Abstract
Rationale:
The 2010 Deepwater Horizon (DWH) oil spill response and cleanup (OSRC) workers were exposed to airborne total hydrocarbons (THC), benzene, toluene, ethylbenzene, o-, m-, and p-xylenes and n-hexane (BTEX-H) from crude oil and PM2.5 from burning/flaring oil and natural gas. Little is known about asthma risk among oil spill cleanup workers.
Objectives:
We assessed the relationship between asthma and several oil spill-related exposures including job class, THC, individual BTEX-H chemicals, the BTEX-H mixture, and PM2.5 using data from the GuLF Long-Term Follow-up Study, a prospective cohort of 24,937 cleanup workers and 7,671 nonworkers following the DWH disaster.
Methods:
Our analysis largely focused on the 19,018 workers without asthma before the spill who had complete exposure, outcome, and covariate information. We defined incident asthma 1–3 years following exposure using both self-reported wheeze and self-reported physician diagnosis of asthma. THC and BTEX-H were assigned to participants based on measurement data and work histories while PM2.5 used modeled estimates. We used modified Poisson regression to estimate risk ratios (RR) and 95% confidence intervals (CIs) for associations between spill-related exposures and asthma and a quantile-based g-computational approach to explore the joint effect of the BTEX-H mixture on asthma risk.
Results:
Oil spill workers had greater asthma risk than nonworkers (RR: 1.60, 95% CI: 1.38, 1.85). Higher estimated THC exposure levels were associated with increased risk in an exposure-dependent manner (linear trend test p<0.0001). Asthma risk also increased with increasing exposure to individual BTEX-H chemicals and the chemical mixture: A simultaneous quartile increase in the BTEX-H mixture was associated with an increased asthma risk of 1.45 (95% CI: 1.35,1.55). With fewer cases, associations were less apparent for physician-diagnosed asthma alone.
Conclusions:
THC and BTEX-H were associated with increased asthma risk defined using wheeze symptoms as well as a physician diagnosis.
Keywords: asthma, BTEX-H, volatile organic compounds, total hydrocarbons, oil spills, mixtures
INTRODUCTION
Following the 2010 Deepwater Horizon (DWH) disaster, which released ~4.9 million barrels of crude oil, concerns were raised for the short- and long- term health of the tens of thousands of oil spill response and cleanup (OSRC) workers who aided in cleanup efforts on land and on water. OSRC workers were exposed to airborne contaminants, including volatile and non-volatile petroleum hydrocarbons, directly from the leaking crude oil and from the combustion of crude oil stemming from removal efforts of burning/flaring oil and natural gas. Observational epidemiologic studies of health effects following other oil spill exposures have found that cleanup workers are at higher risk of adverse respiratory health effects (1). However, most studies have not had quantitative measures of oil spill exposures, and none have assessed specific chemical components related to obstructive pulmonary disease.
Volatile components of liquid crude oil are commonly referred to as total hydrocarbons (THC). We follow here this naming convention, although our measurements represent measured total petroleum hydrocarbons (TPH). TPH includes only those hydrocarbons commonly found in crude oil that are liquids in their pure state at ambient temperatures (2–4). This chemical mixture can contaminate the environment through the production and use of petroleum products. Additionally, the general population can be exposed to THC from cigarette smoke (5), traffic pollution (6), and from off-gassing of building materials, paint, and furniture in indoor environments (7–10). While health effects of THC are variable and depend on dose, duration, and the specific chemicals comprising THC, limited studies suggests that inhalation exposures to certain THC mixtures may lead to adverse respiratory health outcomes (11).
A notable subset of the THC volatiles DWH OSRC workers were exposed to included benzene, toluene, ethylbenzene, o-, m-, and p-xylenes, and n-hexane, collectively referred to here as BTEX-H. These chemicals have been classified as hazardous air pollutants according to the US Clean Air Act. BTEX-H chemicals have been linked with adverse health outcomes including cancer and lung disease (12, 13). Some studies suggest BTEX-H exposure can lead to adverse respiratory health outcomes such as asthma (14), reduced lung function (15) and lung inflammation (16), adverse respiratory symptoms (17), and emergency department visits for asthma (18). However, findings on the link between BTEX-H and respiratory health are mixed and no studies have assessed this in the context of oil spill cleanup related exposures. A systematic review of the effects of VOCS (which included BTEX-H chemicals) on asthma development and exacerbation found an equal number of studies showing increased risks of adverse outcomes as studies showing no adverse effects (19). Authors of that review highlighted the limited quality of existing studies, citing inadequate personal air sampling as well as a lack of adjustment for confounders. Further, no prior studies have considered the total effect of BTEX-H as a mixture, which is of increasing interest in exposure-health assessments, despite their typical co-occurrence in the environment (20). In addition to crude oil, these OSRC workers were exposed to PM2.5, a US EPA criteria air pollutant and well-established risk factor for asthma (21). PM2.5 is thought to induce proinflammatory effects in the respiratory tract (22). Evidence linking PM2.5 to asthma has been demonstrated in both general population studies of the ambient environment (23, 24) and in occupational settings (25–27). However, asthma risks linked to PM2.5 vary by its composition (28, 29). Consequently, despite established links in other settings, little is known about asthma risks associated with the PM2.5 generated from DWH oil burning/flaring activities.
To address gaps in the literature on asthma following oil spill cleanup-related exposures, we evaluated the primary inhalation hazards experienced by OSRC workers following the DWH disaster, including THC, individual BTEX-H chemicals, BTEX-H as a mixture, and PM2.5 from the burning/flaring oil and gas.
METHODS
Study design and participants
We analyzed data from the Gulf Long-term Follow Up Study (GuLF Study), which included 32,608 adults ≥21 years of age who participated in oil spill response and cleanup work (OSRC workers n=24,937) and those who were trained but not hired (non-workers n=7,671) (30). Participants enrolled by completing a computer assisted telephone interview between March 2011 and March 2013. Interviews collected detailed information on oil spill work histories, demographics, health, and lifestyle characteristics.
Of the 32,608 potentially eligible for inclusion in the analysis, we excluded participants who completed only a brief questionnaire for Vietnamese only speakers (n=999); those who reported an asthma diagnosis prior to April 20, 2010, the onset date of the disaster (n=2,886); and those with missing critical analysis variables (did not report asthma status (n=103), did not report the date of asthma diagnosis (n=403), or reported a diagnosis in 2010 did not report information on the month in which they were diagnosed (n=30)). We also excluded participants with missing information on factors applied to asthma classification (for participants not identified as having asthma using any of the criteria): bronchitis (N=105), emphysema (N=61), smoking status (N=387), or wheeze at either time point (N=407). The overlap among these variables resulted in 916 participants being excluded. We further excluded an additional 2,668 participants missing other covariate information (85% of whom were missing information on smoking pack-years). An additional 152 workers had missing DWH job/activity information. The final analytic sample comprised of N=24,603 (19,018 workers; 5,585 nonworkers). Except for an analysis comparing workers to nonworkers, the primary analytic sample comprised the 19,018 OSRC workers, who had clean-up related exposure estimates and had not been diagnosed with asthma prior to the oil spill. This study was approved by the Institutional Review Board of the National Institute of Environmental Health Sciences (NIEHS). All study participants provided informed consent.
