Abstract
Background:
Dupilumab, a monoclonal antibody targeting IL-4Rα, improves upper and lower airway symptoms in patients with aspirin-exacerbated respiratory disease (AERD), but the mechanisms leading to clinical improvement are not fully elucidated.
Objective:
To identify the mechanistic basis of clinical improvement in AERD patients treated with dupilumab.
Methods:
Twenty-two patients with AERD were treated with dupilumab for 3 months for severe asthma and/or chronic rhinosinusitis with nasal polyps. Clinical outcomes were assessed at baseline and at one and three months after initiation of dupilumab. Nasal fluid, urine, blood, and inferior turbinate scrapings were collected at the three time points for mediator levels, cellular assays, and RNA-sequencing.
Results:
Participants had rapid improvement in clinical measures including sense of smell, sinonasal symptoms, and lung function after 1 month of dupilumab, which were sustained after 3 months of dupilumab. Baseline severity of smell loss correlated with lower nasal prostaglandin E2 levels. Dupilumab increased nasal prostaglandin E2 and decreased levels of nasal albumin, nasal and urinary leukotriene E4, and serum and nasal IgE. Transcripts related to epithelial dysfunction and leukocyte activation and migration were downregulated in inferior turbinate tissue after treatment with dupilumab. There were no dupilumab-induced changes in nasal eosinophilia.
Conclusion:
Inhibition of IL-4Rα in AERD led to rapid improvement in respiratory symptoms and smell, with a concomitant improvement in epithelial barrier function, a decrease in inflammatory eicosanoid levels, and an increase in the anti-inflammatory eicosanoid prostaglandin E2. The therapeutic effects of dupilumab are likely due to decreased IL-4Rα signaling on respiratory tissue granulocytes, epithelial cells, and B cells.
Keywords: Aspirin-exacerbated respiratory disease, AERD, interleukin 4Rα, interleukin 4, interleukin 13, nasal polyp, dupilumab, anosmia, leukotriene E4, prostaglandin E2
Capsule Summary:
Dupilumab induces rapid clinical improvements in olfaction, congestion, lung function, and quality of life in AERD, which correspond mechanistically to dupilumab-induced decreased leukotriene E4, increased prostaglandin E2, and improved nasal epithelial barrier.
Introduction:
The advent of targeted respiratory biologic therapies for the treatment of asthma and chronic rhinosinusitis with nasal polyps (CRSwNP) has yielded significant improvement in control of these diseases and in patient quality-of-life.1 In aspirin-exacerbated respiratory disease (AERD), the triad of asthma, CRSwNP and respiratory reactions to cyclooxygenase (COX) 1 inhibitors, the upper and lower airway inflammation is often difficult to treat, with many patients failing first-line therapies.2, 3
Dupilumab, a fully human monoclonal antibody targeting interleukin (IL)-4Rα, inhibits signaling of both IL-4 and IL-13. It is approved for both moderate-to-severe eosinophilic asthma and inadequately controlled CRSwNP,4–7 as well as for atopic dermatitis, and it is also under investigation for other type 2 inflammatory diseases.8, 9 Prior studies have shown that within 16 weeks of treatment, dupilumab leads to robust improvements in upper and lower respiratory tract symptoms in AERD patients, including increased sense of smell, reduction in nasal polyp size, and improvements in lung function.10, 11 In our experience, we have noted an even more rapid onset of clinical improvement with dupilumab, often within the first month of initiating treatment.
Inhibiting the type 2 cytokines IL-4 and IL-13 has broad impacts on chronic inflammation including IgE class switching, Th2 lymphocyte differentiation, mast cell activation, mucus secretion, and airway smooth muscle proliferation.12–14 Indeed, IL-4Rα is expressed on multiple cell types that are potentially relevant to AERD, including mast cells, eosinophils, B cells, and epithelial cells, and it is not yet known which of these cells are causative effectors of the chronic disease in AERD, nor which of them are most affected by dupilumab and underlie the mechanism of benefit of IL-4Rα inhibition.15 Dupilumab is known to induce a decrease in both serum IgE6 and urinary leukotriene (LT)E4,16 but medications that target only IgE (omalizumab) or only leukotriene production (zileuton) may be less efficacious compared to dupilumab in many patients with AERD, suggesting that there are additional mechanisms by which IL-4Rα inhibition affords therapeutic benefit.
Elucidating which dupilumab-induced cellular changes lead to the drug’s dramatic clinical benefit in AERD will allow for increased understanding of the underlying pathobiology of the disease and may help teach us which effector cell(s) cause the chronic inflammation in AERD. With this study we sought to determine the impact of IL-4Rα blockade in AERD, with parallel examination of early treatment timepoints (1 month and 3 months) for both clinical and mechanistic outcomes.
Methods:
Patient Characterization
Participants between the ages of 18 and 75 years were recruited from the Brigham and Women’s Hospital (Boston, MA) Allergy and Immunology Clinics from 2018–2020 (Table 1). The Mass General Brigham Institutional Review Board approved the study and all participants provided written informed consent. Patients with AERD all had asthma, CRSwNP, and had a diagnosis of AERD previously confirmed via a physician-observed oral challenge to aspirin which induced objectively-defined upper and/or lower respiratory symptoms including nasal congestion, rhinorrhea, sneezing, wheezing, dyspnea, and/or fall in forced expiratory volume in 1 second (FEV1).
TABLE 1.
Patient Characteristics.
| (N = 22) | |
|---|---|
| Age in years (Mean ± SD) | 52.6 ± 13.4 |
| Gender (N, % Female) | 12, 54.5% |
| Race (N, % White) | 19, 86.4% |
| Lifetime endoscopic sinus surgeries (Mean ± SD) | 2.4 ± 1.1 |
| Prior use of respiratory biologic (N, %)ϕ | 3, 13.6% |
| Current use of daily aspirin therapy at study entry* (N, %) | 8, 36.3% |
| Current use of montelukast (N, %) | 11, 50% |
| FEV1 % Predicted (Mean ± SD) | 75.7 ± 19.6 |
| Baseline SNOT-22 (Mean ± SD) | 48.7 ± 22.3 |
| Baseline ACQ-6 (Mean ± SD) | 1.6 ± 1.3 |
| Baseline UPSIT score (Mean ± SD) | 15.5 ± 9.6 |
Participants previously treated with respiratory biologic had minimum of 6-month washout period
Aspirin therapy: either 650 mg daily or 1300 mg daily
Patients with AERD who met criteria for the FDA-approved use of every-other-week dupilumab (300 mg subcutaneously) for the treatment of moderate-to-severe eosinophilic asthma or CRSwNP were prescribed dupilumab by their pulmonologist or allergist/immunologist as part of their usual clinical care. Patients were recruited prior to initiation of their first dose of dupilumab. Participants were followed for this observational study at three visits: a baseline visit just prior to their first dose of dupilumab (baseline), after 1 month (2 doses) on dupilumab (month 1), and again after 3 months on dupilumab (month 3). Participants were excluded if they received any other biologic therapy within 6 months of enrollment.
Clinical Procedures and Specimen Procurement
At each of the three study visits, clinical parameters were collected, including sense of smell (University of Pennsylvania Smell Identification Test (UPSIT)), peak nasal inspiratory flow (PNIF), nasal polyp size as assessed with an otoscope (unilateral polyp score of 0 if not visible, 1 if visible above middle turbinate, and 2 if visible below middle turbinate, for total maximum bilateral score of 4), spirometry (FEV1 and forced vital capacity (FVC)), and patient-reported outcome measures including sinonasal outcome test (SNOT)-22 and Asthma Control Questionnaire-6 (ACQ-6) scores. Biological specimens including urine, blood, nasal fluid, and nasal cells from the inferior turbinate were collected at the same timepoints. All measurements for a single participant were done together in the same batch. For full details, see the Methods section in this article’s Online Repository.
Flow Cytometry
Flow cytometry measurement of peripheral blood eosinophils, basophils, neutrophils, and surface CRTH2 expression was performed as previously described.17 The presence of platelet-adherent leukocytes was assessed as previously described.18 For full details, see the Methods section in this article’s Online Repository and Supplemental Table E1.
Mediator Quantification
Nasal fluid and serum were measured for levels of total IgG (Invitrogen, Waltham, MA), IgA (Invitrogen), IgE (Invitrogen for serum, Abcam, Waltham, MA for nasal secretions) and IgG4 (eBioscience, San Diego, CA) by ELISA, and for total tryptase at Virginia Commonwealth University. Nasal secretions were further analyzed for albumin (Abcam) and eosinophilic cationic protein (ECP) by ELISA (Lifespan Biosciences, Seattle, WA). Urinary eicosanoids (Vanderbilt University) and nasal eicosanoids (University of California San Diego) were measured by mass spectrometry.19
Inferior Nasal Turbinate Epithelial Cell Bulk RNA-sequencing
For bulk RNA-sequencing (RNA-seq), cells were scraped from the inferior turbinate and immediately preserved in RNAprotect®. Sequencing libraries were generated using a modified low-input bulk RNA-seq pipeline, as described.17 Differential expression analysis was conducted with DESeq2.20 WebGestalt online tool was used to identify the distribution of pre-ranked DEGs involved in specific signaling pathways in Gene Ontology Biological Processes database. R package “ggplot2” was used to plot pathways with FDR < 0.01 in either of the comparisons. For full details, see the Methods section in this article’s Online Repository.
