factors and those with 5 or 6 syndemic factors were each collapsed into a single category, with final categories including 0/1, 2, 3, 4, or 5/6 syndemic factors.
A logistic regression model was conducted to assess for associations between syndemic scores and BV diagnosis. Syndemic levels were dummy coded and compared with the reference category of 0/1 syndemic factors. Odds ratios were estimated as effect sizes, and estimated effects are reported with 95% confidence intervals. A stacked barplot was generated in ggplot and presented the percent of positive BV for each syndemic factor score against the fitted probability positive for each syndemic factor from the logistic regression. Statistical significance was determined by α = 0.05 and analyses were conducted using R Version 4.0.4.
Results
Sociodemographic Characteristics, Risk factors, and Intravaginal Practices with Bacterial Vaginosis by Nugent Score
Table 1 compares sociodemographic characteristics, sexual behaviors, substance use, and IVP between women with and without BV diagnosis. A total of 166 women were enrolled in the study, of which more than half self-identified as non-Hispanic (72.9%) and Black or African American (61.4%). Many women reported a low household monthly income (<$1,000 was reported by 53.6%) and had low education (only 46.4% completed high school or higher) and engaged in IVP (68.7%).
Table 1.
Sociodemographic, Sexual Behaviors, Substance use, and Intravaginal Practices by Diagnosis of Bacterial Vaginosis
| No Bacterial Vaginosis | Bacterial Vaginosis | Test Statistic | P | |
|---|---|---|---|---|
|
| ||||
| Total N=166 a | N=66 (39.8) | N=100 (60.2) | ||
|
| ||||
| Sociodemographic | ||||
|
| ||||
| Age, mean ± SD | 32.7 ± 8.1 | 32.4 ± 7.6 | 0.25 | 0.80 |
| Ethnicity b | 4.00 | 0.04 | ||
| Hispanic | 24 (53.3) | 21 (46.7) | ||
| Non-Hispanic | 42 (34.7) | 79 (65.3) | ||
| Race b | 21.69 | <0.01 | ||
| Black/African American | 28 (27.5) | 74 (72.5) | ||
| White | 34 (66.7) | 17 (33.3) | ||
| Other | 4 (30.8) | 9 (69.2) | ||
| Education b | 2.63 | 0.01 | ||
| Less High School | 5 (17.2) | 24 (82.8) | ||
| High School | 22 (36.7) | 38 (63.3) | ||
| Household Monthly Income | 5.94 | 0.05 | ||
| <$1000 | 28 (31.5) | 61 (68.5) | ||
| 1000 to $3000 | 24 (45.3) | 29 (54.7) | ||
| >$3000 | 13 (56.5) | 10 (43.5) | ||
|
| ||||
| Sexual Behaviors c | ||||
|
| ||||
| Number of male partners, mean ± SD | 1.35 ± 1.70 | 1.34 ± 1.3 | 0.03 | 0.98 |
| Number of new sexual partners, mean ± SD | 0.35 ± 1.09 | 0.28 ± 0.9 | 0.39 | 0.70 |
| Use of male condoms c | 1.40 | 0.72 | ||
| Always | 15 (46.9) | 17 (53.1) | ||
| Sometimes | 7 (33.3) | 14 (66.7) | ||
| Never | 30 (38.0) | 49 (62.0) | ||
| Did not have vaginal sex | 3 (50.0) | 3 (50.0) | ||
| History of Sex Work d | NA | 1.00 | ||
| Yes | 4 (36.4) | 7 (63.6) | ||
| No | 62 (40.0) | 93 (60.0) | ||
| Type of Sex b c | 6.49 | 0.04 | ||
| Vaginal and Oral | 41 (48.2) | 44 (51.8) | ||
| Vaginal Only | 14 (26.4) | 39 (73.6) | ||
| No Sex | 11 (39.3) | 17 (60.7) | ||