Asthma classification
In the enrollment interview, participants who reported a physician diagnosis of asthma were asked the month and year of, or age at, first diagnosis. Participants were similarly asked about physician diagnoses of bronchitis and emphysema. Participants were also asked whether they experienced wheeze while working on the oil spill, and separately, within 30 days of the enrollment interview. Reponses included “All of the time”, “Most of the time”, “Sometimes”, “Rarely”, and “Never”. We considered a response of “all of the time” or “most of the time” as being positive for wheeze in line with asthma.
Due to concerns of possible underreporting of asthma diagnoses among participants (attributed to lack of health care access), we chose to characterize asthma using both self-reported wheeze and self-reported physician diagnosis of asthma. This choice was supported by comparing differences between those reporting wheeze only and those reporting an asthma diagnosis. On average, compared to those with diagnosed asthma, those reporting wheeze only were slightly younger (40 years vs 42 years); had lower educational attainment (<high school/equivalent 67% vs 50%); and were more likely to self-identify as Black (40% vs 31%).
We defined three groups of incident asthma cases to maximize identification of cases following methods previously reported (31). First, incident asthma cases were identified if participants reported “yes” to “Has a doctor ever told you that you have asthma?” with a date of first diagnosis after April 20, 2010 and “no” to “Has a doctor ever told you that you have emphysema?” and “no” to “Has a doctor ever told you that you have bronchitis?” (N=127). Second, to identify potential undiagnosed cases, we included participants who: 1) reported wheeze all or most of the time at the time of the spill or within 30 days prior to enrollment; 2) were never smokers; and 3) did not report ever having a doctor diagnosed chronic bronchitis or emphysema (N=826). Finally, because asthma, bronchitis, and emphysema can co-occur, we included a group of participants who reported a physician-diagnosis of incident asthma and either bronchitis or emphysema if they were never smokers (N=30). The non-cases comprised those who did not report incident asthma or wheeze all or most of time at the time of the spill or within 30 days prior to enrollment. Using this definition, we identified 983 individuals with asthma representing incident cases arising between the spill and the date of interview (1–3 years after the oil spill).
Work status and job classification
Participants were classified as OSRC workers (yes/no) based on whether they worked ≥ 1 day on oil spill cleanup efforts as reported at the enrollment interview (32). Since participants reported working multiple DWH jobs, participants were classified hierarchically by the type of work performed such that they were assigned to one of six job classes that had the highest approximate exposure to THC based on industrial hygienist review of self-reported job histories and external information on THC exposures during the clean-up effort. Of note, job classes with lower average THC exposure could have had other relevant respiratory exposures. The six job categories (ordered from highest to lowest exposed) were: response, operations, cleanup on water, decontamination, cleanup on land, and support work (32).
Total hydrocarbons and BTEX-H exposure estimates
THC and BTEX-H exposure estimates were derived from > 28,000 full-shift personal air samples taken with passive organic vapor dosimeters at the time of OSRC efforts and analyzed for THC (measured as total petroleum hydrocarbons in ppm-days) and BTEX-H (in ppb-days). A full description of the exposure assessment can be found elsewhere (32) and in the Supplement.
Burning/flaring oil and natural gas and modeled PM2.5
During the spill, mitigation efforts included 3 activities that generated exposures to crude oil combustion byproducts: 1) Burning/flaring oil and natural gas at the wellhead (referred to as the ‘hot zone’, 2) in-situ burning of oil, and 3) diesel fuel exhaust from vessel and mechanical equipment engines on water and land. Industrial hygienists characterized in ordinal categories workers’ potential exposure to the former two based on geographic areas of work in relation to burning/flaring activities. Workers were considered to have ‘high’ exposure if they worked on a vessel in the hot zone during the time that flaring occurred; ‘medium’ if they worked on a vessel within approximately 5 nautical miles of the hot zone (‘source’ area) and reported seeing burning, and ‘low’ if they worked on a vessel that burned or helped burn oil in situ (32). Due to the inability to assign diesel exhaust exposures, the third PM2.5 source was not considered. In addition to ordinal categories of potential burning/flaring oil/gas exposures we also considered modeled PM2.5 (μg/m3-days) exposure estimates. A detailed account of modeled PM2.5 data is described elsewhere (33) and in the Supplement.
Covariates
We selected covariates using a directed acyclic graph (34). The covariates used were the minimally sufficient adjustment set identified by the DAG (Supplemental Figure 1). Detailed information on covariates can be found in the Supplement.
Statistical analysis
We generated descriptive statistics for characteristics of workers in the analytic sample as well as for workers compared to nonworkers. We also calculated Pearson coefficients for correlations between cumulative exposure metrics of 1) individual BTEX-H chemicals and 2) cumulative exposure metrics Cum1max THC and the individual BTEX-H chemicals where, as described in the Supplement, Cum1max is the sum of maximum daily job/activity-specific exposures across the time-period worked. We used multivariable modified Poisson regression with robust error variance to calculate risk ratios (RR) and 95% confidence intervals (CIs) for associations between individual oil spill exposures (work status, hierarchical job class, THC, BTEX-H, ordinal burning/flaring oil/gas categories, and PM2.5) and incident asthma. Modified Poisson was chosen over log binomial regression due to model convergence issues (35). Primary models adjusted for all covariates, while secondary models investigating exposure to THC and BTEX-H additionally adjusted for exposure to burning/flaring oil/gas and models of either the ordinal burning/flaring oil or quantitative PM2.5 exposures were adjusted for THC. Models using information on either ordinal burning/flaring of oil and gas or PM2.5 did not include N=389 participants due to missing information on this exposure. For BTEX-H chemicals, we assessed both 5 single chemical models as well as the mixture of all 5 BTEX-H chemicals using modified Poisson regression and quantile-based g-computation (see Supplement for details on method) to estimate a joint exposure-response for all chemicals in the mixture effects (36). To complement reporting of risks with specific oil spill components we conducted a linear trend test with p<0.05 as the threshold for statistical significance. We performed descriptive analyses and individual BTEX-H chemical analyses using SAS version 9.4 (SAS Institute, Inc., Cary NC). We carried out the mixtures analysis using R version 3.5.2 with R package ‘qgcomp’.