Statistical Analysis
Mixed effect analysis was used to assess change over time for numerical clinical outcomes. The model included a fixed categorical effect of visit as month and random intercept. An unstructured covariance structure was used to model the within-participant errors. P-values were reported for overall time effect, month 1 compared to baseline, and month 3 compared to baseline. The p values for two pair-wise comparisons (month 1 compared to baseline and month 3 compared to baseline) were Tukey-Kramer adjusted. Categorical clinical outcomes were analyzed using the Generalized Estimating Equations model. Wilcoxon signed-rank tests were used for biomarker analysis. A reduced p-value of 2.5% was used to test significance level for biomarker analyses due to analysis of multiple biomarkers. Correlation between biomarkers was assessed using Pearson/Spearman correlation coefficient. Analysis was performed using SAS version 9.4 (Cary, NC) and figures were prepared in GraphPad Prism version 9.2.0 (GraphPad, La Jolla, CA).
Results:
Study Population and Demographics
Twenty-two patients with physician-diagnosed AERD who initiated treatment with dupilumab as an add-on asthma or CRSwNP therapy participated in this observational study (Table 1). No patients were on systemic corticosteroids, 11/22 patients were on montelukast, and 15/22 patients used nasal budesonide irrigations, with the remaining patients using over-the-counter intranasal steroid sprays for at least one month prior to entering the study. Eight of 22 participants were on daily aspirin therapy after desensitization for 6 months or more prior to starting dupilumab, but did not achieve sufficient control of asthma or CRSwNP on aspirin therapy and thus elected to pursue treated with dupilumab. There were no baseline differences in baseline number of endoscopic sinus surgeries, patient reported rate of polyp regrowth, and FEV1 % predicted in participants who were on high-dose daily aspirin compared to those who were not. All participants previously underwent endoscopic sinus surgery. All baseline medications were kept consistent through the study period.
Dupilumab-Induced Changes in Clinical Outcomes
The UPSIT score was significantly improved after 1 and 3 months of dupilumab treatment (Figure 1A, mean change of 11.3 and 11.9, respectively, P<0.0001 at both time points). Based on UPSIT scores, 16 of 22 participants were classified as anosmic (UPSIT score <19) at baseline, but after 1 month of treatment only 4 participants were still anosmic (Figure 1B, Odds Ratio OR (95%CI)=0.12 (0.04–0.36), P=0.0002), and this effect was sustained after 3 months of dupilumab (Odds Ratio (95%CI)=0.08 (0.02–0.28), P<0.0001). Improvement in the SNOT-22 beyond the minimal clinically important difference of 8.9 points21 was observed after 1 month of dupilumab (mean change −34.4, P<0.0001) and was sustained at the 3-month time point (mean change −34.5, P<0.0001) (Figure 1C). The PNIF was increased after 1 and 3 months of dupilumab (Figure 1D, mean change 31.4 mL (P=0.0023) and 36.5 ml (P=0.0007), respectively). Nasal polyp scores also improved with dupilumab, with significant reduction in nasal polyp burden identified after just 1 month of treatment (Figure 1E, P<0.0001).
Figure 1: Dupilumab-induced changes in clinical upper respiratory outcomes.
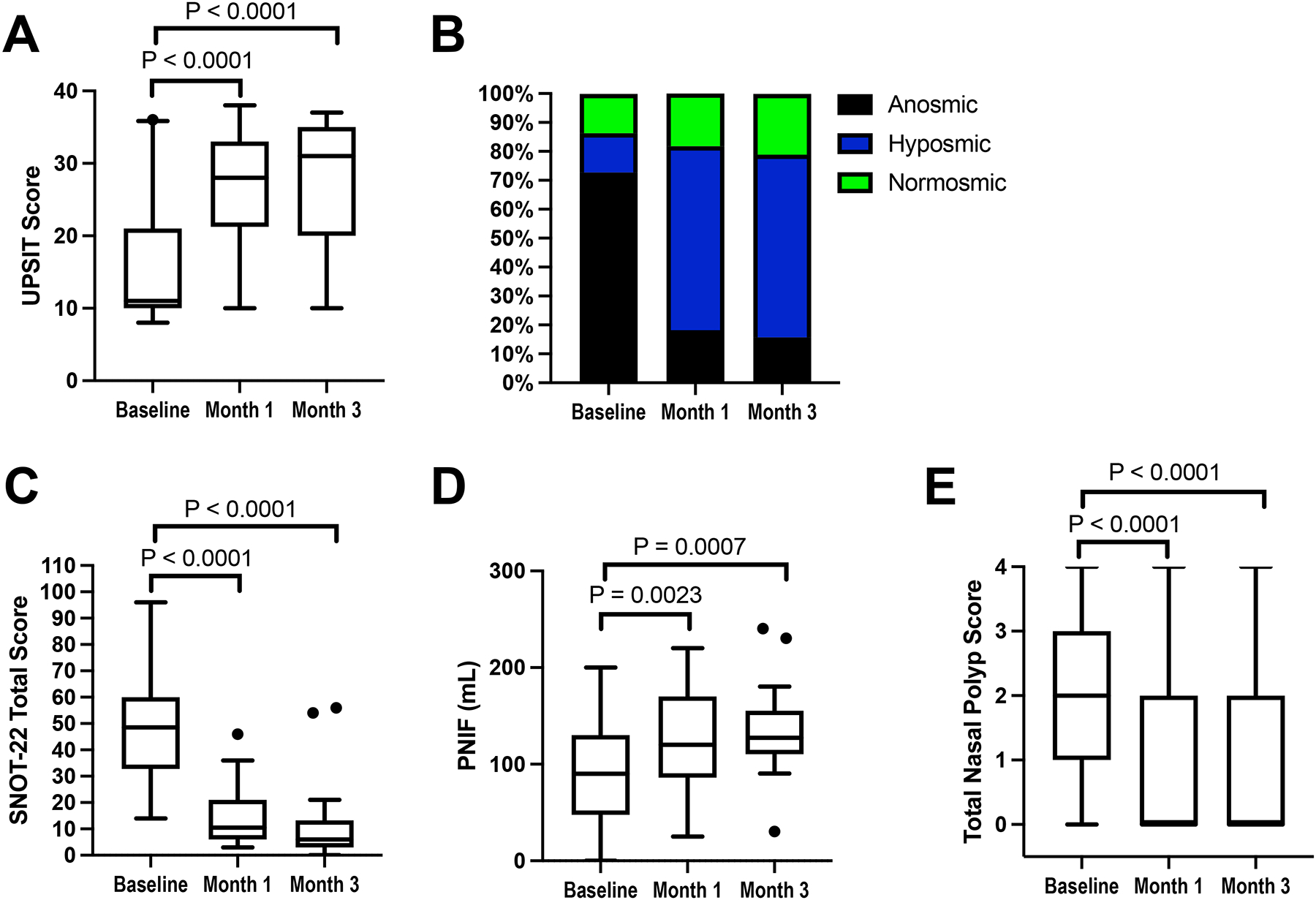
Smell identification level as measured by UPSIT is shown as raw scores (A) or as summarized levels of anosmia, hyposmia, or normosmia (B) at the pre-dupilumab baseline, and after 1 and 3 months of treatment with dupilumab. SNOT-22 scores (C), peak nasal inspiratory flow (PNIF) (D), and otoscopically scored bilateral nasal polyp scores (E) are shown for the same timepoints. Data in A, C-E are shown as Tukey’s box-and-whisker plots, N = 22 participants.
Lower airway assessments also showed improvements in lung function and asthma control. The FEV1% predicted improved by a mean of 12.6% (P=0.0002) and 12.1% (P=0.0015) at months 1 and 3, respectively (Figure 2B), and there were also significant improvements in the total liters of FEV1 and FVC at both visits (Figure 2A and C). Patient-reported asthma control, assessed by ACQ-6, significantly improved after 1 month of dupilumab (mean change −1.3, P<0.0001), which was sustained after 3 months of treatment (Figure 2D).
Figure 2: Dupilumab-induced changes in pulmonary outcomes.