|
| ||||
| Substance use | ||||
|
| ||||
| Cannabis – Last Use b | 13.56 | 0.01 | ||
| In the past year or later | 14 (66.7) | 7 (33.3) | ||
| In the past month | 10 (50.0) | 10 (50.0) | ||
| In the past week | 1 (11.1) | 8 (88.9) | ||
| In the past day | 4 (28.6) | 10 (71.4) | ||
| Today | 2 (16.7) | 10 (83.3) | ||
| Cocaine – Last Use d | NA | 0.84 | ||
| In the past year or later | 6 (46.2) | 7 (53.8) | ||
| In the past month | 1 (50.0) | 1 (50.0) | ||
| In the past week | 0 (0.0) | 2 (100.0) | ||
| Today | 0 (0.0) | 1 (100.0) | ||
| Alcohol Use – Frequency d | NA | 0.25 | ||
| Less than one drink a month | 7 (53.8) | 6 (46.2) | ||
| Less than 7 drinks a week | 31 (44.9) | 38 (55.1) | ||
| Daily | 2 (20.0) | 8 (80.0) | ||
|
| ||||
| Intravaginal Practices | ||||
|
| ||||
| History of IVP c | 46 (40.4) | 68 (59.6) | <0.01 | 0.87 |
| Reasons for IVP b d | NA | <0.01 | ||
| To clean myself | 32 (34.8) | 60 (65.2) | ||
| To please my sex partner | 13 (76.5) | 4 (23.5) | ||
| To prevent or cure infections | 1 (20.0) | 4 (80.0) | ||
Note: T-tests or Mann-Whitney tests were used for median/mean comparison of groups unless otherwise specified.
N (%) unless otherwise specified
Variables included in the Syndemic Score
Timeframe refers to the previous month
Fisher’s exact test
Overall, 60.2% of women were found to have BV. BV was more common among non-Hispanic women than Hispanic women (Table 1). It was also commonly found in Black women and women reporting race as other compared to White women. Women who did not complete high school (38) had also higher proportions of BV compared to women who completed high school or other higher education. Participants who reported vaginal sex in the previous month, and who reported recent cannabis use were more likely to be diagnosed with BV (Table 1). Lastly, there was a higher proportion of BV in women who engaged in hygiene reasons for IVP compared to pleasing their sexual partner or for health reasons (preventing or cure infections).
Syndemic Factors and Associations with Bacterial Vaginosis by Nugent Score
Race, ethnicity, education, vaginal sex, recent cannabis use, and reasons for IVP were included in the syndemic score. The total syndemic score had a range of 0–6. Syndemic scores by BV outcome are reported in Table 2 and Figure 1. There were a greater proportion of women with more syndemic factors in the BV group, compared to women without BV.
Table 2.
Syndemic Scores by Diagnosis of Bacterial Vaginosis
| Score | No Bacterial Vaginosis N (%) | Bacterial Vaginosis N (%) |
|---|---|---|
| 0 | 4 (80) | 1 (20) |
| 1 | 8 (61) | 5 (38) |
| 2 | 18 (51) | 17 (49) |
| 3 | 12 (35) | 22 (65) |
| 4 | 3 (20) | 12 (80) |
| 5 | 1 (9) | 10 (91) |
| 6 | 0 (0) | 1 (100) |
Note. A total of 166 were enrolled in the study. Syndemic factors included were race, ethnicity, education, vaginal sex, recent cannabis use, and reasons for IVP.
Figure 1. Probability of a Positive Diagnosis of Bacterial Vaginosis by Syndemic Score.