In sensitivity analyses we restricted to workers with no exposure to burning/flaring oil/gas (N=16,880) due to the small number of workers exposed. We also tested associations using an alternative THC exposure metric (Cum2ave), which summed the average of the exposure estimates of multiple tasks within a day across the entire work period, to assess a different exposure mechanism. We also conducted analyses excluding subjects reporting wheeze only at the time of the spill (N=18,320) because it is possible that irritation or other stressors at time of cleanup work led people to conflate other respiratory symptoms with wheeze. Finally, we repeated associations with asthma defined only as physician-diagnosed asthma after the spill (N=127) (i.e., without consideration of wheeze reporting).
RESULTS
Select characteristics of workers in the analytic sample are summarized in Table 1. Characteristics comparing workers versus nonworkers are summarized in Supplemental Table 1. Workers were on average 42 years old and predominantly White (66%), non-Hispanic (93%) and male (83%) with highest educational attainment of some college/2-year degree or less (75%). While 53% of workers were never-smokers, the average pack-years among ever smokers was 16 (SD:18). At the enrollment interview, a small percentage reported having had a physician diagnosis of bronchitis (5%) or emphysema (1%). Some participants had worked on other oil spill cleanup efforts (13%) or had previous experience in the oil industry (16%).
Table 1.
Characteristics workers in analytic sample (N=19,018)
| Characteristic | Mean(SD) |
|---|---|
| Age, years | 42(12) |
| Pack-years (among smokers) | 16(18) |
| N(%) | |
| Gender | |
| Female | 3,300(17) |
| Male | 15,718(83) |
| Race | |
| White | 12,629(66) |
| Black | 4,448(23) |
| Other | 1,941(10) |
| Hispanic ethnicity | |
| Hispanic | 1,290(7) |
| Non-Hispanic | 17,728(93) |
| Highest educational attainment | |
| Less than high school/equivalent | 2,924(15) |
| High school diploma/GED | 5,639(30) |
| Some college/2-year degree | 5,681(30) |
| 4-year college graduate or more | 4,774(25) |
| Lifetime smoking quantity | |
| Heavy current | 2,036(10) |
| Light current | 3,935(21) |
| Former | 2,979(16) |
| Never | 10,068(53) |
| Self-reported physician diagnosis of bronchitis | |
| Yes | 925(5) |
| No | 18,093(95) |
| Self-reported physician diagnosis of emphysemab | |
| Yes | 176(1) |
| No | 18,841(99) |
| Previous oil cleanup work | |
| Yes | 2,433(13) |
| No | 16,585(87) |
| Previous oil industry work | |
| Yes | 2,990(16) |
| No | 16,028(84) |
Excluding all workers with self-reported physician’s diagnosis of asthma prior to oil spill date
N=1 missing information on self-reported physician diagnosis of emphysema (but included in complete case analysis based on asthma classification)
Pearson correlation coefficients of Cum1max THC and individual BTEX-H chemicals were high (r=0.72 to 0.86) (Supplemental Table 2a). Among BTEX-H chemicals, correlations were also generally high (r=0.49 to 0.96) (Supplemental Table 2b). Distributions of quantitative exposure metrics are shown in Table 2. All chemical distributions demonstrated a right skew, whereby the median value was less than the mean value for each chemical.
Table 2.
Distributions of total hydrocarbons and BTEX-H cumulative quantitative exposure estimates (N=19,018)a
| Exposure (units) | Mean | SD | Min | 25th percentile | 50th percentile | 75th percentile | Max |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Total hydrocarbons, ppm-days | 101.43 | 135.74 | 0.01 | 11.79 | 50.40 | 135.44 | 1243.76 |
| Benzene, ppb-days | 360.66 | 489.99 | 0.01 | 44.07 | 184.52 | 492.44 | 7744.08 |
| Toluene, ppb-days | 1271.01 | 1538.86 | 0.14 | 155.25 | 724.24 | 1886.73 | 18067.89 |
| Ethylbenzene, ppb-days | 276.23 | 367.81 | 0.005 | 37.43 | 152.69 | 380.84 | 8225.82 |
| Xylenes, ppb-days | 1748.60 | 1734.43 | 2.35 | 550.24 | 1240.47 | 2402.48 | 24413.06 |
| n-Hexane, ppb-days | 1214.47 | 3405.28 | 0.06 | 73.31 | 294.80 | 1167.89 | 62438.68 |
Cumulative quantitative exposure estimates were generated by summing over maximum daily exposure estimates (i.e. when individuals had multiple jobs/activities in a given day, the estimate for the exposure group with the highest estimated exposure)
Associations of asthma with work status, hierarchical job class, and Cum1max THC are shown in Tables 3a and 3b. We found that working on the oil spill cleanup effort in any capacity was associated with a higher risk of incident asthma (adjusted RR: 1.60, 95% CI: 1.38, 1.85). Compared with support workers, asthma risk was increased in all other hierarchal job classes, with the highest risks seen for “operations” (RR: 4.29; 95% CI: 3.16,5.82) and “response” (RR: 3.80, 95% CI: 2.42,5.99). Compared to the lowest THC quintile, those in the highest THC quintile had an increased risk for asthma of 2.95 (95% CI: 2.33, 3.74). A linear trend was observed for increasing THC across quintiles, with p<0.0001 for the fully adjusted model (Model 2).
Table 3a.
Associations between oil spill cleanup work and incident asthma among GuLF Study workers vs. non-workers (N=24,603)a
| Asthma n(% total cases) | Risk RatioCRUDE (95% CI) | Risk RatioMODEL1(95% CI)b | |
|---|---|---|---|
| OSRC work | |||
| No | 196(17) | Referent | Referent |
| Yes | 983(83) | 1.47(1.26,1.71) | 1.60(1.38,1.85) |
Excluding all workers with self-reported physician’s diagnosis of asthma prior to oil spill date
OSRC= Oil spill response and cleanup
Model 1: age, gender, race, ethnicity, smoking status, pack-years, previous oil spill cleanup work, previous oil industry experience, education
Table 3b.