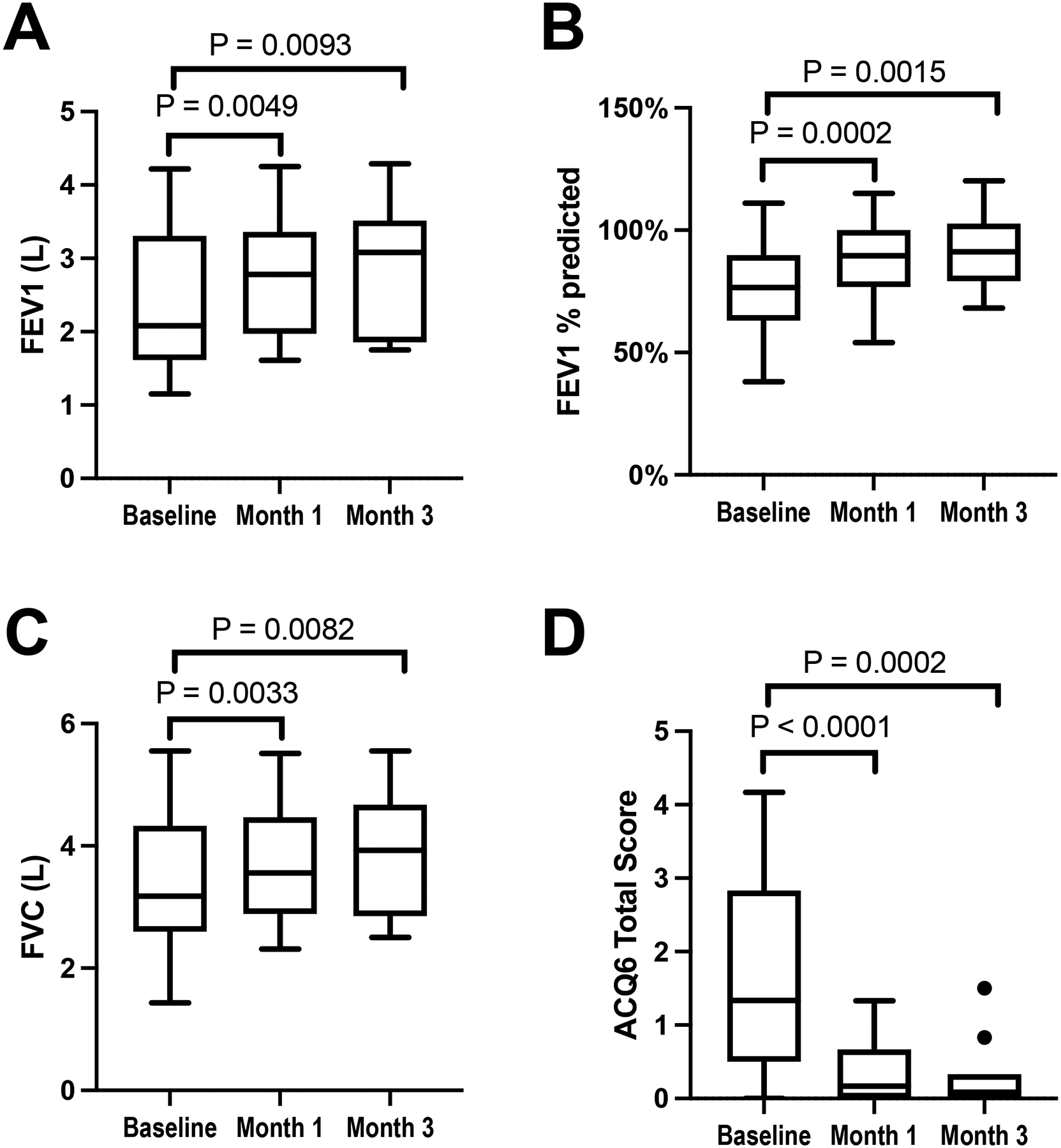
Pulmonary function and asthma control as measured by liters of FEV1 (A), FEV1 % predicted (B), liters of FVC (C), and ACQ-6 (D) are shown at the pre-dupilumab baseline, and after 1 and 3 months of treatment with dupilumab. Data are shown as Tukey’s box-and-whisker plots, N = 22 participants.
Effects of Dupilumab on Nasal and Urinary Eicosanoid Levels
Urinary levels of LTE4 significantly decreased after 1 and 3 months of dupilumab (Figure 3A, P<0.0001 and P=0.0095, respectively), and nasal LTE4 decreased by more than 7-fold from baseline to month 1 (P=0.0256) and even further at month 3 (P=0.0020) (Figure 3D). There were no significant changes in urinary levels of the prostaglandin (PG)D2 metabolite tetranor-PGD-M, though there was a trend toward a decrease at month 1 (Figure 3B), and there were no dupilumab-induced changes in nasal levels of the PGD2 metabolite, DHKPGD2 (Figure 3E). Urinary PGE-M levels did not change with dupilumab (Figure 3C). However, there was a significant dupilumab-induced increase in nasal fluid PGE2, with nearly a 2-fold increase after 1 and 3 months of treatment (Figure 3F, P = 0.0103 and 0.0063, respectively). There was no significant difference in baseline levels of urinary LTE4, urinary PGE-M, nasal DHKPGD2, or nasal PGE2 in the participants on or off of high-dose daily aspirin (data not shown). However, patients on high-dose aspirin at the start of the study had baseline lower urinary PGD-M compared to the patients not on high-dose aspirin (mean of 1.1 ng/mL vs 3.1 ng/mL, P < 0.01).
Figure 3: Dupilumab-induced changes in nasal and urinary eicosanoids.
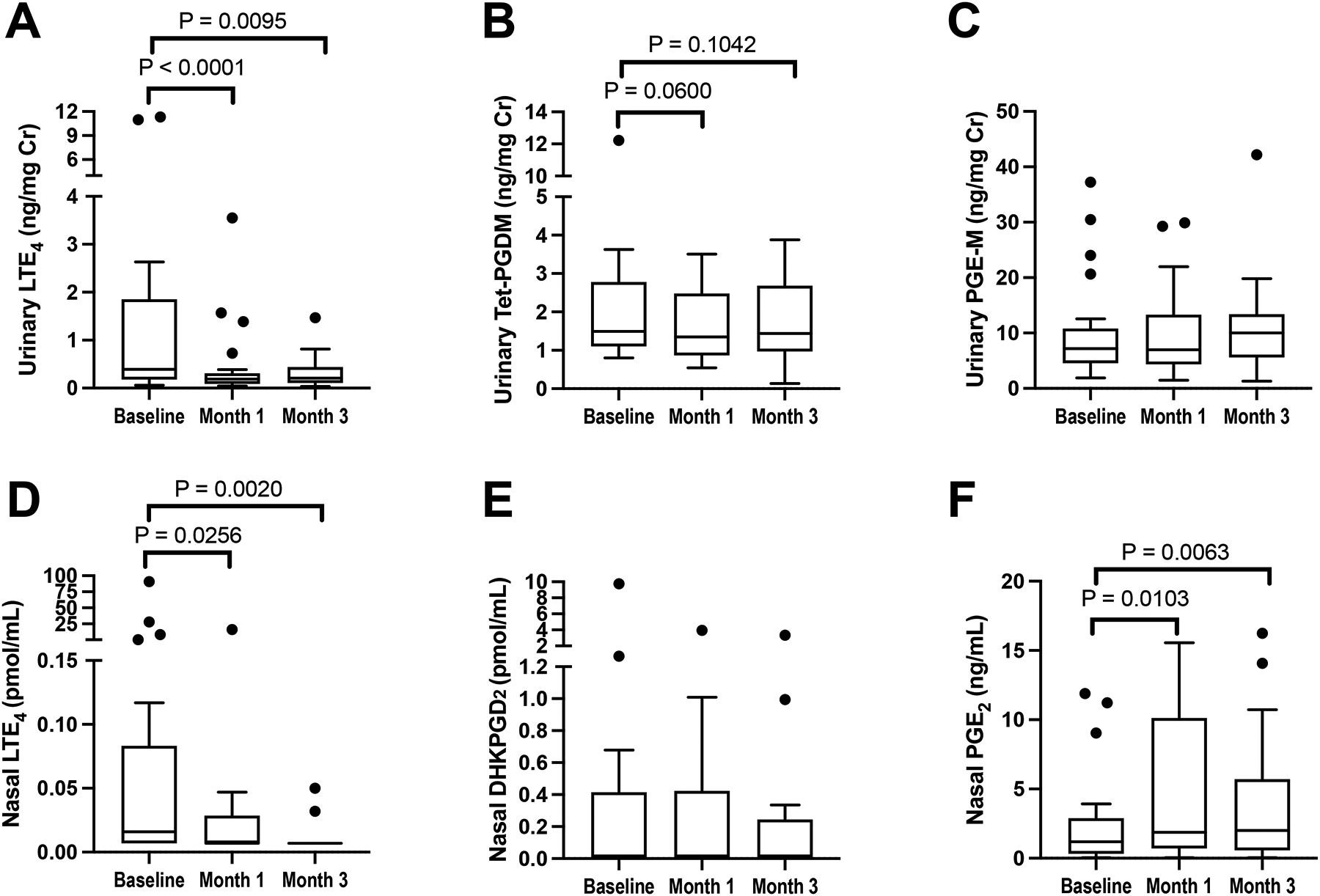
Urinary levels of LTE4, tetranor-PGDM, and PGE-M (A-C), and nasal fluid levels of LTE4, DHKPGD2, and PGE2, (D-E) are shown at the pre-dupilumab baseline, and after 1 and 3 months of treatment with dupilumab. Data are shown as Tukey’s box-and-whisker plots, analysis with Wilcoxon signed-rank test, N = 22 participants.
We then assessed for a relationship between PGE2 and LTE4 levels and sense of smell and identified an association between baseline nasal PGE2 levels and UPSIT score, but not LTE4 levels and UPSIT score. At baseline 6/22 participants had an UPSIT score of UPSIT ≥19 and were classified as normosmic/hyposmic, while 16/22 participants had an UPSIT score <19 and were classified as anosmic. The anosmic participants had significantly lower nasal PGE2 at baseline compared to the hyposmic/normosmic participants, with a mean nasal PGE2 level of 1.60 ng/mL vs. 5.11 ng/mL, P=0.0168 (Figure 4A). Further, we observed a positive correlation between the baseline UPSIT score and nasal PGE2 levels (r=0.503, P=0.0171) and a trend toward a positive correlation between the dupilumab-induced change in nasal PGE2 between baseline and month 1, and the corresponding change in UPSIT (Figure 4B and C).