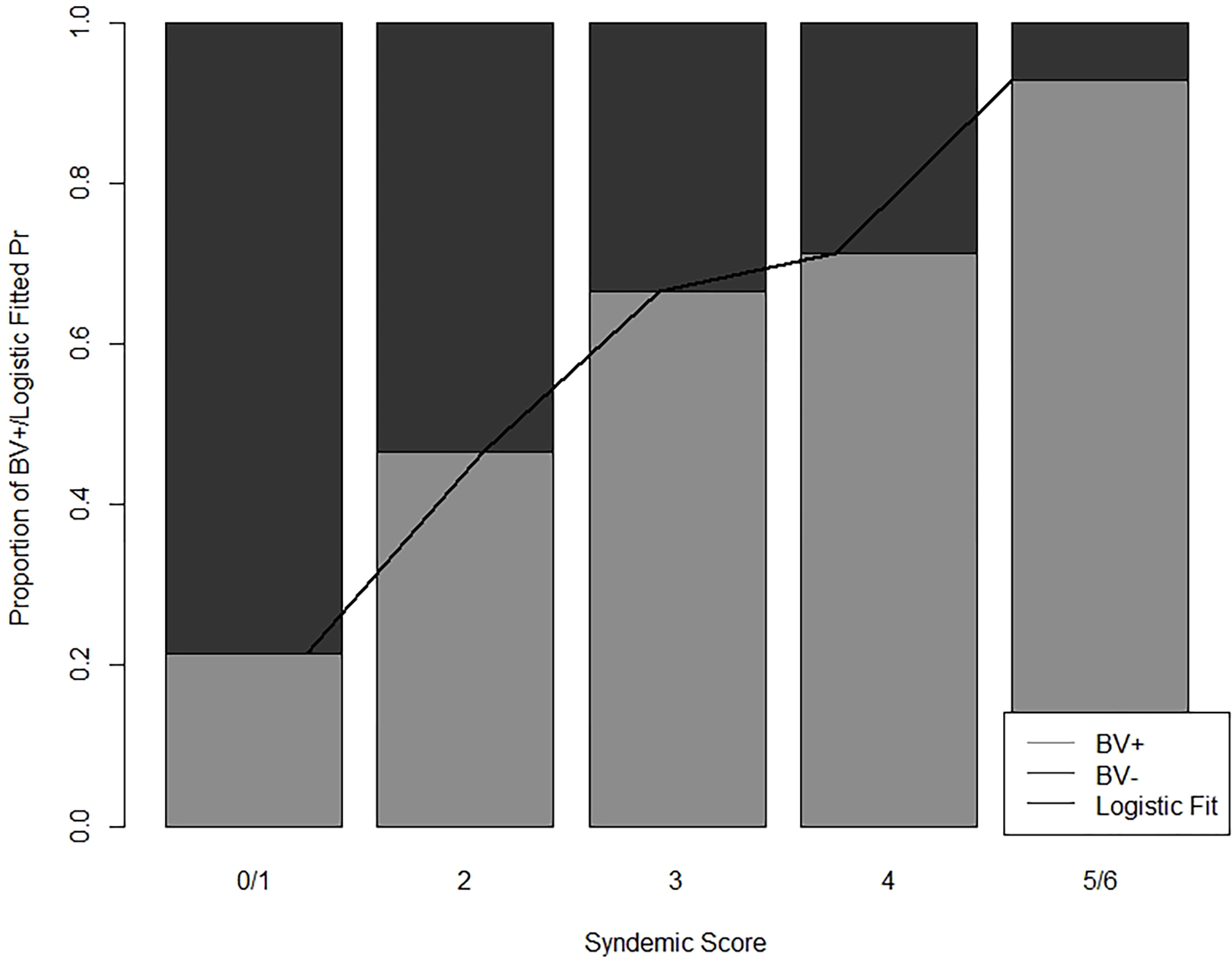
A Stacked Barplot illustrating the percent BV positive for each syndemic level and the fitted probability positive for each syndemic score from the logistic regression. Syndemic factors included: Race, Ethnicity, Education, Vaginal Sex, Recent Cannabis Use, Reasons for Intravaginal Practices. A higher syndemic score was associated with increased odds of having BV among women of reproductive age
The odds of BV in women with a score of 3 (Odds Ratio [OR] 3.67; 95% Confidence Interval [CI)] 1.13–12.97; p=0.04) was higher than the odds of BV in women with a syndemic score of 0/1 group. Compared to women with 0/1 syndemic conditions, women with a score of 4 (OR 8.00; 95% CI 1.77–46.40; p=0.01), and with a score of 5/6 (OR 21.99 95% CI 3.18–453.71; p=0.01) had higher odds of BV. No difference in the odds of being diagnosed with BV was found between women with 0/1 syndemic factors and 2 syndemic factors (OR 1.89 95% CI 0.59–6.49; p=0.29). Generally, in predicting BV using syndemic scores as seen in Table 3, we found increasingly higher odds of BV compared to reference group.
Table 3.