Associations between hierarchical job classes, total hydrocarbons, and incident asthma among workers (N=19,018)
| Exposure | Asthma cases n(%) | Risk RatioCRUDE (95% CI) | Risk RatioMODEL1 (95% CI) | Risk RatioMODEL2 (95% CI) |
|---|---|---|---|---|
| Hierarchical job class | ||||
| Support | 47(5) | Referent | Referent | Referent |
| Land cleanup | 122(12) | 3.43(2.47,4.79) | 2.40(1.72,3.35) | 2.41(1.73,3.37) |
| Decontamination | 136(14) | 4.08(2.94,5.66) | 3.13(2.26,4.35) | 3.15(2.27,4.36) |
| Water cleanup | 152(16) | 2.22(1.60,3.06) | 2.39(1.74,3.30) | 2.42(1.75,3.34) |
| Operations | 368(37) | 5.15(3.81,6.95) | 4.29(3.16,5.82) | 4.29(3.16,5.82) |
| Response | 158(16) | 5.23(3.80,7.21) | 4.44(3.22,6.11) | 3.80(2.42,5.99) |
| Total hydrocarbons (Cum1max; ppm-days) | ||||
| Quintile 1 | 97(10) | Referent | Referent | Referent |
| Quintile 2 | 160(16) | 1.65(1.29,2.11) | 1.48(1.16,1.89) | 1.50(1.17,1.92) |
| Quintile 3 | 184(19) | 1.90(1.49,2.42) | 1.74(1.36,2.21) | 1.76(1.38,2.25) |
| Quintile 4 | 230(23) | 2.37(1.88,2.99) | 2.18(1.72,2.75) | 2.15(1.70,2.72) |
| Quintile 5 | 312(32) | 3.22(2.57,4.02) | 2.99(2.37,3.76) | 2.95(2.33,3.74) |
Model 1: age, gender, race, ethnicity, smoking status, pack-years, previous oil spill cleanup work, previous oil industry experience, education
Model 2: Model 1+ burning/flaring oil/gas (ordinal categories)
Associations between asthma and individual BTEX-H and burning/flaring oil/gas exposures are shown in Table 4. In fully adjusted models for each individual BTEX-H chemical, the highest versus lowest quartile was associated with increased risk for asthma in an exposure-dependent manner (p-test for linear trend <0.0001 for each chemical), even after adjustment for ordinal categories of burning/flaring oil/gas.
Table 4.
Associations of BTEX-H, burning/flaring oil/gas, PM2.5 with incident asthma among GuLF Study workers (N=19,018)
| Exposure | Asthma cases n (%) | Risk RatioCRUDE (95% CI) | Risk RatioMODEL1 (95% CI) | Risk RatioMODEL2 (95% CI)a |
|---|---|---|---|---|
| Benzene (ppb-days) | ||||
| Quartile 1 | 129(13) | Referent | Referent | Referent |
| Quartile 2 | 231 (24) | 1.79(1.45,2.21) | 1.60(1.30,1.98) | 1.60(1.30,1.98) |
| Quartile 3 | 267(27) | 2.07(1.68,2.54) | 1.97(1.60,2.40) | 1.91(1.55,2.35) |
| Quartile 4 | 356(36) | 2.76(2.26,3.36) | 2.71(2.22,3.32) | 2.67(2.17,3.28) |
| p-trend <0.0001 | ||||
| Toluene (ppb-days) | ||||
| Quartile 1 | 130(13) | Referent | Referent | Referent |
| Quartile 2 | 214(22) | 1.65(1.33,2.04) | 1.50(1.22,1.85) | 1.49(1.21,1.84) |
| Quartile 3 | 248(25) | 1.91(1.55, 2.35) | 1.86(1.51,2.29) | 1.80(1.46,2.23) |
| Quartile 4 | 391(40) | 3.01(2.48,3.65) | 3.01(2.47,3.68) | 2.99(2.44,3.66) |
| p-trend <0.0001 | ||||
| Ethylbenzene (ppb-days) | ||||
| Quartile 1 | 132(13) | Referent | Referent | Referent |
| Quartile 2 | 225(23) | 1.70(1.38,2.10) | 1.53(1.24,1.89) | 1.55(1.26,1.91) |
| Quartile 3 | 279(28) | 2.11(1.72,2.59) | 1.91(1.56,2.34) | 1.91(1.56,2.34) |
| Quartile 4 | 347(35) | 2.63(2.16,3.20) | 2.69(2.20,3.28) | 2.66(2.16,3.27) |
| p-trend <0.0001 | ||||
| Xylenes (ppb-days) | ||||
| Quartile 1 | 151(15) | Referent | Referent | Referent |
| Quartile 2 | 194(20) | 1.28(1.04,1.58) | 1.24(1.01,1.51) | 1.25(1.02,1.53) |
| Quartile 3 | 252(26) | 1.67(1.37,2.03) | 1.56(1.29,1.90) | 1.56(1.28,1.90) |
| Quartile 4 | 386(39) | 2.56(2.13,3.07) | 2.62(2.18,3.15) | 2.62(2.16,3.17) |
| p-trend <0.0001 | ||||
| n-Hexane (ppb-days) | ||||
| Quartile 1 | 125(13) | Referent | Referent | Referent |
| Quartile 2 | 248(25) | 1.98(1.61,2.45) | 1.66(1.34,2.05) | 1.66(1.34,2.05) |
| Quartile 3 | 272(27) | 2.18(1.77,2.68) | 2.08(1.69,2.56) | 2.08(1.69,2.56) |
| Quartile 4 | 338(34) | 2.70(2.21,3.31) | 2.79(2.27,3.43) | 2.71 (2.19,3.37) |
| p-trend <0.0001 | ||||
| Potential exposure to burning oil/gas (ordinal categories) | ||||
| No | 822(83) | Referent | Referent | Referent |
| Low/Medium | 123(13) | 1.62(1.35,1.95) | 1.48(1.25,1.77) | 1.08(0.90,1.29) |
| High | 21(2) | 2.22(1.47,3.35) | 1.67(1.16,2.40) | 1.36(0.94,1.96) |
| PM2.5 (μg/m3) | ||||
| Non-water workers | 546(56) | 1.12(.98,1.29) | 0.87(0.76,1.00) | 1.18(1.02,1.37) |
| Low-exposed water workers and In situ (10 μg/m3) | 306(31) | Referent | Referent | Referent |
| Source (29 μg/m3) and Hot zone (97 μg/m3) | 131(13) | 1.73(1.41,2.13) | 1.37(1.12,1.68) | 1.16(0.95,1.42) |
Models with ordinal burning/flaring oil/gas are missing N=389 participants due to lack of information on this exposure
Model 1: age, gender, race, ethnicity, smoking, pack-years, previous oil spill cleanup work, previous oil industry experience, highest educational attainment
Model 2: Model 1+ potential exposure to burning/flaring crude oil for THC analyses (or THC for ordinal categories of burning/PM2.5 analyses)
In addition, participants with the highest potential exposure to burning/flaring oil/gas based on industrial hygiene review had an elevated risk (RR: 1.67, 95% CI: 1.16, 2.40) for asthma compared with the unexposed workers prior to adjustment for THC but risk was attenuated after adjustment (RR: 1.36(0.94,1.96) (Table 4). We saw increased asthma risk associated with the maximum 12-hour PM2.5 exposure (RR: 1.37, 95% CI: 1.12, 1.68) prior to THC adjustment (Model 1). After adjustment for THC, the risk was attenuated to 1.16 (95% CI: 0.95,1.42) (Model 2).
The overall BTEX-H mixture was associated with an estimated forty five percent increase in asthma risk per quartile (RR per quartile: 1.45, 95% CI: 1.35–1.55). Table 5 shows the joint effects of the BTEX-H mixture on asthma risk for each higher quartile compared to the lowest quartile. Of the chemicals positively weighted in the overall mixture effect, the largest positive weight was for toluene while of those negatively weighted, the largest negative weight was for ethylbenzene (Figure 1).