Figure 4: Relationship between nasal PGE2 and sense of smell.
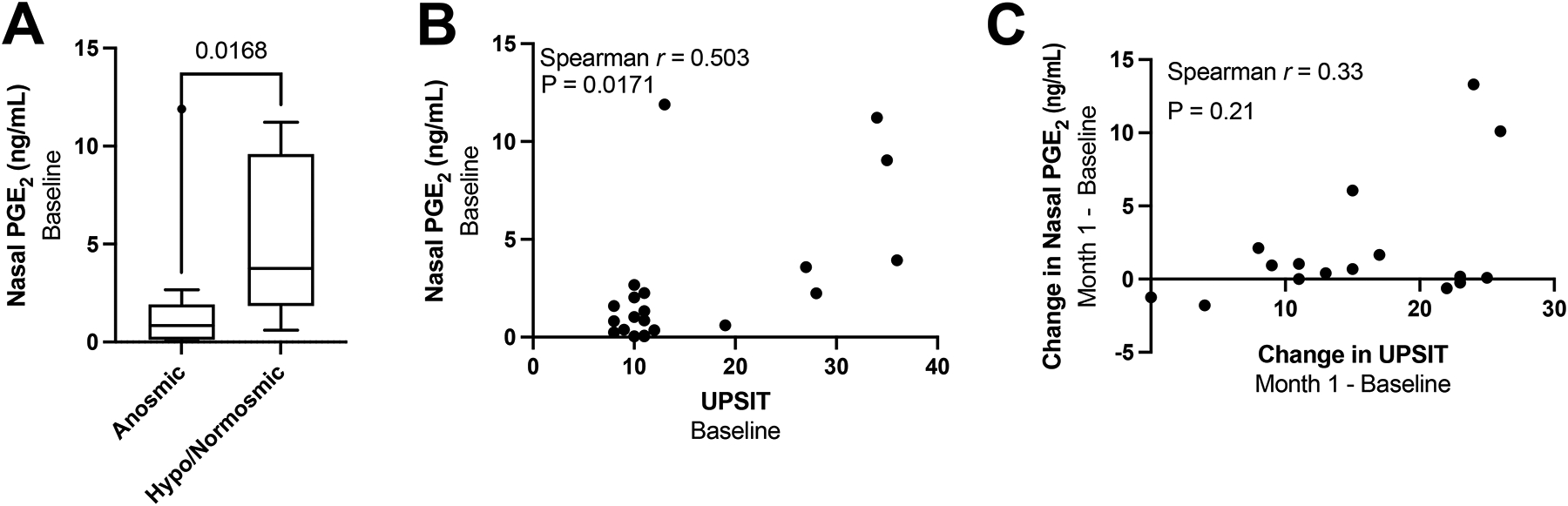
Nasal fluid PGE2 levels of patients who were anosmic (could identify <19 items on the UPSIT) or hyposmic/normosmic (could identify ≥19 items on the UPSIT) at baseline are compared (A). Correlations between baseline nasal PGE2 levels and baseline UPSIT score (B) and between the dupilumab-induced change from baseline to month 1 in nasal PGE2 and UPSIT score (C) are shown with Spearman correlation coefficients.
Effects of Dupilumab on Nasal Albumin, Eosinophilic Cationic Protein and Nasal and Serum Tryptase
Nasal albumin levels significantly fell after 1 month of dupilumab (Figure 5A, P=0.0149) which was sustained at the 3-month timepoint (P=0.0160). There was a slight trend toward decreased nasal ECP levels after 1 and 3 months of dupilumab (Figure 5B), and there was no significant dupilumab-induced change in serum or nasal tryptase (data not shown).
Figure 5: Dupilumab-induced decrease in nasal albumin and ECP.
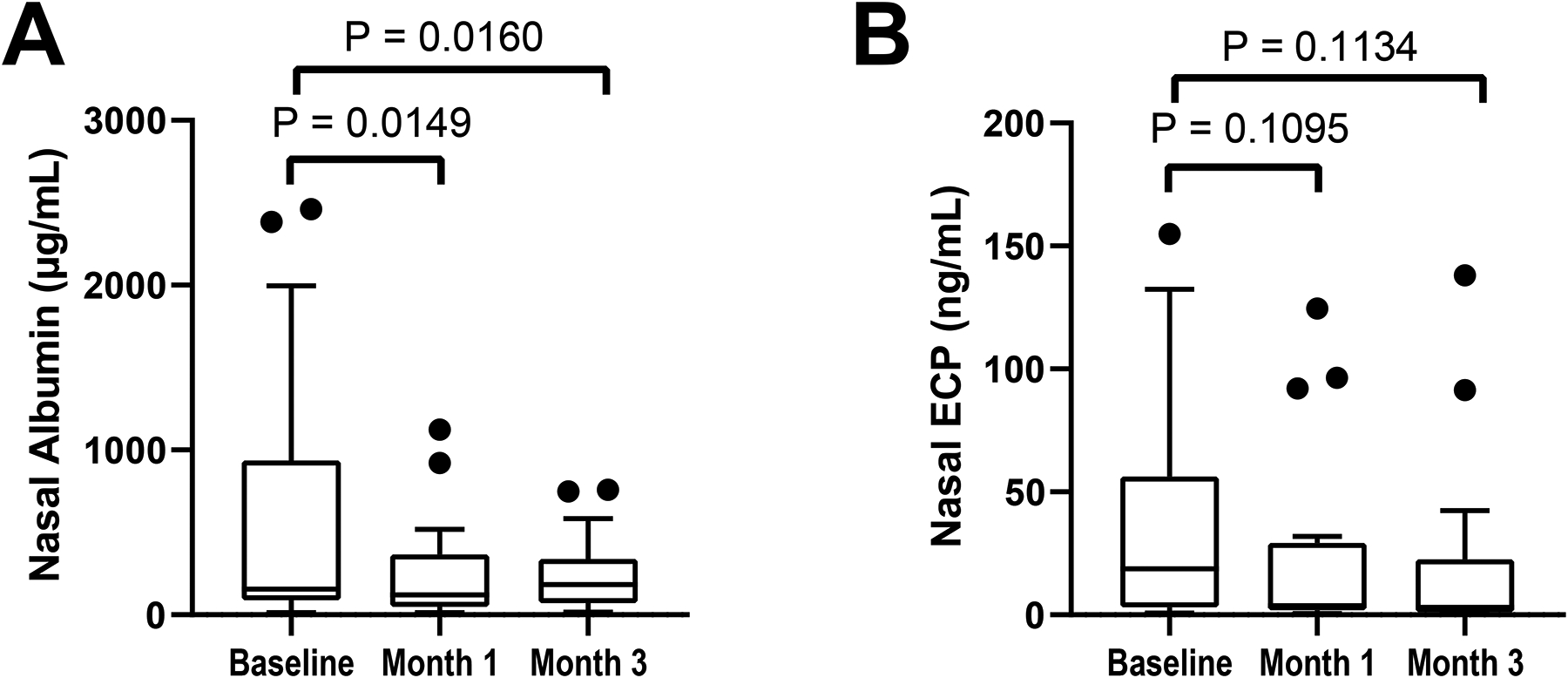
Nasal fluid levels of albumin (A) and eosinophilic cationic protein (ECP) (B) are shown at the pre-dupilumab baseline, and after 1 and 3 months of treatment with dupilumab. Data are shown as Tukey’s box-and-whisker plots.
Effects of Dupilumab on Nasal and Serum Antibody Levels
Both serum and nasal IgE levels decreased after 1 month of dupilumab (P<0.0001 and P=0.0003, respectively), which was sustained after 3 months (Figure 6A and B, P<0.0001). There were no significant changes in nasal IgA, total nasal IgG, or nasal or serum IgG4 measured at these timepoints (data not shown).
Figure 6: Dupilumab-induced decrease in IgE.