Relationship of Syndemic Scores with Diagnosis of Bacterial Vaginosis
| Estimate | Standard Error | Z | p | Odds Ratio [95% Confidence Interval] | |
|---|---|---|---|---|---|
| Intercept a | −0.69 | 0.50 | −1.39 | 0.17 | 0.50 [0.17–1.29] |
| 2 Syndemic Factors | 0.64 | 0.60 | 1.05 | 0.29 | 1.89 [0.59–6.49] |
| 3 Syndemic Factors | 1.30 | 0.62 | 2.11 | 0.04 | 3.67 [1.13–12.97] |
| 4 Syndemic Factors | 2.08 | 0.82 | 2.55 | 0.01 | 8.00 [1.77–46.40] |
| 5 to 6 Syndemic Factors | 3.09 | 1.16 | 2.67 | 0.01 | 21.99 [3.18–453.71] |
Reference group is 0–1 Syndemic Factors
Syndemic factors included were race, ethnicity, education, vaginal sex, recent cannabis use, and reasons for IVP.
Discussion
This study used a syndemic approach to identify demographic and behavioral factors that correlate with BV among racially and ethnically diverse women of reproductive age living in Miami, Florida, a city with high prevalence of women with HIV. Race, ethnicity, education, vaginal sex, cannabis use, and reasons for IVP were independently associated with BV. However, when analyzed together, women with three or more syndemic factors were at increased odds of having BV compared to women with 0/1. Our findings are in alignment with previous research supporting the use of a syndemic approach for understanding involvement of risky behaviors and poor health outcomes (39). This study is the first to incorporate demographic and behavioral factors in a syndemic score to predict BV among reproductive age women.
Previous studies focused on understanding the etiology of BV have also found multiple factors associated with BV, which is helpful in identifying potential risk factors in isolation. Previous research reveal that Black women have higher rates of BV than other racial groups. BV is more common in Black women of African ancestry, and biological differences have been observed in their vaginal microbial profiles when compared to women of European ancestry (40, 41). In addition, several biological and behavioral factors are correlated with differences in the vaginal microbiome and the relative abundance of BV-associated species (3, 5, 41). Examples include body mass index, diet, smoking, alcohol use, number of sexual partners, and socioeconomic status as potentially associated with BV. However, examining several syndemic factors provides a better understanding of how the combination of multiple interrelated risk factors increase women’s vulnerability to BV.
The relationship between sociodemographic and behavioral factors with BV suggests interventions may be needed to reduce BV risk, especially those with high syndemic scores (e.g., women identifying as non-Hispanic Black with less education). African American women have been found to be more likely than Latina or Caucasian women to learn about IVP for cleaning purposes from their mothers (1), which suggests education, cultural norms, and motivations for IVP may intersect with race and ethnicity. As such, any behavioral interventions should be culturally sensible and consider sociocultural factors (e.g., educational attainment, cultural/family norms) that could contribute to BV risk or barriers to change in health behaviors. For example, implementing patient education on reducing cleansing behavior should use plain language, while aware of the patient’s health literacy, and use the patient’s primary language. Furthermore, it should also consider cultural beliefs to try to improve adherence. Discussion of the gynecological and obstetric implications, and HIV risk in the context of BV could be conducted during routine clinical visits. Of note, most women knowledge about vaginal cleansing comes from family members or friends (42, 43), which suggests that conducting outreach to mothers of young girls may be one avenue for the delivery of interventions. However, due to the link between low educational attainment and BV, delivering interventions in schools may not reach those most at risk
On the other hand, while symptomatic BV would compel gynecological evaluation, more than 50% cases of BV are asymptomatic, which still poses a risk for women’s health (44). Our findings support further research to develop a screening tool that incorporates demographic and behavioral practices such as IVP and substance use to identify those at higher risk for BV (45).
Interestingly, our results show that engaging in IVP for cleansing purposes was associated with BV, but the overall use of IVP was not. Potential reasons could be related to the frequency of IVP use. For instance, women who engage in IVP for cleansing purposes may be engaging in IVP more often than women who engage in IVP for different purposes (46). Furthermore, the use of specific products (e.g., soap) and techniques used for cleaning purposes could be more abrasive than products used by women engaging in IVP for other reasons (8, 43). Finally, there may be reverse directionality; women who have symptoms of BV may engage in IVP to manage symptoms associated with cleanliness, such as odor and discharge. Longitudinal research is needed to examine whether IVP occurs prior to BV or if BV symptoms prompt IVP.