Table 5.
Joint effects of BTEX-H mixture on asthma risk among GuLF Study workers (N=19,018)
| BTEX-H Mixture (ppb-days) | Risk Ratio (95% CI)a |
|---|---|
| Quartile 1 | Referent |
| Quartile 2 | 1.45(1.36,1.54) |
| Quartile 3 | 2.10(1.86,2.38) |
| Quartile 4 | 3.05(2.53,3.67) |
| Overall average per quartile increase | 1.45(1.35,1.55) |
Adjusted for age, gender, race, ethnicity, smoking, pack-years, previous oil spill cleanup work, previous oil industry experience, highest educational attainment
FIGURE 1. Results from quantile g-computational BTEX-H mixture analysis with asthma risk.
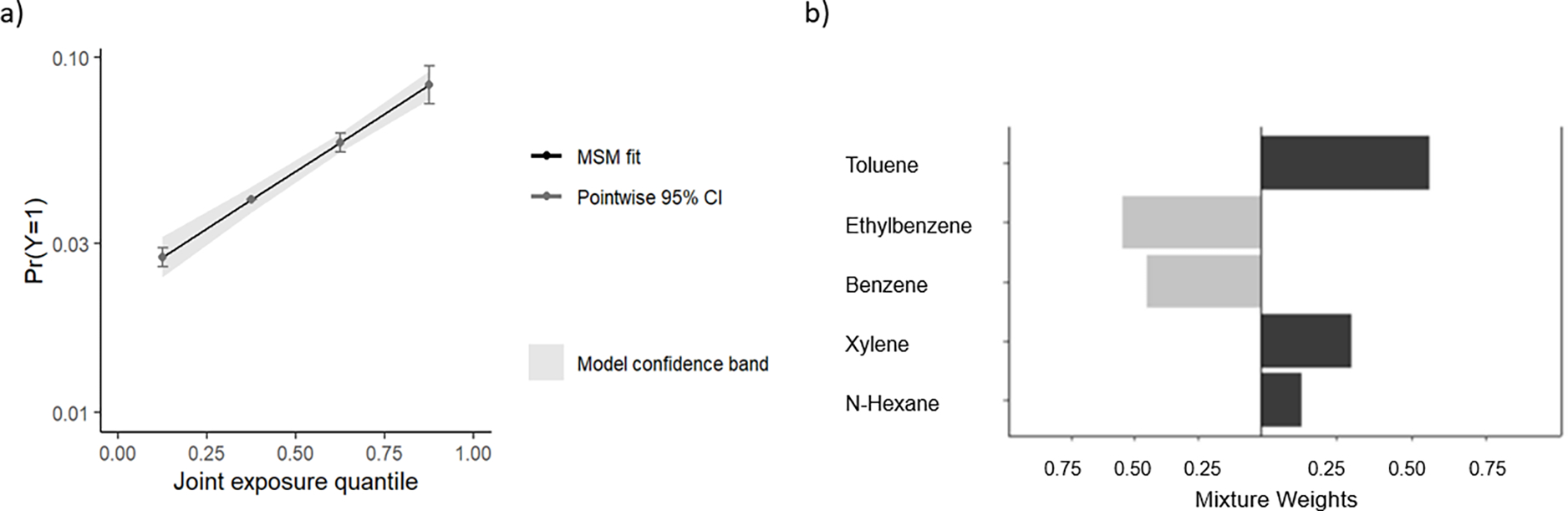
a) Shows the marginal structural model (MSM) fit (dark gray) for the association between increasing mixture exposure quartiles and asthma risk. The x-axis represents quartiles of the BTEX-H mixture and the y-axis represents the log odds of asthma. b) Weight representing contribution to the mixture effect of each chemical component. When all components are considered together, the relative contribution of toluene, xylene, and n-hexane have the greatest contribution to the mixture effect. Coefficients for each chemical component from the underlying model are as follows: Benzene (Beta:−0.07, SE:0.08, p-value:0.36), Toluene (Beta:0.34, SE:0.08, p-value:1.60 × 10−5), Ethylbenzene (Beta:−0.11, SE:0.09, p-value: 0.23), Xylene (Beta:0.19, SE:0.08, p-value:0.02), n-Hexane (Beta:0.08, SE:0.06, p-value:0.20).
Secondary analyses excluding those with exposure to burning/flaring oil/gas exposures showed no substantive differences (reflected in similar point estimates and confidence intervals) for associations between asthma with both THC and BTEX-H (Supplemental Table 4). Sensitivity analyses assessing an alternative THC exposure metric, Cum2ave, also showed associations similar to those in the primary analysis (Supplemental Table 5). Additional sensitivity analyses restricting to those who did not report wheeze at the time of the spill also showed similar associations seen in the primary analysis (Supplemental Tables 6 and 7).
In analyses where asthma was defined based only on a physician’s diagnosis associations between asthma and oil spill exposures were largely attenuated. Associations with toluene and n-hexane remained, but exposure-responses were no longer apparent for any associations, although the sample sizes were much smaller compared to the primary analysis (Supplemental Table 8).
DISCUSSION
In this study, we assessed the relationship between asthma incidence and oil spill exposures among OSRC workers following the DWH disaster. We observed increased risks of asthma—where asthma was defined either by wheeze and or a physician’s diagnosis—associated with exposures to THC and individual BTEX-H chemicals, even after adjusting for exposure to burning/flaring oil/gas. We also observed that exposure to particulates from burning/flaring, estimated both ordinally and quantitatively, was associated with increased wheeze-defined asthma risk.
In sensitivity analyses that included only those with physician-diagnosed asthma, associations between asthma and THC or the BTEX-H chemicals, were largely attenuated. This difference in findings suggests that the observed associations may be driven largely by wheeze symptoms rather than asthma per se, or may reflect both differences between those with and without access to medical care, and a true undercounting of clinical asthma in this population.
The literature on health effects of oil spills is limited. We were able to find only one study of oil spill-related BTEX exposures and asthma, which focused on exposure and asthma among children living near the Hebei oil spill (14). While the physiology of disease differs between children and adults, and there were likely differences in exposure levels and duration (including possible dermal exposure), our results generally agreed (both in direction and magnitude of association) with the Hebei Spirit study findings, in which the highest versus lowest BTEX-exposed children were 1.9 (95% CI: 1.1–3.1) times as likely to develop asthma.
Epidemiologic studies in other occupational settings also generally support the link between BTEX-H and indicators of asthma (37). In occupational settings, researchers have observed respiratory impairment (measured as respiratory symptoms and percent predicted values for forced expiratory and forced vital capacity) linked with BTEX-H among paint manufacturing workers (38–40). In the general population, studies have found increased asthma and wheeze (14, 41), reduced lung function (15), lung inflammation (16), and emergency department visits for asthma (18) associated with higher levels of various BTEX-H chemicals compared to lower levels. Other studies found insufficient evidence of a link between some BTEX exposures and physician diagnosed asthma or symptoms of asthma (19, 41) or reduced pulmonary function (42). Discrepancies in findings across studies may be attributable to differences in study populations, limited exposure measures, and limited control of confounding.