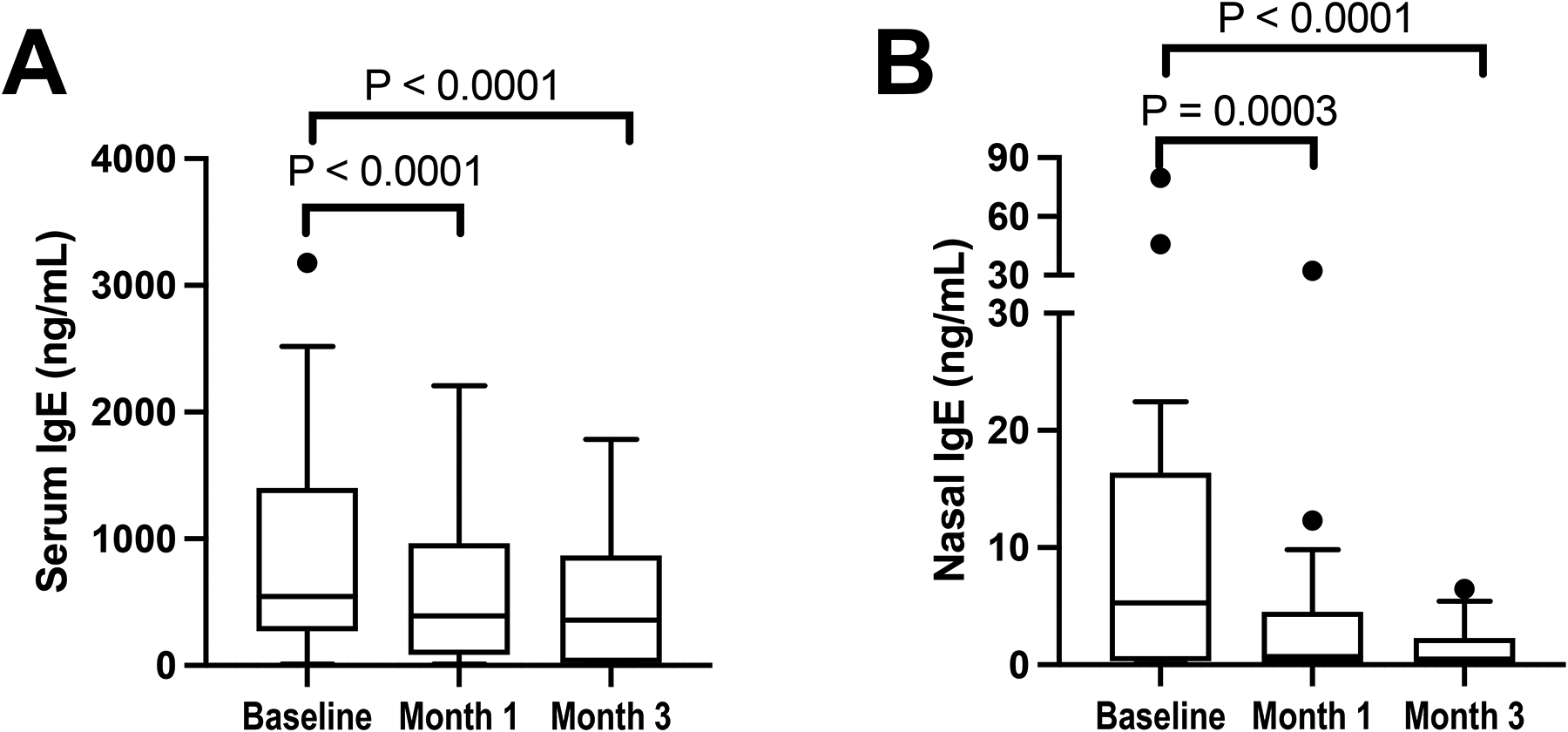
Serum IgE (A) and nasal fluid IgE (B) levels are shown at the pre-dupilumab baseline, and after 1 and 3 months of treatment with dupilumab. Data are shown as Tukey’s box-and-whisker plots.
Blood granulocyte levels, their CRTH2 expression, and the levels of leukocyte-platelet aggregates are unchanged by dupilumab
Dupilumab did not significantly change the peripheral blood levels of eosinophils, basophils, or neutrophils, measured as a percent of total CD45+ cells (Supplemental Figure E1, A–C), and it did not change the percentages of any platelet-adherent leukocyte subsets (Supplemental Figure E1, D–G). Dupilumab also did not significantly change the surface expression of CRTH2 on either blood eosinophils or basophils, calculated as a median fluorescence intensity (Supplemental Figure E2).
Inferior Turbinate RNA-sequencing
Analysis of the inferior turbinate scraping RNA-seq samples revealed that after one month of dupilumab there were 32 upregulated and 25 downregulated transcripts, and after three months there were 34 transcripts upregulated and 86 downregulated that passed the false discovery rate with an adjusted p-value of <0.05 (Figure 7A and B, Table E2) when including long noncoding RNA. Two transcripts related to mucus overproduction and epithelial dysfunction (MUC5B22–24 and PTHLH25) were significantly downregulated after the first month of dupilumab treatment. Based on prior scRNA-seq, we recover mostly basal, secretory and ciliated epithelial cells, but also capture some lymphocytes, granulocytes, and myeloid cells from inferior turbinate scrapings.15 Gene ontogeny (GO) analyses of the data suggest downregulation of 24 pathways and upregulation of four pathways after 1 or 3 months of treatment with dupilumab (Figure 7C). Among others, there was dupilumab-induced downregulation of GO pathways involved in leukocyte activation and migration, differentiation, and cell-cell adhesion (Table E3). Notably, there were no differences in epithelial transcripts known to be upregulated or downregulated by topical corticosteroid use26 in patients who used intranasal budesonide irrigations vs fluticasone nasal spray.
Figure 7: Dupilumab-induced differntial gene expression and gene enrichment analyses.
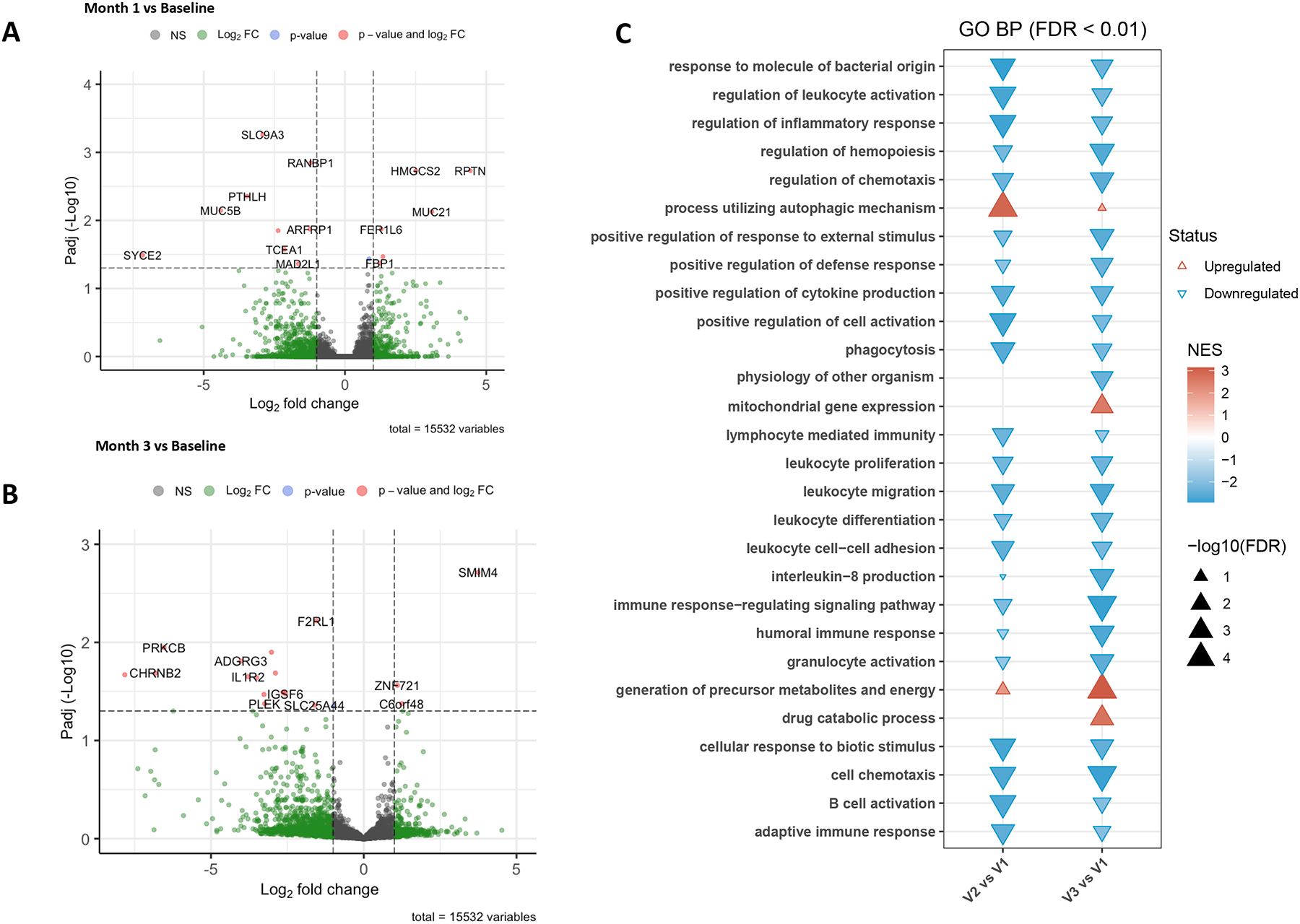
Volcano plots showing dupilumab-induced differential gene expression at month 1 compared to baseline (A) and month 3 compared to baseline (B). Gene ontogeny analysis reveals upregulation and downregulation of specific pathways by dupilumab treatment after 1 and 3 months of treatment (C).