On the other hand, not finding a link between IVP and BV is in accordance with recent literature. Despite several studies finding an association between IVPs and negative vaginal health outcomes, conclusive evidence is still conflicting, and results were not replicated in other studies. A recent systematic review showed that while 6 studies found an association between vaginal douching and BV, STIs, and HIV, results were mixed or found no association in several others (47). Relevant potentials reasons listed as responsible for introducing bias were 1) study design (e.g., cross sectional and retrospective instead of prospective); 2) sampling conditions (e.g., populations that are known to have an increased risk for negative vaginal outcomes; and 3) not controlling for relevant confounders (definition, frequency, intensity, and reasons for IVP) (8, 47).
There are several limitations to the study that warrant mention. Cross-sectional data was presented from a longitudinal study evaluating factors associated with BV recurrence. Thus, the sample size for this analysis was not calculated a priori. Due to the small sample size, we were not able to evaluate interactions between syndemic factors, and collinearity might also contribute to the wide confidence intervals for the odds ratio. Data collected relied on self-report regarding behavior, and responses may have been affected by recall bias and social desirability. There may be a bi-directional relationship wherein women with BV may have been experiencing more vaginal discharge and malodor, leading to higher levels of IVP. In addition, there are multiple sociocultural mechanisms though which demographics such as race may be associated with BV (e.g., chronic stress due to racism); although the current study did not collect such information. Due to the high prevalence of racial and ethnic minorities and results may be not generalizable to other populations with different demographic characteristics.
Conclusions
Our study is the first to use a syndemic approach to better understand how the interaction of demographic and behavioral factors contribute to the development of BV among reproductive-aged women (43, 48). Results support the development of multilevel interventions to reduce the incidence of BV as a strategy to reduce HIV transmission (42, 48, 49). Such interventions would be particularly relevant for women at greater risk for HIV infection.
Acknowledgements
Funding Statement
This work was supported by National Institutes of Health (50) grants to the University of Miami Center for AIDS Research grant [P30A1073961 to M.L.A.] and the Center for HIV and Research in Mental Health [P30MH116867 to D.L.J.]. VJR’s work on this study was partially supported by a Ford Foundation Fellowship, administered by the National Academies of Science, a PEO Scholar Award from the PEO Sisterhood, and a grant from the National Institute of Mental Health of the NIH [R36MH127838].
Funding:
Research reported in this publication was supported by R01AI138718 to MLA, the University of Miami Center for AIDS Research, grant No. P30A1073961, and grant No. P30MH116867 through the Center for HIV and Research in Mental Health from the National Institutes of Health (NIH). VJR’s work on this study was partially supported by a Ford Foundation Fellowship, administered by the National Academies of Science, a PEO Scholar Award from the PEO Sisterhood, and a grant from the National Institute of Mental Health of the NIH [R36MH127838]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or these Institutions.
Footnotes
Conflicts of Interest
All authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest beyond research funding related to the work presented in this manuscript.
Ethics approval: The questionnaire and methodology for this study was approved by the Institutional Review Boards of the University of Miami. This study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki.
Consent: Informed consent was obtained from all individuals participants included in the study.
Consent for Publication: NA
Availability of data and material: The data underlying this article will be shared on reasonable request to the corresponding author.
Code Availability: The custom code utilized will be shared on reasonable request to the corresponding author.