There is also support for this link from animal studies, which have shown that exposure to toluene, for example, can induce an inflammatory response (43), as well as activate immune functions including CD4+T cells and neurotrophin production (44). Other animal studies suggest oxidative stress mechanisms may also be involved (45–47).
We employed a g-Computation approach to estimate a joint exposure-response for all chemicals in the BTEX-H mixture. Results from this approach can be interpreted as a generalized linear regression model (here, a log-binomial model) with a coefficient that in our case corresponds to the expected change in the log-odds outcome per simultaneous single quartile increase in all exposures. We assumed linearity and additivity on the log-odds scale.
The g-computational method we used quantifies which components contribute either more, or less to the total observed mixture risk relative to the other components evaluated. In our case, weights showed that toluene had the largest positive weight while benzene showed the largest negative weight. In the underlying model, coefficients for singular components suggest that toluene (Beta: 0.34, SE:0.08, p-value=1.5 × 10−5) was a significant contributor, while benzene (Beta: −0.07, SE:0.08, p-value=0.36) and ethylbenzene (Beta: −0.11, SE:0.09; p-value=0.23) were not.
One explanation for this is that, based on the air monitoring data from the time of cleanup, toluene was shown to be higher by weight and by volume than benzene in the crude oil mixture (48). This could mean that in the g-computation model, a one quartile increase in benzene does not contribute greatly to asthma as compared to toluene, due to the larger absolute increase in exposure level represented by toluene. It is also possible the difference is due to an artefact of the proportions of measurements below the limit of detection for benzene and ethylbenzene (which are higher than that of toluene) used in the exposure prediction model (49). This could lead to greater measurement uncertainty. It is also possible that, if there is a true dose-response association between benzene, g-computation method may smooth benzene’s potential effect at the highest level of exposure due to the skew in the exposure distribution.
The discrepancy in results between these approaches may be a result of the high correlation between chemicals which is one motivation behind using mixture analyses. Notably, ethylbenzene and xylene, both individually associated with asthma risk, were very highly correlated in our sample (r=0.96) but had opposite contributions due to much smaller contribution of ethylbenzene to THC compared to xylene. The high correlation is expected because both chemicals have the same molecular weight and virtually the same vapor pressure.
Strengths
This study has several strengths. Of note, our study estimated relationships between asthma risk and a BTEX-H mixture, which to our knowledge has not been assessed in prior studies. Assessment of health effects related to mixtures has been identified as being crucial in understanding realistic exposure scenarios given the oftentimes high correlation chemicals in the environment or workplace; it is thought to identify risk factors more accurately for diseases with environmental origins (20, 36) and single pollutant models can be misleading (36). Another strength is the statistical power afforded by the large study sample and detailed covariate data, which increased our ability to detect differences in asthma risk and control for numerous potential confounders. The exposure metrics were based on air monitoring data at the time of exposure which improves upon exposure definitions found in other oil spill studies (1, 50). It is also important to note that exposure assessment methods used in this study are consistent with the state-of-the-art industrial hygiene approaches and the amount of exposure monitoring data used to inform estimates is unprecedented. Finally, our asthma characterization evaluated incidence which is preferred over prevalence estimates.
Limitations
While our study reports novel associations of oil spill cleanup related exposures with respect to asthma risk, this same attribute limits the generalizability of our study results to other THC or BTEX-H exposure situations. The generalizability of our findings may be limited due to the unique exposures faced by the GuLF Study workers, though job-related health risks may inform future targeted interventions to further protect cleanup workers in a similar scenario. The characterization of asthma employed the use of both self-reported wheeze and self-reported physician diagnosis of asthma (51). Though similar approaches have been used in other studies (31, 52, 53), this asthma definition may suffer from misclassification.
A large majority of those defined as having asthma in our study (87% (856/983) of identified cases) did not have a physicians’ diagnosis. This definition may have overestimated the incidence of true asthma in our population. On the other hand, asthma incidence based on a physician’s diagnosis would underestimate the true asthma incidence, given this cohort’s limited access to healthcare and likely differential access among participants to that care. While, we do not have external validation of our case definition, we have previously shown in a subset of this population that those with high versus low THC exposure had suggestively lower lung function in line with obstructive lung disease patterns (51).
Self-reported cigarette smoking was also used in analyses and while proven valid (54), may still contribute to bias from residual confounding by those misrepresenting lifestyle behaviors. Our study could also suffer bias from unmeasured confounding (such as from lack of information on environmental smoking exposure, a primary source of BTEX-H) that could explain the larger effect sizes seen in our study compared to other studies that were able to account for environmental tobacco exposure. Effect magnification has also been raised as a concern for novel associations when exposures are rare, and sample sizes are small (55) –although these concerns probably don’t apply to our study.
Limitations of THC and BTEX-H exposure characterization include lack of individual monitoring results for many persons in our study. Although the >28,000 samples were collected on the DWH OSRC workers, the numbers of measurements were insufficient to develop participant-based exposure estimates. Therefore, the measurements were grouped based on oil spill job or activity via a job-exposure matrix and assigned to study participants based on their self-reported jobs/activities (32, 48). As a result, we expect some measurement error/uncertainty in our estimates, but not likely bias (32). For THC and BTEX-H exposures we reported p-values <0.0001 associated with a linear test for trend. We conducted a linear test for trend specifically, based on initial monotonic patterns displayed by the effect estimates. We recognize that this does not necessarily mean that a dose-response truly exists but believe this is supportive evidence in favor of such a relationship. For PM2.5 exposures, we did not observe an exposure-response pattern with effect estimates for the asthma outcomes. This may be explained by limited power to detect differences in the high category, or by the fact that those exposed to burning/flaring may also have been the healthiest prior to the spill (healthy worker effect). Performing a complete-case analysis of participants excluded on missing data that are not missing at random could induce selection bias. However, characteristics of those included in the analysis did not differ substantively compared to characteristics of those without full covariate information.
Conclusions
We assessed the asthma risk among OSRC workers exposed to THC, BTEX-H and PM2.5. We observed that THC, the individual BTEX-H chemicals, and the BTEX-H mixture were each associated with increased risk of asthma in an exposure-dependent manner. Our study provides the first evidence of increased asthma risk associated with exposures to individual crude oil components and the BTEX-H mixture following the DWH disaster.
Supplementary Material
ACKNOWLEDGEMENTS
Author would like to thank Mark Bodkin for the data management of this project.
Funding sources
This research was funded by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences (Z01 ES 102945).