Discussion:
Consistent with previous studies,10, 16 our open-label observational trial of dupilumab in 22 adult patients with AERD showed that inhibition of IL-4Rα led to significant improvement in sense of smell, nasal congestion, nasal polyp size, asthma symptoms, and lung function. Our nasal polyp scoring system was limited in that it was done by otoscopic examination, not nasal endoscopy, but we identified significant changes in polyp size nonetheless. We saw near-universal improvement in our well phenotyped AERD cohort, while studies of severe asthma and CRSwNP show more variability in response, likely reflecting heterogeneity of those patient populations and non-type 2 mechanisms of disease. Ours is the first study to show that the majority of the clinical benefit afforded by dupilumab in AERD, including the striking olfactory improvement, actually occurs within the first month of treatment (Figures 1 and 2), and is sustained at 3 months, suggesting that 3 months is an adequate trial of dupilumab in patients with AERD. Given the widespread expression of IL-4Rα on multiple potentially relevant cell types, we suspect that the rapid clinical improvement noted in this study is likely due to several concomitant mechanistic changes, including a reduction in cysteinyl leukotrienes (cysLTs), an increase in local nasal PGE2, an improvement in airway epithelial barrier integrity, and transcriptional changes in epithelial cell dysfunction and leukocyte differentiation and proliferation pathways within the sinus tissue.
Changes in eicosanoids, specifically the increases in nasal PGE2 and decreases in LTE4 (Figure 3) may underlie a major portion of the clinical benefit seen with dupilumab treatment in AERD. PGE2, which is generated dominantly by macrophages, epithelial cells, and other stromal cells through the COX-2 pathway,27 with some contribution from COX-1, plays a critical protective role in AERD and is considered to largely function as an anti-inflammatory mediator in the airway. COX-2 mRNA expression is diminished in NP epithelial cells from AERD patients, and subsequent PGE2 production is also reduced.28 Of note, high levels of IL-4 within the tissues may be a direct cause of the reduction in COX-2 and PGE2 in AERD, as IL-4 can inhibit PGE2 production through selective inhibition of COX-2 mRNA,29, 30 though the specific enzymatic pathways that were affected by dupilumab and led to the increase in PGE2 are not yet clear. Deficient levels of PGE2 likely have a major role in AERD disease pathogenesis, as PGE2 signaling inhibits 5-lipoxygenase (5-LO) function, preventing the synthesis of cysLTs,31 and PGE2 also has direct protective effects on mast cells.32 Thus, the rise in nasal PGE2 and subsequent restoration of respiratory tissue levels of PGE2 we observed with dupilumab could contribute to the significant decrease seen in nasal and urinary LTE4 through suppression of mast cell activation and through 5-LO inhibition of cysLT-producing granulocytes (Figure 3 A and D). However, IL-4 also directly induces expression of leukotriene C4 synthase by human mast cells and primes them for cysLT generation33 and therefore, direct inhibition of mast cell IL-4Rα could account for the dupilumab-induced decrease in LTE4. Whether the decreases in LTE4 are due to direct (inhibition of IL-4Rα on 5-LO- and leukotriene C4 synthase-expressing granulocytes) or indirect (inhibition of IL-4Rα on respiratory epithelial cells, allowing for increased PGE2 production and 5-LO inhibition) effects of dupilumab cannot be determined by this study, but warrant further investigation with single-cell analysis techniques. Although there was a trend towards dupilumab-induced decrease in PGD2 metabolites (Figure 3B), this was not statistically significant. It is not known whether IL-4 or IL-13 signaling on mast cells directly regulates their production of PGD2, and there are tissue-specific mast cell differences (mucosal mast cells vs connective tissue-type mast cells) in their metabolism of arachidonic acid to leukotrienes or prostaglandins.34 Nonetheless, PGD2 is the primary eicosanoid product of mast cells, and considering the dramatic dupilumab-induced decrease in LTE4, but lack of change in tryptase or PGD2, we suspect that dupilumab either led to selective changes in mast cell phenotype or activation, or to changes in other cysLT-producing granulocyte activation levels. CysLTs are known to mediate much of the chronic inflammation and the acute aspirin-induced reactions in AERD35, 36 and PGE2 has an additional role in limiting eosinophil migration and enhancing smooth muscle relaxation through EP2 receptor signaling37–39, both which have important pathogenic implications in AERD. Therefore, the changes we noted in this study in both PGE2 and LTE4 levels likely underlie much of the clinical benefit afforded by dupilumab, including the improvements in lung function and asthma control (Figure 2).
Chronic upper airway symptoms and loss of smell contribute deeply to quality of life impairment in AERD.40 Patients with AERD have even more severe anosmia than aspirin-tolerant patients with CRSwNP,41 but the underlying etiology of the abnormality in olfaction is not known. We found that the baseline nasal fluid PGE2 levels of participants who were anosmic were more than 4-fold lower than those of participants who were normosmic or only hyposmic, (Figure 4A), and that the baseline nasal fluid PGE2 level correlated positively with the number of scents each participant could identify (Figure 4B). In our study, sense of smell dramatically improved for most patients after just one month of dupilumab (Figure 1 A and B), which was sustained at the three-month visit. There were four patients who remained anosmic after treatment with dupilumab, possibility due to permanent damage to olfactory neurons or pathways. Nasal PGE2 also increased during this time frame (Figure 3F), with a trend towards a positive correlation with improvement in sense of smell (Figure 4C). To our knowledge, there is no known direct link between PGE2 and either olfaction or the function of olfactory receptor neurons or sustentacular supporting cells, and olfactory neurons have not been shown to express any PGE2 receptors. Further, administration of high-dose aspirin may reduce PGE2 production, though paradoxically it is a treatment that has been shown to improve sense of smell in some patients with AERD. Therefore, our findings could be correlative but not causative, as perhaps a dupilumab-induced restoration of epithelial-derived PGE2 is a reflection of overall improvement in the epithelial health within the sinuses, an improvement which may extend to the olfactory epithelium.
CRSwNP tissue has abnormal expression of epithelial intracellular junction proteins, in part driven by high local levels of IL-4 and IL-13, leading to a “leaky” epithelial barrier and subsequent exudation of plasma proteins into nasal secretions.42, 43 Nasal fluid albumin, a marker of plasma exudation and edema, is higher in patients with CRSwNP than in patients without NPs, and is reduced by corticosteroids.44, 45 In vivo administration of IL-4 causes nasal congestion and edema, which may be due to an induction of epithelial permeability.46 We found that dupilumab decreased nasal albumin levels (Figure 5A), and downregulated transcripts related to glandular cell hyperplasia (MUC5B, encoding a member of the mucin family and PTHLH, encoding parathyroid hormone-like hormone),22–25 after just one month of treatment, as it concurrently improved nasal airflow and decreased congestion (Figure 1D), suggesting an improvement in the “leakiness” of the epithelial barrier.
The treatment-induced decrease in serum and nasal IgE levels confirms previous findings that inhibition of IL-4/IL-4Rα signaling on antibody secreting cells reduces IgE production, possibly by inhibiting IgE class-switching (Figure 6).47 Rates of clinical atopy in AERD are on par with the general population and many AERD patients are skin test negative to all common environmental allergens.48 Furthermore, the NSAID-induced reactions are not associated with drug-specific IgE. Nonetheless, IgE inhibition with omalizumab has some effect as a treatment for AERD, leading to reductions in urinary levels of both LTE4 and PGD2 metabolites,49 and pre-treatment with omalizumab can inhibit or lessen aspirin-induced reactions.50 The role that the dupilumab-induced reduction in local and systemic IgE levels plays in the improvement in clinical symptoms is unclear, but given that IgE receptor crosslinking induces mast cell release of cysLTs,51 dupilumab may help to indirectly reduce mast cell cysLT release through downregulation of IgE synthesis.
We did not identify changes in blood or nasal eosinophilia (as measured by nasal ECP levels, Figure 5B) induced by dupilumab. Given that dupilumab is efficacious in patients with CRSwNP regardless of patient eosinophilic status,52 and that near-complete depletion of eosinophils from within the blood and NP tissue does not necessarily provide symptomatic improvement or reduction in NP size in patients with CRSwNP,53 eosinophils are likely not the main effector cells that drive inflammation in AERD. Unlike our recent finding that mepolizumab treatment in AERD leads to decreased nasal and urinary PGD2 metabolites, and a subsequent increase of the surface expression of the PGD2 receptor CRTH2,17 we did not observe a significant dupilumab-induced decrease in PGD2 metabolite levels or a change in granulocyte CRTH2 expression, reflecting the divergent mechanistic consequences of inhibition of IL-5 vs IL-4Rα. However, we do see transcriptional changes in the nasal tissue showing a decrease in pathways involved in the activation and migration of leukocytes as well as B cell activation (Figure 7), which may underlie some of the benefit afforded by dupilumab, allowing for decreased recruitment of activated inflammatory leukocytes to the respiratory tissues. The limited number of differentially expressed genes was in part due to patient heterogeneity, batch effect, and depth of sequencing reads. Highly powered studies and/or single-cell RNA-seq will help to address patient-to-patient heterogeneity. Additionally, by sampling the accessible and relatively normal inferior turbinate tissue rather than the inflamed nasal polyp tissue, we may have obscured our ability to see changes in the type 2 gene signature with dupilumab. Future studies focused specifically on the highly inflamed nasal polyp tissue will allow for greater understanding of dupilumab-induced changes.