References
- 1.Brown JM, Poirot E, Hess KL, Brown S, Vertucci M, Hezareh M. Motivations for Intravaginal Product Use among a Cohort of Women in Los Angeles. PLoS One. 2016;11(3):e0151378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sha BE, Chen HY, Wang QJ, Zariffard MR, Cohen MH, Spear GT. Utility of Amsel criteria, Nugent score, and quantitative PCR for Gardnerella vaginalis, Mycoplasma hominis, and Lactobacillus spp. for diagnosis of bacterial vaginosis in human immunodeficiency virus-infected women. J Clin Microbiol. 2005;43(9):4607–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Onderdonk AB, Delaney ML, Fichorova RN. The Human Microbiome during Bacterial Vaginosis. Clin Microbiol Rev. 2016;29(2):223–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kairys N, Garg M. Bacterial Vaginosis. StatPearls. Treasure Island (FL) 2021. [PubMed] [Google Scholar]
- 5.Greenbaum S, Greenbaum G, Moran-Gilad J, Weintraub AY. Ecological dynamics of the vaginal microbiome in relation to health and disease. Am J Obstet Gynecol. 2019;220(4):324–35. [DOI] [PubMed] [Google Scholar]
- 6.Tapia GR, Glynn TR, Miller C, Manuzak JA, Broedlow CA, Mcgaugh A, et al. Syndemics and preexposure prophylaxis are independently associated with rectal immune dysregulation in sexual minority men. AIDS. 2021;35(8):1295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown JM, Hess KL, Brown S, Murphy C, Waldman AL, Hezareh M. Intravaginal Practices and Risk of Bacterial Vaginosis and Candidiasis Infection Among a Cohort of Women in the United States. Obstetrics & Gynecology. 2013;121(4):773–80. [DOI] [PubMed] [Google Scholar]
- 8.Low N, Chersich MF, Schmidlin K, Egger M, Francis SC, van de Wijgert JH, et al. Intravaginal practices, bacterial vaginosis, and HIV infection in women: individual participant data meta-analysis. PLoS Med. 2011;8(2):e1000416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Myer L, Kuhn L, Stein ZA, Wright TC, Jr., Denny L. Intravaginal practices, bacterial vaginosis, and women’s susceptibility to HIV infection: epidemiological evidence and biological mechanisms. Lancet Infect Dis. 2005;5(12):786–94. [DOI] [PubMed] [Google Scholar]
- 10.Alcaide ML, Rodriguez VJ, Fischl MA, Jones DL, Weiss SM. Addressing intravaginal practices in women with HIV and at-risk for HIV infection, a mixed methods pilot study. Int J Womens Health. 2017;9:123–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diclemente RJ, Young AM, Painter JL, Wingood GM, Rose E, Sales JM. Prevalence and correlates of recent vaginal douching among African American adolescent females. J Pediatr Adolesc Gynecol. 2012;25(1):48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang J, Hatch M, Zhang D, Shulman J, Harville E, Thomas AG. Frequency of douching and risk of bacterial vaginosis in African-American women. Obstet Gynecol. 2004;104(4):756–60. [DOI] [PubMed] [Google Scholar]
- 13.Korte JE, Shain RN, Holden AE, Piper JM, Perdue ST, Champion JD, et al. Reduction in sexual risk behaviors and infection rates among African Americans and Mexican Americans. Sex Transm Dis. 2004;31(3):166–73. [DOI] [PubMed] [Google Scholar]
- 14.Grimley DM, Annang L, Foushee HR, Bruce FC, Kendrick JS. Vaginal douches and other feminine hygiene products: women’s practices and perceptions of product safety. Matern Child Health J. 2006;10(3):303–10. [DOI] [PubMed] [Google Scholar]
- 15.Cherpes TL, Hillier SL, Meyn LA, Busch JL, Krohn MA. A delicate balance: risk factors for acquisition of bacterial vaginosis include sexual activity, absence of hydrogen peroxide-producing lactobacilli, black race, and positive herpes simplex virus type 2 serology. Sex Transm Dis. 2008;35(1):78–83. [DOI] [PubMed] [Google Scholar]
- 16.Gupta A, Cox C, Dunivan GC, Gaskins JT, Rogers RG, Iglesia CB, et al. Desire for Continued Pessary Use Among Women of Hispanic and Non-Hispanic Ethnic Backgrounds for Pelvic Floor Disorders. Female Pelvic Med Reconstr Surg. 2019;25(2):172–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ness RB, Hillier S, Richter HE, Soper DE, Stamm C, Bass DC, et al. Can known risk factors explain racial differences in the occurrence of bacterial vaginosis? J Natl Med Assoc. 2003;95(3):201–12. [PMC free article] [PubMed] [Google Scholar]