Footnotes
Conflict of Interest
All authors report no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Aguilera F, Méndez J, Pásaro E, Laffon B. Review on the effects of exposure to spilled oils on human health. Journal of Applied Toxicology. 2010;30(4):291–301. [DOI] [PubMed] [Google Scholar]
- 2.Health) NNIfOSa. NIOSH method 1501 (hydrocarbons, aromatic) NIOSH manual of analytical methods. 4th edition. 1994. [Available from: https://www.cdc.gov/niosh/docs/2003-154/pdfs/1501.pdf. [Google Scholar]
- 3.Health) NNIfOSa. NIOSH method 1500 (hydrocarbons, BP 36°– 216°C). NIOSH manual of analytical methods. 4th edition. 1994. [Google Scholar]
- 4.Health) NNIfOSa. NIOSH method 1550 (naphtha’s). NIOSH manual of analytical methods. 4th edition. 1994. [Google Scholar]
- 5.Wallace L, Pellizzari E, Hartwell TD, Perritt R, Ziegenfus R. Exposures to benzene and other volatile compounds from active and passive smoking. Archives of Environmental Health: An International Journal. 1987;42(5):272–9. [DOI] [PubMed] [Google Scholar]
- 6.Cozzarelli IM, Baehr A. Volatile fuel hydrocarbons and MTBE in the environment. TrGeo. 2003;9:612. [Google Scholar]
- 7.Dehghani MH, Baghani AN, Fazlzadeh M, Ghaffari HR. Exposure and risk assessment of BTEX in indoor air of gyms in Tehran, Iran. Microchemical journal. 2019;150:104135. [Google Scholar]
- 8.Baltrėnas P, Baltrėnaitė E, Šerevičienė V, Pereira P. Atmospheric BTEX concentrations in the vicinity of the crude oil refinery of the Baltic region. Environmental monitoring and assessment. 2011;182(1–4):115–27. [DOI] [PubMed] [Google Scholar]
- 9.Wallace LA. The exposure of the general population to benzene. Cell Biol Toxicol. 1989;5(3):297–314. [DOI] [PubMed] [Google Scholar]
- 10.Wallace LA, Pellizzari ED, Hartwell TD, Davis V, Michael LC, Whitmore RW. The influence of personal activities on exposure to volatile organic compounds. Environmental research. 1989;50(1):37–55. [DOI] [PubMed] [Google Scholar]
- 11.Agency for Toxic Substances and Disease Registry. Toxicological profile for total petroleum hydrocarbons (TPH). 1999. [PubMed]
- 12.Loomis D, Guyton KZ, Grosse Y, El Ghissassi F, Bouvard V, Benbrahim-Tallaa L, et al. Carcinogenicity of benzene. The Lancet Oncology. 2017;18(12):1574–5. [DOI] [PubMed] [Google Scholar]
- 13.Bolden AL, Kwiatkowski CF, Colborn T. New look at BTEX: are ambient levels a problem? Environmental science & technology. 2015;49(9):5261–76. [DOI] [PubMed] [Google Scholar]
- 14.Noh SR, Kim J-A, Cheong H-K, Ha M, Jee Y-K, Park M-S, et al. Hebei Spirit oil spill and its long-term effect on children’s asthma symptoms. Environmental Pollution. 2019;248:286–94. [DOI] [PubMed] [Google Scholar]
- 15.Arif AA, Shah SM. Association between personal exposure to volatile organic compounds and asthma among US adult population. International archives of occupational and environmental health. 2007;80(8):711–9. [DOI] [PubMed] [Google Scholar]
- 16.Greenwald R, Sarnat SE, Raysoni AU, Li W-W, Johnson BA, Stock TH, et al. Associations between source-indicative pollution metrics and increases in pulmonary inflammation and reduced lung function in a panel of asthmatic children. Air Quality, Atmosphere & Health. 2013;6(2):487–99. [Google Scholar]
- 17.Chen L, Hu G, Fan R, Lv Y, Dai Y, Xu Z. Association of PAHs and BTEX exposure with lung function and respiratory symptoms among a nonoccupational population near the coal chemical industry in Northern China. Environment international. 2018;120:480–8. [DOI] [PubMed] [Google Scholar]
- 18.Lemke LD, Lamerato LE, Xu X, Booza JC, Reiners JJ, Raymond DM Iii, et al. Geospatial relationships of air pollution and acute asthma events across the Detroit–Windsor international border: Study design and preliminary results. Journal of exposure science & environmental epidemiology. 2014;24(4):346–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nurmatov UB, Tagiyeva N, Semple S, Devereux G, Sheikh A. Volatile organic compounds and risk of asthma and allergy: a systematic review. European Respiratory Review. 2015;24(135):92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taylor KW, Joubert BR, Braun JM, Dilworth C, Gennings C, Hauser R, et al. Statistical approaches for assessing health effects of environmental chemical mixtures in epidemiology: lessons from an innovative workshop. Environmental health perspectives. 2016;124(12):A227–A9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li T, Hu R, Chen Z, Li Q, Huang S, Zhu Z, et al. Fine particulate matter (PM2. 5): The culprit for chronic lung diseases in China. Chronic diseases and translational medicine. 2018;4(03):176–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nel AE, Diaz-Sanchez D, Li N. The role of particulate pollutants in pulmonary inflammation and asthma: evidence for the involvement of organic chemicals and oxidative stress. Current Opinion in Pulmonary Medicine. 2001;7(1):20–6. [DOI] [PubMed] [Google Scholar]
- 23.Fann N, Lamson AD, Anenberg SC, Wesson K, Risley D, Hubbell BJ. Estimating the national public health burden associated with exposure to ambient PM2. 5 and ozone. Risk Analysis: An International Journal. 2012;32(1):81–95. [DOI] [PubMed] [Google Scholar]
- 24.Hwang S-L, Lin Y-C, Lin C-M, Hsiao K-Y. Effects of fine particulate matter and its constituents on emergency room visits for asthma in southern Taiwan during 2008–2010: A population-based study. Environmental Science and Pollution Research. 2017;24(17):15012–21. [DOI] [PubMed] [Google Scholar]
- 25.Toskala E, Kennedy DW, editors. Asthma risk factors. International forum of allergy & rhinology; 2015: Wiley Online Library. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carlton CS. Personal PM2. 5 exposures for firefighters doing prescribed forest burns in the Southeastern United States: University of Georgia; 2004. [Google Scholar]
- 27.Holt NR, Gao CX, Borg BM, Brown D, Broder JC, Ikin J, et al. Long-term impact of coal mine fire smoke on lung mechanics in exposed adults. Respirology (Carlton, Vic). 2021;26(9):861–8. [DOI] [PubMed] [Google Scholar]
- 28.Lavigne É, Talarico R, van Donkelaar A, Martin RV, Stieb DM, Crighton E, et al. Fine particulate matter concentration and composition and the incidence of childhood asthma. Environment international. 2021;152:106486. [DOI] [PubMed] [Google Scholar]