Major limitations of our study are that it was an open-label observational study without a placebo arm. However, the efficacy of dupilumab in patients with asthma and CRSwNP is well established.4, 7 Further, the longitudinal clinical and mechanistic repeated measures allowed for each participant to serve as their own control as our goal was to understand the early dupilumab-induced mechanistic changes. Dupilumab leads to rapid improvement in both upper and lower airway symptoms, sense of smell, and lung function, and to reduction in nasal polyp burden in patients with AERD. While there are multiple potential mechanisms by which dupilumab may lead to clinical improvement in patients with AERD, we cannot yet determine which mechanistic changes are the principal drivers of disease resolution, nor which are the result of direct vs indirect effects of IL-4Rα blockade. We conclude that the mechanistic changes underlying the clinical improvements of IL-4Rα inhibition with dupilumab primarily involve effects on tissue mast cells, and possibly other granulocytes, B cells, and epithelial barrier dysfunction.
Supplementary Material
Key Messages:
In an open-label trial of 22 AERD patients treated with dupilumab for three months, dramatic improvements in sense of smell, nasal congestion, nasal polyp size, lung function, asthma control, and quality of life were seen within the first month of therapy.
Baseline loss of smell correlated with lower nasal prostaglandin E2 levels. Dupilumab treatment increased nasal prostaglandin E2, decreased inflammatory eicosanoids, improved the nasal epithelial barrier, and downregulated nasal transcripts involved in epithelial dysfunction, which paralleled the rapid time course of the therapeutic benefits.
The therapeutic effects of dupilumab in AERD are likely due to decreased IL-4Rα signaling on local respiratory tissue granulocytes and epithelial cells.
Funding:
This work was supported by Regeneron, the National Institutes of Health (NIH grant nos U19AI095219, K23AI139352, R01HL128241), and by generous contributions from the Vinik and Kaye Families. J.O.M is a New York Stem Cell Foundation – Robertson Investigator. J.O.M was supported by the Richard and Susan Smith Family Foundation, the AGA Research Foundation’s AGA-Takeda Pharmaceuticals Research Scholar Award in IBD – AGA2020-13-01, the HDDC Pilot and Feasibility P30 DK034854, the Food Allergy Science Initiative, the Leona M. and Harry B. Helmsley Charitable Trust, The Pew Charitable Trusts Biomedical Scholars, and The New York Stem Cell Foundation.
Abbreviations:
- 5-LO
5-lipoxygenase
- ACQ-6
Asthma Control Questionnaire-6
- AERD
aspirin-exacerbated respiratory disease
- COX
cyclooxygenase
- CRSwNP
chronic rhinosinusitis with nasal polyps
- CRTH2
chemoattractant receptor-homologous molecule expressed on Th2 cells
- CysLT
cysteinyl leukotriene
- ESS
endoscopic sinus surgery
- ECP
eosinophilic cationic protein
- FEV1
Forced expiratory volume in 1 second
- FVC
Forced vital capacity
- GO
gene ontogeny
- IL
interleukin
- ILC2
type 2 innate lymphoid cells
- LT
leukotriene
- PG
prostaglandin
- PNIF
peak nasal inspiratory flow
- SNOT
sinonasal outcome test
- TXB2
thromboxane B2
- UPSIT
University of Pennsylvania Smell Identification Test
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: T Laidlaw has served on scientific advisory boards for GlaxoSmithKline, AstraZeneca, Sanofi-Genzyme, and Regeneron. K Buchheit has served on scientific advisory boards for AstraZeneca, Sanofi-Genzyme, Regeneron, and GlaxoSmithKline. J Bensko has served on scientific advisory boards for GlaxoSmithKline. J Ordovas-Montanes. reports compensation for consulting services with Cellarity and Hovione.
References.
- 1.Bachert C, Desrosiers MY, Hellings PW, Laidlaw TM. The Role of Biologics in Chronic Rhinosinusitis with Nasal Polyps. J Allergy Clin Immunol Pract. 2021;9(3):1099–106. [DOI] [PubMed] [Google Scholar]
- 2.Morales DR, Guthrie B, Lipworth BJ, Jackson C, Donnan PT, Santiago VH. NSAID-exacerbated respiratory disease: a meta-analysis evaluating prevalence, mean provocative dose of aspirin and increased asthma morbidity. Allergy. 2015;70(7):828–35. [DOI] [PubMed] [Google Scholar]
- 3.Stevens WW, Peters AT, Hirsch AG, Nordberg CM, Schwartz BS, Mercer DG, et al. Clinical Characteristics of Patients with Chronic Rhinosinusitis with Nasal Polyps, Asthma, and Aspirin-Exacerbated Respiratory Disease. J Allergy Clin Immunol Pract. 2017;5(4):1061–70 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castro M, Corren J, Pavord ID, Maspero J, Wenzel S, Rabe KF, et al. Dupilumab Efficacy and Safety in Moderate-to-Severe Uncontrolled Asthma. N Engl J Med. 2018;378(26):2486–96. [DOI] [PubMed] [Google Scholar]
- 5.Rabe KF, Nair P, Brusselle G, Maspero JF, Castro M, Sher L, et al. Efficacy and Safety of Dupilumab in Glucocorticoid-Dependent Severe Asthma. The New England journal of medicine. 2018;378(26):2475–85. [DOI] [PubMed] [Google Scholar]
- 6.Bachert C, Mannent L, Naclerio RM, Mullol J, Ferguson BJ, Gevaert P, et al. Effect of Subcutaneous Dupilumab on Nasal Polyp Burden in Patients With Chronic Sinusitis and Nasal Polyposis: A Randomized Clinical Trial. JAMA. 2016;315(5):469–79. [DOI] [PubMed] [Google Scholar]
- 7.Bachert C, Han JK, Desrosiers M, Hellings PW, Amin N, Lee SE, et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): results from two multicentre, randomised, double-blind, placebo-controlled, parallel-group phase 3 trials. Lancet. 2019;394(10209):1638–50. [DOI] [PubMed] [Google Scholar]
- 8.Simpson EL, Akinlade B, Ardeleanu M. Two Phase 3 Trials of Dupilumab versus Placebo in Atopic Dermatitis. N Engl J Med. 2017;376(11):1090–1. [DOI] [PubMed] [Google Scholar]
- 9.Hirano I, Dellon ES, Hamilton JD, Collins MH, Peterson K, Chehade M, et al. Efficacy of Dupilumab in a Phase 2 Randomized Trial of Adults With Active Eosinophilic Esophagitis. Gastroenterology. 2020;158(1):111–22 e10. [DOI] [PubMed] [Google Scholar]
- 10.Laidlaw TM, Mullol J, Fan C, Zhang D, Amin N, Khan A, et al. Dupilumab improves nasal polyp burden and asthma control in patients with CRSwNP and AERD. J Allergy Clin Immunol Pract. 2019;7(7):2462–5 e1. [DOI] [PubMed] [Google Scholar]
- 11.Bavaro N, Gakpo D, Mittal A, Bensko JC, Laidlaw TM, Buchheit KM. Efficacy of dupilumab in patients with aspirin-exacerbated respiratory disease and previous inadequate response to anti-IL-5 or anti-IL-5Ralpha in a real-world setting. J Allergy Clin Immunol Pract. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steinke JW, Borish L. Th2 cytokines and asthma. Interleukin-4: its role in the pathogenesis of asthma, and targeting it for asthma treatment with interleukin-4 receptor antagonists. Respir Res. 2001;2(2):66–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Minty A, Chalon P, Derocq JM, Dumont X, Guillemot JC, Kaghad M, et al. Interleukin-13 is a new human lymphokine regulating inflammatory and immune responses. Nature. 1993;362(6417):248–50. [DOI] [PubMed] [Google Scholar]
- 14.Zurawski G, de Vries JE. Interleukin 13, an interleukin 4-like cytokine that acts on monocytes and B cells, but not on T cells. Immunol Today. 1994;15(1):19–26. [DOI] [PubMed] [Google Scholar]
- 15.Ordovas-Montanes J, Dwyer DF, Nyquist SK, Buchheit KM, Vukovic M, Deb C, et al. Allergic inflammatory memory in human respiratory epithelial progenitor cells. Nature. 2018;560(7720):649–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mustafa SS, Vadamalai K, Scott B, Ramsey A. Dupilumab as Add-on Therapy for Chronic Rhinosinusitis With Nasal Polyposis in Aspirin Exacerbated Respiratory Disease. Am J Rhinol Allergy. 2021;35(3):399–407. [DOI] [PubMed] [Google Scholar]