- 18.Koumans EH, Sternberg M, Bruce C, McQuillan G, Kendrick J, Sutton M, et al. The prevalence of bacterial vaginosis in the United States, 2001–2004; associations with symptoms, sexual behaviors, and reproductive health. Sex Transm Dis. 2007;34(11):864–9. [DOI] [PubMed] [Google Scholar]
- 19.Fethers KA, Fairley CK, Hocking JS, Gurrin LC, Bradshaw CS. Sexual risk factors and bacterial vaginosis: a systematic review and meta-analysis. Clin Infect Dis. 2008;47(11):1426–35. [DOI] [PubMed] [Google Scholar]
- 20.Paul K, Boutain D, Manhart L, Hitti J. Racial disparity in bacterial vaginosis: the role of socioeconomic status, psychosocial stress, and neighborhood characteristics, and possible implications for preterm birth. Soc Sci Med. 2008;67(5):824–33. [DOI] [PubMed] [Google Scholar]
- 21.Nansel TR, Riggs MA, Yu KF, Andrews WW, Schwebke JR, Klebanoff MA. The association of psychosocial stress and bacterial vaginosis in a longitudinal cohort. Am J Obstet Gynecol. 2006;194(2):381–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jain JP, Bristow CC, Pines HA, Harvey-Vera A, Rangel G, Staines H, et al. Factors in the HIV risk environment associated with bacterial vaginosis among HIV-negative female sex workers who inject drugs in the Mexico-United States border region. BMC Public Health. 2018;18(1):1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singer M, Bulled N, Ostrach B, Mendenhall E. Syndemics and the biosocial conception of health. Lancet. 2017;389(10072):941–50. [DOI] [PubMed] [Google Scholar]
- 24.Singer M, Clair S. Syndemics and public health: reconceptualizing disease in bio-social context. Med Anthropol Q. 2003;17(4):423–41. [DOI] [PubMed] [Google Scholar]
- 25.Singer MC, Erickson PI, Badiane L, Diaz R, Ortiz D, Abraham T, et al. Syndemics, sex and the city: understanding sexually transmitted diseases in social and cultural context. Soc Sci Med. 2006;63(8):2010–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention (CDC). Estimated HIV incidence and prevalence in the United States, 2014–2018: CDC. HIV Surveillance Supplemental Report 2020;25(No. 1); 2020 [Available from: https://www.cdc.gov/hiv/library/reports/hiv-surveillance.html. [Google Scholar]
- 27.Massad LS, Daubert EM, Evans CT, Minkoff H, Kassaye S, Dionne-Odom J, et al. Trends in Bacterial Vaginosis Prevalence in a Cohort of U.S. Women with and at Risk for HIV. J Womens Health (Larchmt). 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Colonna C, Steelman M. Amsel Criteria. StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2021, StatPearls Publishing LLC.; 2021. [PubMed] [Google Scholar]
- 29.Atashili J, Poole C, Ndumbe PM, Adimora AA, Smith JS. Bacterial vaginosis and HIV acquisition: a meta-analysis of published studies. AIDS (London, England). 2008;22(12):1493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol. 1991;29(2):297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Menard JP. Antibacterial treatment of bacterial vaginosis: current and emerging therapies. Int J Womens Health. 2011;3:295–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coudray MS, Madhivanan P. Bacterial vaginosis-A brief synopsis of the literature. Eur J Obstet Gynecol Reprod Biol. 2020;245:143–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Friedman MR, Stall R, Plankey M, Wei C, Shoptaw S, Herrick A, et al. Effects of syndemics on HIV viral load and medication adherence in the multicenter AIDS cohort study. AIDS (London, England). 2015;29(9):1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harkness A, Bainter SA, O’Cleirigh C, Mendez NA, Mayer KH, Safren SA. Longitudinal effects of syndemics on ART non-adherence among sexual minority men. AIDS and Behavior. 2018;22(8):2564–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harkness A, Bainter SA, O’Cleirigh C, Albright C, Mayer KH, Safren SA. Longitudinal Effects of Syndemics on HIV-Positive Sexual Minority Men’s Sexual Health Behaviors. Archives of sexual behavior. 