- 29.Kurai J, Watanabe M, Sano H, Hantan D, Shimizu E. The effect of seasonal variations in airborne particulate matter on asthma-related airway inflammation in mice. International Journal of Environmental Research and Public Health. 2016;13(6):579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kwok RK, Engel LS, Miller AK, Blair A, Curry MD, Jackson WB, et al. The GuLF STUDY: A Prospective Study of Persons Involved in the Deepwater Horizon Oil Spill Response and Clean-Up. Environmental Health Perspectives. 2017;125(4):570–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Young MT, Sandler DP, DeRoo LA, Vedal S, Kaufman JD, London SJ. Ambient Air Pollution Exposure and Incident Adult Asthma in a Nationwide Cohort of U.S. Women. American journal of respiratory and critical care medicine. 2014;190(8):914–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stewart PA, Stenzel MR, Ramachandran G, Banerjee S, Huynh TB, Groth CP, et al. Development of a total hydrocarbon ordinal job-exposure matrix for workers responding to the Deepwater Horizon disaster: The GuLF STUDY. Journal of exposure science & environmental epidemiology. 2018;28(3):223–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pratt GC, Stenzel MR, Kwok RK, Groth CP, Banerjee S, Arnold SF, et al. Modeled Air Pollution from In Situ Burning and Flaring of Oil and Gas Released Following the Deepwater Horizon Disaster. Annals of Work Exposures and Health. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Greenland S, Pearl J, Robins JM. Causal Diagrams for Epidemiologic Research. Epidemiology. 1999;10(1):37–48. [PubMed] [Google Scholar]
- 35.Zou G A modified poisson regression approach to prospective studies with binary data. American journal of epidemiology. 2004;159(7):702–6. [DOI] [PubMed] [Google Scholar]
- 36.Keil AP, Buckley JP, O’Brien KM, Ferguson KK, Zhao S, White AJ. A quantile-based g-computation approach to addressing the effects of exposure mixtures. Environmental health perspectives. 2020;128(4):047004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Delfino RJ. Epidemiologic evidence for asthma and exposure to air toxics: linkages between occupational, indoor, and community air pollution research. Environmental health perspectives. 2002;110(suppl 4):573–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zailina H, Mohd Hafiz Syazwan T, Naing L, Jamal H, Rusli N. Occupational xylene exposure and respiratory impairment of paint manufacturing workers. International Journal of Medicine and Medical Sciences. 2013;5(5):214–20. [Google Scholar]
- 39.Ojo TO, Onayade AA, Akinyemi PA, Adesanmi AJ. Environmental working conditions, lung function and total serum bile acids of spray painters exposed to organic solvents in Ile-Ife, Nigeria. Journal of Health and Pollution. 2017;7(13):2–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gam KB, Kwok RK, Engel LS, Curry MD, Stewart PA, Stenzel MR, et al. Lung Function in Oil Spill Response Workers 1–3 Years After the Deepwater Horizon Disaster. Epidemiology. 2018;29(3):315–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gordian ME, Stewart AW, Morris SS. Evaporative gasoline emissions and asthma symptoms. Int J Environ Res Public Health. 2010;7(8):3051–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elliott L, Longnecker MP, Kissling GE, London SJ. Volatile Organic Compounds and Pulmonary Function in the Third National Health and Nutrition Examination Survey, 1988–1994. Environmental Health Perspectives. 2006;114(8):1210–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tin Tin Win S, Yamamoto S, Nakajima D, Furuyama A, Fukushima A, Ahmed S, et al. Modulation of neurological related allergic reaction in mice exposed to low-level toluene. Toxicology and Applied Pharmacology. 2007;222(1):17–24. [DOI] [PubMed] [Google Scholar]
- 44.Fujimaki H, Win-Shwe T-T, Yamamoto S, Nakajima D, Goto S. Role of CD4+ T cells in the modulation of neurotrophin production in mice exposed to low-level toluene. Immunopharmacology and immunotoxicology. 2009;31(1):146–9. [DOI] [PubMed] [Google Scholar]
- 45.Fujimaki H, Yamamoto S, Hojo R, Sato F, Kunugita N, Arashidani K. Effect of long-term exposure to low-level toluene on airway inflammatory response in mice. Toxicology letters. 2007;168(2):132–9. [DOI] [PubMed] [Google Scholar]
- 46.Wang F, Li C, Liu W, Jin Y. Effect of exposure to volatile organic compounds (VOCs) on airway inflammatory response in mice. Journal of Toxicological Sciences. 2012;37(4):739–48. [DOI] [PubMed] [Google Scholar]
- 47.Wang F, Li C, Liu W, Jin Y. Oxidative damage and genotoxic effect in mice caused by sub-chronic exposure to low-dose volatile organic compounds. Inhalation toxicology. 2013;25(5):235–42. [DOI] [PubMed] [Google Scholar]
- 48.Stenzel MR, Groth CP, Huynh TB, Ramachandran G, Banerjee S, Kwok RK, et al. Exposure Group Development in Support of the NIEHS GuLF Study. Annals of Work Exposures and Health. 2022;66(Supplement_1):i23–i55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stenzel MR, Groth CP, Banerjee S, Ramachandran G, Kwok RK, Engel LS, et al. Exposure Assessment Techniques Applied to the Highly Censored Deepwater Horizon Gulf Oil Spill Personal Measurements. Annals of Work Exposures and Health. 2021;66(Supplement_1):i56–i70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Laffon B, Pasaro E, Valdiglesias V. Effects of exposure to oil spills on human health: Updated review. Journal of toxicology and environmental health Part B, Critical reviews. 2016;19(3–4):105–28. [DOI] [PubMed] [Google Scholar]
- 51.Gam KB, Kwok RK, Engel LS, Curry MD, Stewart PA, Stenzel MR, et al. Exposure to Oil Spill Chemicals and Lung Function in Deepwater Horizon Disaster Response Workers. J Occup Environ Med. 2018;60(6):e312–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carnes MU, Hoppin JA, Metwali N, Wyss AB, Hankinson JL, O’Connell EL, et al. House dust endotoxin levels are associated with adult asthma in a US farming population. Annals of the American Thoracic Society. 2017;14(3):324–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hoppin JA, Umbach DM, London SJ, Henneberger PK, Kullman GJ, Coble J, et al. Pesticide use and adult-onset asthma among male farmers in the Agricultural Health Study. European Respiratory Journal. 2009;34(6):1296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Patrick DL, Cheadle A, Thompson DC, Diehr P, Koepsell T, Kinne S. The validity of self-reported smoking: a review and meta-analysis. American Journal of Public Health. 1994;84(7):1086–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ioannidis JP. Why most discovered true associations are inflated. Epidemiology. 2008;19(5):640–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