- 17.Buchheit KM, Lewis E, Gakpo D, Hacker J, Sohail A, Taliaferro F, et al. Mepolizumab targets multiple immune cells in aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laidlaw TM, Cahill KN, Cardet JC, Murphy K, Cui J, Dioneda B, et al. A trial of type 12 purinergic (P2Y12) receptor inhibition with prasugrel identifies a potentially distinct endotype of patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2019;143(1):316–24 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laidlaw TM, Kidder MS, Bhattacharyya N, Xing W, Shen S, Milne GL, et al. Cysteinyl leukotriene overproduction in aspirin-exacerbated respiratory disease is driven by platelet-adherent leukocytes. Blood. 2012;119(16):3790–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hopkins C, Gillett S, Slack R, Lund VJ, Browne JP. Psychometric validity of the 22-item Sinonasal Outcome Test. Clin Otolaryngol. 2009;34(5):447–54. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, Derycke L, Holtappels G, Wang XD, Zhang L, Bachert C, et al. Th2 cytokines orchestrate the secretion of MUC5AC and MUC5B in IL-5-positive chronic rhinosinusitis with nasal polyps. Allergy. 2019;74(1):131–40. [DOI] [PubMed] [Google Scholar]
- 23.Ding GQ, Zheng CQ. The expression of MUC5AC and MUC5B mucin genes in the mucosa of chronic rhinosinusitis and nasal polyposis. Am J Rhinol. 2007;21(3):359–66. [DOI] [PubMed] [Google Scholar]
- 24.Viswanathan H, Brownlee IA, Pearson JP, Carrie S. MUC5B secretion is up-regulated in sinusitis compared with controls. Am J Rhinol. 2006;20(5):554–7. [DOI] [PubMed] [Google Scholar]
- 25.Wu X, Peters-Hall JR, Ghimbovschi S, Mimms R, Rose MC, Pena MT. Glandular gene expression of sinus mucosa in chronic rhinosinusitis with and without cystic fibrosis. Am J Respir Cell Mol Biol. 2011;45(3):525–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woodruff PG, Boushey HA, Dolganov GM, Barker CS, Yang YH, Donnelly S, et al. Genome-wide profiling identifies epithelial cell genes associated with asthma and with treatment response to corticosteroids. Proc Natl Acad Sci U S A. 2007;104(40):15858–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brock TG, McNish RW, Peters-Golden M. Arachidonic acid is preferentially metabolized by cyclooxygenase-2 to prostacyclin and prostaglandin E2. The Journal of biological chemistry. 1999;274(17):11660–6. [DOI] [PubMed] [Google Scholar]
- 28.Roca-Ferrer J, Garcia-Garcia FJ, Pereda J, Perez-Gonzalez M, Pujols L, Alobid I, et al. Reduced expression of COXs and production of prostaglandin E(2) in patients with nasal polyps with or without aspirin-intolerant asthma. The Journal of allergy and clinical immunology. 2011;128(1):66–72 e1. [DOI] [PubMed] [Google Scholar]
- 29.Sugiyama E, Taki H, Kuroda A, Mino T, Yamashita N, Kobayashi M. Interleukin-4 inhibits prostaglandin E2 production by freshly prepared adherent rheumatoid synovial cells via inhibition of biosynthesis and gene expression of cyclo-oxygenase II but not of cyclo-oxygenase I. Ann Rheum Dis. 1996;55(6):375–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Han R, Smith TJ. T helper type 1 and type 2 cytokines exert divergent influence on the induction of prostaglandin E2 and hyaluronan synthesis by interleukin-1beta in orbital fibroblasts: implications for the pathogenesis of thyroid-associated ophthalmopathy. Endocrinology. 2006;147(1):13–9. [DOI] [PubMed] [Google Scholar]
- 31.Narayanankutty A, Resendiz-Hernandez JM, Falfan-Valencia R, Teran LM. Biochemical pathogenesis of aspirin exacerbated respiratory disease (AERD). Clin Biochem. 2013;46(7–8):566–78. [DOI] [PubMed] [Google Scholar]
- 32.Rastogi S, Willmes DM, Nassiri M, Babina M, Worm M. PGE2 deficiency predisposes to anaphylaxis by causing mast cell hyperresponsiveness. J Allergy Clin Immunol. 2020;146(6):1387–96 e13. [DOI] [PubMed] [Google Scholar]
- 33.Jiang Y, Kanaoka Y, Feng C, Nocka K, Rao S, Boyce JA. Cutting edge: Interleukin 4-dependent mast cell proliferation requires autocrine/intracrine cysteinyl leukotriene-induced signaling. J Immunol. 2006;177(5):2755–9. [DOI] [PubMed] [Google Scholar]
- 34.Heavey DJ, Ernst PB, Stevens RL, Befus AD, Bienenstock J, Austen KF. Generation of leukotriene C4, leukotriene B4, and prostaglandin D2 by immunologically activated rat intestinal mucosa mast cells. J Immunol. 1988;140(6):1953–7. [PubMed] [Google Scholar]
- 35.Christie PE, Tagari P, Ford-Hutchinson AW, Charlesson S, Chee P, Arm JP, et al. Urinary leukotriene E4 concentrations increase after aspirin challenge in aspirin-sensitive asthmatic subjects. Am Rev Respir Dis. 1991;143(5 Pt 1):1025–9. [DOI] [PubMed] [Google Scholar]
- 36.Sestini P, Armetti L, Gambaro G, Pieroni MG, Refini RM, Sala A, et al. Inhaled PGE2 prevents aspirin-induced bronchoconstriction and urinary LTE4 excretion in aspirin-sensitive asthma. Am J Respir Crit Care Med. 1996;153(2):572–5. [DOI] [PubMed] [Google Scholar]
- 37.Torres R, Picado C, de Mora F. The PGE2-EP2-mast cell axis: an antiasthma mechanism. Mol Immunol. 2015;63(1):61–8. [DOI] [PubMed] [Google Scholar]
- 38.Sastre B, del Pozo V. Role of PGE2 in asthma and nonasthmatic eosinophilic bronchitis. Mediators Inflamm. 2012;2012:645383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sturm EM, Schratl P, Schuligoi R, Konya V, Sturm GJ, Lippe IT, et al. Prostaglandin E2 inhibits eosinophil trafficking through E-prostanoid 2 receptors. J Immunol. 2008;181(10):7273–83. [DOI] [PubMed] [Google Scholar]
- 40.Ta V, White AA. Survey-Defined Patient Experiences With Aspirin-Exacerbated Respiratory Disease. J Allergy Clin Immunol Pract. 2015;3(5):711–8. [DOI] [PubMed] [Google Scholar]
- 41.Gudziol V, Michel M, Sonnefeld C, Koschel D, Hummel T. Olfaction and sinonasal symptoms in patients with CRSwNP and AERD and without AERD: a cross-sectional and longitudinal study. Eur Arch Otorhinolaryngol. 2017;274(3):1487–93. [DOI] [PubMed] [Google Scholar]
- 42.Wise SK, Laury AM, Katz EH, Den Beste KA, Parkos CA, Nusrat A. Interleukin-4 and interleukin-13 compromise the sinonasal epithelial barrier and perturb intercellular junction protein expression. Int Forum Allergy Rhinol. 2014;4(5):361–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Biewenga J, Stoop AE, van der Heijden HA, van der Baan S, van Kamp GJ. Albumin and immunoglobulin levels in nasal secretions of patients with nasal polyps treated with endoscopic sinus surgery and topical corticosteroids. J Allergy Clin Immunol. 1995;96(3):334–40. [DOI] [PubMed] [Google Scholar]
- 44.Rudack C, Sachse F, Alberty J. Primary role of growth-related oncogene-alpha and granulocyte chemotactic protein-2 as neutrophil chemoattractants in chronic rhinosinusitis. Clin Exp Allergy. 2006;36(6):748–59. [DOI] [PubMed] [Google Scholar]
- 45.Moloney JR, Oliver RT. HLA antigens, nasal polyps and asthma. Clin Otolaryngol Allied Sci. 1980;5(3):183–9. [DOI] [PubMed] [Google Scholar]
- 46.Emery BE, White MV, Igarashi Y, Mullol J, Berkebile C, Peden D, et al. The effect of IL-4 on human nasal mucosal responses. J Allergy Clin Immunol. 1992;90(5):772–81. [DOI] [PubMed] [Google Scholar]
- 47.Corren J, Castro M, O’Riordan T, Hanania NA, Pavord ID, Quirce S, et al. Dupilumab Efficacy in Patients with Uncontrolled, Moderate-to-Severe Allergic Asthma. J Allergy Clin Immunol Pract. 2020;8(2):516–26. [DOI] [PubMed] [Google Scholar]
- 48.Johns CB, Laidlaw TM. Elevated total serum IgE in nonatopic patients with aspirin-exacerbated respiratory disease. Am J Rhinol Allergy. 2014;28(4):287–9. [DOI] [PubMed] [Google Scholar]
- 49.Hayashi H, Mitsui C, Nakatani E, Fukutomi Y, Kajiwara K, Watai K, et al. Omalizumab reduces cysteinyl leukotriene and 9alpha, 11beta-prostaglandin F2 overproduction in aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2016;137(5):1585–7 e4. [DOI] [PubMed] [Google Scholar]
- 50.Lang DM, Aronica MA, Maierson ES, Wang XF, Vasas DC, Hazen SL. Omalizumab can inhibit respiratory reaction during aspirin desensitization. Ann Allergy Asthma Immunol. 2018;121(1):98–104. [DOI] [PubMed] [Google Scholar]
- 51.Kanaoka Y, Boyce JA. Cysteinyl leukotrienes and their receptors: cellular distribution and function in immune and inflammatory responses. Journal of immunology. 2004;173(3):1503–10. [DOI] [PubMed] [Google Scholar]
- 52.Fujieda S, Matsune S, Takeno S, Ohta N, Asako M, Bachert C, et al. Dupilumab efficacy in chronic rhinosinusitis with nasal polyps from SINUS-52 is unaffected by eosinophilic status. Allergy. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Laidlaw TM, Prussin C, Panettieri RA, Lee S, Ferguson BJ, Adappa ND, et al. Dexpramipexole depletes blood and tissue eosinophils in nasal polyps with no change in polyp size. Laryngoscope. 2019;129(2):E61–E6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