2019;48(4):1159–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Glynn TR, Safren SA, Carrico AW, Mendez NA, Duthely LM, Dale SK, et al. High Levels of Syndemics and Their Association with Adherence, Viral Non-suppression, and Biobehavioral Transmission Risk in Miami, a U.S. City with an HIV/AIDS Epidemic. AIDS Behav. 2019;23(11):2956–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sullivan KA, Messer LC, Quinlivan EB. Substance abuse, violence, and HIV/AIDS (SAVA) syndemic effects on viral suppression among HIV positive women of color. AIDS patient care and STDs. 2015;29(S1):S42–S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chahine A, Koru-Sengul T, Feaster DJ, Dilworth SE, Antoni MH, Klatt N, et al. Blue Monday: Co-occurring Stimulant Use and HIV Persistence Predict Dysregulated Catecholamine Synthesis. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2021;86(3):353–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Santos G-M, Do T, Beck J, Makofane K, Arreola S, Pyun T, et al. Syndemic conditions associated with increased HIV risk in a global sample of men who have sex with men. Sexually Transmitted Infections. 2014;90(3):250–3. [DOI] [PubMed] [Google Scholar]
- 40.Ellington K, Saccomano SJ. Recurrent bacterial vaginosis. Nursing. 2021;51(3):48–52. [DOI] [PubMed] [Google Scholar]
- 41.Fettweis JM, Brooks JP, Serrano MG, Sheth NU, Girerd PH, Edwards DJ, et al. Differences in vaginal microbiome in African American women versus women of European ancestry. Microbiology (Reading). 2014;160(Pt 10):2272–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alcaide ML, Chisembele M, Malupande E, Rodriguez VJ, Fischl MA, Arheart K, et al. A bio-behavioral intervention to decrease intravaginal practices and bacterial vaginosis among HIV infected Zambian women, a randomized pilot study. BMC Infect Dis. 2017;17(1):338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alcaide ML, Chisembele M, Malupande E, Arheart K, Fischl M, Jones DL. A cross-sectional study of bacterial vaginosis, intravaginal practices and HIV genital shedding; implications for HIV transmission and women’s health. BMJ Open. 2015;5(11):e009036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Klebanoff MA, Schwebke JR, Zhang J, Nansel TR, Yu KF, Andrews WW. Vulvovaginal symptoms in women with bacterial vaginosis. Obstet Gynecol. 2004;104(2):267–72. [DOI] [PubMed] [Google Scholar]
- 45.Nelson DB, Bellamy S, Odibo A, Nachamkin I, Ness RB, Allen-Taylor L. Vaginal symptoms and bacterial vaginosis (BV): how useful is self-report? Development of a screening tool for predicting BV status. Epidemiol Infect. 2007;135(8):1369–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lewis FMT, Diesel J. Intravaginal Practices Among Women Attending a Sexually Transmitted Disease Clinic—Philadelphia, 2017. Sexually Transmitted Diseases. 2021;48(5):e64–e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rael CT, Das D, Bauermeister J, Lentz C, Carballo-Diéguez A, Giguere R, et al. Understanding Women’s Vaginal Douching Behaviors and Practices for Consideration in the Development of a Potential Future Vaginal Microbicide Douche for HIV Prevention: A Systematic Review of the Literature. AIDS Behav. 2021;25(9):2992–3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alcaide ML, Cook R, Chisembele M, Malupande E, Jones DL. Determinants of intravaginal practices among HIV-infected women in Zambia using conjoint analysis. Int J STD AIDS. 2016;27(6):453–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alcaide ML, Strbo N, Romero L, Jones DL, Rodriguez VJ, Arheart K, et al. Bacterial Vaginosis Is Associated with Loss of Gamma Delta T Cells in the Female Reproductive Tract in Women in the Miami Women Interagency HIV Study (WIHS): A Cross Sectional Study. PLoS One. 2016;11(4):e0153045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sun J, Brown TT, Tong W, Samuels D, Tien P, Aissani B, et al. African Mitochondrial DNA Haplogroup L2 Is Associated With Slower Decline of β-cell Function and Lower Incidence of Diabetes Mellitus in Non-Hispanic, Black Women Living With Human Immunodeficiency Virus. Clin Infect Dis. 2020;71(8):e218–e25. [DOI] [PMC free article] [PubMed] [Google Scholar]


